Abstract
Respiratory infections can be spread via ‘contact’ with droplets from expiratory activities such as talking, coughing and sneezing, and also from aerosol-generating clinical procedures. Droplet sizes predominately determine the times they can remain airborne, the possibility of spread of infectious diseases and thus the strategies for controlling the infections. While significant inconsistencies exist between the existing measured data on respiratory droplets generated during expiratory activities, a food dye was used in the mouth during measurements of large droplets, which made the expiratory activities ‘unnatural’. We carried out a series of experiments using glass slides and a microscope as well as an aerosol spectrometer to measure the number and size of respiratory droplets produced from the mouth of healthy individuals during talking and coughing with and without a food dye. The total mass of respiratory droplets was measured using a mask, plastic bag with tissue and an electronic balance with a high precision. Considerable subject variability was observed and the average size of droplets captured using glass slides and microscope was about 50–100 µm. Smaller droplets were also detected by the aerosol spectrometer. More droplets seemed to be generated when a food dye was used.
Keywords: droplets, talking, coughing, airborne infection
1. Introduction
During human expiratory activities such as talking, laughing, coughing and sneezing, many droplets of saliva and other secretions are expelled from the respiratory tract (the mouth and nose). It is now known that respiratory infections can be spread by these droplets and their residues after evaporation (Garner 1996). Larger droplets may rapidly settle out of the air and thus contribute to disease transmission to individuals in close proximity; smaller droplets may remain suspended for a long time and contribute to disease transmission over larger distances. Their sizes predominately determine the times they can remain airborne and thus the possibility of spread of infectious diseases if these respiratory droplets contain infectious pathogens (Wells 1934; Wan & Chao 2007; Xie et al. 2007). Moreover, the size distribution of such droplets influences the type of microorganisms that may be carried as well as the strategies for controlling the infections. Previous studies of hospital ventilation and infection control reveal a need for a more accurate measurement of respiratory droplets (Li et al. 2005; Qian et al. 2006). In the literature, many researchers have investigated droplet generation during expiratory activities using different methods (Jennison 1942; Duguid 1945, 1946; Loudon & Roberts 1967; Fairchild & Stamper 1987; Papineni & Rosenthal 1997; Fennelly et al. 2004; Yang et al. 2007), of which at least three detailed measured data on respiratory droplets exist (Duguid 1946; Loudon & Roberts 1967; Papineni & Rosenthal 1997). However, there exist significant inconsistencies between the existing data, as reviewed by Nicas et al. (2005) and Morawska (2006).
Difficulties in capturing and subsequent sizing of respiratory droplets are mainly due to the small sizes and rapid evaporation of droplets after release, and evaporation loss can not be neglected because of the typical size range of 0–200 µm. There exist significant inconsistencies between the existing data using different methods, which failed to consider the effect of evaporation, which is critical. Different methods and instruments employed are likely to have contributed to the inconsistency in the findings. Moreover, we noticed that when the deposition method was applied, a dye was used in the mouth to easily distinguish the droplets (Duguid 1945, 1946; Loudon & Roberts 1967). The introduction of the dye may influence the formation of secretion in the mouth and thus the number or size of droplets produced. Even though the subjects were told that they could swallow their saliva or spit it out and try to make their expiratory activities as natural as possible, they may still have had psychological effects that could change saliva secretion. The number and size of droplets expelled without food dye were not studied. We considered whether the introduction of a dye influences the production of droplets during talking or coughing. We demonstrated that droplet stain marks even without dye could be distinguished on clean glass slides under a microscope, hence we could measure the naturally expelled droplets.
The studies reported here were undertaken to determine the number and size of respiratory droplets emitted by different healthy people during talking and coughing. Experiments both with and without food dye were conducted. To determine the number and size, subjects were told to speak and cough into a small chamber which was constructed according to the design by previous investigators. Large droplets produced were recovered under a microscope from the glass slides placed inside the chamber by the method of impaction and settling. The concentration of small droplets was monitored by a portable dust monitor by the method of air sampling. As to the measurements of total mass, respiratory droplets were collected with a surgical face mask and plastic bag with tissue inside, which were weighed before and after the collection with a high-precision electronic balance.
2. Material and Methods
2.1. Measurements of droplet sizes and numbers
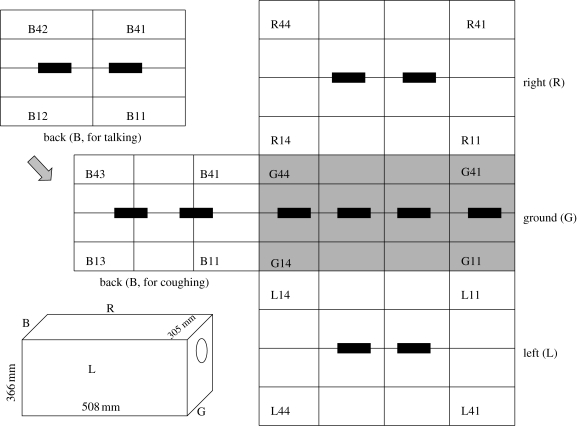
Experiments were undertaken to determine the number and size of respiratory droplets emitted by a group of healthy people during talking and coughing. A small air-tight box was constructed with the same dimensions as the design of Loudon & Roberts (1967), which was 366 mm (14.4 inches) by 508 mm (20 inches) by 305 mm (12 inches) inside. In the following text, we refer to the six walls as the front wall, back wall, left wall, right wall, ceiling and ground. The box was made of stainless steel except for the ceiling which was made of Perspex. At about two-thirds of the height of the front wall, an entry hole of 102 mm (4 inches) in diameter was cut for the purpose of respiratory droplet expulsion from a subject's mouth. A detachable air-tight door was screwed onto the entry hole. The left and right walls were detachable. When droplets were expelled into the box from the entry hole, large ones would quickly settle or impact on the surfaces nearby and leave stain marks, while small ones would totally evaporate into droplet nuclei, which could remain suspended in the air. To collect the large droplets, microscope glass slides and strips of water-sensitive paper (WSP) both of standard size of 76 by 26 mm were attached to four walls of the box prior to each test, i.e. the back wall, left wall, right wall and ground. WSP is a special slide strip made of specially coated yellow paper that turns blue when exposed to water droplets. These strips can give us a quick and visual indication of droplet size and density.
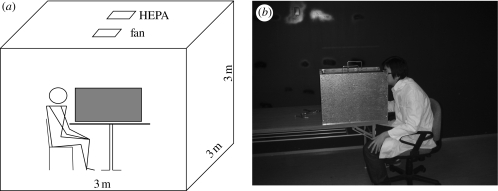
Small droplets or droplet nuclei suspended in the air were measured by a 16-channel dust monitor (Grimm 1.108, Germany), which could provide real-time size measurements of particles from 0.3 to 20 µm. The sampling flow rate was 1.2 l min−1 and reproducibility was ±2 per cent. A small hole was drilled in the centre of left and right walls to insert the air inlet of the dust monitor. The box was placed in a clean room (3 m × 3 m × 3 m) equipped with a high-efficiency particulate air (HEPA) filtration system. We also put another dust monitor away from the box to monitor the particle concentration in the clean room. A schematic diagram and a photo of the experimental setup are shown in figure 1.
Figure 1.
(a) Schematic diagram and (b) photo of the experimental setup.
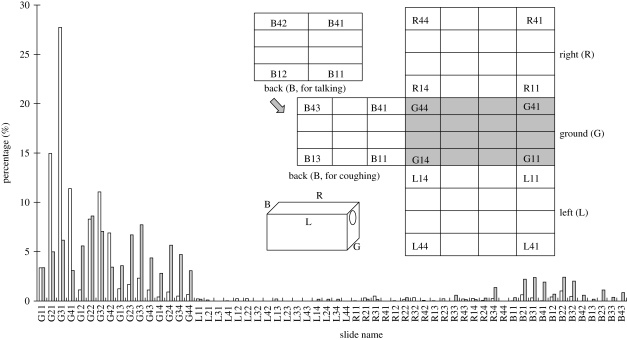
All the subjects were from our research group, non-smoking and around 20–40 years old. We studied two expiratory activities, i.e. talking and coughing. During each talking experiment, the subject counted from 1 to 100 loudly and slowly, and 56 slides would be used. As for each coughing experiment, 20 coughs were made into the box and 60 slides would be used. Usually 10 pieces of WSP were used in each experiment, four on the ground and two on each of the other three walls. The placement of these slides and WSP (in black) is shown in figure 2.
Figure 2.
Schematic diagram of slide placement in the test box.
Experiments were conducted with the following procedures.
(i) Before each experiment, all the slides were cleaned first with 75 per cent alcohol and then with distilled water, rubbed with cloth to make them dry and clean and stored inside the slide boxes to partition them off. The air-tight box was cleaned and then put into the clean room in which the HEPA system was always on.
(ii) The glass slides and WSPs were loaded into the box, and the dust monitor was turned on inside and outside the box to monitor the particle concentration. After about 2 h, the particle concentration would be very low compared with that outside the room.
(iii) The subject was asked to go into the clean room, sit on the chair before the box and wait for 20 min. As the indoor particle concentration would increase due to door opening and subject entering, it took some time for the HEPA system to dilute the particle concentration.
(iv) The subject carried out the planned expiratory activity into the box through the entry hole. As soon as this was completed, the subject screwed the entry hole to seal the box. The subject was asked to wait for another 20 min before going out for particle concentration measurement inside the box, because opening the door would influence the indoor particle concentration.
(v) After another 2 h, the researchers could go inside the room, open the box and collect the glass slides and WSP cards, which would be labelled as to position. The box was then cleared for the next experiment.
To test the effect of food dye on droplet generation, experiments were conducted. Food dye powder (Lemon Yellow Powder H1794) was dissolved in distilled water and mass concentration was prepared at about 2.5 per cent. Before expiratory activities, the subject was asked to gargle using the food dye solution two or three times to stain the saliva. The subject could spit out the excessive saliva if he/she felt that there was too much saliva in the mouth. During talking or coughing, the subject could swallow or spit the excessive saliva if he/she felt there was too much saliva in the mouth, in order to make the expiratory activity as natural as possible. After certain times of counting or coughing, the subject was asked to gargle using the food dye solution again.
These slides were completely scanned under a microscope (Leica DM1000, Leica Microsystems, Germany) visually. We distinguished droplet stain marks by morphology. Usually, a ‘ring’ could be observed surrounding the dried residue of a droplet. Every droplet stain mark was photographed with a high-resolution CCD camera (Leica DFC320, Leica Microsystems) connected to the microscope. The sizes of these stain marks were analysed using an image-processing software developed in the laboratory. The particle concentration in different size ranges produced during expiratory activities could be obtained by subtracting the concentration outside the box from that inside the box.
2.2. Measurements of total mass of droplets
It is known that droplets evaporate quickly after they are produced. To accurately measure the total mass of droplets produced during talking and coughing, evaporation should be avoided as much as possible. In our experiments, an air-tight plastic bag with tissue inside was used to collect the droplets. A subject put his/her mouth inside the bag and finished the requested expiratory manoeuvres, i.e. counting from 1 to 100 or coughing 20 times. During this process, condensation of exhaled breath would occur. So we also used a mask to collect the droplets. Subjects wore a surgical face mask and then counted or coughed. The mask or plastic bag with tissue was weighed before and immediately after the collection with an analytical balance with an accuracy of 0.1 mg (Shimadzu AUW 220, Japan). The total mass of droplets collected using these two methods could then be estimated and compared.
3. Results
3.1. Results of respiratory droplet sizes and numbers
Three male subjects and four female subjects participated in the measurements of droplet sizes, of which three male subjects and two female subjects were all Chinese-speaking healthy adults and two female subjects (F3 and F4) were English-speaking healthy adults. Experiments with and without food dye were carried out for talking, while only experiments without food dye were done for coughing. The food dye solution has a taste like sea water. In order to make the subjects feel not so bad to have the dye in their mouth, we added sugar in three trials of the experiments with food dye. In experiments with the food dye used, the subjects slowly counted aloud from 1 to 100 into the box. In the experiment without food dye, the subject slowly counted aloud from 1 to 100 into the box three times. That was done because in the test not so many droplets were observed from the strips of WSP after one trial of talking. For each coughing experiment, the subjects were asked to cough 20 times.
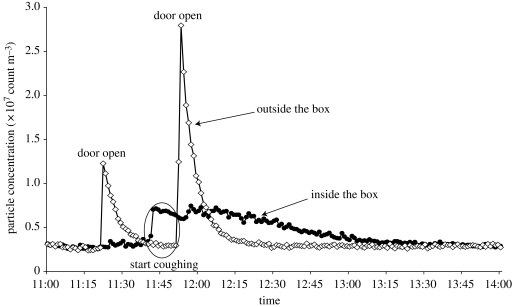
Particle concentrations both inside and outside the box were monitored by dust monitors during the experiments. Figure 3 shows the particle concentration history during one trial of coughing. When the door was opened, the particle concentration inside the room but outside the test box would first increase and then decrease quickly because the HEPA filter in the room was on, which could remove particles. Before the subject's expiratory activities, particle concentration inside the air-tight box would not be higher than outside the air-tight box. Figure 3 shows that when the subject started to cough into the box, a higher concentration inside the box was monitored, which means small droplets/particles (more than 0.5 µm) were generated. However, only in some experiments was an obvious higher concentration inside the box observed than that outside the box for some size channels during the time period of talking or coughing. From those data, it was very difficult to calculate the numbers of small droplets and droplet nuclei produced. Thus, only results of the measured deposited droplets obtained from glass slides were reported subsequently.
Figure 3.
Particle concentration history inside and outside the box during the entire process of one trial of coughing experiment conducted on 25 October 2006. Filled circle, diameter greater than 0.50 µm (outside the box); open diamond, diameter greater than 0.50 µm (inside the box).
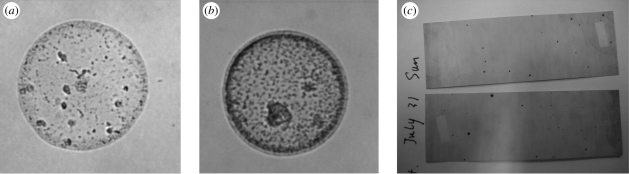
Droplet stain marks on the slides were scanned under a microscope and images were taken, one example of which is shown in figure 4a (no dye) and figure 4b (with dye). Droplet spots on WSP strips are shown in figure 4c. The numbers of droplet stain marks observed on the slides in different experiments are summarized in table 1. A great individual variability was observed. The average number of droplets found in seven subjects' talking experiments without the food dye was 323 during the process of counting from 1 to 100 for three times, i.e. 108 if counting from 1 to 100 only once. Averages of 315 and 273 were found during the process of counting from 1 to 100 when food dye and food dye with sugar solution were used, respectively. For 20 coughs, the average value was only 108. Figure 5 shows the number percentages of droplet stain marks observed on different slides during talking and coughing, which were calculated from the data of all experiments. We found that most of the droplets were deposited on the ground. For talking experiments, 93.7 per cent of the large droplets were deposited on the ground. Among the slides on the ground, 57.4 per cent of the droplets deposited on the slides in the first row (about 0.1 m away from the mouth) and 27.4 per cent deposited on the slides in the second row (about 0.2 m away). Almost 90 per cent fell within a distance of 0.3 m. For coughing experiments, 80.9 per cent of the droplets were deposited on the ground, which were almost evenly distributed on the four rows. Fifteen per cent of the droplets could reach the back wall, which is more than 0.5 m away. This deposition pattern agrees well with that of droplet spots on WSP and also that in experiments of Loudon & Roberts (1967). Compared with talking, droplets from coughing dispersed longer and in a lager area.
Figure 4.
Droplet stain mark: (a) on the slide surface when no food dye was used; (b) on the slide surface when food dye was used; (c) on a WSP.
Table 1.
Total number of droplet stain marks observed on all slides (56 slides used for talking and 60 slides used for coughing) in different experiments.
| talking (counting from 1 to 100) |
coughing (20 times) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no food dye (three repeats)a | food dye | food dye with sugar | no food dye | |||||||||||||
| subject | M1 | M2 | M3 | F1 | F2 | F3 | F4 | M1 | F1 | M1 | M3 | F1 | M1 | M2 | M3 | F1 |
| number | 456 | 1173 | 41 | 96 | 395 | 40 | 58 | 518 | 113 | 303 | 335 | 183 | 169 | 133 | 38 | 91 |
| average | 323 (108 for each talking) | 315 (for each talking) | 273 (for each talking) | 108 (for each coughing) | ||||||||||||
aThese data were for subjects who counted from 1 to 100 for three times, while the rest of the experiments were done with one time talking (counting from 1 to 100 only once) or one time coughing (20 coughs) only.
Figure 5.
Number percentages of droplet stain marks observed on different slides. Unfilled bar, talking; filled bar, coughing.
The images of droplet stain marks were analysed using our image-processing software, and the sizes of the droplet stain marks could be calculated. As we know, the shapes of these stain marks will not be totally circular. Here we choose an area-equivalent diameter to represent the size of the droplet stain mark. We also need to determine the relation between the sizes of the droplets while in their original spherical state and the sizes of the stain marks that the droplets leave on evaporation after impinging and flattening upon a slide. Based on the work of Duguid (1946), we assumed that the stain marks left on glass slides were about three times the diameter of the original droplets. We also carried out simple experiments to confirm this ratio.
We also assumed that the droplet number distribution on each wall was the same as that on the slides attached to this wall. Then the total numbers of droplets inside the box were estimated from the numbers of droplet stain marks counted on the slides. The total numbers of droplets produced by each subject in different size intervals during talking (counting from 1 to 100) and coughing (20 times) are summarized in table 2. Having these data, we also calculated the percentage of droplets in different diameter ranges and cumulative percentage of droplets less than the stated diameter ranges produced in different sets of experiments, which are summarized in table 3. For talking experiments, almost all the droplets recovered were less than 500 µm. When food dye with sugar was used, a small fraction of droplets less than 5 µm were recovered. It is obvious that more droplets were produced when the food dye solution was used. During the process of counting from 1 to 100, the number of droplets produced without a food dye (average: 760) was only about one-third of the droplets produced when a food dye was used (average: 2273). In all the coughing experiments, no food dye was used and many droplets larger than 500 µm were observed. No droplets less than 5 µm were detected. An average of 800 droplets could be observed from 20 coughs.
Table 2.
Estimated total numbers of droplets in different diameter ranges emitted during talking or coughing (M, male subject; F, female subject; the sizes of droplets used the values at sampling positions).
| size range (µm) | each talking (counting from 1 to 100) |
each coughing (20 times) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no food dye |
food dye |
food dye with sugar |
no food dye |
|||||||||||||
| M1 | M2 | M3 | F1 | F2 | F3 | F4 | M1 | F1 | M1 | M3 | F1 | M1 | M2 | M3 | F1 | |
| 0–5 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 66 | 92 | 0 | 0 | 0 | 0 | 0 |
| 5–10 | 0 | 0 | 5 | 0 | 0 | 6 | 7 | 24 | 0 | 303 | 309 | 115 | 0 | 44 | 0 | 0 |
| 10–15 | 2 | 11 | 24 | 0 | 0 | 0 | 2 | 14 | 0 | 158 | 208 | 138 | 0 | 7 | 0 | 7 |
| 15–20 | 12 | 35 | 11 | 0 | 9 | 7 | 0 | 165 | 14 | 82 | 108 | 79 | 7 | 15 | 0 | 0 |
| 20–25 | 14 | 86 | 13 | 7 | 28 | 0 | 4 | 230 | 28 | 87 | 72 | 72 | 0 | 0 | 0 | 0 |
| 25–30 | 28 | 154 | 7 | 12 | 32 | 0 | 7 | 280 | 43 | 115 | 93 | 43 | 7 | 44 | 36 | 21 |
| 30–35 | 40 | 187 | 0 | 2 | 58 | 3 | 7 | 345 | 43 | 122 | 86 | 57 | 7 | 28 | 0 | 14 |
| 35–40 | 65 | 239 | 4 | 0 | 79 | 0 | 0 | 302 | 36 | 72 | 93 | 43 | 7 | 85 | 0 | 14 |
| 40–45 | 84 | 229 | 0 | 0 | 65 | 2 | 9 | 338 | 50 | 72 | 57 | 43 | 42 | 71 | 28 | 50 |
| 45–50 | 50 | 246 | 0 | 9 | 65 | 2 | 9 | 259 | 43 | 152 | 86 | 57 | 7 | 50 | 14 | 21 |
| 50–75 | 271 | 854 | 16 | 57 | 236 | 20 | 31 | 763 | 237 | 230 | 446 | 216 | 218 | 281 | 57 | 158 |
| 75–100 | 256 | 369 | 7 | 62 | 147 | 7 | 19 | 420 | 159 | 299 | 316 | 180 | 253 | 180 | 100 | 172 |
| 100–150 | 180 | 233 | 7 | 48 | 103 | 29 | 24 | 335 | 100 | 251 | 259 | 161 | 387 | 63 | 21 | 129 |
| 150–200 | 54 | 58 | 2 | 14 | 56 | 6 | 14 | 146 | 28 | 121 | 36 | 28 | 145 | 43 | 7 | 28 |
| 200–250 | 15 | 23 | 0 | 4 | 25 | 2 | 0 | 74 | 21 | 61 | 28 | 53 | 66 | 13 | 8 | 21 |
| 250–300 | 9 | 14 | 2 | 2 | 7 | 2 | 2 | 7 | 7 | 0 | 36 | 7 | 17 | 0 | 0 | |
| 300–350 | 4 | 4 | 2 | 2 | 2 | 2 | 15 | 0 | 92 | 30 | 58 | 20 | 13 | |||
| 350–400 | 7 | 4 | 4 | 2 | 7 | 0 | 8 | 7 | 0 | |||||||
| 400–450 | 0 | 2 | 2 | 0 | 0 | 17 | 0 | |||||||||
| 450–500 | 0 | 2 | 14 | 8 | 10 | 0 | ||||||||||
| 500–1000 | 3 | 14 | 69 | 8 | ||||||||||||
| 1000–1500 | 7 | |||||||||||||||
| total | 1091 | 2749 | 100 | 225 | 918 | 100 | 135 | 3738 | 809 | 2213 | 2425 | 1322 | 1331 | 952 | 271 | 648 |
| average | 760 | 2273 | 1986 | 800 | ||||||||||||
Table 3.
Percentage of droplets in different diameter ranges emitted during talking or coughing (at sampling position).
| talking (counting from 1 to 100) |
coughing (20 times) |
talking (counting from 1 to 100) |
coughing (20 times) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| size range (µm) | no food dye (T-N) (%) | food dye (T-D) (%) | food dye with sugar (T-S) (%) | no food dye (C-N) (%) | size range (µm) | no food dye (T-N) (%) | food dye (T-D) (%) | food dye with sugar (T-S) (%) | no food dye (C-N) (%) |
| 0–5 | 0.2 | 0 | 2.7 | 0 | <5 | 0.2 | 0 | 2.7 | 0 |
| 5–10 | 0.3 | 0.5 | 12.2 | 1.4 | <10 | 0.6 | 0.5 | 14.8 | 1.4 |
| 10–15 | 0.7 | 0.3 | 8.5 | 0.4 | <15 | 1.3 | 0.8 | 23.3 | 1.8 |
| 15–20 | 1.4 | 3.9 | 4.5 | 0.7 | <20 | 2.7 | 4.8 | 27.8 | 2.5 |
| 20–25 | 2.9 | 5.7 | 3.9 | 0 | <25 | 5.5 | 10.4 | 31.7 | 2.5 |
| 25–30 | 4.5 | 7.1 | 4.2 | 3.4 | <30 | 10.1 | 17.6 | 35.9 | 5.9 |
| 30–35 | 5.6 | 8.5 | 4.4 | 1.5 | <35 | 15.6 | 26.1 | 40.4 | 7.4 |
| 35–40 | 7.3 | 7.4 | 3.5 | 3.3 | <40 | 22.9 | 33.5 | 43.8 | 10.7 |
| 40–45 | 7.3 | 8.5 | 2.9 | 6.0 | <45 | 30.2 | 42.0 | 46.7 | 16.7 |
| 45–50 | 7.2 | 6.6 | 4.9 | 2.9 | <50 | 37.4 | 48.7 | 51.7 | 19.6 |
| 50–75 | 27.9 | 22.0 | 15.0 | 22.3 | <75 | 65.3 | 70.7 | 66.6 | 41.8 |
| 75–100 | 16.3 | 12.7 | 13.3 | 22.0 | <100 | 81.6 | 83.4 | 80.0 | 63.9 |
| 100–150 | 11.7 | 9.6 | 11.3 | 18.7 | <150 | 93.4 | 93.0 | 91.2 | 82.6 |
| 150–200 | 3.8 | 3.8 | 3.1 | 7.0 | <200 | 97.2 | 96.8 | 94.3 | 89.6 |
| 200–250 | 1.3 | 2.1 | 2.4 | 3.4 | <250 | 98.5 | 98.9 | 96.7 | 92.9 |
| 250–300 | 0.7 | 0.3 | 0.7 | 0.5 | <300 | 99.2 | 99.2 | 97.4 | 93.5 |
| 300–350 | 0.3 | 0.3 | 2.0 | 2.8 | <350 | 99.5 | 99.5 | 99.5 | 96.3 |
| 350–400 | 0.3 | 0.2 | 0.1 | 0.2 | <400 | 99.8 | 99.7 | 99.6 | 96.5 |
| 400–450 | 0.1 | 0 | 0 | 0.5 | <450 | 99.9 | 99.7 | 99.6 | 97.1 |
| 450–500 | 0 | 0.3 | 0.1 | 0.3 | <500 | 99.9 | 100 | 99.8 | 97.4 |
| 500–1000 | 0.1 | 0 | 0.2 | 2.4 | <1000 | 100 | 100 | 100 | 99.8 |
| 1000–1500 | 0 | 0 | 0 | 0.2 | <1500 | 100 | 100 | 100 | 100 |
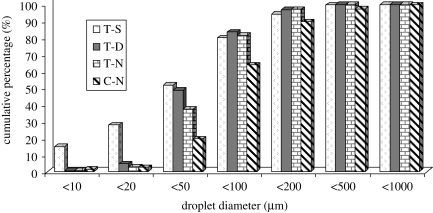
The cumulative percentages of large droplets expelled during talking and coughing are shown in figure 6. For talking experiments, when food dye with sugar was used, about 15 per cent of the droplets were less than 10 µm, 52 per cent of the droplets less than 50 µm and 80 per cent of the droplets less than 100 µm. When food dye was used, about 5 per cent of the droplets were less than 20 µm, 49 per cent of the droplets less than 50 µm and 83 per cent of the droplets less than 100 µm. When no food dye was used, only about 3 per cent of the droplets were less than 20 µm, 37 per cent of the droplets less than 50 µm and 82 per cent of the droplets less than 100 µm. More small droplets were recovered when food dye was used, especially when sugar was added. This may be because of the introduction of a food dye and sugar into the mouth. On the one hand, they would stimulate the secretion of saliva. On the other hand, droplet evaporation rate decreases if food dye and sugar were added. For all the coughing experiments, no food dye was used. About 2.5 per cent of the droplets were less than 20 µm and 1.4 per cent less than 10 µm. Only 20 per cent of the droplets were less than 50 µm and 64 per cent of the droplets less than 100 µm. Compared with talking, a higher percentage of droplets larger than 500 µm were observed. The difference in droplet size distribution may lie in the difference of droplet generation mechanism between talking and coughing.
Figure 6.
Cumulative percentage of droplets less than the stated diameter produced by talking and coughing (T-S, talking experiment with food dye with sugar used; T-D, talking experiment with food dye used; T-N, talking experiment without food dye; C-N, coughing experiment without food dye).
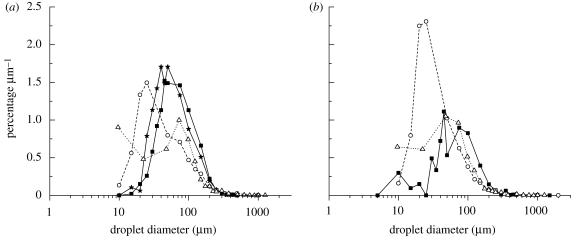
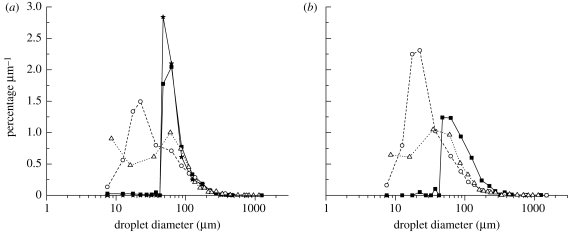
Size distribution information can be presented in many forms. Here we also divided the percentage of droplets in each interval by the width of that interval and plotted the figure of percentage µm−1 versus droplet diameter (figure 7), which includes the results in the studies of Duguid (1946; labelled as ‘T-Duguid’ and ‘C-Duguid’) and Loudon & Roberts (1967; labelled as ‘T-L&R’ and ‘C-L&R’). In this figure, only the data of droplets sampled by the surface deposition method were considered in the percentage calculation. The medium droplet size in each interval was used as the characteristic droplet diameter. This droplet size distribution curve was the graphical representation of the frequency function or probability density function. From figure 7a, we could find that the peak diameter lay between 35 and 50 µm in current talking experiments without food dye, between 30 and 45 µm in current talking experiments with food dye, between 15 and 25 µm in Duguid's (1946) study. In the study of Loudon & Roberts (1967), droplets of 8 and 100 µm in diameter almost have the same frequency. Figure 7b shows the results of coughing. The commonest diameter lay between 35 and 100 µm in current coughing experiments without food dye, between 15 and 25 µm in Duguid's (1946) study and between 22 and 73 µm in Loudon & Roberts (1967). We notice that a larger proportion of droplets between 5 and 20 µm could be generated during talking than coughing when no food dye was used, which also can be seen in figure 6. However, there is no great difference between the size distributions of droplets produced by the different types of expiratory activities. As to the effect of food dye, because the introduction of food dye slows down the droplet evaporation, the peak of the frequency function moves towards larger droplet diameter when no food dye was used, especially when compared with Duguid's (1946) results. This large difference may also be explained by the different droplet collection methods used. Droplets were collected inside a box in the current study, while in Duguid's (1946) study droplet spray was directed at a slide in front of the mouth. Droplets experienced different evaporation times during their aerial transport.
Figure 7.
Percentage µm−1 versus droplet diameter detected on the sampling slides: (a) talking (T-N, talking experiment without food dye (filled square); T-D, talking experiment with food dye used (filled star); T-Duguid, talking experiment in Duguid (1946) (open circle); T-L&R, talking experiment in Loudon & Roberts (1967) (open triangle)); (b) coughing (C-N, coughing experiment without food dye (filled square); C-Duguid, coughing experiment in Duguid (1946) (open circle); C-L&R, coughing experiment in Loudon & Roberts (1967) (open triangle)). (Note: see the discussion in the text on the calculation of the vertical axis value.)
3.2. Total mass of droplets
The total mass of droplets collected using surgical face mask and plastic bag with tissue inside is shown in table 4. Considerable subject variability was observed, which coincides with that in droplet size distribution measurements. An average of 22.9 mg of fluid was obtained during 20 coughs using the surgical face mask method and 85 mg of fluid using a plastic bag with tissue. And averages of 18.7 and 79.4 mg of fluid were measured using mask and plastic bag, respectively, during counting to 100.
Table 4.
Total mass of droplets collected using surgical face mask and plastic bag with tissue inside.
| weight (mg) |
average (mg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| activity | M1 | M2 | M3 | M4 | M5 | M6 | M7 | F1 | F2 | |
| talking (counting from 1 to 100) | ||||||||||
| mask | 3.7 | 41.8 | 61.3 | — | — | — | — | 15.7 | 1 | 18.7 |
| 5.5 | 12.6 | 7.7 | ||||||||
| bag | 69.4 | 48.8 | 121.7 | — | — | — | — | 113.7 | 66.2 | 79.4 |
| 38.6 | 151.5 | |||||||||
| coughing (20 times) | ||||||||||
| mask | 44.9 | 30.4 | 17.5 | — | — | — | — | 15.1 | 4.6 | 22.9 |
| 31.5 | ||||||||||
| 16.2 | ||||||||||
| bag | 154.5 | 87.8 | 85.8 | 62.7 | 67.4 | 41.8 | 91.9 | 41.4 | 55.4 | 85.0 |
4. Discussion
The droplet numbers and sizes presented above were obtained on glass slides at different sampling positions. From the droplet origin (mouth) to the sampling position, a droplet would evaporate and its size would shrink. So it is not enough to know the size distribution of droplets detected on sampling slides, which is not the real size distribution of droplets generated during expiratory activities. We need to know the droplet size at the origin. How much one droplet loses water is predominantly determined by the time it takes to fly from the droplet origin (mouth) to the sampling position, which we could call the ‘residence time’. Because of the limitation of the experimental design, we could not know the exact value of residence time for each droplet. To roughly estimate the droplet size change, we assume that the residence time equals the time for the droplet freely falling to the same height of sampling position. During the experiments, we also recorded the air temperature and relative humidity inside the box. The average temperature was 28°C and relative humidity was 70 per cent. Using the evaporation model for a freely falling pure liquid droplet described in Xie et al. (2007), we could roughly back-calculate the droplet size at the origin.
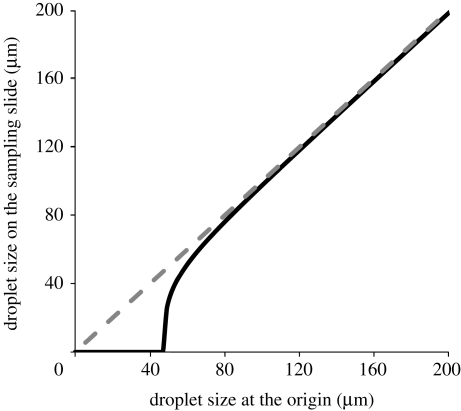
Figure 8 shows the relationship between droplet size at the origin and droplet size on the sampling slide when the sampling slide is placed on the ground of the box. Because of droplet evaporation, the droplet size at the sampling position is smaller than the droplet size at the origin. That is why in figure 8 the black line is below the dashed line. When released from the same height, larger droplets would quickly reach the ground and their size would not change too much, while smaller droplets would evaporate quickly during the relatively slow falling process. From figure 8, we can see that if the droplet size at the origin is less than 47 µm, the droplet would dry out before it reaches the ground and could not form a droplet stain mark on the glass slide. When the droplet size at the origin is larger than 80 µm, droplet size changes very little.
Figure 8.
Relationship between droplet size at the origin and droplet size on the sampling slide. Black line, ground wall of the box; dashed line, Y = X.
Figure 5 shows that almost all the droplets were detected on the ground. Among the droplets detected on the box walls, more than 65 per cent of the droplets produced by talking are smaller than 75 µm. More than 40 per cent of the droplets produced during coughing are smaller than 75 µm. According to figure 8, these droplets should have larger sizes at the origin, i.e. the mouth. Table 5 summarizes the measured size distribution of droplets detected at the sampling positions and the estimated size distribution of droplets at the origin. The percentages and cumulative percentages of droplets in different diameter ranges were calculated, respectively. We can see the size shift. At the origin, more droplets fall into a size range of 50–100 µm. For talking, only less than 10 per cent of the droplets were less than 50 µm and more than 50 per cent of the droplets were in the size range of 50–75 µm when no food dye was used. And for coughing, about 7 per cent of the droplets were less than 50 µm and more than 30 per cent of the droplets in the size range of 50–75 µm. We could also plot the figure of percentage µm−1 versus droplet diameter (figure 9) using the estimated data of droplet sizes at the origin. The peak diameter lay between 45 and 75 µm in current talking and coughing experiments. It indicated that using the current deposition method only large droplets generated from the expiratory activities could be sampled. A methodology that can cover the whole size range is needed.
Table 5.
Percentage of droplets in different diameter ranges emitted at the origin (i.e. mouth, estimated by using a simple evaporation model) during talking or coughing.
| size range (µm) | talking (counting from 1 to 100) |
coughing (20 times) |
||||||
|---|---|---|---|---|---|---|---|---|
| no food dye (T-N) |
food dye (T-D) |
food dye with sugar (T-S) |
no food dye (C-N) |
|||||
| sampling position (%) | mouth origin (%) | sampling position (%) | mouth origin (%) | sampling position (%) | mouth origin (%) | sampling position (%) | mouth origin (%) | |
| 1–2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2–4 | 0.1 | 0 | 0 | 0 | 0.9 | 0 | 0 | 0 |
| 4–8 | 0.3 | 0.1 | 0.4 | 0 | 7.4 | 0 | 1.1 | 0 |
| 8–16 | 1.1 | 0.3 | 0.3 | 0 | 15.8 | 0.3 | 0.9 | 0 |
| 16–24 | 3.3 | 0.1 | 6.6 | 0 | 6.9 | 0.1 | 0.7 | 0.3 |
| 24–32 | 7.6 | 0.2 | 11.1 | 0 | 5.7 | 0 | 4.0 | 0 |
| 32–40 | 10.4 | 0.2 | 12.3 | 0 | 6.6 | 0.4 | 4.0 | 0.5 |
| 40–50 | 14.4 | 8.9 | 16.6 | 14.2 | 8.1 | 34.2 | 8.9 | 6.2 |
| 50–75 | 27.5 | 51.1 | 22.8 | 52.5 | 14.6 | 28.4 | 22.2 | 30.8 |
| 75–100 | 16.5 | 19.3 | 12.5 | 15.2 | 14.0 | 15.4 | 22.0 | 23.4 |
| 100–125 | 7.7 | 8.3 | 6.3 | 6.3 | 6.6 | 7.3 | 12.3 | 15.0 |
| 125–150 | 4.2 | 4.6 | 3.9 | 4.4 | 4.9 | 5.0 | 6.4 | 5.9 |
| 150–200 | 3.9 | 4.0 | 4.0 | 4.0 | 3.4 | 3.6 | 7.0 | 7.4 |
| 200–250 | 1.3 | 1.3 | 2.1 | 2.3 | 2.8 | 2.8 | 3.4 | 3.4 |
| 250–500 | 1.6 | 1.6 | 1.1 | 1.1 | 2.2 | 2.2 | 4.5 | 4.5 |
| 500–1000 | 0.1 | 0.1 | 0.4 | 0.4 | 2.4 | 2.4 | ||
| 1000–2000 | 0.2 | 0.2 | ||||||
Figure 9.
Percentage µm−1 versus droplet diameter estimated at the origin: (a) talking (T-N, talking experiment without food dye (filled square); T-D, talking experiment with food dye used (filled star); T-Duguid, talking experiment in Duguid (1946) (open circle); T-L&R, talking experiment in Loudon & Roberts (1967) (open triangle)); (b) coughing (C-N, coughing experiment without food dye (filled square); C-Duguid, coughing experiment in Duguid (1946) (open circle); C-L&R, coughing experiment in Loudon & Roberts (1967) (open triangle)). (Note: see the discussion in the text on the calculation of the vertical axis value.)
In the literature, Duguid (1945, 1946) found that the average number of expelled droplets was 250 by counting aloud from 1 to 100. And an average of 1764 droplets were obtained by Loudon & Roberts (1967) for the same expiratory activity, in which 1652 droplets were recovered from droplet stain marks. Food dye was used in both studies. In our experiments, we obtained an average of 760 droplets for talking without food dye (2273 when food dye was used) using glass slides. The average number of droplets produced during talking when food dye was used did not differ greatly from that noted by Loudon & Roberts (1967), but was higher than that recorded by Duguid (1946), even when no food dye was used. This may be because of the vigour and loudness of the talking as pointed out by Loudon & Roberts (1967) or the different types of food dye that perhaps could influence the secretion of saliva. In our experiments, five subjects are Chinese and only two subjects are native English speakers. The latter two non-Chinese subjects produced relatively fewer droplets than all other subjects. Difference in pronunciation may induce the number difference of droplet generation. Inouye (2003) suggested that the efficiency of transmission of severe acute respiratory syndrome (SARS) by talking might be affected by the spoken language. The aspiration pronunciation system in different languages is different, and aspiration could generate droplets. More studies and more samples are needed to support this hypothesis.
As to coughing, an average of 5000 droplets by a cough with mouth initially closed was reported in Duguid (1945, 1946), and 464 by one ‘natural’ cough recorded in Loudon & Roberts (1967), of which 237 were recovered from droplet stain marks. Food dye was used in both studies. On average, we only found 40 droplets in one natural cough without food dye, which is far less than those found by Duguid (1946) and Loudon & Roberts (1967). As mentioned in Loudon & Roberts (1967), many factors have effects on the numbers of droplets, such as the amount of secretion present in the mouth and its location, and the placement and movement of lips, tongue and teeth during the cough. Based on the results of talking, the usage of food dye would be a reason to explain the above difference. Moreover, in Duguid's (1946) study, the health status of test subjects was not described. The subjects were healthy both in the study of Loudon & Roberts (1967) and in the current study. As we know, it may be difficult for healthy people to produce violent coughs. The violence of coughs would be different for patients with respiratory diseases and thus has an effect on droplet generation, as well as more secretions of fluids on airway surfaces and higher frequency of coughing (Papineni & Rosenthal 1997). All these factors are difficult to control and to quantify.
In the measurements of total droplet mass generated during talking and coughing, the average value obtained with a surgical mask was much less than that when a plastic bag with a tissue was used. This could be explained by the condensation of water vapour in the exhaled breath when a plastic bag was used. In the measurements of droplet size distribution, we obtained the size data of each droplet captured at the sampling position. We also back-calculated the corresponding droplet size at the origin. Having these two droplet diameters, and by assuming the density of these droplets as the same as pure water droplets, we could estimate the mass of each droplet both at sampling position and at the origin. Adding the mass of all the droplets captured together, we have the total droplet mass data for each experiment which are summarized in table 6. ‘S’ means the total mass of droplets detected at sampling position and ‘O’ means the total mass of droplets at the origin. Compared with the estimated droplet mass data in table 6, the measured results using surgical face mask were much larger, even if we doubled the density of the droplets.
Table 6.
Total mass of droplets calculated using measured droplet number and size data during talking or coughing (S, the total mass of droplets detected at sampling position; O, the total mass of droplets at the origin).
| talking (counting from 1 to 100) |
coughing (20 times) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| no food dye |
food dye |
food dye with sugar |
no food dye |
|||||||||||||
| M1 | M2 | M3 | F1 | F2 | F3 | F4 | M1 | F1 | M1 | M3 | F1 | M1 | M2 | M3 | F1 | |
| droplet number | 1091 | 2749 | 100 | 225 | 918 | 100 | 135 | 3738 | 809 | 2213 | 2425 | 1322 | 1331 | 952 | 271 | 648 |
| weight (mg) S (O) | 0.94 (1.00) | 1.41 (1.53) | 0.29 (0.30) | 0.49 (0.50) | 0.97 (1.02) | 0.15 (0.15) | 0.11 (0.12) | 3.23 (3.43) | 0.52 (0.56) | 3.67 (3.80) | 3.56 (3.71) | 1.36 (1.43) | 20.44 (20.56) | 1.62 (1.66) | 0.13 (0.14) | 0.55 (0.58) |
| average number | 760 | 2273 | 1986 | 800 | ||||||||||||
| average weight (mg) S (O) | 0.59 (0.63) | 1.88 (2.00) | 2.86 (2.98) | 5.68 (5.74) | ||||||||||||
It is difficult to capture all the droplets produced during expiratory activities using the methods in the current study and previous studies. If we have the size distribution, the total number of droplets produced may be estimated from the total mass measurement. In the literature, we only found one study that measured the total mass. Zhu et al. (2006) reported that an average of 6.7 mg of saliva per cough was collected on the mask. In the current study, an average of 1.1 mg of fluid was obtained per cough using the surgical face mask method and 4.2 mg of fluid using plastic bag with tissue, less than 6.7 mg reported by Zhu et al. (2006). And averages of 18.7 and 79.4 mg of fluid were measured using mask and plastic bag, respectively, during counting from 1 to 100. Actually, both methods have their limitations. The mask and plastic bag could not be fitted to the face perfectly. There would be gaps and droplets may have escaped from these gaps during coughing. Droplet evaporation also occurs in the process. Saliva on the lips may touch the mask or plastic bag. Condensation of water vapour in the exhaled breath occurs in the plastic bag. Droplets may be re-inhaled in the plastic bag. All these factors would influence the results.
However, as seen in tables 4 and 6, we found that the total mass measured from experiments was much larger than that calculated from measurements of droplet numbers and sizes. This could be explained as both experiments have limitations. In the experiments of measuring droplet numbers and sizes, only part of the droplets expelled was captured, which was indicated from the size range covered, and in the experiments of measuring droplet total mass, maybe more respiratory droplets were involved.
5. Conclusions
This study demonstrates the feasibility of measuring the respiratory droplets produced during talking and coughing without a dye, by which expiratory activities are natural compared with the subject's behaviour when a food dye is used. The glass slide method shows considerable promise, although scanning and analysing the droplet stain marks are very time consuming. The study also supports the belief that talking and coughing play important roles in the generation of respiratory droplets and provides more information about respiratory droplets produced by healthy subjects. More droplets were generated when food dye was used. There was no great difference about the size distributions of droplets produced when food dye was used or not used, nor between talking and coughing. More small droplets were produced in the more violent activity of coughing.
Acknowledgements
Ethical approval for the experimental study was obtained from the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster.
The work was supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (project no. HKU 7150/06). We thank post graduate student volunteers at the Department of Mechanical Engineering for participating in the tests.
Footnotes
One contribution of 10 to a Theme Supplement ‘Airborne transmission of disease in hospitals’.
References
- Duguid J. F. 1945. The numbers and the sites of origin of the droplets expelled during expiratory activities. Edinburgh Med. J. 52, 385–401. [PMC free article] [PubMed] [Google Scholar]
- Duguid J. F. 1946. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. J. Hyg. 4, 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild C. I., Stamper J. F. 1987. Particle concentration in exhaled breath. Am. Ind. Hyg. Assoc. J. 48, 948–949. [DOI] [PubMed] [Google Scholar]
- Fennelly K. P., Martyny J. W., Fulton K. E., Orme I. M., Cave D. M., Heifets L. B. 2004. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am. J. Resp. Crit. Care Med. 169, 604–609. ( 10.1164/rccm.200308-1101OC) [DOI] [PubMed] [Google Scholar]
- Garner J. S. 1996. Guideline for isolation precautions in hospitals. The Hospital Infection Control Practices Advisory Committee. Infect. Control Hosp. Epidemiol. 17, 53–80. ( 10.1086/647190) [DOI] [PubMed] [Google Scholar]
- Inouye S. 2003. SARS transmission: language and droplet production. Lancet 362, 170 ( 10.1016/S0140-6736(03)13874-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennison M. W. 1942. Atomizing of mouth and nose secretions into the air as revealed by high speed photograph. Aerobiology 17, 106–128. [Google Scholar]
- Li Y. G., Huang X., Yu I. T. S., Wong T. W., Qian H. 2005. Role of air distribution in SARS transmission during the largest nosocomial outbreak in Hong Kong. Indoor Air 15, 83–95. ( 10.1111/j.1600-0668.2004.00317.x) [DOI] [PubMed] [Google Scholar]
- Loudon R. G., Roberts R. M. 1967. Droplet expulsion from the respiratory tract. Am. Rev. Resp. Dis. 95, 435–442. [DOI] [PubMed] [Google Scholar]
- Morawska L. 2006. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air 16, 335–347. ( 10.1111/j.1600-0668.2006.00432.x) [DOI] [PubMed] [Google Scholar]
- Nicas M., Nazaroff W. W., Hubbard A. 2005. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J. Occup. Environ. Hyg. 2, 143–154. ( 10.1080/15459620590918466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papineni R. S., Rosenthal F. S. 1997. The size distribution of droplets in the exhaled breath of healthy human subjects. J. Aerosol Med. 10, 105–116. ( 10.1089/jam.1997.10.105) [DOI] [PubMed] [Google Scholar]
- Qian H., Li Y., Nielsen P. V., Hyldgaard C. E., Wong T. W., Chwang A. T. Y. 2006. Dispersion of exhaled droplet nuclei in a two-bed hospital ward with three different ventilation systems. Indoor Air 16, 111–128. ( 10.1111/j.1600-0668.2005.00407.x) [DOI] [PubMed] [Google Scholar]
- Wan M. P., Chao C. Y. H. 2007. Transport characteristics of expiratory droplets and droplet nuclei in indoor environments with different ventilation airflow patterns. J. Biomech. Eng. 129, 341–353. ( 10.1115/1.2720911) [DOI] [PubMed] [Google Scholar]
- Wells W. F. 1934. On air-borne infection. Study II. Droplets and droplet nuclei. Am. J. Hyg. 20, 611–618. [Google Scholar]
- Xie X. J., Li Y. G., Chwang A. T. Y., Ho P. L., Seto W. H. 2007. How far droplets can move in indoor environments—revisiting the Wells evaporation–falling curve. Indoor Air 17, 211–225. ( 10.1111/j.1600-0668.2007.00469.x) [DOI] [PubMed] [Google Scholar]
- Yang S., Lee G. W. M., Chen C. M., Wu C. C., Yu K. P. 2007. The size and concentration of droplets generated by coughing in human subjects. J. Aerosol Med. 20, 484–494. ( 10.1089/jam.2007.0610) [DOI] [PubMed] [Google Scholar]
- Zhu S. W., Kato S., Yang J. H. 2006. Study on transport characteristics of saliva droplets produced by coughing in a calm indoor environment. Build. Environ. 41, 1691–1702. ( 10.1016/j.buildenv.2005.06.024) [DOI] [Google Scholar]