Abstract
Well-focused X-ray beams, generated by advanced synchrotron radiation facilities, yielded high-resolution diffraction data from crystals of ribosomes, the cellular nano-machines that translate the genetic code into proteins. These structures revealed the decoding mechanism, localized the mRNA path and the positions of the tRNA molecules in the ribosome and illuminated the interactions of the ribosome with initiation, release and recycling factors. They also showed that the ribosome is a ribozyme whose active site is situated within a universal symmetrical region that is embedded in the otherwise asymmetric ribosome structure. As this highly conserved region provides the machinery required for peptide bond formation and for ribosome polymerase activity, it may be the remnant of the proto-ribosome, a dimeric pre-biotic machine that formed peptide bonds and non-coded polypeptide chains. Synchrotron radiation also enabled the determination of structures of complexes of ribosomes with antibiotics targeting them, which revealed the principles allowing for their clinical use, revealed resistance mechanisms and showed the bases for discriminating pathogens from hosts, hence providing valuable structural information for antibiotics improvement.
Keywords: evolving ribosomes, peptide bond formation, synchrotron radiation
1. Introduction
Ribosomes, the universal cellular riboprotein assemblies, are the nano-machines that translate the genetic code into proteins by providing the framework for proper positioning of the other participants in this fundamental process, thus enabling decoding, successive peptide bond formation and the protection of the nascent protein chains. Ribosomes act rapidly and efficiently, producing proteins on a continuous basis at an incredible speed of approximately 20 peptide bonds per second. Within the framework of living cells, ribosomes are giant assemblies composed of many different proteins (r-proteins) and long ribosomal RNA (rRNA) chains. The ratio of rRNA to r-proteins (approx. 2 : 1) is maintained throughout evolution, with the exception of the mammalian mitochondrial ribosome, in which almost half of the bacterial rRNA is replaced by r-proteins. All ribosomes are constituted by two unequal subunits. In prokaryotes, the small subunit, denoted 30S, contains an RNA chain (16S) of about 1500 nucleotides and 20–21 different proteins, whereas the large subunit (called 50S in prokaryotes) has two RNA chains (23S and 5S RNA) of about 3000 nucleotides in total and 31–35 different proteins. In all organisms, the two subunits exist independently and associate to form functionally active ribosomes. In each, the ribosomal proteins are entangled within the complex rRNA conformation, thus maintaining a striking dynamic architecture that is ingeniously designed for ribosome functions: precise decoding, substrate-mediated peptide bond formation and efficient polymerase activity.
Other players in the process are messenger RNA (mRNA), which carries the genetic code, and transfer RNA (tRNA) molecules, which bring the cognate amino acids to the ribosome. For increasing the efficiency, a large number of ribosomes act simultaneously as polymerases synthesizing proteins by one-at-a-time addition of amino acids to a growing peptide chain while translocating along the mRNA template. While the elongation of the nascent chain proceeds, the two subunits perform cooperatively. The small subunit provides the path along which the mRNA progresses, the decoding centre and the mechanism controlling translation fidelity, and the large subunit contains the site for the main ribosomal catalytic function, polymerization of amino acids and the protein exit tunnel.
Currently, many of the mechanisms involved in ribosome functions are rather well understood, owing to the crystal structures of ribosomes and their complexes, which became available at the turn of the millennium. Among those are the decoding mechanism (reviewed in Ogle et al. 2003), the mRNA progression mode (Yusupova et al. 2006), the relative positions of the A-, P- and E-tRNAs (Yusupov et al. 2001), the way the initiation and the termination of the elongation cycle are modulated by initiation factors (Carter et al. 2001; Pioletti et al. 2001), release (Laurberg et al. 2008; Weixlbaumer et al. 2008) and recycling factors (Wilson et al. 2005; Borovinskaya et al. 2007) and the provision of the architectural and dynamic elements required for amino acid polymerization (Bashan et al. 2003; Bashan & Yonath 2008b).
The involvement of RNA-rich particles in genetic expression was suggested over five decades ago, when the so-called ‘Palade particles’ were located within RNA-rich regions, in close association with the membrane of the endoplasmic reticulum (Palade 1955; Watson 1963), in accordance with the idea that the ribosome ancestor was made exclusively of RNA (Crick 1968). The localization of the cellular translation site and the extensive biochemical research that followed yielded illuminating findings about the overall nature of the ribosome function, but detailed functional information was not available because of the lack of three-dimensional structures, and hence led to several common wisdom hypotheses that underwent significant alterations once the structures became available. Striking examples of conceptual revolutions in the understanding of the ribosomal function (reviewed in Wekselman et al. 2008) relate to the functional contribution of the different ribosomal components and the path taken by nascent chains. Originally, it was assumed that decoding of the genetic code and peptide bond formation are performed by r-proteins, while rRNA provides the ribosome scaffold (Garrett & Wittmann 1973). This assumption was challenged (Noller et al. 1992), and met with scepticism, although the major roles played by RNA molecules in various life processes became evident around this period. Shifting the focus from the r-proteins to the rRNA was proved to be right a decade later, when the high-resolution structures showed that both the decoding centre and the site of peptide bond formation (called peptidyl transferase centre or PTC) reside in rRNA predominant environments.
Another assumption was that nascent proteins reside on the ribosome surface until maturation. Even after biochemical experiments indicating nascent chain protection by the ribosome (Malkin & Rich 1967; Sabatini & Blobel 1970) and visualizing this tunnel in EM reconstructions from two-dimensional sheets (Milligan & Unwin 1986; Yonath et al. 1987), doubt was publicly expressed (Moore 1988; Ryabova et al. 1988) for almost a decade, until verified by cryo-electron microscopy (Frank et al. 1995; Stark et al. 1995). Remarkably, once a tunnel of dimensions matching those predicted in the 1960s (Malkin & Rich 1967) was observed in high-resolution crystal structures, it was suggested to be of a Teflon-like character, with no dynamics and/or chemical properties allowing interactions with progressing nascent chains (Ban et al. 2000; Nissen et al. 2000), although this assumption conflicted with previous observations (e.g. Nagano et al. 1991; Crowley et al. 1993; Walter & Johnson 1994). However, further results of biochemical, microscopical and computational experiments clearly showed that this tunnel participates actively in nascent chain progression, arrest and cellular signalling (e.g. Gabashvili et al. 2001; Gong & Yanofsky 2002; Nakatogawa & Ito 2002; Berisio et al. 2003, 2006; Gilbert et al. 2004; Johnson & Jensen 2004; Woolhead et al. 2004, 2006; Amit et al. 2005; Ziv et al. 2005; Cruz-Vera et al. 2006; Kaiser et al. 2006; Mankin 2006; Mitra et al. 2006; Tenson & Mankin 2006; Voss et al. 2006; Deane et al. 2007; Schaffitzel & Ban 2007; Petrone et al. 2008), and that in eubacteria, nascent proteins progress along this tunnel and emerge into a shelter formed by chaperones, preventing aggregation and misfolding (Baram et al. 2005; Schluenzen et al. 2005).
This review describes selected events in the chronological progress of ribosomal crystallography, the innovation procedures and the crucial role played by the big facilities (reviewed in Gluehmann et al. 2001). It focuses on the structural and dynamic properties of the ribosome that enable its function as an efficient nano-machine, mentions how antibiotics can hamper its function and addresses issues relating to the origin of ribosomes.
2. From in-house X-ray generators to advanced synchrotron radiation facilities
Ribosomes have been considered non-crystallizable owing to their high degree of internal mobility, flexibility, functional heterogeneity, marked tendency to deteriorate, chemical complexity, large size and asymmetric nature. Nevertheless, because of the major significance of ribosomes for cell vitality, attempts at the crystallization of ribosomal particles have been made worldwide for over two decades since they were discovered and chemically characterized, all of which were found to be unproductive. The first three-dimensional micro-crystals of ribosomal particles, diffracting to relatively high resolution, 3.5 Å, were obtained at the beginning of the 1980s (Yonath et al. 1980). This breakthrough was based on the presumptions that the higher the sample homogeneity, the better the crystals, and that the preferred conformation is that of the functionally active ribosomes. Consequently, highly active ribosomes of bacteria that grow under robust conditions were selected. The first crystals were obtained from the large ribosomal subunits from Bacillus stearothermophilus (B50S), a source considered to be almost an extremophile at the beginning of the 1980s. A few years later, crystals were obtained from the large ribosomal subunits of the extreme halophilic bacterium Halobacterium marismortui, which lives in the Dead Sea (Shevack et al. 1985). In 1987, 7 years after the first crystallization of ribosomal particles, parallel efforts led to the growth of crystals of the small ribosomal subunit (Yusupov et al. 1987) and of the entire ribosome (Trakhanov et al. 1987) from the extreme thermophilic bacterium Thermus thermophilus.
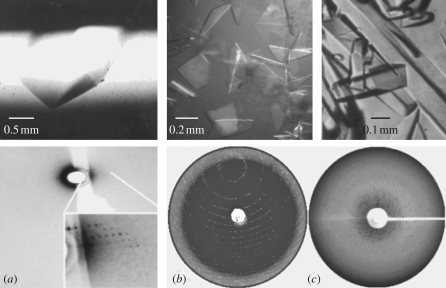
At that time, it was widely assumed that ribosome structure may never be determined because it was clear that alongside the improvement of ribosome crystals, ribosome crystallography required the development of innovative methodologies. Thus, because of the weak diffraction power of the ribosome crystals, even the most advanced rotating anode generators were not sufficiently powerful to yield suitable diffraction patterns and synchrotron radiation was at its embryonic stages. Hence, only a few diffraction spots could be recorded (Yonath et al. 1984) even when irradiating extremely large crystals (approx. 2 mm in length) with their X-ray beams (figure 1a). When more suitable synchrotron sources became available, the severe radiation sensitivity of the ribosomal crystals caused extremely fast crystal decay. Hence, pioneering cryo-data collection became crucial (Hope et al. 1989), and once established, it yielded interpretable diffraction patterns at high resolution even from extremely thin crystals (figure 1b). Additionally, multi-heavy atom clusters suitable for phasing were identified (Thygesen et al. 1996). One of these clusters, originally used for providing anomalous phasing power, was found to play a dual role in the determination of the structure of the small ribosomal subunit from T. thermophilus (T30S). Thus, post-crystallization treatment with these clusters dramatically increased the resolution from the initial 7–9 (Yonath et al. 1988) to 3 Å (figure 1c; Schluenzen et al. 2000), presumably by minimizing the internal flexibility required for facilitating mRNA binding and progression through the ribosome (Bashan & Yonath 2008a).
Figure 1.
From poor to useful diffraction of ribosomal crystals: (a) the tip of an approximately 2 mm long crystal of B50S and its diffraction pattern obtained in 1984 at the EMBL beam line at DESY/Hamburg at 4°C. (b) Crystals of H50S and their diffraction pattern obtained in 1998 at ID13 ESRF at −180°C. Note that the diffraction extends to 2.8 Å, although the crystals are extremely thin. (c) Diffraction pattern obtained from a multi-tungsten-cluster treated T30S crystal in 1998 at 19ID/APS/ANL at −180°C.
Continuous efforts aimed at improving crystals included the assessment of the influence of the relative concentrations of mono- and di-valent ions (von Bohlen et al. 1991) on crystal properties, which led to dramatic improvements in the quality of the crystals from the large ribosomal subunits from H. marismortui (H50S). Also, constant refinements of bacterial growth (Auerbach-Nevo et al. 2005) alongside a thorough investigation on crystallization conditions (Zimmerman & Yonath 2009) indicated a noteworthy correlation between the conditions under which these ribosomes function and the quality of the resulting crystals. Along these lines, it is worth mentioning that flexible regions were detected in electron-density maps obtained from ribosomal crystals grown under close to physiological conditions (Harms et al. 2001) whereas the same regions were highly disordered in crystals obtained under conditions far from their physiological environment (Ban et al. 2000). An alternative strategy for crystal refinement was to crystallize complexes of ribosomes with substrates, inhibitors and/or factors that can trap them at preferred orientations. Indeed, the initial diffracting crystals of the whole ribosome from T. thermophilus (T70S) with mRNA and tRNA molecules diffracted to rather low resolution (Hansen et al. 1990). The advances of the brightness and collimation of synchrotron radiation X-ray beams, the installation of advanced detectors and the introduction of cryo-bio-crystallographic techniques (Hope et al. 1989) yielded impressive advances in resolution from many crystal forms, including of functional complexes (Yusupov et al. 2001; Korostelev et al. 2006; Selmer et al. 2006; Yusupova et al. 2006; Voorhees et al. 2009). Also, these techniques enabled structure determination of ribosomes trapped at a specific, albeit not necessarily functional, conformation (Schuwirth et al. 2005).
In parallel, the favourable properties and the high quality of the currently available X-ray beam lines enabled the determination of structures of over two dozen complexes of ribosomes with the antibiotics targeting them. These revealed the principles allowing for clinical use, illuminated mechanisms for acquiring resistance and showed the bases for discrimination between pathogens and host cells, and hence provided the structural bases for antibiotics improvement. Owing to space limitations, only the main principles of this very important topic are mentioned here. It was found that antibiotics target ribosomes at distinct locations within functionally relevant sites, mostly composed solely of rRNA. They exert their inhibitory action by diverse modes, including competing with substrate binding, interfering with ribosomal dynamics, minimizing ribosomal mobility, facilitating miscoding, hampering the progression of the mRNA chain and blocking the nascent protein exit tunnel. Although the ribosomes are highly conserved organelles, they possess subtle sequence and/or conformational variations, which enable drug selectivity, thus facilitating clinical usage.
The structural implications of these differences were deciphered by comparisons of high-resolution structures of complexes of antibiotics with ribosomal particles from eubacteria resembling pathogens, Deinococcus radiodurans, and of an archaeon that shares properties with eukaryotes (Yonath & Bashan 2004; Yonath 2005). The various antibiotic-binding modes detected in these structures demonstrate that members of antibiotic families possessing common chemical elements with minute differences might bind to ribosomal pockets in significantly different modes, governed by their chemical properties, even when the nucleotide determining binding was mutated to resemble eukaryotes (Tu et al. 2005). On the other hand, the same binding pockets may accommodate chemically different antibiotics. Similarly, the nature of seemingly identical mechanisms of drug resistance is dominated, directly or via cellular effects, by the antibiotics' chemical properties (Davidovich et al. 2007, 2008). The observed variability in antibiotic-binding and inhibitory modes justifies expectations for structurally based improved properties of existing compounds as well as for the discovery of novel drug classes. Detailed accounts can be found in several recent reviews (e.g. Auerbach et al. 2004; Yonath & Bashan 2004; Poehlsgaard & Douthwaite 2005; Yonath 2005; Bottger 2006, 2007; Tenson & Mankin 2006).
3. Ribosome polymerase activity
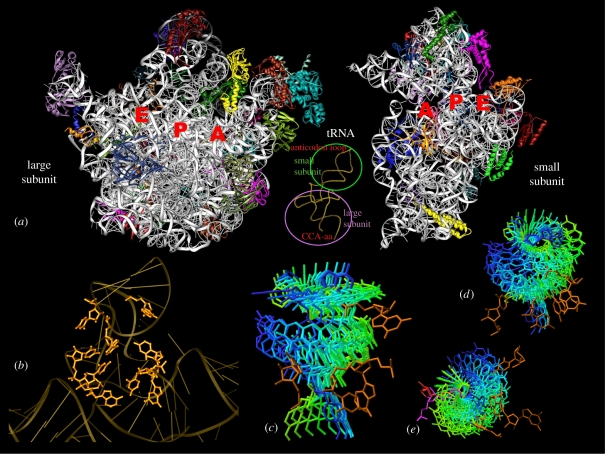
The recent availability of crystal structures of bacterial ribosomes and their complexes, all obtained by advanced synchrotron radiation, enabled a quantum jump in the understanding of the machinery of protein biosynthesis. These structures showed that the interface surfaces of both ribosomal subunits are outstandingly rich in RNA, and its two active sites—the decoding region and the PTC—are made exclusively of RNA components. Hence, the ribosome is a ribozyme. The PTC is situated within a highly conserved universal symmetrical region (figure 2) that is embedded in the otherwise asymmetric structure, and this region provides the machinery required for peptide bond formation and for ribosome polymerase activity, the latter being of particular significance for smooth production of the nascent proteins. The substrates for this reaction are aminoacylated or peptidylated tRNA molecules. The three-dimensional structures of all tRNA molecules from all living cells across evolution are alike, although each of them is specific to its amino acid. They are built mainly of double-helical L-shaped molecules in a stem–elbow–stem organization, and contain a loop complementing the three-nucleotide codes on the mRNA (figure 2). About 70 Å away, at their 3′ends, they contain a single strand with the universal sequence CCA, to which the cognate amino acid is bound by an ester bond. The tRNA molecules are the non-ribosomal entities combining the two subunits, as each of their three binding sites, A-(aminoacyl), P-(peptidyl) and (exit), resides on both subunits. At the A- and P-sites, the tRNA anticodon loops interact with the mRNA on the small subunit, and the acceptor stem with the aminoacylated or peptidylated 3′end is located on the large subunit. A- to P-site tRNA translocation comprises two highly correlated motions: sideways shift and a ribosomal navigated rotatory motion (figure 2; Agmon et al. 2003, 2005, 2006, 2009; Bashan et al. 2003; Sato et al. 2006; Bashan & Yonath 2008b), during which peptide bonds are being formed (Gindulyte et al. 2006). This process also involves the translocation of the tRNA 3′end from the A- to the P-site, the detachment of the P-site tRNA from the growing polypeptide chain, the passage of the deacylated tRNA molecule to the E-site and its subsequent release.
Figure 2.
The PTC and the passage of the tRNA 3′end by rotatory motion. (a) The interface faces as seen in the 3 Å structures of the two ribosomal subunits of the eubacterium D. radiodurans (rRNA is shown in silver, and each of the r-proteins is shown in a different colour). Note that these interfaces are rich in RNA. Inset: the backbone of a tRNA molecule. The circles designate the regions interacting with each of the ribosomal subunits. (b) A view into the PTC backbone. The nucleotides located in proximity to the substrates are shown in detail. (c–e) Snapshots of the tRNA 3′end passage from A- to P-site, represented by the transition from the A-site aminoacylated tRNA (in blue) to the P-site (in green), obtained computationally by successive rotations by 15° each around the bond connecting the 3′end to the rest of the tRNA, with the ribosomal nucleotides that interact with the motion (in gold). (a) An orthogonal view and (d,e) two side views are shown. (e) The two flexible nucleotides (A2602 and U2585) that seem to anchor and propel this motion are shown in red and magenta, respectively.
Although aminoacylated tRNA molecules are the natural substrates of ribosomes, ‘minimal substrates’ or ‘fragment reaction substrates’, which are capable of forming single peptide bonds, are the substrate analogues commonly used in biochemical experiments. Despite being small and consequently presumed to be readily diffused into their locations within the ribosome, the reactions with these compounds are significantly slower compared with those of full-size tRNA. The mystery of the increased duration of peptide bond formation by these single-bond substrate analogues was recently clarified, as it was shown that the excessive time is due to conformational rearrangement of the substrates, as well as of specific PTC components (Yonath 2003; Selmer et al. 2006). Consistently, it was found that the peptidyl transfer reaction is modulated by conformational changes at the active site (Schmeing et al. 2005b; Brunelle et al. 2006; Beringer & Rodnina 2007), and this process consumes time. The fragment reaction substrate analogues are basically derivatives of puromycin. Although they are capable of producing only single peptide bonds, they were overestimated to be suitable to mimic the natural ribosome function. Complexes of H50S with minimal substrates obtained under far-from-optimal functional conditions led to the initial suggestion that three specific rRNA nucleotides catalyse peptide bond formation by the general acid/base mechanism that was based on the crystal structure of complexes of H50S with such minimal substrates (Nissen et al. 2000), which was challenged almost instantaneously by a battery of biochemical and mutational studies (e.g. Barta et al. 2001; Polacek et al. 2001; Thompson et al. 2001; Polacek & Mankin 2005; Bieling et al. 2006), as well as by structural comparisons which showed that the H50S active site contains key PTC components in orientations that differ significantly from those observed in functional complexes of the T70S ribosome (Korostelev et al. 2006; Selmer et al. 2006). Notably, it should be kept in mind that although single peptide bonds can be produced solely by RNA, the polymerase activity of the ribosome, namely the subsequent occurrence of peptidyl transfer by rRNA, has not been fully demonstrated (Anderson et al. 2007), and it is conceivable that the r-protein L2 is involved in the efficient elongation of the nascent chain (Cooperman et al. 1995).
It appears that the choice of substrate analogues may be the reason for the misinterpretation. The structure of the large ribosomal subunit from D. radiodurans (D50S) in complexes with a substrate analogue mimicking the A-site tRNA part interacting with the large subunit, called ASM, advanced the comprehension of peptide bond formation by showing that ribosomes position their substrates in stereochemistry suitable for peptide bond formation, thus providing the machinery for peptide bond formation and tRNA translocation (Bashan et al. 2003; Agmon et al. 2005). Furthermore, the ribosomal architecture, which facilitates positional catalysis of peptide bond formation, promotes substrate-mediated chemical acceleration in accord with the requirement of full-length tRNAs for rapid and smooth peptide bond formation, observed by various methods, including the use of chemical (Weinger et al. 2004; Brunelle et al. 2006; Weinger & Strobel 2006), mutagenesis (Sato et al. 2006), computational (Sharma et al. 2005; Gindulyte et al. 2006; Trobro & Aqvist 2006) and kinetic procedures (Beringer et al. 2005; Wohlgemuth et al. 2006; Beringer & Rodnina 2007; Rodnina et al. 2007). The current consensus view is consistent with ribosomal positional catalysis that allows for chemical catalysis by its P-site tRNA substrate. The importance of the accurate positioning of the substrates within the ribosome frame, accompanied by the key role that the tRNA interactions with 23S rRNA play in peptide bond formation on the ribosome, is currently widely accepted (e.g. Beringer & Rodnina 2007; Bashan & Yonath 2008b) even by those who originally suggested that the ribosome catalyses peptide bond formation by acid/base mechanism (Simonovic & Steitz 2008).
4. Motions within the peptidyl transferase centre
Both ribosomal tasks, formation of peptide bonds and the processivity of this reaction, namely for amino acid polymerization, are governed by the ribosomal striking architecture, which contains a highly conserved region of 180 nucleotides, related by pseudo-twofold symmetry, the rRNA fold, but not the sequences. This sizable intra-ribosomal symmetrical region is located within the otherwise asymmetric ribosome and has been identified in all known ribosome structures, regardless of their source, their functional state or their kingdom of life (Agmon et al. 2003; Bashan et al. 2003; Zarivach et al. 2004; Baram & Yonath 2005). Particularly, the same substructure was identified in the cores of ribosomes from mesophilic, thermophilic, radiophilic and halophilic bacteria from eubacteria and archaea, in assembled empty ribosomes or in complexes of them with substrates, in unbound and complexed large subunit, including complexes with ribosomal antibiotics and non-ribosomal factors involved in protein biosynthesis (Agmon et al. 2005, 2006). Thus, despite size differences between ribosomes of the various kingdoms of life, the functional regions are well conserved, with the highest level of sequence conservation at their central core, and the largest structural differences at the periphery (Mears et al. 2002; Thompson & Dahlberg 2004). Although there is no sequence symmetry, the sequences of the nucleotides constructing the symmetrical region are highly conserved throughout evolution (Agmon et al. 2006, 2009; Davidovich et al. in press), indicating low or no sensitivity to environmental conditions. This symmetrical region includes the PTC and its environments, and connects all ribosomal functional regions involved in amino acid polymerization, namely the tRNA entrance/exit dynamic stalks, the PTC, the nascent protein exit tunnel and the bridge connecting the PTC cavity with the vicinity of the decoding centre in the small subunit. As it is located at the heart of the ribosome, it can serve as the central feature for signalling between all the functional regions involved in protein biosynthesis that are located remotely from each other (up to 200 Å away), but must ‘talk’ to each other during elongation (Uemura et al. 2007).
The PTC is located at the midst of this symmetrical region in the bottom of a V-shaped cavity and is built as an arched void. tRNA acceptor stem interacts extensively with the cavity's walls, as observed for the complex D50S-ASM (Bashan et al. 2003). Although the PTC has significant tolerance in the positioning of fragment reaction substrates (Yonath 2003), the interactions of the tRNA acceptor stem seem to be crucial for accurate substrate positioning in the PTC at the configuration allowing for peptide bond formation, in accord with the finding that the tRNA core region is functionally important for its dynamic interactions with the ribosome (Pan et al. 2006). The linkage between the elaborate architecture of the symmetrical region and the position of the A-site tRNA indicates that the translocation of the tRNA 3′end is performed by a combination of independent, albeit synchronized motions: a sideways shift, performed as a part of the overall mRNA/tRNA translocation, and a rotatory motion of the A-tRNA 3′end along a path confined by the PTC walls.
This rotatory motion is navigated and guided by the ribosomal architecture, mainly the PTC rear wall that confines the rotatory path and two flexible nucleotides that seem to anchor and propel it (figure 2). Hence, the ribosomal architecture and its mobility provide all structural elements enabling the ribosome to function as an amino acid polymerase, including the formation of two symmetrical universal base pairs between the tRNAs and the PTC (Bashan et al. 2003; Agmon et al. 2005), a prerequisite for substrate-mediated acceleration (Weinger & Strobel 2006) and for the direction of the nascent protein into the exit tunnel. Importantly, all nucleotides involved in this rotatory motion have been classified as essential by a comprehensive genetic selection analysis (Sato et al. 2006). Furthermore, the rotatory motion positions the proximal 2′-hydroxyl of P-site tRNA A76 in the same position and orientation found in crystals of the entire ribosome with mRNA and tRNAs, as determined independently in two laboratories (Korostelev et al. 2006; Selmer et al. 2006), and allows for chemical catalysis of peptide bond formation by A76 of the P-site tRNA (Weinger & Strobel 2006).
Simulation studies indicated that during this motion, the rotating moiety interacts with ribosomal components, confining the rotatory path along the ‘PTC rear wall’ (Agmon et al. 2005, 2006). Consistently, quantum mechanical calculations, based on D50S structural data, indicated that the transition state (TS) of this reaction, namely peptide bond formation, is formed during the rotatory motion and is stabilized by hydrogen bonds with rRNA nucleotides (Gindulyte et al. 2006) and is located between the A- and the P-sites at a position similar to that found experimentally in the crystal structure of a complex made of the large subunit from a ribosome from a different source, H50S, with a chemically designed TS analogue (Schmeing et al. 2005a). The correlation between the rotatory motion and amino acid polymerization rationalizes the apparent contradiction associated with the location of the growing protein chain. Thus, the traditional biochemical methods for the detection of ribosome activity were based on the reaction between substrate analogues designed for producing a single peptide bond and do not involve A- to P-site translocation, whereas nascent protein elongation by substrates suitable for performing the A- to P-site passage occurs close to the P-site in a position close to that of properly designed TS analogues (Schmeing et al. 2005a) near the P-site.
5. Is the ribosomal core an optimized ancient entity?
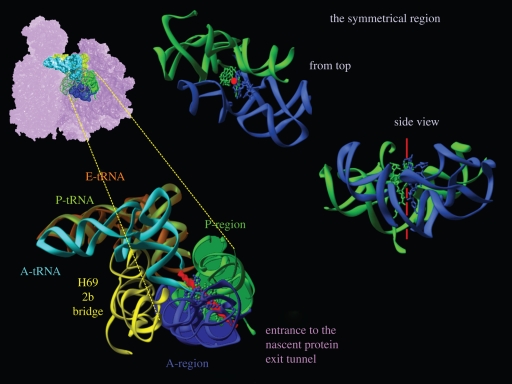
Remarkably, the high level of conservation of components of the symmetrical region that was detected even in mitochondrial ribosomes, in which half the rRNA is replaced by proteins also indicates the ability of the symmetrical region to provide all structural elements required for performing polypeptide elongation. Hence, we suggest that the modern ribosome evolved from a simpler entity, which can be described as a pro-ribosome, by gene fusion or gene duplication (Baram & Yonath 2005). In particular, the preservation of the three-dimensional structure of the two halves of the ribosomal frame, regardless of the sequence, emphasizes the superiority of functional requirement over sequence conservation and indicates that the PTC has evolved by gene fusion. In particular, it demonstrates the rigorous requirements of accurate substrate positioning in stereochemistry supporting peptide bond formation. This as well as the universality of the symmetrical region led to the assumption that the ancient ribosome was composed of a pocket confined by two RNA chains, which formed a dimer, and this pocket is still embedded in the modern ribosome and appears as its symmetrical region (figure 3).
Figure 3.
The ribosomal symmetrical region and suggested proto-ribosome. In all, the region hosting A-site tRNA is shown in blue and that hosting the P-site tRNA in green. Similarly, the A-site tRNA mimic (Bashan et al. 2003) is shown in blue, and the derived P-site tRNA (by the rotatory motion) is shown in green. The imaginary symmetrical axis is shown in red. Top left: the symmetrical region within the ribosome and its details. The A-region is shown in blue, the P-region in green and the non-symmetrical extensions are shown as pink dots on green ‘ropes’. A- and P-site tRNAs are shown in cyan and green-yellow. A zoom into the symmetrical region is shown in bottom left. The bridge to the decoding centre and A-, P- and E-site tRNA molecules were docked, based on Yusupov et al. (2001).
Based on this observation, we have proposed (Agmon et al. 2006, 2009; Davidovich et al. in press) that the ancient machinery that could form peptide bonds was made exclusively from RNA molecules, using substituents available in the primordial soup, such as short RNA chains that could acquire stable conformations, which were sufficiently stable to survive changing evolution stresses. These surviving ancient RNA chains could fold spontaneously and then dimerize. The products of the dimerization yielded three-dimensional structures with a symmetrical pocket that could accommodate two small substrates (e.g. amino acids conjugated with mono- or oligo-RNA nucleotides in a stereochemistry suitable for spontaneous reaction of peptide bond formation). Hence, they could become the ancestors of the RNA chains that construct the symmetrical region in the contemporary ribosome. The most appropriate pockets for accommodating this reaction survived. As RNA chains can act as gene-like molecules coding for their own reproduction (Lincoln & Joyce 2009), the surviving ancient pockets became the templates for the ancient ribosomes. At a later stage, these initial RNA genes underwent optimization to produce more defined, relatively stable pockets, and when the correlation between the amino acid and the growing peptidyl sites was established, each of the two halves was further optimized for its task so that their sequences evolved differently. The entire ribosome could have evolved gradually around these symmetrical regions until it acquired its final shape (Bokov & Steinberg 2009).
The substrates of the ancient ribosomes, which were initially spontaneously produced amino acids conjugated with single or short oligo-nucleotides (Ilangasekare et al. 1995; Lehmann et al. 2007), could have evolved in parallel to allow accurate binding, as occurs for aminoacylated CCA 3′end. Later on, these were converted into longer and more compounds with a contour that could complement the inner surface of the reaction pocket. For increasing specificity, these short RNA segments were extended to larger structures by their fusion with additional RNA features, thus forming the ancient tRNA molecules capable of storing, selecting and transferring instructions for producing useful proteins. Subsequently, the decoding process was combined with peptide bond formation. Adding a feature similar to the modern anticodon loop allowed some genetic control, presumably after polypeptides capable of enzymatic function were created. Analysis of substrate-binding modes to inactive and active ribosomes led to similar conclusions (Johansson et al. 2008).
In short, the ancient ribosome appears to be a dimeric ribozyme that produced peptide bonds sporadically. As the products of this reaction may act as substrates, elongation of the dipeptides could occur. Once these polypeptides acquired the capacity to perform enzymatic tasks, the information about their desired structure was stored in genes. Consequently, molecules capable of decoding this information simultaneously with transporting the cognate substrates (tRNA) evolved. The size and the complexity of the proto-ribosome were increased until it reached the size and shape for hosting the newly developed tRNA molecules and acquired the properties enabling smooth translation of genetic information into proteins.
Acknowledgements
Thanks are due to all members of the ribosome group at the Weizmann Institute for their experimental efforts and illuminating discussion. Support was provided by the US National Institutes of Health (GM34360), the German Ministry for Science and Technology (BMBF 05-641EA) and the Kimmelman Center for Macromolecular Assemblies. A.Y. holds the Martin and Helen Kimmel Professorial Chair. X-ray diffraction data were collected from the EMBL and MPG beam lines at DESY; F1/CHESS, Cornell University; SSRL/Stanford University; ESRF/EMBL, Grenoble; BL26/PF/KEK, Japan; and APS/Argonne National Laboratory.
Footnotes
One contribution of 13 to a Theme Supplement ‘Biological physics at large facilities’.
References
- Agmon I., et al. 2003. On peptide bond formation, translocation, nascent protein progression and the regulatory properties of ribosomes. Eur. J. Biochem. 270, 2543–2556. ( 10.1046/j.1432-1033.2003.03634.x) [DOI] [PubMed] [Google Scholar]
- Agmon I., Bashan A., Zarivach R., Yonath A. 2005. Symmetry at the active site of the ribosome: structural and functional implications. Biol. Chem. 386, 833–844. ( 10.1515/BC.2005.098) [DOI] [PubMed] [Google Scholar]
- Agmon I., Bashan A., Yonath A. 2006. On ribosome conservation and evolution. Isr. J. Ecol. Evol. 52, 359–379. ( 10.1560/IJEE_52_3-4_359) [DOI] [Google Scholar]
- Agmon I., Davidovich C., Bashan A., Yonath A. 2009. Identification of the prebiotic translation apparatus within the contemporary ribosome. See http://precedings.nature.com/documents/2921/version/1. [Google Scholar]
- Amit M., Berisio R., Baram D., Harms J., Bashan A., Yonath A. 2005. A crevice adjoining the ribosome tunnel: hints for cotranslational folding. FEBS Lett. 579, 3207–3213. ( 10.1016/j.febslet.2005.03.023) [DOI] [PubMed] [Google Scholar]
- Anderson R. M., Kwon M., Strobel S. A. 2007. Toward ribosomal RNA catalytic activity in the absence of protein. J. Mol. Evol. 64, 472–483. ( 10.1007/s00239-006-0211-y) [DOI] [PubMed] [Google Scholar]
- Auerbach T., Bashan A., Yonath A. 2004. Ribosomal antibiotics: structural basis for resistance, synergism and selectivity. Trends Biotechnol. 22, 570–576. ( 10.1016/j.tibtech.2004.09.006) [DOI] [PubMed] [Google Scholar]
- Auerbach-Nevo T., Zarivach R., Peretz M., Yonath A. 2005. Reproducible growth of well diffracting ribosomal crystals. Acta Crystallogr. D Biol. Crystallogr. 61, 713–719. ( 10.1107/S0907444905006311) [DOI] [PubMed] [Google Scholar]
- Ban N., Nissen P., Hansen J., Moore P. B., Steitz T. A. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science 289, 905–920. ( 10.1126/science.289.5481.905) [DOI] [PubMed] [Google Scholar]
- Baram D., Yonath A. 2005. From peptide-bond formation to cotranslational folding: dynamic, regulatory and evolutionary aspects. FEBS Lett. 579, 948–954. ( 10.1016/j.febslet.2004.11.063) [DOI] [PubMed] [Google Scholar]
- Baram D., Pyetan E., Sittner A., Auerbach-Nevo T., Bashan A., Yonath A. 2005. Structure of trigger factor binding domain in biologically homologous complex with eubacterial ribosome reveals its chaperone action. Proc. Natl Acad. Sci. USA 102, 12 017–12 022. ( 10.1073/pnas.0505581102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta A., Dorner S., Polacek N. 2001. Mechanism of ribosomal peptide bond formation. Science 291, 203 ( 10.1126/science.291.5502.203a) [DOI] [PubMed] [Google Scholar]
- Bashan A., Yonath A. 2008a. The linkage between ribosomal crystallography, metal ions, heteropolytungstates and functional flexibility. J. Mol. Struct. 890, 289–294. ( 10.1016/j.molstruc.2008.03.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan A., Yonath A. 2008b. Correlating ribosome function with high-resolution structures. Trends Microbiol. 16, 326–335. ( 10.1016/j.tim.2008.05.001) [DOI] [PubMed] [Google Scholar]
- Bashan A., et al. 2003. Structural basis of the ribosomal machinery for peptide bond formation, translocation, and nascent chain progression. Mol. Cell 11, 91–102. ( 10.1016/S1097-2765(03)00009-1) [DOI] [PubMed] [Google Scholar]
- Beringer M., Rodnina M. V. 2007. The ribosomal peptidyl transferase. Mol. Cell 26, 311–321. ( 10.1016/j.molcel.2007.03.015) [DOI] [PubMed] [Google Scholar]
- Beringer M., Bruell C., Xiong L., Pfister P., Bieling P., Katunin V. I., Mankin A. S., Bottger E. C., Rodnina M. V. 2005. Essential mechanisms in the catalysis of peptide bond formation on the ribosome. J. Biol. Chem. 280, 36 065–36 072. ( 10.1074/jbc.M507961200) [DOI] [PubMed] [Google Scholar]
- Berisio R., Schluenzen F., Harms J., Bashan A., Auerbach T., Baram D., Yonath A. 2003. Structural insight into the role of the ribosomal tunnel in cellular regulation. Nat. Struct. Biol. 10, 366–370. ( 10.1038/nsb915) [DOI] [PubMed] [Google Scholar]
- Berisio R., Corti N., Pfister P., Yonath A., Bottger E. C. 2006. 23S rRNA 2058A→G alteration mediates ketolide resistance in combination with deletion in L22. Antimicrob. Agents Chemother. 50, 3816–3823. ( 10.1128/AAC.00767-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieling P., Beringer M., Adio S., Rodnina M. V. 2006. Peptide bond formation does not involve acid–base catalysis by ribosomal residues. Nat. Struct. Mol. Biol. 13, 423–428. ( 10.1038/nsmb1091) [DOI] [PubMed] [Google Scholar]
- Bokov K., Steinberg S. V. 2009. A hierarchical model for evolution of 23S ribosomal RNA. Nature 457, 977–980. ( 10.1038/nature07749) [DOI] [PubMed] [Google Scholar]
- Borovinskaya M. A., Pai R. D., Zhang W., Schuwirth B. S., Holton J. M., Hirokawa G., Kaji H., Kaji A., Cate J. H. 2007. Structural basis for aminoglycoside inhibition of bacterial ribosome recycling. Nat. Struct. Mol. Biol. 14, 727–732. ( 10.1038/nsmb1271) [DOI] [PubMed] [Google Scholar]
- Bottger E. C. 2006. The ribosome as a drug target. Trends Biotechnol. 24, 145–147. ( 10.1016/j.tibtech.2006.02.005) [DOI] [PubMed] [Google Scholar]
- Bottger E. C. 2007. Antimicrobial agents targeting the ribosome: the issue of selectivity and toxicity—lessons to be learned. Cell Mol. Life Sci. 64, 791–795. ( 10.1007/s00018-007-6431-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle J. L., Youngman E. M., Sharma D., Green R. 2006. The interaction between C75 of tRNA and the A loop of the ribosome stimulates peptidyl transferase activity. RNA 12, 33–39. ( 10.1261/rna.2256706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A. P., Clemons W. M., Jr, Brodersen D. E., Morgan-Warren R. J., Hartsch T., Wimberly B. T., Ramakrishnan V. 2001. Crystal structure of an initiation factor bound to the 30S ribosomal subunit. Science 291, 498–501. ( 10.1126/science.1057766) [DOI] [PubMed] [Google Scholar]
- Cooperman B. S., Wooten T., Romero D. P., Traut R. R. 1995. Histidine 229 in protein L2 is apparently essential for 50S peptidyl transferase activity. Biochem. Cell Biol. 73, 1087–1094. ( 10.1139/o95-117) [DOI] [PubMed] [Google Scholar]
- Crick F. H. 1968. The origin of the genetic code. J. Mol. Biol. 38, 367–379. ( 10.1016/0022-2836(68)90392-6) [DOI] [PubMed] [Google Scholar]
- Crowley K. S., Reinhart G. D., Johnson A. E. 1993. The signal sequence moves through a ribosomal tunnel into a noncytoplasmic aqueous environment at the ER membrane early in translocation. Cell 73, 1101–1115. ( 10.1016/0092-8674(93)90640-C) [DOI] [PubMed] [Google Scholar]
- Cruz-Vera L. R., Gong M., Yanofsky C. 2006. Changes produced by bound tryptophan in the ribosome peptidyl transferase center in response to TnaC, a nascent leader peptide. Proc. Natl Acad. Sci. USA 103, 3598–3603. ( 10.1073/pnas.0600082103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovich C., Bashan A., Auerbach-Nevo T., Yaggie R. D., Gontarek R. R., Yonath A. 2007. Induced-fit tightens pleuromutilins binding to ribosomes and remote interactions enable their selectivity. Proc. Natl Acad. Sci. USA 104, 4291–4296. ( 10.1073/pnas.0700041104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovich C., Bashan A., Yonath A. 2008. Structural basis for cross resistance to ribosomal PTC antibiotics. Proc. Natl Acad. Sci. USA 105, 20 665–20 670. ( 10.1073/pnas.0810826105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovich C., Belousoff M., Bashan A., Yonath A. In press The evolving ribosome: from non-coded peptide bond formation to sophisticated translation machinery. Res. Microbiol. [DOI] [PubMed] [Google Scholar]
- Deane C. M., Dong M., Huard F. P., Lance B. K., Wood G. R. 2007. Cotranslational protein folding—fact or fiction? Bioinformatics 23, i142–i148. ( 10.1093/bioinformatics/btm175) [DOI] [PubMed] [Google Scholar]
- Frank J., et al. 1995. A model of protein synthesis based on cryo-electron microscopy of the E. coli ribosome. Nature 376, 441–444. ( 10.1038/376441a0) [DOI] [PubMed] [Google Scholar]
- Gabashvili I. S., Gregory S. T., Valle M., Grassucci R., Worbs M., Wahl M. C., Dahlberg A. E., Frank J. 2001. The polypeptide tunnel system in the ribosome and its gating in erythromycin resistance mutants of L4 and L22. Mol. Cell 8, 181–188. ( 10.1016/S1097-2765(01)00293-3) [DOI] [PubMed] [Google Scholar]
- Garrett R. A., Wittmann H. G. 1973. Structure of bacterial ribosomes. Adv. Protein Chem. 27, 277–347. ( 10.1016/S0065-3233(08)60450-7) [DOI] [PubMed] [Google Scholar]
- Gilbert R. J., Fucini P., Connell S., Fuller S. D., Nierhaus K. H., Robinson C. V., Dobson C. M., Stuart D. I. 2004. Three-dimensional structures of translating ribosomes by cryo-EM. Mol. Cell 14, 57–66. ( 10.1016/S1097-2765(04)00163-7) [DOI] [PubMed] [Google Scholar]
- Gindulyte A., Bashan A., Agmon I., Massa L., Yonath A., Karle J. 2006. The transition state for formation of the peptide bond in the ribosome. Proc. Natl Acad. Sci. USA 103, 13 327–13 332. ( 10.1073/pnas.0606027103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluehmann M., et al. 2001. Ribosomal crystallography: from poorly diffracting microcrystals to high-resolution structures. Methods 25, 292–302. ( 10.1006/meth.2001.1241) [DOI] [PubMed] [Google Scholar]
- Gong F., Yanofsky C. 2002. Instruction of translating ribosome by nascent peptide. Science 297, 1864–1867. ( 10.1126/science.1073997) [DOI] [PubMed] [Google Scholar]
- Hansen H. A., Volkmann N., Piefke J., Glotz C., Weinstein S., Makowski I., Meyer S., Wittmann H. G., Yonath A. 1990. Crystals of complexes mimicking protein biosynthesis are suitable for crystallographic studies. Biochim. Biophys. Acta 1050, 1–7. ( 10.1016/0167-4781(90)90132-L) [DOI] [PubMed] [Google Scholar]
- Harms J., Schluenzen F., Zarivach R., Bashan A., Gat S., Agmon I., Bartels H., Franceschi F., Yonath A. 2001. High resolution structure of the large ribosomal subunit from a mesophilic eubacterium. Cell 107, 679–688. ( 10.1016/S0092-8674(01)00546-3) [DOI] [PubMed] [Google Scholar]
- Hope H., et al. 1989. Cryocrystallography of ribosomal particles. Acta Crystallogr. B 45, 190–199. ( 10.1107/S0108768188013710) [DOI] [PubMed] [Google Scholar]
- Ilangasekare M., Sanchez G., Nickles T., Yarus M. 1995. Aminoacyl-RNA synthesis catalyzed by an RNA. Science 267, 643–647. ( 10.1126/science.7530860) [DOI] [PubMed] [Google Scholar]
- Johansson M., Bouakaz E., Lovmar M., Ehrenberg M. 2008. The kinetics of ribosomal peptidyl transfer revisited. Mol. Cell 30, 589–598. ( 10.1016/j.molcel.2008.04.010) [DOI] [PubMed] [Google Scholar]
- Johnson A. E., Jensen R. E. 2004. Barreling through the membrane. Nat. Struct. Mol. Biol. 11, 113–114. ( 10.1038/nsmb0204-113) [DOI] [PubMed] [Google Scholar]
- Kaiser C. M., Chang H. C., Agashe V. R., Lakshmipathy S. K., Etchells S. A., Hayer-Hartl M., Hartl F. U., Barral J. M. 2006. Real-time observation of trigger factor function on translating ribosomes. Nature 444, 455–460. ( 10.1038/nature05225) [DOI] [PubMed] [Google Scholar]
- Korostelev A., Trakhanov S., Laurberg M., Noller H. F. 2006. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell 126, 1065–1077. ( 10.1016/j.cell.2006.08.032) [DOI] [PubMed] [Google Scholar]
- Laurberg M., Asahara H., Korostelev A., Zhu J., Trakhanov S., Noller H. F. 2008. Structural basis for translation termination on the 70S ribosome. Nature 454, 852–857. ( 10.1038/nature07115) [DOI] [PubMed] [Google Scholar]
- Lehmann J., Reichel A., Buguin A., Libchaber A. 2007. Efficiency of a self-aminoacylating ribozyme: effect of the length and base-composition of its 3′ extension. RNA 13, 1191–1197. ( 10.1261/rna.500907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln T. A., Joyce G. F. 2009. Self-sustained replication of an RNA enzyme. Science 323, 1229–1232. ( 10.1126/science.1167856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin L. I., Rich A. 1967. Partial resistance of nascent polypeptide chains to proteolytic digestion due to ribosomal shielding. J. Mol. Biol. 26, 329–346. ( 10.1016/0022-2836(67)90301-4) [DOI] [PubMed] [Google Scholar]
- Mankin A. 2006. Antibiotic blocks mRNA path on the ribosome. Nat. Struct. Mol. Biol. 13, 858–860. ( 10.1038/nsmb1006-858) [DOI] [PubMed] [Google Scholar]
- Mears J. A., Cannone J. J., Stagg S. M., Gutell R. R., Agrawal R. K., Harvey S. C. 2002. Modeling a minimal ribosome based on comparative sequence analysis. J. Mol. Biol. 321, 215–234. ( 10.1016/S0022-2836(02)00568-5) [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Unwin P. N. 1986. Location of exit channel for nascent protein in 80S ribosome. Nature 319, 693–695. ( 10.1038/319693a0) [DOI] [PubMed] [Google Scholar]
- Mitra K., Schaffitzel C., Fabiola F., Chapman M. S., Ban N., Frank J. 2006. Elongation arrest by SecM via a cascade of ribosomal RNA rearrangements. Mol. Cell 22, 533–543. ( 10.1016/j.molcel.2006.05.003) [DOI] [PubMed] [Google Scholar]
- Moore P. B. 1988. The ribosome returns. Nature 331, 223–227. ( 10.1038/331223a0) [DOI] [PubMed] [Google Scholar]
- Nagano K., Takagi H., Harel M. 1991. The side-by-side model of two tRNA molecules allowing the alpha-helical conformation of the nascent polypeptide during the ribosomal transpeptidation. Biochimie 73, 947–960. ( 10.1016/0300-9084(91)90136-O) [DOI] [PubMed] [Google Scholar]
- Nakatogawa H., Ito K. 2002. The ribosomal exit tunnel functions as a discriminating gate. Cell 108, 629–636. ( 10.1016/S0092-8674(02)00649-9) [DOI] [PubMed] [Google Scholar]
- Nissen P., Hansen J., Ban N., Moore P. B., Steitz T. A. 2000. The structural basis of ribosome activity in peptide bond synthesis. Science 289, 920–930. ( 10.1126/science.289.5481.920) [DOI] [PubMed] [Google Scholar]
- Noller H. F., Hoffarth V., Zimniak L. 1992. Unusual resistance of peptidyl transferase to protein extraction procedures. Science 256, 1416–1419. ( 10.1126/science.1604315) [DOI] [PubMed] [Google Scholar]
- Ogle J. M., Carter A. P., Ramakrishnan V. 2003. Insights into the decoding mechanism from recent ribosome structures. Trends Biochem. Sci. 28, 259–266. ( 10.1016/S0968-0004(03)00066-5) [DOI] [PubMed] [Google Scholar]
- Palade G. E. 1955. A small particulate component of the cytoplasm. J. Biophys. Biochem. Cytol. 1, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Kirillov S., Zhang C. M., Hou Y. M., Cooperman B. S. 2006. Rapid ribosomal translocation depends on the conserved 18–55 base pair in P-site transfer RNA. Nat. Struct. Mol. Biol. 13, 354–359. ( 10.1038/nsmb1074) [DOI] [PubMed] [Google Scholar]
- Petrone P. M., Snow C. D., Lucent D., Pande V. S. 2008. Side-chain recognition and gating in the ribosome exit tunnel. Proc. Natl Acad. Sci. USA 105, 16 549–16 554. ( 10.1073/pnas.0801795105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioletti M., et al. 2001. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J. 20, 1829–1839. ( 10.1093/emboj/20.8.1829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlsgaard J., Douthwaite S. 2005. The bacterial ribosome as a target for antibiotics. Nat. Rev. Microbiol. 3, 870–881. ( 10.1038/nrmicro1265) [DOI] [PubMed] [Google Scholar]
- Polacek N., Mankin A. S. 2005. The ribosomal peptidyl transferase center: structure, function, evolution, inhibition. Crit. Rev. Biochem. Mol. Biol. 40, 285–311. ( 10.1080/10409230500326334) [DOI] [PubMed] [Google Scholar]
- Polacek N., Gaynor M., Yassin A., Mankin A. S. 2001. Ribosomal peptidyl transferase can withstand mutations at the putative catalytic nucleotide. Nature 411, 498–501. ( 10.1038/35078113) [DOI] [PubMed] [Google Scholar]
- Rodnina M. V., Beringer M., Wintermeyer W. 2007. How ribosomes make peptide bonds. Trends Biochem. Sci. 32, 20–26. ( 10.1016/j.tibs.2006.11.007) [DOI] [PubMed] [Google Scholar]
- Ryabova L. A., Selivanova O. M., Baranov V. I., Vasiliev V. D., Spirin A. S. 1988. Does the channel for nascent peptide exist inside the ribosome? Immune electron microscopy study. FEBS Lett. 226, 255–260. ( 10.1016/0014-5793(88)81434-0) [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Blobel G. 1970. Controlled proteolysis of nascent polypeptides in rat liver cell fractions. II. Location of the polypeptides in rough microsomes. J. Cell Biol. 45, 146–157. ( 10.1083/jcb.45.1.146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N. S., Hirabayashi N., Agmon I., Yonath A., Suzuki T. 2006. Comprehensive genetic selection revealed essential bases in the peptidyl-transferase center. Proc. Natl Acad. Sci. USA 103, 15 386–15 391. ( 10.1073/pnas.0605970103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffitzel C., Ban N. 2007. Generation of ribosome nascent chain complexes for structural and functional studies. J. Struct. Biol. 158, 463–471. ( 10.1016/j.jsb.2007.01.005) [DOI] [PubMed] [Google Scholar]
- Schluenzen F., et al. 2000. Structure of functionally activated small ribosomal subunit at 3.3 angstroms resolution. Cell 102, 615–623. ( 10.1016/S0092-8674(00)00084-2) [DOI] [PubMed] [Google Scholar]
- Schluenzen F., et al. 2005. The binding mode of the trigger factor on the ribosome: implications for protein folding and SRP interaction. Structure 13, 1685–1694. ( 10.1016/j.str.2005.08.007) [DOI] [PubMed] [Google Scholar]
- Schmeing T. M., Huang K. S., Kitchen D. E., Strobel S. A., Steitz T. A. 2005a. Structural insights into the roles of water and the 2’ hydroxyl of the P site tRNA in the peptidyl transferase reaction. Mol. Cell 20, 437–448. ( 10.1016/j.molcel.2005.09.006) [DOI] [PubMed] [Google Scholar]
- Schmeing T. M., Huang K. S., Strobel S. A., Steitz T. A. 2005b. An induced-fit mechanism to promote peptide bond formation and exclude hydrolysis of peptidyl-tRNA. Nature 438, 520–524. ( 10.1038/nature04152) [DOI] [PubMed] [Google Scholar]
- Schuwirth B. S., Borovinskaya M. A., Hau C. W., Zhang W., Vila-Sanjurjo A., Holton J. M., Cate J. H. D. 2005. Structures of the bacterial ribosome at 3.5 A resolution. Science 310, 827–834. ( 10.1126/science.1117230) [DOI] [PubMed] [Google Scholar]
- Selmer M., Dunham C. M., Murphy IV F. V., Weixlbaumer A., Petry S., Kelley A. C., Weir J. R., Ramakrishnan V. 2006. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313, 1935–1942. ( 10.1126/science.1131127) [DOI] [PubMed] [Google Scholar]
- Sharma P. K., Xiang Y., Kato M., Warshel A. 2005. What are the roles of substrate-assisted catalysis and proximity effects in peptide bond formation by the ribosome? Biochemistry 44, 11 307–11 314. ( 10.1021/bi0509806) [DOI] [PubMed] [Google Scholar]
- Shevack A., Gewitz H. S., Hennemann B., Yonath A., Wittmann H. G. 1985. Characterization and crystallization of ribosomal particles from Halobacterium marismortui. FEBS Lett. 184, 68–71. ( 10.1016/0014-5793(85)80655-4) [DOI] [Google Scholar]
- Simonovic M., Steitz T. A. 2008. Peptidyl-CCA deacylation on the ribosome promoted by induced fit and the O3′-hydroxyl group of A76 of the unacylated A-site tRNA. RNA 14, 2372–2378. ( 10.1261/rna.1118908) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark H., Mueller F., Orlova E. V., Schatz M., Dube P., Erdemir T., Zemlin F., Brimacombe R., van Heel M. 1995. The 70S Escherichia coli ribosome at 23 A resolution: fitting the ribosomal RNA. Structure 3, 815–821. ( 10.1016/S0969-2126(01)00216-7) [DOI] [PubMed] [Google Scholar]
- Tenson T., Mankin A. 2006. Antibiotics and the ribosome. Mol. Microbiol. 59, 1664–1677. ( 10.1111/j.1365-2958.2006.05063.x) [DOI] [PubMed] [Google Scholar]
- Thompson J., Dahlberg A. E. 2004. Testing the conservation of the translational machinery over evolution in diverse environments: assaying Thermus thermophilus ribosomes and initiation factors in a coupled transcription–translation system from Escherichia coli. Nucleic Acids Res. 32, 5954–5961. ( 10.1093/nar/gkh925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Kim D. F., O'Connor M., Lieberman K. R., Bayfield M. A., Gregory S. T., Green R., Noller H. F., Dahlberg A. E. 2001. Analysis of mutations at residues A2451 and G2447 of 23S rRNA in the peptidyltransferase active site of the 50S ribosomal subunit. Proc. Natl Acad. Sci. USA 98, 9002–9007. ( 10.1073/pnas.151257098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygesen J., Weinstein S., Franceschi F., Yonath A. 1996. The suitability of multi-metal clusters for phasing in crystallography of large macromolecular assemblies. Structure 4, 513–518. ( 10.1016/S0969-2126(96)00057-3) [DOI] [PubMed] [Google Scholar]
- Trakhanov S. D., Yusupov M. M., Agalarov S. C., Garber M. B., Ryazantsev S. N., Tischenko S. V., Shirokov V. A. 1987. Crystallization of 70S ribosome and 30S ribosomal subunits from Thermus thermophilus. FEBS Lett. 220, 319–322. ( 10.1016/0014-5793(87)80838-4) [DOI] [Google Scholar]
- Trobro S., Aqvist J. 2006. Analysis of predictions for the catalytic mechanism of ribosomal peptidyl transfer. Biochemistry 45, 7049–7056. ( 10.1021/bi0605383) [DOI] [PubMed] [Google Scholar]
- Tu D., Blaha G., Moore P. B., Steitz T. A. 2005. Structures of MLSBK antibiotics bound to mutated large ribosomal subunits provide a structural explanation for resistance. Cell 121, 257–270. ( 10.1016/j.cell.2005.02.005) [DOI] [PubMed] [Google Scholar]
- Uemura S., Dorywalska M., Lee T. H., Kim H. D., Puglisi J. D., Chu S. 2007. Peptide bond formation destabilizes Shine–Dalgarno interaction on the ribosome. Nature 446, 454–457. ( 10.1038/nature05625) [DOI] [PubMed] [Google Scholar]
- von Bohlen K., et al. 1991. Characterization and preliminary attempts for derivatization of crystals of large ribosomal subunits from Haloarcula marismortui diffracting to 3 A resolution. J. Mol. Biol. 222, 11–15. ( 10.1016/0022-2836(91)90730-T) [DOI] [PubMed] [Google Scholar]
- Voorhees R. M., Weixlbaumer A., Loakes D., Kelley A. C., Ramakrishnan V. 2009. Insights into substrate stabilization from snapshots of the peptidyl transferase center of the intact 70S ribosome. Nat. Struct. Mol. Biol. 16, 528–533. ( 10.1038/nsmb.1577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss N. R., Gerstein M., Steitz T. A., Moore P. B. 2006. The geometry of the ribosomal polypeptide exit tunnel. J. Mol. Biol. 360, 893–906. ( 10.1016/j.jmb.2006.05.023) [DOI] [PubMed] [Google Scholar]
- Walter P., Johnson A. E. 1994. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu. Rev. Cell Biol. 10, 87–119. ( 10.1146/annurev.cb.10.110194.000511) [DOI] [PubMed] [Google Scholar]
- Watson J. D. 1963. Involvement of RNA in the synthesis of proteins. Science 140, 17–26. ( 10.1126/science.140.3562.17) [DOI] [PubMed] [Google Scholar]
- Weinger J. S., Strobel S. A. 2006. Participation of the tRNA A76 hydroxyl groups throughout translation. Biochemistry 45, 5939–5948. ( 10.1021/bi060183n) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger J. S., Parnell K. M., Dorner S., Green R., Strobel S. A. 2004. Substrate-assisted catalysis of peptide bond formation by the ribosome. Nat. Struct. Mol. Biol. 11, 1101–1106. ( 10.1038/nsmb841) [DOI] [PubMed] [Google Scholar]
- Weixlbaumer A., Jin H., Neubauer C., Voorhees R. M., Petry S., Kelley A. C., Ramakrishnan V. 2008. Insights into translational termination from the structure of RF2 bound to the ribosome. Science 322, 953–956. ( 10.1126/science.1164840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekselman I., Davidovich C., Agmon I., Zimmerman E., Rozenberg H., Bashan A., Berisio R., Yonath A. 2008. Ribosome's mode of function: myths, facts and recent results. J. Pept. Sci. 15, 122–130. ( 10.1002/psc.1077) [DOI] [PubMed] [Google Scholar]
- Wilson D. N., Schluenzen F., Harms J. M., Yoshida T., Ohkubo T., Albrecht R., Buerger J., Kobayashi Y., Fucini P. 2005. X-ray crystallography study on ribosome recycling: the mechanism of binding and action of RRF on the 50S ribosomal subunit. EMBO J. 24, 251–260. ( 10.1038/sj.emboj.7600525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth I., Beringer M., Rodnina M. V. 2006. Rapid peptide bond formation on isolated 50S ribosomal subunits. EMBO Rep. 7, 699–703. ( 10.1038/sj.embor.7400732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolhead C. A., McCormick P. J., Johnson A. E. 2004. Nascent membrane and secretory proteins differ in FRET-detected folding far inside the ribosome and in their exposure to ribosomal proteins. Cell 116, 725–736. ( 10.1016/S0092-8674(04)00169-2) [DOI] [PubMed] [Google Scholar]
- Woolhead C. A., Johnson A. E., Bernstein H. D. 2006. Translation arrest requires two-way communication between a nascent polypeptide and the ribosome. Mol. Cell 22, 587–598. ( 10.1016/j.molcel.2006.05.021) [DOI] [PubMed] [Google Scholar]
- Yonath A. 2003. Ribosomal tolerance and peptide bond formation. Biol. Chem. 384, 1411–1419. ( 10.1515/BC.2003.156) [DOI] [PubMed] [Google Scholar]
- Yonath A. 2005. Antibiotics targeting ribosomes: resistance, selectivity, synergism, and cellular regulation. Annu. Rev. Biochem. 74, 649–679. ( 10.1146/annurev.biochem.74.082803.133130) [DOI] [PubMed] [Google Scholar]
- Yonath A., Bashan A. 2004. Ribosomal crystallography: initiation, peptide bond formation, and amino acid polymerization are hampered by antibiotics. Annu. Rev. Microbiol. 58, 233–251. ( 10.1146/annurev.micro.58.030603.123822) [DOI] [PubMed] [Google Scholar]
- Yonath A., Muessig J., Tesche B., Lorenz S., Erdmann V. A., Wittmann H. G. 1980. Crystallization of the large ribosomal subunit from B. stearothermophilus. Biochem. Int. 1, 315–428. [Google Scholar]
- Yonath A., Bartunik H. D., Bartels K. S., Wittmann H. G. 1984. Some X-ray diffraction patterns from single crystals of the large ribosomal subunit from B. stearothermophilus. J. Mol. Biol. 177, 201–206. ( 10.1016/0022-2836(84)90066-4) [DOI] [PubMed] [Google Scholar]
- Yonath A., Leonard K. R., Wittmann H. G. 1987. A tunnel in the large ribosomal subunit revealed by three-dimensional image reconstruction. Science 236, 813–816. ( 10.1126/science.3576200) [DOI] [PubMed] [Google Scholar]
- Yonath A., Glotz C., Gewitz H. S., Bartels K. S., von Bohlen K., Makowski I., Wittmann H. G. 1988. Characterization of crystals of small ribosomal subunits. J. Mol. Biol. 203, 831–834. ( 10.1016/0022-2836(88)90216-1) [DOI] [PubMed] [Google Scholar]
- Yusupov M. M., et al. 1987. Crystallization of the 30S subunits of Thermus thermophilus ribosomes. Dokl. Akad. Nauk (USSR) 292, 1271–1274. [Google Scholar]
- Yusupov M. M., Yusupova G. Z., Baucom A., Lieberman K., Earnest T. N., Cate J. H., Noller H. F. 2001. Crystal structure of the ribosome at 5.5 A resolution. Science 292, 883–896. ( 10.1126/science.1060089) [DOI] [PubMed] [Google Scholar]
- Yusupova G., Jenner L., Rees B., Moras D., Yusupov M. 2006. Structural basis for messenger RNA movement on the ribosome. Nature 444, 391–394. ( 10.1038/nature05281) [DOI] [PubMed] [Google Scholar]
- Zarivach R., et al. 2004. Functional aspects of ribosomal architecture: symmetry, chirality and regulation. J. Phys. Org. Chem. 17, 901–912. ( 10.1002/poc.831) [DOI] [Google Scholar]
- Zimmerman E., Yonath A. 2009. Biological implications of the ribosome's stunning stereochemistry. ChemBioChem 10, 63–72. ( 10.1002/cbic.200800554) [DOI] [PubMed] [Google Scholar]
- Ziv G., Haran G., Thirumalai D. 2005. Ribosome exit tunnel can entropically stabilize α-helices. Proc. Natl Acad. Sci. USA 102, 18 956–18 961. ( 10.1073/pnas.0508234102) [DOI] [PMC free article] [PubMed] [Google Scholar]