Abstract
Young adult Purkinje cell degeneration (pcd) mutant mice, with complete loss of cerebellar cortical Purkinje cells, are impaired in delay eyeblink classical conditioning. In the delay paradigm, the conditioned stimulus (CS) overlaps and coterminates with the unconditioned stimulus (US), and the cerebellar cortex supports normal acquisition. The ability of pcd mutant mice to acquire trace eyeblink conditioning in which the CS and US do not overlap has not been explored. Recent evidence suggests that cerebellar cortex may not be necessary for trace eyeblink classical conditioning. Using a 500 ms trace paradigm for which forebrain structures are essential in mice, we assessed the performance of homozygous male pcd mutant mice and their littermates in acquisition and extinction. In contrast to results with delay conditioning, acquisition of trace conditioning was unimpaired in pcd mutant mice. Extinction to the CS alone did not differ between pcd and littermate control mice, and timing of the conditioned response was not altered by the absence of Purkinje cells during acquisition or extinction. The ability of pcd mutant mice to acquire and extinguish trace eyeblink conditioning at levels comparable to controls suggests that the cerebellar cortex is not a critical component of the neural circuitry underlying trace conditioning. Results indicate that the essential neural circuitry for trace eyeblink conditioning involves connectivity that bypasses cerebellar cortex.
Eyeblink classical conditioning is of demonstrated utility as a model system for the study of neurobiological mechanisms underlying associative learning and memory. A substantial body of data has demonstrated that the cerebellar interpositus nucleus ipsilateral to the conditioned eye is essential for the acquisition and maintenance of eyeblink conditioning (see Christian & Thompson, 2003 for a review). In eyeblink conditioning, conditioned stimulus (CS) and unconditioned stimulus (US) information are transmitted to the cerebellum via mossy fibers originating in the pontine nuclei and climbing fibers originating in the inferior olive, respectively (Mauk, Steinmetz, & Thompson, 1986; Steinmetz, Rosen, Chapman, Lavond, & Thompson, 1986, Steinmetz et al., 1987, Steinmetz, Lavond, & Thompson, 1989). This CS and US information converge upon (1) Purkinje cells in the cerebellar cortex and (2) the cerebellar interpositus nucleus (Gould, Sears, & Steinmetz, 1993; Steinmetz & Sengelaub, 1992; Thompson, 1986; Tracy, Thompson, Krupa & Thompson, 1998). Repeated pairings of this convergent information are hypothesized to yield robust synaptic plasticity (e.g., long-term depression - LTD; long-term potentiation - LTP) within each cerebellar region, resulting in learning of the contingent CS-US relationship (Hansel, Linden, & D’Angelo, 2001; Linden & Conner, 1995; Nores, Medina, Steele, & Mauk, 2000; Pugh & Raman, 2006). Whereas it is widely-accepted that the cerebellar interpositus nucleus is essential for all forms of eyeblink classical conditioning, there is debate about the role of cerebellar cortical integrity in normal acquisition.
Lesions of the cerebellar cortex have produced dramatically different results, ranging from mild impairments to complete abolition of the eyeblink conditioned response (CR; Lavond, Steinmetz, Yokaitis, & Thompson, 1987, Lavond & Steinmetz, 1989; Yeo, Hardimann, & Glickstein, 1985). A mutant mouse model – the Purkinje cell degeneration (pcd) mouse - has provided valuable data for addressing this debate. Mice homozygous for the pcd mutation are born with Purkinje cells, but by the fourth postnatal week all Purkinje cells have been eliminated (Mullen, Eicher, & Sidman, 1976). Importantly, the integrity of the interpositus nucleus is maintained in these mice (Chen, Bao, Lockard, Kim, & Thompson, 1996). Since Purkinje cells represent the sole output of the cerebellar cortex, these mice exhibit a “functional lesion” of the entire cerebellar cortex that obviates potential methodological pitfalls inherent with traditional lesion methods. Delay eyeblink conditioning – a paradigm in which the CS overlaps and coterminates with the US - is impaired in young adult pcd mice relative to controls, though pcd mice produce low levels of conditioning (Chen et al., 1996). CR levels during delay eyeblink conditioning in pcd mice do not appear to represent pseudoconditioning, as unpaired presentations of the CS and US in pcd mice yield significantly lower CR percentages (< 20%) than counterparts given paired CS-US training (Chen et al., 1999). Further evidence that eyeblink conditioning impairments in pcd mice are associative in nature is provided by standard performance measures, as UR amplitudes (Chen et al., 1996) and tone-induced activity in cochlear nuclei (Chen, Bao, & Thompson, 1999) do not differ between pcd and control mice. Additionally, lesions of the interpositus nucleus in pcd mice abolish conditioned eyeblink responses (Chen et al., 1999). Impairments in delay eyeblink conditioning have also been shown in “waggler”, a mutant mouse which lacks brain-derived neurotrophic factor (BDNF) in cerebellar granule cells but appears normal in morphological features of cerebellar deep nuclei (Bao, Chen, Qiao, Knusel, & Thompson, 1998b). These findings suggest that the cerebellar cortex is normally involved in delay eyeblink conditioning but is not essential.
Similar findings of impaired (but not abolished) eyeblink conditioning in the delay paradigm have been shown in various mutant and transgenic mouse models exhibiting impaired cerebellar cortical LTD. Specifically, glial fibrillary acidic protein (GFAP; Shibuki et al., 1996), PTPMEG (a cytoplasmic protein-tyrosine phosphatase expressed in Purkinje cells; Kina et al., 2007), and δ2 glutamate receptor (GluRδ2; Kakegawa et al., 2008) knockout mice as well as phospholipase C β4 (PLCβ4; Kishimoto et al., 2001a) and metabotropic glutamate receptor 1 (mGluR1; Aiba et al., 1994) mutant mice all show impairments in cerebellar cortical LTD and delay eyeblink conditioning. Recently, Lee, Chatila, Ram, & Thompson (2009) demonstrated impaired retention but unimpaired acquisition in calcium/calmodulin-dependent protein kinase type IV (CaMKIV) knockout mice, behavioral effects that parallel findings of normal acquisition but impaired maintenance of LTD in mice deficient in CaMKIV (Ho et al., 2000). Furthermore, genetically modified mice that exhibit alterations in cerebellar cortical functioning without impairing LTD are unimpaired in delay eyblink conditioning (see Endo et al., 2009; Tanaka et al. 2008). These findings provide substantial evidence that LTD at Purkinje cell synapses is the primary cerebellar cortical mechanism by which normal acquisition and retention of delay eyeblink conditioning is produced and maintained.
Trace eyeblink conditioning is a variant of eyeblink conditioning in which a stimulus free (“trace”) period – usually 250 – 1000 ms (depending on the species that is tested) – occupies the interval between the offset of the CS and the onset of the US. When sufficiently long trace intervals are used, acquisition of trace eyeblink conditioning requires the integrity of forebrain areas such as the hippocampus (Kim, Clark, & Thompson, 1995; Moyer, Deyo, & Disterhoft, 1990; Solomon, Vander Schaaf, Thompson, & Weisz, 1986) and prefrontal cortex (Oswald, Knuckley, Mahan, Snaders, & Powell, 2006; Weible, McEchron, & Disterhoft, 2000) in addition to the cerebellum. Consistent with findings in delay eyeblink conditioning paradigms, lesions of the cerebellar interpositus nucleus abolish trace eyeblink conditioning in rabbits (Pakaprot, Kim, & Thompson, 2009; Woodruff-Pak, Lavond, & Thompson, 1985) whereas lesions of the cerebellar cortex produce only transient impairments in retention of trace eyeblink CRs (Woodruff-Pak et al., 1985). Recent findings in humans further suggest that trace eyeblink conditioning is not dependent on cerebellar cortical integrity, as patients with cerebellar cortical lesions displayed comparable levels of conditioning to normal controls in the acquisition of a forebrain-dependent trace eyeblink conditioning task (Gerwig et al., 2008).
Mounting evidence indicates that the cerebellar cortex may be differentially engaged in delay relative to trace eyeblink conditioning. In a recent study, rats trained in a delay eyeblink conditioning task showed higher levels of metabolic activity in regions of the cerebellar cortex compared to rats trained in a trace eyeblink conditioning task (Plakke, Freeman & Poremba, 2007). Perhaps the most compelling evidence for differential engagement of cerebellar cortical mechanisms between these tasks, however, comes from studies comparing delay and trace eyeblink conditioning in various mutant and transgenic mouse models. Specifically, the aforementioned GluRδ2 knockout and PLCβ4 mutant mice (with selective deficiencies of cerebellar cortical components critical for the induction of LTD at the parallel fiber-Purkinje cell synapse) showed robust impairments in delay eyeblink conditioning while trace eyeblink conditioning was unimpaired (Kishimoto et al., 2001a, b, c). Similarly, a mouse strain with a selective knockout of Purkinje cell Scn8a sodium channels – a condition that disrupts normal firing patterns of Purkinje cells (Raman, Sprunger, Meisler, & Bean, 1997) – was impaired in delay but not in trace eyeblink conditioning (Woodruff-Pak, Green, Levin, & Meisler, 2006). Differences in performance of delay and trace eyeblink conditioning were evident when the tasks were matched for the interstimulus interval (ISI) between CS and US onset (Woodruff-Pak et al., 2006). Additionally, GluRδ2 knockout mice were impaired in delay eyeblink conditioning both when the CS-US interval was short (252 ms) and when it was long (852 ms), but they were not impaired when the CS-US interval was 852 ms long and included a 500 ms trace period (Kishimoto et al., 2001b). These findings suggest that cerebellar cortical integrity is important for delay, but not for trace eyeblink conditioning (see Woodruff-Pak & Disterhoft, 2008 for a review).
The present study used young adult homozygous male pcd mutant mice to assess whether a functional lesion of the entire cerebellar cortex, namely the complete absence of Purkinje cells, is capable of impairing hippocampus-dependent trace eyeblink conditioning (c.f., Tseng, Guan, Disterhoft, & Weiss, 2004). Previous eyeblink conditioning studies with homozygous pcd mutant mice used the delay paradigm (Chen et al., 1996, 1999). Whereas aspects of cerebellar cortical functioning were compromised in transgenic mice previously shown to be unimpaired in trace eyeblink conditioning (Kishimoto et al., 2001a, b, c; Woodruff-Pak et al., 2006), the possibility exists that other, intact, cerebellar cortical functions (or regions) contributed to these high levels of performance. Demonstration of unimpaired trace eyeblink conditioning in pcd homozygous mice would provide considerable support for our contention that the cerebellar cortex is not important for the acquisition and maintenance of trace eyeblink conditioning.
Methods
Subjects
A total of 21 young adult male mice were tested. Nine mice were homozygous pcd mutant mice (Strain name: B6.BR-Agtpbp1pcd/J; complete loss of cerebellar Purkinje cells by the fourth postnatal week) and the remaining 12 mice were littermate wildtype controls of the C57BL/6J strain (Jackson Laboratories). All mice weighed between 16 and 40 grams at the time of surgery, with the pcd mutant mice of notably smaller size and a mean weight of 19.8 (s.d. = 3.7) g. in comparison to the mean of 32.3 (s.d. = 6.9) g. of the wildtype littermates. At 4–5 months of age mice began eyeblink classical conditioning training. Mice were group-housed in standard polycarbonate cages and had ad libitum access to sterile food and water. Room lighting was timed for a 12:12-hr light-dark cycle. All research methods were approved by Temple University’s Institutional Animal Care and Use Committee.
Eyeblink Classical Conditioning
Surgery
All mice received surgery to implant recording and stimulating electrodes for eyeblink classical conditioning. For anesthesia, a “non-rebreathing” Isoflurane administration system was used. Anesthesia was induced in a chamber with O2 + 3% Isoflurane at a flow rate of 1 L per minute. The Isoflurane was then reduced to 2.5% as the mouse was placed on a surgical platform and fitted with a nose cone for anesthesia maintenance throughout the procedure. Opthalmic ointment was applied to each eye to prevent drying, and mice were covered with gauze strips to maintain normal thermoregulation. After a small surgical incision was made to expose the top of the skull, four Teflon-coated stainless steel wires (0.003-in bare, 0.0045-in coated; A-M Systems, Everet, WA) soldered to a four-pin male header (Jameco Electronics, Belmont, CA) were implanted intramuscularly in the orbicularis oculi of the left upper eyelid. Wires were stripped of Teflon and carefully placed such that only the muscle-embedded wire was bare. To ensure that the wires would not move or recede back into the periorbital cavity, wires were glued to the skull. The two wires most rostral were used to record differential electromyography (EMG) activity, and the two most caudal were used to deliver the eyeblink-eliciting stimulus. When all wires were placed, the four-pin headstage was cemented to the skull and the incision was closed. Following surgery, mice were given Baytril antibiotic (85 mg/kg sc) to prevent infection and Buprenex (0.075 mg/kg sc) for analgesia. Mice were allowed a minimum of 5 days to recover from surgery.
Eyeblink conditioning procedure
The conditioned eyeblink training apparatus consisted of four sound- and light-attenuating chambers (Med Associates, St. Albans, Vermont). Each chamber contained a beaker in which the mouse was placed (depth = 3.25″, length and width = 5″), a copper Faraday cage covering the beaker, a ventilation fan, and a wall-mounted speaker. A shielded four-conductor wire was attached to the mouse’s headstage and was used to deliver a blink-eliciting stimulus to the orbicularis oculi and to record EMG activity. EMG activity was passed through a 300–5,000 Hz filter and amplified by 10 K. The signal was then integrated and digitized before being read into a system compatible with IBM (White Plains, New York) described by G. Chen and Steinmetz (1998) for processing. Data were collected in RAM and saved to a hard drive for offline analyses.
A subgroup of the mice (N = 3 pcds; 4 controls) underwent 2 days of pre-training exposure to the experimental apparatus. Pre-training was identical in design to acquisition training (e.g., duration of 1 hour; presentation of background noise by ventilation fan) with the exception that the discrete CS (tone) and US (periorbital shock) were not delivered. Days 3–12 of training for mice that received apparatus pre-exposure training (or days 1–10 for those that did not receive pre-exposure training) were devoted to daily trace eyeblink classical conditioning training. Each training session was controlled by a program written in C + + language (G. Chen & Steinmetz, 1998) and run on an IBM-PC compatible 386 computer. The intertrial interval was random, ranging from 15 s to 30 s at 1-s intervals. Mice were tested in groups of four. Each session lasted approximately 1 hour and mice were allowed to move about freely within the cage during testing. The ventilation fan generated a 70-dB background noise. Each daily session (10 sessions of acquisition total) consisted of 100 trials (presented in blocks of 10). Each 10-trial block consisted of 9 paired trials and 1 CS-alone test trial. Trace eyeblink conditioning paired trials included a 250-ms, 85-dB, 1-kHz tone CS, followed 500 ms after its onset (and 250 ms after its offset) by a 100-ms 0.5 mA DC pulse stimulation US generated by an Isostim™ Stimulator/Isolator (4 separate pulses 20 ms in duration – 5 ms spacing between pulses). The 250 ms period between CS offset and US onset represented a stimulus-free “trace” interval. It was determined by observation that a 0.5 mA stimulus was sufficient to cause a blink/head jerk in all mice. With the exception of four controls, mice underwent five daily extinction trials following acquisition training. During extinction (5 session total), conditions were identical to acquisition with the exception that the US was not presented. Thus, each session of extinction consisted of 100 trials of a 250-ms 85-dB, 1-kHz tone CS with no US presentations.
Each session was computer-scored with a macro written in Visual Basic, which analyzed each trial individually for responses. Whenever EMG activity in the orbicularis oculi exceeded five standard deviations above baseline, a response was considered to have occurred. If a response took place in the first 100 ms prior to the CS onset, then the trial was excluded as a bad trial. A startle (or “alpha”) response was scored if the response occurred in the first 60 ms after CS onset. A CR was scored if a response occurred after the 60 ms startle period and before the US onset (500 ms after CS onset). Responses occurring after US onset were recorded as URs. Data was analyzed with SPSS statistical package with significance levels set at p < .05.
Histology
Following eyeblink training, all mice were deeply anesthetized with isoflurane and perfused through the heart with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were stored in this fixative until sectioning. Shortly before sectioning, brains were transferred to 70% alcohol. Sections were cut in the coronal plane through the entire cerebellum with a Vibratome 3000 Sectioning System (Vibratome Co., St. Louis, MO, US) at a thickness of 70 μm. This yielded 45–55 sections, all of which were mounted on glass slides and stained with Thionin. To identify Purkinje cells in the cerebellar cortex, samples were observed and recorded at 60 × magnification with a Nikon Optiphot 2 light microscope with a motorized stage and an Optronics MicroFire color digital camera (Optronics, Goleta, CA, USA).
Results
The major aim of this study was to examine performance of homozygous male pcd mutant mice and their normal control littermates in a 500 ms trace eyeblink classical conditioning paradigm that was demonstrated to be forebrain-dependent in mice (Tseng et al., 2004; see Figure 1 for sample electromyography recordings from a pcd and a control mouse). Furthermore, CS-alone extinction training was conducted following paired CS-US training as a within-subjects control for non-associative contributions to acquisition training. Previous findings from our laboratory using young adult C57BL/6J mice receiving explicitly unpaired presentations of the CS and US have revealed significant decreases in responding during CS presentations relative to counterparts receiving paired CS-US training (Vogel, Ewers, Ross, Gould, & Woodruff-Pak, 2002).
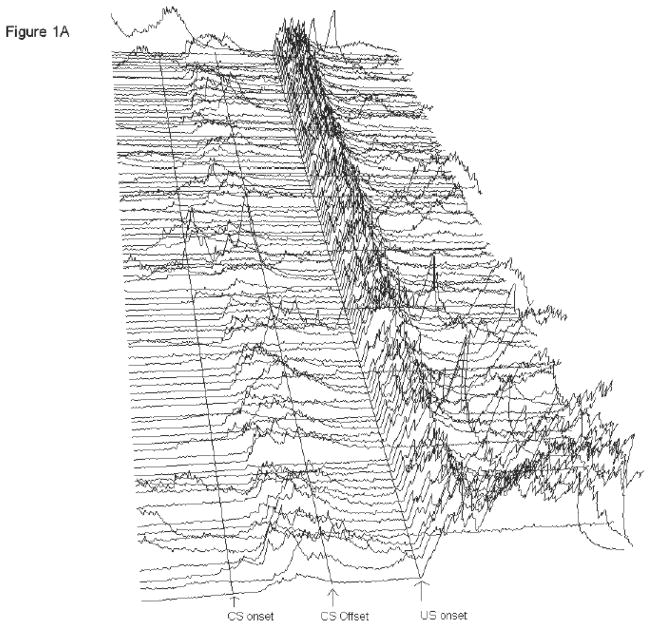
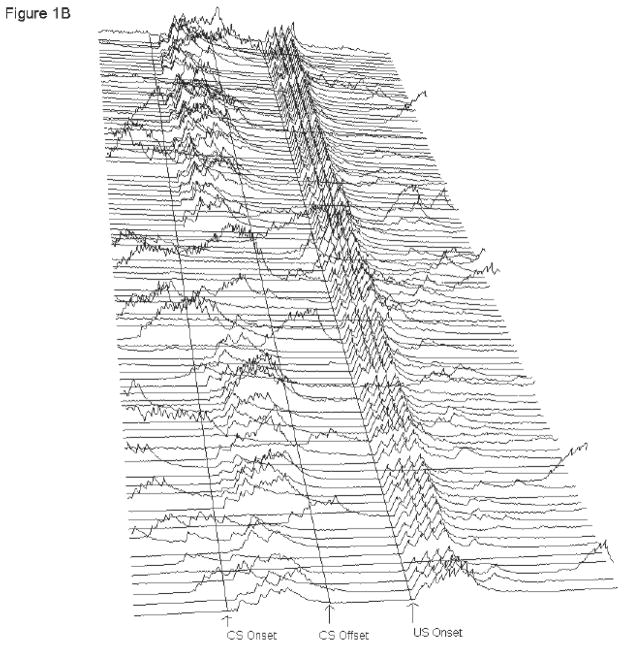
Figure 1.
A and B: Electromyography (EMG) recorded from eye muscles (orbicularis oculi) of the left upper eyelid during trace eyeblink conditioning. Each line represents EMG activity from an individual trial (1–100, with Trial 1 represented at the bottom of each figure and Trial 100 represented at the top of each figure) from Session 6 of 500-ms trace eyeblink classical conditioning. Total trial length was 1,350 ms. Lines are drawn to approximate the onset of the conditioned stimulus (CS) and unconditioned stimulus (US). There were 249 ms in the pre-CS period before CS onset. CS onset is marked, and then there were 500 ms between CS onset and US onset (marked). There were 601 ms in the post-US period. A response was scored if it exceeded mean pre-CS activity by five standard deviations. Performance is shown for a pcd mouse (A) and a wildtype littermate (B) that were representative of their respective groups. For the pcd mouse shown here (A), 90 of the 100 trials were usable for analysis; the remaining 10 trials were excluded due to excessive pre-CS EMG activity. During paired CS-US trials this subject (A) performed 48% conditioned responses (CRs). Short-latency alpha (or ‘startle’) responses (0–60 ms after CS onset) occurred in 3% of the paired CS-US trials. For the wildtype littermate (B), 87 of the 100 trials were usable for analysis. During paired CS-US trials there were 68% CRs. Consistent with the higher percentage of short-latency alpha responses emitted in wildtypes relative to pcds, alpha responses occurred during 54% of the paired CS-US trials.
Acquisition – CR percentage, CR peak amplitude, and CR latency
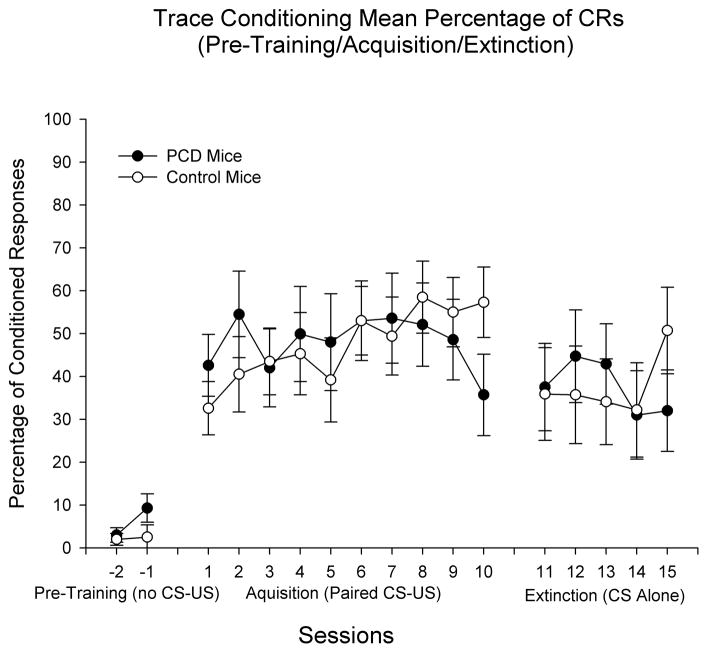
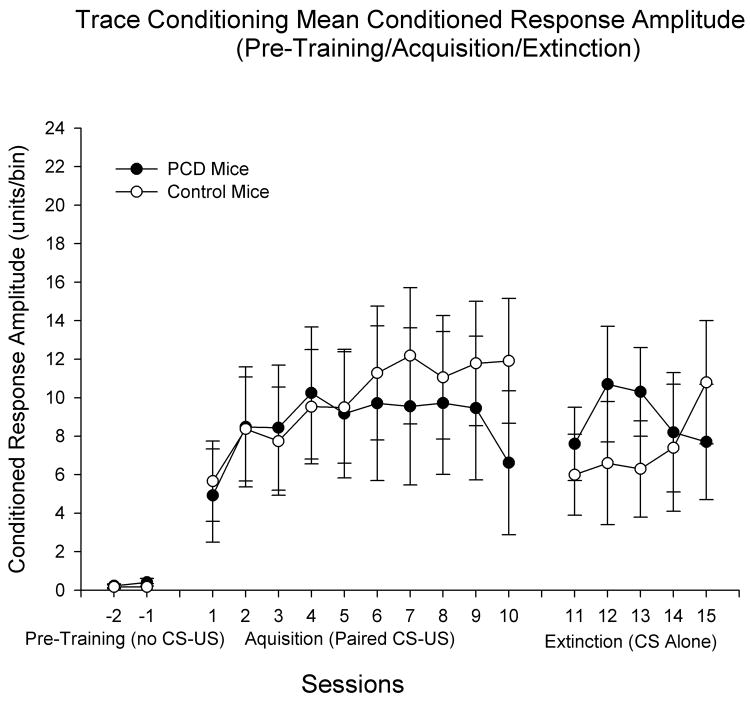
Separate 2 (Genotype) × 10 (Training Sessions) repeated measures ANOVA was performed on the dependent measures of percentage of CRs, amplitude of CRs, and latency of CRs to examine effects of genotype on acquisition of the CR in the 500-ms trace procedure. Homozygous pcd mutant mice did not differ from littermate controls in any of the dependent measures observed. For the percentage of CR measure neither the effect of genotype nor the Genotype × Training Sessions interaction were statistically significant, indicating that mice lacking cerebellar Purkinje cells acquired CRs as well as normal mice (see Figure 2). The effect of sessions was statistically significant (F(9, 171) = 2.107, p < 0.03), indicating that both groups increased the percentage of CRs over the 10-day acquisition period. Similar results were observed in the CR peak amplitude measure, as homozygous pcd mutant mice did not differ from littermates (no significant effects of genotype or Genotype × Training Sessions for CR peak amplitude; see Figure 3). The effect of sessions in the peak amplitude of CR measure was statistically significant (F(9, 171) = 3.053, p < 0.003). These data indicate that pcd mice are not impaired in acquisition of trace eyeblink conditioning. Comparison of the present data with previous studies using unpaired CS and US presentations (CR levels < 20% across training; Bao et al., 1998a; Chen et al., 1996; Tseng et al., 2004) provides further evidence that CR levels during acquisition in the present study reflect associative learning of the CS-US relationship. Separate analyses showed an increase in the percentage of CRs between the first block of training (9 CS-US trials) at Session 1 and the last three blocks of CS-US trials from Session 1 in in pcd subjects: Block 1 = 33% CRs; blocks 8–10 = 49%, 49%, and 40% CRs, respectively. A similar analysis in the controls showed some decrease in in the percentage of CRs between the first and last three trial blocks of Session 1: Block 1 = 39% CRs; blocks 8–10 = 27%, 25%, and 31% CRs, respectively.
Figure 2.
Trace eyeblink classical conditioning in Purkinje cell degeneration (pcd) mutant mice and wildtype littermate control mice. Percentage of conditioned responses (CRs) are displayed across the three phases of training: (1) pre-training (2 sessions) in which the animals were placed into the experimental apparatus without presentations of the CS or unconditioned stimulus (US); (2) acquisition training (10 daily 100-trial sessions) in which the CS preceded and reliably predicted the US; and (3) extinction training (5 daily 100-trial sessions) in which the CS was presented as in acquisition training, but without US presentations. The duration of pre-training sessions was equal to that of acquisition and extinction sessions (approximately 1 hour). CR incidence at all three phases of training does not differ as a function of group. As expected, modest declines in CR incidence are evident during CS-alone extinction training. Data in panels represent means plus and minus the standard error of the mean. The Ns differ in each phase: Phase 1 (pre-training) – N = 3 pcd; 4 control; Phase 2 (acquisition training) – N = 9 pcd; 12 control; Phase 3 (extinction training) – N = 9 pcd; 8 control.
Figure 3.
Trace eyeblink classical conditioning in Purkinje cell degeneration (pcd) mutant mice and wildtype littermate control mice. Peak amplitude of conditioned responses (CRs) are displayed across the three phases of training: (1) pre-training (2 sessions) in which the animals were placed into the experimental apparatus without presentations of the CS or unconditioned stimulus (US); (2) acquisition training (10 daily 100-trial sessions) in which the CS preceded and reliably predicted the US; and (3) extinction training (5 daily 100-trial sessions) in which the CS was presented as in acquisition training, but without US presentations. The duration of pre-training sessions was equal to that of acquisition and extinction sessions (approximately 1 hour). As in the CR percentage measure, CR amplitude does not differ as a function of group in any of the three phases of training. Unlike controls, CR amplitude levels during extinction did not return to initial acquisition levels in pcd mice. Data represent means plus and minus the standard error of the mean. The Ns differ in each phase: Phase 1 (pre-training) – N = 3 pcd; 4 control; Phase 2 (acquisition training) – N = 9 pcd; 12 control; Phase 3 (extinction training) – N = 9 pcd; 8 control.
Measures of CR timing – onset and peak latency – did not differ between homozygous pcd mice and controls (data not shown), consistent with our previous report comparing mice with cerebellar cortical disruption with normal controls (see Woodruff-Pak et al., 2006). The effect of sessions was not statistically significant, indicating that CR latency did not change across sessions.
Extinction – CR percentage, CR peak amplitude, and CR latency
Separate 2 (Genotype) × 5 (Extinction Training Sessions) repeated measures ANOVA was performed on the dependent measures of percentage of CRs, amplitude of CRs, and latency of CRs to examine effects of genotype on extinction of the CR in the 500-ms trace procedure. Similar to effects reported for acquisition, homozygous pcd mutant mice did not differ from littermate controls in percentage of CR during extinction (Figure 2, right panel). Furthermore, the effect of sessions was not statistically significant. During extinction homozygous pcd mutant mice did not differ from littermates in peak amplitude of CR (Figure 3, right panel). As with percentage of CR, in the peak amplitude of CR measure the effect of sessions failed to achieve statistical significance. Consistent with the acquisition data, these findings suggest that pcd mice are not impaired in extinction of forebrain-dependent trace eyeblink conditioning. While neither group showed significant decreases in CR levels across days during CS-alone extinction training, CR levels during extinction in both groups were numerically lower than CR levels during the latter half of acquisition (with the exception of Session 10 for pcds – see Figures 2 and 3). Under the present conditions, additional CS-alone sessions may be necessary to demonstrate statistically significant decreases in CR levels following CS-US acquisition training (see Discussion). The timing of CRs (onset and peak latency) during extinction did not differ between homozygous pcd mice and controls (data not shown) and the effect of sessions was not statistically significant.
Performance Measures
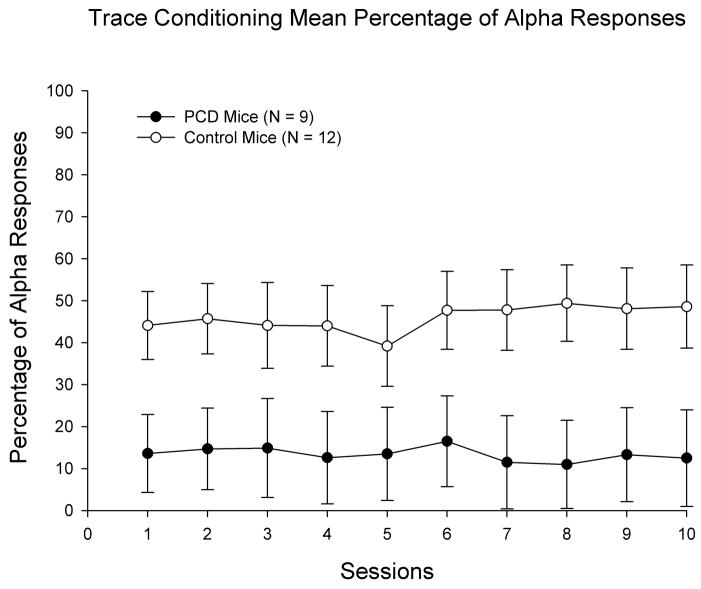
Levels of alpha responding (responses that exceeded threshold within the first 60 ms of CS onset) differed significantly across groups during acquisition – but not extinction – training. Specifically, the percentage of alpha responding during acquisition was significantly higher in controls relative to homozygous pcd mice as indicated by a statistically significant genotype effect (F(1, 19) = 5.846, p < 0.027). This effect was due to enhanced levels of alpha responses across training in controls relative to pcd mice (Figure 4). There were no significant main or interactive effects of sessions or Genotype × Sessions in the percentage of alpha responding. Furthermore, there were no significant effects involving genotype in the percentage of alpha responding measure during extinction training.
Figure 4.
Percentage of alpha responding (responses occurring within the first 60 ms of CS onset) during acquisition training in Purkinje cell degeneration (pcd) mutant mice and wildtype littermate control mice. Robust group differences are evident, as the percentage of alpha responding in controls is substantially higher than in pcd mutant mice. Data represent means plus and minus the standard error of the mean. The Ns for each group are the same as reported in acquisition phases for the CR percentage and CR peak amplitude measures.
Analyses of unconditioned response (UR) amplitudes were conducted on the first 10 trials of Session 1. Later trials were not analyzed for UR amplitude due to the possibility of contamination of UR topography via CR-UR summation effects (c.f., Weisz & McInerney, 1990). Consistent with previous reports (Chen et al., 1996, 1999), UR levels were comparable across groups (pcd: mean= 34.5 units, S.D. = 21.4; control: mean = 36.8 units; S.D. = 26.6).
Neuroanatomical Assessment of Purkinje Cells
Histological analyses of the cerebella of homozygous pcd mutant mice and littermate controls confirmed the absence of Purkinje cells in the brains of the mutant mice (see Figure 5). As expected, cerebellar sections from pcd mice are devoid of Purkinje cells while Purkinje cells are clearly visible in the control sections.
Figure 5.
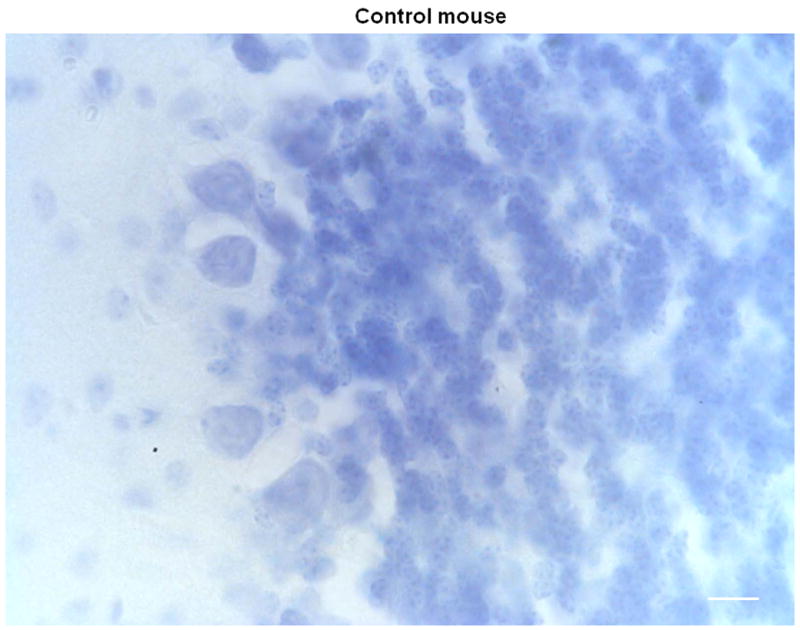
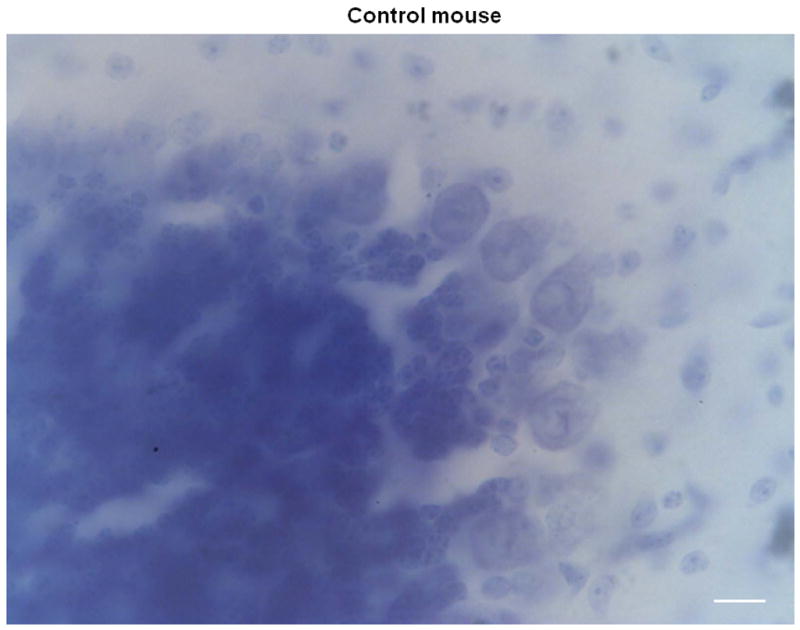
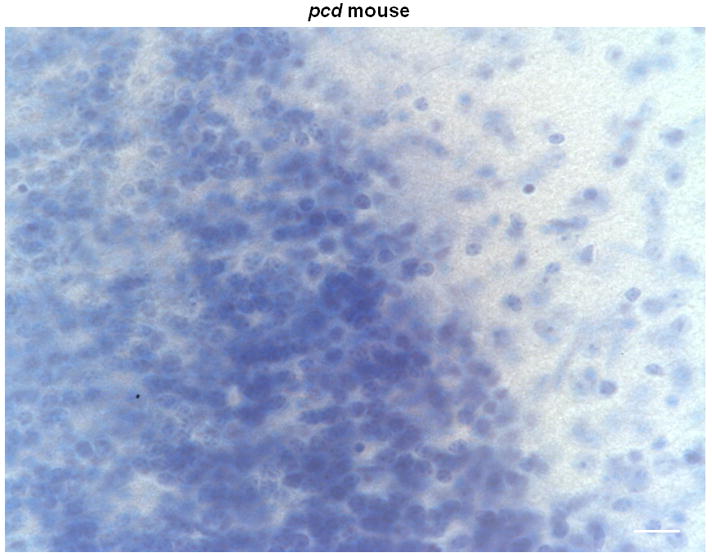
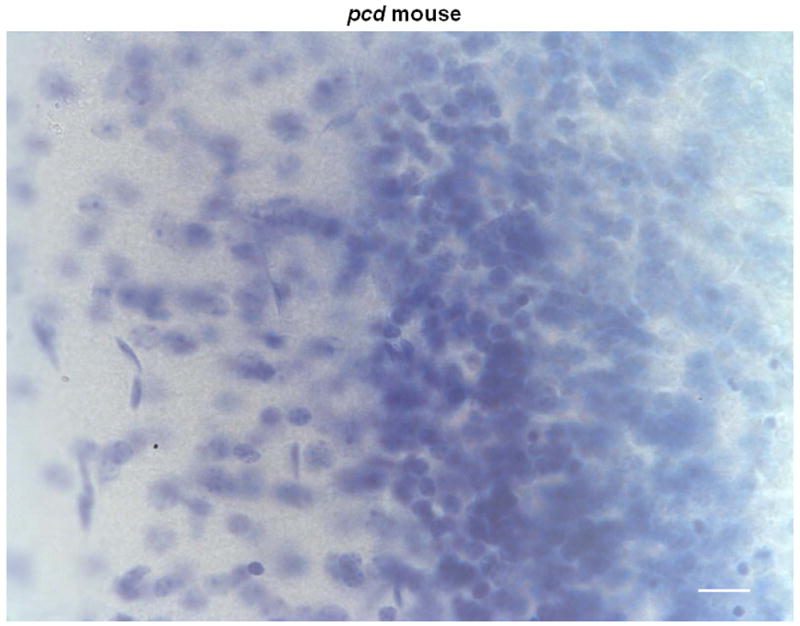
Representative coronal sections of the cerebellar cortex stained with Thionin in 2 control (A, B) and 2 pcd mice (C, D) magnified at 60x. Note the absence of Purkinje cells in the pcd sections (C, D), while Purkinje cells are abundant in the control sections (A, B). Scale bars = 20 μm.
Discussion
The present data support and extend previous findings of unimpaired trace eyeblink conditioning in mice with compromised cerebellar cortical functioning (Kishimoto et al., 2001a, b, c; Woodruff-Pak et al., 2006). Consistent with our hypothesis, the absence of Purkinje cells in pcd mutant mice did not impair their ability to acquire a trace eyeblink conditioning task that is dependent on forebrain (e.g., hippocampus; portions of the prefrontal cortex) integrity (Kalmbach, Ohyama, Kreider, Riusech, & Mauk, 2009; Tseng et al. 2004; Weible et al., 2000). Purkinje cell axons provide the only cerebellar cortical connections to the deep cerebellar nuclei. Thus, in the absence of Purkinje cells, the cerebellar cortex is disconnected from the cerebellar deep nuclei. Homozygous pcd mice did not differ from normal controls in measures of CR incidence, CR amplitude, or CR latency during either acquisition or extinction training. These results suggest that cerebellar cortical integrity is not essential for the acquisition or extinction of forebrain-dependent trace eyeblink conditioning.
It is widely-accepted that forebrain-cerebellar interactions underlie most forms of trace eyeblink conditioning (Kalmbach et al., 2009; Woodruff-Pak & Disterhoft, 2008). Given the functional disconnection of the cerebellar cortex in pcd mutant mice, their ability to acquire a trace eyeblink conditioning task at levels comparable to normal controls suggests continued forebrain interactions with functionally intact cerebellar regions. The cerebellar interpositus nucleus is essential for all forms of eyeblink conditioning (Christian & Thompson, 2003) and remains functionally intact in young adult pcd mice (Chen et al., 1996). Synaptic inputs from putative CS and US pathways (mossy fibers and climbing fibers, respectively) input directly to and converge upon the cerebellar deep nuclei (including the interpositus nucleus). Additionally, neuronal plasticity necessary for learning conditioned eyeblink responses has been demonstrated in cerebellar deep nuclei (Kleim et al., 2002; Ohyama, Nores, Medina, Riusech, & Mauk, 2006). Therefore, forebrain-interpositus interactions that bypass the cerebellar cortex may drive trace eyeblink conditioning performance observed in pcd mutant mice in the present study (c.f., Woodruff-Pak & Disterhoft, 2008). Identifying critical pathways between forebrain structures implicated in trace eyeblink conditioning and cerebellar deep nuclei may provide clues as to how trace eyeblink conditioning is acquired in the absence of cerebellar cortical involvement.
One such pathway may involve projections from regions of the prefrontal cortex to pontine regions transmitting CS-related information to the cerebellum. Involvement of components of the prefrontal cortex in trace eyeblink conditioning has been verified with electrophysiological recording (Weible, Weiss, & Disterhoft, 2003) and lesion methods (Kronforst-Collins & Disterhoft, 1998; McLaughlin, Skaggs, Churchwell, & Powell, 2002; Oswald et al., 2006; Weible et al., 2000). Direct projections from regions of the medial prefrontal cortex to pontine nuclei have been identified (Buchanan, Thompson, Maxwell, & Powell, 1994; Weible, Weiss, & Disterhoft, 2007) and these connections may provide the anatomical basis for modulation of CS-related input to the cerebellar interpositus nucleus critical for trace eyeblink conditioning. Specifically, forebrain-mediated activity in pontine regions may be necessary to prolong transmission of the CS signal to the cerebellum beyond the point of CS offset. This mechanism may therefore allow for temporal overlap of CS and US input in the cerebellum during extended (stimulus-free) trace intervals (c.f., Woodruff-Pak & Disterhoft, 2008). Without such a mechanism, cerebellar-brainstem circuits alone may be insufficient to support continued transmission of CS input for extended periods beyond CS offset.
Using a dual delay/trace paradigm, Kalmbach et al. (2009) demonstrated that projections from particular pontine regions (lateral pontine nuclei) to the cerebellum are essential for trace – but not delay – eyeblink conditioning, thus suggesting that critical CS-related inputs to the cerebellum associated with trace eyeblink conditioning may be anatomically separate from those mediating delay eyeblink conditioning. This proposed differential CS-related input may account for findings of enhanced metabolic activation of the posterior interpositus nucleus in rats trained in a trace eyeblink conditioning task relative to a group trained in a delay task matched for the ISI between CS and US onset (Plakke et al., 2007). Further anatomical and electrophysiological studies are required to support the existence of differential CS-related input. However, it may be that differential activation as a function of task may result from anatomically distinct inputs to different regions of the interpositus nucleus. The aforementioned proposal does not directly implicate the hippocampus in interactions with the cerebellum during trace conditioning, but essential hippocampal influence on cerebellar functioning during trace eyeblink conditioning may be exerted via extensive interconnections with prefrontal regions.
While the effect of sessions was not significant during CS-alone extinction training, an overall decrease in CR levels during extinction (relative to the latter half of CS-US training, with the exception of Session 10) was evident in both groups. Previous reports investigating extinction of trace eyeblink conditioning in normal mice using training conditions similar to those used in the present study have yielded inconsistent findings, ranging from a lack of significant decreases in CR levels across extinction training (e.g., Kishimoto et al., 2001a; Woodruff-Pak et al., 2006) to reports indicating some evidence of significant decreases in CR levels across CS-alone extinction training (e.g., Kishimoto et al., 2006; Kishimoto and Kano, 2006). Extending the number of CS-alone extinction sessions in future studies using this paradigm may therefore be necessary to obtain consistent, significant decreases in CR levels between early and late extinction sessions. The present findings illustrating lack of impairments in both CR timing and during CS-alone extinction training in pcd mice relative to controls replicates and extends previous trace eyeblink conditioning findings from our laboratory using a transgenic mouse with a selective knockout of sodium channel Scn8A in Purkinje cells (Woodruff-Pak et al., 2006). Cerebellar cortical integrity therefore does not appear to be necessary for precise CR timing or for executing typical CR patterns during extinction in trace eyeblink conditioning. However, the cerebellar cortex has been hypothesized to govern response timing features of the eyeblink CR as well as the execution of proper responding during post-CS-US training in extinction paradigms (Perrett, Ruiz, & Mauk, 1993, Perrett & Mauk, 1995). Briefly, LTD at the parallel fiber-Purkinje cell synapse in the cerebellar cortex is hypothesized to mediate timing-dependent disinhibition of deep nuclear cells to produce well-timed CRs (Nores et al., 2000). Extinction of the CR has been proposed to reflect potentiation (LTP) of cerebellar cortical parallel fiber-Purkinje cell synapses due to continued CS presentations in the absence of US input. While our present findings using homozygous pcd mutant mice devoid of Purkinje cells may appear to challenge popular theories of cerebellar cortical control of CR timing and extinction, these hypotheses have been generated using pharmacological disconnection or lesions of the cerebellar cortex with standard delay eyeblink conditioning paradigms in rabbits (e.g., Perrett et al., 1993, Perrett & Mauk, 1995), conditions that differ from those employed in the present study. Therefore, we cannot rule out the possibility that species differences and/or compensatory developmental mechanisms resulting from the gradual loss of Purkinje cells early in the postnatal period in our pcd mice may account for these discrepancies. Additionally, aforementioned forebrain-pontine projections that appear to be selectively utilized in trace eyeblink conditioning may be sufficient to activate cerebellar deep nuclear cells in a temporal fashion essential for proper CR timing. These connections may also allow for decreases in deep nuclear responsiveness to continued CS presentations in the absence of US input during extinction.
Acquisition levels in the present study are consistent with previous findings of trace eyeblink conditioning in mice trained under similar experimental conditions. Previous studies using the same mouse strain (C57BL/6J) and eyeblink recording technique (EMG) with similar CS-US parameters (tone CS frequency at 1–2 kHz and 80–85 db; CS durations between 250–352 ms; US duration of 100 ms; trace intervals between 250–500 ms) have yielded Session 1 and late-session acquisition levels (typically 20–35% and 50–70% CRs, respectively) in control mice (see Ewers, Morgan, Gordon, & Woodruff-Pak, 2006; Kishimoto et al., 2001b, 2002b, 2006; Ohno, Tseng, Silva, & Disterhoft, 2005; Tseng et al., 2004; Weiss, Sametsky, Sasse, Speiss, & Disterhoft, 2005; Woodruff-Pak et al., 2006) similar to those reported in the present study (Session 1 levels of approximately 30% in controls and 40% in pcds; late-session levels of approximately 55–60% in controls and 50% in pcds). The relatively high CR percentage levels found early in training may be attributable (in part) to enhanced arousal levels experienced by the subset of animals in each group that did not receive eyeblink chamber preexposure prior to CS- US training. CR peak amplitude provides another useful measure of the strength of the CS-US relationship. In the present study, acquisition reflected in the peak amplitude of the CR followed a pattern similar to those shown in delay-conditioned wild-type C57 mice used in previous studies employing experimental conditions similar to those in the present study (see Chen et al., 1996, 1999). The primary exception in the pattern of CR levels during acquisition between the present study and previous studies investigating eyeblink conditioning in mice is the dramatic decrease in CR levels between acquisition Sessions 9–10 for pcd mice. We cannot account for the cause of this unexpected decrease, but aside from this aberrant session CR levels – particularly in the CR peak amplitude measure – display gradual increases across many of the acquisition sessions.
Consistent with the lack of group differences in CR levels across acquisition (and extinction), pre-training CR levels were extremely low (< 10%) in both groups, and URs during the first block of training did not differ between pcd and control mice. Control and pcd mice did differ, however, in the percentage of startle (or ‘alpha’) responses (responses exceeding threshold within the first 60 ms after CS presentation). Normal controls showed significantly higher startle response levels relative to the pcd mice. These findings are inconsistent with those of Chen et al. (1996), who reported no significant differences between pcd and control mice in percentages of startle responding to a white noise burst. Group differences do not appear to be driven by excessively low levels of startle responding in pcd mice, as (approximately) 10% of the recorded trials showed a startle. These startle response percentages are comparable to (alpha) response levels recorded from the first 28–60 ms following CS onset in normal mice using tone CS decibel levels (80–85 db) and frequencies (1–2-kHz) similar to those used in the present study (see Bao, Chen & Thompson., 1998a; Kato et al., 2005; Kishimoto et al., 1997; Tseng et al., 2004; Woodruff-Pak et al., 2006). For reasons that are unclear, the startle response percentage is significantly higher (~40 – 50% across training) in normal controls in the present study. Enhanced startle responding in our controls may reflect unusually heightened sensitivity to the tone CS and could be expected to translate to enhanced CR levels. However, sample tracings provided in Figure 1 (particularly Figure 1B) indicate that the topography of alpha responses are distinct from CRs. CRs are significantly higher in amplitude and response duration relative to early onset alpha responses. It therefore appears that the unusually high percentage of alpha responding in controls is not significantly contributing to responses occurring in the CR period.
The role of the cerebellar cortex in trace eyeblink conditioning has gained much interest over the past decade (see Woodruff-Pak & Disterhoft, 2008). In contrast to delay eyeblink conditioning which persists following ablation of various forebrain circuitry (Ivkovich & Thompson, 1997; Oakley & Russell, 1972; Schmaltz & Theios, 1972; Solomon, Solomon, Vander Schaff, & Perry, 1983), neural mechanisms that underlie trace eyeblink conditioning require the functional integrity of forebrain circuits (e.g., hippocampus; prefrontal cortex; Moyer et al., 1990; Oswald et al., 2006; Solomon et al., 1986; Weible et al., 2000) in addition to cerebellar circuitry underlying standard delay eyeblink conditioning (Pakaprot et al., 2009). The cerebellar cortex was initially assumed to play a significant role in the acquisition of trace eyeblink conditioning (see Woodruff-Pak et al., 1985), though recent findings – including those in the present study - converge on the conclusion that cerebellar cortical integrity is not important for trace eyeblink conditioning (Gerwig et al., 2008; Kishimoto et al., 2001a, b, c; Woodruff-Pak et al., 2006). It may be the case that anatomically distinct (rather than hierarchical) extra-cerebellar pathways contribute to delay versus trace eyeblink conditioning. This proposal has strong support from recent findings of Kalmbach et al. (2009), who showed that a forebrain-mediated CS pathway in the lateral pontine region is necessary for trace but not delay eyeblink conditioning. This possibility underscores the need for further investigation of forebrain-cerebellar interactions in trace eyeblink conditioning.
Acknowledgments
The authors thank Max Chae, Sung Kim, and Jordan Zach for technical assistance with surgeries to implant electrodes and behavioral training. The authors also thank Kim Nguyen for assistance with histology and microscopy, and Andrey Mavrichev for designing the software used for visualization of EMG activity from individual sessions. This research was supported by grants from the National Institute on Aging, 1 R01 AG021925 and 1 R01 AG023742 to DSW-P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Bao S, Chen L, Thompson RF. Classical eyeblink conditioning in two strains of mice: conditioned responses, sensitization, and spontaneous eyeblinks. Behavioral Neuroscience. 1998a;112:714–718. doi: 10.1037//0735-7044.112.3.714. [DOI] [PubMed] [Google Scholar]
- Bao S, Chen L, Qiao X, Knusel B, Thompson RF. Impaired eye-blink conditioning in waggler, a mutant mouse with cerebellar BDNF deficiency. Learning and Memory. 1998b;5:355–364. [PMC free article] [PubMed] [Google Scholar]
- Buchanan SL, Thompson RH, Maxwell BL, Powell DA. Efferent connections of the medial prefrontal cortex in the rabbit. Experimental Brain Research. 1994;100:469–483. doi: 10.1007/BF02738406. [DOI] [PubMed] [Google Scholar]
- Chen L, Bao S, Lockard JM, Kim JJ, Thompson RF. Impaired classical eyeblink conditioning in cerebellar-lesioned and Purkinje cell degeneration (pcd) mutant mice. The Journal of Neuroscience. 1996;16:2829–2838. doi: 10.1523/JNEUROSCI.16-08-02829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Bao S, Thompson RF. Bilateral lesions of the interpositus nucleus completely prevent eyeblink conditioning in Purkinje cell-degeneration mutant mice. Behavioral Neuroscience. 1999;113:204–210. doi: 10.1037//0735-7044.113.1.204. [DOI] [PubMed] [Google Scholar]
- Chen G, Steinmetz JE. A general-purpose computer system for behavioral conditioning and neural recording experiments. Behavioral Research Methods, Instruments, & Computers. 1998;30:384–391. [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learning and Memory. 2003;11:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Endo S, Shuto F, Le Dinh T, Okamoto T, Ikeda T, Suzuki M, Kawahara S, Yanagihara D, Sato Y, Yamada K, Sakamoto T, Kirino Y, Hartell NA, Yamaguchi K, Itohara S, Nairn A, Greengard P, Nagao S, Ito M. Dual involvement of G-substrate in motor learning revealed by gene deletion. Proceedings of the National Academy of the Sciences (USA) 2009;106:3525–3530. doi: 10.1073/pnas.0813341106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Morgan DG, Gordon MN, Woodruff-Pak DS. Associative and motor learning in 12-month-old transgenic APP+PS1 mice. Neurobiology of Aging. 2006;27:1118–1128. doi: 10.1016/j.neurobiolaging.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Gerwig M, Eβer AC, Guberina H, Frings M, Kolb FP, Forsting M, Aurich V, Beck A, Timmann D. Trace eyeblink conditioning in patients with cerebellar degeneration: comparison of short and long trace intervals. Experimental Brain Research. 2008;187:85–96. doi: 10.1007/s00221-008-1283-2. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Sears LL, Steinmetz JE. Possible CS and US pathways for rabbit classical eyelid conditioning: electrophysiological evidence for projections from the pontine nuclei and inferior olive to cerebellar cortex and nuclei. Behavioral and Neural Biology. 1993;60:172–185. doi: 10.1016/0163-1047(93)90285-p. [DOI] [PubMed] [Google Scholar]
- Hansel C, Linden DJ, D’Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nature Neuroscience. 2001;4:467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- Ho N, Liauw JA, Blaeser F, Wei F, Hanissian S, Muglia LM, Wozniak DF, Nardi A, Arvin KL, Holtzman DM, Linden DJ, Zhou M, Muglia LJ, Chatila TA. Impaired synaptic plasticity and cAMP response element-binding protein activation in Ca2+/calmodulin-dependent protein kinase type IV/Gr-deficient mice. The Journal of Neuroscience. 2000;20:6459–6472. doi: 10.1523/JNEUROSCI.20-17-06459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivkovich D, Thompson RF. Motor cortex lesions do not affect learning or performance of the eyeblink response in rabbits. Behavioral Neuroscience. 1997;111:727–738. doi: 10.1037//0735-7044.111.4.727. [DOI] [PubMed] [Google Scholar]
- Kakegawa W, Miyazaki T, Emi K, Matsuda K, Kohda K, Motohashi J, Mishina M, Kawahara S, Watanabe M, Yuzaki M. Differential regulation of synaptic plasticity and cerebellar motor learning by the C-terminal PDZ-binding motif of GluRδ2. The Journal of Neuroscience. 2008;28:1460–1468. doi: 10.1523/JNEUROSCI.2553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Kreider JC, Riusech F, Mauk MD. Interactions between prefrontal cortex and cerebellum revealed by trace eyelid conditioning. Learning and Memory. 2009;16:86–95. doi: 10.1101/lm.1178309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Takatsuki K, Kawahara S, Fukunaga S, Mori H, Mishina M, Kirino Y. N-Methyl-D-Aspartate receptors play important roles in acquisition and expression of the eyeblink conditioned response in glutamate receptor subunit δ2 mutant mice. Neuroscience. 2005;135:1017–1023. doi: 10.1016/j.neuroscience.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behavioral Neuroscience. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kina SI, Tezuka T, Kusakawa S, Kishimoto Y, Kakizawa S, Hashimoto K, Ohsugi M, Kiyama Y, Horai R, Sudo K, Kakuta S, Iwakura Y, Lino M, Kano M, Manabe T, Yamamoto T. Involvement of protein-tyrosine phosphatase PTPMEG in motor learning and cerebellar long-term depression. European Journal of Neuroscience. 2007;26:2269–2278. doi: 10.1111/j.1460-9568.2007.05829.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Kirino Y, Kadotani H, Nakamura Y, Ikeda M, Yoshioka T. Conditioned eyeblink response is impaired in mutant mice lacking NMDA receptor subunit NR2A. Neuroreport. 1997;8:3717–3721. doi: 10.1097/00001756-199712010-00012. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Hirono M, Sugiyama T, Kawahara S, Nakao K, Kishio M, Katsuki M, Yoshioka T, Kirino Y. Impaired delay but normal trace eyeblink conditioning in PLCβ4 mutant mice. Neuroreport. 2001a;12:2919–2922. doi: 10.1097/00001756-200109170-00033. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Kano M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. The Journal of Neuroscience. 2006;26:8829–8837. doi: 10.1523/JNEUROSCI.1236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Suzuki M, Mori H, Mishinia M, Kirino Y. Classical conditioning in glutamate receptor subunit δ2 mutant mice is impaired in the delay paradigm but not in the trace paradigm. European Journal of Neuroscience. 2001b;13:1249–1253. doi: 10.1046/j.0953-816x.2001.01488.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Fujimichi R, Mori H, Mishina M, Kirino Y. Impairment of eyeblink conditioning in GluRδ2-mutant mice depends on the temporal overlap between conditioned and unconditioned stimuli. European Journal of Neuroscience. 2001c;14:1515–1521. doi: 10.1046/j.0953-816x.2001.01772.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Fujimichi R, Araishi K, Kawahara S, Kano M, Aiba A, Kirino Y. mGluR1 in cerebellar Purkinje cells is required for normal association of temporally contiguous stimuli in classical conditioning. European Journal of Neuroscience. 2002;16:2416–2424. doi: 10.1046/j.1460-9568.2002.02407.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Nakazawa K, Tonegawa S, Kirino Y, Kano M. Hippocampal CA3 NMDA receptors are crucial for adaptive timing of trace eyeblink conditioned response. The Journal of Neuroscience. 2006;26:1562–1570. doi: 10.1523/JNEUROSCI.4142-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Freeman JH, Bruneau R, Nolan BC, Cooper NR, Zook A, Walters D. Synapse formation is associated with memory storage in the cerebellum. Proceedings of the National Academy of the Sciences (USA) 2002;99:13228–13231. doi: 10.1073/pnas.202483399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Disterhoft JF. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiology of Learning and Memory. 1998;69:147–162. doi: 10.1006/nlme.1997.3818. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Steinmetz JE, Yokaitis MH, Thompson RF. Reacquisition of classical conditioning after removal of cerebellar cortex. Experimental Brain Research. 1987;67:569–593. doi: 10.1007/BF00247289. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Steinmetz JE. Acquisition of classical conditioning without cerebellar cortex. Behavioural Brain Research. 1989;33:113–164. doi: 10.1016/s0166-4328(89)80047-6. [DOI] [PubMed] [Google Scholar]
- Lee KH, Chatila TA, Ram RA, Thompson RF. Impaired memory of eyeblink conditioning in CaMKIZ KO mice. Behavioral Neuroscience. 2009;123:438–442. doi: 10.1037/a0014724. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Connor JA. Long-term synaptic depression. Annual Review of Neuroscience. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Steinmetz JE, Thompson RF. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proceedings of the National Academy of the Sciences (USA) 1986;83:5349–5353. doi: 10.1073/pnas.83.14.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, Skaggs H, Churchwell J, Powell DA. Medial prefrontal cortex and pavlovian conditioning: trace versus delay conditioning. Behavioral Neuroscience. 2002;116:37–47. [PubMed] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behavioral Neuroscience. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Eicher EM, Sidman RL. Purkinje cell degeneration, a new neurological mutation in the mouse. Proceedings of the National Academy of the Sciences (USA) 1976;73:208–212. doi: 10.1073/pnas.73.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nores WL, Medina JF, Steele PM, Mauk MD. Relative contributions of the cerebellar cortex and cerebellar nucleus to eyelid conditioning. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink Classical Conditioning, Vol. 2, Animal Models. Boston: Kluwer Academic Publishers; 2000. pp. 205–229. [Google Scholar]
- Oakley DA, Russell IS. Neocortical lesions and Pavlovian conditioning. Physiology and Behavior. 1972;8:915–926. doi: 10.1016/0031-9384(72)90305-8. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Medina JF, Riusech FA, Mauk MD. Learning-induced plasticity in deep cerebellar nucleus. The Journal of Neuroscience. 2006;26:12656–12663. doi: 10.1523/JNEUROSCI.4023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald B, Knuckley B, Mahan K, Snaders C, Powell DA. Prefrontal control of trace versus delay eyeblink conditioning: role of the unconditioned stimulus in rabbits (Oryctolagus cuniculus) Behavioral Neuroscience. 2006;120:1033–1042. doi: 10.1037/0735-7044.120.5.1033. [DOI] [PubMed] [Google Scholar]
- Pakaprot N, Kim S, Thompson RF. The role of the cerebellar interpositus nucleus in short and long term memory for trace eyeblink conditioning. Behavioral Neuroscience. 2009;123:54–61. doi: 10.1037/a0014263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning- dependent timing of conditioned eyelid responses. The Journal of Neuroscience. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett SP, Mauk MD. Extinction of conditioned eyelid responses requires the anterior lobe of cerebellar cortex. The Journal of Neuroscience. 1995;15:2074–2080. doi: 10.1523/JNEUROSCI.15-03-02074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plakke B, Freeman JH, Poremba A. Metabolic mapping of the rat cerebellum during delay and trace eyeblink conditioning. Neurobiology of Learning and Memory. 2007;88:11–18. doi: 10.1016/j.nlm.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron. 2006;51:113–123. doi: 10.1016/j.neuron.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 1997;19:881–891. doi: 10.1016/s0896-6273(00)80969-1. [DOI] [PubMed] [Google Scholar]
- Schmaltz LW, Theios J. Acquisition and extinction of a classically conditioned response in hippocampectomized rabbits (Oryctolagus cuniculus) Journal of Comparative and Physiological Psychology. 1972;79:328–333. doi: 10.1037/h0032531. [DOI] [PubMed] [Google Scholar]
- Shibuki K, Gomi H, Chen L, Bao S, Kim JJ, Wakatsuki H, Fujisaki T, Fujimoto K, Katoh A, Ikeda T, Chen C, Thompson RF, Itohara S. Deficient long-term depression, impaired eyeblink conditioning, and normal motor coordination in GFAP mutant mice. Neuron. 1996;16:587–599. doi: 10.1016/s0896-6273(00)80078-1. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Solomon SD, Vander Schaff E, Perry HE. Altered activity in the hippocampus is more detrimental to classical conditioning than removing the structure. Science. 1983;220:329–331. doi: 10.1126/science.6836277. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Rosen DJ, Chapman PF, Lavond DG, Thompson RF. Classical conditioning of the rabbit eyelid response with a mossy-fiber stimulation CS: I pontine nuclei and middle cerebellar peduncle stimulation. Behavioral Neuroscience. 1986;100:878–887. doi: 10.1037//0735-7044.100.6.878. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of the Sciences (USA) 1987;84:3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3:225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Sengelaub DR. Possible conditioned stimulus pathway for classical eyelid conditioning in rabbits. Behavioral and Neural Biology. 1992;57:103–115. doi: 10.1016/0163-1047(92)90593-s. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Yamaguchi K, Tatsukawa T, Nishioka C, Nishiyama H, Theis M, Willecke K, Itohara S. Lack of connexin43-mediated Bergmann glial gap junctional coupling does not affect cerebellar long-term depression, motor coordination, or eyeblink conditioning. Frontiers in Behavioral Neuroscience. 2008;2:1–14. doi: 10.3389/neuro.08.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF. The neurobiology of learning and memory. Science. 1986;233:941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Tracy JA, Thompson JK, Krupa DJ, Thompson RF. Evidence of plasticity in the pontocerebellar conditioned stimulus pathway during classical conditioning of the eyeblink response in the rabbit. Behavioral Neuroscience. 1998;112:267–285. doi: 10.1037//0735-7044.112.2.267. [DOI] [PubMed] [Google Scholar]
- Tseng W, Guan R, Disterhoft JF, Weiss C. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus. 2004;14:58–65. doi: 10.1002/hipo.10157. [DOI] [PubMed] [Google Scholar]
- Vogel RW, Ewers M, Ross C, Gould TJ, Woodruff-Pak DS. Age-related impairment in the 250-millisecond delay eyeblink classical conditioning procedure in C57BL/6 mice. Learning and Memory. 2002;9:321–336. doi: 10.1101/lm.50902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, McEchron MD, Disterhoft JF. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behavioral Neuroscience. 2000;114:1058–1067. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Activity profiles of single neurons in caudal anterior cingulate cortex during trace eyeblink conditioning in the rabbit. Journal of Neurophysiology. 2003;90:599–612. doi: 10.1152/jn.01097.2002. [DOI] [PubMed] [Google Scholar]
- Weible AP, Weiss C, Disterhoft JF. Connections of the caudal anterior cingulate cortex in rabbit: neural circuitry participating in the acquisition of trace eyeblink conditioning. Neuroscience. 2007;145:288–302. doi: 10.1016/j.neuroscience.2006.11.046. [DOI] [PubMed] [Google Scholar]
- Weiss C, Sametsky E, Sasse A, Spiess J, Disterhoft JF. Acute stress facilitates trace eyeblink conditioning in C57BL/6 mice and increases the excitability of their CA1 pyramidal neurons. Learning and Memory. 2005;12:138–143. doi: 10.1101/lm.89005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz DJ, McInerney J. An associative process maintains reflex facilitation of the unconditioned nictitating membrane response during the early stages of training. Behavioral Neuroscience. 1990;104:21–27. doi: 10.1037//0735-7044.104.1.21. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Disterhoft J. Where is the trace in trace conditioning? Trends in Neurosciences. 2008;31:105–112. doi: 10.1016/j.tins.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Green JT, Levin SI, Meisler MH. Inactivation of sodium channel Scn8A (Nav1.6) in Purkinje neurons impairs learning in Morris water maze and delay but not trace eyeblink classical conditioning. Behavioral Neuroscience. 2006;120:229–240. doi: 10.1037/0735-7044.120.2.229. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF. Trace conditioning: abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Research. 1985;348:249–260. doi: 10.1016/0006-8993(85)90443-3. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardimann MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit II. lesions of the cerebellar cortex. Experimental Brain Research. 1985;60:99–113. doi: 10.1007/BF00237023. [DOI] [PubMed] [Google Scholar]