Abstract
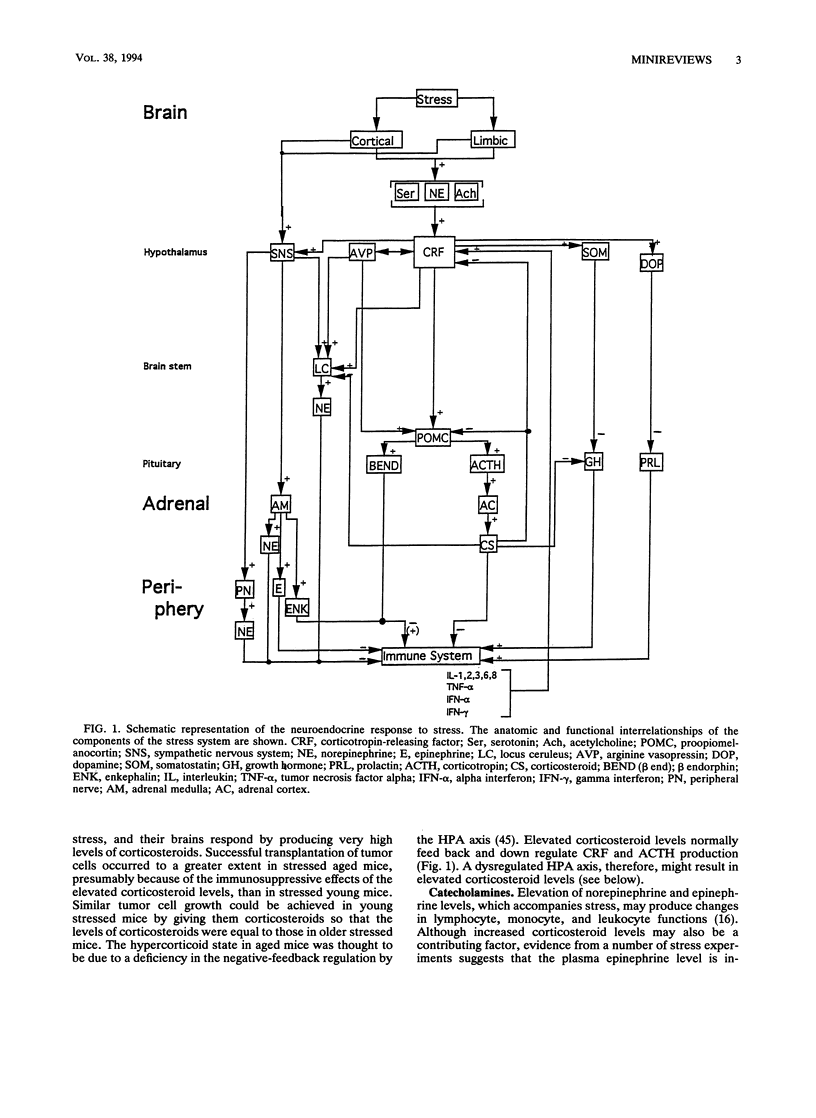
Psychoneuroimmunology is a relatively new discipline which deals with CNS-immune system interactions. The evidence for such interactions was reviewed, as was the neuroendocrinologic response to stress. Recent evidence indicates that the behavioral, nervous system, and neuroendocrine responses to stress are mediated by hypothalamic CRF, which acts on both the sympathetic nervous system and the HPA axis, resulting in increased levels of corticosteroids, catecholamines, and certain opiates, substances which are generally immunosuppressive. Concentrations of growth hormone and prolactin, which are immunoenhancing, are elevated early during the response to stress but are later suppressed. Although several other neuromediators may also be released with stress, the net effect of a variety of acute stressors is down regulation of the immune system function. In the following minireview, I consider whether stress alters the resistance of the host to infection as well as the immunomodulatory effects of released immune system mediators on the brain.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ader R., Cohen N. Behaviorally conditioned immunosuppression. Psychosom Med. 1975 Jul-Aug;37(4):333–340. doi: 10.1097/00006842-197507000-00007. [DOI] [PubMed] [Google Scholar]
- Arnason B. G. Nervous system-immune system communication. Rev Infect Dis. 1991 Jan-Feb;13 (Suppl 1):S134–S137. [PubMed] [Google Scholar]
- Baroni C. Thymus, peripheral lymphoid tissues and immunological responsiveness of the pituitary dwarf mouse. Experientia. 1967 Apr 15;23(4):282–283. doi: 10.1007/BF02135688. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Meltzer M. S., Holaday J. W. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science. 1988 Jan 22;239(4838):401–404. doi: 10.1126/science.3122324. [DOI] [PubMed] [Google Scholar]
- Blalock J. E. A molecular basis for bidirectional communication between the immune and neuroendocrine systems. Physiol Rev. 1989 Jan;69(1):1–32. doi: 10.1152/physrev.1989.69.1.1. [DOI] [PubMed] [Google Scholar]
- Brummitt C. F., Sharp B. M., Gekker G., Keane W. F., Peterson P. K. Modulatory effects of beta-endorphin on interferon-gamma production by cultured peripheral blood mononuclear cells: heterogeneity among donors and the influence of culture medium. Brain Behav Immun. 1988 Sep;2(3):187–197. doi: 10.1016/0889-1591(88)90021-9. [DOI] [PubMed] [Google Scholar]
- Chelmicka-Schorr E., Arnason B. G. Nervous system-immune system interactions. Res Publ Assoc Res Nerv Ment Dis. 1990;68:67–90. [PubMed] [Google Scholar]
- Chelmicka-Schorr E., Kwasniewski M. N., Thomas B. E., Arnason B. G. The beta-adrenergic agonist isoproterenol suppresses experimental allergic encephalomyelitis in Lewis rats. J Neuroimmunol. 1989 Dec;25(2-3):203–207. doi: 10.1016/0165-5728(89)90138-0. [DOI] [PubMed] [Google Scholar]
- Chrousos G. P., Gold P. W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992 Mar 4;267(9):1244–1252. [PubMed] [Google Scholar]
- Clevenger C. V., Russell D. H., Appasamy P. M., Prystowsky M. B. Regulation of interleukin 2-driven T-lymphocyte proliferation by prolactin. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6460–6464. doi: 10.1073/pnas.87.16.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., Kelley K. W. Stress and immunity: an integrated view of relationships between the brain and the immune system. Life Sci. 1989;44(26):1995–2008. doi: 10.1016/0024-3205(89)90345-7. [DOI] [PubMed] [Google Scholar]
- Dardenne M., Kelly P. A., Bach J. F., Savino W. Identification and functional activity of prolactin receptors in thymic epithelial cells. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9700–9704. doi: 10.1073/pnas.88.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A. J., Berridge C. W. Is corticotropin-releasing factor a mediator of stress responses? Ann N Y Acad Sci. 1990;579:183–191. doi: 10.1111/j.1749-6632.1990.tb48360.x. [DOI] [PubMed] [Google Scholar]
- Edwards C. K., 3rd, Yunger L. M., Lorence R. M., Dantzer R., Kelley K. W. The pituitary gland is required for protection against lethal effects of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2274–2277. doi: 10.1073/pnas.88.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felten D. L., Felten S. Y., Bellinger D. L., Carlson S. L., Ackerman K. D., Madden K. S., Olschowki J. A., Livnat S. Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol Rev. 1987 Dec;100:225–260. doi: 10.1111/j.1600-065x.1987.tb00534.x. [DOI] [PubMed] [Google Scholar]
- Felten D. L., Felten S. Y. Sympathetic noradrenergic innervation of immune organs. Brain Behav Immun. 1988 Dec;2(4):293–300. doi: 10.1016/0889-1591(88)90031-1. [DOI] [PubMed] [Google Scholar]
- Fiatarone M. A., Morley J. E., Bloom E. T., Benton D., Makinodan T., Solomon G. F. Endogenous opioids and the exercise-induced augmentation of natural killer cell activity. J Lab Clin Med. 1988 Nov;112(5):544–552. [PubMed] [Google Scholar]
- Irwin M., Hauger R. L., Jones L., Provencio M., Britton K. T. Sympathetic nervous system mediates central corticotropin-releasing factor induced suppression of natural killer cytotoxicity. J Pharmacol Exp Ther. 1990 Oct;255(1):101–107. [PubMed] [Google Scholar]
- Janković B. D. Neuroimmunomodulation: facts and dilemmas. Immunol Lett. 1989 May;21(2):101–118. doi: 10.1016/0165-2478(89)90046-1. [DOI] [PubMed] [Google Scholar]
- Kelley K. W. Cross-talk between the immune and endocrine systems. J Anim Sci. 1988 Aug;66(8):2095–2108. doi: 10.2527/jas1988.6682095x. [DOI] [PubMed] [Google Scholar]
- Kelley K. W. Growth hormone, lymphocytes and macrophages. Biochem Pharmacol. 1989 Mar 1;38(5):705–713. doi: 10.1016/0006-2952(89)90222-0. [DOI] [PubMed] [Google Scholar]
- Khansari D. N., Murgo A. J., Faith R. E. Effects of stress on the immune system. Immunol Today. 1990 May;11(5):170–175. doi: 10.1016/0167-5699(90)90069-l. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Cacioppo J. T., Malarkey W. B., Glaser R. Acute psychological stressors and short-term immune changes: what, why, for whom, and to what extent? Psychosom Med. 1992 Nov-Dec;54(6):680–685. doi: 10.1097/00006842-199211000-00008. [DOI] [PubMed] [Google Scholar]
- Lightman S. L., Young W. S., 3rd Influence of steroids on the hypothalamic corticotropin-releasing factor and preproenkephalin mRNA responses to stress. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4306–4310. doi: 10.1073/pnas.86.11.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melia K. R., Duman R. S. Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8382–8386. doi: 10.1073/pnas.88.19.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar D. B., Hough C. J., Mazorow D. L., Gootenberg J. E. Beta-endorphin's modulation of lymphocyte proliferation is dose, donor, and time dependent. Brain Behav Immun. 1990 Sep;4(3):232–242. doi: 10.1016/0889-1591(90)90025-l. [DOI] [PubMed] [Google Scholar]
- Munck A., Guyre P. M. Glucocorticoid physiology, pharmacology and stress. Adv Exp Med Biol. 1986;196:81–96. doi: 10.1007/978-1-4684-5101-6_6. [DOI] [PubMed] [Google Scholar]
- Munck A., Guyre P. M., Holbrook N. J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984 Winter;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nordlind K., Mutt V., Sundström E. Effect of neuropeptides and monoamines on lymphocyte activation. Brain Behav Immun. 1988 Dec;2(4):282–292. doi: 10.1016/0889-1591(88)90030-x. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Chao C. C., Molitor T., Murtaugh M., Strgar F., Sharp B. M. Stress and pathogenesis of infectious disease. Rev Infect Dis. 1991 Jul-Aug;13(4):710–720. doi: 10.1093/clinids/13.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Sharp B., Gekker G., Brummitt C., Keane W. F. Opioid-mediated suppression of interferon-gamma production by cultured peripheral blood mononuclear cells. J Clin Invest. 1987 Sep;80(3):824–831. doi: 10.1172/JCI113140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto J., Subirá M. L., Castilla A., Arroyo J. L., Serrano M. Opioid peptides modulate the organization of vimentin filaments, phagocytic activity, and expression of surface molecules in monocytes. Scand J Immunol. 1989 Apr;29(4):391–398. doi: 10.1111/j.1365-3083.1989.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Rogers T. J., Taub D. D., Eisenstein T. K., Geller E. B., Adler M. W. Immunomodulatory activity of kappa-, mu-, and delta-selective opioid compounds. NIDA Res Monogr. 1990;105:82–88. [PubMed] [Google Scholar]
- Sapolsky R. M., Donnelly T. M. Vulnerability to stress-induced tumor growth increases with age in rats: role of glucocorticoids. Endocrinology. 1985 Aug;117(2):662–666. doi: 10.1210/endo-117-2-662. [DOI] [PubMed] [Google Scholar]
- Savino W., Gagnerault M. C., Bach J. F., Dardenne M. Neuroendocrine control of thymic hormonal production. II. Stimulatory effects of endogenous opioids on thymulin production by cultured human and murine thymic epithelial cells. Life Sci. 1990;46(23):1687–1697. doi: 10.1016/0024-3205(90)90384-4. [DOI] [PubMed] [Google Scholar]
- Solomon G. F., Amkraut A. A. Psychoneuroendocrinological effects on the immune response. Annu Rev Microbiol. 1981;35:155–184. doi: 10.1146/annurev.mi.35.100181.001103. [DOI] [PubMed] [Google Scholar]
- Spanagel R., Herz A., Shippenberg T. S. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsagarakis S., Holly J. M., Rees L. H., Besser G. M., Grossman A. Acetylcholine and norepinephrine stimulate the release of corticotropin-releasing factor-41 from the rat hypothalamus in vitro. Endocrinology. 1988 Oct;123(4):1962–1969. doi: 10.1210/endo-123-4-1962. [DOI] [PubMed] [Google Scholar]
- Valentino R. J. CRH effects on central noradrenergic neurons: relationship to stress. Adv Exp Med Biol. 1988;245:47–64. doi: 10.1007/978-1-4899-2064-5_5. [DOI] [PubMed] [Google Scholar]
- Valentino R. J. Corticotropin-releasing factor: putative neurotransmitter in the noradrenergic nucleus locus ceruleus. Psychopharmacol Bull. 1989;25(3):306–311. [PubMed] [Google Scholar]
- Wiedemann K., von Bardeleben U., Holsboer F. Influence of human corticotropin-releasing hormone and adrenocorticotropin upon spontaneous growth hormone secretion. Neuroendocrinology. 1991 Nov;54(5):462–468. doi: 10.1159/000125937. [DOI] [PubMed] [Google Scholar]


