Abstract
Objectives
Evaluate the role of serotonin receptors 1B and 2A, thromboxane synthase and receptor and phospholipases A2 and C in response to cardiopulmonary bypass in patients.
Methods
Atrial tissue was harvested from patients before and after cardiopulmonary bypass with cardioplegia (n=13). Coronary microvessels were assessed for vasoactive response to serotonin with and without inhibitors of 5-HT1B and 5-HT2A receceptors, phospholipase A2 and C. Expression of 5-HT1B and 5-HT2A mRNA was determined by RT-PCR. Expression of 5-HT1B, 5-HT2A, Thromboxane A2 receptor and synthase protein was determined by immunoblotting and immunohistochemistry.
Results
Exposure of microvessels to serotonin elicited a 7.3 ± 2% relaxation response pre-bypass, changing to a strong contraction response of -19.2 ± 2% after bypass (p<0.001). Addition of either a specific 5-HT1B antagonist or inhibitor of PLA2 resulted in a significant decrease in the contractile response to -8.6 ±1% (p<0.001) and 2.8 ± 3% (p= 0.001), respectively.
5-HT1B receptor mRNA expression increased 1.82 ± 0.34 fold after bypass (p=0.044), while 5-HT2A mRNA expression did not change. 5-HT1B receptor, but not 5-HT2A, protein expression increased after bypass by 1.35 ± 0.7 fold (p=0.0413). Neither thromboxane synthase nor thromboxane receptor expression changed after bypass. Immunohistochemistry demonstrated 5-HT1B receptor increased mainly in the arterial smooth muscle. There was no appreciable difference in arterial expression of either thromboxane synthase or receptor.
Conclusion
These data indicate that 5-HT-induced vascular dysfunction after cardiopulmonary bypass with cardioplegia may be mediated by increased expression of 5-HT1B receptor and subsequent PLA2 activation in myocardial coronary smooth muscle.
Mini Abstract
The expression of 5-HT1B receptor protein and mRNA were increased in the atrial myocardium after cardioplegia and cardiopulmonary bypass (CP-CPB). Serotonin elicited a strong contraction response of atrial microvessels after CPB which was mitigated by specific 5-HT1B receptor blockers and phospholipase A2 inhibitors. This suggests that coronary microvascular contraction after CP-CPB is related to increased expression of the 5-HT1B receptor and subsequent phospholipase A2 signaling.
Introduction
Endothelial dysfunction occurs in many cardiovascular diseases including hypertension, diabetes mellitus, hyperlipidemia and atherosclerosis (1-3). It is also estimated to occur in a clinically relevant manner in up to 2.5 % of patients after cardiac surgery (4) with EKG changes occurring in up to 8% of patients (5). These clinical events are thought to be secondary to altered vasomotor regulation due to coronary artery and microvascular spasm. This altered response has been implicated as a cause of myocardial ischemia in a number of clinical situations such as angina, acute coronary syndrome and vasospasm after coronary artery bypass graft (CABG) utilizing cardioplegia and cardiopulmonary bypass (CP-CPB). A number of vasoactive substances have been implicated in this process. These include thrombin (5), endothelin (6), Adenosine diphosphate, thromboxane A2 (TXA2) (7), acetylcholine (8), nitric oxide (NO) (9), adenosine (10), reactive oxygen species (which interfere with endothelium dependent relaxation) (11), and serotonin (12).
Serotonin (5-hydoxytryptamine, 5-HT) is a vasoreactive amine with numerous actions affecting the circulation of various organs, and its actions are mediated by multiple receptor subtypes. There are two major 5-HT receptor subtypes, 1B and 2A, expressed in heart tissue that can have opposing effects on the coronary vasculature. 5-HT2A often acts as a vasodilator, while 5-HT1B acts as a vasoconstrictor (13-15). In a recent study Shimizu et al infused the 5-HT1B agonist sumatriptan into the coronary arteries of 9 patients, 5 with confirmed variant angina. Sumatriptan elicited coronary artery spasm in all patients with variant angina (16). Similarly, Dahlof and Mathew reviewed the literature on reports of cardiovascular complications of patients taking sumatriptan for treatment of migraines. They noted that 3-5% of patients report chest tightness or pressure after taking the drug, although no EKG or echocardiography data support that this sensation is of cardiac origin, the authors felt there was enough anecdotal data to recommend caution in using 5-HT1 agonists in patients at high risk for coronary artery disease or vasospasm (17).
In previous studies we have shown that myocardial dysfunction after CP-CPB is associated with increased coronary contraction mediated by 5-HT (18) and a switch from serotonin-induced coronary vasodilation pre-CP-CPB to contraction post-CP-CPB.
Serotonin has been shown to activate phosopholipase A2 (PLA2) which is known to release arachidonic acid, the precursor to a number of inflammatory mediators including thromboxane (TXA2) (19). Activation of PLA2, by 5-HT or other receptors, may contribute to post-CP-CPB vasoconstriction.
Our hypothesis is that the differential response to 5-HT may be caused by a shift in the proportion of receptor subtype expressed. Thromboxane synthase (TS) and the thromboxane receptor (TR) have also been implicated in leading to vasoconstriction post-CP-CPB, and in conjunction with serotonin may lead to further exacerbation of the vessel contraction. It is clear this is a complex and multifactorial process and this study was designed to specifically examine CP-CPB induced changes in the 5HT receptor subtypes, TS, TR, phospholipase C (PLC) and PLA2 as they may contribute to a vasoconstrictive local milieu post-CP-CPB.
Methods
Collection of Tissue
Right atrial tissue was harvested from patients undergoing CABG with cardioplegia and cardiopulmonary bypass. Aspirin was discontinued 24 hours prior to surgery, but other medications were continued up to the time of surgery. Double cannulation sutures were placed in the atrial appendage. The first excised portion of right atrial tissue was harvested after heparinization but before initiation of CP-CPB (pre). The second portion of atrium was harvested after cross clamp removal and termination of CPB, but before protamine administration (post). Cold-blood cardioplegia solution was used. Tissue in the pre and post groups was immediately frozen in liquid nitrogen after harvest in preparation for molecular studies, was placed in cold (5°C to 10°C) Krebs' buffer solution for vascular reactivity studies or fixed in 10% formalin overnight and embedded in paraffin for immunohistochemistry.
The study was approved by the clinical research committee of Beth Israel Deaconess Medical Center, and informed consent was obtained from each patient.
In Vitro Atrial Microvascular Studies
Coronary microvascular reactivity was examined in the ischemic territory as previously described (20). Briefly, coronary arterioles (70 to 180 μm) were dissected with a 40x microscope. Microvessels were mounted on dual glass micropipettes and examined in a pressurized isolated microvessel chamber. Relaxation responses of microvessels were examined after development of spontaneous tone with supplemental precontraction by the thromboxane A2 analog U46619. Vascular responses to serotonin (5-HT, 10-9 to 10-4 mol/L) alone and in the presence of a specific 5-HT1B receptor antagonist, SB224289, (10-6) before and after CP-CPB were examined. Vessel responses to inhibitors of PLA2 (quinacrine; 10-6 mol/L) and PLC (U73122; 10-6 mol/L) before and after CP-CPB were also examined. All reagents from Sigma-Aldrich, St Louis MO.
Expression of 5-HT1B and 2A mRNA
For 5HT-1B and 5HT-2A mRNA studies, semi-quantitative reverse transcriptase polymerase chain reaction (RT-PCR) was performed. Primers were designed on the basis of published sequences (14). The 5-HT1A primers of the sense 5'-acagaattcatggatgtgctcagccctggtca-3'and the antisense 5'-tctgtcgactcactggcggcagaagttacacttaat-3' were used. For 5-HT2A, the primer of sense 5'-gcgggtaccatgcaattaaatgatgacaccaggctc-3' and the antisense 5'-tatctcgagtccacagttgccacggcaacta-3' were used. An equal amount of total RNA was used. For quantification, GAPDH was amplified from the same amount of RNA to correct for variation of different samples. The PCR products were loaded in a 1% agarose gel containing ethidium bromide and scanned and quantified with Image-Quant software (Molecular Dynamics, Sunnyvale, CA).
Expression of 5HT-1B, 2A, TS and TR protein
Myocardial samples were homogenized in RIPA buffer (Boston Bioproducts, Worcester, MA) and protein concentration determined by BCA assay (Pierce, Rockford, IL). Total protein (40 μg/lane) was fractionated on 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Immobilon-P, Millipore, Bedford, MA). Equal protein loading was determined by Ponceau staining. The membranes were incubated with either anti- 5-HT1B at 1:100 (vol/vol) dilution, anti- 5-HT2A (both from BD Biosciences, San Jose, CA) at 1:100 dilution, anti- thromboxane A2 synthase at 1:300 dilution or with anti- thromboxane A2 receptor (both from Santa Cruz, Santa Cruz, CA) at 1:100. After washing, the membranes were incubated in appropriate secondary antibody. Peroxidase activity was visualized with an enhanced chemiluminescence substrate system (Amersham, Fairfield, CT) and exposure to X-ray film. Densitometry of digitized x-ray films (ScanJet 4c, Hewlett Packard, Palo Alto, CA) was performed with Image-Quant software (Molecular Dynamics Inc, Sunnyvale, CA). Data are presented as mean ± SEM in arbitrary density units.
Immunohistochemistry
Formalin fixed tissue samples were deparafinized, rehydrated and incubated with 3% hydrogen peroxide. Antibodies against 5-HT1B or 5-HT2A (2 μg / ml) (BD Biosciences) were applied to the sections and isotype-matched control antibody (negative control) was applied in the same dilution for 2 hours at room temperature. Following a PBS wash, immunoreactivity was detected with a biotinylated goat anti-mouse secondary antibody and the avidin-biotin-peroxidase complex (Vector Laboratories, Burlingame, CA). Color was developed using diaminobenzidine substrate (1mg/ml in PBS and 0.03% H2O2). Sections were then counterstained with hematoxilin, dehydrated and mounted. Photomicrographs were taken with a Zeiss Axiolab microscope (Carl Zeiss Inc, Thornwood, NY) equipped with digital camera (Photodoc, Upland, CA).
Statistics
Microvascular reactivity data were analyzed using two-way repeated measures ANOVA. Densitometry data were analyzed using one way ANOVA (Systat, San Jose, CA). Data are reported as mean ± SEM and p< 0.05 was considered significant.
Results
Atrial tissue was obtained from 13 patients. All were male with an average age of 66.8 ± 4 years. Seventy seven percent of the patients had hypertension, 54% had hyperlipidemia and 23% had diabetes mellitus. Four were smokers at the time of operation. The average pre-operative ejection fraction was 46.4 ± 5.8%. Mean cardioplegia time was 58 ± 7 minutes, and the mean time from cross clamp removal until second tissue harvest was 10 ± 3 minutes. None of the patients in this study suffered any major clinical adverse events, including overt evidence of myocardial ischemia, unexpected hemodynamic instability or cardiac depression.
Microvessel characteristics
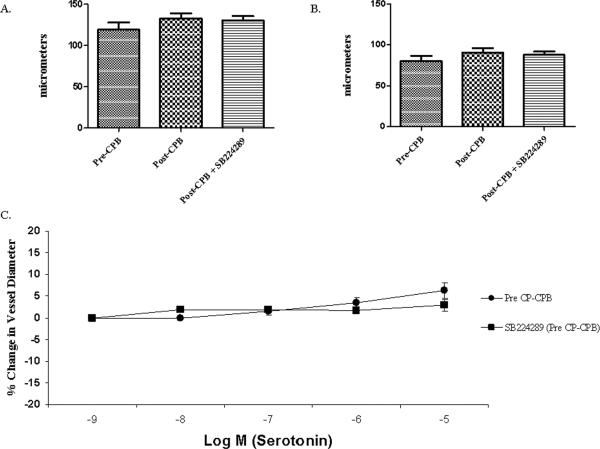
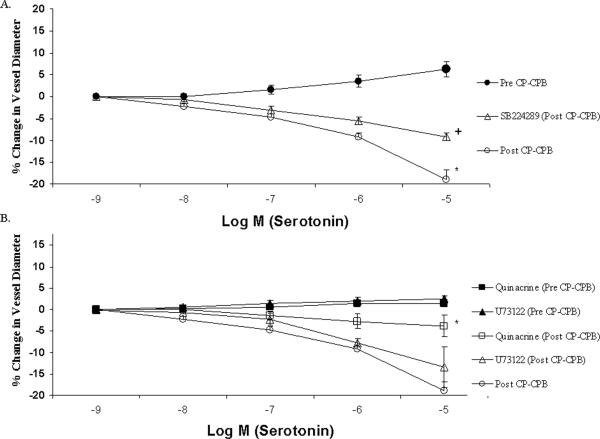
Atrial microvessel internal diameter ranged from 70 to 180 μm, averaging 106±4 μm in the pre-CP-CPB group and 123 ± 13 μm in the post-CP-CPB group (figure 1A and B). The pre-CP-CPB vessels demonstrated a relaxation response that was not affected by the presence of the 5-HT1B receptor antagonist, SB224289 (figure 1C). Post-CP-CPB vessels demonstrated a strong contraction response. This contraction response was diminished in the presence of SB224289 (figure 2A). Addition of quinacrine, a selective PLA2 inhibitor, had no effect on the pre-CP-CPB microvessels, but significantly inhibited the contraction response post-CP-CPB. Addition of the PLC inhibitor resulted in no change in the contraction response (figure 2B).
Figure 1. Coronary microvascular response to serotonin in vitro.
A. Diameter of microvessels before pre-contraction. There was no difference in baseline vessel diameter among the groups (p=0.4).
B. Diameter of microvessels after pre-contraction. Vessels were pre-contracted with U46619, a thromboxane A2 analog. There was no difference among the groups prior to addition of serotonin (p=0.31).
C. Serotonin response of vessels harvested before CP-CPB with and without selective 5-HT1B receptor blocker, SB224289. SB224289 administered in the presence of 5HT to the pre-CP-CPB microvessels resulted in a response similar to the 5HT alone (p= 0.44).
Figure 2. Serotonin-inducecd coronary microvascular response to 5-HT1B, PLC and PLA2 inhibitors in vitro.
A. Coronary microvascular response to serotonin demonstrates a relaxation response prior to cardiopulmonary bypass. After bypass the microvessels switched to a strong contraction response. After addition of a 5-HT1B receptor blocker (SB224289) the post bypass contraction response was significantly diminished. (* pre- to post-bypass p< 0.001) (+ post-bypass to post-bypass + antagonist p< 0.001).
B. Microvascular response to serotonin in the presence of the PLC inhibitor, Quinacrine and PLA2 inhibitor, U73122, prior to CP-CPB. (Quinacrine to 5HT alone p= 0.17 and U73122 to 5HT alone p= 0.38). Post-CP-CPB microvessels subjected to Quinacrine yielded a significant reduction in the contractile response as compared to the post-CP-CPB response with 5HT alone (* p< 0.001). The contractile response was not significantly changed when U73122 was applied to post-CP-CPB microvessels as compared to 5HT alone (p= 0.58).
5-HT receptor and thromboxane synthase and receptor expression
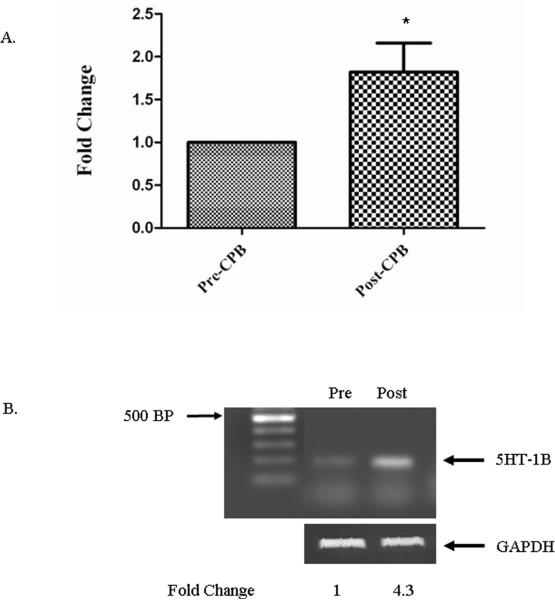
Expression of 5HT receptors 1B and 2A were analyzed by semiquantitative RT-PCR. Serotonin 5-HT1B receptor mRNA expression increased by an average of 1.82 ± 0.34 fold post-CP-CPB (figures 3A and B), while 5-HT2A mRNA expression was unchanged (p=0.509) (not shown).
Figure 3. 5-HT1B mRNA expression by RT-PCR.
A. Expression of 5-HT1B mRNA was increased (* p= 0.044) fold after CP-CPB.
B. Agarose- ethidium bromide gel (1%) showing the PCR amplification of 5HT-1B Post-CP-CPB as compared to pre-CP-CPB normalized to 1. GAPDH was used as a loading control. Data presented as fold change ± SEM.
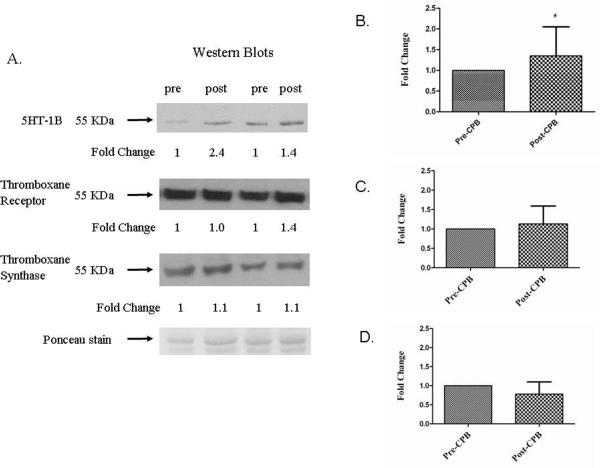
Western blotting showed that 5-HT1B protein expression was increased 1.35 ± 0.7 fold following CP-CPB (figures 4A and B), while 5-HT2A receptor did not change (p= 0.951) (not shown). Likewise TR and TS protein levels were unchanged (figures 4A, C, and D).
Figure 4. 5-HT1B, TR and TS protein expression by western blotting.
A. Expression of 5-HT1B receptor protein was found to increase after CP-CPB. The expression of TR and TS was not significantly changed the myocardial tissue. B. 5-HT1B receptor protein expression. Post-CP-CPB is compared to pre-CP-CPB normalized to 1 (* p= 0.041). C. Thromboxane receptor protein expression. (p= 0.62). D. Thromboxane synthase protein expression. (p= 0.18). Ponceau stain is used for loading/transfer monitoring. Data presented as fold change ± SEM.
Immunohistochemistry
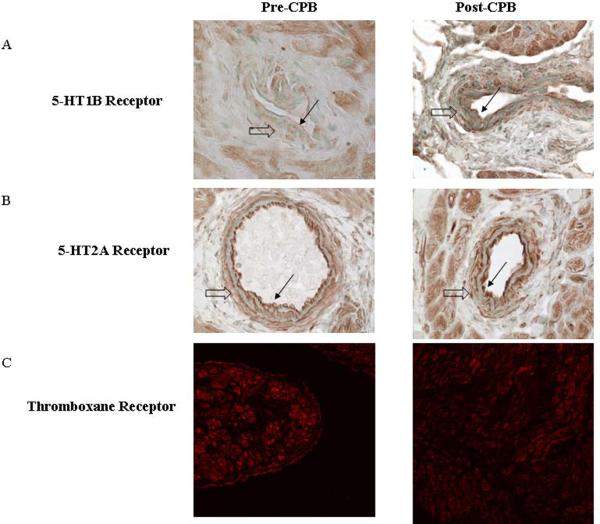
Immunohistochemistry demonstrated increased in 5-HT1B expression post-CP-CPB and 5-HT2A appeared unchanged (figure 5A and B), particularly in coronary vessels. 5-HT1B expression was increased in the arterial media smooth muscle. There was no apparent change in TR (figure 5C) or TS (not shown).
Figure 5. Localization of 5-HT1B, 5-HT2A receptors and thromboxane receptor by Immunohistochemistry and immunofluorescence, respectively.
A. Expression of 5-HT1B receptor is upregulated in the vascular smooth muscle cells (VSMC). B. Expression of 5-HT2A receptor is unchanged after CP-CPB. C. Expression of thromboxane receptor is unchanged between pre and post CP-CPB Magnification: immunohistochemoistry 400x, immunofluorescence 200x. (Large open arrow indicates VSMC, small black arrow indicates endothelium).
Discussion
In this study we examined coronary microvascular responses before and after CP-CPB. Atrial microvessels harvested prior to CP-CPB displayed a small relaxation response to 5-HT. In contrast, the coronary vessels isolated after CP-CPB displayed a strong contractile response to 5-HT. These findings indicate changes in the vasomotor pathways after surgery, and possibly a shift in receptor subtype expression to account for this functional change. The addition of a 5-HT1B blocker significantly diminished the contractile response, suggesting the post-CP-CPB vasoconstriction is at least in part mediated by 5-HT1B. Addition of a PLA2 inhibitor also significantly attenuated the post-CP-CPB contractile response to serotonin, but the PLC inhibitor was ineffective. There was no significant difference in the tissue expression of TR or TS.
Coronary microcirculation provides significant control over myocardial perfusion (21). It has been shown that the microvascular response to neurohumoral substances, such as serotonin and norepinephrine, is often different in magnitude or opposite to that observed in large vessels (22, 23). Thus, the study of microvascular regulation is likely more physiologically relevant than alterations in larger vessels.
To further explore a possible altered serotonin receptor expression, we examined the transcriptional and translational products of the two most commonly implicated receptor subtypes (24). We demonstrated upregulation of 5-HT1B receptor mRNA and protein expression by RT-PCR and western blotting. 5-HT2A expression was unchanged. These findings support the concept of a shift in 5-HT receptor subtypes in response to CP-CPB, and that 5-HT1B contributes to the change in microvascular response.
An upregulation of the 5-HT1B receptor occurred after surgery. Changes in 5-HT1B receptor expression have been demonstrated in other studies in a similarly brief time period. In a study of the cerebral vasculature during ischemia/reperfusion, it was noted that 5-HT1B receptor expression was upregulated in the vascular smooth muscle cells within 60 minutes of the onset of ischemia (25). It was hypothesized the expression was controlled by MEK1 activating ERK1/2, which in turn activated the transcription factor Elk1. In that study, administration of a MEK1 inhibitor diminished the upregulation of 5-HT1B receptor. The dual endothelin 1 receptor antagonist bosentan, used in the treatment of pulmonary hypertension, has been shown to also partially inhibit 5-HT1B receptors and thus may have a role in treatment of post CP-CPB vasoconstriction, via antagonist effects on 5-HT receptors as well as endothelin (6, 26).
Coronary vasoconstriction has been implicated in myocardial ischemia in a number of clinical situations including stable and variant angina, acute coronary syndrome and after cardiac surgery (27, 28). Other studies have indicated serotonin may contribute to post-ischemic coronary vascular constriction (8, 11, 13, 14, 18). It has been postulated that atherosclerosis may cause a shift in serotonin receptor subtypes in vascular smooth muscle cells (VSMC) by increased receptor mediated Ca++ mobilization (24). Serotonin receptor subtypes have various, and at times opposing, effects on different vascular beds in different species (7, 13, 29). Both 5-HT1B and 2A are expressed on the VSMC, but mediate their responses by different mechanisms. 5-HT1B receptors work through PLA2 and adenylyl cyclase, in contrast 5-HT2A receptors are positively coupled to phospholipase C (29, 30). Although our evidence strongly supports increased PLA2 dependent 5-HT1B signaling, we can not definitively rule out that changes in microvessel reactivity may be due to alternative changes in second messengers or other unaccounted for receptor subtypes.
TS and the TR have also been implicated in causing vasoconstriction after CP-CPB. We have previously shown that COX-2 expression is increased after cardiac operations, and the presence of a COX-2 inhibitor or a thromboxane synthase inhibitor markedly diminishes the contractile response to serotonin in this setting. (12). From that study it appears 5HT elicits the synthesis and release of thromboxane A2. This may be mediated by activation of PLA2 which releases arachidonic acid and leads to production of, among other products, TXA2 (31-33). In the current study we found no significant difference in TS or TR expression, though activity was not measured. The improvement in post-CP-CPB contraction by the PLA2 inhibitor may be secondary to production of less TXA2. Studies measuring levels of TXA2 locally in the presence of a PLA2 inhibitor would be needed to support this.
In addition, the role of platelets and other circulating cells in this process is not accounted for, and they have been shown to be critical (34). It is possible the primary source of 5-HT in the post-cardiac surgery setting is from activated platelets. In a study characterizing the expression of TS it was found to be most abundant in circulating cells, to a lesser degree in spleen, liver and lung, and essentially undetectable in heart tissue (35). Although there does not appear to be a change in TS and TR expression in the atrial microcirculation, circulating blood components, mostly platelets, may be delivering a higher concentration of thromboxane and 5-HT to the vessel and affecting a greater response.
In this study we found an approximately 50% reduction in the vasoconstrictive response using a specific 5-HT1B receptor blocker and 84% reduction with an inhibitor of the second messenger PLA2, both of which should result in significant hemodynamic improvement. However, it also indicates there are other factors contributing to the vasoconstriction. Studies are currently underway to further characterize other mediators of post CP-CPB vasomotor dysfunction.
Limitations
This study has shown the potential role of changes in serotonin receptor expression in coronary vasospasm after cardiac surgery. However, a limitation of this study is the relatively small sample size. There are multiple and varying risk factors in each patient that can have an impact on the results. It is possible that there is a difference depending on the presence of diabetes, hypercholesterolemia and other co-existing conditions, or an effect of medications. Future studies, using a larger number of patients, could be undertaken to further elucidate how specific variables such as comorbidities and medications (eg. β-blockers, amiodarone, ACE inhibitors and insulin) contribute to the observed process. Future studies will also further examine the second messengers involved with the 5-HT1B receptor as they may be different before and after CP-CPB leading to the divergent vasomotor responses. In addition, right atrial tissue was examined as a surrogate for ventricular myocardium. However, previously we have shown that human atrial myocardial microvessels respond similarly to what is observed in the ventricular microcirculation.
Clinical implications
The contraction response demonstrated in this experiment has been implicated as a cause of myocardial ischemia in a number of clinical situations such as angina, acute coronary syndrome and vasospasm after cardiac surgery. It is clearly a complex and multifactorial process mediated by a number of pathways. Antagonizing the increased vasoconstrictive 5-HT1B receptor along with other components in the signaling pathways may allow clinicians to alleviate myocardial ischemia in the post surgical setting.
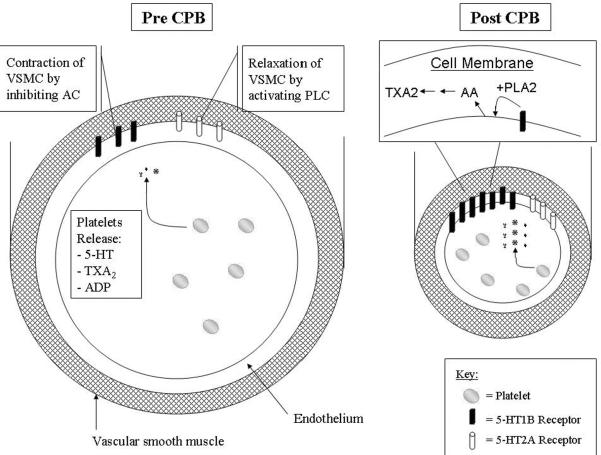
Figure 6. Summary of 5-HT receptor expression before and after cardiopulmonary bypass (CP-CPB).
The expression of 5-HT1B receptor is significantly increased after bypass and associated with vasoconstriction, while expression of 5-HT2A is unchanged after bypass. Serotonin (5-HT), along with TXA2, ADP and other mediators, are released from platelets. Platelets are activated after CP-CPB and degranulate releasing many substances. The endothelium also produces 5-HT. The 5-HT1B receptors are negatively coupled to adenylyl cyclase and the 5-HT2A receptors are positively coupled to phospholipase C. Serotonin activates PLA2 and may lead to further increases in TXA2. All of these signals influence the vascular smooth muscle cells (VSMC) to respond.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sellke FW, Li J, Stamler A, Lopez JJ, Thomas KA, Simons M. Angiogenesis induced by acidic fibroblast growth factor as an alternative method of revascularization for chronic myocardial ischemia. Surgery. 1996 Aug;120(2):182–8. doi: 10.1016/s0039-6060(96)80286-8. [DOI] [PubMed] [Google Scholar]
- 2.Luscher TF, Tanner FC, Tschudi MR, Noll G. Endothelial dysfunction in coronary artery disease. Annu Rev Med. 1993;44:395–418. doi: 10.1146/annurev.me.44.020193.002143. [DOI] [PubMed] [Google Scholar]
- 3.Perrault LP, Mahlberg F, Breugnot C, Bidouard J-P, Villeneuve N, Vilaine J-P, et al. Hypercholesterolemia Increases Coronary Endothelial Dysfunction, Lipid Content, and Accelerated Atherosclerosis After Heart Transplantation. Arterioscler Thromb Vasc Biol. 2000 March 1;20(3):728–36. doi: 10.1161/01.atv.20.3.728. 2000. [DOI] [PubMed] [Google Scholar]
- 4.Buxton AE, Hirshfeld JW, Jr., Untereker WJ, Goldberg S, Harken AH, Stephenson LW, et al. Perioperative coronary arterial spasm: long-term follow-up. Am J Cardiol. 1982 Sep;50(3):444–51. doi: 10.1016/0002-9149(82)90308-3. [DOI] [PubMed] [Google Scholar]
- 5.Skarvan K, Graedel E, Hasse J, Stulz P, Pfisterer M. Coronary artery spasms after coronary artery bypass surgery. Anesthesiology. 1984 Sep;61(3):323–7. doi: 10.1097/00000542-198409000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990 Jul 5;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 2.Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. J Vet Pharmacol Ther. 2008 Jun;31(3):187–99. doi: 10.1111/j.1365-2885.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- 8.Sun H, Mohri M, Shimokawa H, Usui M, Urakami L, Takeshita A. Coronary microvascular spasm causes myocardial ischemia in patients with vasospastic angina. J Am Coll Cardiol. 2002 Mar 6;39(5):847–51. doi: 10.1016/s0735-1097(02)01690-x. [DOI] [PubMed] [Google Scholar]
- 9.Stowe DF, Ebert TJ. Neural and endothelial control of the peripheral circulation--implications for anesthesia: Part II, Endothelium-mediated effects in the normal and diseased circulation. J Cardiothorac Vasc Anesth. 1996 Jan;10(1):159–71. doi: 10.1016/s1053-0770(96)80191-1. [DOI] [PubMed] [Google Scholar]
- 10.Sellke FW, Boyle EM, Jr., Verrier ED. Endothelial cell injury in cardiovascular surgery: the pathophysiology of vasomotor dysfunction. Ann Thorac Surg. 1996 Oct;62(4):1222–8. doi: 10.1016/0003-4975(96)00538-3. [DOI] [PubMed] [Google Scholar]
- 11.Sellke FW, Friedman M, Dai HB, Shafique T, Schoen FJ, Weintraub RM, et al. Mechanisms causing coronary microvascular dysfunction following crystalloid cardioplegia and reperfusion. Cardiovasc Res. 1993 Nov;27(11):1925–32. doi: 10.1093/cvr/27.11.1925. [DOI] [PubMed] [Google Scholar]
- 12.Metais C, Bianchi C, Li J, Simons M, Sellke FW. Serotonin-induced human coronary microvascular contraction during acute myocardial ischemia is blocked by COX-2 inhibition. Basic Res Cardiol. 2001 Feb;96(1):59–67. doi: 10.1007/s003950170078. [DOI] [PubMed] [Google Scholar]
- 13.Cote F, Fligny C, Fromes Y, Mallet J, Vodjdani G. Recent advances in understanding serotonin regulation of cardiovascular function. Trends Mol Med. 2004 May;10(5):232–8. doi: 10.1016/j.molmed.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Ishida T, Hirata K, Sakoda T, Kawashima S, Akita H, Yokoyama M. Identification of mRNA for 5-HT1 and 5-HT2 receptor subtypes in human coronary arteries. Cardiovasc Res. 1999 Jan;41(1):267–74. doi: 10.1016/s0008-6363(98)00162-x. [DOI] [PubMed] [Google Scholar]
- 15.Chester AH, Martin GR, Bodelsson M, Arneklo-Nobin B, Tadjkarimi S, Tornebrandt K, et al. 5-Hydroxytryptamine receptor profile in healthy and diseased human epicardial coronary arteries. Cardiovasc Res. 1990 Nov;24(11):932–7. doi: 10.1093/cvr/24.11.932. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu M, Hata K, Takaoka H, Kanazawa K, Shinke T, Matsumoto H, et al. Sumatriptan provokes coronary artery spasm in patients with variant angina: possible involvement of serotonin 1B receptor. Int J Cardiol. 2007 Jan 8;114(2):188–94. doi: 10.1016/j.ijcard.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Dahlof CG, Mathew N. Cardiovascular safety of 5HT1B/1D agonists--is there a cause for concern? Cephalalgia. 1998 Oct;18(8):539–45. doi: 10.1046/j.1468-2982.1998.1808539.x. [DOI] [PubMed] [Google Scholar]
- 18.Metais C, Li J, Simons M, Sellke FW. Serotonin-induced coronary contraction increases after blood cardioplegia-reperfusion: role of COX-2 expression. Circulation. 1999 Nov 9;100(19 Suppl):II328–34. doi: 10.1161/01.cir.100.suppl_2.ii-328. [DOI] [PubMed] [Google Scholar]
- 19.Berg KA, Clarke WP. Regulation of 5-HT(1A) and 5-HT(1B) receptor systems by phospholipid signaling cascades. Brain Res Bull. 2001 Nov 15;56(5):471–7. doi: 10.1016/s0361-9230(01)00645-1. [DOI] [PubMed] [Google Scholar]
- 20.Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, et al. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg. 2008 May;33(5):906–13. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chilian WM, Layne SM, Klausner EC, Eastham CL, Marcus ML. Redistribution of coronary microvascular resistance produced by dipyridamole. Am J Physiol. 1989 Feb;256(2 Pt 2):H383–90. doi: 10.1152/ajpheart.1989.256.2.H383. [DOI] [PubMed] [Google Scholar]
- 21.Lamping KG, Kanatsuka H, Eastham CL, Chilian WM, Marcus ML. Nonuniform vasomotor responses of the coronary microcirculation to serotonin and vasopressin. Circ Res. 1989 Aug;65(2):343–51. doi: 10.1161/01.res.65.2.343. [DOI] [PubMed] [Google Scholar]
- 23.Chilian WM, Layne SM, Eastham CL, Marcus ML. Heterogeneous microvascular coronary alpha-adrenergic vasoconstriction. Circ Res. 1989 Feb;64(2):376–88. doi: 10.1161/01.res.64.2.376. [DOI] [PubMed] [Google Scholar]
- 24.Ishida T, Kawashima S, Hirata K, Sakoda T, Shimokawa Y, Miwa Y, et al. Serotonin-induced hypercontraction through 5-hydroxytryptamine 1B receptors in atherosclerotic rabbit coronary arteries. Circulation. 2001 Mar 6;103(9):1289–95. doi: 10.1161/01.cir.103.9.1289. [DOI] [PubMed] [Google Scholar]
- 25.Maddahi A, Edvinsson L. Enhanced expressions of microvascular smooth muscle receptors after focal cerebral ischemia occur via the MAPK MEK/ERK pathway. BMC Neurosci. 2008;9:85. doi: 10.1186/1471-2202-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rondelet B, Van Beneden R, Kerbaul F, Motte S, Fesler P, McEntee K, et al. Expression of the serotonin 1b receptor in experimental pulmonary hypertension. Eur Respir J. 2003 Sep;22(3):408–12. doi: 10.1183/09031936.03.00036203. [DOI] [PubMed] [Google Scholar]
- 27.Maseri A, L'Abbate A, Baroldi G, Chierchia S, Marzilli M, Ballestra AM, et al. Coronary vasospasm as a possible cause of myocardial infarction. A conclusion derived from the study of “preinfarction” angina. N Engl J Med. 1978 Dec 7;299(23):1271–7. doi: 10.1056/NEJM197812072992303. [DOI] [PubMed] [Google Scholar]
- 28.Willerson JT, Golino P, Eidt J, Campbell WB, Buja LM. Specific platelet mediators and unstable coronary artery lesions. Experimental evidence and potential clinical implications. Circulation. 1989 Jul;80(1):198–205. doi: 10.1161/01.cir.80.1.198. [DOI] [PubMed] [Google Scholar]
- 29.Villalon CM, Centurion D. Cardiovascular responses produced by 5-hydroxytriptamine:a pharmacological update on the receptors/mechanisms involved and therapeutic implications. Naunyn Schmiedebergs Arch Pharmacol. 2007 Oct;376(12):45–63. doi: 10.1007/s00210-007-0179-1. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Jian B, Chu R, Lu Z, Li Q, Dunlop J, et al. Serotonin mechanisms in heart valve disease II: the 5-HT2 receptor and its signaling pathway in aortic valve interstitial cells. Am J Pathol. 2002 Dec;161(6):2209–18. doi: 10.1016/S0002-9440(10)64497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hassantash SA, Omrani GR, Givtaj N, Afrakhteh M. Pharmacological prevention of the deleterious effects of cardiopulmonary bypass. Asian Cardiovasc Thorac Ann. 2007 Jun;15(3):218–24. doi: 10.1177/021849230701500309. [DOI] [PubMed] [Google Scholar]
- 32.Heying R, van Oeveren W, Wilhelm S, Schumacher K, Grabitz RG, Messmer BJ, et al. Children undergoing cardiac surgery for complex cardiac defects show imbalance between pro- and anti-thrombotic activity. Crit Care. 2006;10(6):R165. doi: 10.1186/cc5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang H, Yao T, Wang W, Zhu D, Zhang W, Chen H, et al. Continuous ultrafiltration attenuates the pulmonary injury that follows open heart surgery with cardiopulmonary bypass. Ann Thorac Surg. 2003 Jul;76(1):136–40. doi: 10.1016/s0003-4975(03)00264-9. [DOI] [PubMed] [Google Scholar]
- 34.Shen RF, Tai HH. Thromboxanes: synthase and receptors. J Biomed Sci. 1998;5(3):153–72. doi: 10.1007/BF02253465. [DOI] [PubMed] [Google Scholar]
- 35.Miyata A, Yokoyama C, Ihara H, Bandoh S, Takeda O, Takahashi E, et al. Characterization of the human gene (TBXAS1) encoding thromboxane synthase. Eur J Biochem. 1994 Sep 1;224(2):273–9. doi: 10.1111/j.1432-1033.1994.00273.x. [DOI] [PubMed] [Google Scholar]