Abstract
Behavioural experiments for magnetoreception in eusocial insects in the last decade are reviewed. Ants and bees use the geomagnetic field to orient and navigate in areas around their nests and along migratory paths. Bees show sensitivity to small changes in magnetic fields in conditioning experiments and when exiting the hive. For the first time, the magnetic properties of the nanoparticles found in eusocial insects, obtained by magnetic techniques and electron microscopy, are reviewed. Different magnetic oxide nanoparticles, ranging from superparamagnetic to multi-domain particles, were observed in all body parts, but greater relative concentrations in the abdomens and antennae of honeybees and ants have focused attention on these segments. Theoretical models for how these specific magnetosensory apparatuses function have been proposed. Neuron-rich ant antennae may be the most amenable to discovering a magnetosensor that will greatly assist research into higher order processing of magnetic information. The ferromagnetic hypothesis is believed to apply to eusocial insects, but interest in a light-sensitive mechanism is growing. The diversity of compass mechanisms in animals suggests that multiple compasses may function in insect orientation and navigation. The search for magnetic compasses will continue even after a magnetosensor is discovered in eusocial insects.
Keywords: compass, orientation cue, magnetic particles, magnetic sense, Hymenoptera, Isoptera
1. Introduction
Magnetoreception is the sensory ability to perceive magnetic cues, transduce them and transfer them to the nervous system and to the brain, where processing and interpretation occurs. Behavioural experiments have demonstrated that animals can use the geomagnetic field as a cue for spatial orientation (Wiltschko & Wiltschko 2005), although the biophysical mechanisms of transduction are unclear, the magnetoreceptors have not been identified convincingly and the knowledge of the neural substrate relating magnetic mechanisms remains very poor (Němec et al. 2005). Histological studies can localize candidate magnetoreceptive structures, but their involvement in magnetoreception cannot be tested. From a neurobiological perspective, there are few electrophysiological studies of specific brain structures related to processing of magnetic information, such as in molluscs (Cain et al. 2005) and in birds (Beason 2005). Research objectives should include detection of the ability to sense magnetic cues by an animal, location and characterization of the sensory structure, measurement of neural activity in the sensory tissue and understanding of the neural processing of the information for behavioural decisions.
Here, we review behavioural experiments that test magnetic navigation and orientation as in migration, homing, foraging in eusocial insects and the waggle dance of honeybees. This review is restricted to studies on orientation behaviours, as magnetoreception is mostly used in the literature, in contrast with the more general concept of magnetic sensitivity, for which any behaviour (e.g. nest construction) is considered (Walker 1997; Wiltschko & Wiltschko 2005). For the first time, the use of physical techniques to determine the magnetic properties of the nanoparticles found in eusocial insects as the magnetosensor candidate is presented. Twelve years from the reviews of insects (Vácha 1997) and arthropods (Walker 1997), published research on these subjects has not increased as rapidly as in vertebrates, particularly in birds (Mouritsen & Ritz 2005).
A magnetic compass was first demonstrated in migratory birds. The migration phenomenon has imparted great interest in magnetoreception studies. Migration is also observed in some insect species. Monarch butterflies seasonally migrate from summer habitats in North America to overwintering sites in Mexico (Mouritsen & Frost 2002), neotropical butterflies Aphrissa statira cross the isthmus of Panama in abundance during May–July (Srygley et al. 2006) and colonies of Pachycondyla marginata ants migrate over 2 days, covering distances from 2 to 97 m in a southeast semideciduous forest of Brazil (Leal & Oliveira 1995).
The majority of insect species live solitarily, while eusocial insects live in well-organized colonies in which they are in permanent contact with other individuals. This organization relies on a considerable degree of complexity of tasks. Communication and foraging can be accomplished via different sensory abilities based on visual, acoustic, tactile, chemical or magnetic information (Billen 2006). Eusocial insects, such as honeybees and desert ants, navigate over relatively short distances in straight lines back to their nests after complicated feeding excursions. In addition to their efficient way of homing, eusocial insects are an interesting group to study magnetic orientation because of the large number of individuals in colonies, allowing the collection of many observations in a short time. The widespread occurrence of magnetosensitivity among magnetotactic bacteria and animals indicates ancient origins of magnetoreception. Complex social organizations, including castes and differentiated tasks, of termites, ants and some of the bees and wasps originated during the Cretaceous times (Holldobler & Wilson 1990), which suggests that magnetoreception in these insects may have had an adaptive significance.
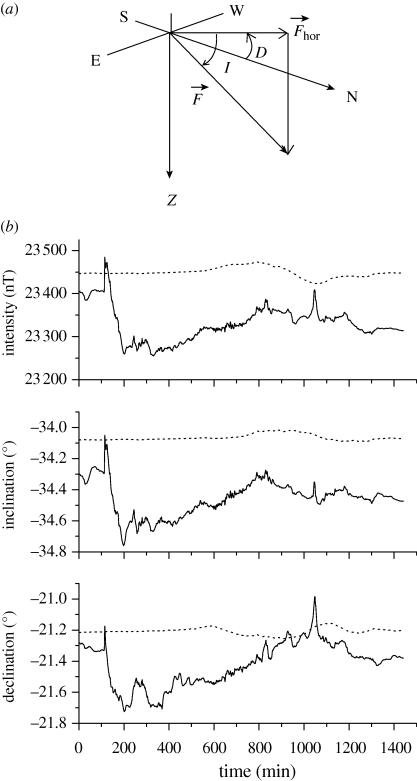
The geomagnetic field is analogous to a large magnet with its poles localized near to the geographical ones. This field vector is commonly defined by its total intensity (F), the declination angle of the horizontal component (D) relative to geographical North–South and its inclination angle (I) relative to the horizontal component (figure 1a). These components vary both spatially and temporally (figure 1b). Animals might use information from intensity gradients or from the directional cues, which is characteristic of the horizontal polarity and inclination angle. The polarity compass (or magnetic compass) is the ability to sense the magnetic North and South axis from the horizontal polarity. The inclination compass, as derived from studies of migratory orientation in birds, is the ability to sense poleward from equatorward directions based on the polarity of the vertical component (down or upward) (Wiltschko & Wiltschko 2005). In addition, local field anomalies can be generated by high concentrations of magnetic material in the Earth's crust. These spatial anomalies are characterized by a gradient of about 102 γ m−1 (1/4π A m−2), much greater than the main geomagnetic field gradient of about 10−1 γ m−1 (10−3/4π A m−2). Insects that move slowly over short distances within or through anomalies might use their distinct properties for orientational or navigational strategies. These can also be temporally modified by the electromagnetic radiation produced by solar flares, causing geomagnetic storms. These storms cause small variations in its intensity, larger than the daily variations (figure 1b).
Figure 1.
(a) The geomagnetic field vector F and the positive convention for the declination (D) and inclination (I) angles relative to its horizontal component (Fhor) and the geographical North (N), South (S), West (W) and East (E). Z is the gravity vector (Northern Hemisphere condition). (b) Geomagnetic field components F, D and I as a function of time of day on 5 May 2001 (dotted line) and of a magnetic storm day (6 November 2001, solid line). Data from the National Observatory, Vassouras, Brazil.
2. Behavioural evidence for magnetoreception
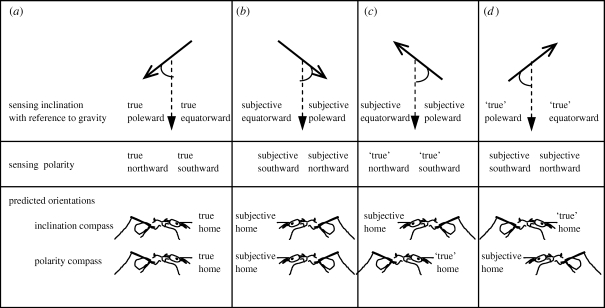
Studies to date have identified some common characteristics of animal magnetoreception. If orientation reverses when the horizontal component of the magnetic field is reversed, the animal may have either a compass that senses magnetic polarity or an inclination compass, which distinguishes poleward from equatorward orientations (figure 2a,b). Inclination compasses are observed to be light sensitive in birds (Wiltschko & Wiltschko 2005), whereas the polarity compass is light independent (Ritz et al. 2002). Nevertheless, light sensitivity in magnetic orientation of a beetle appeared contradictory. The change of the horizontal component when the vertical component was cancelled resulted in light-independent changes in orientation (Arendse 1978), but changes in orientation were light dependent in the natural vertical condition (Vácha & Soukopová 2004). Another option to distinguish between the two possible compasses is an experiment reversing the vertical component of the magnetic field while maintaining the natural horizontal component (figure 2c). This will result in reversal of orientation if the animal possesses an inclination compass alone, but will not result in reversal if the animal possesses only a polarity compass. Reversing both the vertical and horizontal components of the magnetic field will result in reversal of orientation if the animal possesses a polarity compass, but will not result in reversal if the animal possesses an inclination compass (figure 2d). For eusocial insects, most behavioural experiments testing magnetoreception have not followed the experimental protocols applied to migratory songbirds. Some were prior to modern orientation concepts; some are field observations; and the majority concern local rather than long-distance navigational orientation.
Figure 2.
Experimentally distinguishing an inclination compass from a polarity compass. The example given is for an ant colony located in the Northern Hemisphere (north of the magnetic equator). (a) The natural condition; (b–d) three experimental conditions: (b) polarity reversed, inclination unchanged; (c) polarity unchanged, inclination reversed; (d) polarity reversed, inclination reversed. Top box: the poleward and equatorward directions sensed with an inclination compass in each treatment; middle box: the northward and southward directions sensed with a polarity compass; and bottom box: the predicted orientation of homing ants using an inclination compass or a polarity compass when the true direction of the nest is to the south and towards the equator. (c,d) ‘True’ indicates a magnetic orientation under the altered magnetic field vector that is indistinguishable from that in natural conditions. Solid arrows, orientations of the magnetic field; vertical dashed arrows, gravity; curved lines indicate the poleward directions relative to gravity that are predicted to be sensed by an inclination compass.
2.1. Bees
Honeybees (Apis mellifera) are by far the most studied eusocial insect. There is evidence for magnetoreception from several activities including the waggle dance, foraging and flight. Foragers perform the waggle dance on the honeycomb to inform other bees of the direction of a food source. Their orientations are affected by changes in magnetic field intensity and polarity and are also sensitive to changes in light wavelengths (Lindauer & Martin 1972; Gould et al. 1980; Leucht 1984; Leucht & Martin 1990). Conditionally trained bees discriminated local magnetic anomalies and alternating magnetic fields cycling at different frequencies (Walker & Bitterman 1985, 1989a,b; Kirschvink & Kobayashi 1991; Walker 1997). Magnetic cues can also contribute to a coordinate frame for visual landmark learning or to discriminate between panoramic patterns in the absence of celestial information (Collett & Baron 1994; Frier et al. 1996).
The ability of bumblebees to follow the trained direction to a food source was observed in the absence of light and chemical cues (Chittka et al. 1999). Although magnetoreception was suggested as a possible light-independent compass, there was neither a direct test, i.e. changing the magnetic field, nor control or obstruction of other cues, such as sound, vibration and temperature.
The magnetic effect on flight orientation was tested as bees exited their nest. In the laboratory, the flight orientation of honeybees A. mellifera changed as predicted when the field was shifted 90° in total darkness (Schmitt & Esch 1993). In the field, the flight direction of stingless bees Schwarziana quadripunctata was measured upon exiting their underground hive during the daylight period of highest foraging activity (Esquivel et al. 2007). No significant differences resulted during six experimental days when alternating between the geomagnetic field and the applied static inhomogeneous field, which was about 10 times that of the geomagnetic field. A surprising statistically different response from all of the other days was obtained on a day when a unique magnetic storm occurred, which presented a variation of about 150 γ (1.50/4π A m−1) in the geomagnetic field intensity (figure 1b). This result, in conjunction with the presence of magnetic nanoparticles (Lucano et al. 2006), suggests that the geomagnetic field is an orientational cue. Considering the magnitude of the temporal variation, the magnetic threshold that can be sensed is lower than the 250 γ (2.50/4π A m−1) observed in honeybee experiments using static magnetic anomalies and alternating magnetic fields (Walker & Bitterman 1985, 1989a,b; Kirschvink & Kobayashi 1991; Kirschvink et al. 1997). Finally, the nest-exiting behaviour of the stingless bee Tetragonisca angustula was tested under three experimental treatments of the vertical component of the local field: null, doubled and reversed. In the presence of other orientation cues, only the reversed vertical field affected the inclination of the flight trajectory, indicating that these bees can sense whether the magnetic field is pointed up or down and, consequently, can distinguish Northern and Southern hemispheres (Esquivel et al. 2008).
2.2. Ants
The subterranean and nocturnal habits of many ants and their movement through cluttered environments obstructing celestial cues make them excellent subjects for orientation by a magnetic compass. Ants are also suitable because they can be trained to forage at a dependable food site from which they return to the nest. Goal-oriented behaviours facilitate predictions of orientation during experimental treatments. However, depending on their habitat, ants may use landmarks, pheromones, vibrations, gravity, sun compass and polarized light to orient. These cues must be carefully masked in order to investigate the exclusive use of a magnetic compass.
Probably the first behavioural experiment testing magnetic orientation was carried out with iron filings attached to the body parts of the ant Myrmica ruginodis. Effects of magnetic fields on ant orientation were observed only when iron was attached to the antennae, with the pedicel being the most sensitive (Vowles 1954).
The strongest evidence for use of a magnetic compass comes from experiments where the polarity of the magnetic field has been reoriented. Çamlitepe et al. (2005) positioned a solenoid over a foraging trail of the black-meadow ant Formica pratensis, and trained ants to climb a pole into the solenoid and enter a four-arm choice arena oriented to the four cardinal directions. The ants were trained to retrieve honey from the North arm. The authors obstructed celestial cues and controlled for the use of landmarks within the solenoid. The orientation of foraging wood ants shifted in accordance with an experimental 90° anticlockwise rotation of the ambient magnetic field in the greenhouse in both artificial light and darkness.
Leaf-cutter ants Atta colombica collect rolled oats or barley and transport the flake back along the trail to the nest. The rolled grain can be grasped to displace the ant without otherwise disturbing its behaviour. Riveros & Srygley (2008) moved leaf-cutter ants to an arena within a Merritt biomagnet that reversed the polarity of the local magnetic field. Seeking to avoid the use of landmarks and celestial cues by the ants, they worked on leaf-cutter ants foraging outdoors at night on moonless nights and cloaked the biomagnet with opaque black tarps. They showed that leaf-cutter ants orient their path-integrated vector with a magnetic compass. In another study, foraging weaver ants Oecophylla smaragdina on their homeward journey reversed their orientation with experimental reversal of the local magnetic field (Jander & Jander 1998).
Outdoor studies on ants in manipulated magnetic fields have resulted in behaviours that do not follow those predicted by shifts in the magnetic field alone. In trials conducted in daylight (Çamlitepe et al. 2005), black-meadow ants oriented uniformly in the natural magnetic field, suggesting conflict with a cue that was not present when trials were conducted in darkness or when the magnetic field was experimentally shifted. Banks & Srygley (2003) worked with leaf-cutter ants during daylight, using a white tarp to obscure landmark and celestial cues. Although polarized light was occluded, the tarp apparently did not prevent the ants from using their sun compass unless the sky was overcast. Control ants and those under clear skies exhibited path integration and headed directly towards home, rather than down the foraging trail from which they had been removed. When the sky was overcast, the ants responded to polar-reversal of the local magnetic field but short of the predicted shift of 180°, which also suggested conflict with another cue. In the experiment that Riveros & Srygley (2008) conducted at night, one-half of the experimental ants in the reversed magnetic field oriented towards their nest, suggesting the use of nocturnal orientation cues, vibrational or otherwise, that the researchers did not obstruct.
If the light sensitivity of the compass is of interest, experimentation in complete darkness is one option. Additional behavioural evidence of the nature of the magnetic compass in ants comes from the application of a brief magnetic pulse to foraging leaf-cutter ants (Riveros & Srygley 2008). The predicted results of pulse magnetization depend on the materials and construction of the compass (Wiltschko & Wiltschko 2005). The strength of the magnetic pulse was sufficient to overcome the coercivity of single-domain (SD), biological magnetite crystals (e.g. a pulse of 55 mT should suffice). A biasing field aligns the magnetite particles in the same direction prior to applying the pulse. For SD magnetite, a magnetic pulse to the magnetite in a parallel biasing field would not result in a change in the magnetic spin within the crystals and the animals' orientations would not be different from the controls, whereas a magnetic pulse in an antiparallel biasing field would result in an orientation opposite to the controls (Kirschvink & Kobayashi 1991). If the magnetite crystals are in the superparamagnetic (SPM) region (see definition in the next section), they can align with an external magnetic field. A cluster of SPM particles is likely to be disrupted by a magnetic pulse (Davila et al. 2005), and the animals would orient randomly in the absence of other cues. Orientation of the leaf-cutter ants was disrupted following exposure to either pulse treatment. This result supports the presence of a compass based on SPM particles (Riveros & Srygley 2008).
A magnetic compass was suggested as a means of orientation during the migration of P. marginata ants. Migrations during the dry/cold season are significantly oriented 13° with the magnetic North–South axis, whereas rainy/hot season migrations do not exhibit a preferred direction (Acosta-Avalos et al. 2001). The observation of magnetic particles in these ants supports the use of the geomagnetic field as an orientation cue for axial migratory routes (Acosta-Avalos et al. 1999; Wajnberg et al. 2000).
Although magnetic sensitivity was observed in nest construction of termites and hornets, there is no evidence of magnetoreception (as defined in §1) to our knowledge.
3. Models of magnetoreception
The magnetoreception mechanisms in insects are poorly understood, but two mechanisms can be considered: ferromagnetic and light-dependent. At present, empirical results are insufficient to formulate specific models aimed at understanding magnetoreception in social insects. While some of the behaviours are very specific to the animal, others are more general and can be used to develop unifying theories.
3.1. Ferromagnetic hypothesis
The ferromagnetic hypothesis derives from observations of the magnetosome of magnetotactic bacteria (Kirschvink & Gould 1981) and was extended to complex animal behaviours. It is postulated that the sensor is composed of magnetic nanoparticles, possibly magnetite, housed in a specialized organ that is coupled to mechanosensitive structures so as to transmit information from the geomagnetic field in the form of a torque or force to the nervous system as an electric signal. Based on this hypothesis, several models for transduction have been put forward for SD magnetite chains from early (Yorke 1979) to more recent models (Walker 2008) and for other magnetic particle systems (Presti & Pettigrew 1980; Kirschvink 1989; Sakaki et al. 1990; Edmonds 1992, 1996; Shcherbakov & Winklhofer 1999; Davila et al. 2003, 2005).
Experimental evidence shows that magnetoreception can be based on orientations of the horizontal and vertical components of the magnetic field and on the field intensity and variations. The components rely on fixed orientation information (magnetic North–South or up–down), but, when combined with intensity, some animals may localize themselves in space and precisely orient to a goal. We use here the term ‘magnetic map’ with no assumption about the nature of the internal spatial representation that a particular animal has (as adopted by Lohmann et al. 2008).
Considering the ferromagnetic hypothesis, interaction and physical response to the magnetic field depend on the size of the magnetic particle involved in magnetoreception. There is a lower size limit for a nanoparticle to be considered magnetically stable, known as a single domain, and its magnetic moment points to a fixed direction relative to the particle. If the size particle is lower than the critical SD size, it becomes unstable owing to thermal fluctuations and is called superparamagnetic. The blocking or critical magnetite volume depends on the grain elongation, magnetic interactions among individual grains or chains and relaxation times (Muxworthy & Williams 2006, 2009). The different magnetic stabilities suggest that SD nanoparticles are involved with the magnetic compass and SPM nanoparticles in the magnetic map mechanism (Johnsen & Lohmann 2005).
Considering the complexity of the magnetic system interactions, a physical model will be better developed if the magnetic properties of the system and its nanoparticles arrangement in the tissue are known. This was the case with models based on SD particles, inspired by the chain structure from bacteria.
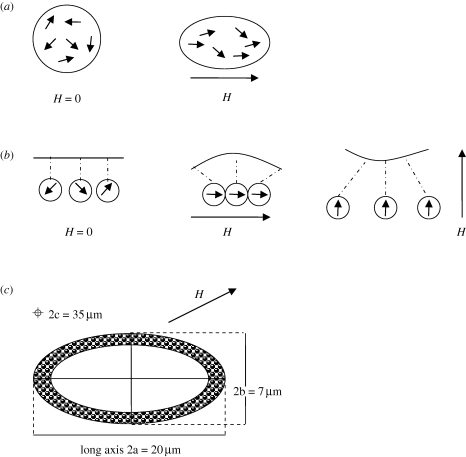
Chains of magnetic material (e.g. SD magnetite) inside sensory cells have been proposed to be involved in detecting the magnetic field assuming the chains are free to rotate about pivots embedded in the cell membrane and linked by microtubule-like strands to mechanically gated ion channels in the membrane of the receptor cell (Walker et al. 2002). Inclusion of a physical link between the chain and cell membrane restricting the motion of the chain to a narrow cone would reduce noise owing to thermal agitation and allow extraction of information on magnetic intensity (Walker 2008). Each cell functions as a single-axis magnetic detector. To detect all the components of the magnetic vector, an array of these oriented cells in different directions is necessary. Although individual SPM particles do not present a stable magnetic remanence, a group of crystals in a special arrangement can collectively interact with the geomagnetic field so that thermal fluctuations can be overcome. Magnetic-field-induced interactions between the SPM particles or aggregates can produce stress on the cellular structures that can then be transduced into the nervous system. Scherbakov & Winklhofer (1999) proposed an osmotic magnetometer based on a single cluster of SPM particles that would deform the cell into a prolate ellipsoid with its long axis parallel to the direction of the magnetic field (figure 3a). Taking into account the observation that the SPM clusters were aggregated in a chain in the pigeon beak (Fleissner et al. 2003), magnetic interactions between clusters were then considered leading to attraction (repulsion) if they were parallel (perpendicular) to the direction of the magnetic field (figure 3b). The relevance of the spatial arrangement of particles is important: for a single cluster, the induced elongation is always parallel to the direction of the magnetic field, whereas for a chain of clusters there is a contraction when the field direction is parallel to the cluster chain.
Figure 3.
Different arrangements of SPM particles based on observations of animal tissues. (a) Magnetic deformation of a cell assuming one single cluster of SPM particles based on Scherbakov & Winklhofer (1999). In the absence of the magnetic field, it is spherical. Under an external field, it is magnetized parallel to the field, inducing a prolate shape with the long axis parallel to the field. Small arrows indicate the magnetic moment of individual particles. (b) A chain of interacting clusters linked to the cell membrane based on Davila et al. (2003). No deformation in the absence of the magnetic field. If the magnetic field is parallel to the chain, the clusters attract each other, shrinking the cell. If the field is perpendicular to the chain, there is elongation of the cell. Small arrows indicate the magnetic moments of the clusters. (c) Diagram of a transverse section of the cell-like structure with particles surrounding it observed in the pedicel–scape joint of the antennae of a migratory ant. The ellipsoid axes 2a, 2b and 2c are shown as in Oliveira et al. (2010). In the presence of a magnetic field oblique to the long axis of the structure, a magnetic torque appears and biases the mechanical one. H, the applied magnetic field.
A magnetic map hypothesis was postulated for the magnetoreception in A. mellifera based on cellular studies. It was observed, for the first time, that the magnetic field induces magnetic granules (MGs) to shrink when parallel to the magnetic field and to enlarge at the vertical direction in the horizontal plane, similar to the model in figure 3. Additionally, it was considered that these size fluctuations of MGs can induce the relaxing and tensing of cytoskeletons to trigger the observed increase in [Ca+2]I, which can further trigger signal transduction for magnetoreception by which honeybees would establish the magnetic map in memory during orientation flights (Hsu et al. 2007).
For eusocial insects, two other models have been specifically proposed for arranged SPM particles as suggested in the dorsal hair of honeybee abdomens (Schiff 1991) and in the antenna of a migratory ant (Oliveira et al. 2010). Schiff & Canal (1993) developed a model based on agglomerated SPM magnetite in the hair of bee abdomens. These particles were considered to be arranged in a rod and a ring, which can be aligned by the geomagnetic field. As a bee moves, the geomagnetic field would cause reorientation of the SPM magnetite inducing an electric field (via Faraday's Law of induction). The increase in the magnetic field produced by the SPM particle was calculated as a function of the distance and of the direction of the magnetic field. The authors theorized that the induced electric field, when a honeybee flies through a magnetic field gradient, could act on the dendrites. Amplification through signal integration of several hairs would be sufficient to stimulate the nerve to generate an action potential.
More recently, the cell-like structure surrounded by magnetic particles found in the pedicel–scape joint of the antenna of the migratory ant P. marginata (Oliveira et al. 2010) was proposed as a magnetosensor based on the ferromembrane model (Winklhofer 1998). A magnetic torque arises when the magnetic field is oblique to the long axis of this anisotropic structure (figure 3c), trying to align it with the magnetic field. Oliveira et al. (2010) estimated that this torque is enough to balance thermal energy at room temperature, even considering SPM haematite particles, a weak magnetic material. The torque acting on this structure would bias the mechanical torque, and by comparing the output of this magnetically coated proprioceptor with a non-magnetic adjacent one, the magnetic torque can be isolated. This recent result suggests that antennae should be one focus of further studies on magnetoreception in eusocial insects.
3.2. Light-dependent magnetoreception
The effect of varying the wavelength of light on magnetic orientation was first tested on honeybee behaviour (Leucht 1984). Sensitivity of the waggle dance to a compensated geomagnetic field decreased from UV to green light (348, 440 and 548 nm). The honeybees showed nearly no reaction in a 2 G (2 × 10−4 T) applied field, about four times the local field intensity. Edmonds (1996) proposed a magnetic compass model, formed by a small quantity of needle-shaped SD nanoparticles in a droplet of nematic liquid crystal (constituted by long molecules that spontaneously orient their long axes parallel, with some degree of anisotropy), adapting it to the animal eye. With cones containing internal liquid media with properties of liquid crystal, optical detection could be easily achieved by inclusion of an array of elongated dye molecules, such as β-carotene, which anisotropically absorb light. Were a subgroup of these oil droplets to become liquid crystalline and to contain some magnetite, a sensitive compass would be formed. The animal would only detect incident light with such a cone when the cone axis is parallel or antiparallel to the direction of the geomagnetic field.
Other light-dependent mechanisms were proposed assuming the influence of magnetic fields on the rate of radical-pair production (Ritz et al. 2002), and it was recently shown that the ultraviolet-A/blue-light photoreceptor cryptochrome is necessary for light-dependent magnetosensitive responses in Drosophila melanogaster flies (Gegear et al. 2008). In honeybees, a similar range of wavelengths was observed to affect waggle dances (Leucht 1984). An alternative model was recently proposed to explain the light-dependent mechanism based on an integration process of a light-independent magnetic compass mechanism and a vision-based skylight colour gradient compass that misperceives compass cues in monochromatic light (Jensen 2009). The model suggests the existence of a single magnetic sense that can explain the light-dependent effects in birds, probably based on magnetic particles. Unfortunately, relevant data for insects are still too few to be interpreted.
4. The search for magnetosensors
Two magnetosensors have been proposed, initially from data available for vertebrates: the ferromagnetic particles and the radical-pair mechanism. Despite the recent interest in verifying the existence of a light-dependent mechanism in insects (Gegear et al. 2008; Vácha et al. 2008) and the pioneer studies in honeybees (Leucht 1984; Leucht & Martin 1990), there is still no direct identification of the radical-pair mediator in eusocial insects. In contrast, the first step towards confirming the ferromagnetic hypothesis, which is to demonstrate the presence of magnetic nanoparticles in or around innervated structures in tissues, has been conducted to a reasonable degree.
Biomineralization occurs in almost every biological phylum from unicellular organisms to mammals (Bauerlein 2005), and it forms a wide variety of biominerals. Magnetite, the most common magnetic source in biological systems (Gould 2008), and maghemite, a cation-deficient form of magnetite, frequently appear biomineralized together (Fleissner et al. 2007). The biological functions of these iron oxides in animals are mainly associated with magnetoreception, but additional functions remain poorly known.
Looking for low concentrations of magnetic particles dispersed in an insect body is still a needle-in-the-haystack task (Kirschvink et al. 1997; Maher 1998). At least an indication of which body part contains magnetic material is necessary. The use of magnetic techniques can help to restrict the region to look for the sensor, but other magnetic contributions from protein and ingested material can add to the magnetic signal. Despite the complexity arising from unknown compounds and particle orientation in eusocial insects, these tiny nanoparticles have been successfully characterized magnetically, mainly by superconducting quantum interference device (SQUID) magnetometry and ferromagnetic resonance (FMR), and complemented by transmission electron microscopy (TEM) (details of techniques in appendix A).
Magnetic extraction techniques and dehydration of tissue samples were used to concentrate the magnetic materials, eliminating para/diamagnetic biological contributions. However, this is not always possible, and it may affect the material of interest and/or its properties. Because magnetic material is typically found only in very low quantities in insects, it was initially studied in large amounts of crushed material (Esquivel et al. 1999; Wajnberg et al. 2000, 2001; El-Jaick et al. 2001; Abraçado et al. 2005). Orienting body parts improved the signal (Esquivel et al. 2004; Abraçado et al. 2008), and decreasing the amount of sample reduced FMR noise caused by water content. The SQUID sample holder, a capsule commonly used with point-like inorganic samples, causes undesired para/diamagnetic signals owing to the low concentration of magnetic material in biological tissue and the large biological samples. It also results in an undesired asymmetry in the SQUID signal. The tissues can be supported in the sample holder with two inner straws (Hautot et al. 2005) or they can be stuck to a long kapton tape (Lucano et al. 2006). Either will produce an improved signal-to-noise ratio. If the sample length is less than 8 mm, then the magnetization measurement is within 90 per cent of the true value.
4.1. Ferromagnetic resonance and superconducting quantum interference device magnetometry results
4.1.1. Bees
Magnetization of the abdomens of A. mellifera bees was measured separately from heads and thoraxes (anterior segments) as a function of temperature and field (tables 1 and 2). Relatively large magnetite particles (ca 30 nm or more) were found in the abdomens, capable of retaining a small remanent magnetization at room temperature down to 4.2 K (Gould et al. 1980), although these particles were absent from the heads and thoraxes, which showed only a diamagnetic contribution (Takagi 1995). In contrast, thermal decay of remanence (TDR) curves of the anterior segments indicated ferromagnetic particles, although the Verwey transition was not observed. TDR curves confirmed that magnetite particles are predominantly located in bee abdomens (Desoil et al. 2005). Hysteresis curves of A. mellifera abdomens (first three segments) oriented parallel and perpendicular to the applied magnetic field were obtained from 5 to 310 K. At low temperatures, the hysteresis curves indicate a preferential orientation of the magnetic easy axis parallel to the body axis. SPM magnetite was indicated by 50 and 120 K mean blocking temperatures (Esquivel et al. 2002). The presence of SPM magnetite in the trophocyte granules of honeybee abdomens was recently confirmed by SQUID magnetometry among diverse spectroscopic techniques such as atomic force microscopy, magnetic force microscopy (MFM), high-resolution TEM (HRTEM), scanning TEM and energy dispersive X-ray (EDX) with electron spectroscopy for chemical analysis. A high intrinsic coercivity (100–150) ×10−4 T (100–150 G) was shown for purified iron granules (IGs) (Hsu et al. 2007).
Table 1.
Room temperature magnetic parameters of whole eusocial insects and their body parts.
| SQUID |
FMR | |||||||
|---|---|---|---|---|---|---|---|---|
| species | whole worker or body partsa | HC (Oe, 10−3/4π A m−1) | Jr/ Js | Ms (10−6 emu, 10−9 A m2)b | K (104 erg cm−3, 103 J m−3) | references | ||
| bee | A. mellifera | head + antennae | 0.05 | Desoil et al. (2005) | ||||
| thorax | 0.05 | |||||||
| abdomen | 0.09 | |||||||
| −1.9 | El-Jaick et al. (2001) | |||||||
| first three abdomen segments, H // long body axis | 70 | 0.10 | 25 | Esquivel et al. (2002) | ||||
| first three abdomen segments, H ⊥ long body axis | 44 | |||||||
| purified IGs | 100–150c | 0.09 | 2.83 × 102d | Hsu et al. (2007) | ||||
| S. quadripuncta | antennae | 130 | 0.24 | 21 | Lucano et al. (2006) | |||
| head | 32 | 0.12 | 11 | |||||
| thorax | 44 | 0.12 | 7 | |||||
| abdomen | 90 | 0.09 | 9 | |||||
| ant | P. marginata | whole ant, H // long body axis | 30 ± 5 | 0.06 ± 0.02 | 33 ± 1 | Esquivel et al. (2004) | ||
| whole ant, H ⊥ long body axis | 59 ± 6 | 0.14 ± 0.05 | 15 ± 2 | |||||
| antennae | 42 | 0.10 | 54 | Wajnberg et al. (2004) | ||||
| head | 52 | 0.09 | 32 | |||||
| thorax | 61 | 0.13 | 25 | |||||
| abdomen | 64 | 0.13 | 17 | |||||
| 6.4 ± 0.4 | Wajnberg et al. (2000) | |||||||
| S. substituta | head + antennae | 5.1 | Abraçado et al. (2005) | |||||
| abdomen | 4.8 | |||||||
| termite | N. opacus | whole termite, H // long body axis | 100 ± 1 | 0.19 ± 0.02 | 350 ± 5 | Esquivel et al. (2004) | ||
| whole termite, H ⊥ long body axis | 111 ± 4 | 0.19 ± 0.02 | 335 ± 5 | |||||
| whole termite | ||||||||
| as collected | 6.2–6.9 | Oliveira et al. (2008) | ||||||
| 3 days cellulose diet | 3.1–3.3 | |||||||
| 5 days cellulose diet | 50–60 | 0.13–0.19 | 25–29 | Oliveira et al. (2005b) | ||||
| head + antennae | 2.1 ± 0.1 | Alves et al. (2004) | ||||||
| thorax + abdomen, H // long body axis | 2.6 ± 0.1 | |||||||
| thorax + abdomen, H ⊥ long body axis | 3.2 ± 0.2 | |||||||
aH, applied magnetic field.
bMagnetic moment per whole insect or body part.
cOriginal value in G in Hsu et al. (2007).
dValue per 2000 bees.
Table 2.
Size and magnetic domain of particles and magnetic material distribution in whole eusocial insects and their body parts.
|
d (nm) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| species | worker body part | fraction (%) | oriented material | material | magnetic domain | magnetometry | FMR | TEM | references | |
| bee | A. mellifera | whole bee | Y | magnetitea | SPM | 30–35 | Gould et al. (1980) | |||
| head + thorax | — | magnetitea | SPM | <30 | Desoil et al. (2005) | |||||
| antennae, head and thorax | ∼25 | — | magnetitea | large particles and SPM or aggregate | Chambarelli et al. (2008) | |||||
| abdomen | ∼75 | — | ||||||||
| — | magnetitea | SPM and SD or aggregates | <30 (SPM) | Wajnberg et al. (2001) | ||||||
| — | magnetitea | large particles and SPM or aggregate | 12 | El-Jaick et al. (2001) | ||||||
| — | magnetiteb | SD | >30 | Desoil et al. (2005) | ||||||
| first three abdomen segments | Y | magnetitea | SPM | 20–26 | Esquivel et al. (2002) | |||||
| fat body (queen) | — | ferritin-like | 6.4 ± 0.9; 3.9 ± 0.8 | Keim et al. (2002) | ||||||
| abdomen trophocytes | — | magnetite | SPM particles in purified IGs | 9.7 ± 0.2 (SPM in IG) | 10 nm (SPM in IG) | Hsu et al. (2007); Hsu & Li (1994) | ||||
| S. quadripunctata | antennae | 45 ± 5 | — | magnetitea | 37–100 | Lucano et al. (2006) | ||||
| head | 23 ± 3 | ∼220 | ||||||||
| thorax | 15 ± 2 | |||||||||
| abdomen | 19 ± 4 | |||||||||
| S. postica | fat body (queen) | — | ferritin-like | 2.2 ± 0.5; 1.5 ± 0.3 | Keim et al. (2002) | |||||
| ant | P. marginata | whole ant | Y | magnetitea | MD | >220 | Esquivel et al. (2004) | |||
| head + antennae | — | magnetite/maghemite | SPM and SD | 39 ± 2 /26 ± 2c | Acosta-Avalos et al. (1999) | |||||
| antennae | — | iron oxides | 20–100 | Oliveira et al. (2010) | ||||||
| 42 ± 3 | — | magnetitea | PSD or MD | Wajnberg et al. (2004) | ||||||
| head | 24 ± 3 | — | ||||||||
| thorax | 19 ± 3 | — | ||||||||
| — | magnetite/maghemite | SPM and SD | 38 ± 3/27 ± 3c | Acosta-Avalos et al. (1999) | ||||||
| abdomen | — | 39 ± 2/26 ± 1c | ||||||||
| 15 ± 5 | — | magnetitea | MD + PSD or MD + SPM | Wajnberg et.al. (2004) | ||||||
| — | magnetitea | large particles and SPM or aggregate | 13 | Wajnberg et.al. (2000) | ||||||
| — | magnetitea | SPM and SD or aggregates | <30 (SPM) | Wajnberg et.al. (2001) | ||||||
| S. invicta | whole ant | — | magnetitea | SPM and SD or aggregates | <30 (SPM) | |||||
| S. substituta | head + antennae | 55 | — | magnetitea/maghemitea | large particles and SPM or aggregate | 11.0 ± 0.2 | Abraçado et al. (2005) | |||
| thorax | ∼6 | — | bulk like, large aggregates | |||||||
| abdomen | ∼39 | — | large particles and SPM or aggregate | 12.5 ± 0.1 | ||||||
| S. interrupta | antennae | 32 ± 3 | Y | magnetite/maghemitea | large particles and SPM or aggregate | Abraçado et al. (2008) | ||||
| head | 24 ± 2 | Y | ||||||||
| thorax | 21 ± 2 | N | ||||||||
| abdomen | 23 ±1 | N | ||||||||
| termite | N. exitiosus | head + antennae 3 days cellulose diet | 23 | — | magnetitea | Maher (1998) | ||||
| thorax + abdomen 3 days cellulose diet | 77 | — | ||||||||
| A. meridionalis | whole termite 3 days cellulose diet | — | SPM or MD | 10 | ||||||
| N. opacus | whole termite | Y | magnetitea | PSD or PSD + MD | 100–220 | Esquivel et al. (2004) | ||||
| whole termite 3 days cellulose diet | — | magnetitea | SPM | 11.4 ± 0.4 | Oliveira et al. (2008) | |||||
| 14.6 ± 0.6 | ||||||||||
| abdomen + thorax | 97–99 | Y | magnetitea | SPM | 18.5 ± 0.8 | Alves et al. (2004) | ||||
| head | 1–3 | — | ||||||||
Y, angular dependence measured and magnetic anisotropy observed; N, angular dependence measured and magnetic anisotropy not observed. SPM and SD, see definitions in text; PSD, pseudo-single-domain boundary region between SD and multi-domain (MD); MD, large particles with several magnetic domains.
aSuggested.
bObservation of Verwey transition.
cLength/width.
As in SQUID magnetometry, different FMR spectra resulted from similar abdominal samples. FMR temperature-dependence analysis of the high-field (HF) component (defined in appendix A) yielded isolated SPM particles with mean magnetic diameters of 8 nm in A. mellifera abdomens. It is interesting to note that the decrease in the degree of hydration of crushed abdomens resulted in a lower mean magnetic volume of the particles (Wajnberg et al. 2001). Whole abdomens revealed only one component that was considered a paramagnetic species (Takagi 1995), but it was also similar to the ‘envelope component’ associated with amorphous FeOOH observed in crushed abdomens. Crushed abdomens presented a complex FMR spectrum, resulting from the contributions of several ferromagnetic components (El-Jaick et al. 2001), as well as in the spectra of purified and lyophilized IGs from bee trophocytes (Hsu et al. 2007). This spectral difference was related to the difference in material content from one adult individual to another, to the nest locations, to the developmental adult stage or to drying treatments (El-Jaick et al. 2001), but the body part, bee age and preservative solution can also influence the spectra of A. mellifera body parts (Chambarelli et al. 2008). This systematic study has shown, for the first time, the presence of magnetic material in the antennae of this bee in addition to the three other major body parts. The HF intensity of the abdomen is commonly one order of magnitude larger than any other body part, so that it can be difficult to detect the amount of magnetic material in parts other than the abdomen.
SQUID magnetometry and FMR of whole bees and heads, pairs of antennae, thoraxes and abdomens of the stingless bee S. quadripunctata have shown that the distribution in the body parts and the properties of the magnetic material differ from those of A. mellifera, with the antennae presenting the highest amount of magnetic material (table 2; Lucano et al. 2006).
4.1.2. Ants
The first EPR study of six ant species did not attempt to identify ferro(i)magnetic species at g-values greater than 4. It revealed two groups of species: those whose spectra exhibited a strong and narrow signal at g = 2.012 and a weaker one in the range of 3.5–4, and those that gave a strong Mn six-peak signal (Krebs & Benson 1965).
The FMR spectra of abdomens of P. marginata ants as well as of abdomens and head with antennae attached of Solenopsis substituta ants presented two broad lines, the HF and low-field (LF) components (see appendix A). The temperature dependence of the spectral parameters allowed the estimation of KEFF, the effective anisotropy energy density, and of the mean magnetic diameter (Wajnberg et al. 2000; Abraçado et al. 2005). The values are summarized in tables 1 and 2.
The amount of magnetic material and the isolated nanoparticles/aggregates fraction obtained from FMR parameters of Solenopsis species correlate with the geomagnetic field intensity where they were collected, suggesting an adaptation of these ants to their magnetic environment. Isolated nanoparticles were suggested to be involved in long-distance orientation, based on the predominance of the HF component in the spectra of migratory and nomadic ants. FMR is a promising tool for distinguishing migratory from non-migratory ant species (Wajnberg et al. 2005).
Remanent and saturation magnetization ratios calculated from room temperature hysteresis curves of whole P. marginata fall in the range of magnetite multi-domain (MD) particle values (Esquivel et al. 2004). Body parts, oriented parallel to the magnetic field, indicate the strongest saturation magnetization in the antennae (42 ± 3%), suggesting that this structure is also a magnetic sensory organ. The ant abdomens present a wasp-waisted loop with Hcr/Hc of 4.75, a characteristic of a mixed magnetic system (Wajnberg et al. 2004). FMR spectra also showed a higher magnetic fraction in the head with antennae attached than in other body parts of two other ant species, S. substituta and Solenopsis interrupta (Abraçado et al. 2005, 2008).
SQUID magnetometry was used to measure the induced remanent magnetization of the migratory ant P. marginata abdomens and a fire ant Solenopsis sp., from 10 to 300 K. The values at room temperature indicated the presence of SD or aggregates of magnetite nanoparticles, and the upper limit values of 1010 SPM and 109 SD or aggregate particles were estimated in these insects (Wajnberg et al. 2001).
4.1.3. Termites
Twenty years after the first magnetometry result in honeybee A. mellifera abdomen (Gould et al. 1980), magnetic nanoparticles were isolated from Nasutitermes exitiosus and Amitermes meridionalis iron-deprived termites. These termites were washed in double-distilled water to ensure exclusion of extraneous mineral material. The A. meridionalis magnetic measurement indicated the presence of very small concentrations of magnetic material, with three times more in the thorax plus abdomen than in the head. Magnetization of these termites, in progressively increasing, aligning and randomizing magnetic fields, showed a significant interaction between the ferrimagnetic particles, indicating ultrafine (10 nm) and uni-dimensional grains, probably magnetite minerals. Analysis of magnetically extracted grains from these termites provided firm evidence for biogenic function by them (§A.3.; Maher 1998).
Stimulated by the behavioural studies with P. marginata ants that showed well-organized predatory raids towards nests of its only prey, the termite Neocapritermes opacus was studied by FMR (Alves et al. 2004) and SQUID magnetometer in two orientations (Esquivel et al. 2004). FMR revealed oriented magnetic material with only 3 per cent in the head. Only the HF component was observed in the spectra, for which the diameters were estimated (Alves et al. 2004). Later using a different analysis of the data, a smaller diameter was estimated in termites collected directly from the field relative to those that were submitted to a cellulose diet (table 2; Oliveira et al. 2008). The KEFF values were also sensitive to the diet (table 1).
From the hysteresis curves, the remanent and saturation magnetization ratios were calculated in the range of magnetite pseudo-SD or MD particle values (Esquivel et al. 2004). The magnetization in this termite was found to be about two orders of magnitude higher than in the migratory ant. This surprising result was clarified later in comparative magnetic measurements between termites collected from the field and those fed cellulose for 3 days to eliminate other ingested materials. The amount of magnetic material in the latter group declined over 4 days (Oliveira et al. 2005a,b, 2008), but it was still not clear whether all the ingested material was excluded. Sonication in water for a few minutes and further conservation in 70 per cent ethanol reduce the amount of soil particles on the surface of other insects, such as ants. The most important procedure is to keep the insects alive and away from soil (J. F. de Oliveira, D. M. S. Esquivel & E. Wajnberg 2008, unpublished data).
The arrangement of magnetic material in biological samples and a more precise description of magnetic anisotropy can be studied by the angular dependence of FMR spectra in oriented samples. As the amount of magnetic material in the termite N. opacus is higher than in any other eusocial insects studied, it was the first used to quantify the angular dependence of the FMR spectra and the orientation of the magnetic crystalline system relative to the termite body axis (Alves et al. 2009). The presence of an organized ensemble of particles strongly suggested its function as a magnetic sensor.
Localization, even though rough, and characterization of the magnetic material are important for guiding the TEM experiments in the search of the sensor.
4.2. Transmission electron microscopy results
4.2.1. Bees
Evidence of magnetic particles in bee abdomens by TEM became the subject of a controversy in the search for biomineralized magnetic particles. IGs were described as apparently distributed randomly within the cytoplasm of trophocytes that were not innervated in abdomens of A. mellifera workers, which speaks against the hypothesis of magnetoreception (Kuterbach & Walcott 1986). This study, however, could not rule out the possibility that magnetite was present in these granules as they did not have the characteristics of ferritin or haemosiderin. Hsu & Li (1993, 1994) also found IGs containing calcium and phosphorus in an apparently non-crystalline arrangement in the trophocytes. Magnetite was determined by examining the fine structure of the IGs using HRTEM. This result, however, raised some objections (Kirschvink & Walker 1995; Nesson 1995; Nichol & Locke 1995). The IGs of the fat body of A. mellifera and Scaptotrigona postica queens had instead been proposed as formed by degraded forms of holoferritin and phosphorus and calcium precipitated with the ferritin core contents (Keim et al. 2002). A more recent result obtained with a more precise method (Hsu et al. 2007) reaffirms the presence of SPM magnetite in the purified IGs of the honeybee trophocytes. Magnetic and spectroscopy techniques were used to characterize the IGs and to confirm magnetite. IGs containing phosphorus and calcium have also been found in bumblebees (Walcott 1985) and hornets (Hsu 2004). The presence of a possible vesicle membrane surrounding some of the purified IGs was similar to the lipid bilayer membrane found in magnetotactic bacteria. Further evidence for a magnetoreception function came from changes in IG size in the trophocytes that were observed by confocal microscopy upon applying an additional magnetic field to the cells with a concomitant release of calcium ions (Hsu et al. 2007).
Electron-dense material found in rigid hair in or near the cuticle was presumed to be SD and SPM magnetite (Schiff 1991). Forming rings around the dendrites, 10–20 nm electron-dense granules were found along one side and in the base of the hair. These particles were thought to be SPM magnetite, present in large numbers in the bee. Scattered through the cuticle and underlying tissues, 300 nm hexagonal crystalline structures were also present.
4.2.2. Ants
Other investigations for possible magnetoreception sites in eusocial insects have arisen through the years. Prussian blue stain was applied to identify areas of Fe(III) concentration in the body parts of Solenopsis invicta major, media and minor workers, queens and alates (Slowik & Thorvilson 1996). Iron-containing particles were neither consistently found in heads and thoraces nor in queens and alates. The abdomens of S. invicta workers were suggested to be involved in magnetoreception. Major workers were found to have the largest amount of iron. Slowik & Thorvilson (1996) did not mention how the IGs were distributed in the subcuticular region of the abdomen nor whether IGs could be a metabolic product.
Magnetic particles were extracted from the body parts (head, thorax and abdomen) of P. marginata ants using magnetic precipitation methods (Acosta-Avalos et al. 1999). Isolation consisted of maceration in the presence of 5 per cent sodium hypochlorite (NaOCl) solution, centrifugation, sonication and magnetic concentration with an Sm–Co magnet. Iron-containing particles were identified as magnetite/maghemite through electron spectroscopic images and selected-area diffraction patterns. The diffraction patterns were taken from more than one crystal at the same time, which makes the association with a unique crystalline phase difficult. At the same time, the presence of haematite and goethite would not be a surprise because it is known that the activity of NaOCl, an oxidant, can degrade magnetite and produce other iron oxides depending on the reaction time (Towe 1985). It was suggested that the particles were biomineralized for magnetoreception. The method has been used as a first step in detecting magnetic particles in an organism. The amount of extracted particles from abdomens was higher than in other body segments, which differed from the results obtained by SQUID magnetometry of whole body parts. Differences might have resulted from the accumulation of ingested minerals in the digestive system, but extraction methods can also affect differently the magnet material amount of each body part.
Light microscopy and TEM were used for the first time to identify a possible magnetic sensor in the antennae of P. marginata, a promising sensory organ for magnetoreception (Oliveira et al. 2010). Pure Fe/O (or Fe oxides) particles were detected in three main joints, rich in sensorial structures: third segment–pedicel, pedicel–scape and scape–head joints.
Iron oxides have been found in the cuticular knobs. Johnston's organ consists of groups of sensory cells whose nerve fibres run into the two antennal nerves and whose sensory fibres are attached to the cuticular knobs in the articular membrane between the pedicel and the third segment. Localized in the antennal pedicel, Johnston's organ functions as a mechanosensory organ, perceiving alterations of the flagellum in relation to this antennal segment. Other functions are attributed to this organ, so that a magnetic function might be expected. Moreover, it was reported that Johnston's organ is able to perceive gravity and magnetic fields that work as a stimulus for orientation in M. ruginodis and M. laevinodis ants (Vowles 1954).
In the pedicel–scape joint, a cell-like structure was found surrounded by different types of particles, including iron-containing ones. The whole structure was assumed to be a proprioceptor because of its special location. The unknown function of this structure motivated Oliveira et al. (2010) to test it as a magnetosensor of the geomagnetic field. According to the theoretical model, a small amount of incorporated haematite, for example, would be sensitive enough for the detection of the geomagnetic field. The presence of magnetic particles found within the tissue close to mechanosensitive structures in very specific areas along the antenna parts suggests a possible magnetoreception function (Oliveira et al. 2010). The results suggest that ants probably incorporated the magnetic particles from the soil, in these specific parts of the antennae. In ants, the grain-size distribution is broader than in bacteria and the mineralogical composition varies. These observations argue against a biologically controlled growth process. Although these structures are different from the candidate magnetoreceptors of pigeons, trout or magnetotactic bacteria, they may still be functional. Alternatively, the ant particles may be used in the vestibular sense, as iron minerals are dense and thus may provide a good deal of inertia. Magnetite or other magnetic particles in other parts of the ant body have not yet been checked with TEM.
4.2.3. Termites
The search for a magnetoreceptor system has been extended to two termite species (N. exitiosus and A. meridionalis) fed only on cellulose for at least 3 days to clear their guts of any ingested detrital material. The study was based on magnetic measurements of their body parts and TEM analysis of magnetically extracted grains (Maher 1998). Clusters of ultrafine electron-dense material were found in the extracts, and results from the termite extraction were compared with inorganic magnetite of Rendzina soil profile from the UK instead of particles found in the soil where these insects were collected. On average, 10 nm crystals with cubical or hexagonal morphologies and a narrow range of grain-size distribution were found, distinct from the wide distribution of particle size in the soil. Although morphology and size are not enough to elucidate composition, the mineral phase was proposed to be biogenic.
Despite the temptation to recommend a general procedure in the study of magnetic materials in eusocial insects, systematic studies are still missing owing to the variety of parameters that can affect results. Variation among individuals is likely, and statistical analyses are necessary to characterize the sampled population. Furthermore, age of the individual, composition of the colony and annual foraging dynamics might explain differences in S. interrupta (Abraçado et al. 2009). Comparative studies on a variety of species using the same experimental procedure will minimize differences so that similarities in magnetoreception mechanisms can be elucidated. At the same time, the use of a variety of techniques to detail the magnetoreception mechanisms in a few model organisms such as honeybees and migratory ants should also be considered. The study of magnetic material properties in insects is just beginning and deserves the attention required to systematically compare and contrast among them.
5. Conclusions and future directions
Behavioural experiments with eusocial insects remain scarce. In the three ant and three bee genera that have been tested in the past decade, a diversity of orientation mechanisms have been observed. Atta ants present path integration and compass polarity, but bumblebees do not present path integration. Field experiments have shown sensitivity to both a magnetic storm and inversion of the vertical component in two species of the Meliponinae bees and oriented migrations of P. marginata using the geomagnetic field as a cue. Even greater diversity in how eusocial insects sense and process information from magnetic fields is likely to become evident with further sampling.
The geomagnetic field seems to be an important environmental feature to which at least some organisms have evolved adaptations. It would not be surprising then to observe different behaviours and magnetic materials in the same eusocial species sampled from different magnetic fields. In Solenopsis ants, there is evidence that the total amount of magnetic material is associated with the strength of the geomagnetic field at the colony location. It is also interesting that a migratory and a nomadic ant species had more isolated magnetic particles than aggregated ones, suggesting the relevance of the former to the ants' long-distance moving habits.
Behavioural experiments indicate which eusocial insects are likely to possess magnetic sensors, but detailing the magnetic and physical properties of the magnetic particles is fundamental to pinpointing sensory tissues. More directly than behavioural experiments, magnetic techniques and TEM can determine previously unknown localities of magnetic compounds. Different magnetic oxides were observed ranging from SPM to MD particles. These particles were observed in all body parts (antennae, head, thorax and abdomen) of ants and bees, with different distributions in these parts. Net oriented material was observed in whole bodies and parts of termites, bees and ants, as expected for the magnetic sensor.
The abdomen of A. mellifera bees has been suggested as the magnetosensor organ; whereas in ants and S. quadripunctata bees, consistent observations of the highest amount of magnetic material in the head and antennae have pointed to the antenna, which is a tactile, olfactory and gravity sensor, and may also be a magnetic sensor organ. Head and antennae are free of ingested material, whereas it can be present in the thorax and abdomen. The recently observed magnetic particles within the tissue in very specific areas of part of the P. marginata antennae were suggested as being incorporated from the soil during the growing process, with a possible magnetoreception function. So it seems likely that magnetic particles other than biogenic ones can be used in magnetoreceptors. The high quantity of magnetic material found in the N. opacus makes this species an interesting model for magnetic experiments and the prey–predator relation with the migratory ant P. marginata a system for the study of evolutionary aspects of magnetoreception.
A light-dependent mechanism based on a chemical magnetoreceptor and another based on ferromagnetic nanoparticles has been suggested. Most of the behavioural experiments in eusocial insects were not specifically set up to test this mechanism under the current concepts of polarity and inclination compass or map sense. They have mainly been tested for the polarity compass rather than inclination, probably because the latter was associated with navigation of migratory animals over long distances for which hemisphere information is important. Nevertheless, an inclination compass was recently observed for a beetle, Tenebrio molitor. As far as we know, eusocial insects have only been tested for inclination compass in their flight directions when exiting the nest. As bumblebees are able to orient in darkness, the suggested magnetic orientation would be light independent, whereas those observed in the Apis waggle dance and path-integrated homing of Atta are light sensitive, but not dependent. As social insect orientation relies on multiple cues, and different ferromagnetic sensors were observed in different species, it is reasonable to expect that two magnetic mechanisms can coexist. The observation of a magnetic sensor does not exclude the existence of another one. On the contrary, after one has been identified, the search for the other is likely to continue. Furthermore, insects could provide good test animals for the model of compass orientation with cue integration as recently proposed (Jensen 2009).
Considering the diversity of results in the small number of social insects that have been studied, we recommend flexibility in the search for magnetic sensors and mechanisms in social insects. Any oriented nanosized, magnetic compound, biomineralized or not, located in any body part should be tested theoretically and experimentally for magnetoreception. Little is known about the neural mechanisms that underlie magnetoreception even for vertebrates, for which there are numerous behavioural and neurological studies. Although no neurons that respond to magnetic field stimuli have been found in insects, their nervous systems are simpler than those of vertebrates. Neuroethological experiments, such as electroantennograms, will be a promising addition to our understanding of magnetoreception in eusocial insects. Far more work is needed. Considering the complexity of the magnetoreception in these insects, and in animals in general, and the results up to now, more progress can be achieved by joining forces in multidisciplinary groups of behavioural biologists, neurobiologists, molecular biologists and biophysicists and biochemists.
Acknowledgements
We thank Dr H. Lins de Barros (CBPF-MCT) for fruitful discussions and Dr J. Gaskin (USDA-ARS) and anonymous reviewers for suggestions to the manuscript. We are grateful to R. M. Carvalho and Dr L. M. Barreto of the National Observatory (Vassouras, Brazil) for the 2001 geomagnetic field data.
Appendix A: Characterization techniques
A.1. Superconducting quantum interference device magnetometry
The high-precision SQUID magnetometers make possible very accurate measurements of the magnetic material in insects. The sensitivity of modern magnetometers is ca 10−7 emu (10−10 A m2). Biological samples can reduce the precision of the machines by an order of magnitude, but, even so, the SQUID can detect 10 ng (10−12 kg) of magnetite. A great deal of information about magnetic nanoparticles can be obtained from the hysteresis curve (magnetization M as a function of the applied magnetic field H), the saturation magnetization MS, the remanence Mr and the coercive field HC. In a zero field cooling (ZFC) experiment, the sample is cooled to low temperatures under zero field, followed by a low applied field and the net magnetization is measured while heating the sample. Field-cooled (FC) measurements proceed in a similar manner to ZFC, except that the external field is applied while cooling and heating. It is particularly interesting to identify SPM particles, and the temperature at which the ZFC and the FC curves split indicates the onset of blocking for the largest particles, while the maximum temperature in the ZFC curve is the blocking temperature related to the average particle volume (Goya et al. 2003). ZFC and FC experiments provide information on magnetic interactions (Luo et al. 1991). Direct current magnetization and alternating field demagnetization curves can also determine whether magnetic particles interact (Cisowski 1981). Mr/MS × HC diagrams have become relevant in characterizing the magnetic domain of particles in animal tissues (Tauxe et al. 2002).
A.2. Ferromagnetic resonance
FMR is often referred to the application of the EPR technique to ferro(i)magnetic materials, instead of detecting the resonance of individual electronic spins.
Particle shape and size and anisotropy constants can be related to spectral parameters and their angular and temperature dependence. The resonance field, Hr, the value of field of zero crossing of the derivative absorption curve and its effective g-value (gef = hν/βHr), the peak-to-peak linewidth ΔH can be obtained directly from the spectra. The asymmetry ratio, AW = ΔHLF/ ΔHHF, where ΔHLF and ΔHHF are the field difference between the positions of half-maximum intensities ymax/2 in LF and HF in the absorption curve, requires spectral integration (Weiss et al. 2004).
It was demonstrated that the area under the EPR absorption curve, the double-integrated spectra, S, is linearly dependent upon the number of particulate iron of ferrofluid samples (Wilhelm et al. 2002; Gamarra et al. 2008). For ferrimagnetic particles, the FMR area can be measured with good accuracy, corresponding to about 2 × 109 iron particles per sample (Wilhelm et al. 2002). For samples in which the magnetic material is unknown, FMR can be used to measure the relative amount of magnetic material (Lucano et al. 2006; Abraçado et al. 2008).
FMR spectra in social insects are characterized by at least two broad lines: the HF, around g = 2, and the LF, at g > 4, components. The HF component is associated with isolated nanoparticles and the LF one with aggregates of these particles based on demagnetization field contributions to the effective magnetic field. The temperature dependence of Hr and ΔH was used to estimate the mean magnetic particle diameters and the effective anisotropy energy density, KEFF (Wajnberg et al. 2000; El-Jaick et al. 2001; Abraçado et al. 2005). This allows the observation of temperature transitions that are below the expected value for magnetite. These are obtained from changes in the spectral parameters, although the correlation of the changes with magnetic transitions is not well established, even in synthetic samples for which theoretical models and experimental results have different interpretations (Morais et al. 1987; Raikher & Stepanov 1992; Hagiwara & Nagata 1998; Wang et al. 2004). Despite the complexity and wide range of magnetic signals, which strongly depend on size, shape, mineralogy, composition, magnetic anisotropy and particle interactions, these techniques contribute to the study of magnetic particles in eusocial insects.
A.3. Transmission electron microscopy
In TEM, an electron beam passes through the sample and can be detected by a photographic film or a fluorescent screen. Measurements can be made from both the bright- and dark-field imaging modes. The microstructure, grain size and lattice defects can be studied by the bright-image mode, whereas the crystalline structure can be measured by the diffraction mode. TEM requires ultrathin sections of animal tissue, producing an extremely large number of grids to be analysed: a 1 mm3 sample generates 100 000 thin slices.
To identify a magnetic crystal composition by TEM, several steps should be accomplished. It is interesting initially to observe by light microscopy semithin sections specifically stained for iron (Fe). The failure in observing Fe regions does not mean that the element is not present as the regions can be too small to be resolved. Furthermore, sulphide compounds (e.g. greigite) that also can be magnetic need another procedure to be detected.
Three basic steps would then follow: (i) observation of the particle as electron-dense material and confirmation that it has a crystalline structure when the sample is tilted, (ii) identification of the chemical composition of the sample through EDX spectroscopy analysis or electron energy-loss spectroscopy, and (iii) comparison of the diffraction pattern with the spectroscopic data table. For nanosized particles (approx. 20 nm), decreasing the spot size or the aperture to compatible conditions can help in obtaining the diffraction patterns.
A.4. Care with samples
Minute particles of volcanic origin and those raised from the soil that can be found in the atmosphere frequently present magnetic remanence. These particles can be a source of direct contamination on the insect fur or indirect from the food source (leaves, pollen or other insects). Some eusocial insects are in direct contact with soil (termites and ants) or use it in nest construction (hornets and bees), and may also have exogenous magnetic particles adhered to the surface or ingested particles in the abdomen and thorax. Contamination on the body surface can be reduced by careful washing, but it does not guarantee the removal of all extraneous particles and should be considered for extracted particles and whole and body-part samples. A Fe-depleted diet can reduce the magnetic material of the digestive tract (Oliveira et al. 2005a), but a better procedure would be to remove their digestive tract and compare the results. Magnetic particles observed inside cells or organelles cannot be considered as contamination when observed by microscopy techniques.
Footnotes
One contribution to the Theme Supplement ‘Magnetoreception’.
References
- Abraçado L. G., Esquivel D. M. S., Alves O. C., Wajnberg E. 2005. Magnetic material in head, thorax, and abdomen of Solenopsis substituta ants: a ferromagnetic resonance study. J. Magn. Res. 175, 309–316. ( 10.1016/j.jmr.2005.05.006) [DOI] [PubMed] [Google Scholar]
- Abraçado L. G., Esquivel D. M. S., Wajnberg E. 2008. Oriented magnetic material in head and antennae of Solenopsis interrupta ant. J. Magn. Magn. Mater. 320, e204–e206. ( 10.1016/j.jmmm.2008.02.048) [DOI] [Google Scholar]
- Abraçado L. G., Esquivel D. M. S., Wajnberg E. 2009. Solenopsis interrupta ant magnetic material: statistical and seasonal studies. Phys. Biol. 6, 046 012 ( 10.1088/1478-3975/6/4/046012) [DOI] [PubMed] [Google Scholar]
- Acosta-Avalos D., Wajnberg E., Oliveira P. S., Leal I., Farina M., Esquivel D. M. S. 1999. Isolation of magnetic nanoparticles from Pachycondyla marginata ants. J. Exp. Biol. 202, 2687–2692. [DOI] [PubMed] [Google Scholar]
- Acosta-Avalos D., Esquivel D. M. S., Wajnberg E., Lins de Barros H. G. P., Oliveira P. S., Leal I. 2001. Seasonal patterns in the orientation system of the migratory ant Pachycondyla marginata. Naturwissenschaften 88, 343–346. ( 10.1007/s001140100245) [DOI] [PubMed] [Google Scholar]
- Alves O. C., Wajnberg E., Oliveira J. F. d., Esquivel D. M. S. 2004. Magnetic material arrangement in oriented termites: a magnetic resonance study. J. Magn. Res. 168, 246–251. ( 10.1016/j.jmr.2004.03.010) [DOI] [PubMed] [Google Scholar]
- Alves O. C., Acosta-Avalos D., Wajnberg E., Oliveira J. F., Esquivel D. M. S. 2009. Obtaining orientation of organized magnetic particles in Neocapritermes opacus termite by FMR angular dependence. In Magnetic materials: research, technology and applications (ed. Levine J. I.), pp. 297–312. Hauppauge, NY: Nova Science Publishers. [Google Scholar]
- Arendse M. C. 1978. Magnetic field detection is distinct from light detection in the invertebrates Tenebrio and Talitrus. Nature 274, 358–362. ( 10.1038/274358a0) [DOI] [Google Scholar]
- Banks A. N., Srygley R. B. 2003. Orientation by magnetic field in leaf-cutter ants, Atta colombica (Hymenoptera: Formicidae). Ethology 109, 835–846. ( 10.1046/j.0179-1613.2003.00927.x) [DOI] [Google Scholar]
- Bauerlein E. 2005. Biomineralization: progress in biology, molecular biology and application Weinheim, Germany: Wiley-VCH. [Google Scholar]
- Beason C. 2005. Mechanisms of magnetic orientation in birds. Integr. Comput. Biol. 45, 565–573. ( 10.1093/icb/45.3.565) [DOI] [PubMed] [Google Scholar]
- Billen J. 2006. Signal variety and communication in social insects. Proc. Neth. Entomol. Soc. Meet. 17, 9–25. [Google Scholar]
- Cain S. D., Boles L. C., Wang J. H., Lohmann K. J. 2005. Magnetic orientation and navigation in marine turtles, lobsters and molluscs: concepts and conundrums. Integr. Comput. Biol. 45, 539–546. ( 10.1093/icb/45.3.539) [DOI] [PubMed] [Google Scholar]
- Çamlitepe Y., Aksoy V., Neslihan U., Ayse Y., Becenen I. 2005. An experimental analysis on the magnetic field sensitivity of the black-meadow ant Formica pratensis Retzius (Hymenoptera: Formicidae). Acta. Biol. Hung. 56, 215–224. ( 10.1556/ABiol.56.2005.3-4.5) [DOI] [PubMed] [Google Scholar]
- Chambarelli L. L., Pinho M. A., Abraçado L. G., Esquivel D. M. S., Wajnberg E. 2008. Temporal and preparation effects in the magnetic nanoparticles of Apis mellifera body parts. J. Magn. Magn. Mater. 320, e207–e210. ( 10.1016/j.jmmm.2008.02.049) [DOI] [Google Scholar]
- Chittka L., Williams N. M., Rasmussen H., Thomson J. D. 1999. Navigation without vision: bumblebee orientation in complete darkness. Proc. R. Soc. Lond. B 266, 45–50. ( 10.1098/rspb.1999.0602) [DOI] [Google Scholar]
- Cisowski S. 1981. Interacting vs. non-interacting single domain behavior in natural and synthetic samples. Phys. Earth Planet. Int. 26, 56–62. ( 10.1016/0031-9201(81)90097-2) [DOI] [Google Scholar]
- Collett T. S., Baron J. 1994. Biological compasses and the coordinate frame of landmark memories in honeybees. Nature 368, 137–140. ( 10.1038/368137a0) [DOI] [Google Scholar]
- Davila A. F., Fleissner G., Winklhofer M., Petersen N. 2003. A new model for a magnetoreceptor in homing pigeons based on interacting clusters of superparamagnetic magnetite. Phys. Chem. Earth 28, 647–652. ( 10.1016/S1474-7065(03)00118-9) [DOI] [Google Scholar]
- Davila A. F., Winklhofer M., Shcherbakov V. P., Petersen N. 2005. Magnetic pulse affects a putative magnetoreceptor mechanism. Biophys. J. 89, 56–63. ( 10.1529/biophysj.104.049346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoil M., Gillis P., Gossuin Y., Pankhurst Q. A., Hautot D. 2005. Definitive identification of magnetic nanoparticles in the abdomen of the honeybee Apis mellifera. J. Phys. Conf. Ser. 17, 45–49. ( 10.1088/1742-6596/17/1/007) [DOI] [Google Scholar]
- Edmonds D. T. 1992. A magnetite null detector as the migrating bird's compass. Proc. R. Soc. Lond. B 249, 27–31. ( 10.1098/rspb.1992.0079) [DOI] [Google Scholar]
- Edmonds D. T. 1996. A sensitive optically detected magnetic compass for animals. Proc. R. Soc. Lond. B 263, 295–298. ( 10.1098/rspb.1996.0045) [DOI] [PubMed] [Google Scholar]
- El-Jaick L. J., Acosta-Avalos D., Esquivel D. M. S., Wajnberg E., Linhares M. P. 2001. Electron paramagnetic resonance study of honeybee Apis mellifera abdomens. Eur. Biophys. J. 29, 570–586. [DOI] [PubMed] [Google Scholar]
- Esquivel D. M. S., Acosta-Avalos D., El-Jaick L. J., Cunha A. D. M., Malheiros M. G., Wajnberg E., Linhares M. P. 1999. Evidence for magnetic material in the fire ant Solenopsis sp. by electron paramagnetic resonance measurements. Naturwissenschaften 86, 30–32. ( 10.1007/s001140050564) [DOI] [Google Scholar]
- Esquivel D. M. S., Wajnberg E., Cernicchiaro G. R., Acosta-Avalos D., Garcia B. E. 2002. Magnetic material arrangement in Apis mellifera abdomens. Mat. Res. Soc. Symp. Proc. 724, N7.2.1–N7.2.4. [Google Scholar]
- Esquivel D. M. S., Wajnberg E., Cernicchiaro G. R., Alves O. C. 2004. Comparative magnetic measurements of migratory ant and its only termite prey. J. Magn. Magn. Mater. 278, 117–121. ( 10.1016/j.jmmm.2003.12.327) [DOI] [Google Scholar]
- Esquivel D. M. S., Wajnberg E., Nascimento F. S., Pinho M. B., Lins de Barros H. G. P., Eizemberg R. 2007. Do geomagnetic storms change the behavior of the stingless bee guiruçu (Schwarziana quadripunctata)? Naturwissenchaften 94, 139–142. ( 10.1007/s00114-006-0169-z) [DOI] [PubMed] [Google Scholar]
- Esquivel D. M. S., Acosta-Avalos D., Sabagh L. T., Correia A. A. C., Barbosa M. A., Wajnberg E. 2008. Nest-exiting flight angles of stingless bee Tetragonisca angustula: magnetic field effects. In RIN 08: Orientation and navigation: birds, humans and other animals. London, UK: Royal Institute of Navigation. [Google Scholar]
- Fleissner G., Holtkamp-Rötzler E., Hanzlik M., Winklhofer M., Fleissner G., Petersen N., Wiltschko W. 2003. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J. Comput. Neurol. 458, 350–360. ( 10.1002/cne.10579) [DOI] [PubMed] [Google Scholar]
- Fleissner G., Stahl B., Thalau P., Falkenberg G., Fleissner G. 2007. A novel concept of Fe-mineral-based magnetoreception: histological and physicochemical data from the upper beak of homing pigeon. Naturwissenschaften 94, 631–642. ( 10.1007/s00114-007-0236-0) [DOI] [PubMed] [Google Scholar]
- Frier H. J., Edwards E., Smith C., Neale S., Collet T. S. 1996. Magnetic compass cues and visual pattern learning in honeybees. J. Exp. Biol. 199, 1353–1361. [DOI] [PubMed] [Google Scholar]
- Gamarra L. F., et al. 2008. Kinetics of elimination and distribution in blood and liver of biocompatible ferrofluids based on Fe3O4 nanoparticles: an EPR and XRF study. Mater. Sci. Eng. C 28, 519–525. ( 10.1016/j.msec.2007.06.005) [DOI] [Google Scholar]
- Gegear R. J., Casselman A., Waddell S., Reppert S. M. 2008. Cryptochrome mediate light dependent magnetosensitivity in Drosophila. Nature 454, 367–550. ( 10.1038/nature07183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J. L. 2008. Animal navigation: the evolution of magnetic orientation. Curr. Biol. 18, R482–R484. ( 10.1016/j.cub.2008.03.052) [DOI] [PubMed] [Google Scholar]
- Gould J. L., Kirschvink J. L., Deffeyes K. S., Brines M. L. 1980. Orientation of demagnetized bees. J. Exp. Biol. 86, 1–8. [Google Scholar]
- Goya G. F., Berquo T. S., Fonseca F. C., Morales M. P. 2003. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 94, 3520–3528. ( 10.1063/1.1599959) [DOI] [Google Scholar]
- Hagiwara M., Nagata K. 1998. Magnetic behaviors of complex nature found in an oxide glass system containing deposited magnetite clusters at the superparamagnetic state. J. Magn. Magn. Mater. 177, 91–92. [Google Scholar]
- Hautot D., Pankhurst Q. A., Dobson J. 2005. Superconducting quantum interference device measurements of dilute magnetic materials in biological samples. Rev. Sci. Instrum. 76, 045101-1–045101-4. ( 10.1063/1.1868272) [DOI] [Google Scholar]
- Holldobler B., Wilson E. O. 1990. The ants. Cambridge, MA: The Belknap Press of Harvard University. [Google Scholar]
- Hsu C. Y. 2004. The processes of iron deposition in the common hornet (Vespa affınis). Biol. Cell 96, 529–537. ( 10.1016/j.biolcel.2004.05.001) [DOI] [PubMed] [Google Scholar]
- Hsu C. Y., Li C. W. 1993. The ultrastructure and formation of iron granules in the honeybee (Apis mellifera). J. Exp. Biol. 180, 1–13. [Google Scholar]
- Hsu C. Y., Li C. W. 1994. Magnetoreception in honeybees. Science 265, 95–97. ( 10.1126/science.265.5168.95) [DOI] [PubMed] [Google Scholar]
- Hsu C. Y., Ko F. Y., Li C. W., Fann K., Lue J. T. 2007. Magnetoreception system in honeybees (Apis mellifera). PLoS ONE 2, e395–e406. ( 10.1371/journal.pone.0000395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander R., Jander U. 1998. The light and the magnetic compass of the weaver ant, Oecophylla smaragdina (Hymenoptera: Formicidae). Ethology 104, 743–758. [Google Scholar]
- Jensen K. K. 2009. Light-dependent orientation responses in animals can be explained by a model of compass cue integration. J. Theor. Biol. 262, 129–141 ( 10.1016/j.jtbi.2009.09.005) [DOI] [PubMed] [Google Scholar]
- Johnsen S., Lohmann K. J. 2005. The physics and neurobiology of magnetoreception. Nat. Rev. Neurosci. 6, 703–712. ( 10.1038/nrn1745) [DOI] [PubMed] [Google Scholar]
- Keim C. N., Cruz-Landim C., Carneiro F. G., Farina M. 2002. Ferritin in iron containing granules from the fat body of the honeybees Apis mellifera and Scaptotrigona postica. Micron 33, 53–59. ( 10.1016/S0968-4328(00)00071-8) [DOI] [PubMed] [Google Scholar]
- Kirschvink J. L. 1989. Magnetite biomineralization and geomagnetic sensitivity in higher animals: an update and recommendations for future study. Bioelectromagnetics 10, 239–259. ( 10.1002/bem.2250100304) [DOI] [PubMed] [Google Scholar]
- Kirschvink J. L., Gould J. L. 1981. Biogenic magnetite as a basis for magnetic detection in animals. Biosystems 13, 181–201. ( 10.1016/0303-2647(81)90060-5) [DOI] [PubMed] [Google Scholar]
- Kirschvink J. L., Kobayashi A. 1991. Is geomagnetic sensitivity real? Replication of the Walker–Bitterman conditioning experiment in honey bees. Am. Zool. 31, 169–185. [Google Scholar]
- Kirschvink J. L., Walker M. M. 1995. Honeybees and magnetoreception. Science 269, 1889 ( 10.1126/science.269.5232.1889) [DOI] [PubMed] [Google Scholar]
- Kirschvink J. L., Padmanabha S., Boyce C. K., Oglesby J. 1997. Measurements of the threshold sensitivity of honeybees to weak, extremely low-frequency magnetic fields. J. Exp. Biol. 200, 1363–1368. [DOI] [PubMed] [Google Scholar]
- Krebs A. T., Benson B. S. 1965. Electron spin resonance in Formicidae. Nature 207, 1412–1412. ( 10.1038/2071412a0) [DOI] [PubMed] [Google Scholar]
- Kuterbach D. A., Walcott B. 1986. Iron-containing cells in the honey bee (Apis mellifera). J. Exp. Biol. 126, 375–387. [DOI] [PubMed] [Google Scholar]
- Leal I. R., Oliveira P. S. 1995. Behavioral ecology of the neotropical termite-hunting ant Pachycondyla (Termitopone) marginata: colony founding, group-raiding and migratory patterns. Behav. Ecol. Sociobiol. 37, 373–383. ( 10.1007/BF00170584) [DOI] [Google Scholar]
- Leucht T. 1984. Responses to light under varying magnetic conditions in the honeybee, Apis mellifica. J. Comput. Physiol. A 154, 865–870. ( 10.1007/BF00610687) [DOI] [Google Scholar]
- Leucht T., Martin H. 1990. Interactions between e-vector orientation and weak, steady magnetic fields in the honeybee, Apis mellifica. Naturwissenchaften 77, 130–133. ( 10.1007/BF01134475) [DOI] [Google Scholar]
- Lindauer M., Martin H. 1972. Magnetic effects on dancing bees. In Animal orientation and navigation (eds Galler S. R., Schmidt-Koenig K., Jacobs G. J., Belleville R. E.). Washington, DC: US Government Printing Office. [Google Scholar]
- Lohmann K. J., Lohmann C. M. F., Endres C. S. 2008. The sensory ecology of ocean navigation. J. Exp. Biol. 211, 1719–1728. ( 10.1242/jeb.015792) [DOI] [PubMed] [Google Scholar]
- Lucano M. J., Cernhicchiaro G., Wajnberg E., Esquivel D. M. S. 2006. Stingless bee antennae: a magnetic sensory organ? Biometals 19, 295–300. ( 10.1007/s10534-005-0520-4) [DOI] [PubMed] [Google Scholar]
- Luo W., Nagel S. R., Rosenbaum T. F., Rosensweig R. E. 1991. Dipole interactions with random anisotropy in a frozen ferrofluid. Phys. Rev. Lett. 67, 2721–2724. ( 10.1103/PhysRevLett.67.2721) [DOI] [PubMed] [Google Scholar]
- Maher B. A. 1998. Magnetite biomineralization in termites. Proc. R. Soc. Lond. B 265, 733–737. ( 10.1098/rspb.1998.0354) [DOI] [Google Scholar]
- Morais P. C., Lara M. C. F., Skeff Neto K. 1987. Electron spin resonance in superparamagnetic particles dispersed in a non-magnetic matrix. Phil. Magn. Lett. 55, 181–183. ( 10.1080/09500838708207553) [DOI] [Google Scholar]
- Mouritsen H., Frost B. J. 2002. Virtual migration in tethered flying Monarch butterflies reveals their orientation mechanisms. Proc. Natl Acad. Sci. USA 99, 10 162–10 166. ( 10.1073/pnas.152137299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen H., Ritz T. 2005. Magnetoreception and its use in bird navigation. Curr. Opin. Neurobiol. 15, 406–414. ( 10.1016/j.conb.2005.06.003) [DOI] [PubMed] [Google Scholar]
- Muxworthy A. R., Williams W. 2006. Critical single-domain/multidomain grain sizes in noninteracting and interacting elongated magnetite particles: implications for magnetosomes. J. Geophys. Res. 111, B12S12 ( 10.1029/2006JB004588) [DOI] [Google Scholar]
- Muxworthy A. R., Williams W. 2009. Critical superparamagnetic/single-domain grain sizes in interacting magnetite particles: implications for magnetosome crystals. J. R. Soc. Interface 6, 1207–1212. ( 10.1098/rsif.2008.0462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Němec P., Burda H., Oelschläger H. H. A. 2005. Towards the neural basis of magnetoreception: a neuroanatomical approach. Naturwissenschaften 92, 151–157. ( 10.1007/s00114-005-0612-6) [DOI] [PubMed] [Google Scholar]
- Nesson M. H. 1995. Honeybees and magnetoreception. Science 269, 1889–1890. ( 10.1126/science.269.5232.1889-a) [DOI] [PubMed] [Google Scholar]
- Nichol H., Locke M. 1995. Honeybees and magnetoreception. Science 269, 1888–1889. ( 10.1126/science.269.5232.1888) [DOI] [PubMed] [Google Scholar]
- Oliveira J. F., Wajnberg E., Esquivel D. M. S., Alves O. C. 2005a. Magnetic resonance as a technique to magnetic biosensors characterization in Neocapritermes opacus termites. J. Magn. Magn. Mater. 294, e171–e174. ( 10.1016/j.jmmm.2005.03.078) [DOI] [Google Scholar]
- Oliveira J. F., Cernicchiaro G., Winklhofer M., Dutra H., Oliveira P. S., Esquivel D. M. S., Wajnberg E. 2005b. Comparative magnetic measurements on social insects. J. Magn. Magn. Mater. 289, 442–444. ( 10.1016/j.jmmm.2004.11.124) [DOI] [Google Scholar]
- Oliveira J. F., Alves O. C., Esquivel D. M. S., Wajnberg E. 2008. Ingested and biomineralized magnetic material in the prey Neocapritermes opacus termite: FMR characterization. J. Magn. Res. 191, 112–119. ( 10.1016/j.jmr.2007.12.006) [DOI] [PubMed] [Google Scholar]
- Oliveira J. F., Wajnberg E., Esquivel D. M. S., Weinkauf S., Winklhofer S., Hanzlik M. 2010. Ant antennae: are they sites for magnetoreception? J. R. Soc. Interface 7, 143–152. ( 10.1098/rsif.2009.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti D., Pettigrew J. D. 1980. Ferromagnetic coupling to muscle receptors as a basis for geomagnetic field sensitivity in animals. Nature 285, 99–101. ( 10.1038/285099a0) [DOI] [PubMed] [Google Scholar]
- Raikher Y. L., Stepanov V. 1992. Thermal fluctuation effect on the ferromagnetic resonance line shape in disperse ferromagnets. Zh. Eksp. Teor. Fiz. 102, 1409–1423. [Google Scholar]
- Ritz T., Dommer D. H., Phillips J. B. 2002. Shedding light on vertebrate magnetoreception. Neuron 34, 503–506. ( 10.1016/S0896-6273(02)00707-9) [DOI] [PubMed] [Google Scholar]
- Riveros A. J., Srygley R. B. 2008. Do leafcutter ants, Atta colombica, orient their path-integrated home vector with a magnetic compass? Anim. Behav. 75, 1273–1281. ( 10.1016/j.anbehav.2007.09.030) [DOI] [Google Scholar]
- Sakaki Y., Motomiya T., Kato M., Ogura M. 1990. Possible mechanism of biomagnetic sense organ extracted from sockeye salmon. IEEE Trans. Magn. 26, 1554–1556. ( 10.1109/20.104443) [DOI] [Google Scholar]
- Scherbakov V. P., Winklhofer M. 1999. The osmotic magnetometer: a new model for magnetite-based magnetoreceptors in animals. Eur. Biophys. J. 28, 380–392. ( 10.1007/S002490050222) [DOI] [Google Scholar]
- Schiff H. 1991. Modulation of spike frequencies by varying the ambient magnetic field and magnetite candidates in bees (Apis mellifera). Comput. Biochem. Physiol. A 100, 975–985. ( 10.1016/0300-9629(91)90325-7) [DOI] [PubMed] [Google Scholar]
- Schiff H., Canal G. 1993. The magnetic and electric-fields induced by superparamagnetic magnetite in honeybees—magnetoreception—an associative learning. Biol. Cybern. 69, 7–17. [Google Scholar]
- Schmitt D. E., Esch H. E. 1993. Magnetic orientation of honeybees in the laboratory. Naturwissenchaften 80, 41–43. ( 10.1007/BF01139759) [DOI] [Google Scholar]
- Shcherbakov V. P., Winklhofer M. 1999. The osmotic magnetometer: a new model for magnetite-based magnetoreceptors in animals. Eur. Biophys. J. 28, 380–392. ( 10.1007/s002490050222) [DOI] [Google Scholar]
- Slowik T. J., Thorvilson G. 1996. Localization of subcuticular iron-containing tissue in the red imported fire ant. Southwestern Entomol. 21, 247–253. [Google Scholar]
- Srygley R. B., Dudley R., Oliveira E. G., Riveros A. J. 2006. Experimental evidence for a magnetic sense in Neotropical migrating butterflies (Lepidoptera: Pieridae). Anim. Behav. 71, 183–191. ( 10.1016/j.anbehav.2005.04.013) [DOI] [Google Scholar]
- Takagi S. 1995. Paramagnetism of honeybees. J. Physical Soc. Japan 64, 4378–4381. [Google Scholar]
- Tauxe L., Bertram H. N., Seberino C. 2002. Physical interpretation of hysteresis loops: micromagnetic modeling of fine particle magnetite. Geochem. Geophys. Geosyst 3, 1055 ( 10.1029/2001GC000241) [DOI] [Google Scholar]
- Towe K. M. 1985. Studying mineral particulates of biogenic origin by transmission electron microscopy and electron diffraction: some guidelines and suggestions. In Magnetite biomineralization and magnetoreception in organisms. A new biomagnetism (eds Kirschvink J. L., Jones D. S., Macfadden B. J.). New York, NY: Plenum Press. [Google Scholar]
- Vácha M. 1997. Magnetic orientation in insects. Biologia 52, 629–636. [Google Scholar]
- Vácha M., Soukopová H. 2004. Magnetic orientation in the mealworm beetle Tenebrio and the effect of light. J. Exp. Biol. 207, 1241–1248. ( 10.1242/jeb.00874) [DOI] [PubMed] [Google Scholar]
- Vácha M., Drstkova D., Puzova T. 2008. Tenebrio beetles use magnetic inclination compass. Naturwissenschaften 95, 761–765. ( 10.1007/s00114-008-0377-9) [DOI] [PubMed] [Google Scholar]
- Vowles D. M. 1954. The orientation of ants. II. Orientation to light, gravity and polarized light. J. Exp. Biol. 31, 356–375. [Google Scholar]
- Wajnberg E., Acosta-Avalos D., El-Jaick L. J., Abraçado L., Coelho J. L. A., Bazukis A. F., Morais P. C., Esquivel D. M. S. 2000. Electron paramagnetic resonance study of the migratory ant Pachycondyla marginata abdomens. Biophys. J. 78, 1018–1023. ( 10.1016/S0006-3495(00)76660-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajnberg E., Cernicchiaro G., Acosta-Avalos D., El-Jaick L. J., Esquivel D. M. S. 2001. Induced remanent magnetization of social insects. J. Magn. Magn. Mater. 226, 2040–2041. [Google Scholar]
- Wajnberg E., Cernicchiaro G. R., Esquivel D. M. S. 2004. Antennae: the strongest magnetic part of the migratory ant. Biometals 17, 467–470. ( 10.1023/B:BIOM.0000029443.93732.62) [DOI] [PubMed] [Google Scholar]
- Wajnberg E., Alves O. C., Harada A. Y., Esquivel D. M. S. 2005. Brazilian ants diversity and the local geomagnetic field: a ferromagnetic resonance study. Biometals 18, 595–602. ( 10.1007/s10534-005-2995-4) [DOI] [PubMed] [Google Scholar]
- Walcott B. 1985. The cellular localization of particulate iron. In Magnetite biomineralization and magnetoreception in organisms: a new biomagnetism (eds Kirschvink J. L., Jones D. S., MacFadden B. J.). New York, NY: Plenum Press. [Google Scholar]
- Walker M. M. 1997. Magnetic orientation and the magnetic sense in Arthropods. In Orientation and communication in arthropods (ed. Lehrer M.), pp. 187–214. Basel, Switzerland: Birkhauser Verlag. [Google Scholar]
- Walker M. M. 2008. A model for encoding of magnetic field intensity by magnetite-based magnetoreceptor cells. J. Theor. Biol. 250, 85–91. ( 10.1016/j.jtbi.2007.09.030) [DOI] [PubMed] [Google Scholar]
- Walker M. M., Bitterman M. E. 1985. Conditioned responding to magnetic-field by honeybees. J. Comput. Physiol. 157, 67–71. ( 10.1007/BF00611096) [DOI] [Google Scholar]
- Walker M. M., Bitterman M. E. 1989a. Attached magnetics impair magnetic field discrimination by honeybees. J. Exp. Biol 141, 447–451. [Google Scholar]
- Walker M. M., Bitterman M. E. 1989b. Honeybees can be trained to respond to very small changes in geomagnetic field intensity. J. Exp. Biol. 145, 489–494. [Google Scholar]
- Walker M. M., Dennis T. E., Kirschvink J. L. 2002. The magnetic sense and its use in long-distance navigation by animals. Curr. Opin. Neurobiol. 12, 735–744. ( 10.1016/S0959-4388(02)00389-6) [DOI] [PubMed] [Google Scholar]
- Wang J., Chen Q. W., Li X. G., Shi L., Peng Z. M., Zeng C. 2004. Disappearing of the Verwey transition in magnetite nanoparticles synthesized under a magnetic field: implications for the origin of charge ordering. Chem. Phys. Lett 390, 55–58. ( 10.1016/j.cplett.2004.04.005) [DOI] [Google Scholar]
- Weiss B. P., Kim S. S., Kirschvink J. L., Kopp R. E., Sankaran M., Kobayashi A., Komeili A. 2004. Ferromagnetic resonance and low-temperature magnetic tests for biogenic magnetite. Earth Planet. Sci. Lett. 224, 73–89. ( 10.1016/j.espl.2004.04.024) [DOI] [Google Scholar]
- Wilhelm C., Gazeau F., Bacri J. C. 2002. Magnetophoresis and ferromagnetic resonance of magnetically labeled cells. Eur. Biophys. J. 31, 118–125. ( 10.1007/s00249-001-0200-4) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. J. 2005. Magnetic orientation and magnetoreception in birds and other animals. J. Comput. Physiol. A 191, 675–693. ( 10.1007/s00359-005-0627-7) [DOI] [PubMed] [Google Scholar]
- Winklhofer M. 1998. Modelle hypothetischer Magnetfeldrezeptoren auf der Grundlage biogenen Magnetits. Munich, Germany: Ludwig-Maximilians-Universität. [Google Scholar]
- Yorke E. D. 1979. A possible magnetic transducer in birds. J. Theor. Biol. 77, 101–105. ( 10.1016/0022-5193(79)90140-1) [DOI] [PubMed] [Google Scholar]