Abstract
Modifying FTY720, an immunosuppressant modulator, led to a new series of well phosphorylated tetralin analogs as potent S1P1 receptor agonists. The stereochemistry effect of tetralin ring was probed, and (−)-(R)-2-amino-2-((S)-6-octyl-1,2,3,4-tetrahydronaphthalen-2-yl) propan-1-ol was identified as a good SphK2 substrate and potent S1P1 agonist with good oral bioavailability.
Keywords: S1P, FTY720, Multiple sclerosis, SphK2, prodrug, tetralin, X-ray
Multiple sclerosis (MS) is a chronic de-myelinating autoimmune disease that progressively worsens over time, affecting the nerves in the brain, spinal cord, and other parts of the central nervous system.1 MS affects two to three times as many women as men with over 400,000 people in the United States having MS and as many as 2,500,000 people affected worldwide.
Among many oral MS therapeutics under development, FTY720 (1, fingolimod) is interesting as it is the first in a new class of disease-modifying treatments called sphingosine 1-phosphate receptor (S1P-R) modulators and has a novel mode of action. 2 FTY720 is a synthetic analog of myriocin, an antifungal antibiotic isolated from entomopathogenic fungus Isaria sinclairii.3–5 Both myriocin and FTY720 are sphingosine analogs that modulate immune responses in animals studies. Initial results from the two-year Phase III FREEDOMS study show that oral FTY720 was superior to placebo in reducing both relapses and disability progression in patients with relapsing-remitting MS (RRMS), and some adverse effects including bradycardia, skin cancer, liver injury, infections, and increased blood pressure were observed during the clinic trials. 6–9
FTY720 is a prodrug that is phosphorylated in vivo by sphingosine kinase 2 (SphK2) to monophosphate FTY720-P (2) 10 which is an agonist of 4 of the 5 S1P receptors (S1P1, 3, 4, 5) but not S1P2. 11,12 Interaction of FTY720-P with S1P1 causes lymphopenia by sequestering lymphocytes in secondary lymphoid organs. Depletion of lymphocytes from the periphery is thought to be the primary mechanism of action for FTY720. 13 S1P3 activation of FTY720-P is thought to be connected with the adverse effects, such as bradycardia and bronchoconstriction in rodents. 14,15
Here we report our effort to further define the molecular pharmacology of the S1P receptor family and SphK2 enzyme. By restricting the two rotatable bonds between the phenyl ring and the aminodiol head portion of FTY720 (1) with an sp3 hybridized backbone, a conformational restricted tetralin analog 3 (Figure 1) was identified, which evokes a profound, long lasting lymphopenia. 16 However, compound 3 was a mixture of two stereoisomers, and a clear understanding of biological activity of each isomer was necessary. In addition, it is reported that FTY720 is phosphorylated stereo specifically by SphK2 to produce the (S)-phosphate 2. 10 This interesting observation prompted us to design the des-OH analog 5. This report presents the synthesis of the two isomers of 3, all four isomers of 5, and the effect of stereochemistry on their in vitro and in vivo activities.
Figure 1.
Further optimization strategy
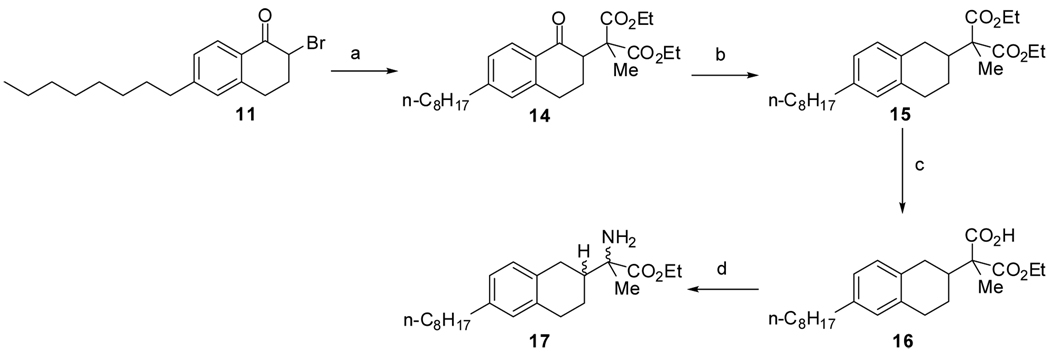
To obtain all isomers of 3 and 5, a convergent route was designed to use a common intermediate 11 (Scheme 1). Starting from commercially available 6-methoxy-3, 4-dihydronaphthalen-1(2H)-one (7), demethylation under HBr gave free phenol 8. The phenol was converted to triflate 9 under mild condition. The C8 tail was installed by Suzuki coupling of 9 with n-octyl-BBN to yield 10. Selective bromination of 10 with CuBr2 in EtOAc/CHCl3 yielded intermediate 11 smoothly.
Scheme 1.
Synthetic scheme for preparation of 11. Reagents and conditions: (a) 48% HBr, 120 °C, 7.5h. 93%; (b) Tf2O, DMAP, 2, 6-Lutidine, CH2Cl2, −30-0 °C, 2h. 96%; (c) i. 9-BBN, 1-octene, THF; ii. 9, KBr, Pd(PPh3)4, K3PO4, H2O, THF, reflux, 3h. 80%; (d) CuBr2, EtOAc, CHCl3, reflux, 2h. 87%.
The diol analog 3 was synthesized as follows (Scheme 2). Displacement of bromide 11 with sodium acetylamino diethylmalonate, followed by reduction gave diester 12. Chiral separation of 12 with ChiralPAK AZ gave (R)-13 and its enantiomer (S) -13. LAH reduction followed by hydrolysis yielded pure (+)-(2’R)-3 and (−)-(2’S)-3. Crystallization of (2’S)-3*HBr in aqueous methanol yielded a single crystal, and its X-ray diffraction established the structure unambiguously (Figure 2). 17
Scheme 2.
Synthetic scheme for preparation of 3. Reagents and conditions: (a) AcNHCH(CO2Et)2, NaH, DMF, 0 °C-rt, overnight, 78%. (b) TiCl4, Et3SiH, CH2Cl2, 0 °C-rt, overnight, 73%. (c) ChiralPAK AZ, Hexane/iPrOH, 92%, 99% ee. (d) LAH, THF, 0 °C -rt, 2h, 59%. (e) LiOH, MeOH/THF/Water, reflux, 5h, 59%.
Figure 2.
X-ray crystal structure of (2’S)-3*HBr salt
The methyl analog 5 was synthesized as illustrated in Scheme 3. Starting from bromide 11, displacement of bromide with sodium methyl diethylmalonate gave ketone 14. Reduction of the arylketone in the presence of the diester was achieved under TiCl4/Et3SiH/DCM condition16 to yield 15 with moderate yield. Other conditions, including hydrogenation (H2/Pd/C with AcOH/MeOH, HCl/EtOH or H2SO4/EtOAc), Zn reduction (with HCl or AcOH), and Et3SiH reduction (with BF3.Et2O or TFA), failed to give acceptable yield of 15, due to either lack of reactivity of the ketone or formation of lactone intermediates. Monohydrolysis of diester 15 under KOH/EtOH yielded acid 16. A very mild Curtius reaction 18 was applied to acid 16 to yield free aminoester 17 directly in one pot.
Scheme 3.
Synthetic scheme for preparation of 17. Reagents and conditions: (a) MeCH(CO2Et)2, NaH, DMF, 0 °C-rt, overnight. 83%; (b) TiCl4, Et3SiH, CH2Cl2, 0 °C-rt. 57%; (c) KOH, EtOH, 60 °C, overnight. 70%; (d) i. DPPA, Et3N, PhMe, reflux, 2h; ii. NaOTMS, THF, 0 °C -rt, overnight. 71%.
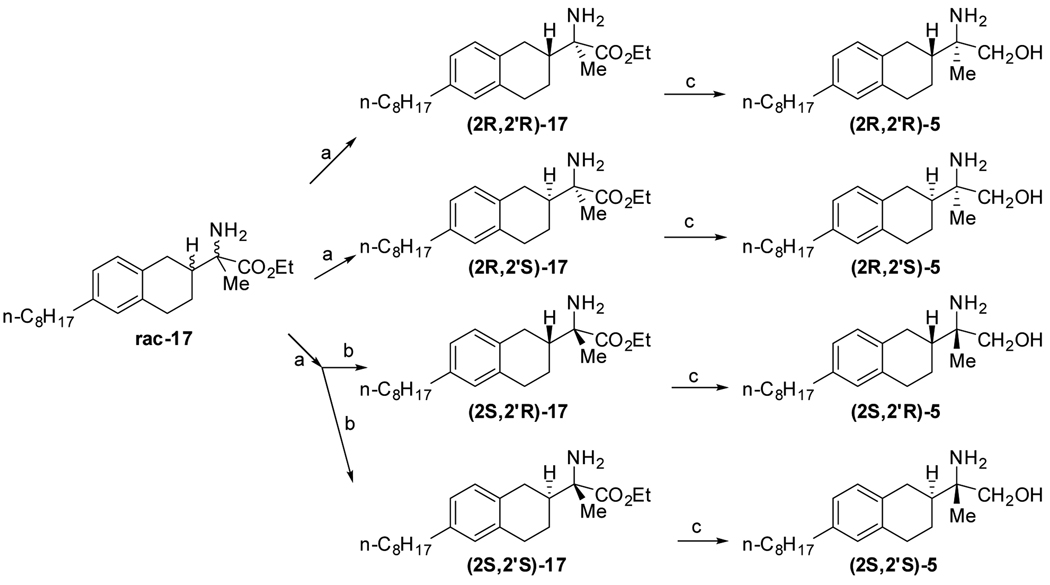
Although 5 could be obtained by direct reduction of aminoester 17, the 4 compounds mixture of 5 couldn’t be separated in our hands, the acid 16 also proved to be problematic. To our delight, a two stages chiral separation can separate all four isomers of 17. Separation with chiral AD-H gave (2R, 2’R)-17, (2R, 2’S)-17 and a mixture of the other two isomers which was further separated with chiral AY to give (2S, 2’R)-17 and (2S, 2’S)-17. (Scheme 4). LAH reduction of pure isomers of 17 yielded (+)-(2R, 2’R)-5, (−)-(2R, 2’S)-5, (+)-(2S, 2’R)-5 and (−)-(2S, 2’S)-5, respectively.20
Scheme 4.
Synthetic scheme for preparation of 5. Reagents and conditions: (a) CHIRALPAK AD-H, ACN/MeOH. 93~98%, 97.6~98% ee; (b) CHIRALPAK AY, Hexane/iPrOH. 79~88%, 96.4~99% ee; (c) LiAlH4, THF, reflux, 3h. 41~85%.
The phosphate of the diol analog 3 was synthesized as illustrated in Scheme 5. The amine (2’R)-3 was protected with Cbz to afford diol 18, which upon phosphorylation under Ag2O/[(BnO)2PO]2O gave the oxazoline 19. Chiral separation of 19 with ChiralPAK AZ gave 20 and its diastereoisomer. Hydrogenation followed by hydrolysis yielded both isomers of (2’R)-3-P.
Scheme 5.
Synthetic scheme for preparation of (2’R)-3-P. Reagents and conditions: (a) Cbz-Cl, KHCO3, EtOAc, H2O, rt, 1.5h, 92%. (b) [(BnO)2PO]2O, Ag2O, hex4NI, CH2Cl2, rt, 3d, 44%. (c) ChiralPAK AZ, ACN/MeOH, 42%, >99.9% de. (d) H2, Pd-C, MeOH, rt, 2h, 91%. (e) LiOH, EtOH/Water, reflux, overnight, 49%.
The phosphate 6 was synthesized as exemplified in Scheme 6. Boc protection of amine 5 followed by phosphorylation and hydrogenation gave Boc-protected phosphoronic acid 24, and acid workup gave phosphate 6.
Scheme 6.
Synthetic scheme for preparation of 6. Reagents and conditions: (a) Boc2O, CHCl3, aq. NaHCO3, rt, overnight. 86%; (b) i. N,N-diethyl-1,5-dihydrobenzo[e][1,3,2]dioxaphosphepin-3-amine, tetrazole,THF, rt, overnight; ii. HOOH, rt, 1h. 74%; (c) Pd-C, H2, MeOH, rt, 1h. 59%; (d) HOAc, HCl, rt, 6h. 86%.
The diol 3 and methyl analog 5 show distinct activity when measuring phosphorylation in vitro on SphK2 (mouse and human) (Table 1). FTY720 is well phosphorylated into (S)-FTY720-P (2). In contrast, both diols (2’R)-3 and (2’S)-3 are poor SphK2 (human) substrates with <10% phosphorylation (9% and 3%, respectively). Phosphorylation of the methyl analogs, however, is much better with (2R, 2’S)-5 showing approximately equal phosphorylation (77% in human and 90% in mouse) to that seen with FTY720. It is interesting to note that (2R, 2’R)-5, the exact methyl analog of better phosphorylated diol (2’R)-3, shows only moderate 38 % phosphorylation by the human kinase. Both (2S, 2’R)-5 and (2S, 2’S)-5 are not substrates for SphK2, this implies that the R configuration of the aminoalcohol head portion is critical for in vivo phosphorylation to convert prodrug (2R, 2’S)-5 into its phosphate (2R, 2’S)-6.
Table 1.
Lymphopenia and phosphorylation of 3 and 5. a
| Compounds | Mouse Lymphopenia ED50, mg/kg |
Mouse PK Phosphoryl ation % |
Phosphoryl ation (mSphK2) % |
Phosphoryl ation (hSphK2) % |
|---|---|---|---|---|
| FTY720 | 0.03 | - | 87 | 76 |
| (2’R)-3 | 0.2 | 3 | 4 | 9 |
| (2’S)-3 | 3.8 | - | - | 3 |
| (2R, 2’S)-5 | 0.1 | 63 | 90 | 77 |
| (2R, 2’R)-5 | 0.8 | - | - | 38 |
| (2S, 2’R)-5 | > 5 | - | - | 0 |
| (2S, 2’S)-5 | > 5 | - | - | 0 |
Assay performed as described in the Reference 19.
The phosphates were also studied in whole cell assay to determine their effect on S1P receptors. It’s known that S1P1 couples to Gi solely and S1P3 couples to Gi and Gq, therefore, both Gi and Gq dependent cellular assays were used to determine agonist/antagonist activity of 3-P and 6 at the human S1P1 or S1P3 receptors (Table 2 and Table 3). In the Gi assay, (2’R)-3-P isomer 1 is similar to (S)-FTY720-P, and is 6 fold less potent at S1P1 (EC50 = 0.077 nM) and 2 fold less potent at S1P3 (EC50 = 0.215 nM). Interestingly, its diastereoisomer (2’R)-3-P isomer 2 is quite active on S1P1 (EC50 = 0.344 nM), while (R)-FTY720-P is less active (EC50 = 23.25 nM). Both (R)-FTY720-P and (2’R)-3-P isomer 2 are not active on S1P3. It should be noted that only (S)-FTY720-P is made in vivo from FTY720. For the methyl analogs at S1P1, compound (2R, 2’S)-6 and (S)-FTY720-P have approximately equal EC50s ( 0.018 nM vs. 0.012 nM, respectively). (2R, 2’R)-6 is also similar to (S)-FTY720-P but less potent. Both (2S, 2’S)-6 and (2S, 2’R)-6 were significantly less active at S1P1 and showed no activity at S1P3. The corresponding aminoalcohols of these two are not substrates for the kinase SphK2. This observation is consistent with FTY720 phosphates. It is interesting that those in vivo disfavored phosphates, (R)-FTY720-P, (2S, 2’S)-6 and (2S, 2’R)-6 show great S1P1/S1P3 selectivity. Clearly, stereochemistry can influence S1P1 and S1P3 activity significantly.
Table 2.
hS1P1 and S1P3 receptors activation on calcium mobilization Gi assay a
| EC50 (nM) | S1P1 | S1P3 |
|---|---|---|
| S1P | 0.027 | 0.449 |
| (S)-FTY720-P | 0.012 | 0.134 |
| (R)-FTY720-P | 23.25 | >5,000 |
| (2’R)-3-P isomer 1 |
0.077 | 0.215 |
| (2’R)-3-P isomer 2 |
0.344 | >5,000 |
| (2R, 2’S)-6 | 0.018 | 0.286 |
| (2R, 2’R)-6 | 0.088 | 0.150 |
| (2S, 2’R)-6 | 19.72 | >5,000 |
| (2S, 2’S)-6 | 13.65 | >5,000 |
Assay performed as described in the Reference 19.
Table 3.
hS1P1-S1P5 receptors activation on calcium mobilization Gq assay a
| EC50 (nM) | S1P1 | S1P2 | S1P3 | S1P4 | S1P5 |
|---|---|---|---|---|---|
| (S)-FTY720-P | 2.02 | >5000 | 27.84 | 22.16 | 0.36 |
| (R)-FTY720-P | >5000 | >5000 | 16.06 | 136.70 | 1988.00 |
| (2’R)-3-P isomer 1 |
1.95 | >5000 | 18.75 | 5.39 | 0.33 |
| (2’R)-3-P isomer 2 |
133.00 | >5000 | 25.72 | 41.45 | 32.96 |
| (2R, 2’S)-6 | 1.79 | >5000 | 97.44 | 15.98 | 1.49 |
| (2R, 2’R)-6 | 5.62 | - | 38.17 | - | - |
| (2S, 2’S)-6 | >5000 | - | 922.10 | - | - |
Assay performed as described in the Reference 19.
For the Gq dependent cellular assay, the trend was similar. (S)-FTY720-P and the (2R) methyl compounds (2R, 2’S)-6, (2R, 2’R)-6 are very active, while their enantiomers (R)-FTY720-P, (2S, 2’S)-6 are not active. Similarly, the diol phosphate (2’R)-3-P isomer 1 (EC50 = 1.95 nM), is as active as (S)-FTY720-P (EC50 = 2.02 nM), and its diastereomer (2’R)-3-P isomer 2 (EC50 = 133 nM) is also active but less so. None of the compounds are active on S1P2 and all are active on S1P3, S1P4 and S1P5. Compared to FTY720, moderate selectivity of S1P1 over S1P3 was observed for (2R, 2’S)-6.
These compounds were investigated further in vivo by measuring their ability to induce mouse lymphopenia (Table 1). Both (2’R)-3 and (2’S)-3 induce lymphopenia, the superior activity of (2’R)-3 (ED50 = 0.2 mg/kg) over (2’S)-3 (ED50 = 3.8 mg/kg) implies (2’R) configuration is favored in the diol context. Interestingly, (2S, 2’R)-5 and (2S, 2’S)-5 are not active, while (2R, 2’S)-5 is very active (ED50 = 0.1 mg/kg), and (2R, 2’R)-5 is less active (ED50 = 0.8 mg/kg). The favored (2’S) configuration in the methyl series is opposite to the diols, the reason for this is not well understood. Clearly, R configuration in the aminoalcohol head portion is necessary for the lymphopenia activity, which is consistent with the observation of FTY720 analogs AAL-R and AAL-S. 11
Both (2’R)-3 and (2R, 2’S)-5 was measured in mouse PK/PD studies at 10 × ED50 for both compounds. 2 mg/kg dose of (2’R)-3 evokes 72 h sustained lymphopenia, with oral bioavailability of 55% and half life t½ = 16 h. Only 3 % conversion of (2’R)-3 to its phosphate was observed in this study which is consistent with the low in vitro phosphorylation (4 % in mouse in vitro assay, Table 1). The low conversion to phosphate for compound (2’R)-3 and low ED50 (0.2 mg/kg) is unexpected, and we do not understand this disconnection. In contrast, 1 mg/kg dose of (2R, 2’S)-5 evokes one-week sustained lymphopenia, with oral bioavailability F = 55%, Vmax = 16 L/Kg, Ci = 5.5 ml/min/kg, half life t½ = 36 h, and 63% phosphorylation. Clearly, (2R, 2’S)-5 is very efficacious with very good oral bioavailability and good phosphorylation.
In summary, a potent, well phosphorylated, orally bioavailable tetralin analog of FTY720 was identified. Stereochemistry on the tetralin ring can significantly influence the phosphorylation and lymphopenia activity, as well as receptor activity. The S1P1 over S1P3 selectivity of this tetralin series was further improved and will be described at a later date.
Supplementary Material
Acknowledgements
These studies were supported in part by a grant from the NIH (R01 GM067958 to KRL and TLM). The authors thank Dr. Douglas M. Ho for performing single-crystal X-ray diffraction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Compston A, Coles A. Lancet. 2008;372:1502. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Hla T. Pharmacol. Res. 2003;47:401. doi: 10.1016/s1043-6618(03)00046-x. [DOI] [PubMed] [Google Scholar]
- 3.Fujita T, Inoue K, Yamamoto S, Ikumoto T, Sasaki S, Toyama R, Chiba K, Hoshino Y, Okumoto T. J. Antibiot. 1994;47:208. doi: 10.7164/antibiotics.47.208. [DOI] [PubMed] [Google Scholar]
- 4.Adachi K, Kohara T, Nakao N, Arita M, Chiba K, Mishina T, Sasaki S, Fujita T. Bioorg. Med. Chem. Lett. 1995;5:853. [Google Scholar]
- 5.Kiuchi M, Adachi K, Kohara T, Minoguchi M, Hanano T, Aoki Y, Mishina T, Arita M, Nakao N, Ohtsuki M, Hoshino Y, Teshima K, Chiba K, Sasaki S, Fujita T. J. Med. Chem. 2000;43:2946. doi: 10.1021/jm000173z. [DOI] [PubMed] [Google Scholar]
- 6.Fujino M, Funeshima N, Kitazawa Y, Kimura H, Amemiya H, Suzuki S, Li XK. J. Pharmacol. Exp. Ther. 2003;305:70. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- 7.Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. N. Eng. J. Med. 2006;355:1124. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, Pohlmann H, Kappos L. Neurology. 2009;72:73. [Google Scholar]
- 9.Novartis. FTY720 FREEDOMS study: Initial results. 2009 [cited; Available from: http://www.novartis.com/newsroom/news/2009-09-30_spotlight-fty720.shtml.
- 10.Albert R, Hinterding K, Brinkmann V, Guerini D, Hartwieg C-M, Knecht H, Simeon C, Streiff M, Wagner T, Welzenbach K, Zecri F, Zollinger M, Cooke N, Francotte E. J. Med. Chem. 2005;48:5373. doi: 10.1021/jm050242f. [DOI] [PubMed] [Google Scholar]
- 11.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. J. Biol. Chem. 2002;277:21453. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 12.Mandala SM, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei G-J, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Science. 2002;296:346. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 13.Marsolais D, Rosen H. Nat. Rev. Drug Disc. 2009;8:297. doi: 10.1038/nrd2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrest M, Sun SY, Hajdu R, Bergstrom J, Card D, Doherty G, Hale J, Keohane C, Meyers C, Milligan J, Mills S, Nomura N, Rosen H, Rosenbach M, Shei G-J, Singer II, Tian M, West S, White V, Xie J, Proia RL, Mandala S. J. Pharmacol. Exp. Ther. 2004;309:758. doi: 10.1124/jpet.103.062828. [DOI] [PubMed] [Google Scholar]
- 15.Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J, Rosen H. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9270. doi: 10.1073/pnas.0501997102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Lynch KR, Macdonald TL. W.O. Patent 2007092638, 2007. Chem. Abstr. 2007;147:235303. [Google Scholar]
- 17.Crystallographic details of (2’S)-3 have been deposited at the Cambridge Crystallographic Data Center and allocated the deposition number CCDC 759847.
- 18.Ma B, Lee W-C. Tetrahedron Lett. 2010;51:385. [Google Scholar]
- 19.Lynch KR, Macdonald TL, Guckian K, Lin EY-S, Ma B. W.O. Patent 2009023854, 2009. Chem. Abstr. 2009;150:259836. [Google Scholar]
-
20.Absolute configuration of (+)-(2R, 2’R)-5 was established by a new synthesis listed in the following scheme from an intermediate (+)-(R)-4-((R)-6-hydroxy-1,2,3,4-tetrahydronaphthal-en-2-yl)-4-methyloxazolidin-2-one (25), whose structure was confirmed by X-ray crystal structure of a brominated derivative (unpublished results). The other isomers were assigned by careful comparison of spectroscopic data.
 Conditions: (a). Tf2O, py, CH2Cl2, rt; Pd(dppf)Cl2, tBuNH2, iPrOH, H2O, n-C6H13CH=CHBF3K, 100 °C. (b). Pd/C, H2, EtOH, rt; LiOH, EtOH, H2O, reflux. 26: 1H NMR (400MHz, CDCl3) δ = 7.12 (d, J = 7.8 Hz, 1 H), 7.06 (s, 1 H), 7.01 (d, J = 7.9 Hz, 1 H), 6.32 (d, J = 15.8 Hz, 1 H), 6.18 (dd, J = 6.7, 15.8 Hz, 1 H), 4.34 (d, J = 8.6 Hz, 1 H), 4.09 (d, J = 8.6 Hz, 1 H), 2.96 - 2.71 (m, 3 H), 2.62 - 2.51 (m, 1 H), 2.19 (q, J = 6.8 Hz, 2 H), 2.00 - 1.84 (m, 2 H), 1.52 - 1.43 (m, 2 H), 1.41 (s, 3 H), 1.31 (br. s., 7 H), 0.93 - 0.85 (m, 3 H). 13H NMR (100MHz, CDCl3) δ = 158.8, 135.89, 135.87, 133.40, 130.7, 129.37, 129.28, 126.2, 123.6, 74.4, 60.0, 43.5, 33.0, 31.7, 30.0, 29.5, 29.4, 28.9, 24.1, 23.4, 22.6, 14.1. LCMS: m/z = 342.20 ([M+1], 100%).
Conditions: (a). Tf2O, py, CH2Cl2, rt; Pd(dppf)Cl2, tBuNH2, iPrOH, H2O, n-C6H13CH=CHBF3K, 100 °C. (b). Pd/C, H2, EtOH, rt; LiOH, EtOH, H2O, reflux. 26: 1H NMR (400MHz, CDCl3) δ = 7.12 (d, J = 7.8 Hz, 1 H), 7.06 (s, 1 H), 7.01 (d, J = 7.9 Hz, 1 H), 6.32 (d, J = 15.8 Hz, 1 H), 6.18 (dd, J = 6.7, 15.8 Hz, 1 H), 4.34 (d, J = 8.6 Hz, 1 H), 4.09 (d, J = 8.6 Hz, 1 H), 2.96 - 2.71 (m, 3 H), 2.62 - 2.51 (m, 1 H), 2.19 (q, J = 6.8 Hz, 2 H), 2.00 - 1.84 (m, 2 H), 1.52 - 1.43 (m, 2 H), 1.41 (s, 3 H), 1.31 (br. s., 7 H), 0.93 - 0.85 (m, 3 H). 13H NMR (100MHz, CDCl3) δ = 158.8, 135.89, 135.87, 133.40, 130.7, 129.37, 129.28, 126.2, 123.6, 74.4, 60.0, 43.5, 33.0, 31.7, 30.0, 29.5, 29.4, 28.9, 24.1, 23.4, 22.6, 14.1. LCMS: m/z = 342.20 ([M+1], 100%).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.