Abstract
Melatonin is a hormone secreted from the pineal gland specifically at night and contributes to a wide array of physiological functions in mammals. Melatonin is one of the most well understood output of the circadian clock located in the suprachiasmatic nucleus. Melatonin synthesis is controlled distally via the circadian clock located in the suprachiasmatic nucleus and proximally regulated by norepinephrine released in response to the circadian clock signals. To understand melatonin synthesis in vivo, we have performed microdialysis analysis of the pineal gland, which monitors melatonin as well as the precursor (serotonin) and intermediate (N-acetylserotonin) of melatonin synthesis in freely moving animals in realtime at high resolution. Our data revealed a number of novel features of melatonin production undetected using conventional techniques, which include (1) large inter-individual variations of melatonin onset timing; (2) circadian regulation of serotonin synthesis and secretion in the pineal gland; and (3) a revised view on the rate-limiting step of melatonin formation in vivo. This article will summarize the main findings from our laboratory regarding melatonin formation in mammals.
Keywords: Melatonin, Serotonin (5-HT), Pineal gland, Microdialysis
1 Introduction
The pineal gland synthesizes and secretes melatonin with a dramatic circadian rhythm. Precise daily regulation of the melatonin biosynthetic pathway results in rhythmic secretion patterns conserved across vertebrate species [1, 2]. Melatonin plays an important role in a multitude of physiological functions [3, 4] and may be involved in human disorders including autism [5], cancer [6], and diabetes [7]. Thus, it is important to understand how melatonin synthesis is regulated both in vivo and in vitro.
Melatonin synthetic mechanisms have been studied in a number of laboratories over the years and are summarized in several excellent review articles published recently. Simonneaux and Ribelayga [2] offer a comprehensive review of pineal physiology and melatonin formation up to 2003. A concise review of arylalkylamine N-acetyltransferase (AANAT), the key enzyme in melatonin formation, can be found in Klein [8]. Maronde and Stehle [9] outline the potential role of a number of transcription factors controlling melatonin synthesis, and Falcon et al. [10] offer a review of the structural and functional evolution of pineal melatonin synthesis. In this paper, we will complement the existing reviews in the field by summarizing in vivo studies of melatonin production regulation, focusing on experiments conducted in our laboratory using pineal microdialysis.
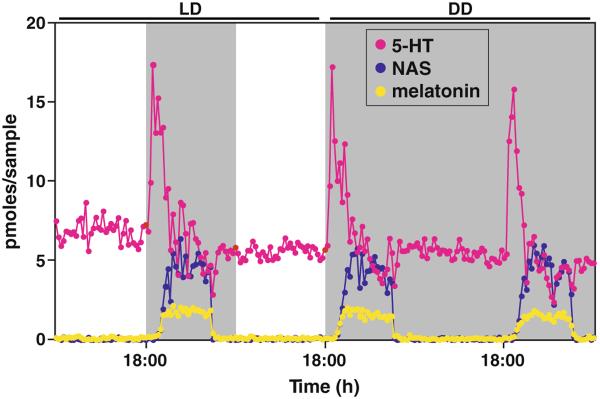
Melatonin synthesis commences with the active uptake of amino acid tryptophan into the pineal gland. Tryptophan is subsequently hydroxylated and decarboxylated to serotonin (5-hydroxytryptamine, 5-HT). 5-HT is N-acetylated by serotonin N-acetyltransferase (or Arylalkylamine N-acetyltransferase, AANAT) to form N-acetylserotonin (NAS), which is converted to melatonin (N-acetyl-5-methoxytryptamine) by the enzyme hydroxyindole-O-methyltransferase (HIOMT) [1]. The three components of the melatonin synthetic pathway, namely 5-HT, NAS, and melatonin, all display dramatic circadian rhythms in nocturnal rodents (Fig. 1). Such rhythms persist in constant darkness, indicating that a circadian clock drives the pineal secretion of these indoles.
Fig. 1.
Secretion profiles of 5-HT, NAS and melatonin from pineal microdialysis of a rat. All three compounds display marked circadian rhythms in both light and dark (LD) and constant dark (DD) conditions with nocturnal increase in secretion
2 Timing of melatonin secretion
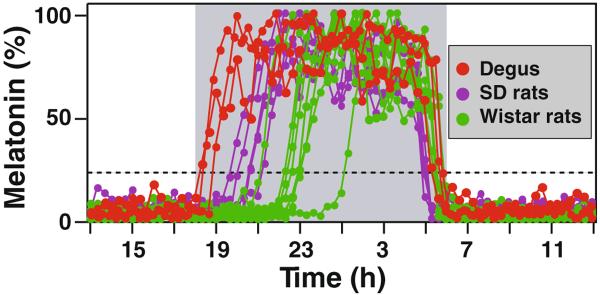
A large inter-individual variation in timing of melatonin secretion exists between animals in the same strain and between different species [11]. Recent analysis of degu pineal gland indicates that melatonin onset is much earlier in this species than in rats (Fig. 2) or hamsters [11]. Indeed, in animals entrained in light and dark cycle of 12h, melatonin onset timing ranges from 0.2 to 6.5 hrs after the onset of darkness. The variable timing of melatonin secretion within the same species of rats is not due to an altered regulation of melatonin synthesis, since exogenously applied activators of beta-adrenergic receptors elicit melatonin production in these animals with identical kinetics [11]. The timing of melatonin increase is tightly associated with the timing of 5-HT surge [11]. It remains to be determined if the timing of 5-HT and melatonin release strictly correlates with the timing of norepinephrine release from the superior cervical ganglion. In human studies, the onset timing of melatonin secretion was shown to be associated with the circadian period length [12]; however, a similar association was not found in rats (data not published).
Fig. 2.
Inter-individual and inter-species variation of melatonin onset timing. Three Degus (red dots), 5 Sprague Dawley rats (purple dots), and 7 Wistar rats (green dots) are shown. The onset timing is defined as when melatonin reaches 20% of daily maximum level, which is marked by the dashed line
In contrast to the variable timing of melatonin onset, the decline of melatonin secretion at the end of each night occurs with little individual variations. When animals are entrained, the decline of melatonin release always precedes the lights-on by about one to two hours, indicating a circadian control of both onset and offset of melatonin production [13].
3 Circadian regulation of 5-HT, the melatonin precursor
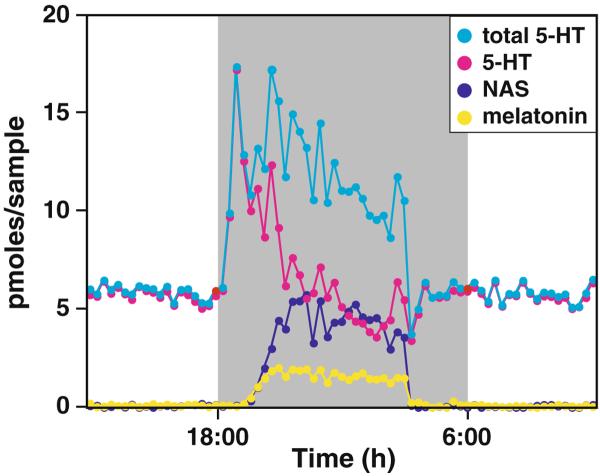
One of our initial findings using the pineal microdialysis technique was the novel discovery that 5-HT secretion level surges early in the night in all rodents examined (Fig. 1) [11, 14, 15]. In fact, the minimum level of 5-HT production as reflected by the sum of the extracellular 5-HT, NAS, and melatonin concentrations is far greater than the daytime 5-HT level (Fig. 3)[15]. Thus, in contrast to the common notion of 5-HT being produced constitutively, such results indicate that 5-HT synthesis increases at night through active diurnal regulation. The daily increase in 5-HT production is independent of melatonin secretion, but is dependent on the adrenergic signaling at night [14]. By blocking melatonin formation, the total content and secretion of 5-HT increases at night since 5-HT is not converted to NAS [14]. The nighttime increase of 5-HT synthesis is mediated by the increased beta-adrenergic and cAMP signals [15]; however, elevated 5-HT secretion is independent of beta-adrenergic receptors [14]. The increased 5-HT synthesis may be necessary to meet the demand of nocturnal NAS and melatonin production, and to compensate for the loss of intracellular 5-HT from increased secretion at night.
Fig. 3.
The total 5-HT output displays marked circadian rhythm with high levels at night. The total 5-HT is calculated as the sum of 5-HT, NAS (N-acetylated 5-HT), and melatonin (final metabolic product of 5-HT in the pineal)
5-HT is synthesized from amino acid tryptophan first by the rate-limiting enzyme tryptophan hydroxylase (TPH), and by the aromatic amino acid decarboxylase (AADC). Recently, we have shown that TPH1, the peripheral isoform of the enzyme, is phosphorylated at serine 58 (S58) residue in the pineal gland at night, and this phosphorylation occurs in parallel with the nocturnal increase in TPH1 protein content [15]. Phosphorylation at the S58 is responsible for cAMP induced increase in TPH1 protein content in vitro. Furthermore, in vivo termination of nocturnal cAMP surge in the pineal gland from nighttime light exposure abolishes the nocturnal increase in TPH1 protein content [15]. These results indicate that the surge in 5-HT synthesis at night is mediated by posttranslational modification of TPH1 protein.
In contrast to the rapid surge in 5-HT output, melatonin and NAS secretion rises with a persistent delay following the 5-HT increase in rats. The interval between the onset of 5-HT and melatonin is remarkably conserved in individuals of the same species regardless of strains, and is about 50 min for rats and 4 hrs for hamsters [11]. The long delay in melatonin surge in hamster pineal gland may indicate an alternative mechanism of Aanat activation or melatonin synthesis in this species [16]. The delay in melatonin rise following the 5-HT surge is presumably due to the time required for transcriptional activation of Aanat mRNA levels [17] in response to the increased cAMP signaling [18]. We reasoned if AANAT activation at night is controlled at posttranscriptional levels, the surge of 5-HT secretion might not be detectable. To test this hypothesis, we have analyzed melatonin synthesis in Octodon degus, a diurnal mammal that belong to the infraorder Hystricognathi and are distinct from the members of Myomorpha suborder that include nocturnal rodents such as laboratory rats, mice, hamsters, and diurnal rodents including grass rats and sand rats. Melatonin release surges early in Octodon degus compared to rats (Fig. 2) and is controlled by posttranscriptional activation of AANAT [19]. As predicted, the early surge of 5-HT at night was not detected in this species (data not shown). These results indicate that 5-HT surge at early night is detectable only when melatonin synthetic rate is slower than that of 5-HT.
Importantly, these data indicate that the nocturnal increase in 5-HT synthesis functions to support maximum levels of melatonin induction in vivo. It remains to be seen if melatonin output is reduced when diurnal rise of 5-HT synthesis is blocked in future studies.
4 The rate-limiting step of melatonin synthesis
In contrast to the consistently high level of 5-HT released during daytime, NAS and melatonin secretions are minimal during daytime and increases dramatically at night, reaching maximum levels within one hour following the onset of darkness in all rats examined. While secretion of 5-HT from the pineal gland is regulated [14], release of NAS and melatonin appears to be constitutive. This suggests that extracellular levels of NAS and melatonin monitored by the microdialysis technique (Fig. 1) are proportional to the synthesis of the two compounds inside the pineal cells.
For many years, AANAT, which converts 5-HT to NAS, has been considered the rate-limiting enzyme of melatonin production, which implies that NAS is present in smaller molar quantity compared to melatonin at night. While this is true for daytime and the initial hour of melatonin synthesis at night (Fig. 3), our data clearly demonstrate that melatonin synthesis is limited by the speed of conversion from NAS to melatonin for the bulk of night, which is mediated by HIOMT activities [20]. This conclusion is based on three lines of evidence: (1) Melatonin levels remained unchanged when AANAT activity was reduced by 10 fold in a mutant strain of rat; (2) the continuous increase in NAT protein levels at late night does not produce a proportional increase in melatonin; and (3) an increase in NAS in the same animal over several circadian cycles do not result in corresponding increase in melatonin output [20]. These results strongly suggest that AANAT is not the rate-limiting enzyme of melatonin formation at night. The predominant feature of AANAT in the pineal gland of most species is its large nocturnal increase in activity that drives the daily rhythm in melatonin secretion, and as such should be considered the “melatonin rhythm-generating enzyme.”
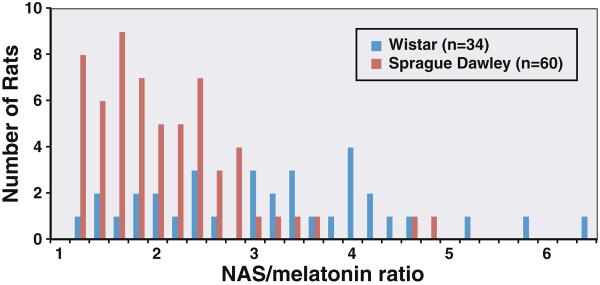
In addition to the three lines of evidence presented, we have also found that NAS, the enzymatic product of AANAT, is almost always present in vast molar excess to melatonin in the pineal at night [20]. To further illustrate this point, we analyzed the ratio of NAS to melatonin from rats of different strains (Fig. 4). For Sprague Dawley rats, majority of the animals display a NAS/melatonin ratio between 1.2–2.4, whereas Wistar rats display a ratio that is more widespread in the 1.2–6.4 range. Longitudinal study of the same animal show that NAS peak levels can vary dramatically from day to day due to parameters such as surgical stress, while melatonin levels rarely display parallel changes unless NAS is decreased to a level lower than melatonin [20]. These studies are consistent with the earlier in vitro finding that levels of NAS released from norepinephrine-stimulated pinealocytes are much higher than that of melatonin [21]. These results suggest that the rate-limiting step in melatonin production of rats is actually the conversion of melatonin from NAS through methylation. In light of the fact that human NAS levels in circulation is also much higher than melatonin levels [22], the conversion of NAS to melatonin may also be the rate-limiting step of melatonin synthesis in humans.
Fig. 4.
NAS is secreted in molar excess of melatonin in all outbred rats tested
While it is clear that the conversion of NAS to form melatonin limits the rate of melatonin synthesis at night, there are also supporting factors for HIOMT as the rate-limiting enzyme of melatonin synthesis. Recent identification of missense mutations in HIOMT from patients who suffer from autism spectrum disorders supports the notion that HIOMT is the rate-limiting enzyme in melatonin formation as these patients display lower levels of melatonin secretion [5]. In addition to HIOMT, methionine adenosyltransferase (MAT) may also be important in determining the rate of melatonin formation. MAT is essential for synthesis of (S)-Adenosylmethionine, which acts as the methyl donor required for HIOMT activity [23]. Future studies are warranted to further investigate the rate-limiting step of melatonin formation in vivo.
5 In vivo stability of AANAT, a key enzyme of melatonin formation
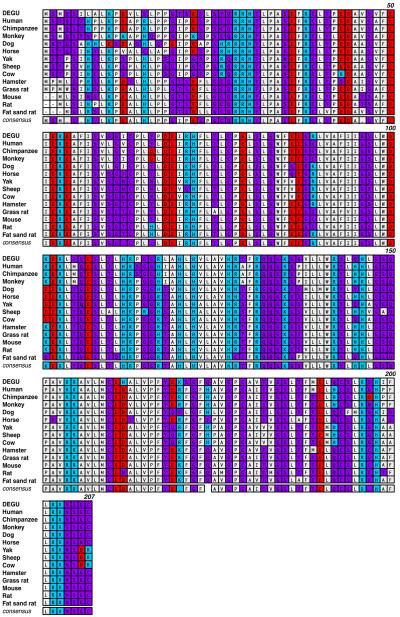
Mammalian AANAT protein is a polypeptide consisting of either 204 (Fat sand rat), 205 (laboratory rats and mice), or 207 (all primates, ungulates, and degus) amino acids (Fig. 5) (also see [24]). The N-termini (amino acids 1–27 in degu AANAT) is the most divergent among all AANATs identified to date (Fig. 5), while the following phosphorylation motif (RRHT31 in degus and RRHT29 in rats) is absolutely conserved. Phosphorylation of the threonine residue leads to 14-3-3 interaction and stabilization of AANAT in vitro [24]. Within this motif, it is rather peculiar that there exists an invariant histidine residue not required for the phosphorylation at threonine to occur. The identification of a natural mutation of the histidine to tyrosine at this position, which exhibits reduced protein stability, informed us of the potential function of this residue in supporting AANAT function in vivo as will be discussed below [25, 26].
Fig. 5.
Amino acid sequence comparison of AANAT from degu and other mammalian species. The sequence comparison was performed using MacVector ClustalW alignment program and was color-coded based on functionality of the amino acids. Acidic residues were marked in red, basic residues in blue, hydrophobic residues in gray, and hydrophilic residues in purple. The numbers indicated on the upper right lines denote the amino acid positions based on the degu AANAT sequence
In our earlier studies we have identified a pineal night-specific ATPase (PINA), a novel splice variant of the ATP7B gene disrupted in Wilson disease [27]. PINA expression exhibits a dramatic diurnal rhythm in both pineal gland and retina with 100-fold greater expression at night than at day. Further study with Long Evans Cinnamon (LEC) rats, which lack PINA in the pineal gland, revealed an additional defect in AANAT [28]. Linkage studies confirm that the AANAT phenotype is entirely independent of PINA mutation in the pineal gland of LEC rats, and sequence analysis demonstrates that AANAT defect is due to a point mutation in AANAT coding region [28]. While analyzing the melatonin synthetic pathways of LEC rats, we discovered that AANAT activity and protein levels are greatly reduced, and that the highly conserved histidine 28 is mutated to tyrosine (H28Y) [25]. To study the effect of H28Y, we isolated a new strain of rat (termed LPN) defective in AANAT but normal for both PINA and coat color. Compared to control rats, the LPN rats display lower NAT protein levels and enzyme activities. Furthermore, when expressed in vitro, AANAT-H28Y is very unstable in both bacteria and mammalian cells, indicating that the H28Y mutation is the cause of reduced AANAT activity and protein levels in vivo [25]. To date, the H28Y mutation is the only missense mutation identified in animals or humans that affect AANAT protein levels.
The H28 residue is conserved in all known AANAT from mammalian species (Fig. 5) and is adjacent to the similarly conserved threonine residue at the 29th position (31st in degus). The T29 residue in the RRH28T29 motif is phosphorylated in vivo and in vitro by PKA and PKC [24, 29]. Upon phosphorylation of AANAT at T29 residue, AANAT interacts with 14-3-3 proteins, which in vitro leads to a stabilization of AANAT protein [24, 26, 30]. Accordingly, mutations that affect either AANAT phosphorylation at the T29 position or a subsequent interaction with 14-3-3 would affect AANAT protein stability. Recent study from our lab clearly demonstrates that the decreased stability of the AANAT-H28Y mutant in vivo is due to inefficient interaction of the H28Y mutant with the 14-3-3, and provides a platform for further understanding of molecular mechanism of AANAT protein degradation [26].
6 A new animal model for in vivo understanding of melatonin formation
While AANAT is the key enzyme in melatonin synthesis, and responsible for the dramatic diurnal rhythms of melatonin secretion in all species analyzed, significant differences exist between the molecular regulations of Aanat in various species. In nocturnal rodents commonly used in laboratory investigations, melatonin production is rate limited by cAMP-dependent transcriptional activation of Aanat [18, 20, 31]. In diurnal mammals, however, posttranscriptional control of AANAT by PKA dominantly regulates melatonin production since Aanat mRNA levels display very little diurnal variation [32–34]. The differential mechanisms of AANAT control result in marked differences in the dynamics of melatonin secretion at night. In nocturnal animals such as rats and hamsters, the onset of melatonin secretion is markedly delayed after dark onset [11]. In contrast, melatonin in humans rapidly surges following dark onset without latency [35]. The rapid release of melatonin following dark onset in non-rodent diurnal mammals and the expression of significant quantities of Aanat mRNA in the day demonstrate that AANAT is posttranscriptionally controlled during the diurnal cycle, likely by cAMP mediated protection of AANAT from proteasome-dependent degradation [36]. To understand melatonin synthesis in humans, it is thus desirable to establish a laboratory animal model with a similar mode of melatonin regulation as in humans.
Recently, we have analyzed melatonin synthesis in Octodon degus, a small diurnal mammal classified in the Hystricognath suborder that is distinct from nocturnal rodents such as all common laboratory rats, mice, and hamsters, and diurnal rodents such as grass rats and fat sand rats [19]. We have found that (1) degu Aanat is constitutively expressed in the pineal gland with little diurnal variation; (2) degu melatonin onset is much earlier than in rats and hamsters, and is similar to the rapid onset of melatonin found in primates and ungulates; (3) degu AANAT protein sequence reads more like those of primates and ungulates than those of the nocturnal rodents (Fig. 5). Combined with the small size of this species, the degu proves to be a valuable laboratory animal model that can be used to understand melatonin synthesis in non-rodent diurnal mammals including humans.
7 Conclusion
Our studies in the past few years have demonstrated that a large inter-individual variation in timing of melatonin secretion exists within rats from the same strain, between rats from different strains, and in animals from different species. Using microdialysis technique, we have also found that 5-HT synthesis and secretion display marked circadian rhythms with high levels at night, which contrasts the traditional notion of constitutive production. Moreover, we have discovered that AANAT is not the rate-liming enzyme of melatonin synthesis at night. Additionally, we have demonstrated that AANAT protein stability depends critically on the integrity of the RRH28T29 motif whose phosphorylation at the T29 residue and its subsequent interaction with 14-3-3 is important for AANAT stability. These studies reveal new features of in vivo melatonin formation, which will guide further understanding of melatonin formation in mammals both in vivo and in vitro.
Acknowledgement
Authors wish to thank Dr. Lijun Wang for help with animal care, and Ms Yaxi Chen for laboratory assistance, and Dr Michael Wang and Ms Soo Jung Lee for help with degu AANAT cloning. This work was support by grant NS057583 (to JB).
References
- 1.Borjigin J, Li X, Snyder SH. The pineal gland and melatonin: molecular and pharmacologic regulation. Annual Reviews in Pharmacology and Toxicology. 1999 doi: 10.1146/annurev.pharmtox.39.1.53. [DOI] [PubMed] [Google Scholar]
- 2.Simonneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 2003;55(2):325–95. doi: 10.1124/pr.55.2.2. [DOI] [PubMed] [Google Scholar]
- 3.Reiter RJ. The melatonin rhythm: both a clock and a calendar. Experientia. 1993;49(8):654–64. doi: 10.1007/BF01923947. [DOI] [PubMed] [Google Scholar]
- 4.Skene DJ, Arendt J. Human circadian rhythms: physiological and therapeutic relevance of light and melatonin. Ann Clin Biochem. 2006;43(Pt 5):344–53. doi: 10.1258/000456306778520142. [DOI] [PubMed] [Google Scholar]
- 5.Melke J, Goubran Botros H, Chaste P, Betancur C, Nygren G, Anckarsater H, et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol Psychiatry. 2008;13(1):90–8. doi: 10.1038/sj.mp.4002016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jasser SA, Blask DE, Brainard GC. Light during darkness and cancer: relationships in circadian photoreception and tumor biology. Canc Causes Contr. 2006;17(4):515–23. doi: 10.1007/s10552-005-9013-6. [DOI] [PubMed] [Google Scholar]
- 7.Peschke E. Melatonin, endocrine pancreas and diabetes. J Pineal Res. 2008;44(1):26–40. doi: 10.1111/j.1600-079X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 8.Klein DC. Arylalkylamine N-acetyltransferase: “the Timezyme”. J Biol Chem. 2007;282(7):4233–7. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- 9.Maronde E, Pfeffer M, Glass Y, Stehle JH. Transcription factor dynamics in pineal gland and liver of the Syrian hamster (Mesocricetus auratus) adapts to prevailing photoperiod. J Pineal Res. 2007;43(1):16–24. doi: 10.1111/j.1600-079X.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- 10.Falcon J, Besseau L, Fuentes M, Sauzet S, Magnanou E, Boeuf G. Structural and functional evolution of the pineal melatonin system in vertebrates. Ann N Y Acad Sci. 2009;1163:101–11. doi: 10.1111/j.1749-6632.2009.04435.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu T, Borjigin J. Relationship between nocturnal serotonin surge and melatonin onset in rodent pineal gland. Journal of Circadian Rhythms. 2006 doi: 10.1186/1740-3391-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gronfier C, Wright KP, Jr, Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc Natl Acad Sci U S A. 2007;104(21):9081–6. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perreau-Lenz S, Kalsbeek A, Van Der Vliet J, Pevet P, Buijs RM. In vivo evidence for a controlled offset of melatonin synthesis at dawn by the suprachiasmatic nucleus in the rat. Neuroscience. 2005;130(3):797–803. doi: 10.1016/j.neuroscience.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Sun X, Deng J, Liu T, Borjigin J. Circadian 5-HT production regulated by adrenergic signaling. Proceedings of the National Academy of Sciences. 2002 doi: 10.1073/pnas.062585499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Z, Liu T, Chattoraj A, Ahmed S, Wang MM, Deng J, et al. Posttranslational regulation of TPH1 is responsible for the nightly surge of 5-HT output in the rat pineal gland. J Pineal Res. 2008;45(4):506–14. doi: 10.1111/j.1600-079X.2008.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simonneaux V, Sinitskaya N, Salingre A, Garidou ML, Pevet P. Rat and Syrian hamster: two models for the regulation of AANAT gene expression. Chronobiol Int. 2006;23(1–2):351–9. doi: 10.1080/07420520500521962. [DOI] [PubMed] [Google Scholar]
- 17.Borjigin J, Wang MM, Snyder SH. Diurnal variation in mRNA encoding serotonin N-acetyltransferase in pineal gland. Nature. 1995 doi: 10.1038/378783a0. [DOI] [PubMed] [Google Scholar]
- 18.Roseboom PH, Coon SL, Baler R, McCune SK, Weller JL, Klein DC. Melatonin synthesis: analysis of the more than 150-fold nocturnal increase in serotonin N-acetyltransferase messenger ribonucleic acid in the rat pineal gland. Endocrinology. 1996;137(7):3033–45. doi: 10.1210/endo.137.7.8770929. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Liu T, Chattoraj A, Zhang LS, Wang L, Lee TM, Wang MM, Borjigin J. Posttranscriptional regulation of pineal melatonin synthesis in Octodon degus. J Pineal Res. 2009;46(5) doi: 10.1111/j.1600-079X.2009.00690.x. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu T, Borjigin J. N-acetyltransferase is not the rate-limiting enzyme of melatonin synthesis at night. Journal of Pineal Research. 2005 doi: 10.1111/j.1600-079X.2005.00223.x. [DOI] [PubMed] [Google Scholar]
- 21.Miguez JM, Simonneaux V, Pevet P. The role of the intracellular and extracellular serotonin in the regulation of melatonin production in rat pinealocytes. J Pineal Res. 1997;23(2):63–71. doi: 10.1111/j.1600-079x.1997.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 22.Arendt J. Melatonin in humans: it's about time. J Neuroendocrinol. 2005;17(8):537–8. doi: 10.1111/j.1365-2826.2005.01333.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim JS, Coon SL, Blackshaw S, Cepko CL, Moller M, Mukda S, et al. Methionine adenosyltransferase:adrenergic-cAMP mechanism regulates a daily rhythm in pineal expression. J Biol Chem. 2005;280(1):677–84. doi: 10.1074/jbc.M408438200. [DOI] [PubMed] [Google Scholar]
- 24.Klein DC. Evolution of the vertebrate pineal gland: the AANAT hypothesis. Chronobiol Int. 2006;23(1–2):5–20. doi: 10.1080/07420520500545839. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z, Deng J, Borjigin J. A novel H28Y mutation in LEC rats leads to decreased NAT protein stability in vivo and in vitro. J Pineal Res. 2005;39(1):84–90. doi: 10.1111/j.1600-079X.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 26.Huang Z, Chattoraj A, Li X, Snyder SH, Borjigin J. The increased degradation of NAT-H28Y mutant protein is due to a reduced interaction with 14-3-3. J Pineal Res. 2009;46(1):119–20. doi: 10.1111/j.1600-079X.2008.00619.x. [DOI] [PubMed] [Google Scholar]
- 27.Borjigin J, Payne AS, Deng J, Li X, Wang MM. A novel pineal night-specific ATPase encoded by the Wilson Disease Gene. Journal of Neuroscience. 1999 doi: 10.1523/JNEUROSCI.19-03-01018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed S, Deng J, Borjigin J. A new strain of rat for functional analysis of PINA. Molecular Brain Research. 2005 doi: 10.1016/j.molbrainres.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Choi BH, Chae HD, Park TJ, Oh J, Lim J, Kang SS, et al. Protein kinase C regulates the activity and stability of serotonin N-acetyltransferase. J Neurochem. 2004;90(2):442–54. doi: 10.1111/j.1471-4159.2004.02495.x. [DOI] [PubMed] [Google Scholar]
- 30.Li X. The regulation of tissue-specific and rhythmic expression of serotonin N-acetyltransferase. Neuroscience. The Johns Hopkins University; Baltimore, MD: 2000. p. 90. [Google Scholar]
- 31.Borjigin J, Wang MM, Snyder SH. Diurnal variation in mRNA encoding serotonin N-acetyltransferase in pineal gland. Nature. 1995;378(6559):783–5. doi: 10.1038/378783a0. [DOI] [PubMed] [Google Scholar]
- 32.Coon SL, Roseboom PH, Baler R, Weller JL, Namboodiri MA, Koonin EV, et al. Pineal serotonin N-acetyltransferase: expression cloning and molecular analysis. Science. 1995;270(5242):1681–3. doi: 10.1126/science.270.5242.1681. [DOI] [PubMed] [Google Scholar]
- 33.Craft CM, Murage J, Brown B, Zhan-Poe X. Bovine arylalkylamine N-acetyltransferase activity correlated with mRNA expression in pineal and retina. Brain Res Mol Brain Res. 1999;65(1):44–51. doi: 10.1016/s0169-328x(98)00336-2. [DOI] [PubMed] [Google Scholar]
- 34.Ackermann K, Bux R, Rub U, Korf HW, Kauert G, Stehle JH. Characterization of human melatonin synthesis using autoptic pineal tissue. Endocrinology. 2006;147(7):3235–42. doi: 10.1210/en.2006-0043. [DOI] [PubMed] [Google Scholar]
- 35.Wehr TA. The durations of human melatonin secretion and sleep respond to changes in daylength (photoperiod) J Clin Endocrinol Metab. 1991;73(6):1276–80. doi: 10.1210/jcem-73-6-1276. [DOI] [PubMed] [Google Scholar]
- 36.Schomerus C, Korf HW, Laedtke E, Weller JL, Klein DC. Selective adrenergic/cyclic AMP-dependent switch-off of proteasomal proteolysis alone switches on neural signal transduction: an example from the pineal gland. J Neurochem. 2000;75(5):2123–32. doi: 10.1046/j.1471-4159.2000.0752123.x. [DOI] [PubMed] [Google Scholar]