Our study implicated the role of cellular stress through the production of oxidative and nitrative lipid, DNA, and protein damage followed closely by accelerated and increased apoptosis as a key mechanism for the synergistic effects of radiofrequency ablation and liposomal doxorubicin.
Abstract
Purpose:
To determine if oxidative and nitrative stress and/or apoptosis contribute to increased coagulation when combining radiofrequency (RF) ablation with liposomal doxorubicin.
Materials and Methods:
Animal care committee approval was obtained. R3230 mammary adenocarcinomas in Fischer rats were treated with either RF ablation (n = 43), 1 mg of intravenously injected liposomal doxorubicin (n = 26), or combined therapy (n = 30) and were compared with control subjects (n = 11). A subset of animals receiving combination therapy (n = 24) were treated in the presence or absence of N-acetylcysteine (NAC) administered 24 hours and 1 hour before RF ablation. Tumors were analyzed 2 minutes to 72 hours after treatment to determine the temporal range of response by using immunohistochemical staining of the apoptosis marker cleaved caspase-3, phosphorylated γH2AX, and HSP70 and of markers of oxidative and nitrative stress (8-hydroxydeoxyguanosine [8-OHdG], 4-hydroxynonenal [4-HNE]–modified proteins, and nitrotyrosine [NT]). Statistical analyses, including t tests and analysis of variance for comparisons where appropriate, were performed.
Results:
By 4 hours after RF ablation alone, a 0.48-mm ± 0.13 (standard deviation) peripheral band with 57.0% ± 7.3 cleaved caspase-3 positive cells was noted at the ablation margin, whereas a 0.73-mm ± 0.18 band with 77.7% ± 6.3 positivity was seen for combination therapy (P < .03 for both comparisons). Combination therapy caused increased and earlier staining for 4-HNE–modified proteins, 8-OHdG, NT, and γH2AX with colocalization to cleaved caspase-3 staining. A rim of increased HSP70 was identified peripheral to the area of cleaved caspase-3. Parameters of oxidative and nitrative stress were significantly inhibited by NAC 1 hour following RF ablation, resulting in decreased cleaved caspase-3 positivity (0.28-mm ± 0.09 band of 25.9% ± 7.4 positivity vs 0.59-mm ± 0.11 band of 62.9% ± 6.0 positivity, P < .001 for both comparisons).
Conclusion:
Combining RF ablation with liposomal doxorubicin increases cell injury and apoptosis in the zone of increased coagulation by using a mechanism that involves oxidative and nitrative stress that leads to accelerated apoptosis.
© RSNA, 2010
Introduction
Radiofrequency (RF) ablation is a rapidly maturing minimally invasive image-guided tumor therapy that is increasingly utilized for the treatment of focal hepatic, renal, bone, and lung tumors (1–5). However, RF treatment efficacy is hindered by its inability to effectively treat tumors greater than 3–5 cm in diameter (2,6). This limitation has led to the development of combination therapies that aim to increase coagulation by modulating biophysical properties such as tumor electrical and thermal conductivity as well as blood flow (7–10) or through coupling the effect of RF ablation with that of chemotherapeutic agents (11–14). Along these lines, Monsky et al (15) first reported increased intratumoral drug accumulation in tissues treated with RF ablation and intravenous administration of liposomal doxorubicin. Tissues undergoing combined treatment also exhibited increased tumor necrosis in animal models (16,17) and patients (14) in the transient hyperthermic zone of red coagulation surrounding the zone of white coagulation (ie, heat-induced tumor death) (18).
Although combined therapies have demonstrated increased tumor destruction, particularly in the periphery of the ablation zone, to our knowledge, the underlying mechanisms that cause increased tumor destruction have not been sufficiently explored. One potential avenue, implicating cellular oxidative stress, has been suggested by the unexpected finding of increased coagulation when combining RF thermal ablation with blank liposomes (12,19). Indeed, it is known that the thermal dosimetry at which this increased coagulation is observed is only slightly higher than that at hyperthermia where oxidative stress has been shown to play a role at activating tumoricidal pathways involving both apoptosis and necrosis (20–25). Hence, we hypothesized that the synergistic effects of RF ablation combined with liposomal doxorubicin are caused by cellular oxidative stress which leads to increased apoptosis and/or reduced heat-shock protein production (26–28). As an alternative, we hypothesized that combination therapy accelerates damage not only to proteins but also to other cellular constituents such as DNA (29). Accordingly, the goal of our study was to determine the underlying mechanisms behind increased coagulation when combining RF ablation with liposomal doxorubicin by assessing the temporal expression of morphologic and cellular changes, including levels of oxidative and nitrative stress, damage to DNA, activation of apoptotic pathways, and heat-shock protein expression.
Materials and Methods
Experiment Overview
The study was performed in two phases. In phase 1, we examined the underlying mechanisms behind the synergistic effects of combining RF ablation and liposomal doxorubicin. Ninety-nine animals were randomly assigned into three treatment groups: RF ablation alone (n = 43), intravenous administration of liposomal doxorubicin (n = 26), or combined therapy (liposomal doxorubicin given 15 minutes after RF ablation, n = 30), with an additional 11 animals serving as control subjects. Tumors were harvested at nine time points (three to six animals per time point and treatment): 2 and 30 minutes and 1, 2, 4, 8, 12, 24, and 72 hours after treatment to determine the temporal range of response. Tissues were stained with hematoxylin-eosin for gross pathologic examination and were also stained for the presence of cleaved caspase-3 (a product of the apoptotic pathway [30]), 8-hydroxydeoxyguanosine (8-OHdG) (an oxidized DNA nucleoside [31]), 4-hydroxynonenal (4-HNE)–modified proteins (a product of lipid peroxidation [32]), nitrotyrosine (NT) (an indicator of nitrative stress and oxidative damage [32]), phosphorylated γH2AX (result of double-stranded DNA breaks [29]), and a heat shock protein marker, HSP70 (33,34). In phase 2 of the study, a subset of animals (n = 24) receiving combined RF ablation and liposomal doxorubicin therapy was used to examine the outcomes of blocking the effects of cellular stress. N-acetylcysteine (NAC) (American Regent, Shirley, NY), a thiol antioxidant (35), was administered at a dose of 300 mg per kilogram of body weight by using intraperitoneal injection (n = 12) at 24 hours and 1 hour prior to RF application. Tissues were harvested at 30 minutes, 60 minutes, and 4 hours after ablation (four animals at each time point) and examined for evidence of 8-OHdG, NT, and cleaved caspase-3.
Animal Model
The protocol was approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center. For all experiments and procedures, anesthesia was induced by using intraperitoneal injection with a mixture of ketamine (50 mg/kg, Ketaject; Phoenix Pharmaceutical, St Joseph, Mo) and xylazine (5 mg/kg, Rompun; Bayer, Shawnee Mission, Kan). When animals demonstrated signs of arousal, booster anesthesia injections at one-tenth the dose were intraperitoneally administered every 30–60 minutes. The well-established R3230 mammary adenocarcinoma model (19) was used to produce tumors in female Fischer rats (mean weight, 150 g ± 20 [standard deviation]; age range, 7–9 weeks) (Taconic Farms, Germantown, NY); this was the strain in which the tumor was originally derived.
All animal studies were performed by researchers trained in the use of this specific model and in small-animal RF ablation techniques (S.A.S., R.S., S.R., S.N.G.). Tumors measuring 1.0–1.2 cm in diameter were initially harvested from a live carrier by using an aseptic technique. Within 30 minutes of tumor dissection and removal, the tumor was homogenized (PowerGen 125; Fisher Scientific, Pittsburgh, Pa) and suspended in 7 mL of Roswell Park Memorial Institute medium 1640 1× (Mediatech, Manassas, Va). Results of prior control experiments in our laboratory have shown that this produces a concentration of 1 × 108 cells per milliliter, with greater than 95% cellular viability (S.N.G., unpublished data, 2000). Under direct visualization, 0.2–0.3 mL of the tumor suspension was injected slowly with an 18-gauge needle subcutaneously into the mammary fat pad at the left abdomen. Animals were monitored, and tumor diameter was measured every 3–4 days. Tumors grew for 14–24 days until the desired treatment size was achieved. Solid nonnecrotic tumors (as determined with ultrasonography [US]) measuring 1.3–1.6 cm in diameter were used for these studies.
RF Application
In a manner similar to that at prior experiments (10,11), a 500-kHz RF generator (ML-1; Valleylab, Boulder, Colo) was used to apply conventional monopolar RF (S.A.S., R.S., S.R. S.N.G.). To complete the RF circuit, each animal was placed on a standardized grounding pad (Valleylab). The contact area of each animal was shaved, and US gel was applied to ensure proper contact. A 21-gauge 0.81-mm-diameter insulated electrode with a 1.0-cm exposed tip was placed in the center of the tumor with US guidance. The limited exposure of the electrode (1.0-cm tip) allowed focused energy deposition. RF energy was applied for 5 minutes, with the generator output titrated to maintain a designated tip temperature of 70°C ± 2.
Liposomal Doxorubicin Administration
Polyethylene glycol–stabilized long-circulating liposomal doxorubicin (Doxil; Alza Pharmaceuticals, Palo Alto, Calif) was administered with tail vein injection by using a dose of 8 mg/kg (1 mg in 500 μL dilution). This dose was chosen on the basis of results of previous studies demonstrating a marked coagulation increase to 13.5 mm during the 48 hours following RF ablation by using prior parameters (19). Liposomal doxorubicin injection occurred approximately 15 minutes after RF application. This injection time was on the basis of time course studies demonstrating maximal synergistic effect with this protocol (19).
Pathologic Examination
Tissues were harvested at 2 and 30 minutes and at 1, 2, 4, 8, 12, 24, and 72 hours after RF ablation (three to six specimens per parameter). Tissues receiving NAC injection were harvested at 30 minutes, 60 minutes, and 4 hours. Tumors were sliced perpendicularly to the direction of electrode insertion and placed in cassettes containing the central section of tumor. Tissue was fixed in 10% formalin overnight at 4°C, embedded in paraffin, and sliced at a thickness of 5 µm. Tissues were stained with hematoxylin-eosin for gross pathologic examination.
Immunohistochemical Examination
Pathologic preparation, immunohistochemistry, and slide analysis were performed by researchers trained in these specific techniques (R.S., S.R., W.Y.). Five-micrometer slices were placed on charged plus slides (Fisher Scientific) and incubated overnight at 37°C and 1 hour at 65°C. At paraffin removal and tissue rehydration, slides were heated in a microwave oven at 97°C for 10 minutes for retrieval of antigenic activity. Slices were incubated with hydrogen peroxide at room temperature for 10 minutes to inactivate endogenous peroxidase. Immunohistochemical assays were performed by using antibodies on each sample to detect cleaved caspase-3 (Cell Signaling Technologies, Danvers, Mass), γH2AX phospho-histones (Cell Signaling Technologies), NT (Chemicon, Temecula, Calif), and 8-OHdG (Genox, Baltimore, Md), as well as 4-HNE–modified proteins (Cell Signaling Technologies) and HSP70 (Assay Designs, Ann Arbor, Mich). Slices were incubated with primary antibodies at room temperature for 60 minutes and washed with phosphate-buffered saline. Slices were then incubated with species-matched secondary antibodies at room temperature for 30 minutes. Protein expression was visualized by using diaminobenzidine as substrate. Nuclear counterstaining was performed lightly with hematoxylin. Slices were dehydrated, and cover slips were applied (Permount; Richard-Allan Scientific, Kalamazoo, Mich). As an additional control to ensure uniformity of staining, whenever direct comparisons were made, immunohistochemical staining was repeated with all relevant comparison slides stained at the same time.
Double immunohistochemical staining protocol included the following: (a) endogenous peroxidase block and pretreatment similar to single antibody staining; (b) protein block using protein block serum-free solution (Dako, Glostrup, Denmark); (c) application of the primary antibody cocktail, consisting of the diluted two primary antibodies (cleaved caspase-3 and HSP70 or cleaved caspase-3 and 8-OHdG) in one container for 60 minutes at room temperature; (d) application of the secondary antibody cocktail (Polymer Detection Kit #1, Mouse AP + Rabbit HRP; Biocare Medical, Concord, Calif) for 30 minutes; (e) application of diaminobenzidine chromogen (Dako Cytomation, Glostrup, Denmark) for 5 minutes followed by Vulcan Fast Red Chromogen (Biocare Medical) for 10 minutes; and (f) eosin counterstaining.
Analysis
Slides were imaged and analyzed by using a microscope (Micromaster I; Westover Scientific, Mill Creek, Wash) and imaging software (Micron; Westover Scientific). Temporal evolution of cellular morphology and the spatial distribution of protein expression were determined in relation to the central zone of RF coagulation and to one another. Quantitative analysis was performed by using three metrics: the average thickness of the rim of staining, the percentage of cells that stain per high-powered (×40) field within the defined zone, and the product of rim thickness multiplied by percentage of positive cells. Five random high-powered fields were analyzed for a minimum of three specimens for each parameter and scored in a blinded fashion to remove observer bias. Multivariate analysis of variance was performed for each of the immunohistochemical examinations separately for each of the endpoints (average thickness of the rim of staining, percentage of cells that stain per high-powered field within the defined zone, and the product of rim thickness multiplied by percentage of positive cells) to determine the effects of RF ablation alone versus liposomal doxorubicin alone versus combination therapy. Paired t tests were then performed for specific comparisons at specified times if and only if the analysis of variance achieved statistical significance (SAS, SAS Institute, Cary, NC; Excel, Microsoft, Redmond, Wash). For experiments in which NAC was administered, changes in immunohistochemical staining were compared with control tumors by using similar endpoints and techniques. For all statistical comparisons, P values less than .05 were considered to indicate significant differences.
Results
Control Tumors and Liposomal Doxorubicin Alone
Control tumors that received no treatment exhibited an absence of discreet focal staining for all examined immunohistochemical stains. Likewise, tumors treated with liposomal doxorubicin alone exhibited an absence of focal staining up to 12 hours. Although minimal scattered cellular apoptosis was seen at 24 hours, this was not accompanied by an increase in staining for cellular stress, γH2AX, or heat shock proteins in a defined spatial distribution pattern. Additionally, no differences in the characteristic histopathologic appearance of this tumor was noted at hematoxylin-eosin staining for up to 72 hours in tumors receiving liposomal doxorubicin alone.
RF Ablation Alone
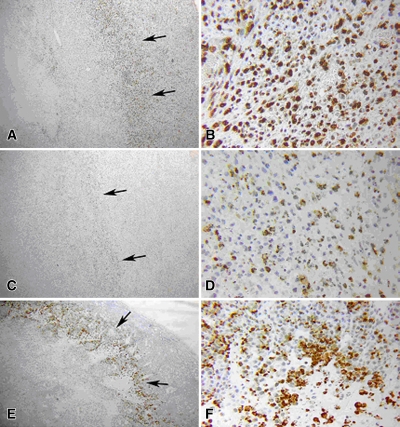
At hematoxylin-eosin staining, tissues treated with RF ablation alone exhibited the characteristic tumor necrosis in the zone of white coagulation surrounded by a process of vascularization and early inflammatory reaction that progressed until 24 hours (Fig 1) (36). By 24 hours, the classic findings of coagulation necrosis, including streaming cytoplasm and pyknotic nuclei, were evident in the ablation zone on all samples.
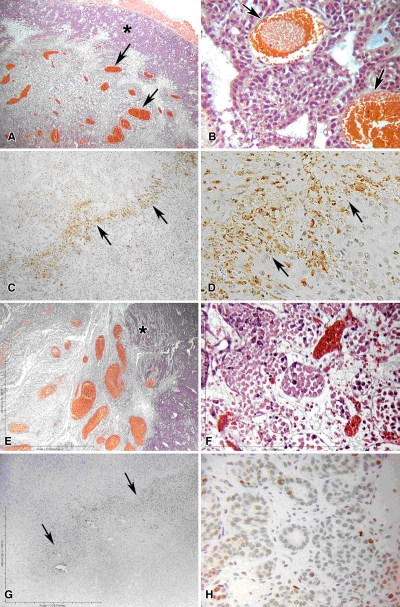
Figure 1:
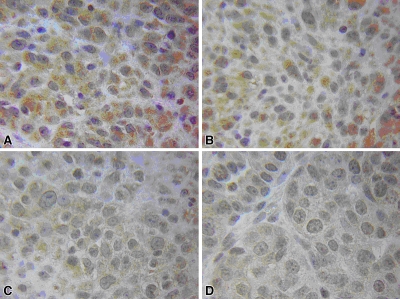
Results of RF ablation alone at 4 hours in rat mammary adenocarcinoma model. A, B, Hematoxylin-eosin staining exhibits typical disease of the ablation zone, including pyknotic condensed nuclei of tumor and marginal hyperemia and neovasculature (arrows). C, D, Tissue stained for cleaved caspase-3 demonstrates up-regulation of apoptotic pathways in a thin periablational margin (arrows). E , F, By 24 hours, much of the coagulation has converted to frank coagulative necrosis. G, H, Near total absence of the cleaved caspase-3 staining. Arrows in G = periablational zone. * = peripheral normal viable tumor. (Original magnification in A, C, E, and G, ×4; in B, D, F, and H, ×40.)
Immunohistochemical results revealed bands of discreet staining for the different antibodies that were situated immediately adjacent to the zone of coagulation and that progressed in a time-dependent fashion. As early as 30 minutes after the ablation, patchy or partial thin rims of 8-OHdG, NT, and γH2AX were noted to surround the ablation zone. Coalescence of these to full rings surrounding the coagulation were noted by 1 hour (Table 1), with a corresponding colocalizing ring of cleaved caspase-3 beginning to appear at 1 hour (Fig 1). Additionally, several patchy areas of 8-OHdG and NT staining were observed at 1 hour within the zone of coagulation within 1–2 mm from the electrode. All of the staining reactions increased progressively, in terms of rim thickness and percentage of positive cells, to a maximum at 4 hours for γH2AX, 8-OHdG, and NT, with maximum cleaved caspase-3 noted at 8 hours, and substantial resolution by 12–24 hours (Table 1, Fig 1). No definitive increase in 4-HNE–modified protein staining was noted at any time point. Colocalization staining demonstrated a ring of HSP70 immediately adjacent and peripheral to the rim of cleaved caspase-3 staining as early as 4 hours after RF ablation (Fig 2). This progressively increased to a maximum at 12 hours, and unlike other stains, persistent HSP70 was detected at 24 hours. Yet, almost no evidence of activity surrounding the central coagulation zone was noted for any stain at 72 hours.
Table 1.
Quantitative Immunohistochemical Analysis
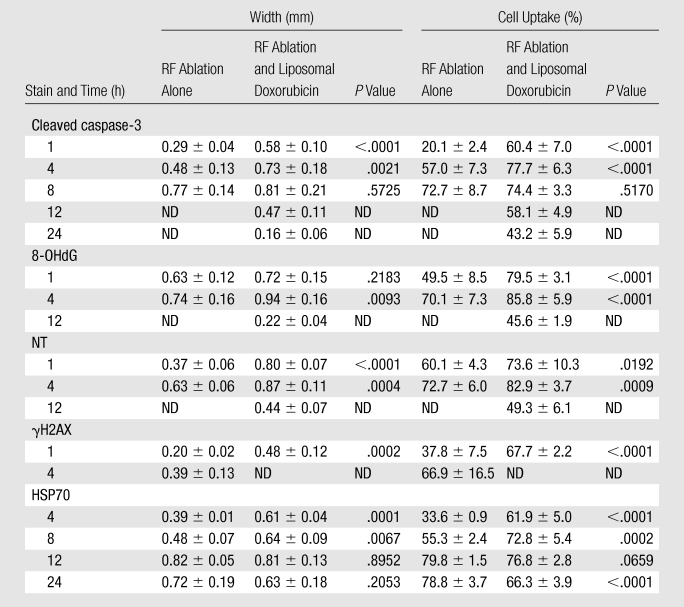
Note.—Data are means ± standard deviations. ND = no data, absence of identifiable expression.
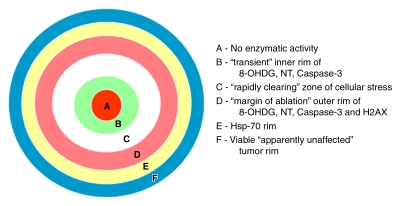
Figure 2:
Spatial distribution of RF heating and tumor destruction in R3230 model. Zones A–D represent regions that undergo white-zone tumor coagulation, with zones B–D showing evidence of cell stress. Apoptosis is most often seen in zone D but can be seen in zone B. Zones B–D are much larger and more pronounced when liposomal doxorubicin is administered as an ablation adjuvant. Zone E represents a hyperthermic region that does not coagulate but rather produces HSP70. This zone likely colocalizes with the red zone of inflammation seen in other tissues. Zone F represents apparently unaffected viable tumor.
Combined Treatment
Hematoxylin-eosin staining for tumors treated with both RF ablation and liposomal doxorubicin exhibited increased periablational vascularization during the first 12 hours (Fig 3), with earlier and more extensive zones of increased tumor coagulation. This included frank coagulative necrosis extending outward into the zone of cleaved-caspase 3 staining at 24 hours.
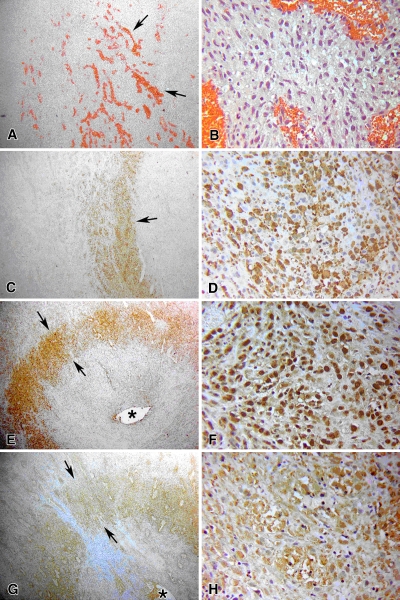
Figure 3:
Results of RF ablation and liposomal doxorubicin treatment at 4 hours. A, B, Hematoxylin-eosin staining exhibits tumor coagulation and increased vascularization in ablated tissue (arrows). C, D, Tissue stained for cleaved caspase-3 demonstrates thicker bands of staining at the periphery of the ablation zone with a greater percentage of positive staining cells compared with RF ablation alone (Fig 1), with marked increase in apoptotic activity (arrow). Staining for (E, F) 8-OHdG and (G, H) NT exhibits distinct rings (arrows) that colocalize with cleaved caspase-3, denoting spatial association between markers of cellular stress and apoptosis. * = site of RF electrode insertion. (Original magnification in A, C, E, and G, ×4; in B, D, F, and H, ×40.)
Combined treatment tissues exhibited an earlier and more substantial temporal response for up-regulation of cellular stress and apoptosis (Fig 3 and 4). Unlike at RF ablation alone, we were able to identify contiguous staining for 4-HNE–modified proteins, NT, and 8-OHdG from approximately 1 mm from the electrode to the margin of tumor destruction (radius, 4.3 mm ± 0.4) at 2–30 minutes after ablation (Fig 5). By 1 hour after RF ablation, evolution in the pattern of up-regulation of 8-OHdG and NT was seen as spatial clearing of some staining that resulted in the appearance of a pronounced double rim of staining (Fig 2). The inner 2–3-mm ring of cellular stress staining was in the central area of coagulation at hematoxylin-eosin staining (ie, cells with pyknotic nuclei and cytoplasmic streaming), whereas the outer rim was at the margin of the ablated tissue. By 4 hours after treatment, further accentuation of up-regulation of cellular stress in tumors receiving combined treatment was apparent with maximum statistically significant increases in oxidative and nitrative damage (in terms of rim thickness, percentage of positive staining cells, and the overall product) at this time (P < .01 in all comparisons) (Table 1, Fig 3 and 4). Compared with RF ablation alone, persistent but progressively reduced staining of the peripheral rim for 8-OHdG and NT was seen at 12 hours. The inner rim of cell stress (8-OHdG, NT) was clearly present in tumors with RF ablation and liposomal doxorubicin treatment at 8–12 hours after ablation but was resolved in all cases by 24 hours.
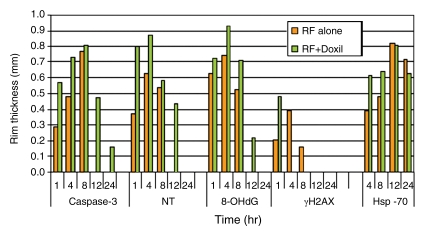
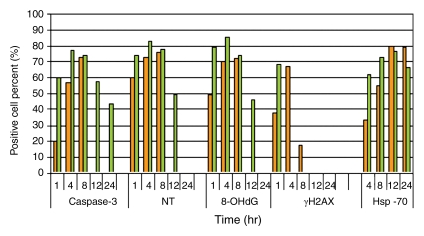
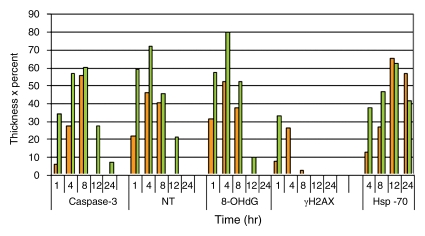
Figure 4a:
Quantitative analysis to compare immunohistochemical staining between tumors treated with RF ablation alone and combined RF ablation and liposomal doxorubicin (Doxil). Bar graphs show immunohistochemical endpoints for RF ablation alone (orange) and combined with liposomal doxorubicin (green). Endpoints of (a) band thickness, (b) percentage of positive cells within the band, and (c) band thickness multiplied by percentage of positive cells all demonstrate earlier and more substantial expression of a peripheral rim for cleaved caspase-3, 8-OHdG, NT, γH2AX, and HSP70.
Figure 5:
Results show early evidence of cell stress for combined RF ablation and liposomal doxorubicin therapy. A, Combined therapy shows contiguous increased cytoplasmic staining for 4-HNE–modified proteins throughout the zone of coagulation at 15 minutes after combined therapy with progressive reduction to baseline at 2 hours. B, Results at 30 minutes. C, Results at 2 hours. D, No staining for 4-HNE–modified proteins is appreciated in specimens treated with RF ablation alone. (D is at 15 minutes after ablation.) (Original magnification, ×100.)
Figure 4b:
Quantitative analysis to compare immunohistochemical staining between tumors treated with RF ablation alone and combined RF ablation and liposomal doxorubicin (Doxil). Bar graphs show immunohistochemical endpoints for RF ablation alone (orange) and combined with liposomal doxorubicin (green). Endpoints of (a) band thickness, (b) percentage of positive cells within the band, and (c) band thickness multiplied by percentage of positive cells all demonstrate earlier and more substantial expression of a peripheral rim for cleaved caspase-3, 8-OHdG, NT, γH2AX, and HSP70.
Figure 4c:
Quantitative analysis to compare immunohistochemical staining between tumors treated with RF ablation alone and combined RF ablation and liposomal doxorubicin (Doxil). Bar graphs show immunohistochemical endpoints for RF ablation alone (orange) and combined with liposomal doxorubicin (green). Endpoints of (a) band thickness, (b) percentage of positive cells within the band, and (c) band thickness multiplied by percentage of positive cells all demonstrate earlier and more substantial expression of a peripheral rim for cleaved caspase-3, 8-OHdG, NT, γH2AX, and HSP70.
Other reactions were also much more rapid and substantial in the combined therapy group. A peripheral rim of cleaved caspase-3 at the margin of ablation was noted as early as 30 minutes, and expression was more substantial than with RF ablation alone during the first 4 hours after ablation (P < .01). Furthermore, in two (33%) of six specimens, a positive inner rim of cleaved caspase-3 staining was identified within the coagulation zone (Fig 6) and was noted to colocalize with the markers of cellular stress. Markers of apoptosis lasted longer in the combined therapy group, with patchy areas of cleaved caspase-3 remaining at 24 hours at the most peripheral edge of its former expression (Table 1).
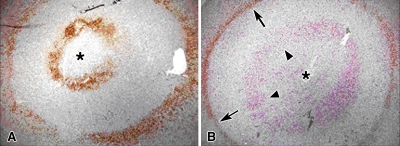
Figure 6:
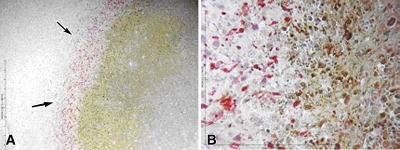
Results show development of a double rim of staining. A, Cleaved caspase-3 staining 4 hours following combined RF ablation and liposomal doxorubicin therapy demonstrates that combination therapy can yield two rings of enzymatic activity. The outermost ring was at the margin of the ablation zone, whereas the inner band occurred within the area of RF ablation–induced coagulation necrosis. The area in between is cleared of activity by 1 hour. B, Double staining for 8-OHdG (red, arrowheads) and cleaved caspase-3 (brown, arrows) in a different specimen obtained 4 hours after combination therapy reveals the preponderance of 8-OHdG staining in the inner rim with colocalization of 8-OHdG and cleaved caspase-3 staining in the outer rim. * = site of RF electrode position.
γH2AX staining was maximum by 1 hour, and unlike RF ablation alone, showed near total absence of staining by 4 hours (Table 1; Figs 4, 7). Likewise, earlier substantial HSP70 expression was noted in the combination therapy group in a rim peripheral to the caspase-3 expression (P < .01 at 4 and 8 hours) (Table 1; Figs 4, 8). Yet, by 24 hours, reduced HSP70 staining when liposomal doxorubicin was added to RF ablation led to a significantly greater number of HSP70 positive cells in the RF ablation alone samples (P < .01).
Figure 7:
Staining for DNA damage (γH2AX). Tissues treated with RF ablation alone compared with combined RF and liposomal doxorubicin treatment at 1 and 4 hours after treatment. A, B, At 1 hour, tissue treated with combined therapy exhibits a single intense band of staining at the margin of ablation (arrows). C, D, At 4 hours, RF ablation and liposomal doxorubicin–treated tissue shows little staining (arrows), suggesting degradation of these phosphorylated products. E, F, Tissue treated with RF ablation alone at 4 hours exhibits a pattern of γH2AX staining similar to that at combination therapy, but with a delayed response (arrows). (Original magnification in A, C, and E, ×4; in B, D, and F, ×40.)
Figure 8:
Results show heat-shock protein (HSP70) expression after RF ablation and liposomal doxorubicin therapy. A, Low- and, B, high-powered staining of tissues harvested 12 hours after treatment with combined therapy were stained for HSP70 (red) and cleaved caspase-3 (brown). A peripheral rim of heat-shock protein expression external to the zone of apoptosis is evident (arrows). (Original magnification in A, ×4; in B, ×40.)
NAC Suppression Experiments
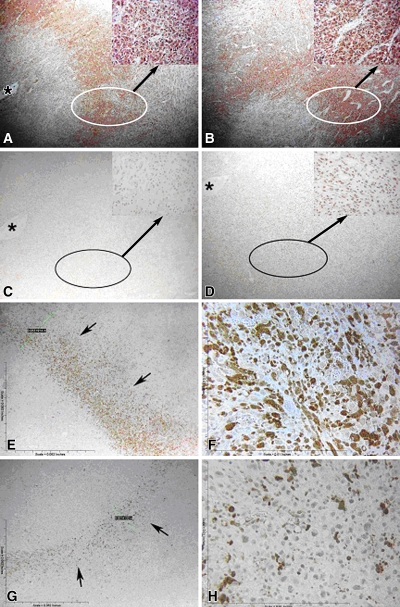
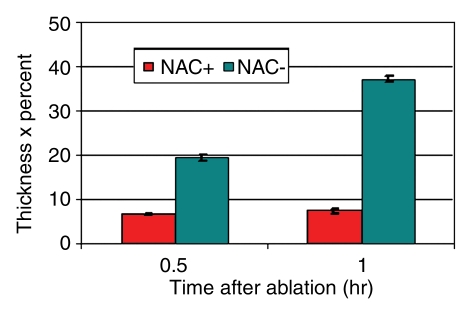
Unlike specimens that received RF ablation and liposomal doxorubicin treatment, tumors in animals pretreated with NAC showed no focal peripheral rim of staining for 8-OHdG, NT, or 4-HNE–modified proteins at 30 or 60 minutes after undergoing RF ablation and receiving liposomal doxorubicin (Fig 9). This was associated with statistically significant reductions in the thickness and percentage of positive cells in the band of cleaved caspase-3 staining in the presence of NAC (P < .01 for all comparisons) (Table 2, Fig 10). However, despite NAC pretreatment, at 4 hours, peripheral rims of staining similar to those at baseline were observed for NT, 8-OHdG, and cleaved caspase-3 at immunohistochemical examination.
Figure 9:
Results show effect of NAC on tumors treated with RF ablation and liposomal doxorubicin. A–D, Immunohistochemical findings of cell stress markers. A, B, Tumors treated with RF ablation and liposomal doxorubicin, but not pretreated with NAC, display similar (A) NT and (B) 8-OHdG staining at 30 minutes compared with previous tumor samples undergoing RF ablation and liposomal doxorubicin therapy. C, D, Tumors pretreated with NAC show an absence of (C) NT and (D) 8-OHdG staining at 30 minutes. Specimens are presented at ×4 magnification with circle and arrow pointing to a ×40-magnification insert in the upper right corner. * = site of electrode insertion. E–H, Cleaved caspase-3 staining at 60 minutes after therapy at (E, G) ×4 and (F, H) ×40 magnification. Staining shows a concomitant decrease in rim thickness (arrows) and percentage of cellular positivity in this rim for tumors (G, H) pretreated with NAC compared with (E, F) control tumors.
Table 2.
Quantitative Data from NAC Suppression
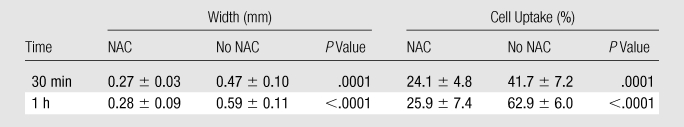
Note.—Data are means ± standard deviations of staining for cleaved caspase-3 activity.
Figure 10:
Effect of NAC before treatment on cleaved caspase-3 expression in tumors treated with RF ablation and liposomal doxorubicin. Bar graphs show cleaved caspase-3 staining in the presence (red) or absence (blue) of NAC at 30 and 60 minutes after therapy. The endpoint of band thickness multiplied by percentage of positive cells demonstrates more substantial expression of a peripheral rim for cleaved caspase-3 in the absence of NAC.
Discussion
The synergistic effects of RF ablation with chemotherapeutic agents such as liposomal doxorubicin have long been observed (11,12,16,17,19), yet the precise mechanisms for increased efficacy have thus far lacked adequate characterization, to our knowledge. Our study implicated the role of cellular stress through the production of oxidative and nitrative lipid, DNA, and protein damage followed closely by accelerated and increased apoptosis as a key mechanism for the synergistic effects of RF ablation and liposomal doxorubicin. These experiments demonstrated that RF ablation alone produces mild damage in a peripheral ring of tissue directly surrounding the area of heat-induced coagulation. However, multiple processes including cellular stress were increased by the addition of liposomal doxorubicin in this hyperthermic zone which has been previously shown to represent the precise area that undergoes frank coagulation (ie, tumor cell death) only when combination therapy is used (17). Indeed, nitrative and oxidative damage to lipids and proteins is much more intense and occurs earlier with combination therapy and was spatially and temporally associated with greater cleaved caspase-3 expression, which further supports the contention that cell stress leads to apoptosis in this system.
After combined therapy, increased cellular stress in the form of lipid damage (ie, increased 4-HNE–modified protein staining) occurred almost immediately after treatment, followed by substantial oxidative damage to DNA (8-OHdG staining), nitrative stress to proteins (NT staining), and apoptosis in the zone of increased coagulation at as early as 1 hour. By contrast, after RF ablation alone, reactions were much less persistent and cellular stress and apoptosis expression lagged, maximally seen around 4 hours.
Our results further demonstrated that pretreating the tumors with the thiol antioxidant NAC at least temporarily blocks and delays the increase in cell stress caused by the addition of liposomal doxorubicin. Although this implicates liposomal doxorubicin in enhancing oxidative stress after RF thermal ablation, nevertheless, we acknowledge that our experimental model does not conclusively prove that oxidative stress is the sole mechanism for the synergistic effects of combined therapy. Indeed, the NAC dose only allowed suppression of the oxidative and nitrative stress response up to 1 hour after treatment. We hypothesize that after this time point, the continuous delivery of long-circulating concentrations of liposomal doxorubicin to the zone of hyperthermic activity created a massive up-regulation of oxidative and nitrative stress that ultimately proved overwhelming for the chosen dose of NAC.
Our study provided insight into tissue changes following ablation. For example, the administration of liposomal doxorubicin unmasked transient persistent cellular function for up to 24 hours in the white coagulation zone, which created a double rim of enzymatic activity. With combination therapy, we found evidence of cell stress and activation of apoptotic pathways in regions that invariably undergo coagulation necrosis (as manifested by absence of all tested cellular functions at 24–48 hours), and, on the basis of known heating profiles exceeding 65°C (36,37), that were previously thought to immediately cease all cellular functions due to massive fulminant protein coagulation. Our results suggest that some cellular metabolic functions involving oxidative and nitrative stress can and will continue for at least a short time, even in moribund cells that have been fatally wounded with high doses of thermal energy. The double rim was not seen in the early specimens, as a much wider uniform zone of cell stress that extended to the outer ablation margin and presumably encompassed both zones was observed.
To account for these spatial and temporal findings, we postulated a model comprising several zones of thermal damage and cellular response based on the radial nonuniform thermal profile created during RF ablation. The innermost zone (zone A) is completely damaged and incapable of any cellular reaction. Surrounding this are at least three zones, B–D (rather than the previously assumed one), that show evidence of cellular stress and ultimately undergo tumor death in the presence of liposomal doxorubicin within 24–48 hours of RF ablation. We speculated that the middle of these three zones (zone C) retains sufficient cellular machinery to rapidly produce protective proteins that prevent proliferation of the cellular stress, whereas the more central inner zone (zone B) has lost the capacity to mount these reactions. The precise nature of these protective mechanisms require exploration in future studies, as the staining patterns for HSP70 did not implicate up-regulation of this candidate pathway in between the two zones of identified cellular stress. Zone D, the outer ring of cell stress, DNA damage, and apoptosis correlates to the ablation margin at 24 hours, and on the basis of prior thermal mapping in this model, occurs when temperatures of 48°C for RF ablation combined with liposomal doxorubicin or 52.4°C for RF ablation alone are achieved (16). Peripheral to this is the region of HSP70 production, zone E, which extends outward to encompass tissues heated up to approximately 1°C lower (46.8°C ± 0.42 when liposomal doxorubicin is added to RF ablation and 51.6°C ± 0.46 for RF ablation alone). This region of HSP70 expression likely also colocalizes with the region of increased inflammatory response that is known to occur in normal tissues (17). It is possible that this inflammatory reaction at the transition zone may contribute to amplified cellular stress and apoptosis in the outermost portion of this ring, but confirmation of this will require future study in models such as normal liver that mount greater inflammatory reaction.
As noted above, manipulating lipid content and metabolism in the area where hyperthermic damage is occurring in the presence of doxorubicin may contribute to enhancement of oxidative stress by causing alterations in lipid peroxidation reactions. This is consistent with our data showing that, in the presence of doxorubicin, immunohistochemical staining for 4-HNE–modified proteins was increased in the area of RF ablation–induced injury. Yet, the mechanism by which lipids and/or doxorubicin reach the inner zone of increased cell stress remains unclear. While increased liposomal doxorubicin in the outer rim is expected (especially with increased blood flow from inflammatory changes) (36), some of the most intense staining occurred close to the electrode. Results of magnetic resonance and computed tomographic perfusion imaging of the coagulation classically suggest an absence of perfusion in this area, possibly from RF heating-induced coagulation of all small vessels less than 3 mm (38). Given the improbability of such large-scale passive diffusion of the 100-nm liposomes, our results suggest that a sufficient number of small vessels or channels may remain open or that lipids may have greater passive diffusion in this region, which correlates to a recent study modeling doxorubicin transport from polymer implantation after RF ablation (39). Accordingly, this may suggest that residual tumor exists not only at the periphery of an ablation zone, but also within it, and bolsters the argument for combining RF ablation with chemoembolic strategies, including lipiodol-based embolization mixtures (40,41) and doxorubicin-eluting beads (42), to maximize the chance of ensuring completeness of ablation and elimination of persistent vascular channels.
We identified a single rim of γH2AX staining that colocalized with the ablation margin and the outermost rim of 8-OHdG and cleaved caspase-3 (zone D) for RF ablation alone or in combination with liposomal doxorubicin. This finding corroborates previously reported breaks in DNA-histone complexes from terminal deoxynucleotidyl transferase–mediated dUTP nick end-labeling staining following RF ablation (43) and provides additional evidence that the DNA damage induced by oxidative stress can be quite severe (44). Regardless, this suggests that damage from RF ablation, particularly with combined treatment (where the reaction was more substantial and ran its course much quicker than with RF ablation alone), may extend beyond protein coagulation to DNA damage. Furthermore, given that radiation therapy is known to produce extensive DNA damage (45), severe DNA damage seen here provides insight into the possible mechanisms by which the combination of RF ablation and radiation therapy lowers the thermal dose of activation and increases animal survival in this model compared with RF ablation and liposomal doxorubicin (46,47). We attribute the absence of γH2AX throughout the rest of the ablation zone to the fact that the phosphorylating proteins (or the cellular machinery that synthesize them) are sufficiently damaged by the therapies and can no longer be identified by our immunohistochemical antibody.
In our tumor model, a single rim of HSP70 expression developed immediately adjacent to the final margin of coagulation both in the RF ablation alone and more quickly in the combination therapy groups. The relatively later time course and spatial distribution of this reaction in an 8-mm well-defined rim occurring in a region of tumor viability correlates with known thermoprotective functions of the heat-shock protein family (26,48). One of our initial hypotheses, that liposomal doxorubicin would reduce heat-shock protein production was disproved. Nevertheless, these findings raise the possibility of potentially extending the ablation zone by eliminating heat-shock protein expression with agents such as quercetin (49).
Our study highlighted both the challenges and potential utility of studying RF ablation. Given the nature of heating from a point source, there is no constant thermal dose. In essence, we are producing an infinite spectrum of thermal doses (50), which underscores the importance of understanding the spatial distribution of a myriad of different mechanisms of cell death and the varied physiologic responses to different levels of thermal insult. On the other hand, it can be argued that rather than resorting to a common strategy of reducing the study of hyperthermia to a single or a set of limited thermal doses (50,51), our model helps us understand what occurs across a wide range of temperatures and thermal doses, a situation which undoubtedly occurs not only in high-temperature ablation, but also more often than not for lower-temperature hyperthermia regimens (52). Regardless, our data support the contention that conceptual models such as the concentric zones highlighted in Figure 2 will be needed to best understand current and future thermal ablation paradigms.
The presence of increased cellular oxidative and nitrative stress and apoptotic activity in tumors following tumor ablation combined with liposomal doxorubicin expands opportunities for future treatment options. These include expanding cellular stress, potentially by altering the lipid or chemotherapeutic formulations or by adding radiation (5,46), and the use of antimitotic cancer drugs such as paclitaxel (53,54), which could lead to a more substantial increase of apoptotic up-regulation and greater tumor necrosis. These findings also encourage exploration of combined treatment for other methods of tumor ablation. Finally, some of these modifications may be necessary for other tumor types which are likely to show both variable thermal dosimetry (50,51) and varied sensitivity to different adjuvants.
In conclusion, though future work is necessary, our evidence supports the hypothesis that combining RF ablation with liposomal doxorubicin accelerates apoptotic activity compared with RF ablation or doxorubicin treatment alone in the zone of increased coagulation. The presence of increased cell oxidative and nitrative stress markers colocalizing with the zone of increased apoptosis and the inhibition of this response by a thiol antioxidant suggest that increased oxidative and nitrative stress are likely to contribute in part to the synergistic effects of RF ablation and liposomal doxorubicin.
Practical applications: Defining the mechanisms behind the synergy between RF ablation and liposomal doxorubicin holds the potential for further improvement of combination therapies for interventional oncology. Our work demonstrated that this synergy is caused in part by cell stress leading to apoptosis, which suggests that combining RF ablation with chemotherapeutic preparations containing agents with greater cell stress and apoptotic potential should be studied. Furthermore, our description of a more peripheral rim of heat-shock protein production points to an additional avenue for increasing the zone of ablation by developing strategies that combine ablation with agents that can block this adaptive response.
Advances in Knowledge.
Cellular oxidative and nitrative stress through the production of oxidative and nitrative lipid and protein damage followed closely by increased apoptosis are key mechanisms underlying the known increased tumor destruction and synergy seen with combined radiofrequency (RF) ablation and intravenous administration of liposomal doxorubicin.
There is up-regulation of markers of oxidative and nitrative stress and apoptosis in portions of the zone of RF ablation–induced coagulation for 24 hours following treatment in areas previously thought to undergo immediate coagulation and hence cessation of all cellular function.
RF ablation alone or in combination with liposomal doxorubicin results in DNA breakage that colocalizes with apoptosis and a ring of heat-shock protein production at the ablation margin.
The white coagulation zone is not homogeneous but is composed of multiple concentric rings denoting a range of different types and extents of thermal damage.
Implication for Patient Care.
Understanding underlying cellular mechanisms and their spatial distribution following the combination of RF ablation and adjuvant chemotherapeutic agents such as liposomal doxorubicin will allow for more tailored drug development to maximize the synergistic effects of combined therapy, thereby enabling increased tumor destruction.
Received July 4, 2009; revision requested August 12; revision received October 12; accepted October 16; final version accepted October 26.
R.S. supported by a fellowship grant from The American Physicians Fellowship for Medicine in Israel. W.Y. supported by a special incubation fund grant of the major research plan from the Beijing Medical Science Technology Commission (no. Z0005190040431). S.N.G. receives sponsored research grants and consults for Angiodynamics (Fremont, Calif).
Funding: This work was supported by National Institutes of Health grants (nos. R01 CA133114, R01 CA100045, and 2R01 HL55519).
See also Science to Practice in this issue.
Abbreviations:
- NAC
- N-acetylcysteine
- NT
- nitrotyrosine
- RF
- radiofrequency
- 8-OHdG
- 8-hydroxydeoxyguanosine
- 4-HNE
- 4-hydroxynonenal
References
- 1.Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 2009;19(5):1206–1213 [DOI] [PubMed] [Google Scholar]
- 2.Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005;234(3):961–967 [DOI] [PubMed] [Google Scholar]
- 3.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma. Part 1. Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol 2005;185(1):64–71 [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann RT, Jakobs TF, Kubisch CH, et al. Radiofrequency ablation in the treatment of osteoid osteoma: 5-year experience. Eur J Radiol Published online January 13, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Dupuy DE, DiPetrillo T, Gandhi S, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest 2006;129(3):738–745 [DOI] [PubMed] [Google Scholar]
- 6.Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology 2000;214(3):761–768 [DOI] [PubMed] [Google Scholar]
- 7.Livraghi T, Goldberg SN, Monti F, et al. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology 1997;202(1):205–210 [DOI] [PubMed] [Google Scholar]
- 8.Goldberg SN, Ahmed M, Gazelle GS, et al. Radio-frequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating and coagulation-phantom and porcine liver study. Radiology 2001;219(1):157–165 [DOI] [PubMed] [Google Scholar]
- 9.Gillams AR, Lees WR. CT mapping of the distribution of saline during radiofrequency ablation with perfusion electrodes. Cardiovasc Intervent Radiol 2005;28(4):476–480 [DOI] [PubMed] [Google Scholar]
- 10.Hines-Peralta A, Sukhatme V, Regan M, Signoretti S, Liu ZJ, Goldberg SN. Improved tumor destruction with arsenic trioxide and radiofrequency ablation in three animal models. Radiology 2006;240(1):82–89 [DOI] [PubMed] [Google Scholar]
- 11.Ahmed M, Lukyanov AN, Torchilin V, Tournier H, Schneider AN, Goldberg SN. Combined radiofrequency ablation and adjuvant liposomal chemotherapy: effect of chemotherapeutic agent, nanoparticle size, and circulation time. J Vasc Interv Radiol 2005;16(10):1365–1371 [DOI] [PubMed] [Google Scholar]
- 12.Ahmed M, Goldberg SN. Combination radiofrequency thermal ablation and adjuvant IV liposomal doxorubicin increases tissue coagulation and intratumoural drug accumulation. Int J Hyperthermia 2004;20(7):781–802 [DOI] [PubMed] [Google Scholar]
- 13.Yamakado K, Nakatsuka A, Takaki H, et al. Early-stage hepatocellular carcinoma: radiofrequency ablation combined with chemoembolization versus hepatectomy. Radiology 2008;247(1):260–266 [DOI] [PubMed] [Google Scholar]
- 14.Goldberg SN, Kamel IR, Kruskal JB, et al. Radiofrequency ablation of hepatic tumors: increased tumor destruction with adjuvant liposomal doxorubicin therapy. AJR Am J Roentgenol 2002;179(1):93–101 [DOI] [PubMed] [Google Scholar]
- 15.Monsky WL, Kruskal JB, Lukyanov AN, et al. Radio-frequency ablation increases intratumoral liposomal doxorubicin accumulation in a rat breast tumor model. Radiology 2002;224(3):823–829 [DOI] [PubMed] [Google Scholar]
- 16.Ahmed M, Monsky WE, Girnun G, et al. Radiofrequency thermal ablation sharply increases intratumoral liposomal doxorubicin accumulation and tumor coagulation. Cancer Res 2003;63(19):6327–6333 [PubMed] [Google Scholar]
- 17.Ahmed M, Liu Z, Lukyanov AN, et al. Combination radiofrequency ablation with intratumoral liposomal doxorubicin: effect on drug accumulation and coagulation in multiple tissues and tumor types in animals. Radiology 2005;235(2):469–477 [DOI] [PubMed] [Google Scholar]
- 18.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 2005;16(6):765–778 [DOI] [PubMed] [Google Scholar]
- 19.Goldberg SN, Girnan GD, Lukyanov AN, et al. Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intravenous liposomal doxorubicin in a rat breast tumor model. Radiology 2002;222(3):797–804 [DOI] [PubMed] [Google Scholar]
- 20.Han SI, Duong HQ, Choi JE, et al. Hyperthermia switches glucose depletion-induced necrosis to apoptosis in A549 lung adenocarcinoma cells. Int J Oncol 2008;32(4):851–860 [PubMed] [Google Scholar]
- 21.Janjetovic K, Misirkic M, Vucicevic L, Harhaji L, Trajkovic V. Synergistic antiglioma action of hyperthermia and nitric oxide. Eur J Pharmacol 2008;583(1):1–10 [DOI] [PubMed] [Google Scholar]
- 22.Wang CC, Chen F, Kim E, Harrison LE. Thermal sensitization through ROS modulation: a strategy to improve the efficacy of hyperthermic intraperitoneal chemotherapy. Surgery 2007;142(3):384–392 [DOI] [PubMed] [Google Scholar]
- 23.Cui ZG, Kondo T, Matsumoto H. Enhancement of apoptosis by nitric oxide released from alpha-phenyl-tert-butyl nitrone under hyperthermic conditions. J Cell Physiol 2006;206(2):468–476 [DOI] [PubMed] [Google Scholar]
- 24.Gabai VL, Budagova KR, Sherman MY. Increased expression of the major heat shock protein Hsp72 in human prostate carcinoma cells is dispensable for their viability but confers resistance to a variety of anticancer agents. Oncogene 2005;24(20):3328–3338 [DOI] [PubMed] [Google Scholar]
- 25.Cook JA, Gius D, Wink DA, Krishna MC, Russo A, Mitchell JB. Oxidative stress, redox, and the tumor microenvironment. Semin Radiat Oncol 2004;14(3):259–266 [DOI] [PubMed] [Google Scholar]
- 26.Calderwood SK, Ciocca DR. Heat shock proteins: stress proteins with Janus-like properties in cancer. Int J Hyperthermia 2008;24(1):31–39 [DOI] [PubMed] [Google Scholar]
- 27.Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: a short introduction to newcomers in the field. Int J Hyperthermia 2006;22(3):191–196 [DOI] [PubMed] [Google Scholar]
- 28.Rylander MN, Feng Y, Bass J, Diller KR. Thermally induced injury and heat-shock protein expression in cells and tissues. Ann N Y Acad Sci 2005;1066:222–242 [DOI] [PubMed] [Google Scholar]
- 29.Bonner M, Kmiec EB. DNA breakage associated with targeted gene alteration directed by DNA oligonucleotides. Mutat Res 2009;669(1-2):85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakob S, Corazza N, Diamantis E, Kappeler A, Brunner T. Detection of apoptosis in vivo using antibodies against caspase-induced neo-epitopes. Methods 2008;44(3):255–261 [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki H, Inoue T, Koizumi M, et al. Urinary 8-hydroxy-2′-deoxyguanosine excretion as a biomarker for estimating DNA oxidation in patients undergoing external radiotherapy and/or brachytherapy. Oncol Rep 2005;13(5):847–851 [PubMed] [Google Scholar]
- 32.Aluise CD, St Clair D, Vore M, Butterfield DA. In vivo amelioration of adriamycin induced oxidative stress in plasma by gamma-glutamylcysteine ethyl ester (GCEE). Cancer Lett 2009;282(1):25–29 [DOI] [PubMed] [Google Scholar]
- 33.Kim JM, Park KH, Kim YJ, Park HJ, Kim DM. Thermal injury induces heat shock protein in the optic nerve head in vivo. Invest Ophthalmol Vis Sci 2006;47(11):4888–4894 [DOI] [PubMed] [Google Scholar]
- 34.Nikfarjam M, Muralidharan V, Su K, Malcontenti-Wilson C, Christophi C. Patterns of heat shock protein (HSP70) expression and Kupffer cell activity following thermal ablation of liver and colorectal liver metastases. Int J Hyperthermia 2005;21(4):319–332 [DOI] [PubMed] [Google Scholar]
- 35.Morrison JP, Coleman MC, Aunan ES, Walsh SA, Spitz DR, Kregel KC. Thiol supplementation in aged animals alters antioxidant enzyme activity after heat stress. J Appl Physiol 2005;99(6):2271–2277 [DOI] [PubMed] [Google Scholar]
- 36.Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer 2000;88(11):2452–2463 [PubMed] [Google Scholar]
- 37.Goldberg SN, Gazelle GS, Dawson SL, Rittman WJ, Mueller PR, Rosenthal DI. Tissue ablation with radiofrequency: effect of probe size, gauge, duration, and temperature on lesion volume. Acad Radiol 1995;2(5):399–404 [DOI] [PubMed] [Google Scholar]
- 38.Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol 2002;178(1):47–51 [DOI] [PubMed] [Google Scholar]
- 39.Weinberg BD, Patel RB, Exner AA, Saidel GM, Gao J. Modeling doxorubicin transport to improve intratumoral drug delivery to RF ablated tumors. J Control Release 2007;124(1-2):11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee MW, Kim YJ, Park SW, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma invisible on both ultrasonography and unenhanced CT: a preliminary study of combined treatment with transarterial chemoembolization. Br J Radiol 2009;82:908–915 [DOI] [PubMed] [Google Scholar]
- 41.Takaki H, Yamakado K, Uraki J, et al. Radiofrequency ablation combined with chemoembolization for the treatment of hepatocellular carcinomas larger than 5 cm. J Vasc Interv Radiol 2009;20(2):217–224 [DOI] [PubMed] [Google Scholar]
- 42.Lencioni R, Crocetti L, Petruzzi P, et al. Doxorubicin-eluting bead-enhanced radiofrequency ablation of hepatocellular carcinoma: a pilot clinical study. J Hepatol 2008;49(2):217–222 [DOI] [PubMed] [Google Scholar]
- 43.Clasen S, Krober SM, Kosan B, et al. Pathomorphologic evaluation of pulmonary radiofrequency ablation: proof of cell death is characterized by DNA fragmentation and apoptotic bodies. Cancer 2008;113(11):3121–3129 [DOI] [PubMed] [Google Scholar]
- 44.Vichi P, Robison S, Tritton TR. Temperature dependence of adriamycin-induced DNA damage in L1210 cells. Cancer Res 1989;49(20):5575–5580 [PubMed] [Google Scholar]
- 45.Prise KM, Schettino G, Folkard M, Held KD. New insights on cell death from radiation exposure. Lancet Oncol 2005;6(7):520–528 [DOI] [PubMed] [Google Scholar]
- 46.Horkan C, Dalal K, Coderre JA, et al. Reduced tumor growth with combined radiofrequency ablation and radiation therapy in a rat breast tumor model. Radiology 2005;235(1):81–88 [DOI] [PubMed] [Google Scholar]
- 47.Solazzo S, Mertyna P, Peddi H, Ahmed M, Horkan C, Goldberg SN. RF ablation with adjuvant therapy: comparison of external beam radiation and liposomal doxorubicin on ablation efficacy in an animal tumor model. Int J Hyperthermia 2008;24(7):560–567 [DOI] [PubMed] [Google Scholar]
- 48.Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 2002;92(5):2177–2186 [DOI] [PubMed] [Google Scholar]
- 49.Wang RE, Kao JL, Hilliard CA, et al. Inhibition of heat shock induction of heat shock protein 70 and enhancement of heat shock protein 27 phosphorylation by quercetin derivatives. J Med Chem 2009;52(7):1912–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mertyna P, Hines-Peralta A, Liu ZJ, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: variability in heat sensitivity in tumors and tissues. J Vasc Interv Radiol 2007;18(5):647–654 [DOI] [PubMed] [Google Scholar]
- 51.Mertyna P, Dewhirst MW, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: the effect of distance and baseline temperature on thermal dose required for coagulation. Int J Hyperthermia 2008;24(7):550–559 [DOI] [PubMed] [Google Scholar]
- 52.Lepock JR. How do cells respond to their thermal environment? Int J Hyperthermia 2005;21(8):681–687 [DOI] [PubMed] [Google Scholar]
- 53.Foland TB, Dentler WL, Suprenant KA, Gupta ML, Jr, Himes RH. Paclitaxel-induced microtubule stabilization causes mitotic block and apoptotic-like cell death in a paclitaxel-sensitive strain of Saccharomyces cerevisiae. Yeast 2005;22(12):971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitzpatrick FA, Wheeler R. The immunopharmacology of paclitaxel (Taxol), docetaxel (Taxotere), and related agents. Int Immunopharmacol 2003;3(13-14):1699–1714 [DOI] [PubMed] [Google Scholar]