Abstract
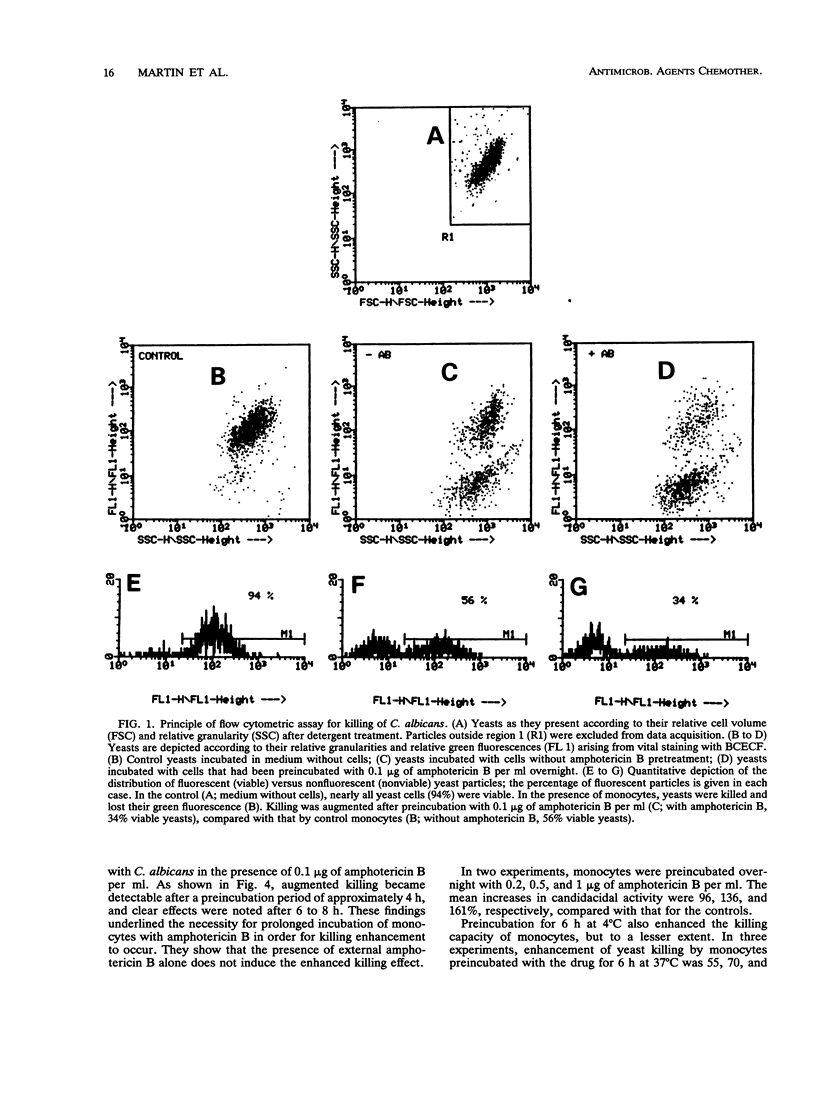
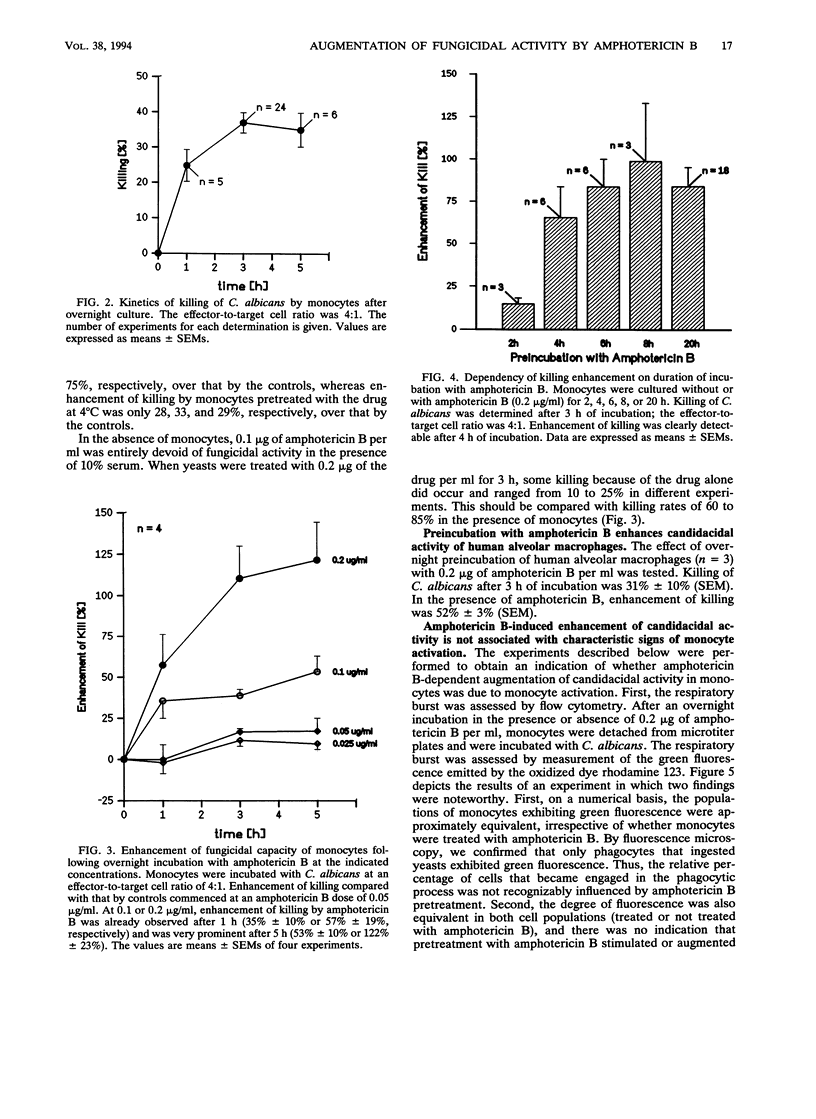
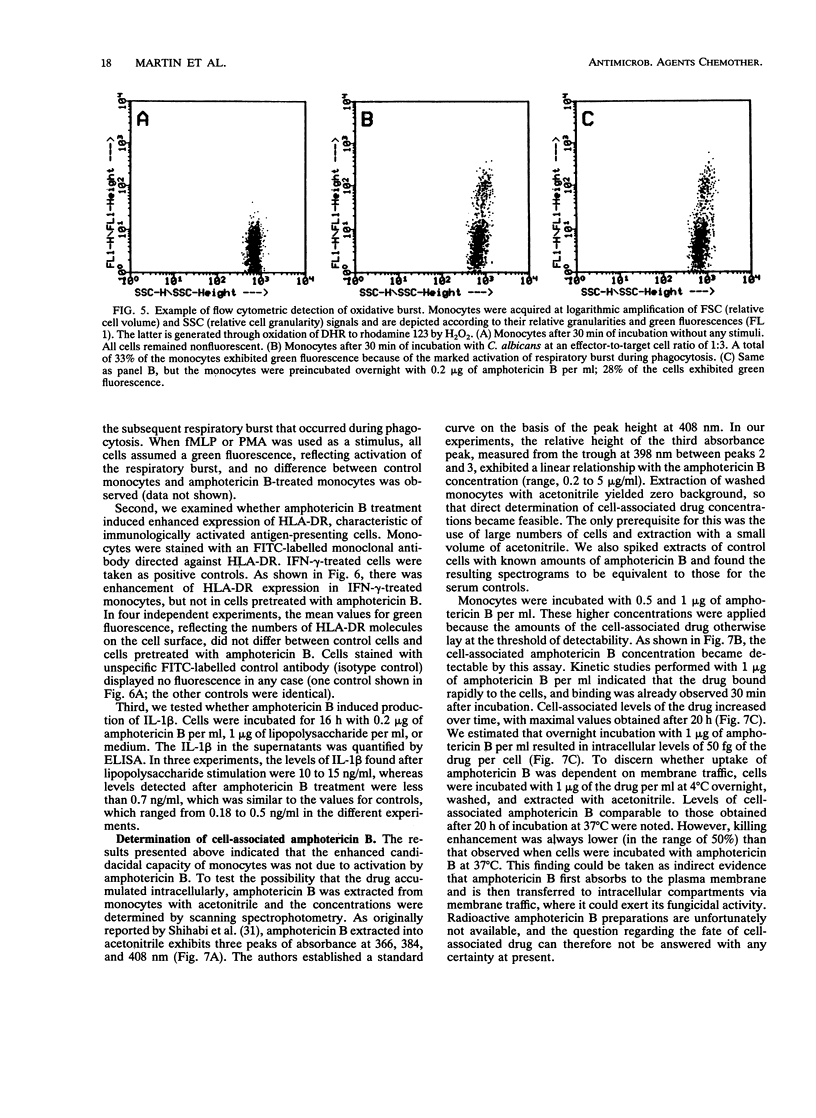
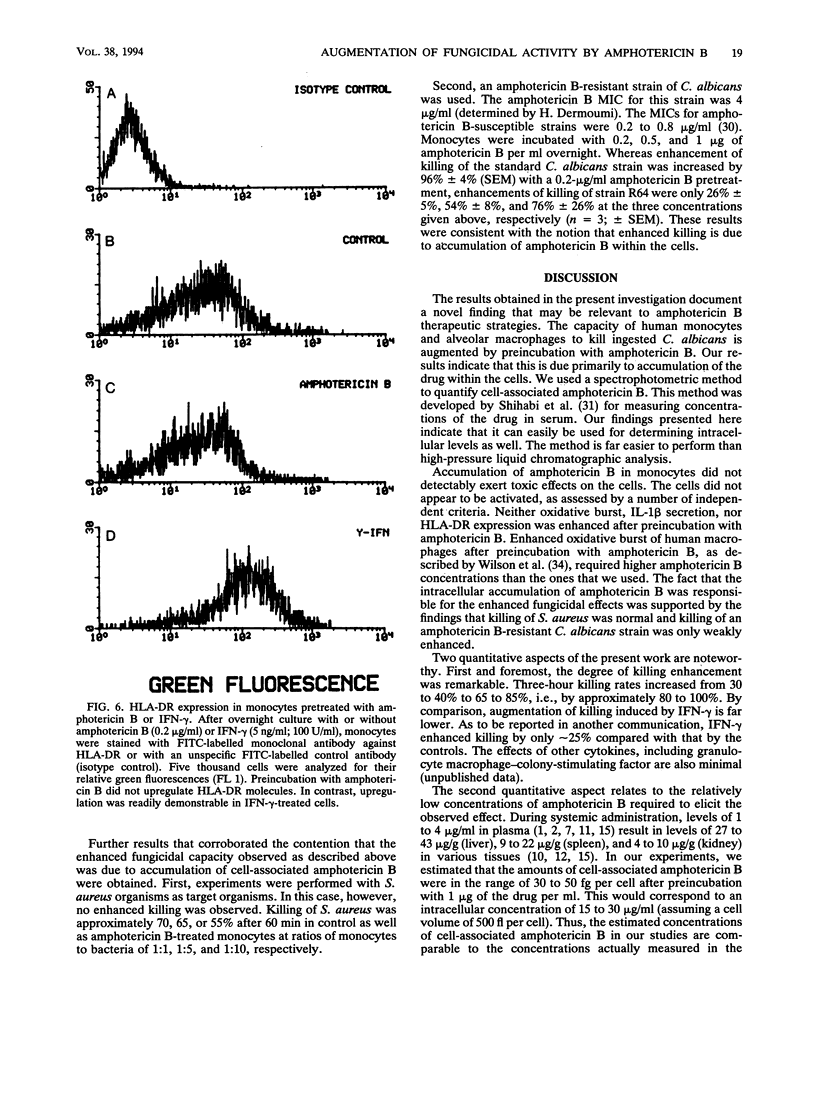
The influence of low doses of amphotericin B on the capacity of human monocytes to kill Candida albicans was investigated. Killing rates were quantified by a novel flow cytometric assay and were found to be 37% +/- 3% (standard error of the mean) after 3 h. Preincubation of monocytes for 6 to 20 h with low concentrations of amphotericin B (0.2 microgram/ml) resulted in a markedly augmented fungicidal capacity. Enhancement of killing was 80% +/- 11% (standard error of the mean) over that by the controls. This effect did not appear to be due to amphotericin B-dependent monocyte activation; the respiratory burst and expression of human leukocyte antigen-DR were unaltered, and no stimulation of interleukin-1 beta release occurred. Cell-associated amphotericin B was extracted with acetonitrile and was quantified by scanning spectrophotometry. Amphotericin B appeared to accumulate in the cells, and intracellular concentrations attained after overnight incubation in 1 microgram of the drug per ml were estimated to be in the range of 50 fg per cell. The fact that intracellular accumulation was responsible for the enhanced fungicidal capacity of monocytes was supported by the findings that killing of Staphylococcus aureus remained normal and enhancement of killing of an amphotericin B-resistant C. albicans strain was minimal. Dramatic enhancement of monocyte fungicidal capacity probably extends to other amphotericin B-susceptible fungi and could represent a hitherto unrecognized determinant underlying the curative properties and prophylactic efficacy of this drug.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson J. M., Nahata M. C. Clinical use of systemic antifungal agents. Clin Pharm. 1988 Jun;7(6):424–438. [PubMed] [Google Scholar]
- Benson J. M., Nahata M. C. Pharmacokinetics of amphotericin B in children. Antimicrob Agents Chemother. 1989 Nov;33(11):1989–1993. doi: 10.1128/aac.33.11.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Martin E. Superoxide generation by human neutrophils induced by low doses of Escherichia coli hemolysin. Infect Immun. 1991 Sep;59(9):2955–2962. doi: 10.1128/iai.59.9.2955-2962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Vecchiarelli A., Mazzolla R., Puccetti P., Marconi P., Garaci E. Immunoadjuvant activity of amphotericin B as displayed in mice infected with Candida albicans. Antimicrob Agents Chemother. 1985 Apr;27(4):625–631. doi: 10.1128/aac.27.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Kobayashi G. S., Medoff G. Toxicity and induction of resistance to Listeria monocytogenes infection by amphotericin B in inbred strains of mice. Infect Immun. 1986 Nov;54(2):303–307. doi: 10.1128/iai.54.2.303-307.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Medoff J., Kobayashi G. S., Schlessinger D., Medoff G. Stimulatory, permeabilizing, and toxic effects of amphotericin B on L cells. Antimicrob Agents Chemother. 1984 Dec;26(6):892–897. doi: 10.1128/aac.26.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Schwartz D. R., Vertut-Croquin A., Schlessinger D., Kobayashi G. S., Medoff G. Involvement of oxidative damage in erythrocyte lysis induced by amphotericin B. Antimicrob Agents Chemother. 1985 Feb;27(2):172–176. doi: 10.1128/aac.27.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot G. G., Pazdur R., Valeriote F. A., Baker L. H. Pharmacokinetics and toxicity of continuous infusion amphotericin B in cancer patients. J Pharm Sci. 1989 Apr;78(4):307–310. doi: 10.1002/jps.2600780409. [DOI] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Hibbs J. B., Jr Modulation of macrophage tumoricidal capability by polyene antibiotics: support for membrane lipid as a regulatory determinant of macrophage function. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4349–4353. doi: 10.1073/pnas.75.9.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia J. K., Pollack M. Amphotericin B induces tumor necrosis factor production by murine macrophages. J Infect Dis. 1989 Jan;159(1):113–116. doi: 10.1093/infdis/159.1.113. [DOI] [PubMed] [Google Scholar]
- Christiansen K. J., Bernard E. M., Gold J. W., Armstrong D. Distribution and activity of amphotericin B in humans. J Infect Dis. 1985 Nov;152(5):1037–1043. doi: 10.1093/infdis/152.5.1037. [DOI] [PubMed] [Google Scholar]
- Collette N., van der Auwera P., Lopez A. P., Heymans C., Meunier F. Tissue concentrations and bioactivity of amphotericin B in cancer patients treated with amphotericin B-deoxycholate. Antimicrob Agents Chemother. 1989 Mar;33(3):362–368. doi: 10.1128/aac.33.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneally E., Cafferkey M. T., Daly P. A., Keane C. T., McCann S. R. Nebulized amphotericin B as prophylaxis against invasive aspergillosis in granulocytopenic patients. Bone Marrow Transplant. 1990 Jun;5(6):403–406. [PubMed] [Google Scholar]
- Denholm E. M., Wolber F. M. A simple method for the purification of human peripheral blood monocytes. A substitute for Sepracell-MN. J Immunol Methods. 1991 Nov 22;144(2):247–251. doi: 10.1016/0022-1759(91)90092-t. [DOI] [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Schlessinger D., Kobayashi G. S. Amphotericin B and filipin effects on L and HeLa cells: dose response. Antimicrob Agents Chemother. 1977 May;11(5):803–808. doi: 10.1128/aac.11.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E., Bhakdi S. Flow cytometric assay for quantifying opsonophagocytosis and killing of Staphylococcus aureus by peripheral blood leukocytes. J Clin Microbiol. 1992 Sep;30(9):2246–2255. doi: 10.1128/jcm.30.9.2246-2255.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E., Bhakdi S. Quantitative analysis of opsonophagocytosis and of killing of Candida albicans by human peripheral blood leukocytes by using flow cytometry. J Clin Microbiol. 1991 Sep;29(9):2013–2023. doi: 10.1128/jcm.29.9.2013-2023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers S. E., Devine S. M., Topper R. L., Ondrey M., Chandler C., O'Toole K., Williams S. F., Larson R. A., Geller R. B. A pilot study of prophylactic aerosolized amphotericin B in patients at risk for prolonged neutropenia. Leuk Lymphoma. 1992 Oct;8(3):229–233. doi: 10.3109/10428199209054909. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Gootee L., Morris R., Bullock W. E. Digestion of Histoplasma capsulatum yeasts by human macrophages. J Immunol. 1992 Jul 15;149(2):574–580. [PubMed] [Google Scholar]
- Nugent K. M., Couchot K. R. Effects of sublethal concentrations of amphotericin B on Candida albicans. J Infect Dis. 1986 Oct;154(4):665–669. doi: 10.1093/infdis/154.4.665. [DOI] [PubMed] [Google Scholar]
- Oehling A., Giron M., Subira M. L. Aerosol chemotherapy in bronchopulmonary candidiasis. Respiration. 1975;32(2):179–184. doi: 10.1159/000193647. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Stewart S. J., Ellner J. J. Amphotericin B-induced resistance to Schistosoma mansoni. J Immunol. 1981 May;126(5):1667–1670. [PubMed] [Google Scholar]
- Pascual A., García I., Conejo C., Perea E. J. Uptake and intracellular activity of fluconazole in human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1993 Feb;37(2):187–190. doi: 10.1128/aac.37.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Granger D. L., Durack D. T. Effects of antifungal agents and gamma interferon on macrophage cytotoxicity for fungi and tumor cells. J Infect Dis. 1987 Aug;156(2):316–323. doi: 10.1093/infdis/156.2.316. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Savani D. V., Durack D. T. Uptake of itraconazole by alveolar macrophages. Antimicrob Agents Chemother. 1993 Apr;37(4):903–904. doi: 10.1128/aac.37.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe G., Emmendörffer A., Oser A., Roesler J., Valet G. Flow cytometric measurement of the respiratory burst activity of phagocytes using dihydrorhodamine 123. J Immunol Methods. 1991 Apr 8;138(1):133–135. doi: 10.1016/0022-1759(91)90074-p. [DOI] [PubMed] [Google Scholar]
- Rousey S. R., Russler S., Gottlieb M., Ash R. C. Low-dose amphotericin B prophylaxis against invasive Aspergillus infections in allogeneic marrow transplantation. Am J Med. 1991 Nov;91(5):484–492. doi: 10.1016/0002-9343(91)90184-y. [DOI] [PubMed] [Google Scholar]
- Shihabi Z. K., Wasilauskas B. L., Peacock J. E., Jr Serum amphotericin-B assay by scanning spectrophotometer. Ther Drug Monit. 1988;10(4):486–489. doi: 10.1097/00007691-198804000-00020. [DOI] [PubMed] [Google Scholar]
- Thomas M. Z., Medoff G., Kobayashi G. S. Changes in murine resistance to Listeria monocytogenes infection induced by amphotericin B. J Infect Dis. 1973 Apr;127(4):373–377. doi: 10.1093/infdis/127.4.373. [DOI] [PubMed] [Google Scholar]
- Wilson E., Thorson L., Speert D. P. Enhancement of macrophage superoxide anion production by amphotericin B. Antimicrob Agents Chemother. 1991 May;35(5):796–800. doi: 10.1128/aac.35.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J. E., Massof S. E. In vivo activation of macrophage oxidative burst activity by cytokines and amphotericin B. Infect Immun. 1990 May;58(5):1296–1300. doi: 10.1128/iai.58.5.1296-1300.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Etten E. W., van de Rhee N. E., van Kampen K. M., Bakker-Woudenberg I. A. Effects of amphotericin B and fluconazole on the extracellular and intracellular growth of Candida albicans. Antimicrob Agents Chemother. 1991 Nov;35(11):2275–2281. doi: 10.1128/aac.35.11.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]


