Abstract
This study was conducted to determine the distribution patterns and duration of stay of Toxocara cati larvae in organs of chickens and to investigate chronic phase and potential zoonotic risk of toxocariasis in chickens. Chickens were orally infected with 1,000 embryonated T. cati eggs and necropsied 240 days post-infection. Organs of the chickens were examined at gross and microscopic levels; tissues were digested to recover larvae. Peribronchiolitis with infiltration of lymphocytes, and hyperplasia of bronchiolar associated lymphatic tissues (BALT) and goblet cells, were evident in the lungs of infected chickens. There were mild hemorrhages and infiltration of lymphocytes and a few eosinophils in the meninges. Larvae were recovered from 30% of the exposed chickens. Larvae recovery indicated that T. cati larvae stay alive for at least 240 days in the chicken brain. Therefore, chickens may potentially act as a paratenic host in nature and transfer T. cati larvae to other hosts.
Keywords: Toxocara cati, pathology, chicken
We have already investigated the tissue migration patterns of Toxocara cati larvae in the visceral organs of chickens and suggested a potential zoonotic risk of this avian species for humans [1]. In this study, we prolonged the observation period till 240 days post-infection (PI) and compared the results with those of the previous study [1]. The main purpose of this study was to determine the larval migration and distribution patterns in chronic toxocariasis in chickens.
Materials used and methods applied were the same as those of the previous paper [1], and the follow-up period was 240 days PI. A total of 15 broiler chickens were divided into 2 groups, with 10 chickens in the experimental group and 5 as controls. Each chicken in the experimental and control groups was housed individually in clean cages. Each chicken in the experimental group was intubated with 1,000 embryonated T. cati eggs. The chickens were killed at day 240 PI. At necropsy, the liver, lungs, kidneys, spleen, heart, and brain of the infected and control chickens were carefully inspected for any gross abnormalities. The skeletal muscles of the limbs, chest, and intercostals were examined in the same way. Histological and larvae recovery methods were the same as the previous paper [1].
No clinical signs were observed in the infected or control groups during the chronic period, and no larvae were recovered from control chickens. However, larvae were recovered from the brain of 3 infected chickens by the squash method at day 240 PI.
Histopathological changes were observed in organs of all the experimentally infected chickens at day 240 PI. Peribronchiolitis with infiltration of lymphocytes, and hyperplasia of bronchiolar associated lymphatic tissues (BALT) and goblet cells, were evident in the lungs of infected chickens (Fig. 1). Mild hemorrhages, lymphocytic infiltrations, and a few eosinophils were observed in the meninges, especially over the cerebellum (Fig. 2). Perivascular infiltration was seen around some of the vessels in the cerebral parenchyma in most of the infected chickens. Mild focal infiltration of lymphocytes was present between cardiac muscle fibers of the infected chickens. No lesions were seen in other tissues of the infected chickens. No abnormality was observed at gross and histological levels in the liver, lungs, kidneys, heart, brain, and other tissues examined of uninfected control chickens.
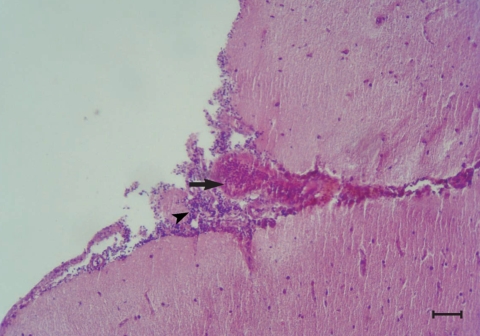
Fig. 1.
Peribronchiolitis, hyperplasia of the bronchiolar associated lymphatic tissues (white arrow) and goblet cells (black arrow) in the parenchyma of the lungs from a chicken infected with 1,000 Toxocara cati eggs on day 240 PI. H-E stain. Scale bar = 135 µm).
Fig. 2.
Congestion (arrow) and infiltration of lymphocytes (arrow head) in the meninges of the brain from a chicken infected with 1,000 T. cati eggs on day 240 PI. H-E stain. Scale bar = 175 µm).
Pathological findings in the lungs of the infected chickens were new and different from the previous short term observation [1]. Hyperplasia of BALT and goblet cells shows the defense mechanism to the chronic infection. The brain lesion was not described in the previous experiment. Since there was only a mild lesion in the brain, there was no behavioral change of the infected chickens. In the present study, no larvae were detected during the chronic phase in the liver, lungs, kidneys, or skeletal muscles in either larval recovery or histological studies. However, larvae were recovered from the brain of 3 infected chickens by the squash method.
The present study is the first report of the experimental infection of chickens with embryonated eggs of T. cati that showed persistence of this ascarid in the brain for at least 240 days PI. In uninfected control chickens, no gross or pathological abnormalities were observed. Therefore, the observed lesions were most likely associated with the migration of T. cati larvae. However, it is unclear why the brain infection was mild and only a few larvae were retrieved. The number of the intubated eggs could be a reason for the absence of the migrating larvae in the liver, muscles, heart, lungs, and kidneys and for the presence of only a few larvae in the brain parenchyma of 30% of the chronically infected chickens. It is reasonable to think that chickens acquire more embryonated eggs and larvae in nature, especially by ingesting earthworms which consume soil contaminated with T. cati eggs. Detailed studies with larger doses of T. cati embryonated eggs are necessary to investigate the larval migration behavior in chickens.
ACKNOWLEDGEMENTS
The authors would like to appreciate the financial support of Shiraz University, Shiraz, Iran. We also would like to thank Mr. L. Shirvani, Mr. G. Yussefi, Mr. S. A. Roueentan, and Mrs. S. Kazemian for their technical assistance.
References
- 1.Azizi S, Oryan A, Sadjjadi SM, Zibaei M. Histopathological changes and larval recovery of Toxocara cati in experimentally infected chickens. Parasitol Res. 2007;102:47–52. doi: 10.1007/s00436-007-0722-5. [DOI] [PubMed] [Google Scholar]