SUMMARY
The BRCA1 gene was identified and cloned in 1994 based on its linkage to early onset breast and ovarian cancer syndromes in women. The tumor suppressor, BRCA1 is known as a major player in the DNA damage response. These are evident from its loss, which causes malignant transformation in breast and ovary, and renders cells to become sensitive to a wide variety of DNA damaging agents. Here, we have implications on functional coupling of the pleiotropic roles of BRCA1, including DNA damage signal networking, DNA repair, transcription, and checkpoint of cell cycle, to tumor suppression by examining the molecular mechanisms and functions of BRCA1.
The breast cancer susceptibility 1 (BRCA1) gene was identified and mapped to chromosome 17q21 by analyzing families at high risk from breast and ovarian cancer, and was first cloned in 1994 (1). The BRCA1 gene encodes a large nuclear protein that is ubiquitously expressed in a number of tissues. BRCA1 shares little structural resemblance to the majority of other known proteins (Fig. 1). Its ortholog is only found in mammals but not in yeast, fly, worm, or zebra fish, indicating that BRCA1 may come later in evolution and it may have more specialized and tissue-specific functions in mammalian cells. Although a number of studies delineating and deciphering the real biological roles of BRCA1 have accumulated, understanding these BRCA1 unique features still remains to be challengingly elucidated.
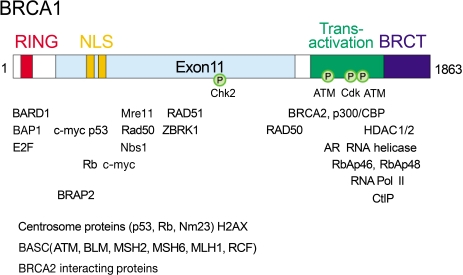
Fig. 1.
A schematic diagram of the BRCA1 polypeptide and its interaction with different proteins. BRCA1 polypeptide has the phosphorylation sites (Ⓟ) shown as the seine residues that are phosphorylated and the kinases responsible. The proteins that are interact with BRCA1 are described below the interacting regions in BRCA1. Centrosome proteins, proteins belonging to BASC, and other BRCA2 interacting proteins have been shown to interact with BRCA1 in vivo. The BRCA1 polypeptide has N-terminal RING motif (red), nuclear localization signal (NLS, orange), and two C-terminal BRCT-domains (blue).
BRCA1 as a tumor suppressor
The BRCA1 mutations account for about 80% of families whose members have a high incidence of both breast and ovarian cancers (2). The BRCA1 gene fits the profile of a classical 'tumor suppressor gene,' since the breast and ovarian cancers that develop in carriers of BRCA1 gene mutations almost always exhibit loss of the wild-type BRCA1 allele (1).
The BRCA1 protein consists of 1863 amino acids (Fig. 1) and is expressed in most proliferating cells (1). The C-terminus of BRCA1 contains an amino-acid sequence motif, now known as a BRCT domain (Fig. 1), recognized in many DNA repair proteins. The BRCT domain of BRCA1 mediates protein interactions that are critical to its role in transcriptional regulation, signaling networking, and the response to DNA damage. The germline mutations in the BRCA1 mutation carriers are frequently found in the BRCT domain, suggesting that the BRCT domain is important for its diverse biological roles. In the N-terminus, there is a ring-finger domain (Fig. 1), also allowing for protein-protein interactions, and thought to be involved in protein ubiquitination. Disruption of the ring domain (by the mutations C61G or C64G) blocked the ability of BRCA1 to repress estrogen receptor-α (ER-α) signaling and to modulate DNA repair, chemosensitivity, and apoptosis. These findings suggest that the ring domain mediates critical protein interactions, and disruption of which contributes to carcinogenesis. Additionally, a variety of structural domains in BRCA1 with these distinct functions hint at diverse roles of BRCA1 in multiple cellular processes.
Recent epidemiologic studies indicate that BRCA1 mutation carriers have a high lifetime risk of breast cancer of up to 80%. In addition to the breast cancer risk, women with BRCA1 mutations have an increased risk of ovarian cancer. These works solidify the concept that BRCA1 functions as a tumor suppressor. Despite the multiple lines of strong evidences supporting the function of BRCA1 as a tumor suppressor, the mechanisms and the precise roles through which BRCA1 loss leads to tumorigenesis remain to be determined.
As a caretaker by maintaining genomic integrity
Tumor suppressor genes are classified into two groups (3): i) caretakers that enable to inhibit or repair DNA damage, and ii) gatekeepers that enable to block the propagation of potential cancer candidate cells by inhibiting cell death (apoptosis) and/or arrest of cell growth (senescence). BRCA1-deficeint cancer or mouse cells show gross chromosomal rearrangements such as translocations, deletions, or fusions of multiple, nonhomologous chromosomes (4). Therefore, we have learned that BRCA1 play a role in maintaining genomic integrity, from the BRCA1-deficeincy.
The genomic instability observed in BRCA1-deficient cells may stem from inappropriate repair or recombination pathways. Both yeast and mammalian mutant models reveal a complex relationship between repair pathways and spontaneous genomic instability (5). Furthermore, the loss of BRCA1 gene expression causes DNA damage repair defects (see later in this review), possibly leading to genomic instability. One of the direct consequences of the absence of genes responsible for DNA damage repair is genetic instability. BRCA1 mutant cells exhibited abnormal cell cycle checkpoint control (see later in this review). Therefore the genetic instability observed in BRCA1 mutant cells is likely a consequence of joint and enforced actions of these defects, i.e. absence of caretaker functions of BRCA1, instead of just impaired DNA damage repair.
During the cell division, genomic instability occurs spontaneously in the BRCA1-deficient cells. It is proposed that an important function of BRCA1 in homologous recombination enable stalled replication forks at template lesions to restart the error-free replication. In addition, this scheme could explain defects in cell proliferation and chromosomal instability.
BRCA1 functions in DNA damage repair
In mammalian cells, double stranded DNA breaks can be repaired by homologous recombination (error-free) through exchange between identical sister chromatids (that is homologous DNA sequences), or nonhomologous end joining (error-prone) through rejoining of different chromosome ends leading to DNA sequence alterations. The other error-prone repair mechanism is single-strand annealing that occurs by a homologous paring with short stretches of single strand and then religation.
BRCA1-null cells are sensitive to double stranded DNA damage (6), suggesting that BRCA1 is important for DNA repair. Indeed, BRCA1 is reported to show a role in error-free homologous recombination, because BRCA1-deficinet mouse cells or human cancers are deficient in homologous recombination, while nonhomologous recombination remains fine (1,6). This can give a plausible scenario that hypothesizes endogenous or exogenous DNA damages in BRCA1-deficient cells can be processed by reprogrammed repair system for the mechanisms that are potentially error-prone, since the better error-free repair mode by homologous recombination is not available.
The further evidence of its role in DNA damage repair is its association with other proteins involved in DNA damage repair including the BRCA2/Rad51 (7) and the hMRE11/hRad50/Nibrin (p95) (MRN complex) complexes (8), which function in homology-directed DNA repair (Fig. 1, 2). The BRCA2 gene, discovered soon after the BRCA1 gene, is also mutated in some heritable breast cancers and pancreatic adenocarcinomas, and encodes another larger protein that is also important for maintaining genome integrity by error-free homologous repair.
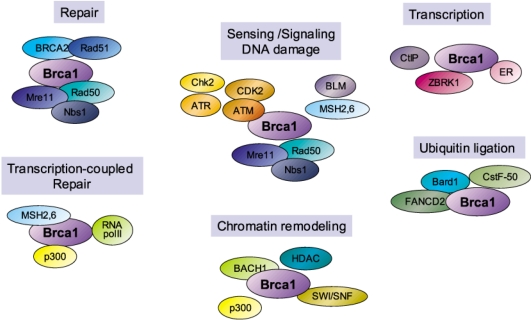
Fig. 2.
Interactions of BRCA1 with a diversity of proteins and its possible roles. The putative cellular functions of protein- protein interactions of BRCA1 are shown in boxes. Their biological significances are not yet clarified.
On the other hand, the recent works with immunoprecipitation and proteomic approaches indicate that BRCA1 is associated with a large complex, called BASC (BRCA1-associated genome surveillance complex), bigger than 2 MD composed of DNA repair proteins such as MSH2, MSH6, MLH1, ATM, BLM and the hRad50/hMRE11/p95 complex (9), which play a role of DNA damage repair, and also BRCA1 is associated with RNA polymerase II, p300/CBP, and HDAC, which function in transcription and chromatin remodeling (Fig. 1, 2). These associations may not prove functional interaction and biochemical identity, but may indirectly address how BRCA1 is implicated in DNA repair. Therefore, these associations have an implication that BRCA1 plays a role in transcription-coupled repair, in addition to homologous recombination-dependent repair. Transcription-coupled repair is the repair of single-stranded DNA damage, presumably via base- excision repair or nucleotide-excision repair, when the DNA damage occurs within transcribed genes (10). The cells lacking BRCA1 is indeed deficient in transcription-coupled repair of DNA damages produced by ionizing radiation or hydrogen peroxide but perform it of UV-induced DNA damage normally (6,10). Together, these suggest BRCA1 can play a critical role of genome stability owing to its ability of DNA repair.
What are biological roles of BRCA1 foci ?
When the BRCA1 gene was first cloned and its amino acid sequences were deduced about ten years ago, the BRCA1 protein showed no homology to other known proteins. Thus, questions concerning its in vivo functions and biochemical activities have dominated the field throughout these years. The first clue to a role for BRCA1 in the DNA-damage response was the finding that BRCA1 associates and co-localizes with Rad51, a DNA recombinase homologous to the bacterial RecA protein, during the S-phase of mitotic cell cycles (7). The BRCA1 proteins are microscopically observed as nuclear dots in subnuclear structures, which are called 'BRCA1 foci' (9,10). Furthermore, the formation of BRCA1 foci is induced upon exposure with DNA damages, either following or almost simultaneously with the rapid subnuclear translocation and phosphorylation events (9,10) (see the detail later in this review). Taken together, these findings suggest roles for BRCA1 in the signaling and/or repair of DNA damages.
BRCA1 colocalizes with phosphorylated proteins of a histone variant, H2AX, (γ-H2AX), after DNA damage (12). Unphosphorylated H2AX is interspersed in chromatin throughout the genome, and following DNA damage, one of the earliest events is the phosphorylation of Ser139 of H2AX in large DNA domains encompassing a million base pairs. γ-H2AX forms discrete foci within 10 minutes of DNA damage with BRCA1 detectable in these foci as fast as within 30 min in some cancer cell lines, as slow as six hours in others. After BRCA1, either Mre11/RAD50/Nbs1 or RAD51/BRCA2, but not both (mutually exclusive), also colocalize with DNA-damage-induced foci (12). BRCA1 proteins can redistribute into distinct and diverse nuclear foci that contain proteins involved in sister chromatid recombination (BLM and/or Rad51/BRCA2), in fixing stalled replication (Mre11/Rad50/Nbs1), or in DNA polymerase complex (PCNA or RFC) (Fig. 2). After BRCA1 foci appearing after DNA damage seem to be aggregates of hundreds or more protein molecules. Though BRCA1-deficient cells have defects in transcription-coupled repair, homologous recombination, nonhomologous end-joining (NHEJ), and microhomology end-joining, they normally have both types of visible repair foci in the absence of BRCA1 proteins (10,13). Therefore, questions concerning the necessity and the indeed roles of BRCA1 in the repair foci and during the repair processes remain elusive.
The BRCA1 protein has also been found to colocalize with another modified histone, macro-H2A1 and H3mK9 (histone H3 methylated at Lys9) at the inactive X chromosome in the female somatic cells. Also, BRCA1 associates with one of markers of the inactive X chromosome, XIST RNA, in a focal XIST RNA dot. The BRCA1-deficient cells show defects in the heterochromatin structure of inactive X chromosome (14). Together, more work will be needed to see whether or what BRCA1 is doing at the inactive X chromosome foci, since how this BRCA1-inactive X chromosome focal formation may assist and work in inactivation of X chromosome in female cells is currently speculative.
What role in cell cycle ?
BRCA1 is found in nuclear foci that form in a cell cycle dependent manner (7,11). BRCA1 mRNA and protein expression rise in mid-late G1 coincident with the rise of cyclin A levels; peak at the G1/S interface or in early-mid S; and decline by late S or G2. In addition, it was reported that BRCA1-deficient cells show a defect in the arrest of DNA synthesis (S) after ionizing radiation damage (4). BRCA1-deficient cells also show a defect in the G2/M phase cell cycle checkpoint, and this phenotype has been associated with radiation sensitivity (4). Brca1 exon 11 isoform-deficient cells show the defect of G2 checkpoint and some of them exhibit amplification of functional centrosomes, leading to unequal segregation of chromosomes and aneuploidy, a major characteristics of many cancer types (4,5). Though these observations suggest that BRCA1 is important in the cell cycle checkpoint at S and G2-M, the exact role of BRCA1 and mechanisms in this process has remained unclear.
Nevertheless, the transcriptional regulation by BRCA1 (see the next section) may functionally link to the cell cycle regulation. p21WAF1/CIP1 was originally identified as a G1 cyclin dependent kinase inhibitor, a target of p53 transcriptional activity, and a growth inhibitor that accumulates during cellular senescence. Subsequently, p21WAF1/CIP1 has been proposed as a tumor suppressor in its own right. Exogenous wild-type BRCA1 was found to transactivate the p21WAF1/CIP1 promoter in human colon cancer cells (15). BRCA1 has also been found to transactivate the cyclin-dependent kinase inhibitor p27KIP1 promoter (16). These cell cycle regulators of which expressions are regulated by BRCA1 can function as mediators between transcription and cell cycle.
BRCA1 in transcriptional regulation
In addition to the association with DNA repair proteins, BRCA1 can be associated with proteins belonging to the transcription factor family. A role in transcription was suggested by the finding that BRCA1 has a conserved acidic domain with transcriptional activity in yeast and mammalian cells. Subsequently, it was found that BRCA1 regulates a variety of transcriptional pathways (Fig. 2, 3). BRCA1 transcription regulatory activity may be mediated, in part, by interaction with the basal transcriptional machinery (RNA helicase A and RNA pol II) and with transcriptional coactivators (p300, CBP) and corepressors (RbAp46/48, HDAC-1/2, and CtIP) (17). BRCA1 can also interact with Brg1, a component of the SWI/SNF chromatin remodeling complex with ATPase activity (17). BRCA1 also associates with tumor suppressors (p53, Rb, BRCA2) (27), which can regulate transcription. Additionally, it is intriguing that BRCA1 shows tissue specific transcriptional activity by interacting with the tissue specific transcription factors, estrogen receptor and androgen receptor. BRCA1 associates with other sequence-specific transcription factors (c-Myc, Oct-1, NF-YA) and cell cycle regulatory transcriptional protein (BARD1, E2F1). It is most likely that BRCA1 may not recognize a specific DNA sequence, but may function as a coactivator or corepressor for other sequence-specific binding proteins by protein-protein interactions. For example, BRCA1 can also function as a corepressor of Gadd45, through its interaction with a novel zinc finger protein, ZBRK1. Together with its association with RNA helicase A, BRCA1 can be a component of the SWI/SNF complex, a large ATP-dependent chromatin remodeling complex (17), aiding transcriptional machinenary in access to DNA. Thus, BRCA1 may gain access to DNA by interacting these chromatin remodeling factors, at least partially (17).
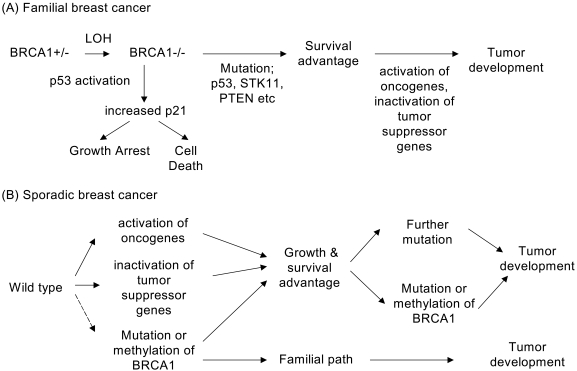
Fig. 3.
Hypothetical breast cancer development models based on previously reported genetic and biochemical data. The plausible roadmaps to breast cancers of (A) BRCA1- associated familial and (B) sporadic cases. (A) In familial breast cancer, one additional somatic mutation in the BRCA1 locus (LOH, loss of heterozygosity) in carriers of one germiline mutant BRCA1 allele (BRCA1+/-) results in a complete loss of BRCA1 function (BRCA1-/-). The BRCA1 deficiency (BRCA1-/-), in turn, causes genomic instability. DNA damage triggered by BRCA1 deficiency-induced genomic instability in BRCA1 null (BRCA1-/-) cells can either activate p53-dependent pathways (downward arrow; leading to cell cycle checkpoint, growth arrest, apoptosis, or senescence) or facilitate further mutations of additional breast cancer-associated susceptibility genes (p53, STK11, PTEN, EGFR, Her2 etc), and other tumor suppressor and oncogenes (rightward arrows, leading to carcinogenesis). Genomic instability for survival/growth advantages can be preferred in breast and ovary tissues, while p53 activation for growth arrest/death can be preferred in other tissues. (B) In sporadic breast cancer, the loss or reduction of BRCA1 expression is not necessary for cancer development. Otherwise, carcinogenesis events can be initiated by activation of oncogenes or inactivation of tumor suppressor genes, but not by somatic mutations or silencing BRCA1 (rare but theoretically possible). At least four somatic mutations are required for the development of sporadic breast cancers.
The BRCT domain in the carboxy-terminus of BRCA1 has transcription activating activity (18), and BRCA1 itself has indeed been shown to increase expression from the GADD45 (18) and p21 promoters (15). On the other hand, BRCA1 exhibit suppression activity for the estrogen receptor α responsive promoter (19) and the IGF-1 receptor promoter. The data about the association of BRCA1 with the diverse transcriptional factors are consistent with the observations that BRCA1 can indeed regulate the transcriptional activity. Although the growing list of many transcriptional factors associated with BRCA1 and discovery of its target genes supports the physiological link of BRCA1's transcriptional activity, the biological significance of its transcriptional activity has been argued and questioned.
BRCA1 regulates cell proliferation and apoptosis
The loss of BRCA1 expression causes embryonic death in mice, possibly by an early (about E7.5) proliferation block accompanied by elevated p21WAF1/CIP1 expression (20) (Fig. 3). This is further supported by the observation that BRCA1 protein is expressed in a wide range of tissues in developing mouse embryos, particularly in proliferating and differentiating cells. These data suggest that BRCA1 plays an important role in cell proliferation. Also, the functional loss of BRCA1 in mammary epithelial cells leads to abnormal blunted ductal morphogenesis and apoptosis (21).
Based on these observations from knockout mice, we can raise a question how the functional loss of BRCA1 in cancer cells should not induce antiproliferation and apoptosis. One explanation for this paradox is that if BRCA1 gene disruption were to occur in normal cells, no survival would be possible (Fig. 3B). In this context, a tempting model is where BRCA1 and p53 genetically and functionally interplay in cancer development (17) (Fig. 3A). It is interesting that p53 transcription is changed in approximately 70% of tested breast tumors tested and mutations in the p53 gene are often observed in BRCA1-associated breast tumors (Fig. 3A). Additionally the introduction of a p53-null allele into BRCA1 conditional knockout mice dramatically promotes and accelerates mammary tumors, of which most have additionally lost their remaining wild type p53 allele (17). In contrast, p53 heterozygous mice rarely develop mammary tumors (17). Together, these data strongly support a role of p53 in BRCA1-associated tumorigenesis, and give us one clue for the paradoxical phenotypes. The p53 loss can lead to block of p53-dependent apoptosis in BRCA1-null cells. At the same time, activation of oncogene and/or inactivation of tumor suppressor induced by increased genomic instability (one consequence of BRCA1 deficiency) can furthermore promote cell proliferation and finally develop cancers (Fig. 3). On the other hand, BRCA1-associated breast cancers without p53 mutations should be equipped with substitute growth advantage mechanisms, such as anti-apoptotic and/or proaoptotic functions for evading antiproliferaion and growth arrest in a p53-independent manner, which is most likely to be induced by genomic instability (Fig. 3). Otherwise, the BRCA1-null cells go to routes for growth arrest or cell death in the absence of additional growth advantageous mutations (Fig. 3A).
What does BRCA1 serve for DNA damage responses ?
Our genetic blueprint, DNA, is constantly assaulted by environmental and cellular causes of DNA damage from environment and endogenous cellular influences, such as: UV, chemical, food, reactive oxygen species from metabolism, DNA replication fork stalling, alterations in telomere shortening and chromatin remodeling. How do cells sense and respond to these stresses in the form of damage to their genetic material, to maintain their genetic integrity? Fortunately, these massive attacks on our DNA are largely counterbalanced by promptly multifaceted and sophisticated surveillance and rescue operations that cells have evolved to deal with DNA damage. Signaling pathways are rapidly activated after exposure to DNA-damaging stresses and proteins are recalled in the active and functional form, in order to efficiently participate in DNA damage responses. These pathways and networks lead to cellular responses such as cell cycle arrest, DNA repair, transcriptional reprogramming, and apoptosis (Fig. 4).
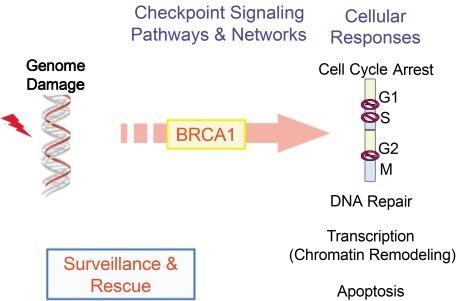
Fig. 4.
The roles of BRCA1 in DNA damage responses. DNA damage triggers kinases-dependent phosphorylation of BRCA1. BRCA1 can process and integrate DNA damage signal by combination of its phosphorylation sites and extent, and BRCA1 transduces the processed signal to other DNA damage responses (arrow). The diverse functions of BRCA1 and other effectors (cell cycle checkpoint, DNA repair, transcription, and apoptosis), in turn, are regulated and tuned by the processed signal i.e. phosphorylation status of BRCA1. Therefore, BRCA1 might function as not only a signal processor but also a coordinator of various activities in DNA damage responses in order to maintain genome integrity.
Phosphorylation of BRCA1 by individual and/or combination of kinases, ATR, ATM, and Chk2 (Fig. 1), occurs on distinct combinatory sets of serine/threonine residues in response to distinct stimuli, suggesting that BRCA1 can serve a specific and fine-tuning function in each distinct case. For example, ATM and Chk2 phosphorylate BRCA1 protein after ionizing irradiation (which mainly generates double stranded DNA breakage) (11,22,23), while ATR phosphorylates it after UV irradiation (nucleotide lesions) or hydroxy urea treatment (replication block by strand gaps). Thus, BRCA1 can play a role as a signal processor and interplayer for selecting suitable responses in each instance.
As we previously describe in this review, BRCA1 has been implicated in one functional consequence, several distinct checkpoints in cell cycle progression. BRCA1 deficient cells fail to arrest the scheduled checkpoints, S, G2, and G2-M transition (4), leading to unresponsiveness to DNA damage, suggesting that BRCA1 is important in control of cell cycle arrest and progression. Depending on different DNA damages, arrest(s) at the several distinct checkpoints throughout cell cycle is/are activated. BRCA1 phosphorylations by ATM are required for the G2 arrest induced by ionizing irradiation (4). Thus, one speculation is that BRCA1 works as a signal processor for the proper cell cycle arrest to occur (Fig. 4).
Also, BRCA1 somehow functions in several different DNA repair events possibly by forming diverse functional repair protein network through protein-protein interactions (Fig. 1, 2), although its precise biochemical role in each process remains unknown. It is known that each distinct DNA repair machinery performs distinct repair process to fix each different DNA damage. Thus, it is postulated that different phosphorylation status of BRCA1 could recruit different DNA repair components and form functionally different DNA repair complexes for each of diverse BRCA1-associated repair complexes to fix distinct DNA damage. Recently, phosphorylation of serine 988 in BRCA1 by Chk2 (23) has reported to be important for activity of Mre11/Rad51/Nbs1 repair complex (24), supporting this idea.
Therefore, the scenario that emerges is one where BRCA1 works in multiple signal transduction pathways by processing signals that are transmitted to cell cycle control and DNA repair systems in the presence of different kinds of DNA damages. Consistent with this idea, BRCA1 associates with many diverse proteins and forms complexes with proteins that can bind to DNA, repair damaged DNA, or/and phosphorylate substrates (kinases) (Fig. 4). It is probable that formation of BRCA1 complexes dynamically varies during cell cycle as well as following different DNA damages, and in turn, different BRCA1 complexes differentially work. Aspects of this story can also be applied to other BRCA1 functions including transcription and apoptosis, in addition to cell cycle control and DNA repair. There is good evidence that BRCA1 phoshorylations occur after DNA damage, and its transcriptional activity on p21WAF1/CIP1 and Gadd45 expression is stimulated. It is likely that BRCA1 interactions with many diverse transcriptional factors very probably change and regulate differentially transcription after different kinds of DNA damage. Increased expression of the cell cycle progression inhibitor p21WAF1/CIP1 renders cell cycle progression to delay by arresting at G1, and DNA-damage induced Gadd45 that functions in DNA repair, cell cycle checkpoint mechanisms, and apoptosis, works in DNA damage responses. The multiple roles of BRCA1 in cell cycle checkpoint, DNA damage repair, transcription and apoptosis (Fig. 4) may be reconciled by postulating a 'signal processor' as well as a 'coordinator of DNA damage responses', in which BRCA1 processes DNA damage signaling through monitoring the circumstances of DNA damage with its distinct patterns of phosphorylation by different kinases, and in turn, can decide and dictate the appropriate pattern of response by coordinating biological responses including cell cycle arrests, DNA repair, transcription, and apoptosis. From this model, the key concept here is then that the differential phosphorylation of BRCA1 determined by kinds and doses of DNA damage renders cells to arrest at appropriate cell cycle checkpoint, to give time for cells to repair DNA damage by forming distinct and specific DNA repair machinery at DNA damaged site. Also, BRCA1 reprograms gene expression pattern by modulating transcription, in order to properly respond to DNA damaged stimuli. In any chance if BRCA1 realizes that these self-guarding machineries are not effective with its phosphorylation status, it directs for cells to die, to keep genome integrity. In other words, BRCA1 stops cell cycle to take time to direct DNA repair when possible, but pushes cells into apoptosis when the damage is too severe to repair, by sensing the global circumstances of DNA damage with distinct phosphorylation pattern.
Together, the scenario presented here gives BRCA1 both upstream (signaling processor and regulatory coordinator) and downstream (effectors) roles in the DNA damage responses (Fig. 2, 4). First, BRCA1 notably participates in dynamic protein complexes that work in sensing and signaling of different types of DNA damage, suggesting that BRCA1 may have functions intrinsic to these upstream roles (Fig. 2). Secondly, BRCA1 works as a cell cycle checkpointer, a DNA damage repairman, a transcriptional regulator, and other downstream effectors (25). Such wide (upstream to downstream) and diverse (most biological DNA damage responses) roles of BRCA1 (Fig. 4), while difficult to accommodate in one BRCA1 gene without direct experimental data, are nonetheless making attractive and implicative sense.
Other BRCA1 functions, something else
Though other functions ascribed to BRCA1 are currently unclear and even primitive, BRCA1's functions intrinsic to ubiquitination and RNA metabolism fall into this category. On the one hand, BARD1 that interacts with BRCA1 through their N-terminal RING domains, has ubiquitnation E3 activity (Fig. 2). Only one known substrate, FANCD2, which is targeted to BRCA1 nuclear foci after DNA damage and works in an intra-S checkpoint and homologous recombination repair responses, is regulated by BRCA1-BARD1, via monoubiquitination (25). Somatic inactivation of FANCD2/BRCA1/BARD1 pathway account for the chromosomal instability of some cancers in the general population, suggesting that this pathway seems to play an important role to keep chromosomal integrity (25). On the other hand, there could be functional interplay between transcription and RNA metabolism, like connections between cell cycle-DNA repair or DNA repair-apoptosis. DNA damage inhibits 3'end processing and polyadeylation of mRNA, in a BARD1 dependent manner (26). A cancer-associated BARD1 mutation lacks this activity, supporting a tempting speculation that ubiquitin activity of BRCA1/BARD1 may be implicated in both protein and RNA processing and this can be a tentative link with tumor suppression. In one way for better understanding this, many groups have been working on what are targeted substrates for BRCA1/BARD1 ubiquitin ligase.
Why specific to breast and ovary cancer predisposition ?
The key concept of BRCA1 is to keep genomic integrity as a caretaker tumor suppressor. The diverse, as mentioned previously, functions of BRCA1 are important for the fundamental general processes, including replication, repair, cell cycle checkpoint, and many other. Thus, an unsolved mystery is the discrepancy between the generality (ubiquitous expression and fundamental role of BRCA1) and the restriction of BRCA1-associated cancers. Why does BRCA1 gene disruption cause cancers only in women? Why only in breast and ovary? The molecular mechanism underlying the gender and tissue specificity of the BRCA1-assoicaited cancer remains puzzling, while several hypotheses may be entertained. BRCA1 disruption may favor tissue specific transformation, in relationships with tissue specific features (for instance, estrogen metabolites, androgen). In the similar context, BRCA1 works as a corepressor for estrogen receptor and as a coactivator for androgen receptor, and thus, BRCA1 deficiency cannot check estrogeninduced growth (mitogenic stimulus of estrogen) and may promote cell growth and antiapoptosis. However, these tissuespecific effects should not be explainable for a caretaker role of BRCA1 in genomic integrity, but for gatekeeper function in growth and death. Also, we have to reconcile one argument against this hypothesis that most BRCA1-associated breast cancers do not express estrogen receptor before going further. In a similar vein, mutation of BRCA1 results in an altered phenotype of X chromosome inactivation, a process by which a major heterochromatin domain is established over one X chromosome, in female cells (14). Additionally, this transcriptional silencing is tissue-specifically regulated in part by homology-dependent chromosome imprinting. The altered mammary gland development and abnormal hormone responsiveness in murine models could reflect such tissue and gender specific function. On the other hand, an alternative hypothesis is tissue-specific LOH frequency. If loss of the second BRCA1 allele occurs with higher frequency in certain tissues, those like breast and ovary, and also prolonged proliferative quiescence in these epithelial tissues changes to strong bursts of proliferation, these tissues can locally develop cancer. Whichever of these possible hypotheses has good reason and sense, very little is known about the precise role of BRCA1 in the biology (more worthwhile and urgently for developmental and cancer biology) of epithelial tissues.
In sporadic cancer ?
Most breast cancers are sporadic and only 5~10% are indeed considered to be familial. Germline mutations of BRCA1 account for only familiar cases, but its somatic mutations are very rare in sporadic breast and ovarian cancers. However, the expression of BRCA1 is often suppressed in many sporadic breast cancers, particularly in thouse that are highly malignant. In some cases, the BRCA1 promoter region is hypermethylated, leading to its reduced expression (27). Together, the gross biological activity of BRCA1 is likely to link to several cancer developmental paths. Thus, BRCA1-mediated tumor suppression and prevention might be compromised in more significant proportion of breast cancers, both heritable as well as sporadic. If this is the case, progress in the understanding of the BRCA1 biology should benefit not only the small number of families carrying the BRCA1 mutation but also the more than 10% of women in the general population. However, the BRCA1 relationship and function in sporadic breast cancers highlight wider gaps in current primitive knowledge and possible model pathways.
ACKNOWLEDGMENTS
We thank members of Lee laboratory for helpful comments on this study. This work was supported by a research grant from the National Cancer Center.
References
- 1.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 2.Neuhausen SL, Marshall CJ. Loss of heterozygosity in familial tumors from three BRCA1-linked kindreds. Cancer Res. 1994;54:6069–6072. [PubMed] [Google Scholar]
- 3.Kinzler KW, Vogelstein B. Cancer-susceptibility genes. Gatekeepers and caretakers. Nature. 1997;386:761–763. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Weaver Z, Linke SP, Li C, Gotay J, Wang XW, Harris CC, Ried T, Deng CX. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell. 1999;3:389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- 5.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 6.Shen SX, Weaver Z, Xu X, Li C, Weinstein M, Chen L, Guan XY, Ried T, Deng CX. A targeted disruption of the murine Brca1 gene causes gamma-irradiation hypersensitivity and genetic instability. Oncogene. 1998;17:3115–3124. doi: 10.1038/sj.onc.1202243. [DOI] [PubMed] [Google Scholar]
- 7.Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, Ashley T, Livingston DM. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 8.Zhong Q, Chen CF, Li S, Chen Y, Wang CC, Xiao J, Chen PL, Sharp ZD, Lee WH. Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science. 1999;285:747–750. doi: 10.1126/science.285.5428.747. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Cortez D, Yazdi P, Neff N, Elledge SJ, Qin J. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 2000;14:927–939. [PMC free article] [PubMed] [Google Scholar]
- 10.Le Page F, Randrianarison V, Marot D, Cabannes J, Perricaudet M, Feunteun J, Sarasin A. BRCA1 and BRCA2 are necessary for the transcription-coupled repair of the oxidative 8-oxoguanine lesion in human cells. Cancer Res. 2000;60:5548–5552. [PubMed] [Google Scholar]
- 11.Scully R, Chen J, Ochs RL, Keegan K, Hoekstra M, Feunteun J, Livingston DM. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–435. doi: 10.1016/s0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 12.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhong Q, Boyer TG, Chen PL, Lee WH. Deficient nonhomologous end-joining activity in cell-free extracts from Brca1-null fibroblasts. Cancer Res. 2002;62:3966–3970. [PubMed] [Google Scholar]
- 14.Ganesan S, Silver DP, Greenberg RA, Avni D, Drapkin R, Miron A, Mok SC, Randrianarison V, Brodie S, Salstrom J, Rasmussen TP, Klimke A, Marrese C, Marahrens Y, Deng CX, Feunteun J, Livingston DM. BRCA1 supports XIST RNA concentration on the inactive X chromosome. Cell. 2002;111:393–405. doi: 10.1016/s0092-8674(02)01052-8. [DOI] [PubMed] [Google Scholar]
- 15.Somasundaram K, Zhang H, Zeng YX, Houvras Y, Peng Y, Zhang H, Wu GS, Licht JD, Weber BL, El-Deiry WS. Arrest of the cell cycle by the tumour-suppressor BRCA1 requires the CDK-inhibitor p21WAF1/CiP1. Nature. 1997;389:187–190. doi: 10.1038/38291. [DOI] [PubMed] [Google Scholar]
- 16.Williamson EA, Dadmanesh F, Koeffler HP. BRCA1 transactivates the cyclin-dependent kinase inhibitor p27(Kip1) Oncogene. 2002;21:3199–3206. doi: 10.1038/sj.onc.1205461. [DOI] [PubMed] [Google Scholar]
- 17.Deng CX, Brodie SG. Roles of BRCA1 and its interacting proteins. Bioessays. 2000;22:728–737. doi: 10.1002/1521-1878(200008)22:8<728::AID-BIES6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Yu X, Wu LC, Bowcock AM, Aronheim A, Baer R. The C-terminal (BRCT) domains of BRCA1 interact in vivo with CtIP, a protein implicated in the CtBP pathway of transcriptional repression. J Biol Chem. 1998;273:25388–25392. doi: 10.1074/jbc.273.39.25388. [DOI] [PubMed] [Google Scholar]
- 19.Fan S, Wang J, Yuan R, Ma Y, Meng Q, Erdos MR, Pestell RG, Yuan F, Auborn KJ, Goldberg ID, Rosen EM. BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science. 1999;284:1354–1356. doi: 10.1126/science.284.5418.1354. [DOI] [PubMed] [Google Scholar]
- 20.Hakem R, de la Pompa JL, Elia A, Potter J, Mak TW. Partial rescue of Brca1 (5-6) early embryonic lethality by p53 or p21 null mutation. Nat Genet. 1997;16:298–302. doi: 10.1038/ng0797-298. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng CX. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- 22.Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- 23.Lee JS, Collins KM, Brown AL, Lee CH, Chung JH. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature. 2000;404:201–204. doi: 10.1038/35004614. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Willers H, Feng Z, Ghosh JC, Kim S, Weaver DT, Chung JH, Powell SN, Xia F. Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair. Mol Cell Biol. 2004;24:708–718. doi: 10.1128/MCB.24.2.708-718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starita LM, Parvin JD. The multiple nuclear functions of BRCA1: transcription, ubiquitination and DNA repair. Curr Opin Cell Biol. 2003;15:345–350. doi: 10.1016/s0955-0674(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 26.Kleiman FE, Manley JL. The BARD1-CstF-50 interaction links mRNA 3 end formation to DNA damage and tumor suppression. Cell. 2001;104:743–753. doi: 10.1016/s0092-8674(01)00270-7. [DOI] [PubMed] [Google Scholar]
- 27.Bianco T, Chenevix-Trench G, Walsh DC, Cooper JE, Dobrovic A. Tumour-specific distribution of BRCA1 promoter region methylation supports a pathogenetic role in breast and ovarian cancer. Carcinogenesis. 2000;21:147–151. doi: 10.1093/carcin/21.2.147. [DOI] [PubMed] [Google Scholar]