Abstract
This paper reviews the directional orientation of birds with the help of the geomagnetic field under various light conditions. Two fundamentally different types of response can be distinguished. (i) Compass orientation controlled by the inclination compass that allows birds to locate courses of different origin. This is restricted to a narrow functional window around the total intensity of the local geomagnetic field and requires light from the short-wavelength part of the spectrum. The compass is based on radical-pair processes in the right eye; magnetite-based receptors in the beak are not involved. Compass orientation is observed under ‘white’ and low-level monochromatic light from ultraviolet (UV) to about 565 nm green light. (ii) ‘Fixed direction’ responses occur under artificial light conditions such as more intense monochromatic light, when 590 nm yellow light is added to short-wavelength light, and in total darkness. The manifestation of these responses depends on the ambient light regime and is ‘fixed’ in the sense of not showing the normal change between spring and autumn; their biological significance is unclear. In contrast to compass orientation, fixed-direction responses are polar magnetic responses and occur within a wide range of magnetic intensities. They are disrupted by local anaesthesia of the upper beak, which indicates that the respective magnetic information is mediated by iron-based receptors located there. The influence of light conditions on the two types of response suggests complex interactions between magnetoreceptors in the right eye, those in the upper beak and the visual system.
Keywords: magnetoreception, compass orientation, fixed-direction responses, radical-pair processes, magnetite-based receptors, monochromatic light
1. Introduction
The existence of a magnetic compass in birds was first demonstrated in European Robins, Erithacus rubecula, with the help of migratory orientation: during the migration season, these birds prefer their migratory direction even in cages, and they responded to a shift in magnetic North with a corresponding change in their headings (Wiltschko, W. 1968). Subsequently, a magnetic compass was also described for several other species of passerine migrants and a shorebird (for a summary, see Wiltschko, W. & Wiltschko, R. 2007). This was also demonstrated in homing pigeons, Columba livia f. domestica (Keeton 1971; Walcott & Green 1974) released under overcast skies, and, recently, by conditioning experiments, in two other species of non-migrants, domestic chickens, Gallus gallus (Freire et al. 2005), and zebra finches, Taeniopygia guttata (Voss et al. 2007).
Experiments based on migratory orientation with robins and conditioning experiments with chickens allowed analysis of the functional properties of this compass mechanism and revealed two surprising characteristics (for a summary, see Wiltschko, W. & Wiltschko, R. 2007; Wiltschko, W. et al. 2007).
The avian magnetic compass is an ‘inclination compass’, which does not rely on the polarity of the magnetic field, but rather on the axial course of the field lines and their inclination, thus distinguishing between ‘poleward’, where the field lines point downward, and ‘equatorward’, where they point upward.
The compass operates spontaneously only within a narrow functional window around the total intensity of the local geomagnetic field; decreasing or increasing the magnetic intensity by about 25–30% results in disorientation.
The processes enabling birds to detect the direction of the magnetic field have long remained enigmatic. Only in recent decades have a number of mechanisms been proposed, with two of these hypotheses being supported by experimental evidence in birds. The first model suggests magnetoreception based on magnetite, a specific form of Fe3O4. Several competing models on the functional mode of magnetite-based receptors have been suggested, some based on magnetic single domains, others on smaller superparamagnetic particles and even others on a combination of both (e.g. Yorke 1979; Kirschvink & Gould 1981; Kirschvink & Walker 1985; Edmonds 1992; Shcherbakov & Winklhofer 1999; Davila et al. 2003; Solov'yov & Greiner 2007, 2009; Walker 2008). Both types of magnetite particles have been described in birds, with single domains suggested to be present in the ethmoid region and the nasal cavity (e.g. Beason & Nichols 1984; Williams & Wild 2001) and superparamagnetic particles reported in distinct structures in the skin of the upper beak (Hanzlik et al. 2000; Winklhofer et al. 2001; Fleissner et al. 2003, 2007; Tian et al. 2007). Behavioural responses of birds to a strong, brief magnetic pulse, designed to alter the magnetization of magnetite, support the involvement of magnetite-based receptors in magnetoreception (e.g. Wiltschko, W. et al. 1994, 2009; Beason et al. 1995, 1997).
The other hypothesis, the ‘radical-pair’ model, first forwarded by Schulten (1982) and later detailed by Ritz et al. (2000), suggests that magnetoreception in birds is based on spin-chemical processes in specialized photopigments. Light-induced photon absorption leads to the formation of a pair of radicals. These radical pairs may be in the singlet or in the triplet state, with the portion of each state and its products depending, among other circumstances, on the alignment of the molecule in the external magnetic field. Such radical pairs could therefore be used to detect magnetic directions. For this mechanism to be viable, birds must be able to compare the amount of singlets or triplets in various spatial alignments. Considering the hemispherical shape of the eyes and their ability to absorb light, the authors suggested that the magnetosensitive processes take place in the eyes, forming centrally symmetric patterns on the retina (Ritz et al. 2000). One prediction of this model is that oscillating magnetic fields in the megahertz (MHz) range would interfere with the singlet–triplet interconversion and thus should disrupt magnetic compass orientation. Data from migratory robins and directionally trained chickens as well as zebra finches show that this is the case (Ritz et al. 2004, 2009; Thalau et al. 2005; Wiltschko, W. et al. 2007; Keary et al. in press), which indicates that the avian magnetic compass is indeed based on a radical-pair mechanism. The prediction that magnetoreception takes place in the eyes is also experimentally supported, revealing a strong lateralization in favour of the right eye (Wiltschko, W. et al. 2002; Roger et al. 2008).
The radical-pair model proposes photon absorption as the first step of magnetoreception; therefore, the response of birds under lights of different wavelengths became of interest. In the present paper, we review the orientation responses of passerine migrants under light of different wavelengths and intensities.
2. Orientation behaviour under various light regimes
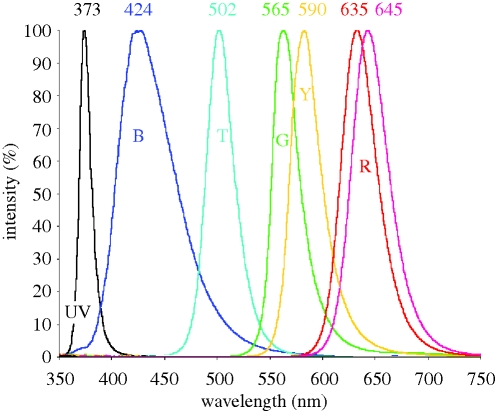
Tests have been performed in monochromatic light produced by light-emitting diodes (LEDs) with a half bandwidth of mostly 30–40 nm (figure 1). A wavelength dependency became evident: magnetic compass orientation requires light from the short-wavelength part of the spectrum. Robins were oriented in their migratory direction under light from 370 nm ultraviolet (UV) up to 565 nm green; in longer wavelength light, they were disoriented (figure 2; Wiltschko, W. & Wiltschko, R. 1999; Muheim et al. 2002; Wiltschko, R. et al. 2007a). The same wavelength dependency was found in Australian silvereyes, Zosterops l. lateralis, and Garden Warblers, Sylvia borin (Wiltschko, W. et al. 1993; Rappl et al. 2000). It is also indicated in homing pigeons and domestic chickens (Wiltschko, R. & Wiltschko, W. 1998; Wiltschko, W. et al. 2007), where green or blue light, respectively, allow orientation, but red light leads to disorientation. These findings suggest that this wavelength dependency may be a general feature of the avian magnetic compass.
Figure 1.
Spectra of the LEDs used in the experiments reported here (figures 2–7). The peak wavelengths are given; the letters indicate the abbreviations used in the figures. Note that the colours are only symbolic.
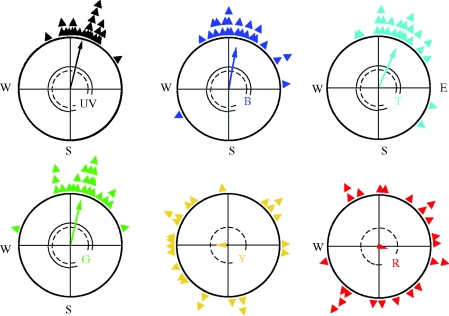
Figure 2.
Orientation of European robins during spring migration under monochromatic light of different wavelengths. The light intensity was 7–8 × 1015 quanta s−1 m−2, except for UV light, which was only 0.8 × 1015 quanta s−1 m−2. The triangles at the periphery of the circle give mean headings of individual birds based on three recordings each; the arrows represent the grand mean vectors with the length proportional to the radius of the circle. The two inner circles mark the 5% (dotted) and the 1% significance border of the Rayleigh test (Batschelet 1981); arrows exceeding these circles indicate significant orientation.
Robins were found to be oriented under red light if they had been exposed to red light for an hour prior to being tested (Wiltschko, W. et al. 2004a). When their behaviour was analysed in detail, it proved not to be normal compass orientation, however.
2.1. Compass orientation under low monochromatic light of short wavelengths
For tests in the various wavelengths described above, the light intensity was equivalent to a quantal flux of 6–9 × 1015 quanta s−1 m−2, except for UV light, where it was only one-tenth of this, namely 0.8 × 1015 quanta s−1 m−2 (Wiltschko, R. et al. 2007a). A quantal flux of 8 × 1015 quanta s−1 m−2 corresponds to the light level of a largely clear sky about 45 min before sunrise or after sunset, or, if only the blue or the green part of the spectrum is considered, to the light level about 38 or 28 min, respectively, before sunrise or after sunset. The light level used for the tests under UV light also corresponds to the respective UV-share of the spectrum about 38 min after sunset.
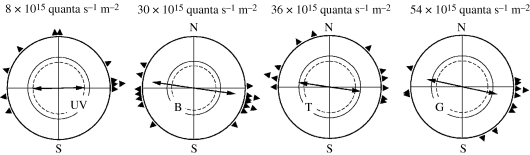
The analysis of the orientation behaviour under short-wavelength monochromatic light at the low intensities described above revealed normal orientation in migratory direction with the help of the inclination compass. Under 370 nm UV, 424 nm blue, 502 turquoise and 565 nm green light, robins preferred the seasonally appropriate southerly directions in autumn and northerly directions in spring (figure 3). When the vertical component of the magnetic field was inverted, they reversed their headings, indicating use of the normal inclination compass (figure 3; Wiltschko, W. et al. 2001; Stapput et al. 2005). Data obtained under green light indicate the existence of a functional window in robins as well as in chickens (Wiltschko, W. et al. 2006a, 2007; Wiltschko, R. et al. 2007a,b).
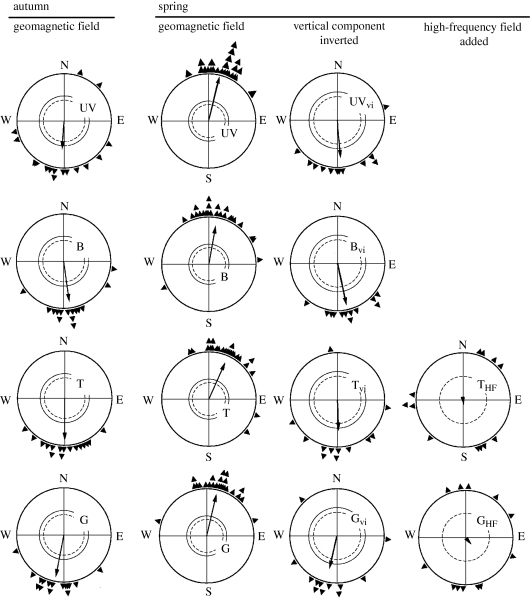
Figure 3.
Orientation by the inclination compass under low-intensity short-wavelength monochromatic light: UV, 373 nm UV; B, 424 nm blue; T, 502 nm turquoise; and G, 565 nm green (figure 1). The light intensities are the same as in figure 2: UV, 0.8 quanta s−1 m−2; blue, turquoise and green, 8 quanta s−1 m−2. In autumn and spring in the local geomagnetic field, the robins prefer their seasonally appropriate southern and northern migratory direction. Inversion of the vertical component of the magnetic field (vi) causes birds to reverse their headings. Treatment with a broadband high-frequency (HF) field including frequencies from 0.1 to 10 MHz at an intensity of 85 nT causes disorientation. Symbols are as in figure 2 (data from Stapput et al. 2005; Thalau et al. 2005; Wiltschko, R. et al. 2005).
In summary, under low-intensity monochromatic lights from UV up to 565 nm green, birds seem to respond as in earlier experiments under ‘white’ light. This, in turn, means that their inclination compass was working normally under the respective light conditions.
Further analyses have addressed the mechanisms of magnetoreception. Orientation broke down when birds were exposed to oscillating magnetic fields in the MHz range, indicating radical-pair processes as the underlying receptive mechanism (figure 3, right). The respective tests were performed under green and turquoise light (Ritz et al. 2004; Thalau et al. 2005; Wiltschko, R. et al. 2005); it can be assumed, however, that tests under UV and blue light would produce the same results, because the responses under these conditions have the same general characteristics. Temporarily deactivating the magnetite-based receptors in the upper beak with a local anaesthetic, in contrast, did not have any effect under green light: European robins as well as Australian silvereyes continued to head into their migratory direction as before (Wiltschko, R. et al. 2007a, 2008; Stapput et al. 2008; see figure 7 below). The same was true for compass orientation in chickens (Wiltschko, W. et al. 2007). Together, these results clearly show that the directional information for the inclination compass originates in the radical-pair processes in the right eye, whereas magnetite-based receptors in the upper beak are not involved.
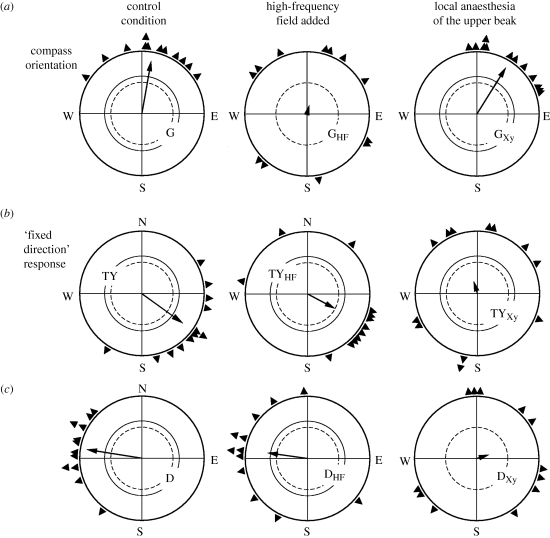
Figure 7.
Effect of high-frequency (HF) fields and of local anaesthesia of the upper beak with the anaesthetic xylocaine in robins on compass orientation under low-intensity green light (a) and the fixed-direction responses observed under bichromatic light combining turquoise and yellow light (b) and in total darkness (c). The HF field disrupts compass orientation and leaves the fixed-direction responses unaffected, whereas anaesthesia of the upper beak (Xy) leaves compass orientation unaffected and disrupts the fixed-direction responses. Symbols are as in figure 2 (data from Thalau et al. 2005; Wiltschko, R. et al. 2007b, 2008; Stapput et al. 2008).
2.2. Different responses under monochromatic lights of higher intensity
When the intensity of monochromatic light is increased, birds show different types of behaviour. This was first observed in Australian silvereyes tested under 565 nm green light of an intensity of about 50 × 1015 quanta s−1 m−2. The birds no longer preferred their migratory direction, but showed northwesterly headings in spring as well as in autumn, i.e. the response under bright green light was ‘fixed’ in the sense that it did not undergo the seasonal change observed in migratory orientation (Wiltschko, W. et al. 2000). This type of response is referred to as a ‘fixed-direction’ response. In silvereyes, another response that looks like a fixed direction was observed under UV lights of about 8 × 1015 quanta s−1 m−2: in southern spring, the birds headed east–northeast instead of south. Data from southern autumn are not available, but this response under UV light shares other characteristics with the fixed-direction responses (see below).
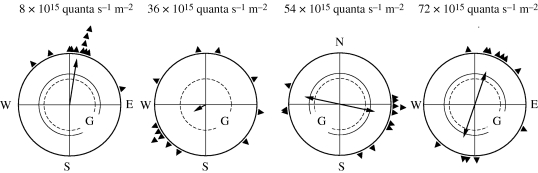
Robins also cease to prefer their migratory direction when the intensity of monochromatic light is increased. They show a variety of responses that seem to follow a certain pattern. Responses under 565 nm green light at different intensities are illustrated in figure 4. Under a low intensity of 8 × 1015 quanta s−1 m−2, robins oriented in their migratory direction; when the intensity was increased, they became disoriented at 36 × 1015 quanta s−1 m−2, then preferred the two ends of an axis that roughly coincided with east–west at 54 × 1015 quanta s−1 m−2 and finally, at 72 × 1015 quanta s−1 m−2, they preferred an axis close to north–south. At other wavelengths, similar axial responses were observed: under 424 nm blue, the robins preferred the north–south axis at the higher light intensities. Under 502 nm turquoise, however, they first preferred the east–west axis, but, at the two higher light intensities, they headed unimodally north (Wiltschko, R. et al. 2007b). This orientation superficially looked like normal compass orientation in spring, but proved to be a fixed-direction response: under bright turquoise light, the robins headed north in spring as well as in autumn (Wiltschko, R. et al. 2005).
Figure 4.
Orientation of robins in spring under 565 nm green light of increasing intensity. At intensities of 36 × 1015 quanta s−1 m−2 and beyond, the birds no longer prefer their northerly migratory direction, but show a pattern of different responses, including axial preferences. The respective quantal flux is indicated above the diagrams. Symbols are as in figure 2 (data from Wiltschko, R. et al. 2007a).
The axial orientation along the east–west axis (figure 4) that seems to be a transient state is observed at different light levels which increase from UV to green light (figure 5). It would have been too great an effort and too time-consuming to test the birds at even more different intensities; hence the critical intensity where orientation in migratory direction is replaced by disorientation or axial responses could not be narrowed down more closely. It is striking, however, that robins under UV, blue and turquoise light orient along the east–west axis at light levels where they, under green, still preferred their migratory direction or were disoriented (Wiltschko, R. et al. 2007b).
Figure 5.
The transient state of axial orientation along the east–west axis in robins is observed at different intensities that increase with increasing wavelengths: UV, 373 nm UV; B, 424 nm blue; T, 502 nm turquoise; and G, 565 nm green. The respective quantal flux is indicated above the diagrams. Symbols are as in figure 2 (data from Wiltschko, R. et al. 2007a).
These tests under higher intensity monochromatic light revealed differences in the responses between European robins and Australian silvereyes. In UV light of 8 × 1015 quanta s−1 m−2 and in green light of 36 × 1015 quanta s−1 m−2, robins oriented axially along the east–west axis, whereas silvereyes showed fixed-direction responses in the same light conditions (see above and below). Whether these are principal differences is unknown. It seems more likely, however, that they represent different phases in the pattern of responses to monochromatic light with increasing light intensities, possibly caused by the different migratory habits of the two species: silvereyes are twilight migrants that migrate at dawn and dusk, in contrast to robins that migrate at night.
It should be emphasized that the highest intensity test lights used in this part of the study were still not very bright; they corresponded to the respective spectral bands found in nature roughly a quarter of an hour before sunrise and after sunset.
2.3. Fixed-direction responses under bichromatic lights and in total darkness
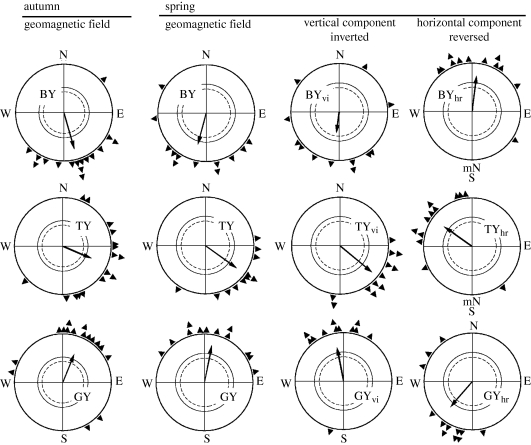
Robins were also tested under a combination of 590 nm yellow light, a wavelength that alone did not allow orientation (figure 2), and blue, turquoise and green light. Here, both components had an equal quantal flux of about 7 × 1015 quanta s−1 m−2, so that the combined bichromatic light totalled a quantal flux of 14–15 × 1015 quanta s−1 m−2. The respective directional preferences are given in figure 6: the birds showed fixed-direction responses that did not change between spring and autumn. The specific fixed directions varied with the wavelength of short-wavelength light; however, northerly headings were observed under green-and-yellow, easterly ones under turquoise-and-yellow and southerly ones under blue-and-yellow (Wiltschko, R. et al. 2004b; Stapput et al. 2005).
Figure 6.
Orientation of robins when 590 nm yellow light is added to 424 nm blue, 502 nm turquoise and 565 nm green light with a quantal flux of about 7 × 1015 quanta s−1 m−2 each, resulting in fixed-direction responses that are different for the different combinations of colours. These responses are not affected by an inversion of the vertical component (vi) of the geomagnetic field, but shift accordingly when the horizontal component is reversed (hr), indicating that they are polar responses to the magnetic field. Symbols are as in figure 2 (data in part from Wiltschko, W. et al. 2004b; Stapput et al. 2005).
Another westerly fixed-direction response was observed under dim red light with a wavelength of 645 nm and a quantal flux of about 3.5 × 1015 quanta s−1 m−2 in robins as well as in silvereyes (Wiltschko, R. et al. 2008). A westerly tendency under dim red light was first described in robins by Muheim et al. (2002), but, because only autumn data were available, it was not recognized as a fixed-direction response. Experiments in total darkness also produced westerly fixed directions that were very similar to those observed under dim red light (Stapput et al. 2008), so that it seems likely that the dim red light in the test cage was so low that it meant ‘darkness’ for the birds and that the two responses observed under dim red light and in darkness are identical.
Further analysis of the fixed-direction responses under bichromatic light showed that they, in contrast to migratory orientation, are polar responses to the magnetic field: they were unaffected by the reversal of the vertical component, but changed accordingly when magnetic north was reversed (figure 6). This also proved true for the fixed-direction responses in total darkness, under dim red and higher intensity turquoise light in robins (Wiltschko, R. et al. 2005, 2008; Stapput et al. 2008) and under bright green light and UV light in silvereyes (Wiltschko, W. et al. 2003). The fixed-direction responses are thus not controlled by the inclination compass. Recent tests under turquoise-and-yellow light and in total darkness revealed another difference to compass orientation: fixed-direction responses are not restricted to a narrow functional window, but also occur in magnetic fields with intensities twice or three times that of the geomagnetic field (Wiltschko, W. et al. 2010).
An analysis of the underlying mechanisms indicated that fixed-direction responses are not affected by oscillating fields in the MHz range; instead, they are disrupted by local anaesthesia of the skin of the upper beak (figure 7). This clearly demonstrates that the respective magnetic information is mediated by the iron-based receptors located there (Wiltschko, R. et al. 2007b, 2008; Stapput et al. 2008).
2.4. Two different types of responses
Analysis of the directional orientation under various light regimes thus reveals two fundamentally different types of responses: (i) normal compass orientation with the inclination compass under white light and under dim monochromatic light from the short-wavelength part of the spectrum and (ii) fixed-direction responses in total darkness, under bright monochromatic light and bichromatic light. The characteristics and the origin of the axial responses have not yet been analysed; however, the circumstances under which they are observed suggest that they are related to fixed-direction responses rather than to compass orientation and probably also originate in the magnetite-based receptors in the beak.
The different properties of compass orientation and fixed-direction responses known so far are listed in table 1. It is surprising that, although both responses involve directional behaviour, the underlying magnetic information originates in different types of receptors based on different physical principles, with compass orientation based on the radical-pair processes and fixed-direction responses based on magnetic information from magnetite-based receptors in the beak.
Table 1.
Difference between compass orientation and fixed-direction responses in birds.
| compass orientation | fixed-direction responses | |
|---|---|---|
| nature of response | axial→inclination compass | polar |
| functional window | narrow window around the total intensity of local geomagnetic field | no intensity window, occur also in stronger fields |
| effect of oscillating fields | disorientation | no effect |
| anaesthesia of the upper beak | no effect | disorientation |
| underlying physical process | radical-pair mechanism | magnetite-based mechanism |
| site of receptors | right eye | skin of the upper beak |
| nerve mediating information | optic nerve | branch of the trigeminal nerve |
| directions preferred | any: migratory direction, home direction or acquired directions | only one specific direction under a given light regime |
3. Interactions between magnetoreceptors and the visual system
The relationship between light conditions and the various responses of birds cannot be explained by one type of specialized magnetoreceptor alone. Rather, complex interactions are suggested between the magnetoreception system in the eye and the photoreceptors, which also involve magnetite-based receptors in the upper beak. This raises a number of questions about the conditions under which compass orientation ceases, the role of the magnetite-based receptors in the beak and the biological significance of the responses. We are still far from understanding the interactions between the various receptor types in detail. Yet some observations point out certain relationships, which might help us to untangle interconnections and interactions within the magnetoreception system.
3.1. The limits of compass orientation
One key observation concerns the transition from compass orientation to other types of response. The inclination compass works under white light in the laboratory as well as outdoors, where it works well in daylight, as demonstrated outdoors by cage experiments with a day migrant (Munro & Wiltschko 1993) and with a nocturnal migrant tested during daytime (Thalau & Wiltschko 1987). Pigeons, too, can use their magnetic compass in bright daylight (e.g. Wiltschko, R. et al. 1981). The inclination compass also works under monochromatic light at short wavelengths up to 565 nm green, but only under low light levels. The latter suggests that magnetoreception in migratory birds requires very little light and that short-wavelength light is crucial.
When the intensity of the test lights was increased, birds ceased to orient in their migratory direction. Migration is a spontaneous behaviour and the motivation to head in the migratory direction is very strong; hence, the observation that birds were active but no longer heading in their migratory direction suggests that they could not locate this direction any longer. This, in turn, indicates that the inclination compass was somehow impaired. The light intensities involved, with a quantal flux up to 7.2 × 1016 quanta s−1 m−2, were still low—light on a clear sunny day is brighter by orders of magnitude. McFarland & Munz (1975) reported that the total light intensity from 400 to 700 nm at midday is in the range of 5.3 × 1020 quanta s−1 m−2, which means that the spectral bands corresponding to our LEDs are roughly one-tenth of this, of the order of 1019 quanta s−1 m−2. Hence, the interference with the inclination compass cannot be attributed to saturation of the crucial receptors. Rather, the reason seems to lie in the narrow bandwidth of the monochromatic test lights. Natural light is composed of wavelengths from all parts of the spectrum. Monochromatic light does not occur under natural conditions; even objects that appear to us as unicoloured and monochromatic, e.g. bright green, reflect a variety of wavelengths, with those of the green range dominating and those of the red part being rarer. It seems to be this unnatural property of our test lights that interferes with the inclination compass.
In monochromatic light, one or two colour receptors are strongly activated, whereas the others have no or only negligible activity. A direct role of the rods and cones in magnetoreception is usually not considered, because opsins do not form the required radical pairs. Instead, cryptochrome, a photopigment with a flavin chromophore, first known from plants, but later also found in animals (e.g. Haque et al. 2002; Möller et al. 2004; Mouritsen et al. 2004; for a review, see Sancar 2003), has been discussed as a promising candidate for the receptor molecule. There is no obvious relationship between the absorption of the avian colour cones and the wavelengths under which the inclination compass operates (figure 8; for details on the spectral sensitivity of birds, see Hart 2001), and an attempt to correlate magnetoreception with the photopigments activated by various test lights remained largely inconclusive (Johnsen et al. 2007). The observation that interference with magnetoreception sets in only above certain light levels suggests that imbalance between the output of the various cone types may be crucial. Colour perception in birds is based on the balance between the outputs of the four types of colour cones, as is recorded, for example, by the retinal ganglion cells where the input from the photoreceptors converges. Natural light will always excite several types of cones. Monochromatic light with only a narrow spectral band, but with marked intensity, may cause excitation of the cones projecting to one colour opponent ganglion cell to become too large to be accepted by the system as normal, and the ganglion cell may no longer produce the appropriate activity, which may, in turn, cause the system to also reject magnetic input.
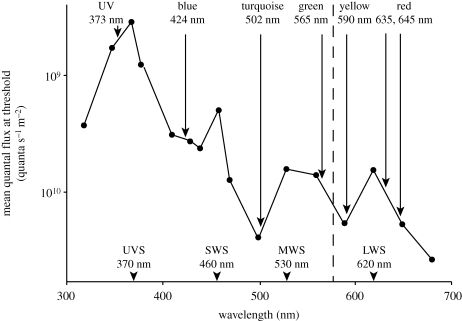
Figure 8.
Spectral sensitivity curve of the Pekin robin, Leiothrix lutea, a passerine species, determined by conditioning experiments (modified from Maier 1992), with wavelengths used in the conditioning tests marked with dots. The peak sensitivity of the four colour receptors is marked below. The peak intensity of the LEDs used to produce the monochromatic and bichromatic lights is also indicated.
The light intensity at which the change to disorientation, axial responses and fixed-direction responses occurs seems to vary with the wavelength of light: it occurs in UV light at a markedly lower quantal flux than in blue, turquoise and green. At the same time, the quantal flux where the transient preference of the east–west axis is observed increases from UV to blue to turquoise to green (figure 5), which suggests a decreasing sensitivity for light of increasing wavelengths. This has an interesting parallel in the relative sensitivity of the avian colour cones, as determined by conditioning experiments with a passerine species: the UV cone proved the most sensitive one, with those responding to longer wavelengths becoming increasingly less sensitive (Burkhardt & Maier 1989; Maier 1992). The responses of birds to monochromatic light thus indicate that the inclination compass seems to work properly only under conditions that do not activate the colour cones beyond a certain level.
In summary, the behaviour of robins in monochromatic light of different intensity suggests an involvement of the visual system in magnetoreception. The visual system seems to gate, i.e. control the transfer of, magnetic input somewhere on its way to the brain area where it is processed. This idea is rather unexpected, and we can only speculate about possible reasons for this inferred relationship between magnetoreception and visual input. A completely independent magnetoreception is theoretically possible, but the magnetoreception system of birds in the right eye probably developed from parts of the visual system. Hence the interrelationship between the two systems could simply have phylogenetic reasons. Yet, it also seems possible that the visual system has an important auxiliary function in magnetoreception, possibly providing important background information for correctly assessing the incoming magnetic information.
The radical-pair model assumes that birds derive directional information from an activation pattern on the retina that is centrally symmetric to the magnetic vector and reflects the amount of singlet or triplet radical pairs—we do not yet know which of these states provides the crucial information. With a maximum difference of about 20 per cent (Ritz et al. 2000), expected differences in the singlet or triplet yield are not large. Ritz et al. (2000) illustrated activation patterns that are assumed to form on the retina, but these regular patterns imply a more or less homogeneous light distribution within the eye. In reality, this will seldom be the case. Normally, the visual field is inhomogeneously illuminated, with the sky brighter than the ground; the distribution of photoreceptors and oil droplets in some birds is adapted to this (Hart 2001). Additionally, parts of the visual field might be shaded, while other parts lie in the sun, with objects reflecting and absorbing different amounts of light, etc. This could mean that the number of radical pairs that is formed, and, with it, the absolute number of singlets and triplets and their products, vary as a function of light intensity. It could modify the activation pattern, rendering it difficult to identify its central symmetry and thus to obtain directional information. Here, the visual system may step in. By providing information about the distribution of light intensity, it may help to compensate for light-induced differences and thus allow birds to correctly interpret the activation pattern. Whether and where these inferred interactions take place—at the receptor level, at the level of the retinal ganglion cells or at higher centres—is still unknown.
3.2. Yellow light interfering with the inclination compass
Another phenomenon that is difficult to explain concerns interactions of 590 nm yellow light with light of shorter wavelengths. On the one hand, there is the very rapid transition from well-oriented behaviour under 565 nm green to disorientation under 590 nm yellow produced by LEDs (figure 2; Wiltschko, W. & Wiltschko, R. 1999). The shift in wavelength is small, and, with a half bandwidth of 32 and 35 nm, respectively, the LED spectra partly overlap (figure 1). Experiments using filters with a narrow bandwidth of only 10 nm indicated disorientation under 567.5 nm light (Muheim et al. 2002), which suggests that it is mainly the short-wavelength flank of the spectrum of the green LEDs below 560 nm that allows orientation.
The wavelength range where the rapid transition to disorientation is observed coincides with the long-wavelength flank of the avian rods. According to the data of Maier & Bowmaker (1993, fig. 1), the rods have half of their maximum sensitivity at 550 nm, about one-third at 565 nm and only about one-sixth at 590 nm, which means that the sensitivity decreases rapidly with increasing wavelength. However, as already mentioned above, the rod pigments themselves can hardly be directly involved in magnetoreception because rhodopsin does not form radical pairs.
The rapid decrease in orientation from green to yellow can likewise not be attributed to the intensity of light falling below a threshold. In this case, an increased light intensity should elicit responses, but increasing the intensity of yellow light about six-fold still produced disorientation (Wiltschko, W. & Wiltschko, R. 2001). Hence, the rapid change to disorientation cannot be attributed to the crucial photopigment no longer absorbing. It rather seems to reflect some antagonistic interactions with receptors activated by longer wavelength light.
The fixed-direction responses observed when short-wavelength light was combined with 590 nm yellow light likewise suggest antagonistic interactions. Yellow light alone does not allow orientation, yet it is not ‘neutral’ in the sense of not being involved in magnetoreception. Although the short-wavelength part of the bichromatic light allows orientation (figure 2), adding yellow light leads to a situation where the inclination compass no longer works properly, which suggests that magnetoreception is disrupted. Interference with the radical-pair processes themselves seems highly unlikely, particularly because recent observations indicate that the crucial radical-pair processes do not take place during photoreduction, but during re-oxidation (Ritz et al. 2009). It therefore seems more likely that the interactions that disrupt the inclination compass occur somewhere higher up during the transmission of magnetic information. The receptors involved in antagonistic interactions and where exactly these take place remains unknown. In the 1980s, electrophysiological recordings from the nucleus of the basal optic root identified two types of units that responded to changes in the direction of the magnetic field, with maxima at different wavelengths. One type had a maximum near 503 nm and already showed a marked decrease at 582 nm, whereas, in the other, the maximum response was observed near 582 nm, with a decrease towards 674 nm (Semm & Demaine 1986). The characteristics of the second unit roughly coincide with the absorption curve of the avian long-wavelength sensitive (LWS) receptor with its maximum mostly between 563 and 567 nm (Maier & Bowmaker 1993; Hart 2001), but it is difficult to see how this LWS-receptor might be involved. Whether there are other units that are possibly activated by long wavelengths, how they might interact and, if so, at what level is not yet known.
4. The role of iron-based receptors in the beak
An unexpected finding was that magnetic information for fixed-direction responses, in contrast to compass orientation, is mediated by magnetite-based receptors in the upper beak described by Hanzlik et al. (2000), Winklhofer et al. (2001) and Fleissner et al. (2003, 2007). This is clearly demonstrated by the observation that temporarily deactivating these receptors with a local anaesthetic leads to a breakdown of the fixed-direction responses, with the birds' headings becoming random (figure 7, right diagrams).
4.1. The output of the magnetite-based receptors
Ever since magnetite was discovered in the ethmoid region and the upper beak, attempts have been made to identify its function. Electrophysiological recordings from the ramus ophthalmicus of the trigeminal nerve that innervates the area where the magnetite-based receptors are found showed responses to changes in the ambient magnetic field, in particular to changes in magnetic intensity (Beason & Semm 1987, 1996; Semm & Beason 1990). It suggests that magnetite-based receptors detect small changes in magnetic intensity, with this information being a component of the navigational ‘map’. This interpretation was supported by anaesthetizing the ophthalmic nerve and the upper beak, which does not disrupt compass orientation in migratory direction (Beason & Semm 1996; Wiltschko, R. et al. 2007a, 2008; Wiltschko, W. et al. 2007; Stapput et al. 2008). Birds have also been subjected to short, strong magnetic pulses to affect the magnetization of the particles in iron-based receptors. In adult, experienced migrants, this led to a deviation of headings by about 90° (Wiltschko, W. et al. 1994, 1998; Beason et al. 1995), whereas the same pulse did not affect the orientation of young, inexperienced migrants that had been caught before they had a chance to establish a map (Munro et al. 1997). The altered headings of experienced birds proved to be controlled by the inclination compass (Wiltschko, R. et al. 2006b). In experienced pigeons, a strong magnetic pulse also led to deviations from the bearings of control birds at distant sites, but not close to the home loft where differences in intensity must be expected to be so small that they would probably be below threshold (Beason et al. 1997). Together, these findings indicate that magnetite-based receptors provide birds with information on magnetic intensity, which constitutes a magnetic component in the multi-modal map system for determining position. It was therefore most surprising to learn that the same receptors additionally provide directing information expressed in fixed-direction responses.
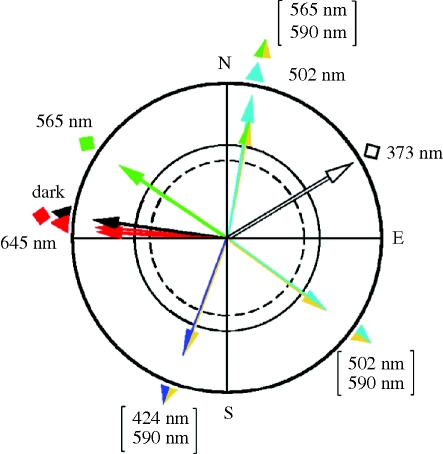
The output of magnetite-based receptors raises several questions. Why are so many different fixed directions observed? A summary of the fixed directions reported so far and the wavelengths under which they are found is given in figure 9; they seem to occur in all directions. One would expect that the receptors in the upper beak provide just one type of directional output, irrespective of light, and hence it is difficult to see how the iron-based receptors could be influenced by different light conditions. Yet, the manifestation of fixed-direction responses clearly depends on the ambient light regime. This suggests interactions between magnetite-based receptors in the beak and the visual system—the visual system seems to modify the output of magnetite-based receptors in a way that causes the different fixed directions to emerge. These interactions seem to take place at higher levels in the brain where input from the trigeminal system that mediates the output of iron-based receptors (Beason & Semm 1996) converges with that from the visual system. The number of observed fixed directions is still limited, and a pattern of what light conditions lead to which fixed direction has not yet become obvious.
Figure 9.
Fixed-direction responses observed so far in European robins (triangles) and Australian silvereyes (diamonds). The wavelength and wavelength combinations are indicated.
4.2. When and how do iron-based receptors control behaviour?
Another question concerns the conditions under which fixed-direction responses occur. They are observed when the normal inclination compass no longer works properly, but they are not observed under all these conditions. Under yellow and red light in the geomagnetic field, birds were disoriented (figure 2), and they were also disoriented under normal light conditions in magnetic fields with intensities about 30 per cent higher or lower than the local geomagnetic field, which indicates the functional window of the inclination compass (e.g. Wiltschko, R. et al. 2006a), although fixed-direction responses can be observed at intensities two or three times of that of the geomagnetic field (Wiltschko, W. et al. 2010). Disorientation is likewise observed in birds exposed to oscillating fields in the MHz range (Ritz et al. 2004, 2009; Thalau et al. 2005; Wiltschko, W. et al. 2007) and when the right eye is covered (Wiltschko, W. et al. 2002). None of these treatments affect iron-based receptors in the beak and one may wonder why, under some conditions, we observe disorientation instead of a fixed-direction response. It is difficult to find a pattern. Disorientation seems to occur under conditions that disrupt the radical-pair processes directly, such as oscillating magnetic fields and covering the right eye to exclude light. Long-wavelength light might no longer be absorbed by the magnetosensitive molecule, thus preventing the formation of the crucial radical pairs. Magnetic intensities outside the functional window, on the other hand, change the activity pattern on the retina, but this disorientation is only temporary, as birds very soon adapt to the new magnetic intensities (Wiltschko, W. et al. 2006a). Fixed-direction responses, in contrast, are mostly observed under extreme light conditions where one would expect radical-pair processes to work properly, but where the imbalance between input from the colour cones appears to interfere with the magnetic compass at a higher level. Fixed-direction responses observed in total darkness (and probably identical ones under dim red light) do not fit this pattern, however, because here, too, radical-pair processes would be suppressed by lack of light; yet in this case, in contrast to covering the right eye, the birds are deprived of all visual input. Although the question of when fixed-direction responses and when disorientation occur cannot yet be answered, the different responses of the birds indicate that an interference with the primary physical processes and interference at higher levels of transmitting and processing magnetic compass information may have different consequences. They imply complex interactions between the two magnetic systems and the visual system that we do not yet understand.
A further question concerns how the conditions under which fixed-direction responses occur affect behaviour. Birds are directed in specific directions, yet input from iron-based receptors does not seem to provide directional information in the sense that birds could use it to locate their migratory direction. Instead, regardless of their motivation to head north or south, the birds always orient in the same direction, which, behaviourally, makes little sense. It looks as if these directions are forced upon the birds by the stimulus situation. In this aspect, the fixed-direction responses appear to be related to alignment responses. However, alignments in the geomagnetic field are usually quadrimodal or axial responses, coinciding with the major axes north–south and east–west (e.g. Martin & Lindauer 1977; Phillips et al. 2002; Begall et al. 2008). This may be true to some extent for axial responses observed under higher intensity short-wavelength monochromatic light, but it does not apply to unimodal fixed-direction responses under other light conditions. The nature of the stimuli produced by the light regime together with input from magnetite-based receptors and what they may mean for birds is as yet unclear.
5. Significance of the two types of responses
The finding that the same type of compass based on radical-pair processes in the eye exists in birds of such distant avian lineages as passerines and galliformes (see Mayr & Clarke (2003) and Ericson et al. (2006) for the phylogenetic relationship among birds) suggests that this mechanism is common to all birds. The inclination compass is a true compass that tells birds where directions lie. Its biological significance is clear: it is the normal compass that birds use to locate compass courses. Migratory birds use it to find their innate migratory direction, pigeons rely on the inclination compass to locate their home course (Keeton 1971; Walcott & Green 1974) and chickens and zebra finches locate directions set by the experimenter (Freire et al. 2005; Voss et al. 2007). In young pigeons, it also serves as a directional reference in the learning processes establishing the sun compass (Wiltschko, W. et al. 1983) and probably also for establishing the navigational map. In summary, the inclination compass provides birds with a general directional reference system and allows them to locate compass courses of all kinds for navigation over great distances as well as for small-scale tasks within the home range.
With fixed-direction responses, the situation is entirely different. So far, they have been observed only under artificial light conditions where the regular magnetic compass no longer seems to work. This means that fixed-direction responses do not occur in nature and are thus not subject to natural selection. Since their manifestation is controlled by the specific light regime that seems to permit only one direction, regardless of the birds' intentions, they cannot be used as a compass to locate the migratory or the home course. They do not seem to be helpful to birds in any way. This leads to the crucial question: why do they exist at all?
At this point, we can only speculate. The directing information for the fixed-direction responses originates in magnetite-based receptors in the beak. Magnetite was first discovered in an orientation context in ‘magnetotactic’ bacteria (Blakemore 1975). It is a product of iron metabolism and has been reported from a wide variety of species from different phyla, including all major groups of vertebrates, where it is mostly found in the ethmoid region (Kirschvink et al. 1985). Mammals, for example, have been shown to have a polarity compass (Marhold et al. 1997a; Wang et al. 2007) that is most probably based on magnetite (Marhold et al. 1997b; Wegner et al. 2006; Holland et al. 2008). The same may apply to fishes (Quinn & Brannon 1982; Walker et al. 1997). For the amphibians and reptiles tested so far, an inclination compass has been reported (Phillips 1986; Light et al. 1993; Lohmann & Lohmann 1993), but the underlying mechanisms have not yet been analysed. Since magnetite-based receptors can also provide an inclination compass (e.g. Kirschvink & Gould 1981; Shcherbakov & Winklhofer 1999; Solov'yov & Greiner 2009), the physical base of these compass mechanisms is still unknown.
Birds, however, have developed a compass mechanism based on a different type of physical process. Iron-based receptors are not involved in the compass. Today, the latter receptors appear to provide information on magnetic intensity used in the navigational map. Yet, in view of the wide distribution of magnetite in vertebrates and its involvement in magnetoreception, it seems possible that it was once involved in an ancient compass used by the birds' distant ancestors. Magnetite-based receptors in birds seem to have now specialized in detecting magnetic intensity and appear to have lost their previous function, which has been taken over by the radical-pair mechanism in the eye. Their directional output could be a phylogenetic relic—an old inheritance that can no longer provide proper compass information. It remains more or less dormant as long as compass information from the radical-pair mechanism is available and becomes effective only when the inclination compass is impaired by extreme light conditions.
The different responses of birds under the various light regimes reviewed here reveal the existence of complex interactions between the two magnetoreception systems—the radical-pair processes in the right eye and the iron-based receptors in the upper beak—and the visual system. Following up and understanding these interrelations will be a challenge for future research.
Acknowledgements
Our work reported here was supported by the Deutsche Forschungsgemeinschaft (grants to R.W. and W.W.) and by the Human Frontier Sciences Program (grant to R.W.). We sincerely thank H.-J. Bischof, Universität Bielefeld, O. Güntürkün, Ruhr-Universität Bochum, and L. Peichl, Max-Planck-Institut für Hirmforschung, Frankfurt, and members of our group for valuable comments and stimulating discussions.
Footnotes
One contribution to the Theme Supplement ‘Magnetoreception’.
References
- Batschelet E. 1981. Circular statistics in biology. New York, NY: Academic Press. [Google Scholar]
- Beason R. C., Nichols J. E. 1984. Magnetic orientation and magnetically sensitive material in a transequatorial migratory bird. Nature 309, 151–153. ( 10.1038/309151a0) [DOI] [Google Scholar]
- Beason R. C., Semm P. 1987. Magnetic responses of the trigeminal nerve system of the Bobolink (Dolichonyx oryzivorus). Neurosci. Lett. 80, 229–234. ( 10.1016/0304-3940(87)90659-8) [DOI] [PubMed] [Google Scholar]
- Beason R. C., Semm P. 1996. Does the avian ophthalmic nerve carry magnetic navigational information? J. Exp. Biol. 199, 1241–1244. [DOI] [PubMed] [Google Scholar]
- Beason R. C., Dussourd N., Deutschlander M. 1995. Behavioural evidence for the use of magnetic material in magnetoreception by a migratory bird. J. Exp. Biol. 198, 141–146. [DOI] [PubMed] [Google Scholar]
- Beason R. C., Wiltschko R., Wiltschko W. 1997. Pigeon homing: effects of magnetic pulse on initial orientation. Auk 114, 405–415. [Google Scholar]
- Begall S., Červeny J., Neef J., Vojtěch O., Burda H. 2008. Magnetic alignment in grazing and resting cattle and deer. Proc. Natl. Acad. Sci. USA 105, 13 451–13 455. ( 10.1073/pnas.0803650105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore R. P. 1975. Magnetotactic bacteria. Science 190, 377–379. ( 10.1126/science.170679) [DOI] [PubMed] [Google Scholar]
- Burkhardt D., Maier E. 1989. The spectral sensitivity of a passerine bird is highest in the UV. Naturwissenschaften 76, 82–83. ( 10.1007/BF00396716) [DOI] [Google Scholar]
- Davila A. F., Fleissner G., Winklhofer M., Petersen N. 2003. A new model for a magnetoreceptor in homing pigeons based on interacting clusters of superparamagnetic magnetite. Phys. Chem. Earth 28, 647–652. [Google Scholar]
- Edmonds D. T. 1992. A magnetite null detector as the migrating bird's compass. Proc. R. Soc. Lond. B 249, 27–31. ( 10.1098/rspb.1992.0079) [DOI] [Google Scholar]
- Ericson P. G. P., et al. 2006. Diversification of neoaves: integration of molecular sequence data and fossils. Biol. Lett. 4, 543–547. ( 10.1098/rsbl.2006.0523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner G., Holtkamp-Rötzler E., Hanzlik M., Winklhofer M., Fleissner G., Petersen N., Wiltschko W. 2003. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J. Comp. Neurol. 458, 350–360. ( 10.1002/cne.10579) [DOI] [PubMed] [Google Scholar]
- Fleissner G., Stahl B., Thalau P., Falkenberg G., Fleissner G. 2007. A novel concept of Fe-mineral-based magnetoreception: histological and physicochemical data from the upper beak of homing pigeons. Naturwissenschaften 94, 631–642. ( 10.1007/s00114-007-0236-0) [DOI] [PubMed] [Google Scholar]
- Freire R., Munro U. H., Rogers L. J., Wiltschko R., Wiltschko W. 2005. Chickens orient using a magnetic compass. Curr. Biol. 15, R620–R621. ( 10.1016/j.cub.2005.08.017) [DOI] [PubMed] [Google Scholar]
- Hanzlik M., Heunemann C., Holzkamp-Rötzler E., Winklhofer M., Petersen N., Fleissner G. 2000. Superparamagnetic magnetite in the upper beak tissue of homing pigeons. BioMetals 13, 325–331. ( 10.1023/A:1009214526685) [DOI] [PubMed] [Google Scholar]
- Haque R., Charausia S. S., Wessel J. H., Iuvone P. M. 2002. Dual regulation of cryptochrome I mRNA expression in chicken retina by light and circadian oscillators. Neuroreport 13, 2247–2251. ( 10.1097/00001756-200212030-00016) [DOI] [PubMed] [Google Scholar]
- Hart N. S. 2001. The visual ecology of avian photoreceptors. Prog. Retin. Eye Res. 20, 675–703. ( 10.1016/S1350-9462(01)00009-X) [DOI] [PubMed] [Google Scholar]
- Holland R. A., Kirschvink J. L., Doak T. G., Wikelski M. 2008. Bats use magnetite to detect the Earth's magnetic field. PLoS ONE 3, e1676 ( 10.1371/journal.pone.0001676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen S., Mattern E., Ritz T. 2007. Light-dependent magnetoreception: quantum catches and opponency mechanisms of possible photosensitive molecules. J. Exp. Biol. 210, 3171–3178. ( 10.1242/jeb.007567) [DOI] [PubMed] [Google Scholar]
- Keary N., Ruploh T., Voss J., Thalau P., Wiltschko R., Wiltschko W., Bischof H.-J. In press Oscillating magnetic field disrupts magnetic orientation in zebra finches, Taeniopygia guttata. Front. Zool. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeton W. T. 1971. Magnets interfere with pigeon homing. Proc. Natl. Acad. Sci. USA 68, 102–106. ( 10.1073/pnas.68.1.102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschvink J. L., Gould J. L. 1981. Biogenic magnetite as a basis for magnetic field detection in animals. BioSystems 13, 181–201. ( 10.1016/0303-2647(81)90060-5) [DOI] [PubMed] [Google Scholar]
- Kirschvink J. L., Walker M. M. 1985. Particle-size considerations for magnetite-based magnetoreceptors. In Magnetite biomineralization and magnetoreception in organisms (eds Kirschvink J. L., Jones D. S., Fadden B. J.), pp. 243–256. New York, NY: Plenum Press. [Google Scholar]
- Kirschvink J. L., Jones D. S., Fadden B. J. (eds) 1985. Magnetite biomineralization and magnetoreception in organisms. New York, NY: Plenum Press. [Google Scholar]
- Light P., Salmon M., Lohmann K. J. 1993. Geomagnetic orientation of loggerhead sea turtles: evidence for an inclination compass. J. Exp. Biol. 182, 1–10. [Google Scholar]
- Lohmann K. J., Lohmann C. M. F. 1993. A light-independent magnetic compass in the leatherback sea turtles. Biol. Bull. 185, 149–151. ( 10.2307/1542138) [DOI] [PubMed] [Google Scholar]
- Maier E. J. 1992. Spectral sensitivities including the ultraviolet of the passeriform bird Leiothrix lutea. J. Comp. Physiol. A 170, 709–714. ( 10.1007/BF00198981) [DOI] [Google Scholar]
- Maier E. J., Bowmaker J. K. 1993. Colour vision in the passeriform bird, Leiothrix lutea: correlation of visual pigment absorbance and oil droplet transmission with spectral sensitivity. J. Comp. Physiol. A 172, 295–301. ( 10.1007/BF00216611) [DOI] [Google Scholar]
- Marhold S., Wiltschko W., Burda H. 1997a. A magnetic polarity compass for direction finding in a subterranean mammal. Naturwissenschaften 84, 421–423. ( 10.1007/s001140050422) [DOI] [Google Scholar]
- Marhold S., Burda H., Kreilos I., Wiltschko W. 1997b. Magnetic orientation in common mole-rats from Zambia. In Orientation and navigation—birds, humans and other animals, pp. 5-1–5-9. Oxford, UK: Royal Institute of Navigation. [Google Scholar]
- Martin H., Lindauer M. 1977. Der Einfluß des Erdmagnetfeldes auf die Schwereorientierung der Honigbiene (Apis mellifica). J. Comp. Physiol. 122, 145–187. ( 10.1007/BF00611888) [DOI] [Google Scholar]
- Mayr G., Clarke J. 2003. The deep divergences of neornithine birds: a phylogenetic analysis of morphological characters. Cladistic 19, 527–553. ( 10.1111/j.1096-0031.2003.tb00387.x) [DOI] [PubMed] [Google Scholar]
- McFarland W. W., Munz F. W. 1975. The visible spectrum during twilight and its implications to vision. In Light as an ecological factor II (eds Evans G. C., Bainbridge R., Rackham O.), pp. 249–270. Oxford, UK: Blackwell. [Google Scholar]
- Möller A., Sagasser S., Wiltschko W., Schierwater B. 2004. Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenschaften 91, 585–588. ( 10.1007/s00114-004-0578-9) [DOI] [PubMed] [Google Scholar]
- Mouritsen H., Janssen-Bienhold U., Liedvogel M., Feenders G., Stalleicken J., Dirks P., Weiler R. 2004. Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation. Proc. Natl Acad. Sci. USA 101, 14 294–14 299. ( 10.1073/pnas.0405968101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muheim R., Bäckman J., Åkesson S. 2002. Magnetic compass orientation in European robins is dependent on both wavelength and intensity of light. J. Exp. Biol. 205, 3845–3856. [DOI] [PubMed] [Google Scholar]
- Munro U., Wiltschko W. 1993. Magnetic compass orientation in the Yellow-faced Honeyeater, Lichenostomus chrysops, a day migrating bird from Australia. Behav. Ecol. Sociobiol. 32, 141–145. ( 10.1007/BF00164047) [DOI] [Google Scholar]
- Munro U., Munro J. A., Phillips J. B., Wiltschko R., Wiltschko W. 1997. Evidence for a magnetite-based navigational ‘map’ in birds. Naturwissenschaften 84, 26–28. ( 10.1007/s001140050343) [DOI] [Google Scholar]
- Phillips J. B. 1986. Two magnetoreception pathways in a migratory salamander. Science 233, 765–767. ( 10.1126/science.3738508) [DOI] [PubMed] [Google Scholar]
- Phillips J. B., Sorland S. C., Freake M. J., Brassart J., Kischvink J. L. 2002. ‘Fixed axis’ magnetic orientation by an amphibian: non-shoreward-directed compass orientation, misdirected homing or positioning a magnetite-based map detector in a consistent alignment relative to the magnetic field. J. Exp. Biol. 205, 3903–3914. [DOI] [PubMed] [Google Scholar]
- Quinn T. P., Brannon E. L. 1982. The use of celestial and magnetic cues by orienting sockeye salmon smolts. J. Comp. Physiol. 147, 547–552. ( 10.1007/BF00612020) [DOI] [Google Scholar]
- Rappl R., Wiltschko R., Weindler P., Berthold P., Wiltschko W. 2000. Orientation of Garden Warblers, Sylvia borin, under monochromatic light of various wavelengths. Auk 117, 256–260. ( 10.1642/0004-8038(2000)117[0256:OBOGWS]2.0.CO;2) [DOI] [Google Scholar]
- Ritz T., Adem S., Schulten K. 2000. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718. ( 10.1016/S0006-3495(00)76629-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T., Thalau P., Phillips J. B., Wiltschko R., Wiltschko W. 2004. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 429, 177–180. ( 10.1038/nature02534) [DOI] [PubMed] [Google Scholar]
- Ritz T., Wiltschko R., Hore P. J., Rodgers C. T., Stapput K., Thalau P., Timmel C. R., Wiltschko W. 2009. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys. J. 96, 3451–3457. ( 10.1016/j.bpj.2008.11.072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger L. J., Munro U., Freire R., Wiltschko R., Wiltschko W. 2008. Lateralized response of chicks to magnetic cues. Behav. Brain Res. 186, 66–71. ( 10.1016/j.bbr.2007.07.029) [DOI] [PubMed] [Google Scholar]
- Sancar A. 2003. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem. Rev. 103, 2203–2237. ( 10.1021/cr0204348) [DOI] [PubMed] [Google Scholar]
- Schulten K. 1982. Magnetic field effects in chemistry and biology. In Festkörperprobleme (ed. Treusch J.), pp. 61–83. Braunschweig, Germany: Vieweg. [Google Scholar]
- Semm P., Beason R. C. 1990. Responses to small magnetic variations by the trigeminal system of the Bobolink. Brain Res. Bull. 25, 735–740. ( 10.1016/0361-9230(90)90051-Z) [DOI] [PubMed] [Google Scholar]
- Semm P., Demaine C. 1986. Neurophysiological properties of magnetic cells in the pigeon's visual system. J. Comp. Physiol. A 159, 619–625. ( 10.1007/BF00612035) [DOI] [PubMed] [Google Scholar]
- Shcherbakov V. P., Winklhofer M. 1999. The osmotic magnetometer: a new model for magnetite-based magnetoreceptors in animals. Eur. Biophys. J. 28, 380–392. ( 10.1007/s002490050222) [DOI] [Google Scholar]
- Solov'yov I. A., Greiner W. 2007. Theoretical analysis of an iron mineral-based magnetoreceptor model in birds. Biophys. J. 93, 1493–1509. ( 10.1529/biophysj.107.105098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solov'yov I. A., Greiner W. 2009. Iron-mineral-based magnetoreceptor in birds: polarity or inclination compass? Eur. Phys. J. D 51, 161–172. ( 10.1140/epjd/e2008-00118-y) [DOI] [Google Scholar]
- Stapput K., Gesson M., Wiltschko R., Wiltschko W. 2005. Light-dependent magnetoreception: behavior of migratory birds under monochromatic and bichromatic Lights. In Animal orientation—bird, humans and other animals, p. 16 Oxford, UK: Royal Institute of Navigation. [Google Scholar]
- Stapput K., Thalau P., Wiltschko R., Wiltschko W. 2008. Orientation of birds in total darkness. Curr. Biol. 18, 602–606. ( 10.1016/j.cub.2008.03.046) [DOI] [PubMed] [Google Scholar]
- Thalau P., Wiltschko W. 1987. Einflüsse des Futterangebots auf die Tagesaktivität von Trauerschnäppern (Ficedula hypoleuca) auf dem Herbstzug. Cour. Forsch. Inst. Senckenberg 97, 67–73. [Google Scholar]
- Thalau P., Ritz T., Stapput K., Wiltschko R., Wiltschko W. 2005. Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwissenschaften 92, 86–90. ( 10.1007/s00114-004-0595-8) [DOI] [PubMed] [Google Scholar]
- Tian L., Xiao B., Lin W., Zhang S., Zhu R., Pan Y. 2007. Testing for the presence of magnetite in the upper-beak skin of homing pigeons. BioMetals 20, 197–203. ( 10.1007/s10534-006-9027-x) [DOI] [PubMed] [Google Scholar]
- Voss J., Keary N., Bischof J. 2007. The use of the geomagnetic field for short distance orientation in zebra finches. NeuroReport 18, 1053–1058. ( 10.1097/WNR.0b013e32818b2a21) [DOI] [PubMed] [Google Scholar]
- Walcott C., Green R. P. 1974. Orientation of homing pigeons altered by a change in the direction of an applied magnetic field. Science 184, 180–182. ( 10.1126/science.184.4133.180) [DOI] [PubMed] [Google Scholar]
- Walker M. M. 2008. A model for encoding of magnetic field intensity by magnetite-based magnetoreceptor cells. J. Theor. Biol. 250, 85–91. ( 10.1016/j.jtbi.2007.09.030) [DOI] [PubMed] [Google Scholar]
- Walker M. M., Diebel C. E., Haugh C. V., Pankhurst P. M., Montgomery J. C., Green C. R. 1997. Structure and function of the vertebrate magnetic sense. Nature 390, 371–376. ( 10.1038/37057) [DOI] [PubMed] [Google Scholar]
- Wang Y., Pan Y., Parsons S., Walker M., Zhang S. 2007. Bats respond to polarity of a magnetic field. Proc. R. Soc. B 274, 2901–2905. ( 10.1098/rspb.2007.0904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner R. E., Begall S., Burda H. 2006. Magnetic compass in the cornea: local anesthesia impairs orientation in a mammal. J. Exp. Biol. 209, 4747–4750. ( 10.1242/jeb.02573) [DOI] [PubMed] [Google Scholar]
- Williams M. N., Wild J. M. 2001. Trigeminally innervated iron-containing structures in the beak of homing pigeons and other birds. Brain Res. 889, 243–246. ( 10.1016/S0006-8993(00)03114-0) [DOI] [PubMed] [Google Scholar]
- Wiltschko R., Wiltschko W. 1998. Pigeon homing: effect of various wavelength of light during displacement. Naturwissenschaften 85, 164–167. ( 10.1007/s001140050476) [DOI] [Google Scholar]
- Wiltschko R., Nohr D., Wiltschko W. 1981. Pigeons with a deficient sun compass use the magnetic compass. Science 214, 343–345. ( 10.1126/science.7280697) [DOI] [PubMed] [Google Scholar]
- Wiltschko R., Ritz T., Stapput K., Thalau P., Wiltschko W. 2005. Two different types of light-dependent responses to magnetic fields in birds. Curr. Biol. 15, 1518–1523. ( 10.1016/j.cub.2005.07.037) [DOI] [PubMed] [Google Scholar]
- Wiltschko R., Stapput K., Bischof H.-J., Wiltschko W. 2007a. Light-dependent magnetoreception in birds: increasing intensity of monochromatic light changes the nature of response. Front. Zool. 4, 5 ( 10.1186/1742-9994-4-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko R., Stapput K., Ritz T., Thalau P., Wiltschko W. 2007b. Magnetoreception in birds: different physical processes for different types of directional responses. HFSP J. 1, 41–48. ( 10.2976/1.2714294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko R., Munro U., Ford H., Stapput K., Wiltschko W. 2008. Light-dependent magnetoreception: orientation behaviour of migratory birds under dim red light. J. Exp. Biol. 211, 3344–3350. ( 10.1242/jeb.020313) [DOI] [PubMed] [Google Scholar]
- Wiltschko W. 1968. Über den Einfluß statischer Magnetfelder auf die Zugorientierung der Rotkehlchen (Erithacus rubecula). Z. Tierpsychol. 25, 536–558. [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 1999. The effect of yellow and blue light on magnetic compass orientation in European Robins, Erithacus rubecula. J. Comp. Physiol. A 184, 295–299. ( 10.1007/s003590050327) [DOI] [Google Scholar]
- Wiltschko W., Wiltschko R. 2001. Light-dependent magnetoreception in birds: the behavior of European Robins, Erithacus rubecula, under monochromatic light of various wavelengths. J. Exp. Biol. 204, 3295–3302. [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 2007. Magnetoreception in birds: two receptors for two different tasks. J. Ornithol. 148(Suppl. 1), S61–S76. ( 10.1007/s10336-007-0233-2) [DOI] [Google Scholar]
- Wiltschko W., Wiltschko R., Keeton W. T., Maddon R. 1983. Growing up in an altered magnetic field affects the initial orientation of young homing pigeons. Behav. Ecol. Sociobiol. 12, 135–142. ( 10.1007/BF00343204) [DOI] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 1993. Red light disrupts magnetic orientation of migratory birds. Nature 364, 525–527. ( 10.1038/364525a0) [DOI] [Google Scholar]
- Wiltschko W., Munro U., Beason R. C., Ford H., Wiltschko R. 1994. A magnetic pulse leads to a temporary deflection in the orientation of migratory birds. Experientia 50, 697–700. ( 10.1007/BF01952877) [DOI] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 1998. Effect of a magnetic pulse on the orientation of Silvereyes, Zosterops l. lateralis, during spring migration. J. Exp. Biol. 201, 3257–3261. [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R., Munro U. 2000. Light-dependent magnetoreception in birds: the effect of intensity of 565-nm green light. Naturwissenschaften 87, 366–369. ( 10.1007/s001140050742) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Gesson M., Wiltschko R. 2001. Magnetic compass orientation of European Robins under 565 nm green light. Naturwissenschaften 88, 387–390. ( 10.1007/s001140100248) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Traudt J., Güntürkün O., Prior H., Wiltschko R. 2002. Lateralisation of magnetic compass orientation in a migratory bird. Nature 419, 467–470. ( 10.1038/nature00958) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 2003. Magnetic orientation in birds: non-compass responses under monochromatic light of increased intensity. Proc. R. Soc. Lond. B 270, 2133–2140. ( 10.1098/rspb.2003.2476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko W., Möller A., Gesson M., Noll C., Wiltschko R. 2004a. Light-dependent magnetoreception in birds: analysis of the behaviour under red light after pre-exposure to red light. J. Exp. Biol. 207, 1193–1203. ( 10.1242/jeb.00873) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Gesson M., Stapput K., Wiltschko R. 2004b. Light-dependent magnetoreception in birds: interaction of at least two different receptors. Naturwissenschaften 91, 130–134. ( 10.1007/s00114-003-0500-x) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Stapput K., Thalau P., Wiltschko R. 2006a. Avian magnetic compass: fast adjustment to intensities outside the normal functional window. Naturwissenschaften 93, 300–304. ( 10.1007/s00114-006-0102-5) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 2006b. Bird navigation: what type of information does the magnetite-based receptor provide? Proc. R. Soc. B 273, 2815–2820. ( 10.1098/rspb.2006.3651) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko W., Freire R., Munro U., Ritz T., Rogers L., Thalau P., Wiltschko R. 2007. The magnetic compass of domestic chicken, Gallus gallus. J. Exp. Biol. 210, 2300–2310. ( 10.1242/jeb.004853) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 2009. Avian orientation: the pulse effect is mediated by the magnetite receptors in the upper beak. Proc. R. Soc. B 276, 2227–2232. ( 10.1098/rspb.2009.0050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko W., Dehe L., Stapput K., Thalau P., Wiltschko R. 2010. Magnetoreception in birds: no intensity window in ‘fixed direction’ responses. Naturwissenschaften 97, 37–42( 10.1007/s00114-009-0608-8) [DOI] [PubMed] [Google Scholar]
- Winklhofer M., Holtkamp-Rötzler E., Hanzlik M., Fleissner G., Petersen N. 2001. Clusters of superparamagnetic magnetite particles in the upper-beak tissue of homing pigeons: evidence of a magnetoreceptor. Eur. J. Miner. 13, 659–669. ( 10.1127/0935-1221/2001/0013-0659) [DOI] [Google Scholar]
- Yorke E. D. 1979. A possible magnetic transducer in birds. Theor. Biol. 77, 101–105. ( 10.1016/0022-5193(79)90140-1) [DOI] [PubMed] [Google Scholar]