Abstract
Titin is an extremely large protein found in highest concentrations in heart and skeletal muscle. The single mammalian gene is expressed in multiple isoforms as a result of alternative splicing. Although titin isoform expression is controlled developmentally and in a tissue specific manner, the vast number of potential splicing pathways far exceeds those described in any other alternatively spliced gene. Over 1 million human splice pathways for a single individual can be potentially derived from the PEVK region alone. A new splicing pattern for the human cardiac N2BA isoform type has been found in which the PEVK region includes only the N2B type exons. The alterations in splicing and titin isoform expression in human heart disease provide impetus for future detailed study of the splicing mechanisms for this giant protein.
1. Introduction
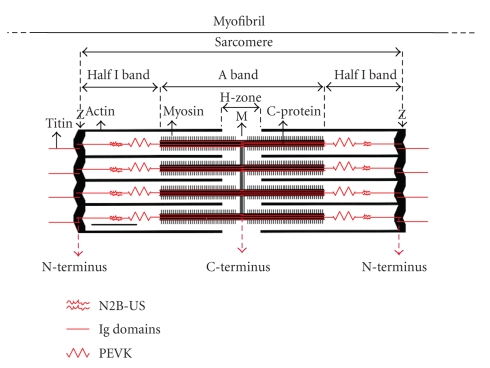
Titin is the third most abundant protein (after myosin and actin) in vertebrate striated muscle, with an average adult human containing ~0.5 kg [1]. This extremely large protein [2], which is also known as connectin [3], spans each half sarcomere from the Z-line to the M-line or center of the sarcomere [4, 5] (Figure 1). The C-terminal A-band segment of titin is attached to the thick filament via multiple binding sites for myosin and C-protein [6] and two C-terminal titin regions from adjacent half-sarcomeres overlap in the M-line region of the sarcomere [7]. Similarly titin's N-terminal segment is anchored in the Z-disk and overlaps another titin N-terminus from the adjacent sarcomere [8]. Titin thus constitutes a continuous filament system along the myofibril. Titin is believed to function as a template in sarcomere assembly and for maintenance of sarcomere integrity [9, 10] (Figure 1). These concepts have been confirmed by recent work showing that titin loss in long-term disuse of skeletal muscle results in the disorganization of the ordered sarcomeric structure [11, 12]. Each end of the thick filament is linked to the nearest Z-disk by titin. This provides axial continuity for the production of resting tension and maintains the thick filament in the center of sarcomere during generation of active force [13]. Titin's several extensible elements establish titin as a critical, multifunctional sarcomeric component. These extensible elements are composed of (1) Tandem Ig segments (consisting of serially linked immunoglobulin-like domains), (2) the PEVK region (so called for its high content of proline (P), glutamate (E), valine (V), and lysine (K) residues), and (3) the cardiac-specific N2B unique sequence (N2B-Us) (Figure 1). In slack sarcomeres the tandem Ig and PEVK segments are collapsed. Upon initial stretch, the collapsed tandem Ig segments are straightened while their individual Ig domains remained folded. With further stretch the PEVK region extends [14, 15]. Modeling tandem Ig and PEVK segments as entropic springs with different bending rigidities indicated that in the physiological SL range (a) the Ig-like domains of the tandem Ig segments remain folded and (b) the PEVK segment behaves as a permanently unfolded polypeptide [16–19]. The cardiac-specific N2B unique sequence (N2B-Us) forms a third spring element in cardiac titin and provides extensibility at the upper range of physiological sarcomere lengths in the heart [17, 20–23].
Figure 1.
Titin location and arrangement in the cardiac sarcomere.
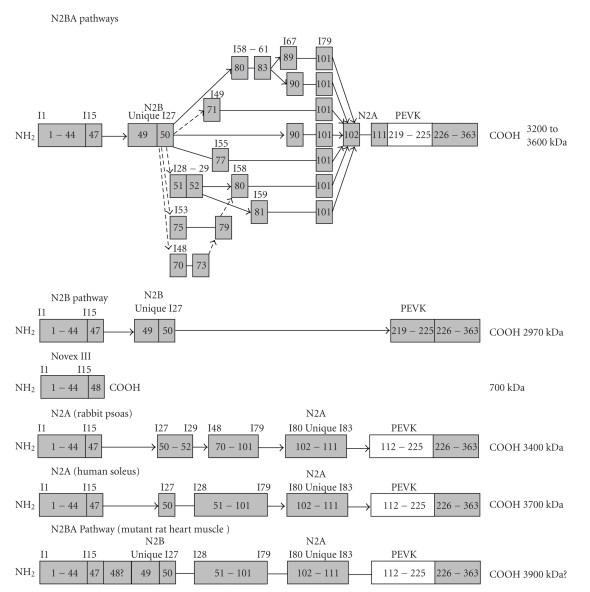
The original description of titin isoforms suggested that the N2B unique sequence occurred in cardiac muscle and a different unique region called N2A was found only in skeletal muscles. Names of the full isoforms in these tissues were then N2B and N2A, respectively [5]. It was later found that the myocardium expresses two major classes of titin isoforms: a smaller N2B and a larger N2BA that contained both the N2B and N2A unique sequences [24, 25] (Figure 2). All these known titin isoforms contain PEVK and tandem Ig segments [5, 25, 26]. The N2B isoform has fewer Ig domains and a short PEVK region; the N2A class of isoforms contains more Ig domains and a somewhat longer PEVK region. Nearly all Z-disk and A-band/M-band titin domains are constitutively expressed in titin isoforms of human cardiac muscle. The main titin differential splicing occurs in the middle Ig and PEVK domains of the I-band-titin segment [24, 27].
Figure 2.
Domain arrangement of different titin isoforms. Arrows indicate the exons spliced, solid line connections denote consecutive exons. Cross-hatch pattern in PEVK indicates variable patterns of skipped exons.
The current review will discuss the isoforms of titin and the alternative splicing patterns that lead to the different forms. A number of excellent reviews should be consulted for further details on the structure and function of titin [6, 19, 28–33].
2. Electrophoresis Detection of Titin
Titin is the biggest protein in the human body, and there are a number of size variants. The earliest reports indicated that there were two electrophoretic bands: a larger T1 (which was the full length version) and T2 (a proteolytic fragment extending from the PEVK region through the carboxyl terminal M line end) [2]. Because of titin's extremely large size, migration is minimal in typical SDS polyacrylamide gels, and it has been difficult to develop a reliable and quantitative gel procedure. An earlier study used 3.3–12% gradient polyacrylamide gels to detect and quantify titin and nebulin from short segments of single muscle fibers [34]. This system separated titin T1 (intact titin, Mr ~3300kDa) and T2 (breakdown product of titin, Mr ~2000kDa). Another group employed 2% polyacrylamide slab gels strengthened with agarose to resolve two T1 bands plus the T2 [35]. More recently 2–9.5% acrylamide gradient gels have been used to separate the large titin isoforms and fragments [25]. These gels showed that the T1 mobility varied greatly between muscle sources, reflecting the difference in molecular mass of the 3.7-MDa soleus titin and the 2.97-MDa rat cardiac titin isoform. The two major T1 bands were ascribed to the titin isoforms N2B and N2BA in cardiac muscle.
However, the gels mentioned above are physically difficult to work with, are more complex to pour, and often undergo distortion or tearing during fixing and staining. It is also very difficult to transfer large proteins out of acrylamide gels for Western blotting. More recently our group has developed a different more reliable and reproducible system called vertical SDS-agarose gel electrophoresis (VAGE) [36]. This method employs Sea Kem Gold agarose and allows high-resolution separation of titin isoforms. In addition the blot transfer efficiency was almost 100% [36]. This system can also be easily adapted for the characterization of other very large proteins from a variety of sources.
The SDS agarose system resolves at least four classes of N2BA titin isoforms. Two rat embryonic/neonatal forms (N2BA-N1; N2BA-N2) with apparent sizes of approximately 3710 and 3590kDa, respectively, are found during late embryonic and immediately after birth [37]. These are gradually replaced by two adult forms (N2BA-A1; N2BA-A2) with sizes of 3390 and 3220. It has been postulated that these shorter versions are due to deletion of large numbers of exons from the exon 50 to 71 and 50 to 90 middle Ig regions (see below) [37].
3. Titin Tissue and Species cDNA Sequence Comparisons
Early cDNA sequencing of titin from human cardiac and soleus indicated that both isoforms were derived from a single gene and obtained by alternative splicing [5, 24]. The single titin gene contained 363 exons [27]. The complete cDNA and genomic sequence determination of human cardiac titin have provided a template for later study of titin splicing variants.
3.1. M Line Region
Kolmerer and coworkers [39] searched for alternatively expressed exons in the M-line region of titin and found that six exons (called Mex1 to 6) coded for the most carboxyl terminus of titin. Message for human cardiac titin always included exon Mex5 while rabbit cardiac titin message contained small amounts with the Mex5 missing and mouse hearts had approximately equal proportions of Mex5 (+) and Mex5 (−). Rabbit skeletal muscles contained varying ratios of Mex5 (+) and Mex5 (−). The proportion of Mex5 (+) containing message (and presumably the titin segment it codes) appears to correlate with the M line fine structure differences that occur between tissues [40–42], developmental stage [43], and species [44].
3.2. Z-Disk Region
The Z-disk region of titin consists of 4 Ig-domains (Z1 to Z4) with large interdomain insertions between Z2 and Z3 and between Z3 and Z4 [5, 45] http://www.ncbi.nlm.nih.gov/pubmed?term=%22Gautel%20M%22%5BAuthor%5D&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVAbstract. The latter insertion contains a series of 45 amino acid residue repeats (Z repeats). Alternative splicing results in the expression of 6 or 7 repeats in human and rabbit heart, 4 to 6 in slow skeletal muscle, and 2 to 4 in fast skeletal muscle [46]. The number of repeats correlates with the Z line thickness [45, 46].
3.3. I-Band Region
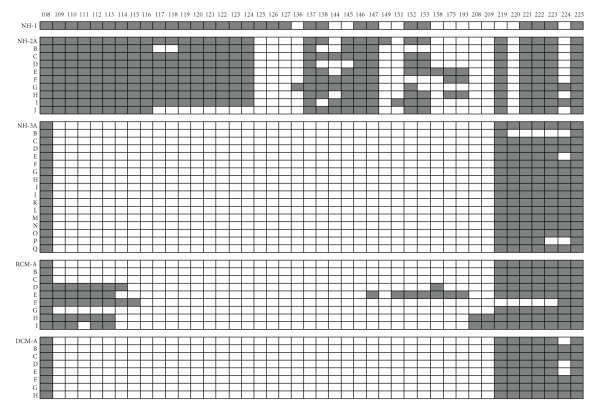
The I-band region of titin is believed to be responsible for the myofibrillar passive tension response to stretch. Surprisingly the splicing patterns of highly diverse titins are exceptionally complex. N2B titin isoforms have the most simple splice plans. Exon 50 (I27) is usually spliced to exon 219. This splicing pattern has been verified in human, rabbit, rat, and pig hearts [38]. PCR amplification with primers from I27 to I84 of rabbit, pig, and rat ventricle is consistent with an expected size of 760 bp (based on the human sequence). Dog heart yielded two fragments (a major 615 bp and a minor 760 bp) and the amino acid sequence deduced from the nucleotide sequences revealed that the dog ventricle short PEVK peptide contains a deletion of 53 amino acid residues from the amino terminal end of the N2B PEVK region (corresponding to exons 219 and 220). The absence of exon 224 also occurs in some clones from human heart (see Figure 3 and discussion below).
Figure 3.
Exon inclusion in clones from the human titin PEVK region. Data from left ventricle samples of five different individuals—three normal (NH-1, NH-2, NH-3), one restrictive cardiomyopathy (RCM), and one dilated cardiomyopathy (DCM). Exon number is listed across the top. Filled boxes denote exon inclusion. Letter designations in each block refer to separate clones from each individual. Clones were obtained by PCR amplification after RT-PCR using primers from exons 108 and 225. Full details on the methods have been published [38]. Data from sample NH-1 is from [38] and for NH-2 clones from [37].
Variable numbers of titin exons between 50 and 219, including the N2A unique region, are expressed in cardiac and skeletal muscle. This splicing pattern gives rise to the N2A protein isoforms in skeletal muscle and the N2BA isoforms in cardiac muscle [24, 27]. N2BA isoforms (containing both N2A and N2B unique elements) have multiple splicing pathways in the I27 to I68 segment (Figure 2). Splice pathways have been identified between I27 (exon 50) and exons 51, 70, 71, 75, 77, 80, and 90 [24, 38]. The expression of more middle Ig domains in fetal rat ventricle is consistent with the larger size of fetal titin N2BA isoforms observed by electrophoresis [37].
The N2B unique exon 49 is excluded in skeletal muscles by splicing together I15 to I27, and only human soleus muscle expresses consecutive exons between 50 and 102 (I27 to I79). Rabbit psoas muscle skips I30 to I47 (Figure 2). Altogether, skeletal titin transcripts always include the N2A segment and exclude the N2B exon; cardiac titins always include the N2B exon [24].
The N2BA PEVK region has an exceptionally varied pattern of splicing (Figure 3). Initial studies with dog cardiac muscle resulted in six clones (A46 A-F) [38], all from the same PCR amplification, having different exon patterns and PEVK lengths (703, 788, 894, 900, 703, and 819 amino acids respectively). Subsequent work with the human PEVK revealed that 10 different clones from a single individual had 10 different patterns of splicing [26]. In addition a completely new pattern of N2BA splicing has been recently obtained in which exon 108, found only in N2BA isoforms, was directly linked to the constitutively expressed PEVK cluster in the 219 to 225 exon region (Figure 3). This so-called “short PEVK N2BA” class of isoforms has been observed in clones from both healthy and diseased hearts. Such a pattern would result in titin molecules with apparently less potential PEVK extension during stretch and thus leads to a stiffer sarcomere. It is not clear whether such message types are expressed into protein and whether there are a significant proportion of such messages transcribed to affect the passive tension. However, if an increased proportion of these splicing products occurred with aging, for example, it might partially explain the increased cardiac stiffness that often occurs during the later period of life.
A mutant rat model has been recently described that results in significant alterations in titin isoform expression [26, 47]. Homozyogous mutants express a giant titin with apparent size on SDS agarose gels of about 3.9 MDa. This is larger than the size of titin from human soleus (3.7 MDa). Since the soleus sequence includes all the middle Ig domains and virtually all the Ig domains from the rest of the gene, it was initially assumed that the mutant rat heart must then express the full PEVK exon cohort plus the N2B unique region. Sequences obtained from PCR clones bridging the exon 108 to 225 region were not much larger than those from wild type [47]. Comparisons of exon expression between wild type and mutants showed some differences, primarily in the exon region between 175 and 219 typically associated with fetal exons (Figure 2) [26, 48]. Thus it appears that more than one mechanism must be involved in titin splicing. The lack of additional PEVK exons in the mutants exacerbates the mass calculation. The inclusion of the N2B unique region would add some mass, but the possible inclusion of exon 48 would add 243 kDa. This idea remains to be verified.
The number of potential PEVK splice variants is huge. Among the ten clones from the normal human 2 in Figure 3 human clones listed, there are 20 exons that are not present in all clones. If all these splicing events were independent, this means that there are 220 combinations, a total of 1,048,576 possible pathways. This far exceeds the approximately 38,016 potential splice variants identified previously with the Dscam (Down syndrome cell adhesion molecule) gene [49, 50]. If all the clones in this table from 5 different individuals are included, then there are 38 differential exons and the number of potential clones exceeds 274 billion.
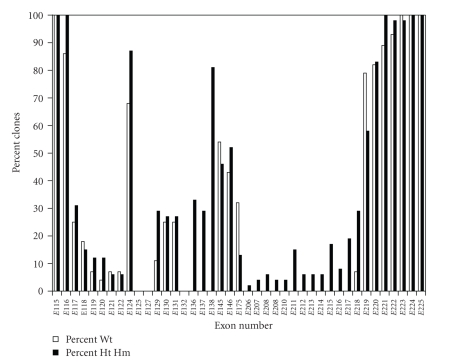
Microarrays have also been used to compare tissue sources for exon expression [48, 51]. Fetal titins express more middle Ig domains and additional PEVK exons. These results are consistent with the cloning and sequencing results [37]. The identification of exon 156 as skeletal muscle specific [48] is also consistent with the absence of this exon from every human (Figure 4) and rat cardiac clone we have sequenced [47]. Microarrays give more global information since all messages from a sample are included. Their disadvantages include the fact that almost no splicing information can be obtained and the results include pooled data from all titins in the sample, including the Novex isoforms. Additionally the duplication of three segments of the titin gene in the region of the PEVK [27] means that some microarray probes are identical. The cloning approach yields actual splice patterns. The major disadvantages include the fact that only possible splice patterns are sampled and that identified clones may not adequately represent, either quantitatively or qualitatively, the actual tissue patterns. However, the cloning approach has verified that titin splicing is highly diverse, and this complicates interpretation of mechanical experiments.
Figure 4.
Exon inclusion in clones from rat left ventricle of wild type and mutant rats. The percent of clones that contain the exons shown is plotted versus exon position. Only exons 115, 224, and 225 were found in all wild type and mutant clones (heterozygote [Ht] and homozygote [Hm]). Data was pooled from four different ages [47].
4. The Ratio of Titin Isoforms and Heart Disease
Titin isoform splicing not only generates diversity of titin isoforms in skeletal muscle [52], but also results in changes of the ratio of titin isoforms (N2BA/N2B) during heart development. Before birth, mammalian heart mainly expresses more compliant N2BA isoforms. During perinatal heart development the larger N2BA isoform is gradually replaced by a smaller isoform N2B, and the N2B isoform becomes the predominant titin isoform in the adult left ventricles (LV) of smaller mammalian species [37, 53, 54]. The N2BA titin isoform prevails over N2B in the adult hearts of larger mammals, including humans [25, 55]. Recent studies have shown that the ratio of titin isoforms is altered in some heart diseases. A canine tachycardia-induced model of dilated cardiomyopathy (DCM) used to understand titin response to a chronic mechanical challenge of the heart indicated that two weeks of pacing gives rise to an exaggerated transmural titin isoform ratio gradient [56] and four weeks of pacing results in a decrease of the N2BA/N2B titin ratio, accompanied by an increase in titin-based passive tension [57]. Another study of spontaneously hypertensive rat model (SHR) also showed a reduced ratio of N2BA/N2B titin in response to pressure overload, consistent with elevated passive tension of heart [58]. More recent analyses showed that the left ventricle biopsies from patients with diastolic heart failure (HF) had a reduced N2BA/N2B titin [59]. Chronically ischemic LVs of coronary-artery-disease (CAD) patients with congestive heart failure (HF) had nearly 50% N2BA titin (compared to N2BA+N2B) while approximate 30% N2BA was found in the LVs of control donor patients [60]. Analysis of explanted nonischaemic human DCM hearts again demonstrated increased proportions of N2BA/N2B [61, 62]. These results are similar to the recently described rat model with lower ejection fraction (unpublished data) which expresses the N2BA isoform almost exclusively [26, 47]. Long-term hypothyroidism (which results in diastolic dysfunction) changed the titin isoform ratios as well. Propylthiouracil (PTU) treatment in rats induced the expression of additional cardiac PEVK and I domain exons similar to those in the large titin isoform of the fetal heart. Consequently, titin-based passive and restoring forces were found to be significantly reduced in cardiac muscle of PTU-treated rats [63, 64]. The mechanisms controlling titin splicing remain unknown, but the ability to manipulate the splicing of this protein has great potential for affecting human health.
Acknowledgment
This work was supported by the University of Wisconsin-Madison College of Agricultural and Life Sciences and a grant from the National Institutes of Health (HL77196).
References
- 1.Labeit S, Kolmerer B, Linke WA. The giant protein titin: emerging roles in physiology and pathophysiology. Circulation Research. 1997;80(2):290–294. doi: 10.1161/01.res.80.2.290. [DOI] [PubMed] [Google Scholar]
- 2.Wang K, McClure J, Tu A. Titin: major myofibrillar components of striated muscle. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(8):3698–3702. doi: 10.1073/pnas.76.8.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maruyama K. Connectin, an elastic protein from myofibrils. Journal of Biochemistry. 1976;80(2):405–407. doi: 10.1093/oxfordjournals.jbchem.a131291. [DOI] [PubMed] [Google Scholar]
- 4.Furst DO, Osborn M, Nave R, Weber K. The organization of titin filaments in the half-sarcomere revealed by monoclonal antibodies in immunoelectron microscopy: a map of ten nonrepetitive epitopes starting at the Z-line extends close to the M line. Journal of Cell Biology. 1988;106(5):1563–1572. doi: 10.1083/jcb.106.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270(5234):293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- 6.Trinick J, Tskhovrebova L. Titin: a molecular control freak. Trends in Cell Biology. 1999;9(10):377–380. doi: 10.1016/s0962-8924(99)01641-4. [DOI] [PubMed] [Google Scholar]
- 7.Obermann WMJ, Gautel M, Steiner F, van der Ven PFM, Weber K, Fürst DO. The structure of the sarcomeric M band: localization of defined domains of myomesin, M-protein, and the 250-kD carboxy-terminal region of titin by immunoelectron microscopy. Journal of Cell Biology. 1996;134(6):1441–1453. doi: 10.1083/jcb.134.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregorio CC, Trombitás K, Centner T, et al. The NH2 terminus of titin spans the Z-disc: its interaction with a novel 19-kD ligand (T-cap) is required for sarcomeric integrity. Journal of Cell Biology. 1998;143(4):1013–1027. doi: 10.1083/jcb.143.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tskhovrebova L, Trinick J. Titin: properties and family relationships. Nature Reviews Molecular Cell Biology. 2003;4(9):679–689. doi: 10.1038/nrm1198. [DOI] [PubMed] [Google Scholar]
- 10.Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circulation Research. 2004;94(3):284–295. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- 11.Udaka J, Ohmori S, Terui T, et al. Disuse-induced preferential loss of the giant protein titin depresses muscle performance via abnormal sarcomeric organization. Journal of General Physiology. 2008;131(1):33–41. doi: 10.1085/jgp.200709888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda N, Granzier HL, Ishiwata S, Kurihara S. Physiological functions of the giant elastic protein titin in mammalian striated muscle. Journal of Physiological Sciences. 2008;58(3):151–159. doi: 10.2170/physiolsci.RV005408. [DOI] [PubMed] [Google Scholar]
- 13.Horowits R, Kempner ES, Bisher ME, Podolsky RJ. A physiological role for titin and nebulin in skeletal muscle. Nature. 1986;322(6084):160–164. doi: 10.1038/323160a0. [DOI] [PubMed] [Google Scholar]
- 14.Linke WA, Ivemeyer M, Olivieri N, Kolmerer B, Ruegg JC, Labeit S. Towards a molecular understanding of the elasticity of titin. Journal of Molecular Biology. 1996;261(1):62–71. doi: 10.1006/jmbi.1996.0441. [DOI] [PubMed] [Google Scholar]
- 15.Trombitás K, Greaser M, Labeit S, et al. Titin extensibility in situ: entropic elasticity of permanently folded and permanently unfolded molecular segments. Journal of Cell Biology. 1998;140(4):853–859. doi: 10.1083/jcb.140.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellermayer MSZ, Smith SB, Granzier HL, Bustamante C. Folding-unfolding transitions in single titin molecules characterized with laser tweezers. Science. 1997;276(5315):1112–1116. doi: 10.1126/science.276.5315.1112. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe K, Nair P, Labeit D, et al. Molecular mechanics of cardiac titin’s PEVK and N2B spring elements. Journal of Biological Chemistry. 2002;277(13):11549–11558. doi: 10.1074/jbc.M200356200. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Linke WA, Oberhauser AF, et al. Reverse engineering of the giant muscle protein titin. Nature. 2002;418(6901):998–1002. doi: 10.1038/nature00938. [DOI] [PubMed] [Google Scholar]
- 19.Granzier H, Labeit S. Cardiac titin: an adjustable multi-functional spring. Journal of Physiology. 2002;541(2):335–342. doi: 10.1113/jphysiol.2001.014381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helmes M, Trombitas K, Centner T, et al. Mechanically driven contour-length adjustment in rat cardiac titin’s unique N2B sequence: titin is an adjustable spring. Circulation Research. 1999;84(11):1339–1352. doi: 10.1161/01.res.84.11.1339. [DOI] [PubMed] [Google Scholar]
- 21.Linke WA, Rudy DE, Centner T, et al. I-band titin in cardiac muscle is a three-element molecular spring and is critical for maintaining thin filament structure. Journal of Cell Biology. 1999;146(3):631–644. doi: 10.1083/jcb.146.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trombitas K, Freiburg A, Centner T, Labeit S, Granzier H. Molecular dissection of N2B cardiac titin’s extensibility. Biophysical Journal. 1999;77(6):3189–3196. doi: 10.1016/S0006-3495(99)77149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trombitas K, Redkar A, Centner T, Wu Y, Labeit S, Granzier H. Extensibility of isoforms of cardiac titin: variation in contour length of molecular subsegments provides a basis for cellular passive stiffness diversity. Biophysical Journal. 2000;79(6):3226–3234. doi: 10.1016/S0006-3495(00)76555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freiburg A, Trombitas K, Hell W, et al. Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circulation Research. 2000;86(11):1114–1121. doi: 10.1161/01.res.86.11.1114. [DOI] [PubMed] [Google Scholar]
- 25.Cazorla O, Freiburg A, Helmes M, et al. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circulation Research. 2000;86(1):59–67. doi: 10.1161/01.res.86.1.59. [DOI] [PubMed] [Google Scholar]
- 26.Greaser ML, Krzesinski PR, Warren CM, Kirkpatrick B, Campbell KS, Moss RL. Developmental changes in rat cardiac titin/connectin: transitions in normal animals and in mutants with a delayed pattern of isoform transition. Journal of Muscle Research and Cell Motility. 2005;26(6–8):325–332. doi: 10.1007/s10974-005-9039-0. [DOI] [PubMed] [Google Scholar]
- 27.Bang M-L, Centner T, Fornoff F, et al. The complete gene sequence of titin, expression of an unusual ≈700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circulation Research. 2001;89(11):1065–1072. doi: 10.1161/hh2301.100981. [DOI] [PubMed] [Google Scholar]
- 28.Wang K. Titin/connectin and nebulin: giant protein rulers of muscle structure and function. Advances in Biophysics. 1996;33:123–134. [PubMed] [Google Scholar]
- 29.Maruyama K. Connectin/titin, giant elastic protein of muscle. FASEB Journal. 1997;11(5):341–345. doi: 10.1096/fasebj.11.5.9141500. [DOI] [PubMed] [Google Scholar]
- 30.Gregorio CC, Granzier H, Sorimachi H, Labeit S. Muscle assembly: a titanic achievement? Current Opinion in Cell Biology. 1999;11(1):18–25. doi: 10.1016/s0955-0674(99)80003-9. [DOI] [PubMed] [Google Scholar]
- 31.LeWinter MM, Wu Y, Labeit S, Granzier H. Cardiac titin: structure, functions and role in disease. Clinica Chimica Acta. 2007;375(1-2):1–9. doi: 10.1016/j.cca.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 32.Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovascular Research. 2008;77(4):637–648. doi: 10.1016/j.cardiores.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 33.Krüger M, Linke WA. Titin-based mechanical signalling in normal and failing myocardium. Journal of Molecular and Cellular Cardiology. 2009;46(4):490–498. doi: 10.1016/j.yjmcc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Granzier HLM, Wang K. Gel electrophoresis of giant proteins: solubilization and silver-staining of titin and nebulin from single muscle fiber segments. Electrophoresis. 1993;14(1-2):56–64. doi: 10.1002/elps.1150140110. [DOI] [PubMed] [Google Scholar]
- 35.Tatsumi R, Hattori A. Detection of giant myofibrillar proteins connectin and nebulin by electrophoresis in 2% polyacrylamide slab gels strengthened with agarose. Analytical Biochemistry. 1995;224(1):28–31. doi: 10.1006/abio.1995.1004. [DOI] [PubMed] [Google Scholar]
- 36.Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight protiens. Electrophoresis. 2003;24(11):1695–1702. doi: 10.1002/elps.200305392. [DOI] [PubMed] [Google Scholar]
- 37.Warren CM, Krzesinski PR, Campbell KS, Moss RL, Greaser ML. Titin isoform changes in rat myocardium during development. Mechanisms of Development. 2004;121(11):1301–1312. doi: 10.1016/j.mod.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Greaser ML, Berri M, Warren CM, Mozdziak PE. Species variations in cDNA sequence and exon splicing patterns in the extensible I-band region of cardiac titin: relation to passive tension. Journal of Muscle Research and Cell Motility. 2002;23(5-6):473–482. doi: 10.1023/a:1023410523184. [DOI] [PubMed] [Google Scholar]
- 39.Kolmerer B, Olivieri N, Witt CC, Herrmann BG, Labeit S. Genomic organization of M line titin and its tissue-specific expression in two distinct isoforms. Journal of Molecular Biology. 1996;256(3):556–563. doi: 10.1006/jmbi.1996.0108. [DOI] [PubMed] [Google Scholar]
- 40.Sjöström M, Squire JM. Fine structure of the A band in cryo sections. The structure of the A band of human skeletal muscle fibres from ultra thin cryo sections negatively stained. Journal of Molecular Biology. 1977;109(1):49–68. doi: 10.1016/s0022-2836(77)80045-4. [DOI] [PubMed] [Google Scholar]
- 41.Edman A-C, Lexell J, Sjöström M, Squire JM. Structural diversity in muscle fibres of chicken breast. Cell and Tissue Research. 1988;251(2):281–289. doi: 10.1007/BF00215835. [DOI] [PubMed] [Google Scholar]
- 42.Podlubnaya ZA, Shpagina MD, Lednev VV. Manifestation of the stripes of minor proteins location in A-bands of rabbit cardiac myofibrils. Journal of Molecular Biology. 1989;210(3):655–658. doi: 10.1016/0022-2836(89)90139-3. [DOI] [PubMed] [Google Scholar]
- 43.Carlsson E, Thornell L-E. Diversification of the myofibrillar M-band in rat skeletal muscle during postnatal development. Cell and Tissue Research. 1987;248(1):169–180. doi: 10.1007/BF01239978. [DOI] [PubMed] [Google Scholar]
- 44.Pask HT, Jones KL, Luther PK, Squire JM. M-band structure, M-bridge interactions and contraction speed in vertebrate cardiac muscles. Journal of Muscle Research and Cell Motility. 1994;15(6):633–645. doi: 10.1007/BF00121071. [DOI] [PubMed] [Google Scholar]
- 45.Gautel M, Goulding D, Bullard B, Weber K, Fürst DO. The central Z-disk region of titin is assembled from a novel repeat in variable copy numbers. Journal of Cell Science. 1996;109(11):2747–2754. doi: 10.1242/jcs.109.11.2747. [DOI] [PubMed] [Google Scholar]
- 46.Sorimachi H, Ishiura S, Suzuki K. Structure and physiological function of calpains. Biochemical Journal. 1997;328, part 3:721–732. doi: 10.1042/bj3280721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greaser ML, Warren CM, Esbona K, et al. Mutation that dramatically alters rat titin isoform expression and cardiomyocyte passive tension. Journal of Molecular and Cellular Cardiology. 2008;44(6):983–991. doi: 10.1016/j.yjmcc.2008.02.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lahmers S, Wu Y, Call DR, Labeit S, Granzier H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circulation Research. 2004;94(4):505–513. doi: 10.1161/01.RES.0000115522.52554.86. [DOI] [PubMed] [Google Scholar]
- 49.Schmucker D, Clemens JC, Shu H, et al. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101(6):671–684. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- 50.Lee C, Kim N, Meenakshi Roy M, Graveley BR. Massive expansions of Dscam splicing diversity via staggered homologous recombination during arthropod evolution. RNA. 2010;16(1):91–105. doi: 10.1261/rna.1812710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Labeit S, Lahmers S, Burkart C, et al. Expression of distinct classes of titin isoforms in striated and smooth muscles by alternative splicing, and their conserved interaction with filamins. Journal of Molecular Biology. 2006;362(4):664–681. doi: 10.1016/j.jmb.2006.07.077. [DOI] [PubMed] [Google Scholar]
- 52.Prado LG, Makarenko I, Andresen C, Krüger M, Opitz CA, Linke WA. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. Journal of General Physiology. 2005;126(5):461–480. doi: 10.1085/jgp.200509364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Opitz CA, Leake MC, Makarenko I, Benes V, Linke WA. Developmentally regulated switching of titin size alters myofibrillar stiffness in the perinatal heart. Circulation Research. 2004;94(7):967–975. doi: 10.1161/01.RES.0000124301.48193.E1. [DOI] [PubMed] [Google Scholar]
- 54.Opitz CA, Linke WA. Plasticity of cardiac titin/connectin in heart development. Journal of Muscle Research and Cell Motility. 2005;26(6–8):333–342. doi: 10.1007/s10974-005-9040-7. [DOI] [PubMed] [Google Scholar]
- 55.Neagoe C, Opitz CA, Makarenko I, Linke WA. Gigantic variety: expression patterns of titin isoforms in striated muscles and consequences for myofibrillar passive stiffness. Journal of Muscle Research and Cell Motility. 2003;24(2-3):175–189. doi: 10.1023/a:1026053530766. [DOI] [PubMed] [Google Scholar]
- 56.Bell SP, Nyland L, Tischler MD, McNabb M, Granzier H, LeWinter MM. Alterations in the determinants of diastolic suction during pacing tachycardia. Circulation Research. 2000;87(3):235–240. doi: 10.1161/01.res.87.3.235. [DOI] [PubMed] [Google Scholar]
- 57.Wu Y, Bell SP, Trombitas K, et al. Changes in titin isoform expression in pacing-induced cardiac failure give rise to increased passive muscle stiffness. Circulation. 2002;106(11):1384–1389. doi: 10.1161/01.cir.0000029804.61510.02. [DOI] [PubMed] [Google Scholar]
- 58.Warren CM, Jordan MC, Roos KP, Krzesinski PR, Greaser ML. Titin isoform expression in normal and hypertensive myocardium. Cardiovascular Research. 2003;59(1):86–94. doi: 10.1016/s0008-6363(03)00328-6. [DOI] [PubMed] [Google Scholar]
- 59.Van Heerebeek L, Borbely A, Niessen HWM, et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113(16):1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 60.Neagoe C, Kulke M, Del Monte F, et al. Titin isoform switch in ischemic human heart disease. Circulation. 2002;106(11):1333–1341. doi: 10.1161/01.cir.0000029803.93022.93. [DOI] [PubMed] [Google Scholar]
- 61.Makarenko I, Opitz CA, Leake MC, et al. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circulation Research. 2004;95(7):708–716. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- 62.Nagueh SF, Shah G, Wu Y, et al. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110(2):155–162. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 63.Wu Y, Peng J, Campbell KB, Labeit S, Granzier H. Hypothyroidism leads to increased collagen-based stiffness and re-expression of large cardiac titin isoforms with high compliance. Journal of Molecular and Cellular Cardiology. 2007;42(1):186–195. doi: 10.1016/j.yjmcc.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 64.Fukuda N, Terui T, Ishiwata S, Kurihara S. Titin-based regulations of diastolic and systolic functions of mammalian cardiac muscle. doi: 10.1016/j.yjmcc.2009.11.013. Journal of Molecular and Cellular Cardiology. In press. [DOI] [PubMed] [Google Scholar]