Abstract
Various infectious agents are known to be transmitted naturally via respiratory aerosols produced by infected patients. Such aerosols may be produced during normal activities by breathing, talking, coughing and sneezing. The schlieren optical method, previously applied mostly in engineering and physics, can be effectively used here to visualize airflows around human subjects in such indoor situations, non-intrusively and without the need for either tracer gas or airborne particles. It accomplishes this by rendering visible the optical phase gradients owing to real-time changes in air temperature. In this study, schlieren video records are obtained of human volunteers coughing with and without wearing standard surgical and N95 masks. The object is to characterize the exhaled airflows and evaluate the effect of these commonly used masks on the fluid-dynamic mechanisms that spread infection by coughing. Further, a high-speed schlieren video of a single cough is analysed by a computerized method of tracking individual turbulent eddies, demonstrating the non-intrusive velocimetry of the expelled airflow. Results show that human coughing projects a rapid turbulent jet into the surrounding air, but that wearing a surgical or N95 mask thwarts this natural mechanism of transmitting airborne infection, either by blocking the formation of the jet (N95 mask), or by redirecting it in a less harmful direction (surgical mask).
Keywords: schlieren, visualization, mask, coughing, airflow, infection control
1. Introduction and literature review
1.1. Airborne infection in hospitals
Modern clinical infection control is performed in indoor environments, mainly those of healthcare institutions such as hospitals, clinics and long-stay residential or nursing homes. These buildings, by nature of containing sick people, have the potential to be major sources of various aerosol-transmitted infections, some of which may be resistant to multiple antimicrobial agents and which may then be transported out into the wider community. These include various viruses (e.g. chickenpox, measles and influenza), bacteria (e.g. tuberculosis, Pseudomonas species and Staphylococcus aureus, including MRSA (methicillin-resistant Staphylococcus aureus)) and fungi (e.g. Aspergillus species, especially their spores). Vulnerable patients, both inpatients and outpatients, may be directly infected by such agents in such healthcare institutions during their admission, or may even be cross-infected by visiting friends and relatives who were infected from the community or from the same or similar healthcare institutions on previous visits.
Hence, it is important to control the transmission of such infections at the source, within these indoor healthcare environments. Hand-washing is a recognized and important part of infection control for agents transmitted by direct contact, but aerosol-transmitted infections have become more important and much debated since the severe acute respiratory syndrome (SARS) outbreaks of 2003 (Wong et al. 2004; Yu et al. 2004, 2005; Li et al. 2005a,b), and during the preparedness for the current influenza pandemic (Tellier 2006, 2007; Brankston et al. 2007; Lemieux et al. 2007; Tang & Li 2007).
Several infections have been shown to be transmitted via short-range and long-range aerosols in community and healthcare settings, including tuberculosis (TB), chickenpox and measles (Cole & Cook 1998; Edwards et al. 2004; Wong & Leung 2004; Tang et al. 2005). Many natural human activities also have the potential to disseminate infectious aerosols, such as breathing, talking, coughing, sneezing and even singing, which may be a particularly effective means of transmitting airborne infection (Cole & Cook 1998; Edwards et al. 2004; Wong & Leung 2004). More recently, there have been several inter-disciplinary reviews involving both microbiologists and engineers (Tang et al. 2006; Li et al. 2007), as well as cross-disciplinary studies involving both physicians and engineers examining the risks of airborne transmission from droplet dispersion and using airflow visualization in different clinical scenarios, including some simulated environments (Qian et al. 2006; Xie et al. 2006, 2007) and others involving ‘real-life’ operating theatres and the workplace (Kim & Flynn 1991; Maynard et al. 2000; Pan et al. 2003; Brohus et al. 2006).
Similarly, artificial respiratory assist devices, such as the oxygen masks and nebulizers used in many hospital wards and the various forms of mechanically assisted ventilation often used on intensive care units, have a greater potential to disseminate airborne infection because of high supply rates of air or oxygen that may be required. These supply rates can be as high as 10–15 l min−1 for an oxygen mask or nebulizer, depending on the needs of the patient. With the use of such masks the exhaled, potentially infectious air is expelled under a greater pressure than normal and may thus reach greater distances from the patient compared with the case of natural breathing (Hui et al. 2006a,b, 2007, 2009; Ip et al. 2007).
Many of the cited studies have involved a mannequin as a patient simulator, or a lung model into which tracer or ‘smoke’ particles have been introduced to track the airflows produced. This has been mainly because such tracer particles may be irritating or toxic to live human volunteers and patients. It is, however, difficult to extrapolate results from a mannequin with simulated respiratory airflows to a live human patient.
1.2. The fluid dynamics of airborne infection spread
Airflows generated by the human body include those due to thermal effects, motion of the body and natural human respiratory activities, e.g. breathing, talking, coughing, sneezing, etc. The temperature difference between human skin and the surrounding air drives a natural-convection boundary layer vertically upwards on the body surface of a standing person, eventually separating from the head and shoulders to form the human thermal plume (Lewis et al. 1969; Clark & Edholm 1985; Craven & Settles 2006). The strong vertical transport of air in this plume can convey floor-level contaminants to the breathing zone, as well as entrain the surrounding air along with any accompanying particle burden.
At walking speeds above approximately 0.2 m s−1, the human thermal plume gives way to the human aerodynamic wake (Edge et al. 2005), an unsteady turbulent airflow that follows a moving person and mixes strongly with the surrounding air owing to entrainment. Thus, hospital staff and patients in motion can transport airborne infectious agents from stationary patients to others by way of the bulk air motion in their aerodynamic wakes. Likewise, aircraft passengers moving in the aisles can spread contamination along the length of the cabin despite the aircraft's ventilation system (Poussou et al. 2008).
As first photographed by Jennison (1942), oral liquids are atomized during speech, coughing and sneezing by the outflow of air from the lungs, generating aerosols that can contain viruses and bacteria. The abrupt release of the air in the lungs by coughing or sneezing projects an impulsively started turbulent jet of air from the mouth or nose with considerable momentum. This aerosol-laden jet, led by a characteristic vortex ring, can penetrate an impressive distance into the surrounding ambient air before finally mixing out owing to turbulent entrainment (Settles 2005, 2006). Originating from an infected respiratory tract, it can expose others in close quarters to infection.
The airflow profile of a cough has been studied (Piirilä & Sovijärvi 1995; McCool 2006), has been measured quantitatively by Khan et al. (2004) (figure 1a), and has been imaged at high speed by Tang & Settles (2008). The first phase is the strong horizontal expulsion of air from the mouth at a flow rate of up to 8 l s−1 during the first 0.1 s. This is followed by a second jet of air directed downwards at about 30° with respect to the first, and with a visible nasal contribution. The final phase of the cough is once again a horizontal expulsion of air, slowly tapering off to conclude the cough with a total duration of just less than 1 s. The total volume of air expelled by the cough, based on figure 1a, is approximately 2 l. For some subjects the cough begins with a downward orientation rather than horizontally, projecting the expelled air jet towards the feet of bystanders (figure 1b). Such cough orientation probably varies between individuals as a matter of personal habit and routine.
Figure 1.
(a) The profile of a typical ‘single forced cough’ in terms of expelled airflow rate versus time, adapted from Khan et al. (2004). (b) Schlieren image of a cough directed downward by a 25-year-old male subject, revealing the character of the cough as a turbulent jet of air.
Given a peak velocity of 13 m s−1 (Khan et al. 2004) and an effective initial diameter of roughly 2 cm, the Reynolds number of the human cough is perhaps 18 000, more than high enough to ensure turbulent flow. Such a turbulent jet spreads after leaving the cougher's mouth by entraining the surrounding air. The accumulated knowledge of a wide range of round turbulent jets—of which the cough jet is a member—indicates that they spread conically with a total included angle of approximately 24° (e.g. Pope 2000).
The main impetus for this study is that, despite the potential for airborne infection, very little attention has been devoted to characterizing the behaviour of the airflow produced by a cough, once it exits the mouth. Here we investigate the cough using the real-time, non-invasive schlieren optical technique with human volunteers.
1.3. The schlieren optical technique applied to biomedical imaging
The optical technique known as schlieren has been used for many years in science and engineering to visualize transparent phenomena non-intrusively, especially the flows of gases and liquids (Settles 2001). The physical principle involved is that transparent phenomena often refract, or bend, a light beam projected through them. Air temperature differences, produced for example by body heat or a cough, are one source of such refraction. The resultant bending of light rays causes the rays to miss a critical focal point in the schlieren optical system, which can be a target as simple as a razor-blade edge that acts as a spatial filter. The razor blade discriminates refracted from unrefracted light rays, thus allowing invisible optical phase disturbances to become visible in the form of grey-scale or colour patterns (e.g. figure 1b).
Visualizing and understanding the human thermal plume (Lewis et al. 1969; Clark & Edholm 1985) was the first practical application of schlieren optics to biomedical problems. Subsequent uses included studying convective flow about animals (Stephens & Start 1972) and imaging the beams produced by medical ultrasound diagnostics (Smith & Thurstone 1974). Imaging the ventilation air currents in hospital operating theatres and related facilities has become an important application (e.g. Whyte & Shaw 1974; Clark & Edholm 1985; Settles 1997, 2001). Davies (1979) introduced the still-unexplored potential of schlieren optics to study speech, respiration and sleep anomalies. Other biomedical applications include observing collateral phenomena during pulsed laser surgery (Bor et al. 1993; Vogel et al. 2006), observing shock-wave lithotripsy (Carnell & Emmony 1995), and the study of olfactory airflows (Settles 2005).
Despite these several examples, much of the potential of schlieren imaging as a non-intrusive diagnostic tool in biomedical imaging remains unexplored.
2. Goal
The goal of the present work is to apply schlieren imaging to the study of airflows generated by the human cough. The unobstructed cough is first observed and quantitative velocimetry is performed by tracking turbulent eddies in the expelled jet of air. Then, the effect of wearing either of two popular mask types (simple surgical and N95 masks) on the cough airflow is assessed by qualitative observation of schlieren video records. Wearing such masks is now recommended as part of everyday personal protective equipment in modern infection control guidelines, particularly recently when dealing with patients potentially infected with pandemic influenza A (H1N1/2009) and other types of airborne-transmitted infections.
3. Material and Methods
3.1. Schlieren optical system
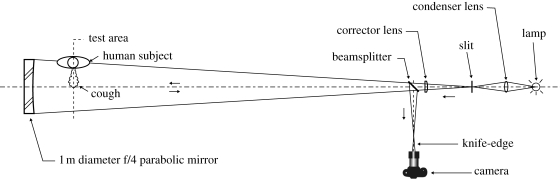
We used a large, sensitive schlieren optical system (figure 2) based on a 1 m diameter parabolic telescope mirror (Settles 2001) to image the airflows associated with the human cough. The 1 m diameter is large enough to visualize the flow about human volunteers and at least the near-field extent of exhaled flows owing to coughing. The human subjects sat on a stool 1 m in front of the mirror and coughed parallel or perpendicular to it, as shown in figure 2. Single-mirror coincident illumination from the filament of an incandescent lamp passed twice through the cough flowfield, on the way to the mirror and returning from it. The corrector lens shown in figure 2 is required because of the difference between the actual parabolic mirror and the perfectly spherical reflector that should be used in this optical arrangement, but was not available. Dispersion in this lens is also responsible for the incidental chromatic effects seen in the present schlieren images.
Figure 2.
Schematic top view of the 1 m aperture schlieren optical system of the Penn State University Gas Dynamics Laboratory, as used in this study (Settles 2001).
The schlieren system is located in a 9 × 11 × 3.2 m laboratory at Penn State University. The thick concrete floor of this laboratory minimizes vibration. Room air-conditioning is turned off while schlieren imaging takes place to provide a quiescent ambient atmosphere. The average air temperature in front of the mirror during testing is 294.5 K and the vertical temperature gradient is 0.5 K m−1. Smaller rooms and various types of operating air distribution system, e.g. displacement ventilation, can change the macroclimate and possibly alter the results (Qian et al. 2006).
High-definition 1280 × 720-pixel, 24 frame s−1 colour video schlieren records of coughs were captured by a Nikon D90 digital SLR camera at the position indicated in figure 2. A 70–300 mm zoom lens was used. Subsequent analysis also yielded high-resolution still colour images with exposure times in the order of 0.04 s from the video records.
A few coughs without masks were also captured using a Photron APX RS black-and-white high-speed digital camera instead of the Nikon D90. In this case schlieren image sequences were obtained at 3000 frames s−1, an individual frame size of 1 megapixel, and a frame exposure of 1 µs.
In this study, the schlieren optical system reveals temperature gradients in the air and integrates this information in the direction of the optical axis in order to produce a planar image of a flow pattern that is actually three-dimensional. Note that it cannot measure airborne particle distributions or sizes, which are not within the objectives of the present study. Other instrumentation, not used here, is specifically required for that purpose.
3.2. Masks
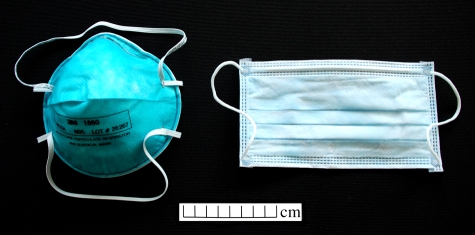
Standard surgical (1826) and N95 (1860) masks manufactured by 3M were used in this study (figure 3). The surgical mask is an inexpensive 9 × 17.5 cm pleated ear-loop face mask. It consists of three layers of fibrous, non-woven filter material. It is not a respirator and is not fitted to the face of the wearer.
Figure 3.
Standard N95 (left) and surgical (right) masks used in this study.
The N95, on the other hand, is designated a ‘particulate respirator and surgical mask’, and is approved by NIOSH (The National Institute for Occupational Safety and Health) (Qian et al. 1998). It provides 95 per cent filtration efficiency against solid and liquid aerosols, and is intended to reduce wearer exposure to particles in the 0.1 µm size range and above. It consists of an outer cover layer, a filter layer and an inner shell. N95 masks of regular size were fitted to the faces of volunteers according to the fitting instructions supplied with the masks.
3.3. Volunteers and cough protocol
Coughing with and without wearing masks was performed by nine healthy volunteers, either individually or in pairs. Six male and four female volunteers were drawn from the age ranges of 20–30 years (five), 30–40 years (two), 40–50 years (one), 50–60 years (one) and 80 years (one). Two of the authors (G. S. Settles and T. J. Liebner) also served as volunteers. All volunteers provided written informed consent to participate in these experiments.
Where two volunteers were simultaneously photographed, the one not coughing wore a surgical mask as a precaution against possible aerosol particles in the cough. Both side and frontal views were imaged in order to reveal any leakage of the cough airflow around the edges of the mask. Also, to assess the motion of exhaled airflows during rest, simulating a patient lying in bed, one 26-year-old male volunteer also lay supine in front of the schlieren mirror while breathing and coughing, both masked and unmasked.
The volunteers were asked to produce a single voluntary cough with force (Khan et al. 2004 and figure 1a). This required inhaling first, then coughing. None of the volunteers had respiratory infections or irritations, and all coughs were voluntary. No particles or extraneous gases were used. The level of non-intrusiveness of the schlieren imaging is equivalent to that of ordinary photography (which it is, technically, except for the additional optical elements shown in figure 2).
4. Results
4.1. ‘Schlieren PIV’ velocimetry of the human cough
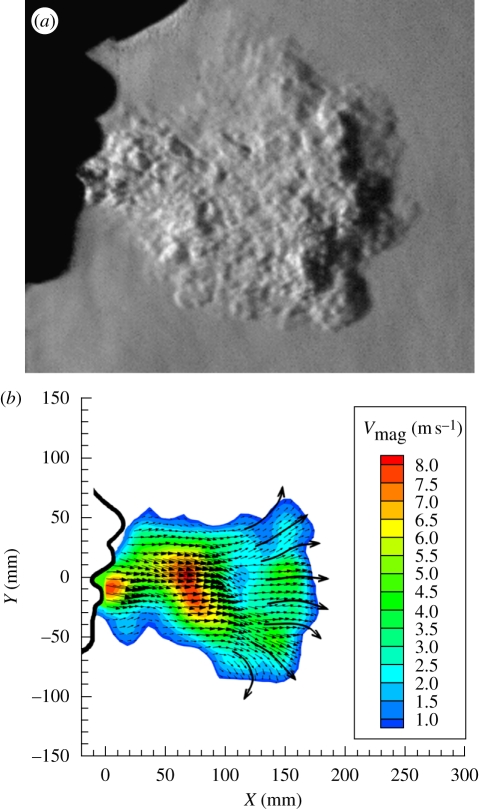
Figure 4 shows the results of a high-speed schlieren video of a single cough by a 57-year-old male volunteer without a mask. The fine-scale turbulence evident without ‘smearing’ in figure 4a attests that the motion of the jet of air from this cough is ‘frozen’ by the 1-µs frame exposure. Consecutive frames 0.00033 s apart can thus be used to trace the motion of the expelled cough. For example, in any two adjacent frames of the hundreds of frames that were recorded, turbulent eddies are observed to shift position incrementally to the right as the cough progresses. Commercial particle image velocimetry (PIV) software (proVISION, Integrated Design Tools, Inc., Tallahasse, FL, USA) was used to process the schlieren image pairs by measuring the shift distance quantitatively, dividing by the time increment between frames, and displaying the result as a velocity-contour and vector map.
Figure 4.
Quantitative results from high-speed video of a cough by a 57-year-old male volunteer. (a) Enlarged schlieren frame no. 300, exposed for 1 µs at 0.1 s from cough onset; (b) instantaneous velocity-magnitude contours and vectors averaged across the cough in the direction perpendicular to the page, obtained by ‘schlieren PIV’ processing of video frames 300 and 301 (figure 4b was first shown by Tang & Settles, 2008–reproduced here with permission from the New England Journal of Medicine).
An example of this for schlieren video frames 300 and 301, at about 0.1 s from the cough onset, is shown in figure 4b. The resulting velocity contours and vectors are averaged across the cough in the direction perpendicular to the page, since the schlieren beam traverses the entire cough in that direction in order to yield images of it. As a result of its similarity to the PIV method, this approach to the velocimetry of turbulence without the traditional tracer particles is known as ‘schlieren PIV’ (Jonassen et al. 2006).
Figure 4b reveals a maximum average airspeed across the early-stage cough of 8 m s−1. The maximum centreline airspeed is certainly greater than that. Further, as described above in §1.2, these velocimetry results also reveal the shape of the vortex ring that leads the motion of the impulsively started turbulent jet produced by coughing.
4.2. Cough visualizations with and without wearing masks
The coughs produced by the present volunteers varied somewhat in force and in direction with respect to the horizontal. Nonetheless, visual similarity was observed in the airflow patterns produced by all unrestricted coughs: all were fully turbulent, and 16 individual measurements from schlieren images yielded an average total jet spreading angle of 23.9° with a s.d. of 3.4° (see §1.2). Unmasked coughs and subsequent coughs with both surgical and N95 masks likewise did not reveal any significant qualitative differences arising from the different volunteers. Thus, the age and gender of the cougher are not seen as significant variables within our small set of volunteers.
The schlieren video results, shot at a normal speed of 24 frames s−1, revealed several important aspects of the dynamics of the human cough. Since these results are not necessarily clear from still images alone, each of figures 5–7 is accompanied by corresponding online digital video footage to assist the interested reader (see the electronic supplementary material). Also, sketches are included here with the corresponding figures to clarify our interpretation of the results.
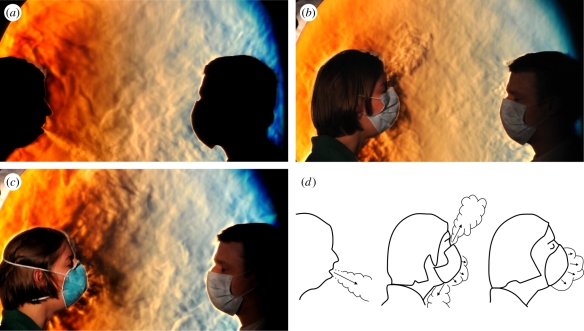
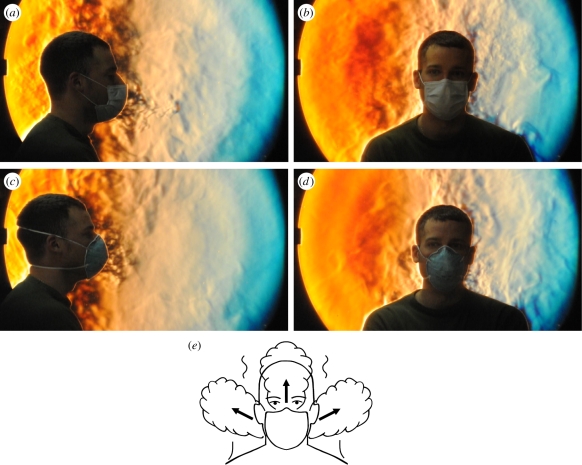
Figure 5.
Schlieren images of two volunteers facing one another. The subject on the right is masked as a precaution. The volunteer on the left coughs in the direction of the other subject first without wearing a mask (a), then while wearing a standard surgical mask (b), and finally while wearing an N95 mask (c). This demonstrates the importance of wearing a mask in reducing the potential for airborne transmission of infection over this distance. Note that in (a) the jet of air produced by the cough plume is directed downwards at roughly a 30° angle, whereas in (b) the expelled air of the cough plume is split into upward and downward components as it exits from the top and bottom edges of the surgical mask. There is also leakage around the side edges of the mask out of the plane of the page, which is shown in the next figure. In (c), the tighter seal of the N95 mask against the face forces more of the expelled cough through the mask. A low-velocity expelled turbulent air mass can be seen just in front of the mask, which does not cross the distance to the other volunteer. The different behaviours of these coughs, sketched in (d), can also be seen more clearly in the accompanying video footage (see the electronic supplementary material Video S1).
Figure 6.
Side and frontal-view schlieren images of coughs by a 26-year-old volunteer while wearing standard surgical mask (a,b) and N95 mask (c,d). The sketch (e) portrays the salient point of this sequence: that massive air leakage occurs around the sides and top of the surgical mask during a cough. The airflows shown here are more clearly seen as dynamic phenomena in the accompanying video footage (see the electronic supplementary material Video S2).
Figure 7.
Schlieren images of a supine volunteer, quietly breathing and coughing. These images demonstrate the effectiveness of standard surgical and N95 masks in containing potentially-infectious exhaled plumes while the subject is lying down (perhaps resting or sleeping) in a supine position. Note the vertical extent of the exhaled breath without a mask in (a). In (b) the effect of the standard surgical mask in the supine position can be seen in splitting the cough, some air passing through the mask and some leaking at its top edge. In comparison, the cough is relatively well contained by the N95 mask in (c). The airflows shown here are more clearly seen as dynamic phenomena in the accompanying video footage (see the electronic supplementary material Video S3). The phenomena in (a) and (b) are also sketched in (d) for clarity.
In figure 5, selected frames are shown from side-view schlieren footage of two volunteers. They first show an unmasked cough (figure 5a), which produces a turbulent jet (directed downwards at about a 30° angle in this case) extending across the schlieren field-of-view towards the second volunteer. The effect of the surgical mask is to block the forward motion of the cough jet (figure 5b) and to redirect it upwards and downwards owing to leakage around the relatively loose fit of the mask. The tighter seal of the N95 mask to the volunteer's face prevents some of this leakage (figure 5c) forcing more of the cough through the front of the mask at a relatively low airspeed. The resulting cough effluent is then transported upwards and away from the second volunteer by the thermal plume of the coughing volunteer.
It is worth noting at this point that leakage or venting of air around the edges of a surgical mask was first observed by Jennison (1942). However, he lacked any means, such as the schlieren optics employed here, to visualize the airflow and thus to determine the fate of the vented air.
Figure 6 demonstrates the leakage of the coughed jet around the side edges of the surgical and N95 masks. Since it does not seal or fit the face tightly, massive air leakage occurs around the sides of the surgical mask during a cough. Again, the dynamics are more clearly illustrated by the online videos accompanying this paper (see the electronic supplementary material).
Figure 7 demonstrates the extent of exhaled airflows produced during normal quiet breathing, such as those that may be produced by a resting or sleeping patient in bed. Without a mask, such exhaled airflows may easily reach beyond the schlieren field-of-view, i.e. more than 0.5 m vertically above the supine subject. The exhaled breath jet is effectively curtailed and contained when wearing either a standard surgical or N95 mask. A cough while wearing the surgical mask shows leakage of air around the nose, but also a significant jet of turbulent air passing through the mask itself. The N95 mask is much more effective in preventing leakage of the cough.
It is important to recognize that the supine human body sheds heat by convection from all horizontal surfaces, thus producing a thermal plume over a horizontal distance comparable to the height of the human subject. Air jets due to breathing and airflows associated with masks become entrained into the upward flow of this thermal plume, produced by the subject's natural body heat (Clark & Edholm 1985).
The qualitative findings from the schlieren video records and extracted images, of relevance to infection control, can be summarized as follows:
(i) Unmasked coughing produces a turbulent air jet extending across the present schlieren field-of-view and probably well beyond it. As already described, this jet may contain infectious aerosolized particles or droplets. The direction of the jet, whether horizontal or at an angle, varies with each human subject as well as with their individually adopted body attitudes, in both standing (figures 1b, 4, 5a and 6g) and supine (figure 7a) positions. The cough jet behaves approximately as a classical round incompressible turbulent jet with a total spreading angle of approximately 24°.
(ii) Wearing a standard surgical mask effectively blocks the forward momentum of the cough jet and its aerosol content, although the loose fit of the mask allows much of the air ejected by the cough to leak around the top, bottom and especially the sides of the mask. This leakage air also has minimal momentum, but is usually entrained into the thermal plume of the cougher rather than being projected in such a way as to affect others (figures 5b,d, 6b,e and 7b). The alternative leakage route around the edges of the mask offers a path of lower resistance to the air ejected by the cough. Therefore, the resulting air jet directly through the front of the mask is much reduced, though this can vary depending on how the mask is worn and its shape and tightness of fit to an individual's face (figures 5b,d, 6a and 7b,d).
(iii) Wearing an N95 mask reduces the leakage around the edges of the mask during coughing, because of its tighter seal to the facial skin (especially with formal fit-testing). However, this improved seal increases the pressure inside the mask when the volunteer coughs, thereby forcing more of the air ejected by the cough directly through the front of the N95 mask while decelerating it significantly (figures 5c,d and 6c). Again, the penetrating mass flow of low-velocity air is swept upwards into the rising thermal plume of the cougher.
(iv) Nonetheless, neither the surgical nor the N95 mask has any possibility of passing or containing all of the 2 l or so of air expelled in less than a second during a cough. Thus, leakage or venting must occur, compromising any existing, originally fit-tested seal between the mask and the face of the wearer.
(v) Even so, both surgical and N95 masks serve an important purpose in preventing airborne virus transmission: they prevent the expelled particle-containing air of a cough from being projected forward as a rapid turbulent jet over distances sufficient to reach the breathing zones of other individuals. Instead, the decelerated and redirected air expelled by the masked cough joins the general upward motion of the cougher's human thermal plume.
5. Discussion
In this study, we have applied schlieren imaging to examine the airflows produced by human volunteers while coughing. Schlieren imaging is safe, non-intrusive and does not require the introduction of any extraneous visualization media (e.g. smoke, other particles or tracer gases). Likewise, mannequins, artificial human-patient simulators, and other types of respiratory experimental apparatus designed to make the airflows visible (Khan et al. 2004; Hui et al. 2006a,b, 2007, 2009; Ip et al. 2007) are not required here. This allows a human volunteer to behave naturally with virtually no confounding effect imposed by the experimental environment. Of course, circumstances still exist where heated mannequins or cough simulators are preferable to human subjects, especially when the use of laser illumination or other potential hazards are involved (Qian et al. 2006; Pantelic et al. 2009).
Previous studies on surgical and N95 masks have found that their efficiency at removing aerosol particles depends upon several factors, including the velocity of the airflow passing through the mask, the degree of leakage around the mask, and the particle size distribution to be filtered (Chen & Willeke 1992; Weber et al. 1993; Chen et al. 1994; Qian et al. 1998; Lee et al. 2008). However, all these studies investigated the mask's efficiency in protecting the wearer from environmental airborne hazards, rather than the reverse problem of containment efficiency when the wearer is the potential source of infectious aerosols. It is arguable that the protective efficiency of the mask should generally apply, regardless of which direction through the mask the contaminated airflows may travel (even though the face seal of the N95 mask is momentarily compromised by a cough).
Surgical and N95 masks have different capture efficiencies for particles with aerodynamic diameters in the sub-micrometre range (Lee et al. 2008). Yet, regardless of the difference in protection factors offered by these two masks, influenza viruses (with sizes in the 0.08–0.12 µm range) and other viruses of similar size are capable of penetrating the mask in either direction. This makes the bulk airflow behaviour of these masks, retarding or diverting the turbulent cough jet into the rising human thermal plume as described in this study, that much more significant in limiting the dissemination of infectious aerosols when these masks are worn by infected, coughing individuals. These aerodynamic aspects of wearing masks have seldom been considered in clinical studies, and are presented here for the first time.
There are many recent studies examining the filtering and other protective aspects of masks. A recent study examined the amount of influenza virus RNA (as measured using a quantitative polymerase chain reaction technique) passing through both surgical and N95 masks when worn by laboratory-confirmed influenza-infected individuals. Both masks were found to be of similar efficacy in this regard (Johnson et al. 2009). Their clinical trials showed that wearing a mask significantly reduced the potential to spread the influenza to others. The present study offers additional reasons, based on the suppression or redirection of the turbulent jet of air from a cough, why this should be the case.
Further studies examining sneezing, normal talking, laughing, sleeping and perhaps even singing are planned. These patient behaviours occur frequently in hospital wards, but the airflows that they produce are poorly understood. In addition, the effects of respiratory assist devices, such as the various types of oxygen masks and nebulizers will also be examined. Different languages may have different levels of risk for producing potentially infectious exhaled aerosols, depending on how certain letters (particularly the consonants or their equivalent) and words are produced (Inouye 2003). This hypothesis can also be examined using the real-time, non-invasive schlieren imaging technique.
Important factors not addressed in this baseline study also need to be considered in future work. For example, the effect of ambient temperature, humidity and ventilation currents on the cough-produced air jets has yet to be studied. Pressure-drop data across the masks we used would also be useful, as would the imaging of bouts of several coughs rather than just the isolated coughs studied here.
It is hoped that this investigation will encourage a broader use of schlieren optics in biomedical studies, especially in those cases where human subjects are involved. At the same time we recognize that airflow visualization is only one step in understanding and quantifying the aerosol transmission of disease. Additional research is required to measure the number and size of droplets produced by various respiratory activities, as well as the presence and quantity of viable infectious agents within the droplets, how many of these are inhaled by a susceptible secondary host (e.g. Xie et al. 2007, 2009), and finally (and perhaps most difficult of all) the mean threshold infectious dose of such agents required to cause disease in the human host.
Acknowledgements
We thank those who volunteered to be photographed for this study, and also the manuscript referees. The assistance of M. J. Hargather, M. J. Madalis and M. E. Staymates is also appreciated.
Footnotes
One contribution of 10 to a Theme Supplement ‘Airborne transmission of disease in hospitals’.
References
- Bor Z., Hopp B., Racz B., Szabo G., Ratkay I., Suveges I., Fust A., Mohay J. 1993. Plume emission, shock wave and surface wave formation during excimer laser ablation of the cornea. Refract Corneal Surg. 9(Suppl. 2), S111–S115. [PubMed] [Google Scholar]
- Brankston G., Gitterman L., Hirji Z., Lemieux C., Gardam M. 2007. Transmission of influenza A in human beings. Lancet Infect. Dis. 7, 257–265. ( 10.1016/S1473-3099(07)70029-4) [DOI] [PubMed] [Google Scholar]
- Brohus H., Balling K. D., Jeppesen D. 2006. Influence of movements on contaminant transport in an operating room. Indoor Air 16, 356–372. ( 10.1111/j.1600-0668.2006.00454.x) [DOI] [PubMed] [Google Scholar]
- Carnell M. T., Emmony D. C. 1995. A schlieren study of the interaction between a lithotripter shock wave and a simulated kidney stone. Ultrasound Med. Biol. 21, 721–724. ( 10.1016/0301-5629(94)00157-9) [DOI] [PubMed] [Google Scholar]
- Chen C. C., Willeke K. 1992. Aerosol penetration through surgical masks. Am. J. Infect. Control 20, 177–184. ( 10.1016/S0196-6553(05)80143-9) [DOI] [PubMed] [Google Scholar]
- Chen S. K., Vesley D., Brosseau L. M., Vincent J. H. 1994. Evaluation of single-use masks and respirators for protection of health care workers against mycobacterial aerosols. Am. J. Infect. Control 22, 65–74. ( 10.1016/0196-6553(94)90116-3) [DOI] [PubMed] [Google Scholar]
- Clark R. P., Edholm O. G. 1985. Man and his thermal environment. London, UK: E. Arnold. [Google Scholar]
- Cole E. C., Cook C. E. 1998. Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies. Am. J. Infect. Control 26, 453–464. ( 10.1016/S0196-6553(98)70046-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven B. A., Settles G. S. 2006. A computational and experimental investigation of the human thermal plume. J. Fluids Eng. 128, 1251–1258. ( 10.1115/1.2353274) [DOI] [Google Scholar]
- Davies T. P. 1979. Schlieren photography: a tool for speech research. Acoust. Lett. 3, 73–75. [Google Scholar]
- Edge B. A., Paterson E. G., Settles G. S. 2005. Computational study of the wake and contaminant transport of a walking human. J. Fluids Eng. 127, 967–977. ( 10.1115/1.2013291) [DOI] [Google Scholar]
- Edwards D. A., Man J. C., Brand P., Katstra J. P., Sommerer K., Stone H. A., Nardell E., Scheuch G. 2004. Inhaling to mitigate exhaled bioaerosols. Proc. Natl Acad. Sci. USA 101, 17383–17388. ( 10.1073/pnas.0408159101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D. S., Hall S. D., Chan M. T., Chow B. K., Tsou J. Y., Joynt G. M., Sullivan C. E., Sung J. J. Y. 2006a. Noninvasive positive-pressure ventilation: an experimental model to assess air and particle dispersion. Chest 130, 730–740. ( 10.1378/chest.130.3.730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D. S., Ip M., Tang J. W., Tang J. W., Wong A. L. N., Chan M. T. V., Hall S. D., Chan P. K. S., Sung J. J. Y. 2006b. Airflows around oxygen masks: a potential source of infection? Chest 130, 822–826. ( 10.1378/chest.130.3.822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D. S., Hall S. D., Chan M. T., Chow B. K., Ng S. S., Gin T., Sung J. J. Y. 2007. Exhaled air dispersion during oxygen delivery via a simple oxygen mask. Chest 132, 540–546. ( 10.1378/chest.07-0636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D. S., Chow B. K., Chu L. C., Ng S. S., Hall S. D., Gin T., Chan M. T. 2009. Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest 135, 648–654. ( 10.1378/chest.08-1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S. 2003. SARS transmission: language droplet production. Lancet 362, 170 ( 10.1016/S0140-6736(03)13874-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip M., et al. 2007. Airflow and droplet spreading around oxygen masks: a simulation model for infection control research. Am. J. Infect. Control 35, 684–689. ( 10.1016/j.ajic.2007.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennison M. W. 1942. Atomizing of mouth and nose secretions into the air as revealed by high-speed photography. In Aerobiology, pp. 106–128. Washington, DC: AAAS. [Google Scholar]
- Johnson D. F., Druce J. D., Birch C., Grayson M. L. 2009. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin. Infect. Dis. 49, 275–277. ( 10.1086/600041) [DOI] [PubMed] [Google Scholar]
- Jonassen D. R., Settles G. S., Tronosky M. D. 2006. Schlieren ‘PIV’ for turbulent flows. Optics Lasers Eng. 44, 190–207. ( 10.1016/j.optlaseng.2005.04.004) [DOI] [Google Scholar]
- Khan T. A., Higuchi H., Marr D. R., Glauser M. N. 2004. Unsteady flow measurements of human micro environment using time-resolved particle image velocimetry. Proc. Room Vent 2004, Coimbra, Portugal. [Google Scholar]
- Kim T., Flynn M. R. 1991. Airflow pattern around a worker in a uniform freestream. Am. Ind. Hyg. Assoc. J. 52, 287–296. [DOI] [PubMed] [Google Scholar]
- Lee S. A., Grinshpun S. A., Reponen T. 2008. Respiratory performance offered by N95 respirators and surgical masks: human subject evaluation with NaCl aerosol representing bacterial and viral particle size range. Ann. Occup. Hyg. 52, 177–185. ( 10.1093/annhyg/men005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux C., Brankston G., Gitterman L., Hirji Z., Gardam M. 2007. Questioning aerosol transmission of influenza. Emerg. Infect. Dis. 13, 173–174. ( 10.3201/eid1301.061202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis H. E., Foster A. R., Mullan B. J., Cox R. N., Clark R. P. 1969. Aerodynamics of the human microenvironment. Lancet 293, 1273–1277. ( 10.1016/S0140-6736(69)92220-X) [DOI] [PubMed] [Google Scholar]
- Li Y., Duan S., Yu I. T., Wong T. W. 2005a. Multi-zone modeling of probable SARS virus transmission by airflow between flats in Block E, Amoy Gardens. Indoor Air 15, 96–111. ( 10.1111/j.1600-0668.2004.00318.x) [DOI] [PubMed] [Google Scholar]
- Li Y., Huang X., Yu I. T., Wong T. W., Qian H. 2005b. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air 15, 83–95. ( 10.1111/j.1600-0668.2004.00317.x) [DOI] [PubMed] [Google Scholar]
- Li Y., et al. 2007. Role of ventilation in airborne transmission of infectious agents in the built environment: a multidisciplinary systematic review. Indoor Air 17, 2–18. ( 10.1111/j.1600-0668.2006.00445.x) [DOI] [PubMed] [Google Scholar]
- Maynard A., Thompson J., Cain J. R., Rajan B. 2000. Air movement visualisation in the workplace: current methods and new approaches. Am. Ind. Hyg. Assoc. J. 61, 51–55. [PubMed] [Google Scholar]
- McCool F. D. 2006. Global physiology and pathophysiology of cough: ACCP evidence-based clinical practice guidelines. Chest 129, 48S–53S. ( 10.1378/chest.129.1_suppl.48S) [DOI] [PubMed] [Google Scholar]
- Pan Y. L., Aptowicz K. B., Chang R. K., Hart M., Eversole J. D. 2003. Characterizing and monitoring respiratory aerosols by light scattering. Opt. Lett. 28, 589–591. ( 10.1364/OL.28.000589) [DOI] [PubMed] [Google Scholar]
- Pantelic J., Sze-To G. N., Tham K. W., Chao C. Y. H., Khoo Y. C. M. 2009. Personalized ventilation as a control measure for airborne transmissible disease spread. J. R. Soc. Interface. 6 S715–S726( 10.1098/rsif.2009.0311.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piirilä A., Sovijärvi A. R. A. 1995. Objective assessment of cough. Euro. Respir. J. 8, 1949–1956. ( 10.1183/09031936.95.08111949) [DOI] [PubMed] [Google Scholar]
- Pope S. B. 2000. Turbulent flows. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Poussou S., Sojka P., Plesniak M. 2008. Experimental model of contaminant transport by a moving wake inside an aircraft cabin. Bull. Am. Phys. Soc. 53, 224–225 (abstract). [Google Scholar]
- Qian H., Li Y., Nielsen P. V., Hyldgaard C. E., Wong T. W., Chwang A. T. 2006. Dispersion of exhaled droplet nuclei in a two-bed hospital ward with three different ventilation systems. Indoor Air 16, 111–128. ( 10.1111/j.1600-0668.2005.00407.x) [DOI] [PubMed] [Google Scholar]
- Qian Y., Willeke K., Grinshpun S. A., Donnelly J., Coffey C. C. 1998. Performance of N95 respirators: filtration efficiency for airborne microbial and inert particles. Am. Ind. Hyg. Assoc. J. 59, 128–132. [DOI] [PubMed] [Google Scholar]
- Settles G. S. 1997. Visualizing full-scale ventilation airflows. ASHRAE J. 39, 19–26. [Google Scholar]
- Settles G. S. 2001. Schlieren and shadowgraph techniques. Visualizing phenomena in transparent media. Berlin, Germany: Springer-Verlag. [Google Scholar]
- Settles G. S. 2005. Sniffers: fluid-dynamic sampling for olfactory trace detection in nature homeland security: the 2004 Freeman Scholar Lecture. J. Fluid. Eng. 127, 189–218. ( 10.1115/1.1891146) [DOI] [Google Scholar]
- Settles G. S. 2006. Fluid mechanics and homeland security. Annu. Rev. Fluid Mech. 38, 87–110. ( 10.1146/annurev.fluid.38.050304.092111) [DOI] [Google Scholar]
- Smith S. W., Thurstone F. L. 1974. Schlieren study of pulsed ultrasound transmission through human skull. J. Clin. Ultrasound 2, 55–59. ( 10.1002/jcu.1870020110) [DOI] [PubMed] [Google Scholar]
- Stephens D. B., Start I. B. 1972. Schlieren photography of the piglet's microenvironment. Cornell Vet. 62, 20–26. [PubMed] [Google Scholar]
- Tang J. W., Eames I., Li Y., Taha Y. A., Wilson P., Bellingan G., Ward K. N., Breuer J. 2005. Door opening motion can potentially lead to a transient breakdown in negative-pressure isolation conditions: the importance of vorticity and buoyancy airflows. J. Hosp. Infect. 61, 283–286. ( 10.1016/j.jhin.2005.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. W., Li Y., Eames I., Chan P. K., Ridgway G. L. 2006. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J. Hosp. Infect. 64, 100–114. ( 10.1016/j.jhin.2006.05.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. W., Li Y. 2007. Transmission of influenza A in human beings. Lancet Infect. Dis. 7, 758 ( 10.1016/S1473-3099(07)70268-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. W., Settles G. S. 2008. Coughing and aerosols. N. Engl. J. Med. 359, e19 ( 10.1056/NEJMicm072576) [DOI] [PubMed] [Google Scholar]
- Tellier R. 2006. Review of aerosol transmission of influenza A virus. Emerg. Infect. Dis. 12, 1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R. 2007. Transmission of influenza A in human beings. Lancet Infect. Dis. 7, 759–760. ( 10.1016/S1473-3099(07)70269-4) [DOI] [PubMed] [Google Scholar]
- Vogel A., Apitz I., Freidank S., Dijkink R. 2006. Sensitive high-resolution white-light Schlieren technique with a large dynamic range for the investigation of ablation dynamics. Optics Lett. 31, 1812–1814. ( 10.1364/OL.31.001812) [DOI] [PubMed] [Google Scholar]
- Weber A., Willeke K., Marchioni R., Myojo T., McKay R., Donnelly J., Liebhaber F. 1993. Aerosol penetration and leakage characteristics of masks used in the health care industry. Am. J. Infect. Control 21, 167–173. ( 10.1016/0196-6553(93)90027-2) [DOI] [PubMed] [Google Scholar]
- Whyte W., Shaw B. H. 1974. The effect of obstructions and thermals in laminar-flow systems. J. Hygiene 72, 415–423. ( 10.1017/S0022172400023652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. C., Leung K. S. 2004. Transmission and prevention of occupational infections in orthopaedic surgeons. J. Bone Joint Surg. Am. 86-A, 1065–1076. [DOI] [PubMed] [Google Scholar]
- Wong T. W., Lee C. K., Tam W., Lau J. T., Yu T. S., Liu S. F., Chan P. K. S., Li Y., Bresee J. S., et al. 2004. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg. Infect. Dis. 10, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Li Y., Zhang T., Fang H. H. 2006. Bacterial survival in evaporating deposited droplets on a Teflon-coated surface. Appl. Microbiol. Biotechnol. 73, 703–712. ( 10.1007/s00253-006-0492-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Li Y., Chwang A. T., Ho P. L., Seto W. H. 2007. How far droplets can move in indoor environments: revisiting the Wells evaporation-falling curve. Indoor Air 17, 211–225. ( 10.1111/j.1600-0668.2007.00469.x) [DOI] [PubMed] [Google Scholar]
- Xie X., Li Y., Sun H., Liu L. 2009. Exhaled droplets due to talking and coughing J. R. Soc. Interface 6S703–S714( 10.1098/rsif.2009.0388.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I. T., Li Y., Wong T. W., Tam W., Chan A. T., Lee J. H. W., Leung D. Y. C., Ho T. 2004. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 350, 1731–1739. ( 10.1056/NEJMoa032867) [DOI] [PubMed] [Google Scholar]
- Yu I. T., Wong T. W., Chiu Y. L., Lee N., Li Y. 2005. Temporal-spatial analysis of severe acute respiratory syndrome among hospital inpatients. Clin. Infect. Dis. 40, 1237–1243. ( 10.1086/428735) [DOI] [PMC free article] [PubMed] [Google Scholar]