Abstract
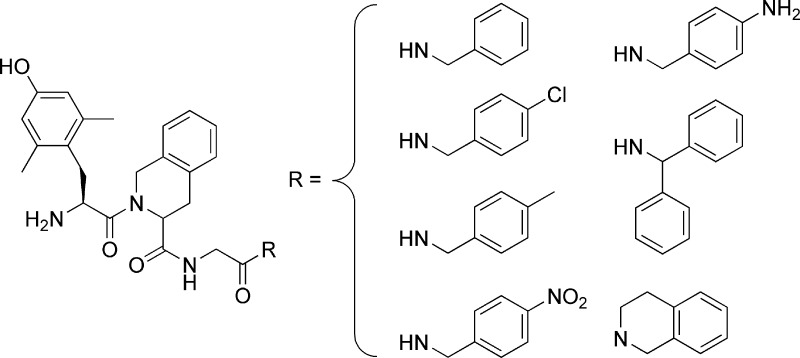
Based on a renewed importance recently attributed to bi- or multifunctional opioids, we report the synthesis and pharmacological evaluation of some analogues derived from our lead μ agonist/δ antagonist, H-Dmt-Tic-Gly-NH-Bzl (Dmt = 2′,6′-dimethyl-l-tyrosine, Tic = 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, Bzl = benzyl). Our previous studies focused on the importance of the C-teminal benzyl function in the induction of such bifunctional activity. The introduction of some substituents in the para position of the phenyl ring (−Cl, −CH3, partially −NO2, inactive −NH2) was found to give a more potent μ agonist/antagonist effect associated with a relatively unmodified δ antagonist activity (pA2 = 8.28−9.02). Increasing the steric hindrance of the benzyl group (using diphenylmethyl and tetrahydroisoquinoline functionalities) substantially maintained the μ agonist and δ antagonist activities of the lead compound. Finally and quite unexpectedly d-Tic2, considered as a wrong opioid message now, inserted into the reference compound in lieu of l-Tic provided a μ agonist/δ agonist better than our reference ligand (H-Dmt-Tic-Gly-NH-Ph; Ph = phenyl) and was endowed with the same pharmacological profile.
Keywords: Bifunctional opioids; Dmt−Tic pharmacophore; opioid peptides; opioid receptors, angiogenesis, tolerance
Recently, bifunctional opioids characterized by a μ agonist/δ antagonist pharmacological profile are gaining renewed importance as potential drugs for the treatment of pain on the basis of their low propensity to induce tolerance and physical dependence (1−3). Furthermore, a project involving the use of our lead μ agonist/δ antagonist H-Dmt-Tic-Gly-NH-Bzl (4), (coded UFP-505) in the treatment of pain resulting from cancer was funded by the University of Leicester (UK) [https://swww2.le.ac.uk/ebulletin/news/press-releases/2000-2009/2008/12/nparticle.2008-12-05.9756874495].
In the 1990s, a series of articles demonstrated the potential utility of bifunctional ligands in pain relief with lower central or peripheral side effects (5−14). Although the majority of opioids act directly on the central nervous system to relieve pain, their activities on peripheral tissues are responsible for many of the secondary complications associated with the management of pain. A recently recognized peripheral effect of opioids and their receptors is the promotion of angiogenesis-dependent tumor growth (15). Opioids at physiologically relevant concentrations promote angiogenesis in vitro, as well as in breast cancer and in wound healing in rodents (16−18). Although naloxone and naltrexone can inhibit tumor growth in rodents (16,19,20), nonselective opioid antagonists cannot be used to counteract the unwanted effects of opioids without also compromising analgesia in a clinical setting. Therefore, it is important to identify agents that maintain the analgesic effect of opioids while inhibiting their angiogenic effect. This goal could be reached, for example, through the coadministration of morphine and the δ selective antagonist naltrindole, instead of the nonselective antagonists naloxone or naltrexone. Considering that bifunctional ligands may have advantages compared with drug combinations, such as more predictable pharmacokinetic and pharmacodynamic relationship as a consequence of the administration of a single drug (1), we focused our attention on the lead μ agonist/δ antagonist H-Dmt-Tic-Gly-NH-Bzl (4) as a potential analgesic displaying a lower induction of tolerance, a lower physical dependence, and a lower angiogenetic propensity compared with morphine. In previous studies regarding the Dmt−Tic opioid pharmacophore, it was demonstrated that the importance of the C-terminal group in the induction of different pharmacological profiles were as follows: -NH-Ph, μ agonist/δ agonist (4); -NH-Bzl, μ agonist/δ antagonist (4); and -NH-CH2-Bid (4), -NH-CH(CH3)-Bid (21), -NH-CH(CH2-COOH))-Bid (21), δ agonists.
On the basis of the new interest in opioid bifunctional μ agonist/δ antagonist ligands, it would be valuable to increase the number of compounds endowed with such a profile for more thorough pharmacological investigations in the future. As a result, H-Dmt-Tic-Gly-NH-Bzl (1) was selected as a starting point for the synthesis of some new analogues modified on the C-terminal benzyl group. A minireview by Schiller et al. (22) prompted us to evaluate the electronic and steric hindrance effects by inserting electron-donating or -withdrawing groups in the para position of the phenyl ring (3−6) and by the employment of benzyl surrogates (7, 8). Finally, although the Tyr/Dmt-d-Tic pharmacophore is considered to contain the wrong stereochemistry (23−25), our curiosity encouraged us to synthesize the diastereomer of the lead compound (1), which resulted in the formation of H-Dmt-d-Tic-Gly-NH-Bzl (2).
Chemistry
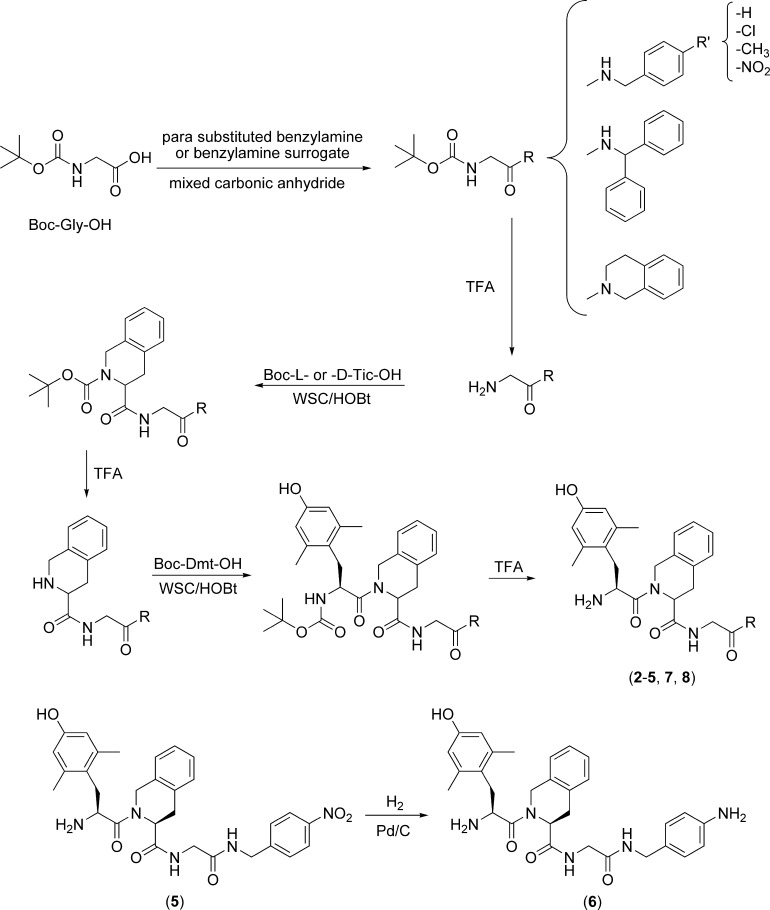
All peptides (2−8) were prepared stepwise in solution using conventional synthetic methods as outlined in Scheme 1. Boc-Gly-OH was condensed with either benzylamine, a para-substituted derivative, or a benzyl surrogate (containing a diphenylmethyl or a tetrahydroisoquinoline functionality) via a mixed carbonic anhydride. Each Boc-protected intermediate was deprotected with TFA and condensed with Boc-Tic-OH or Boc-d-Tic-OH via WSC/HOBt. After Tic deprotection by TFA, the dipeptides were subsequently condensed with Boc-Dmt-OH via WSC/HOBt. Final removal of the N-terminal protecting groups using TFA gave the crude final compounds (2−5, 7, and 8). Compound 6 was obtained by catalytic hydrogenation of the para-nitrobenzyl group of compound 5. All peptides were purified by reverse-phase preparative HPLC.
Scheme 1. General Synthetic Method for Compounds 2−8.
Results and Discussion
Receptor Affinity Analysis
The tripeptides (1−8) were evaluated for their affinity and selectivity for μ, δ, and κ opioid receptors using Chinese hamster ovary (CHO) cell membranes stably expressing the opioid receptors. The data are summarized in Table 1. All compounds (1−8) had nanomolar or subnanomolar affinity for both μ (Ki = 0.068−4.5 nM) and δ (Ki = 0.26−3.2 nM) receptors. Only the para-aminobenzyl analogue (6) resulted in a lower μ affinity (Ki = 34 nM). As expected for peptides containing the Dmt−Tic pharmacophore, κ affinity was low (Ki = 11−750 nM). However, the reference compound (1) and its diastereomer (2) containing the C-terminal unmodified benzyl amide yielded higher κ affinity (39 and 11 nM, respectively). Only diastereomer 2, containing d-Tic, appears to be weakly μ selective (Kiδ/Ki = 47.1) whereas all other compounds (1, 3−8) are substantially less selective (Kiμ/Ki = 1.82−17).
Table 1. Ki Values of the Inhibition of μ, δ and κ Opioid Binding to CHO Membranes.
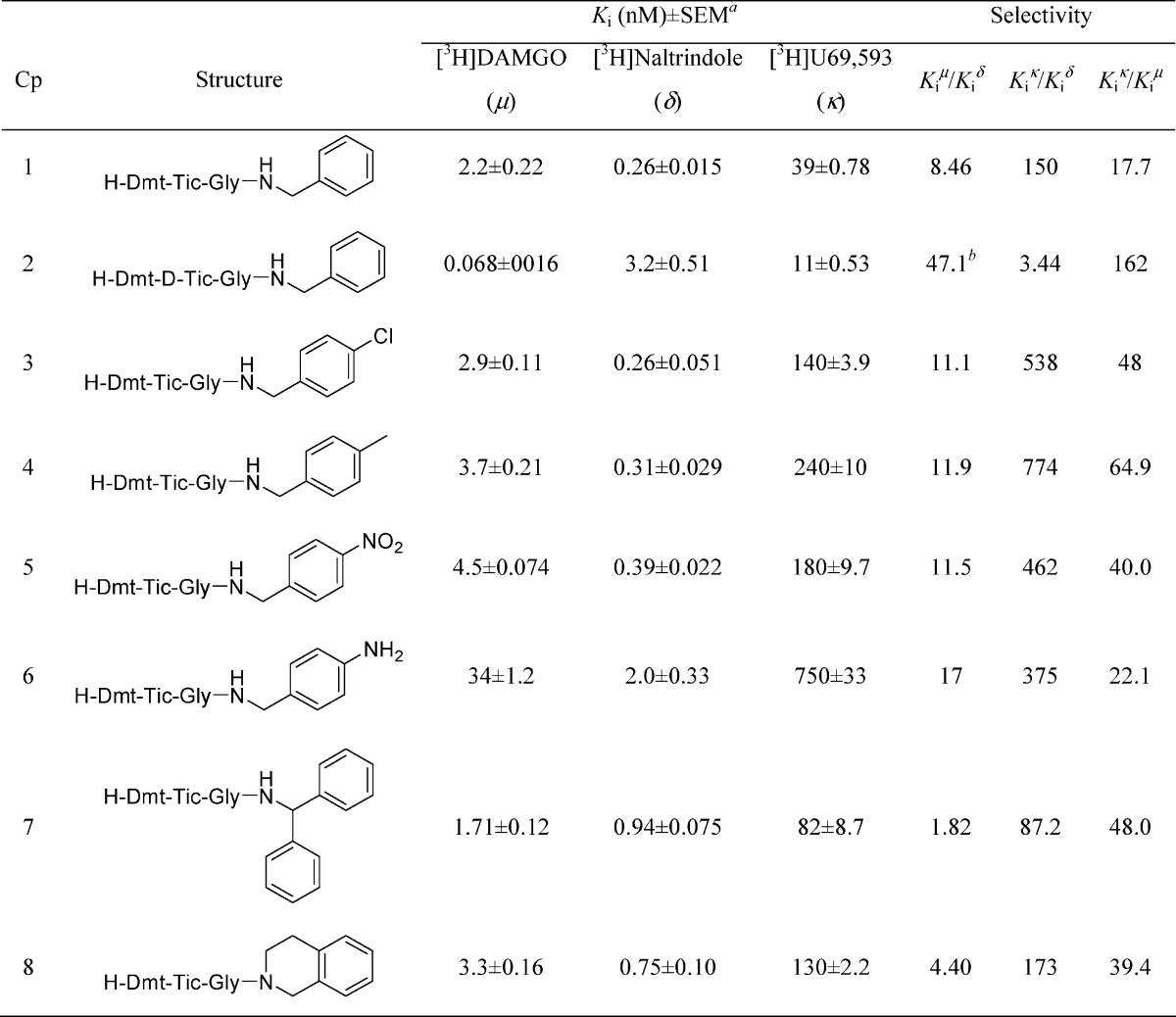 |
See Methods section. The Kd values for [3H]DAMGO, [3H]U69,593, and [3H]naltrindole were 0.56, 0.34, and 0.10 nM, respectively. These values were used to calculate the Ki values.
Selectivity = Kiδ/Ki.
Functional Bioactivity
Table 2 indicates the agonist and antagonist properties of the new compounds (1−8) in stimulating [35S]-GTPγS binding mediated by the μ and δ opioid receptors; [35S]-GTPγS binding mediated by κ receptors is not reported due to their low binding affinity data (Table 1). This new data confirmed the μ agonist (EC50 = 19 nM)/δ antagonist (IC50 = 1.3 nM) activity of the reference compound 1 in comparison with the old data obtained using functional pharmacological assays (GPI, EC50 = 2.69 nM; MVD, Ke =0.56 nM) (4). Compound 3, bearing a chlorine atom at the para position of the benzyl amide, exhibited an agonist effect on μ receptors (EC50 = 33 nM) comparable to other μ agonists containing the Dmt-Tic pharmacophore (26), a low but potentially interesting μ antagonist effect (IC50 = 460 nM), and a potent δ antagonist effect (IC50 = 0.95 nM). Compounds 4 and 5, containing methyl and nitro groups, respectively, in the para position, had a similar but less interesting behavior, especially toward μ receptors. Compound 6 exhibited δ antagonist activity; however the functional bioactivity on μ receptors was not evaluated due to its poor affinity. Compounds 7 and 8 containing C-terminal benzyl amide surrogates revealed a μ agonism/δ antagonism behavior very similar to the reference compound 1. Finally, H-Dmt-d-Tic-Gly-NH-Bzl 2, which incorporated d-Tic, unexpectedly displayed a potent μ agonist (EC50 = 0.9 nM)/δ agonist (EC50 = 2.8 nM) opioid activity, superimposable to μ agonist/δ agonist lead compound H-Dmt-Tic-Gly-Ph (GPI, EC50 = 2.57 nM; MVD, EC50 = 3.02 nM) (4).
Table 2. EC50 and Emax Values for the Stimulation of [35S]GTPγS Binding and IC50 and Imax Values for the Inhibition of Agonist-Stimulated [35S]GTPγS Binding to the Human μ and δ Opioid Receptorsa.
| agonism |
antagonism |
|||||||
|---|---|---|---|---|---|---|---|---|
| μ |
δ |
μ |
δ |
|||||
| compd | EC50 (nM) | Emax (%) | EC50 (nM) | Emax (%) | IC50 (nM) | Imax (%) | IC50 (nM) | Imax (%) |
| 1 | 19 ± 1.9 | 84 ± 10 | c | 6.8 ± 1.8 | b | b | 1.3 ± 0.031 | 95 ± 1.1 |
| 2 | 0.90 ± 0.032 | 92 ± 4.0 | 2.8 ± 0.60 | 44 ± 6.1 | b | b | b | b |
| 3 | 33 ± 2.9 | 52 ± 5.5 | c | 4.5 ± 0.85 | 460 ± 93 | 43 ± 3.3 | 0.95 ± 0.058 | 93 ± 2.2 |
| 4 | 63 ± 3.5 | 34 ± 6.4 | c | 0.4 ± 1.43 | 820 ± 150 | 65 ± 1.1 at 25 μMe | 1.0 ± 0.28 | 86 ± 5.0 |
| 5 | 61 ± 15 | 16 ± 2.5 | c | 3.1 ± 0.43 | 810 ± 27.0 | 74 ± 6.9 | 1.6 ± 0.76 | 79 ± 3.8 |
| 6 | d | d | c | 13 ± 1.0 | d | d | 5.2 ± 0.13 | 78 ± 2.7 |
| 7 | 4.8 ± 0.16 | 45 ± 1.2 | c | −1.9 ± 0.17 | b | b | 8.3 ± 0.26 | 100 ± 7.4 |
| 8 | 22 ± 6.8 | 43 ± 6.6 | c | −1.4 ± 0.14 | b | b | 2.3 ± 0.27 | 99 ± 3.3 |
See Methods section. Data are the mean Emax and EC50 values (SEM from at least three separate experiments, performed in triplicate). For calculation of the Emax values, the basal [35S]GTPγS binding was set at 0%.
No inhibition.
Not active.
Not tested.
Compound 4 at a concentration of 25 μM inhibited 65% ± 1.1% of DAMGO-stimulated [35S]GTPγS binding. An IC50 value was not reported because higher concentrations could not be used without having the DMSO vehicle interfere with the assay. A concentration of 200 nM DAMGO, which gave 96% ± 3.1% stimulation, was used to measure inhibition of DAMGO-stimulated [35S]GTPγS binding. A concentration of 100 nM U50,488, which gave 64% ± 1.9% stimulation, was used to measure inhibition of U50,488-stimulated [35S]GTPγS binding, and 10 nM SNC 80, which gave 66% ± 4.3% stimulation, was used to measure inhibition of [35S]GTPγS binding, mediated by the δ opioid receptor.
Conclusions
In this SAR study, our attention was focused on the elaboration of the C-terminal benzyl amide of our lead μ agonist/δ antagonist H-Dmt-Tic-Gly-NH-Bzl (4). This amide functionality, considered responsible for the pharmacological profile in peptides containing the Dmt-Tic pharmacohore, was modified by the addition of electron-withdrawing or -donating groups in the para position or by increasing its steric hindrance. The pharmacological characterization of these analogues gave some interesting results: (1) affinities for the μ, δ, and κ opioid receptors from CHO cell membranes and agonist/antagonist properties determined by stimulating [35S]-GTPγS binding confirmed the previous data reported for the reference compound 1 using brain P2 synaptosomal membranes for affinity data and GPI and MVD tissues for agonism/antagonism (4); (2) the introduction of substituents in the para position of the phenyl ring and the increase of the steric hindrance further decreased the low κ affinity of the unmodified benzyl amide; (3) compound 3, containing the para-chlorobenzyl amide functionality, displayed the same μ agonist/δ antagonist profile of compound 1 and showed that a low μ antagonism could be useful in the trafficking of these receptors, thus avoiding their internalization without decreasing their analgesic effect (27−29); (4) compound 2 containing d-Tic2 can be considered a derivative of the almost forgotten (Tyr)/Dmt-d-Tic pharmacophore (23−25). Initially, (Tyr)/Dmt-Tic and (Tyr)/Dmt-d-Tic were considered equally important opioid pharmacophores; in fact H-Tyr-Tic-NH224 and H-Tyr-d-Tic-NH224 are commercially available as selective δ antagonist and nonselective μ agonist, respectively. Regarding the (Tyr)/Dmt-d-Tic pharmacophore, to the best of our knowledge only 12 analogues, which were not completely pharmacologically characterized were published from 1992 to 199523−25 potentially due to being considered to contain the wrong stereochemistry. Unexpectedly, the new analogue H-Dmt-d-Tic-Gly-NH-Bzl (2) was endowed with interesting bifunctional μ/δ agonist activity comparable to H-Dmt-Tic-Gly-NH-Ph4 and confirmed the nonselective μ agonist activity evidenced years ago (24). On the basis of the new trend in opioid medicinal chemistry regarding the synthesis of bi- or multifunctional ligands,1−3 the last two compounds mentioned above require more detailed pharmacological in vivo studies. In fact, Negus et al. stated that “delta opioid agonists can selectively enhance the antinociceptive effects of mu opioid agonists without enhancing some other, potentially undesirable mu agonist effects” (30). In summary, it appears that an increase in the steric hindrance of the benzyl amide function does not influence the activity; a substituent in the para position can induce further μ antagonism, and d-Tic2, at least in this case, can reverse a δ antagonism into δ agonism without afffecting the μ activity.
Methods
Chemistry
General Methods
Crude peptides and pseudopeptides were purified by preparative reversed-phase HPLC [Waters Delta Prep 4000 system with Waters Prep LC 40 mm Assembly column C18 (30 cm × 4 cm, 15 μm particle)] and eluted at a flow rate of 20 mL/min with mobile phase solvent A (10% acetonitrile + 0.1% TFA in H2O, v/v), and a linear gradient from 10% to 60% B (60%, acetonitrile + 0.1% TFA in H2O, v/v) in 25 min. Analytical HPLC analyses were performed with a Beckman System Gold (Beckman ultrasphere ODS column, 250 mm × 4.6 mm, 5 μm particle). Analytical determinations and capacity factor (K′) of the products used HPLC in solvents A and B programmed at flow rate of 1 mL/min with linear gradients from 0% to 100% B in 25 min. Analogues had less than 5% impurities at 220 and 254 nm.
TLC was performed on precoated plates of silica gel F254 (Merck, Darmstadt, Germany): (A) 1-butanol/AcOH/H2O (3:1:1, v/v/v); (B) CH2Cl2/toluene/methanol (17:1:2); ninhydrin (1% ethanol, Merck), fluorescamine (Hoffman-La Roche), and chlorine spray reagents. Melting points were determined on a Kofler apparatus and are uncorrected. Optical rotations were assessed at 10 mg/mL in methanol with a Perkin-Elmer 241 polarimeter in a 10 cm water-jacketed cell. Molecular weights of the compounds were determined by a MALDI-TOF analysis (Hewlett-Packard G2025A LD-TOF system mass spectrometer) and α-cyano-4-hydroxycinnamic acid as a matrix. 1H NMR (δ) spectra were measured, when not specified, in DMSO-d6 solution using a Bruker AC-200 spectrometer, and peak positions are given in parts per million downfield from tetramethylsilane as internal standard. The purity of tested compounds was determined by combustion elemental analyses conducted by the Microanalytical Laboratory of the Chemistry Department, University of Ferrara, with a Yanagimoto MT-5 CHN recorder elemental analyzer. All tested compounds possess a purity of at least 95% of the theoretical values.
Boc-d-Tic-Gly-NH-Bzl
To a solution of Boc-d-Tic-OH (0.2 g, 0.72 mmol) and TFA·H-Gly-NH-Bzl (31) (0.2 g, 0.72 mmol) in DMF (10 mL) at 0 °C, NMM (0.08 mL, 0.72 mmol), HOBt (0.12 g, 0.79 mmol), and WSC (0.15 g, 0.79 mmol) were added. The reaction mixture was stirred for 3 h at 0 °C and 24 h at room temperature. After DMF was evaporated, the residue was dissolved in EtOAc and washed with citric acid (10% in H2O), NaHCO3 (5% in H2O), and brine. The organic phase was dried (Na2SO4) and evaporated to dryness. The residue was precipitated from Et2O/Pe (1:9, v/v): yield 0.26 g (85%); Rf(B) 0.72; HPLC K′ 11.02; mp 123−125 °C; [α]D20 +35.2; m/z 425 (M + H)+; 1H NMR (DMSO-d6) δ 1.39−1.41 (d, 9H), 2.92−3.17 (m, 2H), 4.09−4.27 (m, 4H), 4.46−4.92 (m, 3H), 6.96−7.14 (m, 9H).
TFA·H-d-Tic-Gly-NH-Bzl
Boc-d-Tic-Gly-NH-Bzl (0.21 g, 0.5 mmol) was treated with TFA (1.5 mL) for 0.5 h at room temperature. Et2O/Pe (1:1, v/v) were added to the solution until the product precipitated: yield 0.21 g (94%); Rf(A) 0.38; HPLC K′ 6.14; mp 151−153 °C; [α]D20 +30.8; m/z 324 (M + H)+.
Boc-Dmt-d-Tic-Gly-NH-Bzl
To a solution of Boc-Dmt-OH (0.05 g, 0.16 mmol) and TFA·H-d-Tic-Gly-NH-Bzl (0.07 g, 0.16 mmol) in DMF (10 mL) at 0 °C, NMM (0.02 mL, 0.16 mmol), HOBt (0.03 g, 0.18 mmol), and WSC (0.04 g, 0.18 mmol) were added. The reaction mixture was stirred for 3 h at 0 °C and 24 h at room temperature. After DMF was evaporated, the residue was dissolved in EtOAc and washed with citric acid (10% in H2O), NaHCO3 (5% in H2O), and brine. The organic phase was dried (Na2SO4) and evaporated to dryness. The residue was precipitated from Et2O/Pe (1:9, v/v): yield 0.08 g (83%); Rf(B) 0.64; HPLC K′ 9.82; mp 138−140 °C; [α]D20 +20.2; m/z 616 (M + H)+; 1H NMR (DMSO-d6) δ 1.39−1.41 (d, 9H), 2.35 (s, 6H), 2.92−3.17 (m, 4H), 4.09−4.51 (m, 6H), 4.90−4.94 (m, 2H), 6.29 (s, 2H), 6.96−7.14 (m, 9H).
TFA·H-Dmt-d-Tic-Gly-NH-Bzl (2)
Boc-Dmt-d-Tic-Gly-NH-Bzl (0.05 g, 0.08 mmol) was treated with TFA (1 mL) for 0.5 h at room temperature. Et2O/Pe (1:1, v/v) were added to the solution until the product precipitated: yield 0.05 g (97%); Rf(A) 0.41; HPLC K′ 5.21; mp 147−149 °C; [α]D20 +21.5; m/z 516 (M + H)+; 1H NMR (DMSO-d6) δ 2.35 (s, 6H), 2.92−3.17 (m, 4H), 3.93−3.97 (m, 1H), 4.09−4.51 (m, 6H), 4.90−4.94 (m, 1H), 6.29 (s, 2H), 6.96−7.14 (m, 9H). Anal. C32H35F3N4O6: C; H; N.
Boc-Gly-NH-Bzl(pCl)
A solution of Boc-Gly-OH (0.5 g, 2.86 mmol) and NMM (0.31 mL, 2.86 mmol) in DMF (10 mL) was treated at −20 °C with isobutyl chloroformate (IBCF, 0.37 mL, 2.86 mmol). After 10 min at −20 °C, 4-chlorobenzylamine (0.35 mL, 2.86 mmol) was added. The reaction mixture was allowed to stir while slowly warming to room temperature (1 h) and was then stirred for 3 h. The solvent was evaporated, and the residue was partitioned between EtOAc and H2O. The AcOEt layer was washed with citric acid (10% in H2O), NaHCO3 (5% in H2O), and brine. The organic phase was dried (Na2SO4) and evaporated to dryness. The residue was precipitated from Et2O/Pe (1:9, v/v): yield 0.76 g (89%); Rf(B) 0.42; HPLC K′ 4.25; mp 110−112 °C; m/z 300 (M + H)+; 1H NMR (DMSO-d6) δ 1.40 (s, 9H), 3.83−3.87 (d, 2H), 4.46 (s, 2H), 7.00−7.15 (m, 4H).
TFA·H-Gly-NH-Bzl(pCl)
Boc-Gly-NH-Bzl(pCl) (0.71 g, 2.37 mmol) was treated with TFA (2.5 mL) for 0.5 h at room temperature. Et2O/Pe (1:1, v/v) was added to the solution until the product precipitated: yield 0.69 g (93%); Rf(A) 0.39; HPLC K′ 3.67; mp 126−128 °C; m/z 200 (M + H)+.
Boc-Tic-Gly-NH-Bzl(pCl)
This intermediate was obtained by condensation of Boc-Tic-OH with TFA·H-Gly-NH-Bzl(pCl) via WSC/HOBt as reported for Boc-d-Tic-Gly-NH-Bzl: yield 0.52 g (88%); Rf(B) 0.81; HPLC K′ 11.15; mp 131−133 °C; [α]D20 −30.2; m/z 459 (M + H)+; 1H NMR (DMSO-d6) δ 1.38−1.42 (d, 9H), 2.92−3.17 (m, 2H), 4.09−4.27 (m, 4H), 4.46−4.92 (m, 3H), 6.96−7.15 (m, 8H).
TFA·H-Tic-Gly-NH-Bzl(pCl)
Boc-Tic-Gly-NH-Bzl(pCl) was treated with TFA as reported for TFA·H-d-Tic-Gly-NH-Bzl: yield 0.3 g (90%); Rf(A) 0.43; HPLC K′ 6.51; mp 156−158 °C; [α]D20 −28.6; m/z 359 (M + H)+.
Boc-Dmt-Tic-Gly-NH-Bzl(pCl)
This intermediate was obtained by condensation of Boc-Dmt-OH with TFA·H-Tic-Gly-NH-Bzl(pCl) via WSC/HOBt as reported for Boc-Dmt-d-Tic-Gly-NH-Bzl: yield 0.12 g (85%); Rf(B) 0.70; HPLC K′ 9.91; mp 144−146 °C; [α]D20 −20.5; m/z 650 (M + H)+; 1H NMR (DMSO-d6) δ 1.38−1.42 (d, 9H), 2.35 (s, 6H), 2.92−3.17 (m, 4H), 4.09−4.51 (m, 6H), 4.90−4.94 (m, 2H), 6.29 (s, 2H), 6.96−7.15 (m, 8H).
TFA·H-Dmt-Tic-Gly-NH-Bzl(pCl) (3)
Boc-Dmt-Tic-Gly-NH-Bzl(pCl) was treated with TFA as reported for TFA·H-Dmt-d-Tic-Gly-NH-Bzl: yield 0.07 g (96%); Rf(A) 0.46; HPLC K′ 5.36; mp 155−157 °C; [α]D20 −18.6; m/z 550 (M + H)+; 1H NMR (DMSO-d6) δ 2.35 (s, 6H), 2.92−3.17 (m, 4H), 3.95−4.09 (m, 3H), 4.41−4.51 (m, 4H), 4.91−4.93 (m, 1H), 6.29 (s, 2H), 6.96−7.15 (m, 8H). Anal. C32H34ClF3N4O6: C; H; N.
Boc-Gly-NH-Bzl(pMe)
This intermediate was obtained by condensation of Boc-Gly-OH with 4-methylbenzylamine via mixed carbonic anhydride as reported for Boc-Gly-NH-Bzl(pCl): yield 0.72 g (86%); Rf(B) 0.45; HPLC K′ 4.36; mp 119−121 °C; m/z 279 (M + H)+; 1H NMR (DMSO-d6) δ 1.40 (s, 9H), 2.35 (s, 3H), 3.83−3.87 (d, 2H), 4.46 (s, 2H), 6.94−7.13 (m, 4H).
TFA·H-Gly-NH-Bzl(pMe)
Boc-Gly-NH-Bzl(pMe) was treated with TFA as reported for TFA·H-Gly-NH-Bzl(pCl): yield 0.57 g (91%); Rf(A) 0.43; HPLC K′ 3.74; mp 133−135 °C; m/z 179 (M + H)+.
Boc-Tic-Gly-NH-Bzl(pMe)
This intermediate was obtained by condensation of Boc-Tic-OH with TFA·H-Gly-NH-Bzl(pMe) via WSC/HOBt as reported for Boc-d-Tic-Gly-NH-Bzl: yield 0.46 g (87%); Rf(B) 0.84; HPLC K′ 11.23; mp 133−135 °C; [α]D20 −28.4; m/z 439 (M + H)+; 1H NMR (DMSO-d6) δ 1.38−1.42 (d, 9H), 2.35 (s, 3H), 2.92−3.17 (m, 2H), 4.09−4.27 (m, 4H), 4.46−4.92 (m, 3H), 6.94−7.02 (m, 8H).
TFA·H-Tic-Gly-NH-Bzl(pMe)
Boc-Tic-Gly-NH-Bzl(pMe) was treated with TFA as reported for TFA·H-d-Tic-Gly-NH-Bzl: yield 0.27 g (93%); Rf(A) 0.47; HPLC K′ 6.62; mp 163−165 °C; [α]D20 −26.8; m/z 338 (M + H)+.
Boc-Dmt-Tic-Gly-NH-Bzl(pMe)
This intermediate was obtained by condensation of Boc-Dmt-OH with TFA·H-Tic-Gly-NH-Bzl(pMe) via WSC/HOBt as reported for Boc-Dmt-d-Tic-Gly-NH-Bzl: yield 0.11 g (87%); Rf(B) 0.72; HPLC K′ 9.95; mp 149−151 °C; [α]D20 −18.3; m/z 630 (M + H)+; 1H NMR (DMSO-d6) δ 1.38−1.42 (d, 9H), 2.34 (s, 6H), 2.38 (s, 3H), 2.92−3.17 (m, 4H), 4.09−4.51 (m, 6H), 4.90−4.94 (m, 2H), 6.29 (s, 2H), 6.94−7.02 (m, 8H).
TFA·H-Dmt-Tic-Gly-NH-Bzl(pMe) (4)
Boc-Dmt-Tic-Gly-NH-Bzl(pMe) was treated with TFA as reported for TFA·H-Dmt-d-Tic-Gly-NH-Bzl: yield 0.05 g (93%); Rf(A) 0.50; HPLC K′ 5.42; mp 160−162 °C; [α]D20 −17.5; m/z 530 (M + H)+; 1H NMR (DMSO-d6) δ 2.33 (s, 6H), 2.37 (s, 3H), 2.92−3.17 (m, 4H), 3.95−4.09 (m, 3H), 4.41−4.51 (m, 4H), 4.91−4.93 (m, 1H), 6.29 (s, 2H), 6.94−7.02 (m, 8H). Anal. C33H37F3N4O6: C; H; N.
Boc-Gly-NH-Bzl(pNO2) (32)
This intermediate was obtained by condensation of Boc-Gly-OH with 4-nitrolbenzylamine via mixed carbonic anhydride as reported for Boc-Gly-NH-Bzl(pCl): yield 0.96 g (88%); Rf(B) 0.35; HPLC K′ 4.08; mp 117−119 °C; m/z 310 (M + H)+; 1H NMR (DMSO-d6) δ 1.40 (s, 9H), 3.83−3.87 (d, 2H), 4.46 (s, 2H), 7.32−8.07 (m, 4H).
TFA·H-Gly-NH-Bzl(pNO2)
Boc-Gly-NH-Bzl(pNO2) was treated with TFA as reported for TFA·H-Gly-NH-Bzl(pCl): yield 0.85 g (94%); Rf(A) 0.33; HPLC K′ 3.48; mp 134−136 °C; m/z 210 (M + H)+.
Boc-Tic-Gly-NH-Bzl(pNO2)
This intermediate was obtained by condensation of Boc-Tic-OH with TFA·H-Gly-NH-Bzl(pNO2) via WSC/HOBt as reported for Boc-d-Tic-Gly-NH-Bzl: yield 0.73 g (87%); Rf(B) 0.75; HPLC K′ 9.97; mp 136−138 °C; [α]D20 −24.6; m/z 469 (M + H)+; 1H NMR (DMSO-d6) δ 1.38−1.42 (d, 9H), 2.92−3.17 (m, 2H), 4.09−4.27 (m, 4H), 4.46−4.92 (m, 3H), 6.96−8.07 (m, 8H).
TFA·H-Tic-Gly-NH-Bzl(pNO2)
Boc-Tic-Gly-NH-Bzl(pNO2) was treated with TFA as reported for TFA·H-d-Tic-Gly-NH-Bzl: yield 0.61 g (93%); Rf(A) 0.39; HPLC K′ 6.36; mp 161−163 °C; [α]D20 −23.4; m/z 369 (M + H)+.
Boc-Dmt-Tic-Gly-NH-Bzl(pNO2)
This intermediate was obtained by condensation of Boc-Dmt-OH with TFA·H-Tic-Gly-NH-Bzl(pNO2) via WSC/HOBt as reported for Boc-Dmt-d-Tic-Gly-NH-Bzl: yield 0.34 g (85%); Rf(B) 0.62; HPLC K′ 8.85; mp 153−155 °C; [α]D20 −16.6; m/z 661 (M + H)+; 1H NMR (DMSO-d6) δ 1.38−1.42 (d, 9H), 2.35 (s, 6H), 2.92−3.17 (m, 4H), 4.09−4.51 (m, 6H), 4.90−4.94 (m, 2H), 6.29 (s, 2H), 6.96−8.07 (m, 8H).
TFA·H-Dmt-Tic-Gly-NH-Bzl(pNO2) (5)
Boc-Dmt-Tic-Gly-NH-Bzl(pNO2) was treated with TFA as reported for TFA·H-Dmt-d-Tic-Gly-NH-Bzl: yield 0.25 g (95%); Rf(A) 0.38; HPLC K′ 5.03; mp 161−163 °C; [α]D20 −13.3; m/z 561 (M + H)+; 1H NMR (DMSO-d6) δ 2.35 (s, 6H), 2.92−3.17 (m, 4H), 3.95−4.09 (m, 3H), 4.41−4.51 (m, 4H), 4.91−4.93 (m, 1H), 6.29 (s, 2H), 6.96−8.07 (m, 8H). Anal. C32H34F3N5O8: C; H; N.
2TFA·H-Dmt-Tic-Gly-NH-Bzl(pNH2) (6)
To a solution of TFA·H-Dmt-Tic-Gly-NH-Bzl(pNO2) (0.12 g, 0.18 mmol) in methanol (30 mL) was added Pd/C (10%, 0.07 g), and H2 was bubbled for 1 h at room temperature. After filtration, the solution was evaporated to dryness. The residue was crystallized from Et2O/Pe (1:1, v/v): yield 0.12 g (90%); Rf(A) 0.34; HPLC K′ 4.82; mp 169−171 °C; [α]D20 −15.1; m/z 531 (M + H)+; 1H NMR (DMSO-d6) δ 2.35 (s, 6H), 2.92−3.17 (m, 4H), 3.95−4.09 (m, 3H), 4.41−4.51 (m, 4H), 4.91−4.93 (m, 1H), 6.29 (s, 2H), 6.34−7.02 (m, 8H). Anal. C34H37F6N5O8: C; H; N.
tert-Butyl (Benzhydrylcarbamoyl)methylcarbamate [Boc-Gly-NH-Benzhydryl]
This intermediate was obtained by condensation of Boc-Gly-OH with benzhydrylamine via mixed carbonic anhydride as reported for Boc-Gly-NH-Bzl(pCl): yield 0.68 g (85%); Rf(B) 0.58; HPLC K′ 5.51; mp 127−129 °C; m/z 341 (M + H)+; 1H NMR (DMSO-d6) δ 1.40 (s, 9H), 3.83−3.87 (d, 2H), 6.16 (s, 1H), 7.06−7.14 (m, 10H).
TFA·H-Gly-NH-Benzhydryl
Boc-Gly-NH-benzhydryl was treated with TFA as reported for TFA·H-Gly-NH-Bzl(pCl): yield 0.62 g (94%); Rf(A) 0.45; HPLC K′ 4.21; mp 135−137 °C; m/z 241 (M + H)+.
Boc-Tic-Gly-NH-Benzhydryl
This intermediate was obtained by condensation of Boc-Tic-OH with TFA·H-Gly-NH-Benzhydryl via WSC/HOBt as reported for Boc-d-Tic-Gly-NH-Bzl: yield 0.5 g (86%); Rf(B) 0.85; HPLC K′ 11.24; mp 136−138 °C; [α]D20 −20.6; m/z 501 (M + H)+; 1H NMR (DMSO-d6) δ 1.38−1.42 (d, 9H), 2.92−3.17 (m, 2H), 4.09−4.27 (m, 4H), 4.90−4.94 (m, 1H), 6.16 (s, 1H), 7.06−7.14 (m, 10H).
TFA·H-Tic-Gly-NH-Benzhydryl
Boc-Tic-Gly-NH-Benzhydryl was treated with TFA as reported for TFA·H-d-Tic-Gly-NH-Bzl: yield 0.35 g (92%); Rf(A) 0.52; HPLC K′ 7.03; mp 161−163 °C; [α]D20 −21.2; m/z 400 (M + H)+.
Boc-Dmt-Tic-Gly-NH-Benzhydryl
This intermediate was obtained by condensation of Boc-Dmt-OH with TFA·H-Tic-Gly-NH-Benzhydryl via WSC/HOBt as reported for Boc-Dmt-d-Tic-Gly-NH-Bzl: yield 0.15 g (83%); Rf(B) 0.78; HPLC K′ 10.05; mp 149−151 °C; [α]D20 −16.3; m/z 691 (M + H)+; 1H NMR (DMSO-d6) δ 1.38−1.42 (d, 9H), 2.35 (s, 6H), 2.92−3.17 (m, 4H), 4.09−4.51 (m, 4H), 4.90−4.94 (m, 2H), 6.16 (s, 1H), 6.29 (s, 2H), 6.96−7.14 (m, 14H).
TFA·H-Dmt-Tic-Gly-NH-Benzhydryl (7)
Boc-Dmt-Tic-Gly-NH-Benzhydryl was treated with TFA as reported for TFA·H-Dmt-d-Tic-Gly-NH-Bzl: yield 0.08 g (94%); Rf(A) 0.53; HPLC K′ 5.51; mp 161−163 °C; [α]D20 −15.5; m/z 592 (M + H)+; 1H NMR (DMSO-d6) δ 2.35 (s, 6H), 2.92−3.17 (m, 4H), 3.95−4.09 (m, 3H), 4.41−4.51 (m, 2H), 4.91−4.93 (m, 1H), 6.16 (s, 1H), 6.29 (s, 2H), 6.96−7.14 (m, 4H). Anal. C38H39F3N4O6: C; H; N.
tert-Butyl 2-(3,4-Dihydroisoquinolin-2(1H)-yl)-2-oxoethylcarbamate [Boc-Gly-1,2,3,4-tetrahydroisoquinoline] (33)
This intermediate was obtained by condensation of Boc-Gly-OH with 1,2,3,4-tetrahydroisoquinoline via mixed carbonic anhydride as reported for Boc-Gly-NH-Bzl(pCl): yield 0.63 g (83%); Rf(B) 0.51; HPLC K′ 5.24; mp 125−127 °C; m/z 291 (M + H)+; 1H NMR (DMSO-d6) δ 1.40 (s, 9H), 2.79−2.83 (m, 2H), 3.53−3.85 (m, 4H), 4.44−4.48 (m, 2H), 6.96−7.02 (m, 4H).
TFA·H-Gly-1,2,3,4-tetrahydroisoquinoline
Boc-Gly-1,2,3,4-tetrahydroisoquinoline was treated with TFA as reported for TFA·H-Gly-NH-Bzl(pCl): yield 0.54 g (93%); Rf(A) 0.42; HPLC K′ 4.14; mp 130−132 °C; m/z 191 (M + H)+.
Boc-Tic-Gly-1,2,3,4-tetrahydroisoquinoline
This intermediate was obtained by condensation of Boc-Tic-OH with TFA·H-Gly-1,2,3,4-tetrahydroisoquinoline via WSC/HOBt as reported for Boc-d-Tic-Gly-NH-Bzl: yield 0.46 g (88%); Rf(B) 0.82; HPLC K′ 11.00; mp 132−134 °C; [α]D20 −18.6; m/z 451 (M + H)+; 1H NMR (DMSO-d6) δ 1.38−1.42 (d, 9H), 2.81−3.17 (m, 4H), 3.51−3.55 (m, 2H), 4.09−4.27 (m, 4H), 4.44−4.48 (m, 2H), 4.90−4.94 (m, 1H), 6.96−7.02 (m, 8H).
TFA·H-Tic-Gly-1,2,3,4-tetrahydroisoquinoline
Boc-Tic-Gly-1,2,3,4-tetrahydroisoquinoline was treated with TFA as reported for TFA·H-d-Tic-Gly-NH-Bzl: yield 0.31 g (92%); Rf(A) 0.46; HPLC K′ 6.91; mp 153−155 °C; [α]D20 −19.3; m/z 350 (M + H)+.
Boc-Dmt-Tic-Gly-1,2,3,4-tetrahydroisoquinoline
This intermediate was obtained by condensation of Boc-Dmt-OH with TFA·H-Tic-Gly-1,2,3,4-tetrahydroisoquinoline via WSC/HOBt as reported for Boc-Dmt-d-Tic-Gly-NH-Bzl: yield 0.12 g (82%); Rf(B) 0.74; HPLC K′ 9.86; mp 140−142 °C; [α]D20 −17.8; m/z 642 (M + H)+; 1H NMR (DMSO-d6) δ 1.38−1.42 (d, 9H), 2.35 (s, 6H), 2.81−3.17 (m, 6H), 3.53 (m, 2H), 4.09−4.51 (m, 6H), 4.90−4.94 (m, 2H), 6.29 (s, 2H), 6.96−7.02 (m, 8H).
TFA·H-Dmt-Tic-Gly-1,2,3,4-tetrahydroisoquinoline (8)
Boc-Dmt-Tic-Gly-1,2,3,4-tetrahydroisoquinoline was treated with TFA as reported for TFA·H-Dmt-d-Tic-Gly-NH-Bzl: yield 0.06 g (92%); Rf(A) 0.49; HPLC K′ 5.26; mp 157−159 °C; [α]D20 −16.9; m/z 542 (M + H)+; 1H NMR (DMSO-d6) δ 2.35 (s, 6H), 2.81−3.17 (m, 6H), 3.53 (m, 2H), 3.95−4.51 (m, 7H), 4.90−4.94 (m, 1H), 6.29 (s, 2H), 6.96−7.02 (m, 8H). Anal. C34H37F3N4O6: C; H; N.
Pharmacology
Radiolabeled Ligand Binding Assays
Binding assays used to screen compounds are similar to those previously reported (34). Membrane protein from CHO cells that stably expressed one type of the human opioid receptor were incubated with 12 different concentrations of the compound in the presence of either 1 nM [3H]U69,593 (35) (κ), 0.25 nM [3H]DAMGO (36) (μ), or 0.2 nM [3H]naltrindole (37) (δ) in a final volume of 1 mL of 50 mM Tris-HCl, pH 7.5, at 25 °C. Incubation times of 60 min were used for [3H]U69,593 and [3H]DAMGO. Because of a slower association of [3H]naltrindole with the receptor, a 3 h incubation was used with this radioligand. Samples incubated with [3H]naltrindole also contained 10 mM MgCl2 and 0.5 mM phenylmethylsulfonyl fluoride. Nonspecific binding was measured by inclusion of 10 μM naloxone. The binding was terminated by filtering the samples through Schleicher & Schuell no. 32 glass fiber filters using a Brandel 48-well cell harvester. The filters were subsequently washed three times with 3 mL of cold 50 mM Tris-HCl, pH 7.5, and were counted in 2 mL of ScintiSafe 30% scintillation fluid. For [3H]naltrindole and [3H]U69,593 binding, the filters were soaked in 0.1% polyethylenimine for at least 60 min before use. IC50 values were calculated by least-squares fit to a logarithm-probit analysis. Ki values of unlabeled compounds were calculated from the equation Ki = (IC50)/(1 + S) where S = (concentration of radioligand)/(Kd of radioligand) (38). Data are the mean ± SEM from at least three experiments performed in triplicate.
[35S]GTPγS Binding Assays
In a final volume of 0.5 mL, 12 different concentrations of each test compound were incubated with 10 μg (δ) or 7.5 μg (μ) of CHO cell membranes that stably expressed either the human δ or μ opioid receptor. The assay buffer consisted of 50 mM Tris-HCl, pH 7.4, 3 mM MgCl2, 0.2 mM EGTA, 3 μM GDP, and 100 mM NaCl. The final concentration of [35S]GTPγS was 0.080 nM. Nonspecific binding was measured by inclusion of 10 μM GTPγS. Binding was initiated by the addition of the membranes. After an incubation of 60 min at 30 °C, the samples were filtered through Schleicher & Schuell no. 32 glass fiber filters. The filters were washed three times with cold 50 mM Tris-HCl, pH 7.5, and were counted in 2 mL of Ecoscint scintillation fluid. Data are the mean Emax and EC50 values ± SEM from at least three separate experiments, performed in triplicate. For calculation of the Emax values, the basal [35S]GTPγS binding was set at 0%. To determine antagonist activity of a compound at the μ opioid receptors, CHO membranes expressing the μ opioid receptor were incubated with 12 different concentrations of the compound in the presence of 200 nM of the μ agonist DAMGO. To determine whether a compound was an antagonist at δ receptors, CHO membranes expressing the δ receptor were incubated with 12 different concentrations of the test compound in the presence of 10 nM of the δ-selective agonist SNC 80.
Abbreviations
In addition to those of the IUPAC-IUB Commission on Biochemical Nomenclature (J. Biol. Chem. 1985, 1, 14−42), this paper uses the following additional symbols and abbreviations: AcOEt, ethyl acetate; Boc, tert-butyloxycarbonyl; Bzl, benzyl; DAMGO, [d-Ala2,N-Me-Phe4,Gly-ol5]enkephalin; DMF, N,N-dimethylformamide; DMSO-d6, hexadeuteriodimethyl sulfoxide; Dmt, 2′,6′-dimethyl-l-tyrosine; Et2O, diethyl ether; GPI, guinea pig ileum; HOBt, 1-hydroxybenzotriazole; HPLC, high-performance liquid chromatography; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight; MVD, mouse vas deferens; NMM, 4-methylmorpholine; pA2, negative log of the molar concentration required to double the agonist concentration to achieve the original response; Pe, petroleum ether; Ph, phenyl; TFA, trifluoroacetic acid; Tic, 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid; TLC, thin-layer chromatography; WSC, 1-ethyl-3-[(3′-dimethyl)aminopropyl]-carbodiimide hydrochloride.
G.B., S.S., and J.L.N. designed research; C.T. and X.P. synthesized the compounds; B.I.K., J.M.B., and L.H.L. generated and analyzed the pharmacological data; G.B. and J.L.N. wrote the paper.
This work was supported in part by NIH Grants RO1-DA14251 (to J.L.N.) and K05-DA 00360 (to J.M.B.), University of Cagliari (to G.B.), University of Ferrara (to S.S.), and the Intramural Research Program of the NIH and NIEHS (to L.H.L.).
Funding Statement
National Institutes of Health, United States
References
- Schiller P. W. (2009) Bi- or multifunctional opioid peptide drugs. Life Sci. Mar 11 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Zhang J.; Zhang A. (2009) Design of multivalent ligand targeting G-protein-coupled receptors. Curr. Pharm. Des. 15, 682–718. [DOI] [PubMed] [Google Scholar]
- a Lambert D. G. (2008) The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat. Rev. Drug Discovery 7, 694–710. [DOI] [PubMed] [Google Scholar]; b Dietis N.; Guerrini R.; Calò G.; Salvadori S.; Rowbotham D. J.; Lambert D. G. (2009) Simultaneous targeting of multiple opioid receptors: a strategy to improve side-effect profile. Br. J. Anaesth. 103, 38–43. [DOI] [PubMed] [Google Scholar]
- Balboni G.; Guerrini R.; Salvadori S.; Bianchi C.; Rizzi D.; Bryant S. D.; Lazarus L. H. (2002) Evaluation of the Dmt-Tic pharmacophore: Conversion of a potent δ-opioid receptor antagonist into a potent δ agonist and ligands with mixed properties. J. Med. Chem. 45, 713–720. [DOI] [PubMed] [Google Scholar]
- Abdelhamid E. E.; Sultana M.; Portoghese P. S.; Takemori A. E. (1991) Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J. Pharmacol. Exp. Ther. 258, 299–303. [PubMed] [Google Scholar]
- Fundytus M. E.; Schiller P. W.; Shapiro M.; Weltrowska G.; Coderre T. J. (1995) Attenuation of morphine tolerance and dependence with the highly selective delta-opioid receptor antagonist TIPP[psi]. Eur. J. Pharmacol. 286, 105–108. [DOI] [PubMed] [Google Scholar]
- Hepburn M. J.; Little P. J.; Gingras J.; Kuhn C. M. (1997) Differential effects of naltrindole on morphine-induced tolerance and physical dependence in rats. J. Pharmacol. Exp. Ther. 281, 1350–1356. [PubMed] [Google Scholar]
- Rothman R. B.; Danks J. A.; Jacobson A. E.; Burke T. R. Jr.; Rice K. C.; Tortella F. C.; Holaday J. W. (1986) Morphine tolerance increases mu-noncompetitive delta binding sites. Eur. J. Pharmacol. 124, 113–119. [DOI] [PubMed] [Google Scholar]
- Yukhananov R. Y.; Klodt P. M.; Il’Ina A. D.; Zaitsev S. V.; Maisky A. I. (1994) Opiate withdrawal intensity correlates with the presence of DSLET high-affinity binding. Pharmacol., Biochem. Behav. 49, 1109–1112. [DOI] [PubMed] [Google Scholar]
- Kest B.; Lee C. E.; McLemore G. L.; Inturrisi C. E. (1996) An antisense oligodeoxynucleotide to the delta opioid receptor (DOR-1) inhibits morphine tolerance and acute dependence in mice. Brain Res. Bull. 39, 185–188. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; King M. A.; Schuller A. G.; Nitsche J. F.; Reidl M.; Elde R. P.; Unterwald E.; Pasternak G. W.; Pintar J. E. (1999) Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron 24, 243–252. [DOI] [PubMed] [Google Scholar]
- Foxx-Orenstein A. E.; Jin J. G.; Grider J. R. (1998) 5-HT4 receptor agonists and delta-opioid receptor antagonists act synergistically to stimulate colonic propulsion. Am. J. Physiol. 275, G979–G983. [DOI] [PubMed] [Google Scholar]
- Freye E.; Latasch L.; Portoghese P. S. (1992) The delta receptor is involved in sufentanil-induced respiratory depression—opioid subreceptors mediate different effects. Eur. J. Anaesthesiol. 9, 457–462. [PubMed] [Google Scholar]
- Schiller P. W.; Weltrowska G.; Berezowska I.; Nguyen T. M.; Wilkes B. C.; Lemieux C.; Chung N. N. (1999) The TIPP opioid peptide family: development of delta antagonists, delta agonists, and mixed mu agonist/delta antagonists. Biopolymers (Pept. Sci.) 51, 411–425. [DOI] [PubMed] [Google Scholar]
- Farooqui M.; Li Y.; Poonawala T.; Griffin R. J.; Song C. W.; Gupta K. (2007) COX-2 inhibitor celecoxib prevents chronic morphine-induced promotion of angiogenesis, tumor growth, metastasis and mortality, without compromising analgesia. Br. J. Cancer 97, 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K.; Kshirsagar S.; Chang L.; Schwartz R.; Law P. Y.; Yee D.; Hebbel R. P. (2002) Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 62, 4491–4498. [PubMed] [Google Scholar]
- Poonawala T.; Levay-Young B. K.; Hebbel R. P.; Gupta K. (2005) Opioids heal ischemic wounds in the rat. Wound Repair Regen. 13, 165–174. [DOI] [PubMed] [Google Scholar]
- Singleton P. A.; Lingen M. W.; Fekete M. J.; Garcia J. G.; Moss J. (2006) Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: role of receptor transactivation. Microvasc. Res. 72, 3–11. [DOI] [PubMed] [Google Scholar]
- Zagon I. S.; McLaughlin P. J. (1983) Naltrexone modulates tumor response in mice with neuroblastoma. Science 221, 671–673. [DOI] [PubMed] [Google Scholar]
- Koo K. L.; Tejwani G. A.; Abou-Issa H. (1996) Relative efficacy of the opioid antagonist, naltrexone, on the initiation and promotion phases of rat mammary carcinogenesis. Anticancer Res. 16, 1893–1898. [PubMed] [Google Scholar]
- Balboni G.; Salvadori S.; Guerrini R.; Negri L.; Giannini E.; Jinsmaa Y.; Bryant S. D.; Lazarus L. H. (2002) Potent δ-opioid receptor agonists containing the Dmt-Tic pharmacophore. J. Med. Chem. 45, 5556–5563. [DOI] [PubMed] [Google Scholar]
- Schiller P. W.; Weltrowska G.; Schmidt R.; Berezowska I.; Nguyen T. M.; Lemieux C.; Chung N. N.; Carpenter K. A.; Wilkes B. C. (1999) Subtleties of structure-agonist versus antagonist relationships of opioid peptides and peptidomimetics. J. Recept. Signal Transduct. Res. 19, 573–588. [DOI] [PubMed] [Google Scholar]
- Schiller P. W.; Nguyen T. M. −D.; Weltrowska G.; Wilkes B. C.; Marsden B. J.; Lemieux C.; Chung N. N. (1992) Differential stereochemical requirements of μ vs. δ opioid receptors for ligand binding and signal transduction: Development of a class of potent and highly δ-selective peptide antagonists. Proc. Natl. Acad. Sci. U.S.A. 89, 11871–11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temussi P. A.; Salvadori S.; Amodeo P.; Bianchi C.; Guerrini R.; Tomatis R.; Lazarus L. H.; Picone D.; Tancredi T. (1994) Selective opioid dipeptides. Biochem. Biophys. Res. Commun. 198, 933–939. [DOI] [PubMed] [Google Scholar]
- Salvadori S.; Attila M.; Balboni G.; Bianchi C.; Bryant S. D.; Crescenzi O.; Guerrini R.; Picone D.; Tancredi T.; Temussi P. A.; Lazarus L. H. (1995) δ opioidmimetic antagonists: prototypes for designing a new generation of ultraselective opioid peptides. Mol. Med. 1, 678–689. [PMC free article] [PubMed] [Google Scholar]
- Balboni G.; Onnis V.; Congiu C.; Zotti M.; Sasaki Y.; Ambo A.; Bryant S. D.; Jinsmaa Y.; Lazarus L. H.; Trapella C.; Salvadori S. (2006) Effect of lysine at C-terminus of the Dmt-Tic opioid pharmacophore. J. Med. Chem. 49, 5610–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Van Bockstaele E. J.; Liu-Chen L. Y. (2008) In vivo trafficking of endogenous opioid receptors. Life Sci. 83, 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Song B.; Lao L.; Pérez O. A.; Kim W.; Marvizón J. C. (2007) Comparing analgesia and μ-opioid receptors internalization produced by intrathecal enkephalin: requirement for peptidase inhibition. Neuropharmacology 53, 664–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. J.; Tao Y. M.; Li F. Y.; Wang Y. H.; Xu X. J.; Chen J.; Cao Y. L.; Chi Z. Q.; Neumeyer J. L.; Zhang A.; Liu J. G. (2009) Pharmacological characterization of ATPM [(-)3-aminothiazolo[5,4-b]-N-cyclopropylmethylmorphinan hydrochloride], a novel mixed κ-agonist and μ-agonist/-antagonist that attenuates morphine antinociceptive tolerance and heroin self-administration behaviour. J. Pharmacol. Exp. Ther. 329, 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus S. S.; Bear A. E.; Folk J. E.; Rice K. C. (2009) Role of delta opioid efficacy as a determinant of mu/delta opioid interactions in rhesus monkeys. Eur. J. Pharmacol. 602, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournié-Zaluski M. C.; Coulaud A.; Bouboutou B.; Chaillet P.; Devin J.; Waksman G.; Costentin J.; Roques B. P. (1985) New bidentates as full inhibitors of enkephalin-degrading enzymes: synthesis and analgesic properties. J. Med. Chem. 28, 1158–1169. [DOI] [PubMed] [Google Scholar]
- Castillo M. J.; Kurachi K.; Nishino N.; Ohkubo I.; Powers J. C. (1983) Reactivity of bovine blood coagulation factor IXaβ, factor Xaβ, and factor XIa toward fluorogenic peptides containing the activation site sequences of bovine factor IX and factor X. Biochemistry 22, 1021–1029. [DOI] [PubMed] [Google Scholar]
- Tsu H.; Chen X.; Chen C. T.; Lee S. J.; Chang C. N.; Kao K. H.; Coumar M. S.; Yeh Y. T.; Chien C. H.; Wang H. S.; Lin K. T.; Chang Y. Y.; Wu S. H.; Chen Y. S.; Lu I. L.; Wu S. Y.; Tsai T. Y.; Chen W. C.; Hsieh H. P.; Chao Y. S.; Jiaang W. T. (2006) 2-[3-[2-[(2S)-2-Cyano-1-pyrrolidinyl]-2-oxoethylamino]-3-methyl-1-oxobutyl]-1,2,3,4-tetrahydroisoquinoline: A potent, selective, and orally bioavailable dipeptide-derived inhibitor of dipeptidyl peptidase IV. J. Med. Chem. 49, 373–380. [DOI] [PubMed] [Google Scholar]
- Neumeyer J. L.; Zhang A.; Xiong W.; Gu X.; Hilbert J. E.; Knapp B. I.; Negus S. S.; Mello N. K.; Bidlack J. M. (2003) Design and Synthesis of Novel Dimeric Morphinan Ligands for κ and μ Opioid Receptors. J. Med. Chem. 46, 5162. [DOI] [PubMed] [Google Scholar]
- Xia Q.; Tai K. K.; Wong T. M. (1992) Chronic morphine treatment increases the number, but decreases the affinity of [3H]-U 69593 binding sites in the rat heart. Life Sci. 50, 1143–8. [DOI] [PubMed] [Google Scholar]
- Pivovarchik M. V.; Grinevich V. P. (2000) The binding of the opioid receptors of the rat brain to specific ligands under acute alcohol intoxication. Vestsi Nats. Akad. Navuk Belarusi, Ser. Biyal. Navuk 72–74. [Google Scholar]
- Dorn C. R.; Markos C. S.; Dappen M. S.; Pitzele B. S. (1992) Synthesis of tritium-labeled naltrindole. J. Labelled Compd. Radiopharm. 31, 375–80. [Google Scholar]
- Cheng Y. C.; Prusoff W. H. (1973) Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108. [DOI] [PubMed] [Google Scholar]