Abstract
Tolerogenic dendritic cells in the tumor microenvironment can inhibit the generation and maintenance of robust anti-tumor T cell responses. Here, we investigated the effects of local delivery of CD40 ligand (CD40L) by tumor-reactive CD8+ T cells on dendritic cell activation and anti-tumor T cell responses in the TRansgenic Adenocarcimona of the Mouse Prostate (TRAMP) model. To increase the immunostimulatory signal, CD40L was engineered, by deleting the majority of the cytoplasmic domain, to increase both its level of expression and duration on the surface of CD8+ T cells. Tumor-reactive CD8+ T cells expressing the truncated form of CD40L stimulated maturation of dendritic cells in vitro and in the prostate draining lymph nodes (PDLN) in vivo. Following dendritic cell maturation, a significantly higher fraction of adoptively transferred, tumor-reactive (reporter) CD8+ T cells was stimulated to express IFN-γ and infiltrate the prostate tissue. The anti-tumor CD8+ T cell response was further enhanced if TRAMP mice were also immunized with a tumor-specific antigen. These findings demonstrate that augmented T cell responses can be achieved by engineering tumor-reactive T cells to deliver stimulatory signals to dendritic cells in the tumor microenvironment. This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
INTRODUCTION
Harnessing the power of the immune system to destroy cancer has been a long-standing objective of cancer immunotherapy. One widely investigated approach has been adoptive cell transfer (ACT), in which tumor-specific T cells are isolated from patients, expanded ex vivo and reinjected back into the patients to destroy tumor cells. Significant success has been achieved with ACT in treating metastatic melanoma patients, reaching over 50% response rates when ACT is coupled with lymphodepleting preconditioning strategies (1-3). Despite this significant progress, transferred T cells can still be inactivated (tolerized) or deleted, limiting their therapeutic effect. Developing strategies to maximize the function of tumor-reactive T cells in vivo may further increase the clinical impact of T cell-based immunotherapies.
Like most tissue antigens, tumor antigens are cross-presented by specialized antigen-presenting cells, such as dendritic cells (DCs). Mature dendritic cells displaying tumor antigens can initiate productive anti-tumor T cell responses. However, DCs that have been exposed to tumor-derived factors, including VEGF, TGFβ, IL-6, PGE2 and IL-10, tend to anergize T cells (4-9). Such tolerogenic DCs have been found in both tumors and tumor draining lymph nodes (TDLNs). Regardless of their tissue origin, they generally share the ability to induce development of CD4+ and CD8+ regulatory T cells and anergy of antigen-specific T cells (10). Thus, to increase the therapeutic efficacy of adoptively transferred T cells, it is critical to activate tolerogenic DCs in the tumor environment.
CD40 and CD40 ligand (CD40L) are members of the TNF family, and their interaction provides a potent signal for DC activation (11). CD40L expression is tightly regulated, being transiently expressed on the surface of activated CD4+ T cells for less than 24 hrs (11). To explore CD40 ligation as a strategy to activate tolerogenic DCs, systemic administration of agonist anti-CD40 antibodies has been investigated. In mice, such treatment has been shown to mature DCs and replace the need for CD4+ T cell help (12-14). Based on these observations, CD40 ligation has been used to boost the CD8+ T cell response to tumors and to break peripheral self-tolerance (15-17). The consequences of these treatments have proven to be system dependent in murine models, though, as significant immune suppression has been observed as well (18-21). In humans, anti-CD40 monoclonal antibodies (22-26), recombinant soluble CD40L protein (27), and CD40L-expressing autologous tumor cells (28, 29) have been evaluated clinically to treat cancer patients. Although the initial phase I clinical results have shown significant objective anti-tumor responses (30), and no major systemic toxicity has been observed, transient cytokine release syndrome has been a side-effect with several of the agonist anti-CD40 monoclonal antibodies (30). Because elevated CD40 activation has also been implicated in the progression of systemic lupus erythematosus (31), rheumatoid arthritis (32), type 1 diabetes (33), neurodegenerative disorders (34, 35), and allograft rejection (36-38), systemic activation of CD40 could potentially induce autoimmunity. To overcome the variable outcomes and circumvent potential side-effects associated with systemic CD40 ligation, CD40L or anti-CD40 could be delivered locally in the TDLNs and/or tumor tissue.
In this study, we report a new strategy to locally deliver stimulatory CD40L signals using tumor-reactive CD8+ T cells, which naturally traffic to TDLNs. To increase the stimulatory signal, we identified and used a mutant murine CD40L, which lacks the majority of its cytoplasmic domain, to increase both the expression level and duration on the surface of CD8+ T cells. Using an antigen-specific TRAMP model, we show that transferred CD40L-expressing tumor-specific CD8+ T cells can stimulate the maturation of dendritic cells in the PDLNs and augment anti-tumor responses of adoptively transferred, tumor-specific reporter CD8+ T cells. These findings demonstrate that augmented anti-tumor T cell responses can be induced by engineering T cells to deliver a CD40L-mediated stimulatory signal to dendritic cells in the tumor environment.
MATERIALS AND METHODS
Mice, Influenza Virus, Antibodies and Flow Cytometry
TRP-SIY mice were generated by expressing a nominal antigen SIYRYYGL (SIY) in the prostate tissue of TRAMP mice, as reported previously (39). Mice used for all experiments were 3.5 to 4.5 month old heterozygous males. The RAG1-/- 2C TCR transgenic (2C/RAG) mice were maintained on C57BL/6 or C57BL/6 Thy1.1 backgrounds. Recombinant WSN-SIY influenza virus expressing a SIY-neuraminidase fusion protein was described previously (40). For infection, TRP-SIY mice were infected intranasally with 100pfu of WSN-SIY virus in 50μL PBS. Mice were maintained in a specific pathogen-free facility, and all animal experiments were performed in compliance with the institutional guidelines on animal care.
Agonist anti-CD40 antibody (FGK45.5) was a gift from Dr. A. Rolink of the Basal Institute for Immunology, Basel, Switzerland (41). Antibodies to CD40L (eBiosciences), CD8 (BioLegend), Thy1.1 (BioLegend), CD11c (BioLegend), CD80 (BD Pharmingen) and CD86 (BD Pharmingen) were conjugated to either FITC, PE or APC. The 2C TCR was identified using a biotin-conjugated 1B2 clonotypic antibody, detected with streptavidin-APC (BioLegend). Retrovirally-transduced 2C T cells were identified using 1B2 plus an antibody to either Thy1.1 or CD8. Antibody-stained cells were analyzed using a FACSCalibur™ (BD Biosciences) instrument, and the data was processed and evaluated using FlowJo™ software (Tree Star).
Intracellular IFN-γ staining was performed using a BD Cytofix/Cytoperm Kit (BD Biosciences). Briefly, 2C T cells were stimulated in vitro with 1μg/mL SIY peptide for 4hr at 37°C in the presence of BD GolgiPlug™ containing brefeldin A. Cells were stained for 2C TCR, plus CD8 or Thy1.1, and dead cells were marked using a LIVE/DEAD® Fixable Red Dead Cell Stain Kit (Invitrogen). Following fixation and permeabilization, cells were stained intracellularly with a PE-conjugated anti-IFN-γ antibody (BD Pharmingen).
Anti-CD40 Treatment
TRP-SIY mice were injected intraperitoneally with 500μL of either PBS or 200μg anti-CD40 antibody in PBS daily for three days. One day after the first anti-CD40 injection, the treated mice were injected retroorbitally with 100μL PBS containing 1-1.5×106 naïve 2C cells from the spleens and lymph nodes of 2C/RAG mice. Immediately following T cell transfer, mice were infected intranasally with WSN-SIY virus (39). Five and ten days post infection (dpi), 2C T cells from the PDLN, spleens and peripheral lymph nodes (PLN) were analyzed for IFN-γ expression, as above.
Construction of Retroviral Vectors Expressing Wildtype and Mutant CD40L
pMemCD40L, which contains full-length murine CD40L (GenBank accession no. X65453.2) (42), was obtained from Dr. Richard Kornbluth of the University of California at San Diego. A BglII/SalI generated full-length CD40L fragment (wild type) was then subloned into a retroviral vector that expresses Thy1.1. Mutation of tyrosine at amino acid residue 5 to alanine (TAC→GCC) of CD40L (CD40L Y5A) was performed through site-directed mutagenesis, using a QuikChange II Site-Directed Mutagenesis Kit (Stratagene). To delete the first thirteen amino acid residues of the N-terminal cytoplasmic domain of CD40L (CD40L Δ1-13), amino acid residue 12 was first mutated from TCC to TCT through site-directed mutagenesis to generate a Bgl II site. The deletion was then completed by Bgl II digestion and subcloning into the retroviral vector above. Following ligation, a start codon was reintroduced at amino acid 13 (GTG→ATG). Retroviruses expressing wildtype and mutant CD40L were produced in 293FT cells via co-transfection with pCL-Eco (Imgenex), which encodes the retroviral packing components, using TransIT-LT1 (Mirus).
Retrovirus Infection and Generation of In Vitro Memory T Cells
Naïve 2C cells were pooled from the spleens and lymph nodes of 2C/RAG mice following lysis of red blood cells. 2C cells were stimulated for 36 hrs with 1μg/mL SIY peptide in RPMI plus 10%FCS, 50μM 2-mercaptoethanol, 10mM HEPES, 4mM L-glutamine, 100U/mL penicillin, 100ug/mL streptomycin and 50U/mL IL-2. Activated 2C cells were then transduced by spin infection with the indicated retroviral supernatant plus 4μg/mL polybrene (American Bioanalytical), and cultured for another 48 hrs at 5×105 cells/mL in the above medium. Cells were then rinsed and cultured in the same medium, except that IL-2 was substituted with recombinant murine IL-7 (Peprotech), for 6 days to generate in vitro memory T cells.
In Vitro BMDC Maturation Assay
Retrovirally-transduced in vitro memory 2C cells were restimulated by mixing the cells 1:1 with C57BL/6 splenocytes in the presence of 1μg/mL SIY peptide for 48hrs. CD40L-expressing 2C cells were purified by sorting for Thy1.1+ cells. The sorted T cells were then co-cultured at a 10:1 ratio with day 6 immature bone marrow derived dendritic cells (BMDCs). To generate immature BMDCs, bone marrow was harvested from the femurs of C57BL/6 mice. After lysis of red blood cells, the cells were resuspended at 2×105 cells/mL and cultured for 6 days in RPMI plus 10%FCS, 50uM 2-mercaptoethanol, 4mM L-glutamine, 100U/mL penicillin, 100ug/mL streptomycin and J5 cell supernatant containing GM-CSF. As controls, BMDCs were cultured alone, either in the presence or absence of 1μg/mL lipopolysaccharide (LPS). In the absence of LPS, control BMDCs were either left unmanipulated or were taken off the tissue culture plate and concentrated (107 cells/mL), to simulate the other co-culture conditions. Following a 24hr coculture, maturation of BMDCs was evaluated by flow cytometry for CD11c, CD86 and CD80 expression.
In Vivo DC Activation and Reporter T Cell Response
In vitro memory 2C cells either expressing or not expressing CD40L were transferred into TRPSIY mice (1-5 ×106 cells/mouse). A subset of mice was also infected with WSN-SIY. Six days later, the maturation status of the dendritic cells in the PDLN, PLN, mediastenial lymph nodes (MLN) and/or spleens was evaluated by assaying for CD11c, CD11b, CD86 and CD80 expression. Alternatively, 6 days following the transfer of in vitro memory 2C cells, naïve Thy1.1High reporter 2C cells were transferred into the TRP-SIY mice. Five and 10 days post transfer, reporter 2C cells from the PDLN, PLN and spleens were evaluated for IFN-γ expression, as above.
RESULTS
Systemic anti-CD40 treatment breaks tolerance transiently in TRAMP mice
We have previously developed transgenic mice that express a nominal antigen SIYRYYGL (SIY) in the prostate tissue that can be recognized by CD8+ T cells displaying the 2C TCR (39). Introduction of the transgene into TRansgenic Adenocarcimona of the Mouse Prostate (TRAMP) mice results in the generation of double transgenic TRP-SIY mice, which develop SIY-expressing prostate cancer. When TRP-SIY mice are infected intranasally with a SIY-expressing influenza virus (WSN-SIY), adoptively transferred 2C cells become fully activated, infiltrate the prostate tissue, but rapidly lose their function in the tumor tissue (39).
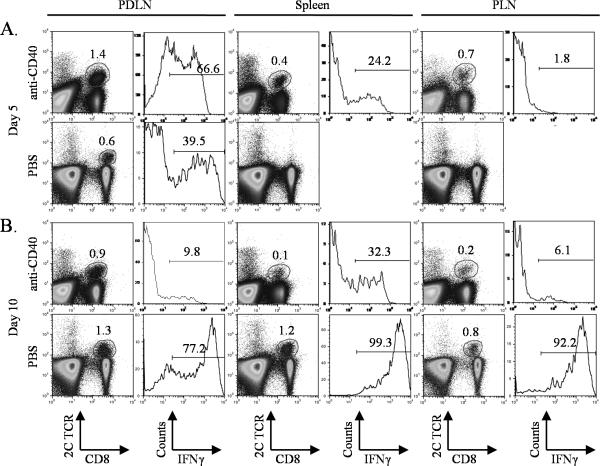
To determine the effects of anti-CD40 treatment on T cell activation and function, TRP-SIY mice were injected with either PBS or an agonist anti-CD40 antibody one day before, on the same day and one day after 2C cell transfer and WSN-SIY infection. Five days post infection (dpi), ~67% of 2C cells from the prostate draining lymph nodes (PDLN) of anti-CD40 treated mice were stimulated to produce IFN-γ, whereas less than 40% of 2C cells from the PDLN of PBS-treated mice produced IFN-γ (Fig. 1A). However, by 10 dpi, only ~10% of 2C cells from the PDLN of anti-CD40-treated mice still expressed IFN-γ, whereas 77% of 2C cells from the PDLN of PBS-treated mice expressed IFN-γ (Fig. 1B). Although anti-CD40 treatment also resulted in an earlier appearance of 2C T cells in the spleens and peripheral lymph nodes (PLN), most of these cells were incapable of producing IFN-γ. In contrast, in PBS-treated mice, almost all 2C cells from the spleens and PLN were able to produce IFN-γ. These results suggest that while systemic anti-CD40 treatment can stimulate a tumor-specific CD8+ T cell response transiently in the PDLN of TRP-SIY mice, it can lead to severe immune suppression in the long-term.
Figure 1. Effect of systemic anti-CD40 treatment on a tumor-reactive CD8+ T cell response in TRP-SY mice.
TRP-SIY mice were injected with PBS or anti-CD40 antibody on days -1, 0 and +1. On day 0, mice were injected with naïve 2C cells and infected with WSN-SIY virus. Five (A) and ten (B) days post infection, single cell suspensions were prepared from the PDLN, spleens and PLN, and cells were restimulated in vitro with SIY peptide for 4hr. Samples were stained for 2C TCR, CD8 and intracellular IFN-γ. Dot plots show 2C TCR versus CD8 staining profiles of live cells. Histograms show IFN-γ expression of 2C TCR+CD8+ cells. Numbers indicate percentage of positive cells. Representative data from one of two similar experiments are shown.
Truncation of the cytoplasmic domain of CD40L leads to increased levels and duration of surface expression
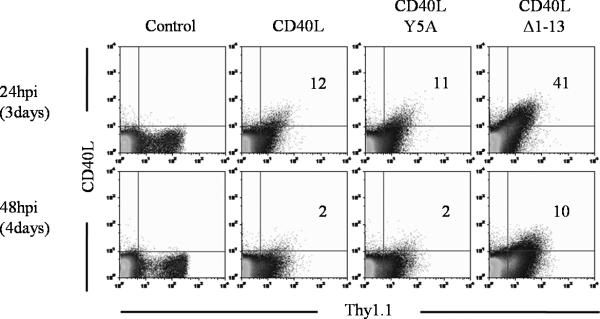
One approach to deliver localized CD40 stimulation is to express CD40L on tumor-reactive CD8+ T cells, which naturally traffic to TDLNs and tumor tissues. Thus, we constructed a retrovirus expressing CD40L and the surface marker Thy1.1. To test CD40L expression on CD8+ T cells, 2C T cells were activated with SIY peptide for 36 hrs, spin-infected with the retrovirus, and were monitored for CD40L and Thy1.1 expression. Twenty-four hrs post retroviral transduction, 12% of Thy1.1+ cells were also CD40L+ (Fig. 2). However, by 48 hrs post transduction, only 2% of Thy1.1+ cells were still CD40L+. Because both CD40L and Thy1.1 expression were driven by the same viral LTR, these results suggest that CD40L expression is tightly regulated post-translationally on the surface of CD8+ T cells, as observed with CD4+ T cells (11).
Figure 2. Surface expression of wildtype and mutant CD40L.
Naïve 2C cells were activated with SIY peptide plus IL-2 for 36hrs. Cells were then transduced with a retrovirus expressing Thy1.1 alone (control), Thy1.1 plus the wild type CD40L, CD40L Y5A or CD40L Δ1-13, and were analyzed for CD40L expression 24hrs and 48hrs later. The time in parentheses corresponds to days post stimulation. Dot plots show CD40L versus Thy1.1 staining profiles of live cells. Numbers indicate the percentage of Thy1.1+ cells that are CD40L+. Transduction efficiencies were 20-25% in all cases, and representative data from one of at least two similar experiments are shown.
To achieve a more sustained and higher level of CD40L expression, we constructed CD40L mutants with potentially reduced susceptibility for receptor-mediated endocytosis (43). In one mutant, referred to as CD40L Y5A, a tyrosine at position 5 was mutated to an alanine, as this residue was thought to be critical for the maintenance of a conserved endocytosis domain (43). However, the mutant displayed expression kinetics and levels comparable to the wild type CD40L (Fig. 2), suggesting this mutation has no significant impact on CD40L expression on CD8+ T cells. In the second mutant, referred to as CD40L Δ1-13, the terminal 13 amino acid residues were deleted from the 22 amino-acid intracellular domain. With this mutant, 41% of Thy1.1+ 2C cells expressed surface CD40L by 24 hrs post retroviral transduction, and 10% of Thy1.1+ 2C cells still expressed CD40L by 48 hrs post transduction (Fig. 2). In addition, the level of CD40L Δ1-13 expression was substantially higher than wildtype CD40L expression at 24 hrs post transduction. Therefore, deletion of the terminal 13 amino acid residues significantly increases the level and duration of CD40L expression on activated CD8+ T cells. CD40L Δ1-13 was used for all subsequent experiments.
CD40L-expressing 2C cells stimulate maturation of dendritic cells in vitro and in vivo
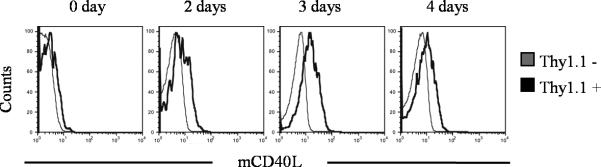
Our strategy for localized CD40 ligation was to engineer tumor-reactive 2C T cells to express CD40L Δ1-13. Because even the truncated form of CD40L is only transiently expressed following T cell activation, an important requirement for the success of this approach is that the retrovirally-transduced CD8+ T cells express CD40L again when they encounter antigen a second time. Thus, retrovirally-transduced 2C cells were transitioned into a memory phenotype in vitro (Supplemental Fig. 1) to ensure they could respond to a second antigen encounter in TRP-SIY mice. In order to demonstrate the ability of these retrovirally-transduced in vitro memory 2C cells to re-express CD40L, the cells were stimulated with SIY peptide 10 days after transduction, long after CD40L had ceased expression. The restimulated 2C cells began to upregulate CD40L two days after restimulation, reached maximal levels of expression three days after restimulation, and began to downregulate expression by four days after restimulation (Fig. 3). Therefore, the retrovirally-transduced in vitro memory CD8+ T cells are capable of re-expressing CD40L when they encounter antigen again.
Figure 3. Kinetics of CD40L Δ1-13 re-expression on retrovirally-transduced in vitro memory 2C cells.
Naive 2C cells were activated with SIY peptide plus IL-2 for 36hrs, and were then transduced with a retrovirus expressing Thy1.1 and CD40L Δ1-13. Cells were cultured for 2 additional days in IL-2 and then transitioned into a memory phenotype by culturing them for 6 days in IL-7. In vitro memory cells were restimulated with SIY peptide plus IL-2 in the presence of a 1:1 mix of C57BL/6 splenocytes, and were stained for Thy1.1, 2C TCR, and CD40L on days 2, 3 and 4. Histograms show expression on 2C TCR+Thy1.1+ transduced cells or 2C TCR+Thy1.1− non-transduced cells. Representative data from one of at least two similar experiments are shown.
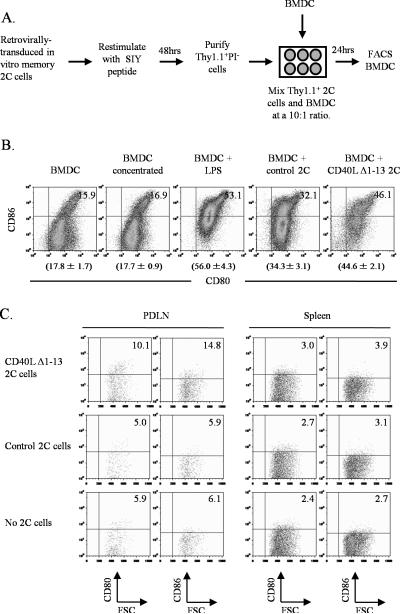
To demonstrate the functionality of CD40L expression on CD8+ T cells, we determined whether CD40L-expressing 2C cells could mature bone marrow-derived dendritic cells (BMDCs) in vitro. 2C T cells were transduced with the CD40L Δ1-13 retrovirus, transitioned in vitro into memory cells, restimulated with SIY peptide for 48hrs, and then co-cultured with immature BMDCs (Fig. 4A). Twenty-four hrs later, the maturation status of the BMDCs was evaluated by assaying for CD80 and CD86 expression. As controls, BMDCs were cultured alone, with control retrovirally-transduced in vitro memory 2C cells that do not express CD40L, or with lipopolysaccharide (LPS). Approximately 15% of BMDCs expressed both CD80 and CD86 when cultured alone, whereas ~55% BMDCs expressed both markers following stimulation with LPS. Although ~34% of BMDCs expressed both CD80 and CD86 when cultured with 2C cells that did not express CD40L, a significantly higher percentage (~45%) of BMDCs expressed both markers when cultured with CD40L-expressing 2C T cells (Fig. 4B). Thus, CD40L-expressing 2C cells can stimulate BMDC maturation in vitro.
Figure 4. CD40L Δ1-13-expressing 2C cells simulate maturation of dendritic cells in vitro and in vivo.
Schematic diagram (A) and results (B) of in vitro BMDC maturation assay. Retrovirally-transduced in vitro memory 2C cells were generated as in Figure 3 and restimulated with SIY peptide for 48hrs. Thy1.1+ transduced 2C cells were purified by cell sorting and cultured with BMDCs for 24 hrs. Cells were stained for CD11c, CD80 and CD86. Dot plots show CD80 and CD86 expression of CD11c+ BMDCs under the indicated conditions. Numbers in graphs indicate the percentage of CD80+CD86+ cells. Numbers below graphs indicate the average percentage of CD80+CD86+ cells ± standard deviation (SD). Representative data from one of at least two similar experiments are shown. P value is <0.05 when percentages of CD80+CD86+ BMDCs were compared between samples stimulated with either control 2C cells or CD40L Δ1-13 2C cells. C, In vivo dendritic cell maturation. In vitro memory 2C cells either expressing or not expressing CD40L were transferred into TRP-SIY mice. Six days later, cells from the PDLN and spleens were pooled from 5 mice and stained for CD11c, CD11b, CD80 and CD86. Dot plots show CD80 and CD86 expression of CD11c+CD11b+ cells. Numbers indicate the percentage of positive cells. Representative data from one of at least two similar experiments are shown.
We also determined if CD40L-expressing (therapeutic) 2C cells could stimulate maturation of dendritic cells in the PDLN of TRP-SIY mice. Retrovirally-transduced in vitro memory 2C cells begin to reexpress CD40L in the PDLN approximately 5 days after transfer (Supplemental Fig. 2). We therefore evaluated the maturation status of CD11c+CD11b+ dendritic cells in the PDLN, spleens and PLN 6 days after transferring in vitro memory 2C cells either expressing or not expressing CD40L (Fig. 4C). A comparable fraction of dendritic cells from the PDLN of mice that received either no cells or control 2C cells expressed CD80 and/or CD86, whereas a higher fraction of dendritic cells from the PDLN of mice that received CD40L-expressing 2C cells expressed CD80 and/or CD86 (Fig. 4C). Minimal difference was seen in the maturation of dendritic cells in the spleens of these mice (Fig. 4C), and no difference was seen in the PLN (data not shown). These results suggest that CD40L-expressing 2C cells can stimulate dendritic cell maturation in the PDLN of TRP-SIY mice, where they encounter tumor-derived antigen.
CD40L-expressing 2C cells stimulate a more robust anti-tumor T cell response
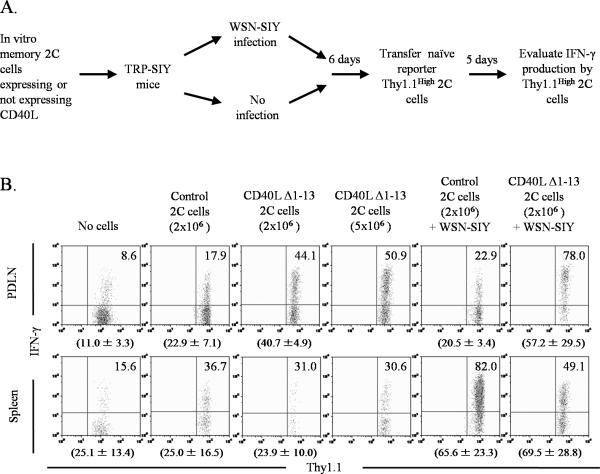
The ability of CD40L-expressing 2C cells to stimulate local DC maturation is expected to stimulate a more robust T cell response in TRP-SIY mice. Thus, TRP-SIY mice were injected with in vitro memory 2C cells either expressing or not expressing CD40L, or were given no cells. Some of the TRP-SIY mice were also infected with WSN-SIY at the time of 2C cell transfer (Fig. 5A). When dendritic cells had matured locally in the PDLN, approximately 6 days following transfer (Fig. 4C), naïve Thy1.1High reporter 2C cells were transferred into the mice. Since Thy1.1 expression on the reporter cells is at least a log higher than on the retrovirally-transduced therapeutic 2C cells, the populations could be clearly differentiated (data not shown). Five days later, reporter 2C cells from the PDLN, spleens and PLN were evaluated for their ability to express IFN-γ following in vitro restimulation (Fig. 5A). Without transfer of therapeutic 2C cells, only ~10% of the reporter 2C cells from the PDLN of TRP-SIY mice were able to express IFN-γ (Fig. 5B). This baseline level increased to ~20% if mice were transferred with control in vitro memory 2C cells, either with or without a WSN-SIY infection. In contrast, the fraction of reporter 2C cells that could express IFN-γ increased to ~40% if the mice were transferred with CD40L-expressing 2C cells. If the mice received a WSN-SIY infection at the time the CD40L-expressing 2C cells were transferred, up to 75% of the reporter 2C cells in the PDLN could express IFN-γ (Fig. 5B). A smaller difference was observed in the percentages of reporter 2C cells that could express IFN-γ in the spleens at this timepoint, consistent with minimal effects of CD40L-expressing 2C cells on the maturation of the dendritic cells in the spleens (Fig. 4C). No significant or consistent trends were observed in the total reporter cell numbers in the PDLN, spleens or PLN between the different treatment groups (data not shown).
Figure 5. CD40L Δ1-13-expressing 2C cells stimulate a more functional anti-tumor T cell response in TRP-SIY mice.
A, Schematic diagram of experimental protocol. In vitro memory 2C cells either expressing or not expressing CD40L Δ1-13 were transferred into TRP-SIY mice. As a control, some TRP-SIY mice were not injected with any in vitro memory 2C cells. The mice were divided into two groups. One group was infected intranasally with WSN-SIY and the other group was not infected. Six days later, naïve Thy1.1High reporter 2C cells were transferred into all mice. Five days after transfer, reporter 2C cells were analyzed for IFNγ expression. B, Reporter 2C T cell responses. Cells from the PDLN and spleens of above treated mice were restimulated with SIY peptide for 4hr, and then stained for Thy1.1, CD8 and intracellular IFN-γ. Dot plots show IFN-γ versus Thy1.1 staining profiles gating on Thy1.1High CD8+ reporter 2C cells. Numbers in graphs indicate the percentage of IFN-γ+ cells. Numbers below graphs indicate the average percentage of IFN-γ+ cells ± SD of the analyzed tissues. Representative data from one of two experiments are shown.
By 10 days after reporter 2C cell transfer, the effector 2C cells had exited the PDLN, and the immune response had begun to contract. Even at this timepoint, more reporter 2C cells were able to produce IFN-γ in the periphery of mice that received CD40L-expressing 2C cells than in those that received control 2C cells (Supplemental Fig. 3). Furthermore, 4-6 times as many reporter 2C cells infiltrated the prostate tissue of TRP-SIY mice that received CD40L-expressing 2C cells than those that received control 2C cells (Supplemental Fig. 3). However, there was no difference in the ability of the reporter 2C cells from the prostate tissue to express IFN-γ.
Together, these results suggest that the transfer of CD40L-expressing therapeutic 2C cells conditions TRP-SIY mice for more robust anti-tumor T cell responses, which can be further enhanced by active immunization.
DISCUSSION
One problem that limits the efficacy of adoptive T cell therapies is rapid tolerization or deletion of transferred tumor-reactive T cells in cancer patients. Although recent studies have shown that younger or central memory-like CD8+ T cells are more potent and persist longer than effector memory-like T cells in the setting of ACT (44, 45), their increased requirement for costimulatory support may heighten the influence of tolerogenic DCs on these transferred cells. Because of its critical and natural role in DC activation, CD40 ligation has been explored to activate tolerogenic DCs in the tumor environment. Although systemic administration of agonist anti-CD40 antibodies has been shown to replace the need for CD4+ T cell help (12-14), boost CD8+ T cell responses to tumors and break peripheral self-tolerance (15-17), there is also evidence that it can induce immune suppression (18-21). Similarly, we found that systemic administration of an agonist anti-CD40 antibody in TRP-SIY mice initially stimulated an anti-tumor CD8+ T cell response, but eventually led to severe immune suppression, in the context of an influenza infection (Fig. 1). In addition, systemic anti-CD40 administration is associated with significant side effects (data not shown). The complicated and variable outcomes of systemic CD40 ligation in immune responses highlight the need to induce CD40 ligation locally in the tumor tissue.
Here, we report a novel strategy to activate tolerogenic DCs by using tumor-reactive CD8+ T cells to deliver an activating CD40L signal in the tumor environment. This strategy allows us to localize the immunostimulatory signal in both time and space. CD40L is normally expressed on activated CD4+ T cells for less than 24 hrs (11), and its expression on activated CD8+ T cells is similarly transient. Because CD40L transcription is driven by a retroviral LTR in our study, the tight regulation of CD40L expression on the surface of CD8+ T cells is likely regulated at the post-translational level. Supporting this notion, deletion of the terminal 13 amino acid residues of the cytoplasmic domain led to a higher level and extended duration of CD40L expression on the surface of CD8+ T cells. This deletion mutant is designed to minimize receptor-mediated endocytosis, but does not likely impact other regulatory mechanisms that also control CD40L expression, such as down regulation of CD40L transcription, proteolytic cleavage, and release of soluble CD40 (11), and thus surface CD40L expression is still transient. We used in vitro memory 2C T cells to deliver CD40L to the correct location, because they recognize a tumor-derived epitope (SIY) and naturally traffic to the TDLNs and tumor tissue. Following adoptive transfer, only those retrovirally-transduced 2C cells that traffic to the PDLN have the opportunity to encounter SIY and re-express CD40L. Even with truncation of the majority of the cytoplasmic domain, CD40L is only expressed on the surface of 2C T cells for 48 to 72 hrs after activation (Fig. 2). Consequently, delivery of the immunostimulatory CD40L signal is limited spatially and temporally. This should minimize the potential autoimmune complications and immune suppression associated with systemic CD40 ligation.
Like CD40L expressed on activated CD4+ T cells, CD40L expressed on the surface of activated CD8+ T cells also stimulates maturation of DCs both in vitro and in vivo (Fig. 4). Co-culture of BMDCs with CD40L-expressing 2C cells for 24 hrs is sufficient to stimulate up-regulation of CD80 and CD86 on DCs, to a similar extent as observed with LPS stimulation (Fig. 4B). Similarly, transfer of CD40L-expressing 2C cells into TRP-SIY mice stimulated up-regulation of CD80 and CD86 on some DCs in the PDLN. Despite the modest observable effect on DC maturation in vivo, the anti-tumor CD8+ T cell response was significantly enhanced, as indicated by the increased percentage of reporter 2C cells that could produce IFNγ in the PDLN and infiltrate the prostate tissue. The enhanced anti-tumor response was even more dramatic when the CD40L-expressing 2C cells were activated by an influenza infection, as opposed to by the tolerizing environment of the tumor (Fig. 5). Following influenza infection, a higher fraction of retrovirally-transduced 2C T cells re-expressed CD40L, which simulated maturation of more DCs in the PDLN (Supplemental Fig. 4). Thus, augmented anti-tumor T cell responses can be induced by engineering T cells to deliver a CD40L-mediated stimulatory signal to dendritic cells in the TDLNs.
There are two critical environments in which tolerance needs to be prevented or broken for adoptive T cell transfer to be most effective: the TDLNs and the tumor itself. If tolerance is broken in the tumor, but not in the TDLNs, adoptively transferred T cells may be tolerized upon initial transfer. This is particularly true for central memory-like T cells that traffic through the secondary lymphoid tissues prior to entering the tumor. If tolerance is broken in the TDLNs, but not in the tumor, adoptively transferred T cells may be tolerized upon entering the tumor tissue. Our approach breaks tolerance in the lymphoid tissues, but not in the tumor itself. As the retrovirus-mediated CD40L expression is transient, the engineered 2C cells no longer express CD40L when they infiltrate the tumor, approximately 7 days post transfer (data not shown, (39)). As a result, neither dendritic cells nor CD40-expressing tumor cells in the prostate tissue are impacted. Thus, following transfer of CD40L-expressing therapeutic 2C cells, adoptively transferred reporter 2C cells become productively primed in the PDLN, maintain their function in the periphery, infiltrate the tumor tissue extensively, but become rapidly tolerized in the tumor (Supplemental Fig. 3). Since the tumor-infiltrating T cells become tolerized, we did not evaluate alterations in disease progression or overall survival of treated mice. To overcome tolerance in both the TDLNs and tumor tissue, our approach needs to be combined with other approaches that break tolerance in the tumor tissue. Alternatively, CD40L could be further engineered to achieve more prolonged surface expression.
Recent pre-clinical and clinical studies have made significant progress in advancing the success of adoptive cell therapy in the clinic. T cell engineering strategies may further augment the promise of such approaches. Our study demonstrates that adoptively transferred T cells can be engineered to deliver functional signals to dendritic cells in the tumor environment, and that those dendritic cells can then stimulate more robust T cell responses. As we better understand the critical role that dendritic cells play in the activation, maintenance and tolerization of T cells in the tumor environment, similar approaches can be explored to engineer tumor-reactive CD8+ T cells to deliver important functional signals to dendritic cells to overcome tumor tolerance.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. A. Rolink for the hydriboma expressing agonist anti-CD40 antibody, Dr. R. Kornbluth for the full-length CD40L cDNA, Anna Morys for technical assistance at the initial stage of the study, Drs. H. Eisen and D. Irvine for critical review and editing of the manuscript, and members of the Chen lab for discussions.
3Abbreviations used in this paper
- SIY
SIYRYYGL
- TRAMP
TRansgenic Adenocarcimona of the Mouse Prostate
- TRP-SIY
TRAMP mice expressing SIY
- WSN-SIY
influenza A/WSN/ expressing SIY in the stalk of neuraminidase
- PDLN
prostate-draining lymph nodes
- TDLNs
tumor draining lymph nodes
- PLN
peripheral lymph nodes
- MLN
mediastenial lymph nodes
Footnotes
This work was supported by a National Defense Science and Engineering Graduate Fellowship and a National Science Foundation Graduate Research Fellowship (to E. M. H.), Koch Research Fund (to J.C.) and grants from the National Institutes of Health CA100875 (to J.C.) and CA97296 (to Hans Schreiber).
References
- 1.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 5.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 6.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 7.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 8.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]
- 9.Kalinski P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J. Immunol. 1998;161:2804–2809. [PubMed] [Google Scholar]
- 10.O'Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–2246. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- 11.van Kooten C, Banchereau J. CD40-CD40 ligand. J. Leukoc. Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 12.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 13.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 14.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 15.French RR, Chan HT, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat. Med. 1999;5:548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 16.Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, Offringa R, Toes RE. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat. Med. 1999;5:774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 17.Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, Fein S, Schoenberger S, Levitsky HI. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat. Med. 1999;5:780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 18.Mauri C, Mars LT, Londei M. Therapeutic activity of agonistic monoclonal antibodies against CD40 in a chronic autoimmune inflammatory process. Nat. Med. 2000;6:673–679. doi: 10.1038/76251. [DOI] [PubMed] [Google Scholar]
- 19.Kedl RM, Jordan M, Potter T, Kappler J, Marrack P, Dow S. CD40 stimulation accelerates deletion of tumor-specific CD8(+) T cells in the absence of tumor-antigen vaccination. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10811–10816. doi: 10.1073/pnas.191371898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGregor CM, Schoenberger SP, Green EA. CD154 is a negative regulator of autoaggressive CD8+ T cells in type 1 diabetes. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9345–9350. doi: 10.1073/pnas.0402807101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartholdy C, Kauffmann SO, Christensen JP, Thomsen AR. Agonistic anti-CD40 antibody profoundly suppresses the immune response to infection with lymphocytic choriomeningitis virus. J. Immunol. 2007;178:1662–1670. doi: 10.4049/jimmunol.178.3.1662. [DOI] [PubMed] [Google Scholar]
- 22.Hussein MA, Berenson JR, Niesvizky R, Munshi NC, Harrop KL, McDonald M, Drachman JG. A phase I humanized anti-CD40 monoclonal antibody (SGN-40) in patients with multiple myeloma. Blood. 2005:106. [absract 2572] [Google Scholar]
- 23.Forero-Torres A, Furman RR, Rosenblatt JD, Younes A, Harrop K, Drachman JG, Advani R. A humanized antibody against CD40 (SGN-40) is well tolerated and active in non-Hodgkin's lymphoma (NHL): Results of a phase I study. J. Clin. Oncol. 2006;24:430s. [Google Scholar]
- 24.Bensinger W, Jagannath S, Becker PS, Anderson KC, Stadtmauer EA, Aukerman L, Fox J, Girish S, Bilic S, Guzy S, Solinger A, Dort S, Wang Y, Hurst D. A phase 1 dose escalation study of a fully human, antagonistic anti-CD40 antibody, HCD122 (formerly CHIR-12.12) in patients with relapsed and refractory multiple myeloma. Blood. 2006:108. [abstract 3575] [Google Scholar]
- 25.Byrd JC, Flinn IW, Khan KD, Kipps TJ, Aukerman L, Fox J, Girish S, Guzy S, Bilic S, Solinger A, Dort S, Wang Y, Hurst D, O'Brien S. Pharmacokinetics and pharmacodynamics from a first-in-human phase 1 dose escalation study with antagonistic anti-CD40 antibody, HCD122 (formerly CHIR-12.12), in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2006:108. [abstract 2837] [Google Scholar]
- 26.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, Green SJ, O'Dwyer PJ, Running KL, Huhn RD, Antonia SJ. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 27.Vonderheide RH, Dutcher JP, Anderson JE, Eckhardt SG, Stephans KF, Razvillas B, Garl S, Butine MD, Perry VP, Armitage RJ, Ghalie R, Caron DA, Gribben JG. Phase I study of recombinant human CD40 ligand in cancer patients. J. Clin. Oncol. 2001;19:3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 28.Wierda WG, Cantwell MJ, Woods SJ, Rassenti LZ, Prussak CE, Kipps TJ. CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood. 2000;96:2917–2924. [PubMed] [Google Scholar]
- 29.Rousseau RF, Biagi E, Dutour A, Yvon ES, Brown MP, Lin T, Mei Z, Grilley B, Popek E, Heslop HE, Gee AP, Krance RA, Popat U, Carrum G, Margolin JF, Brenner MK. Immunotherapy of high-risk acute leukemia with a recipient (autologous) vaccine expressing transgenic human CD40L and IL-2 after chemotherapy and allogeneic stem cell transplantation. Blood. 2006;107:1332–1341. doi: 10.1182/blood-2005-03-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vonderheide RH. Prospect of targeting the CD40 pathway for cancer therapy. Clin. Cancer Res. 2007;13:1083–1088. doi: 10.1158/1078-0432.CCR-06-1893. [DOI] [PubMed] [Google Scholar]
- 31.Early GS, Zhao W, Burns CM. Anti-CD40 ligand antibody treatment prevents the development of lupus-like nephritis in a subset of New Zealand black x New Zealand white mice. Response correlates with the absence of an anti-antibody response. J. Immunol. 1996;157:3159–3164. [PubMed] [Google Scholar]
- 32.Kitagawa M, Suzuki H, Adachi Y, Nakamura H, Yoshino S, Sumida T. Interferon-gamma enhances interleukin 12 production in rheumatoid synovial cells via CD40-CD154 dependent and independent pathways. J. Rheumatol. 2001;28:1764–1771. [PubMed] [Google Scholar]
- 33.Balasa B, Krahl T, Patstone G, Lee J, Tisch R, McDevitt HO, Sarvetnick N. CD40 ligand-CD40 interactions are necessary for the initiation of insulitis and diabetes in nonobese diabetic mice. J. Immunol. 1997;159:4620–4627. [PubMed] [Google Scholar]
- 34.Gerritse K, Laman JD, Noelle RJ, Aruffo A, Ledbetter JA, Boersma WJ, Claassen E. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 1996;93:2499–2504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan J, Town T, Suo Z, Wu Y, Song S, Kundtz A, Kroeger J, Humphrey J, Crawford F, Mullan M. Induction of CD40 on human endothelial cells by Alzheimer's beta-amyloid peptides. Brain Res. Bull. 1999;50:143–148. doi: 10.1016/s0361-9230(99)00122-7. [DOI] [PubMed] [Google Scholar]
- 36.Larsen CP, Alexander DZ, Hollenbaugh D, Elwood ET, Ritchie SC, Aruffo A, Hendrix R, Pearson TC. CD40-gp39 interactions play a critical role during allograft rejection. Suppression of allograft rejection by blockade of the CD40-gp39 pathway. Transplantation. 1996;61:4–9. doi: 10.1097/00007890-199601150-00002. [DOI] [PubMed] [Google Scholar]
- 37.Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, Fechner JH, Jr, Germond RL, Kampen RL, Patterson NB, Swanson SJ, Tadaki DK, TenHoor CN, White L, Knechtle SJ, Harlan DM. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat. Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 38.Geldart T, Illidge T. Anti-CD 40 monoclonal antibody. Leuk. Lymphoma. 2005;46:1105–1113. doi: 10.1080/10428190500085255. [DOI] [PubMed] [Google Scholar]
- 39.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen CH, Ge Q, Talay O, Eisen HN, Garcia-Sastre A, Chen J. Loss of IL-7R and IL-15R expression is associated with disappearance of memory T cells in respiratory tract following influenza infection. J. Immunol. 2008;180:171–178. doi: 10.4049/jimmunol.180.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2 gene product is required for S mu-S epsilon heavy chain class switching. Immunity. 1996;5:319–330. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- 42.Kornbluth RS, Kee K, Richman DD. CD40 ligand (CD154) stimulation of macrophages to produce HIV-1-suppressive beta-chemokines. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5205–5210. doi: 10.1073/pnas.95.9.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yellin MJ, Sippel K, Inghirami G, Covey LR, Lee JJ, Sinning J, Clark EA, Chess L, Lederman S. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell-B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J. Immunol. 1994;152:598–608. [PubMed] [Google Scholar]
- 44.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J. Immunol. 2005;175:7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.