Abstract
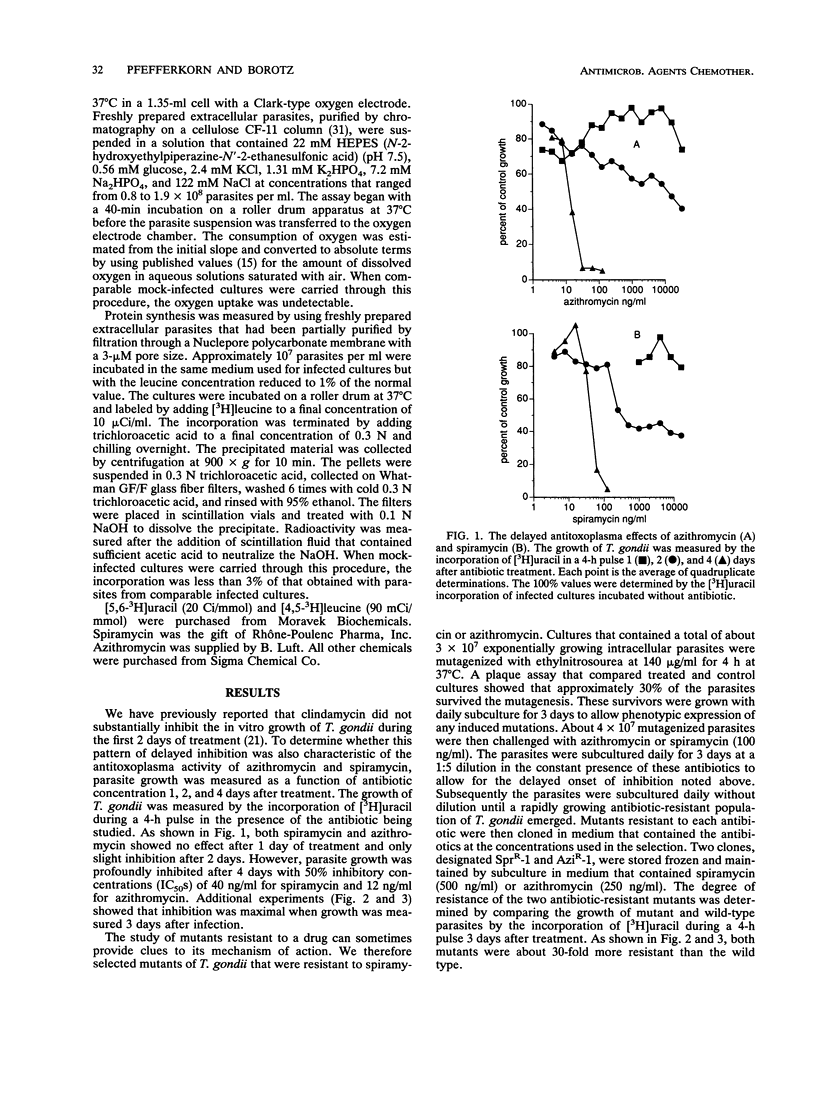
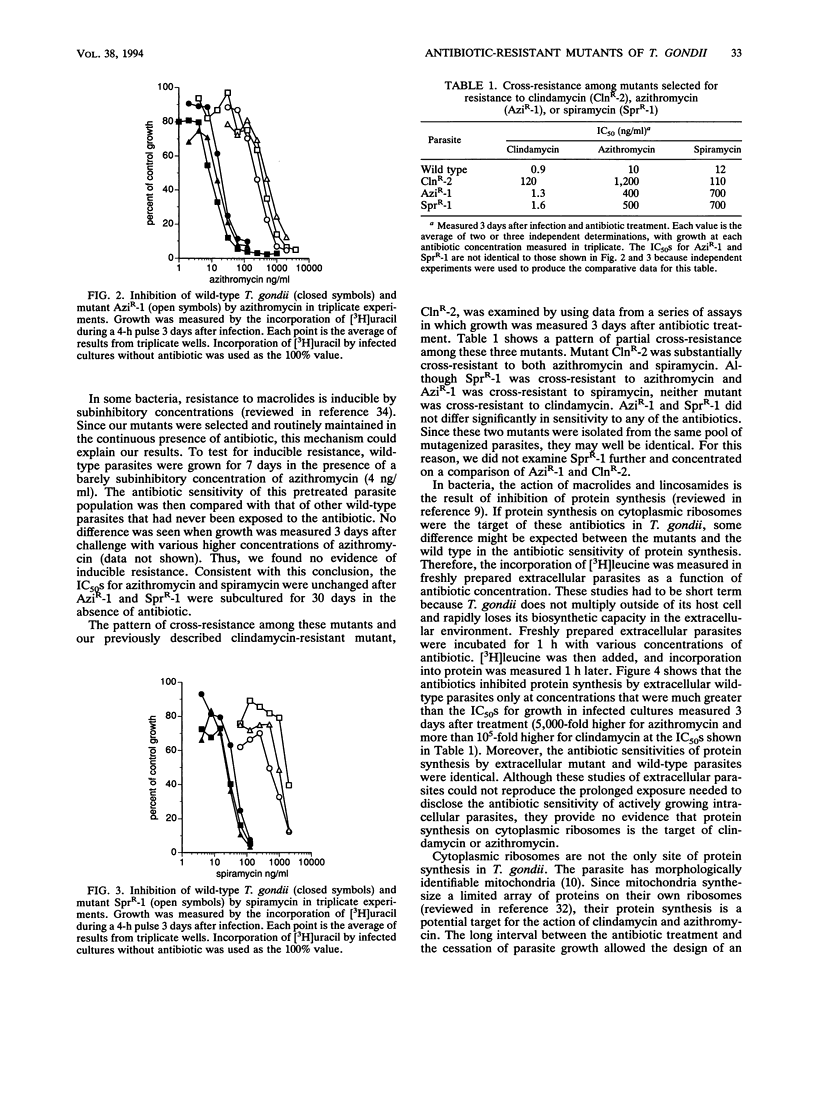
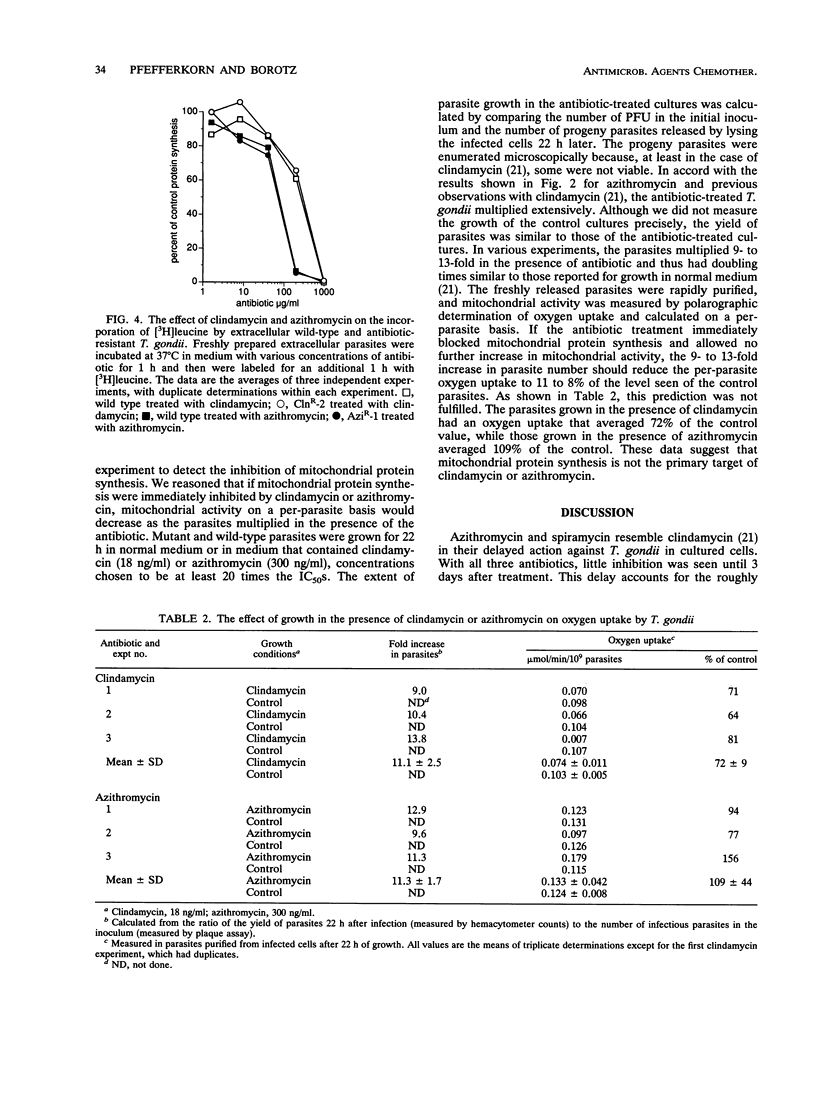
Azithromycin and spiramycin markedly inhibited the growth of Toxoplasma gondii in cultured human fibroblasts. However, 3 days of treatment were required to reveal their full antitoxoplasma activity. This delayed onset of inhibition was similar to that previously reported for clindamycin. Mutants of T. gondii resistant to azithromycin (AziR-1) and spiramycin (SprR-1) were isolated and compared with a previously described mutant resistant to clindamycin (ClnR-2). Mutant ClnR-2 was cross-resistant to all three antibiotics, while AziR-1 was cross-resistant only to spiramycin and SprR-1 was cross-resistant only to azithromycin. In short-term studies of protein synthesis by freshly prepared extracellular parasites, clindamycin and azithromycin were effective only at concentrations much greater than their 50% inhibitory concentrations in infected cultures and the resistant mutants did not differ from the wild type in antibiotic sensitivity. Thus, protein synthesis on cytoplasmic ribosomes of the parasite did not seem to be the target of these antibiotics. To determine whether mitochondrial protein synthesis in T. gondii was inhibited by clindamycin or azithromycin, wild-type parasites were grown in cultured cells in the presence of antibiotic concentrations well above the 50% inhibitory concentrations. Mitochondrial function, measured by oxygen uptake per purified extracellular parasite, did not decrease substantially, after the parasites had multiplied 11-fold in the presence of antibiotic. Thus, mitochondrial protein synthesis did not seem to be the target of clindamycin or azithromycin. An alternative target is protein synthesis in the putative apicomplexan organelle that has a 35-kb genome.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G., Remington J. S. Effect of clindamycin on acute and chronic toxoplasmosis in mice. Antimicrob Agents Chemother. 1974 Jun;5(6):647–651. doi: 10.1128/aac.5.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Shepard R. M., Remington J. S. In vivo activity of the macrolide antibiotics azithromycin, roxithromycin and spiramycin against Toxoplasma gondii. Eur J Clin Microbiol Infect Dis. 1991 Jun;10(6):519–524. doi: 10.1007/BF01963942. [DOI] [PubMed] [Google Scholar]
- Blais J., Garneau V., Chamberland S. Inhibition of Toxoplasma gondii protein synthesis by azithromycin. Antimicrob Agents Chemother. 1993 Aug;37(8):1701–1703. doi: 10.1128/aac.37.8.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Overdulve J. P., Weijers P. J., Fase-Fowler F., Van den Berg M. DNA circles with cruciforms from Isospora (Toxoplasma) gondii. Biochim Biophys Acta. 1984 Feb 24;781(1-2):100–111. doi: 10.1016/0167-4781(84)90128-3. [DOI] [PubMed] [Google Scholar]
- Carlier M. B., Zenebergh A., Tulkens P. M. Cellular uptake and subcellular distribution of roxithromycin and erythromycin in phagocytic cells. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):47–56. doi: 10.1093/jac/20.suppl_b.47. [DOI] [PubMed] [Google Scholar]
- Chamberland S., Kirst H. A., Current W. L. Comparative activity of macrolides against Toxoplasma gondii demonstrating utility of an in vitro microassay. Antimicrob Agents Chemother. 1991 May;35(5):903–909. doi: 10.1128/aac.35.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannemann B., McCutchan J. A., Israelski D., Antoniskis D., Leport C., Luft B., Nussbaum J., Clumeck N., Morlat P., Chiu J. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. The California Collaborative Treatment Group. Ann Intern Med. 1992 Jan 1;116(1):33–43. doi: 10.7326/0003-4819-116-1-33. [DOI] [PubMed] [Google Scholar]
- GAVIN M. A., WANKO T., JACOBS L. Electron microscope studies of reproducing and interkinetic Toxoplasma. J Protozool. 1962 May;9:222–234. doi: 10.1111/j.1550-7408.1962.tb02610.x. [DOI] [PubMed] [Google Scholar]
- Haverkos H. W. Assessment of therapy for toxoplasma encephalitis. The TE Study Group. Am J Med. 1987 May;82(5):907–914. doi: 10.1016/0002-9343(87)90151-3. [DOI] [PubMed] [Google Scholar]
- Hofflin J. M., Remington J. S. Clindamycin in a murine model of toxoplasmic encephalitis. Antimicrob Agents Chemother. 1987 Apr;31(4):492–496. doi: 10.1128/aac.31.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katlama C. Evaluation of the efficacy and safety of clindamycin plus pyrimethamine for induction and maintenance therapy of toxoplasmic encephalitis in AIDS. Eur J Clin Microbiol Infect Dis. 1991 Mar;10(3):189–191. doi: 10.1007/BF01964459. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Styrt B. Clindamycin uptake by human neutrophils. J Infect Dis. 1981 Nov;144(5):472–479. doi: 10.1093/infdis/144.5.472. [DOI] [PubMed] [Google Scholar]
- McMaster P. R., Powers K. G., Finerty J. F., Lunde M. N. The effect of two chlorinated lincomycin analogues against acute toxoplasmosis in mice. Am J Trop Med Hyg. 1973 Jan;22(1):14–17. doi: 10.4269/ajtmh.1973.22.14. [DOI] [PubMed] [Google Scholar]
- Mellors J. W., Debs R. J., Ryan J. L. Incorporation of recombinant gamma interferon into liposomes enhances its ability to induce peritoneal macrophage antitoxoplasma activity. Infect Immun. 1989 Jan;57(1):132–137. doi: 10.1128/iai.57.1.132-137.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Guyre P. M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984 May;44(2):211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Nothnagel R. F., Borotz S. E. Parasiticidal effect of clindamycin on Toxoplasma gondii grown in cultured cells and selection of a drug-resistant mutant. Antimicrob Agents Chemother. 1992 May;36(5):1091–1096. doi: 10.1128/aac.36.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Quantitative studies of the mutagenesis of Toxoplasma gondii. J Parasitol. 1979 Jun;65(3):364–370. [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Specific labeling of intracellular Toxoplasma gondii with uracil. J Protozool. 1977 Aug;24(3):449–453. doi: 10.1111/j.1550-7408.1977.tb04774.x. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Toxoplasma gondii: isolation and preliminary characterization of temperature-sensitive mutants. Exp Parasitol. 1976 Jun;39(3):365–376. doi: 10.1016/0014-4894(76)90040-0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. M., Dayhoff M. O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science. 1978 Jan 27;199(4327):395–403. doi: 10.1126/science.202030. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., LeBlanc A. J., Pfefferkorn E. R., Boothroyd J. C. Generation of a restriction fragment length polymorphism linkage map for Toxoplasma gondii. Genetics. 1992 Dec;132(4):1003–1015. doi: 10.1093/genetics/132.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Morgan E. A. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1984 Jun 11;12(11):4653–4663. doi: 10.1093/nar/12.11.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner R., Cundliffe E., Schmidt F. J. Site of action of a ribosomal RNA methylase responsible for resistance to erythromycin and other antibiotics. J Biol Chem. 1983 Oct 25;258(20):12702–12706. [PubMed] [Google Scholar]
- Vannuffel P., Di Giambattista M., Morgan E. A., Cocito C. Identification of a single base change in ribosomal RNA leading to erythromycin resistance. J Biol Chem. 1992 Apr 25;267(12):8377–8382. [PubMed] [Google Scholar]
- Weisblum B. Inducible resistance to macrolides, lincosamides and streptogramin type B antibiotics: the resistance phenotype, its biological diversity, and structural elements that regulate expression--a review. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):63–90. doi: 10.1093/jac/16.suppl_a.63. [DOI] [PubMed] [Google Scholar]
- Wilson R. J., Gardner M. J., Feagin J. E., Williamson D. H. Have malaria parasites three genomes? Parasitol Today. 1991 Jun;7(6):134–136. doi: 10.1016/0169-4758(91)90276-t. [DOI] [PubMed] [Google Scholar]
- Wittmann H. G., Stöffler G., Apirion D., Rosen L., Tanaka K., Tamaki M., Takata R., Dekio S., Otaka E. Biochemical and genetic studies on two different types of erythromycin resistant mutants of Escherichia coli with altered ribosomal proteins. Mol Gen Genet. 1973 Dec 20;127(2):175–189. doi: 10.1007/BF00333665. [DOI] [PubMed] [Google Scholar]
- Wittner M., Rowin K. S., Tanowitz H. B., Hobbs J. F., Saltzman S., Wenz B., Hirsch R., Chisholm E., Healy G. R. Successful chemotherapy of transfusion babesiosis. Ann Intern Med. 1982 May;96(5):601–604. doi: 10.7326/0003-4819-96-5-601. [DOI] [PubMed] [Google Scholar]
- el Wakeel E. S., Homeida M. M., Ali H. M., Geary T. G., Jensen J. B. Clindamycin for the treatment of falciparum malaria in Sudan. Am J Trop Med Hyg. 1985 Nov;34(6):1065–1068. doi: 10.4269/ajtmh.1985.34.1065. [DOI] [PubMed] [Google Scholar]



