Abstract
Vascular inflammatory disorders are often associated with both decreased NO bioavailability and a lack of responsiveness to NO, a consequence of impaired NO biosynthesis, dysregulated L-arginine metabolism, endothelial nitric oxide synthase (eNOS) uncoupling and NO consumption induced by redox reactions of NO. The latter is mediated via oxidative inflammatory conditions altering NO-dependent endothelial function, including vascular tone and cell proliferation. The redox reactions of NO and byproducts such as nitrite can react to yield electrophilic nitro-fatty acid derivatives (NO2-FA) and exemplifies a biochemical convergence of reactions participating in NO and lipid signaling. NO2-FAs represent a novel therapeutic strategy to treat vascular disorders by improving endothelial dysfunction through enhancing NO signaling and blocking vascular smooth muscle proliferation, inflammation, and maladaptive remodeling.
Introduction
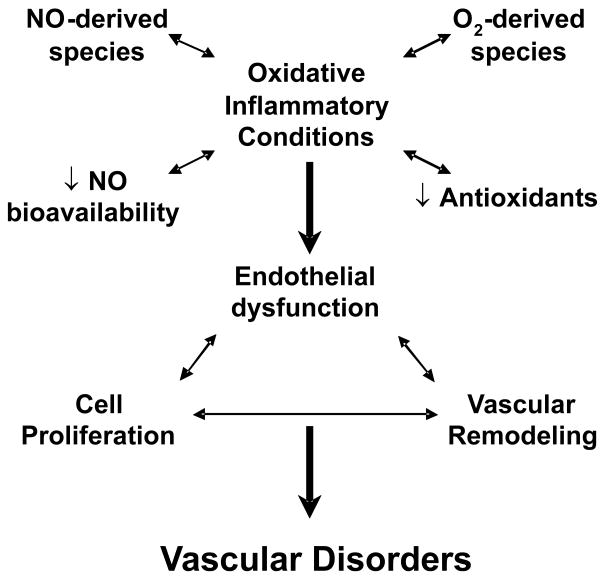
Endothelial dysfunction is a hallmark of vascular inflammatory disorders and plays a central role in mediating structural changes such as lipid accumulation or intimal hyperplasia in the vasculature. Endothelial NO plays a critical role in the regulation of vascular homeostasis by inhibiting inflammatory cell function and smooth muscle proliferation [1, 2]. Oxidative inflammatory conditions, through NO- and O2-derived species, results in oxidative stress, decreased antioxidants, and lower NO bioavailability. This in turn incites a vicious cycle of endothelial dysfunction, vascular cell proliferation and vascular remodeling (Figure 1) increasing susceptibility to atherosclerosis, hypertension, thrombosis, and diabetes mellitus. For example, drugs that make soluble guanylate cyclase (sGC) more responsive to NO and that increase cellular cGMP levels can protect hypoxic mice from developing pulmonary artery hypertension (PAH), but knockout mice lacking eNOS fail to respond as they are incapable of endothelial NO generation [3]. Increasing NO signaling can partially reverse PAH and pulmonary vessel remodeling once PAH has been established [4••, 5••]. Moreover, statins have anti-atherogenic effects mediated, in part, through scavenging superoxide, increasing eNOS expression and NO production, and upregulation of heme oxygenase 1 (HO-1) expression [6, 7]. Since vascular inflammatory disorders are associated with endothelial dysfunction and impaired NO function, the targeting of various aspects of the NO signaling pathway has been proposed as a therapeutic modality. We overview the potential impact of NO2-FA on modulating endothelial gene expression and function as a therapeutic strategy, with these species exhibiting anti-inflammatory cell signaling properties that upregulate HO-1, eNOS and NO production in the vasculature, inhibit VSMC proliferation, and activate PPARγ through Nrf2-dependent and independent processes. Moreover, we address that all of these signaling actions are attributable to the electrophilic nature of NO2-FA.
Figure 1.
Mechanisms for oxidative inflammatory-induced endothelial dysfunction, proliferative effects, and vascular remodeling (vicious cycle) in vascular disorders.
Formation of NO2-FAs
Both NO- and nitrite (NO2−)-derived species yield the nitrating products that mediate the nitration of unsaturated fatty acids [8, 9]. When it was observed that the reaction product of NO and O2.−, peroxynitrite (ONOO−), was a potent biological oxidizing and nitrating agent, the tissue formation and actions of 3-nitro-tyrosine became of interest [10]. The same reactions that nitrate tyrosine also yield NO2-FA, with the prevalence and redundancy of these mechanisms supporting that nitration reactions in part act to transduce NO signaling and tissue inflammatory responses. Both free radical and ionic mechanisms that share nitrogen dioxide (.NO2) as the proximal nitrating species have the ability to generate NO2-FA [11]. NO also rapidly intercepts free and enzyme-bound lipid alkoxyl and peroxyl radicals (k = 2 × 109 M−1s−1) [9, 12] during both autocatalytic and enzymatic fatty acid oxygenation reactions, also yielding nitrated products [9, 13, 14]. Moreover, metabolic, inflammatory and acidic conditions promote fatty acid nitration [15, 16]. For example, cardiac tissue and mitochondrial fatty acid nitration is increased after ischemia-reperfusion (I/R) reactions in vivo [17••]. Importantly, NO2-FA are reversibly-reactive electrophiles that covalently adduct nucleophilic amino acids present in low molecular weight peptides and proteins and that rapidly undergo β-oxidation to shorter chain nitroalkenes and further metabolism to hydroxyl and keto derivatives [17••–19]. Overall, biological fatty acid oxidation and nitration reactions yield an array of NO2-FA regioisomers that display unique chemical reactivities and signaling actions.
Mechanisms of Electrophilic Fatty Acid Reaction
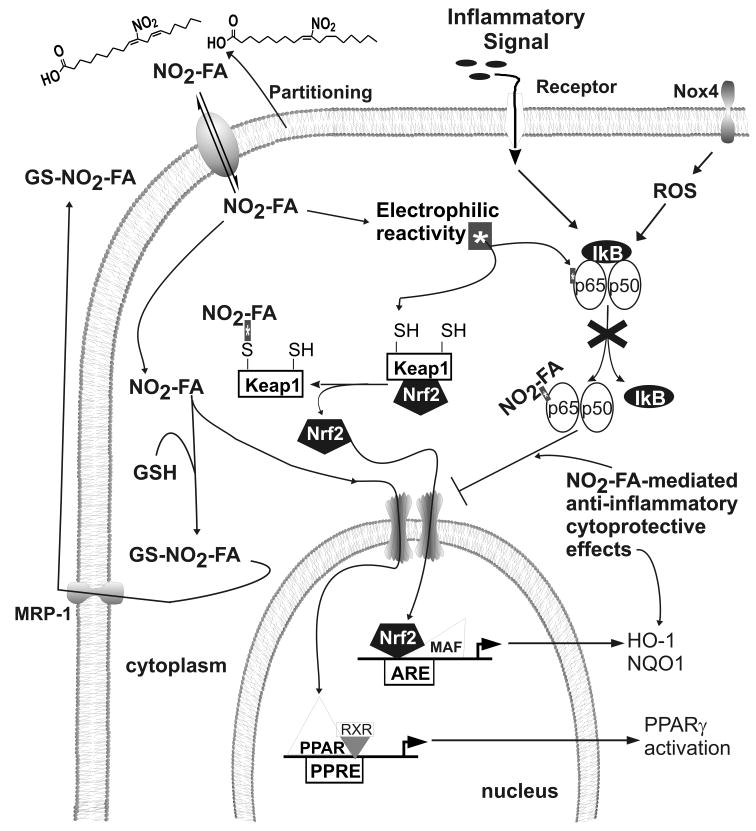
Unsaturated fatty acids can be converted to electrophilic products via enzymatic and non-enzymatic oxidation and nitration reactions [20]. Electrophiles (“electron-lover”) undergo chemical reactions by attacking nucleophiles, accepting an electron pair and forming a chemical bond. Electrophilic species can also be ingested from the diet and are endogenously produced as metabolic byproducts of redox reactions. Some electrophiles permanently and irreversibly modify a target protein and others induce more short-lived and reversible adduction of the target protein [21]. NO2-FAs are Michael acceptors that react with nucleophiles such as the cysteine thiolate, the imidazole moiety of histidine and the ε-amino group of lysine residues. These reactions facilitate the reversible adduction and post-translational modification of proteins to alter structure, trafficking and catalytic activity [20]. Reversibly-reactive electrophiles typically display low or no cytotoxic effects at low concentrations, with these reactions potentially functioning as signaling events that are sensitive to cellular metabolic and redox status. The reversible adduction of cysteine by electrophiles can include inter- or intramolecular exchange reactions between different thiols, with ultimate transfer of the electrophile to GSH and export of the electrophile-GSH adduct from cells through specific multi-drug resistance protein transport mechanisms [22] (Figure 2). This electrophile adduction of GSH can lead to a depletion of GSH pools, thereby altering the redox status of the cell and either directly or indirectly activating compensatory responses (Nrf2 activation) and inducing tissue-protective gene expression. Pharmacologic interventions directed towards activating an integrated system that senses, responds to and controls levels of electrophiles may lead to new strategies for drug discovery and treatment of inflammatory disorders.
Figure 2.
NO2-FA-mediated anti-inflammatory and cytoprotective effects. Electrophilic NO2-FA bind critical nucleophilic residues on p65 and Keap1 thus resulting in inhibition of NFkB-dependent downstream inflammatory signaling and Nrf2-dependent activation of cytoprotective effects, respectively. The disruption of the Keap1/Nrf2 complex by electrophiles leads to Nrf2 translocation to the nucleus forming heterodimeric complexes with small Mafs on the ARE. NO2-FAs bind to PPARγ and partially transactive PPARγ-dependent gene activation. Electrophile-GSH adducts form in the cytoplasm and can be transported out through specific multi-drug resistance proteins (MRP-1).
Electrophile-induced Nrf2 activation
Electrophilic fatty acid derivatives mediate cytoprotective cell signaling reactions via phase 2 gene expression. Biological electrophilic fatty acids, which include NO2-FA, cyclooxygenase-derived 15-deoxy-Δ12,14-PGJ2, a variety of isoprostane derivatives and lipoxygenase-derived α,β-unsaturated ketones [23, 24], are emerging as mediators protectiing against xenobiotic and oxidant injury. The transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2)/Keap1 (Kelch-like ECH-associating protein) pathway mediates phase 2 gene activation. Under normal conditions, Nrf2 localizes to the cytoplasmic supressor protein Keap1 which has several critical cysteine residues that serve as sensors to environmental stresses such as ROS and electrophiles. Keap1 cysteines are oxidized or alkylated, causing a conformational change and liberating Nrf2 to translocate to the nucleus, bind to the cis-acting DNA regulatory antioxidant response element [ARE, also referred to as the electrophile response element (EpRE)], and thereby transactivating Nrf2-dependent gene transcription. This includes enzymes of GSH synthesis and transfer, quinone reductase (NQO1), epoxide hydrolase, thioredoxin, transferrin, catalase, superoxide dismutase, and HO-1 [25]. This widespread mechanism, conserved in both plants and animals, protects against metabolic and inflammatory stress.
NO2-FAs upregulate adaptive protective mediators
NO2-FAs reversibly react with susceptible protein thiols of Keap1 and modulate phase 2 gene expression responses that attenuate vascular anti-inflammatory activity. NO2-FAs induce HO-1, NQO1, and GSH biosynthetic enzyme (GCLM) expression by Nrf2-dependent processes. This induction of HO-1, GCLM and NQO1 mRNA and protein expression was significantly attenuated in cultured human endothelial cells transfected with Nrf2-siRNA [26]. This demonstrates that NO2-FAs mediated induction of tissue-protective responses in endothelial cells is partially regulated by the Nrf2/Keap1 pathway.
NO2-FAs Increase eNOS and HO-1 expression
NO2-FA, as NO and NO2−-derived species, can induce “feedback” regulation of eNOS expression and activity. Administration of OA-NO2 (3 mg/kg/d for 3 d) to mice via a subcutaneously-implanted osmotic mini-pump resulted in an 8-fold increase in plasma OA-NO2 levels compared to oleic acid (OA) controls. OA-NO2 increased aortic eNOS and HO-1 mRNA expression 3-fold, as determined by multiplex real time PCR analysis. Cultured endothelial cells responded similarly to NO2-FAs added to the culture medium, displaying increased eNOS and HO-1 mRNA and protein expression [27, 28]. Additionally, OA-NO2 increased the release of NO in endothelial cells [27]. Thus NO2-FAs induce signaling reactions that increase eNOS–dependent NO production and HO-1 expression, both in vitro and in vivo.
NO2-FAs inhibit VSMC proliferation
NO2-FA inhibited serum-induced VSMC proliferation in a dose-dependent manner with native fatty acids having no effect. Analysis of cell-cycle protein expression revealed upregulation of the cyclin-dependent kinase inhibitor p27kip1 without affecting expression levels of cyclin D1 and E or cyclin-dependent kinase 4. Knock-down of Nrf2 using a si-RNA approach abolished NO2-FA-mediated growth inhibition in VSMCs and the upregulation of p27kip1 protein expression. Conversely, Ad.Nrf2 increased p27kip1 and ectopic expression of Keap1 attenuated the upregulation of p27kip1 by Ad.Nrf2 in a dose-dependent manner [29••]. These data support that NO2-FAs inhibit VSMC proliferation and that this action is, in part, dependent on Nrf2 activation.
NO2-FAs are partial PPAR agonists
Peroxisome proliferator-activating receptor (PPAR) agonists have been plagued by adverse side effects. Partial PPAR agonists that retain their efficacy without adverse side effects appear to be the next generation of signaling activators [30]. One such mechanism of partial PPAR activation focuses on differential recruitment of coactivators and/or corepressors to the receptor, resulting in a tissue-and promoter-selective expression of specific target genes. NO2-FAs and keto-fatty acid derivatives have high binding affinities for all three PPAR isotypes, with PPARγ the most robustly-activated receptor (followed by α and then δ) [31, 32•]. Reporter cell transactivation studies revealed that different regioisomers of NO2-FA behave as partial agonists [33•]. Receptor-ligand-binding analysis indicates that NO2-FA covalently react with the ligand binding domain Cys285 residue of PPARγ in both biochemical reaction systems and cells. While saturation kinetics does not apply to this mode of receptor interaction, comparative studies support a greater EC50 for receptor activation than that of the synthetic PPARγ agonist Rosiglitazone (Rosi) [34•]. NO2-FA transactivation of PPARγ is not induced by similar concentrations of non-electrophilic NO2-FA metabolites, NO donors, native oleic or linoleic acid or oxidized derivatives of these fatty acids in the presence and absence of NO [31]. NO2-FAs act as partial PPARγ agonists in nM ranges, unlike native fatty acid, prostaglandin metabolite and oxidized fatty acid derivatives that only activate PPAR α, γ and δ at non-physiological concentrations of >50 μM [35–37].
Inasmuch as endothelial dysfunction plays a vital role in both systemic and pulmonary vascular diseases [38–40], it is notable that PPARγ agonists increase NO production in EC by post-translational eNOS modifications and not by increased eNOS expression [41–43]. Moreover, endothelial PPARγ−/− mice were hypertensive (significantly higher mean arterial pressure), released less NO, and displayed increased NFkB-DNA binding compared to littermate controls [44]. PPARγ activation is proposed to retard the initiation and development of hypertension [45] and PAH [46] by mechanisms centered on increased NO bioavailability including 1) inhibition of pro-inflammatory NO-consuming signaling reactions, 2) decreased SMC and EC migration and proliferation, and 3) decreased production of reactive species. The therapeutic potential of PPARγ agonists also reveals promise in animal models of PAH and other vascular disorders. For example, Rosi attenuates chronic hypoxia (CH)-induced right ventricular systolic pressure increases (RVSP), right ventricular hypertrophy, vascular remodeling, and Nox4 expression in mice exposed to 10% oxygen for 3–5 weeks. CH-induced Nox4 expression increased superoxide and H2O2 in lung tissue and was blunted by Rosi treatment [47••]. These findings suggest that PPARγ and/or activation by Rosi mediates vascular protective effects by improving endothelial function in part through ROS- and anti-inflammatory-dependent pathways.
NO2-FA suppress pro-inflammatory reactions
Reactive oxygen species induce gene and protein expression by activating transcription factors such as NFkB [48], which in turn play a role in vascular inflammation associated with vascular disorders. Electrophilic NO2-FAs adducted the NFkB p65 subunit, resulting in inhibition of DNA binding and repression of NFkB-dependent target gene expression [49]. Consequently, NO2-FAs attenuate LPS-induced macrophage expression and secretion of pro-inflammatory cytokines, inhibited TNFα-stimulated vascular cell adhesion molecule 1 expression and blocked TNFα-induced adhesion of monocytes to endothelium. Moreover, administration of NO2-FA during or immediately following an ischemic episode induced profound myocardial protection following coronary artery ligation and reperfusion. This protective effect was mediated in part through the inhibition of the p65 subunit of NFkB and the limitation of downstream pro-inflammatory signaling [17••].
Conclusions
NO2-FAs are byproducts of NO and NO2−-dependent oxidative reactions. When exogenously administered at concentrations giving plasma levels 5–10 times greater than found endogenously, these species reduce oxidant stress, inflammation and maladaptive vascular remodeling in a variety of pre-clinical inflammatory injury models. In vitro, NO2-FAs inhibit neutrophil and platelet function, VSMC proliferation, endothelial adhesion molecule expression. LPS-induced macrophage activation and macrophage transmigration [50]. In rodent models the administration of NO2-FAs either by intraperitoneal injection or subcutaneously-implanted osmotic mini-pumps resulted in inhibition of neointimal proliferation following wire-injured vessels [51] and I/R injury to heart and kidney [17••]. Further evaluation of electrophilic nitro- and keto-FAs may reveal a new strategy for limiting the inflammatory reactions and impaired vascular function characteristic of vascular disorders. A broad range of signaling events would be responsible for these effects, since electrophilic lipids act through a multitude of signaling pathways (Figure 2), including increasing eNOS and Nrf2-dependent gene expression, attenuating VSMC proliferation, inhibiting NFkB-induced inflammatory effects and activating PPARγ-dependent gene expression.
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL8115 and HL64937. B.A.F. acknowledges financial interest in Complexa, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Ignarro LJ, Lippton H, Edwards JC, Baricos WH, Hyman AL, Kadowitz PJ, Gruetter CA. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218:739–49. [PubMed] [Google Scholar]
- 2.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–12. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 3.Kobs RW, Chesler NC. The mechanobiology of pulmonary vascular remodeling in the congenital absence of eNOS. Biomech Model Mechanobiol. 2006;5:217–25. doi: 10.1007/s10237-006-0018-1. [DOI] [PubMed] [Google Scholar]
- 4••.Schermuly RT, Stasch JP, Pullamsetti SS, Middendorff R, Muller D, Schluter KD, Dingendorf A, Hackemack S, Kolosionek E, Kaulen C, et al. Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur Respir J. 2008;32:881–91. doi: 10.1183/09031936.00114407. This study demonstrated that idiopathic pulmonary arterial hypertension (IPAH) patients express sGCα1 and β1 proteins in vascular smooth muscle cells of small pulmonary arteries, thus representing a novel potential target for treatment of IPAH. Moreover, BAY 63-2521 activates sGC, mainly through a NO-independent mechanism, and partially reverses right heart hypertrophy and pulmonary vessel remodeling once PAH has been established in both chronic-hypoxic mice and monocrotaline-treated rats (both being well established animal models of PAH) [DOI] [PubMed] [Google Scholar]
- 5••.Dumitrascu R, Weissmann N, Ghofrani HA, Dony E, Beuerlein K, Schmidt H, Stasch JP, Gnoth MJ, Seeger W, Grimminger F, et al. Activation of soluble guanylate cyclase reverses experimental pulmonary hypertension and vascular remodeling. Circulation. 2006;113:286–95. doi: 10.1161/CIRCULATIONAHA.105.581405. Oral administration of soluble guanylate cyclase (sGC) activators attenuated the progression of pulmonary hypertension in monocrotaline-injected rats and chronic hypoxia-exposed (10% O2) mice. Moreover, these sGC activators failed to reverse right ventricular systolic pressure and right heart hypertrophy in the chronic hypoxia-induced eNOS−/− mice. [DOI] [PubMed] [Google Scholar]
- 6.Laufs U. Beyond lipid-lowering: effects of statins on endothelial nitric oxide. European Journal of Clinical Pharmacology. 2003;58:719–731. doi: 10.1007/s00228-002-0556-0. [DOI] [PubMed] [Google Scholar]
- 7.Heeba G, Moselhy ME, Hassan M, Khalifa M, Gryglewski R, Malinski T. Anti-atherogenic effect of statins: role of nitric oxide, peroxynitrite and haem oxygenase-1. British Journal of Pharmacology. 2009;156:1256–1266. doi: 10.1111/j.1476-5381.2009.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubbo H, Darley-Usmar V, Freeman BA. Nitric oxide regulation of tissue free radical injury. Chem Res Toxicol. 1996;9:809–820. doi: 10.1021/tx960037q. [DOI] [PubMed] [Google Scholar]
- 9.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 10.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol Cell Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 11.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d’Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–9. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubbo H, Radi R, Anselmi D, Kirk M, Barnes S, Butler J, Eiserich JP, Freeman BA. Nitric oxide reaction with lipid peroxyl radicals spares alpha-tocopherol during lipid peroxidation. Greater oxidant protection from the pair nitric oxide/alpha-tocopherol than alpha-tocopherol/ascorbate. J Biol Chem. 2000;275:10812–8. doi: 10.1074/jbc.275.15.10812. [DOI] [PubMed] [Google Scholar]
- 13.Gallon AA, Pryor WA. The reaction of low levels of nitrogen dioxide with methyl linoleate in the presence and absence of oxygen. Lipids. 1994;29:171–176. doi: 10.1007/BF02536725. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell VB, Taylor KB, Parthasarathy S, Kuhn H, Koesling D, Friebe A, Bloodsworth A, Darley-Usmar VM, Freeman BA. 15-Lipoxygenase catalytically consumes nitric oxide and impairs activation of guanylate cyclase. J Biol Chem. 1999;274:20083–91. doi: 10.1074/jbc.274.29.20083. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira AM, Ferrari M, Trostchansky A, Batthyany C, Souza JM, Alvarez MN, Lopez GV, Baker PR, Schopfer FJ, O’Donnell V, et al. Macrophage activation induces formation of the anti-inflammatory lipid cholesteryl-nitrolinoleate. Biochem J. 2009;417:223–34. doi: 10.1042/BJ20080701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Donnell VB, Eiserich JP, Chumley PH, Jablonsky MJ, Krishna NR, Kirk M, Barnes S, rley-Usmar VM, Freeman BA. Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem Res Toxicol. 1999;12:83–92. doi: 10.1021/tx980207u. [DOI] [PubMed] [Google Scholar]
- 17••.Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, Baker PR, Ramani R, Freeman BA. Endogenous generation and protective effects of nitro-oleate in a murine model of focal cardiac ischemia and reperfusion. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp275. In press. [Epub ahead of print] This study reveals de novo stimulation of fatty acid nitration in cardiac tissue following ischemia-reperfusion. Exogenous administration of OA-NO2 during an ischemic episode, or even at the time of reperfusion, induced profound myocardial protection following coronary artery ligation and reperfusion. This protective effect was mediated in part through the inhibition of the p65 subunit of NFkB and the limitation of downstream pro-inflammatory signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker LM, Baker PR, Golin-Bisello F, Schopfer FJ, Fink M, Woodcock SR, Branchaud BP, Radi R, Freeman BA. Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J Biol Chem. 2007;282:31085–31093. doi: 10.1074/jbc.M704085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudolph V, Schopfer FJ, Khoo NK, Rudolph TK, Cole MP, Woodcock SR, Bonacci G, Groeger AL, Golin-Bisello F, Chen CS, et al. Nitro-fatty acid metabolome: saturation, desaturation, beta-oxidation, and protein adduction. J Biol Chem. 2009;284:1461–73. doi: 10.1074/jbc.M802298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceaser EK, Moellering DR, Shiva S, Ramachandran A, Landar A, Venkartraman A, Crawford J, Patel R, Dickinson DA, Ulasova E, et al. Mechanisms of signal transduction mediated by oxidized lipids: the role of the electrophile-responsive proteome. Biochem Soc Trans. 2004;32:151–5. doi: 10.1042/bst0320151. [DOI] [PubMed] [Google Scholar]
- 21.Liebler DC. Protein damage by reactive electrophiles: targets and consequences. Chem Res Toxicol. 2008;21:117–28. doi: 10.1021/tx700235t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudolph TK, Freeman BA. Transduction of redox signaling by electrophile-protein reactions. Sci Signal. 2009;2:re7. doi: 10.1126/scisignal.290re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolgar MS, Yang CY, Gaskell SJ. First direct evidence for lipid/protein conjugation in oxidized human low density lipoprotein. J Biol Chem. 1996;271:27999–8001. doi: 10.1074/jbc.271.45.27999. [DOI] [PubMed] [Google Scholar]
- 24.Loidl-Stahlhofen A, Spiteller G. Alpha-Hydroxyaldehydes, products of lipid peroxidation. Biochim Biophys Acta. 1994;1211:156–60. doi: 10.1016/0005-2760(94)90264-x. [DOI] [PubMed] [Google Scholar]
- 25.Holtzclaw WD, Dinkova-Kostova AT, Talalay P. Protection against electrophile and oxidative stress by induction of phase 2 genes: the quest for the elusive sensor that responds to inducers. Adv Enzyme Regul. 2004;44:335–67. doi: 10.1016/j.advenzreg.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Kansanen E, Jyrkkanen H-K, Volger OL, Leinonen H, Kivela AM, Hakkinen S-K, Woodcock SR, Schopfer FJ, Horrevoets AJ, Yla-Herttala S, et al. Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: Identification of heat shock response as the major pathway activated by nitro-oleic acid. J Biol Chem. 2009 doi: 10.1074/jbc.M109.064873. In Press. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoo NKH, Rudolph V, Cole MP, Golin-Bisllo F, Schopfer FJ, Woodcock SR, Batthyany C, Freeman BA. Activation of Vascular Endothelial Nitric Oxide Synthase and Heme Oxygenase-1 Expression by Electrophilc Nitro-Fatty Acids. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2009.10.046. In Press. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright MM, Schopfer FJ, Baker PR, Vidyasagar V, Powell P, Chumley P, Iles KE, Freeman BA, Agarwal A. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proc Natl Acad Sci USA. 2006;103:4299–4304. doi: 10.1073/pnas.0506541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Villacorta L, Zhang J, Garcia-Barrio MT, Chen XL, Freeman BA, Chen YE, Cui T. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol. 2007;293:H770–H776. doi: 10.1152/ajpheart.00261.2007. This study reveals that an Nrf2/Keap1-dependent pathway plays a role in the inhibition of FBS-induced VSMC proliferation. Knock down of Nrf2 using a si-RNA approach abolished NO2-FA-mediated growth inhibition in VSMCs and the upregulation of p27kip1 protein expression. Conversely, Ad.Nrf2 increased p27kip1 and ectopic expression of Keap1 attenuated the upregulation of p27kip1 by Ad.Nrf2 in a dose-dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144:2201–2207. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 31.Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor γ ligand. Proc Natl Acad Sci USA. 2005;102:2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Itoh T, Fairall L, Amin K, Inaba Y, Szanto A, Balint BL, Nagy L, Yamamoto K, Schwabe JWR. Structural basis for the activation of PPARγ by oxidized fatty acids. Nat Struct Mol Biol. 2008;15:924–931. doi: 10.1038/nsmb.1474. Electrophilic oxidized fatty acid derivatives bind to PPARγ (in some cases simultaneously), sense the concentrations of lipid pools, and covalently adduct to the receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Alexander RL, Wright MW, Gorczynski MJ, Smitherman PK, Akiyama TE, Wood HB, Berger JP, King SB, Morrow CS. Differential Potencies of Naturally Occurring Regioisomers of Nitrolinoleic Acid in PPARγ Activation. Biochemistry. 2008;48:492–498. doi: 10.1021/bi8016747. Two regiospecific isomers of LNO2 share similar binding affinities to PPARγ and were ~2-fold higher than that of Rosiglitazone (Rosi) as determined by competition radioligand binding assay. However, transactivation studies demonstrated that the 12-LNO2 derivative had an EC50 of 0.045 μM and similar to Rosi (EC50= 0.067 μM) while the 13-LNO2 had an EC50 value of 0.41 μM, almost a magnitude of order higher. The two LNO2 derivatives bind to PPARg the same but have completely divergent bioactivities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Li Y, Zhang J, Schopfer FJ, Martynowski D, Garcia-Barrio MT, Kovach A, Suino-Powell K, Baker PR, Freeman BA, Chen YE, et al. Molecular recognition of nitrated fatty acids by PPARγ. Nat Struct Mol Biol. 2008;15:865–867. doi: 10.1038/nsmb.1447. This study reveals crystal structure analysis of endogenously-produced nitro-linoleic acid (LNO2) bound to the ligand binding domain of PPARγ. LNO2 covalently reacts with the ligand binding domain Cys285 residue of PPARγ in both biochemical reaction systems and cells and acts as a partial PPARγ agonist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 36.Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, Brown M, Lazar MA. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995;270:23975–83. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 37.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell. 1998;93:229–40. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 38.Vanhoutte PM, Shimokawa H, Tang EHC, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiologica. 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- 39.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–65. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 40.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 41.Cho DH, Choi YJ, Jo SA, Jo I. Nitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment with troglitazone: evidence for involvement of peroxisome proliferator-activated receptor (PPAR) γ-dependent and PPARγ-independent signaling pathways. J Biol Chem. 2004;279:2499–2506. doi: 10.1074/jbc.M309451200. [DOI] [PubMed] [Google Scholar]
- 42.Calnek DS, Mazzella L, Roser S, Roman J, Hart CM. Peroxisome proliferator-activated receptor γ ligands increase release of nitric oxide from endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:52–7. doi: 10.1161/01.atv.0000044461.01844.c9. [DOI] [PubMed] [Google Scholar]
- 43.Polikandriotis JA, Mazzella LJ, Rupnow HL, Hart CM. Peroxisome proliferator-activated receptor γ ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor γ-dependent mechanisms. Arterioscler Thromb Vasc Biol. 2005;25:1810–6. doi: 10.1161/01.ATV.0000177805.65864.d4. [DOI] [PubMed] [Google Scholar]
- 44.Kleinhenz JM, Kleinhenz DJ, You S, Ritzenthaler JD, Hansen JM, Archer DR, Sutliff RL, Hart CM. Disruption of endothelial peroxisome proliferator-activated receptor-γ reduces vascular nitric oxide production. Am J Physiol Heart Circ Physiol. 2009;297:H1647–1654. doi: 10.1152/ajpheart.00148.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPARγ Agonist Rosiglitazone Improves Vascular Function and Lowers Blood Pressure in Hypertensive Transgenic Mice. Hypertension. 2004;43:661–666. doi: 10.1161/01.HYP.0000116303.71408.c2. [DOI] [PubMed] [Google Scholar]
- 46.Matsuda Y, Hoshikawa Y, Ameshima S, Suzuki S, Okada Y, Tabata T, Sugawara T, Matsumura Y, Kondo T. Effects of peroxisome proliferator-activated receptor γ ligands on monocrotaline-induced pulmonary hypertension in rats. Nihon Kokyuki Gakkai Zasshi. 2005;43:283–8. [PubMed] [Google Scholar]
- 47••.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone Attenuates Chronic Hypoxia-induced Pulmonary Hypertension in a Mouse Model. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2008-0132OC. In Press. [Epub ahead of print] PPARγ activation by Rosiglitazone attenuates chronic hypoxia (CH)-induced pulmonary hypertension, right ventricular hypertrophy, and vascular remodeling in mice. The authors demonstrate a novel strategy for attenuating CH-induced proliferative signaling pathways by treatment with Rosiglitazone that in part suppresses NADPH oxidase-derived superoxide and H2O2 generation that stimulates PDGFRβ and inhibits PTEN expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haddad JJ. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal. 2002;14:879–97. doi: 10.1016/s0898-6568(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 49.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, et al. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d’Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cole MP, Rudolph TK, Khoo NKH, Motanya UN, Golin-Bisllo F, Wertz JW, Schopfer FJ, Woodcock SR, Ali MS, Rudolph V, et al. Nitro-Fatty Acid Inhibition of Neointima Formation After Endoluminal Vessel Injury. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.109.199075. In Press. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]