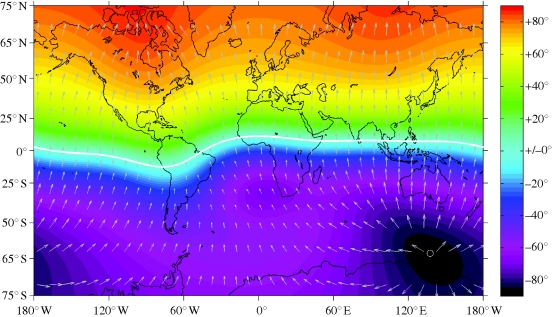
Research into magnetoreception explores the mechanisms and structures by which organisms can detect natural magnetic fields and use them for biologically relevant purposes. The old hypothesis that animals such as birds might use Earth's magnetic field as an orientation cue during migration or homing has been indirectly supported by a wealth of behavioural experiments that demonstrated the capability of various animals to extract directional information from the ambient magnetic field. The local magnetic field vector at any point on Earth can be represented by the total intensity, with directions given by declination and inclination; where declination is the deviation angle of magnetic north from true (geographical) north, reckoned positive eastward, and inclination is the dip angle of the magnetic field lines with respect to the horizontal, reckoned positive downward and negative upward. Owing to the essentially dipolar character of Earth's magnetic field, inclination values are generally positive on the Northern Hemisphere and negative on the Southern Hemisphere (figure 1). Pioneering laboratory experiments by Wiltschko & Wiltschko (1972) revealed a so-called inclination compass response in migratory birds, which depends on both the local magnetic north direction and inclination. The directional responses track a shift in local magnetic north direction for a given inclination I. However, a change of I to −I elicits a change in the preferred direction by 180° for a given magnetic north direction. The latter behaviour is fundamentally different from that of a technical magnetic compass. The inclination compass is invariant to inversion of the magnetic field vector, a transformation that flips the polarity of the field lines, but conserves their axial orientation in space. That a finite inclination is strictly required for the inclination compass to function was shown in experiments mimicking magnetic equator conditions (I = 0°), where migratory birds turned out to be disoriented (Wiltschko & Wiltschko 1972). Like the technical compass, the inclination compass is useless at the poles (I = ±90°), but becomes operational again as soon as the inclination changes from practically vertical (I = 89.7°) to near vertical (I = 88.6°) (Åkesson et al. 2001). This suggests that the detection limit for the horizontal field component is about 0.01 G in a background field of 0.6 G. Assuming a detection threshold of 0.01 G for the vertical field component, too, one can predict a minimum inclination of ±2° for the inclination compass to operate at near-equatorial latitudes, where the background field is about 0.3 G. A gap from −2° to 2° in the useful inclination interval would correspond to a latitudinal gap of ±1°, or ±110 km, either side of the magnetic equator. This figure is obtained from the dipole formula, tan I = 2 tan λ, where λ is magnetic latitude, i.e. the angular distance from the magnetic equator. It is obvious that transequatorial migrants need to rely on cues other than geomagnetic ones for orientation in this gap region. Indeed, birds and other animals seem to take advantage of a number of different environmental cues, which makes it difficult in general to assess the relative importance of geomagnetic cues for animals in the wild. With the aid of lightweight (less than a gram) radiowave transmitters, significant progress has been made recently towards identifying the role of magnetic orientation in free-flying migratory birds: a radio-tracking study of night-migratory birds exposed to either natural or shifted magnetic field during the twilight period before release suggests that these birds select the migratory direction on the basis of twilight cues provided by the sunset direction and/or by associated light polarization patterns, but then maintain their course using geomagnetic cues, obtained from the bird's internal magnetic compass that is calibrated daily from twilight cues (Cochran et al. 2004). For migratory birds that travel over intercontinental distances, covering hundreds of kilometres per night, daily calibration of the biological magnetic compass is a robust strategy for coping with spatial variations of the geomagnetic field owing to non-dipolar components, which otherwise might steer birds off course. Although the geomagnetic reference frame drifts only slowly with time (secular variation1), these changes accumulate from year to year, which again demonstrates the need for periodic calibration of the magnetic compass.
Figure 1.
Simplified representation of the geomagnetic field at Earth's surface, mapped on a Mercator projection. The arrows indicate the local magnetic north direction and total intensity (proportional to the arrow length). The dip angle of the field lines (inclination) are represented by colours. The thick white line delineates the magnetic equator, where inclination is zero. The white circle off East Antarctica shows the position of the south magnetic pole. The north magnetic pole is outside the map, roughly at 85° N, 133° W. Field distribution calculated from World Magnetic Model 2010, see http://www.geomag.bgs.ac.uk/navigation.html for more information and contour charts of the geomagnetic field elements and their annual rate of change. See the electronic supplementary material for dipolar and non-dipolar contributions to the geomagnetic field.
In spite of secular drift and regional variations in the local magnetic north direction, the Earth's magnetic field provides a consistent source of directional and latitudinal information on the global scale owing to its predominantly dipolar character, which it has had for most of the past 2 billion years, as indicated by palaeomagnetic studies on ancient rocks (Evans 2006). From an evolutionary point of view, the existence of a geomagnetic sense is therefore not surprising. That natural selection can bring about biological structures perfectly adapted to be sensitive to the comparatively weak geomagnetic field is evident from the example of magnetotactic bacteria, a polyphyletic group of motile microorganisms that swim along magnetic field lines. They biomineralize magnetosomes, i.e. intracellular membrane-enclosed ferrimagnetic crystals of magnetite Fe3O4 (or greigite Fe3S4), which have exactly the right grain size and arrangement to impart the maximum magnetic dipole moment to the cell body and to effectively align it with the geomagnetic field lines (Frankel et al. 1979). It is plausible that the successful principle of magnetosomes has evolved more than once in organisms and therefore the discovery of magnetic bacteria has triggered a lot of efforts to detect magnetosomes in animals (Kirschvink et al. 1985). Yet, it was not until about a decade ago that the first structural candidates of magnetite-based magnetoreceptors were identified; namely in the olfactory neuroepithelium in the nose of rainbow trout (Walker et al. 1997) and within nerve terminals in the subcutis of the upper beak of homing pigeons (Fleissner et al. 2003). Both candidates appear to be innervated by the ophthalmic branch of the trigeminal nerve, but while the magnetite crystals in fish have magnetic properties similar to the magnetosomes in magnetic bacteria (Diebel et al. 2000), the ones in the pigeon beak have different magnetic properties owing to their much smaller particle sizes (Hanzlik et al. 2000). Thus far, however, we do know enough about the structure and function of the magnetic sense to tie these differences in magnetic properties to potentially different magnetic sensor modalities in these animals.
Current research into magnetoreception is not all about magnetite as a primary agent of geomagnetic-field detection. On the contrary, a second hypothesis has gained rapidly in popularity with the influential paper by Ritz et al. (2000), who proposed the photoreceptor protein cryptochrome in the retina as candidate magnetoreceptor for mediating inclination compass information. Cryptochrome contains two pigment cofactors that upon light excitation may form a transient spin-correlated radical pair, whose reaction rates depend on the strength and axial orientation of the external magnetic field. At the same time, Weaver et al. (2000) have proposed a theoretical model for the transduction of the chemical rate constant of a radical-pair reaction into a nerve signal, assuming neural receptors for a ligand produced by the radical-pair reaction.
This themed collection of papers presents a broad yet faceted overview of current research activities in the vibrant field of magnetoreception. The interdisciplinary nature of the topic brings together experts from behavioural biology, biophysics, biochemistry, chemical physics, cell biology, cognitive science, magnetic materials science, geophysics, mineralogy, neuroscience, physical biology and physiology. Contributors to this Journal of the Royal Society Interface Theme Supplement actively participated in the conference entitled ‘Orientation and Navigation—Birds, Humans and Other Animals’ at Reading University in 2008, organized by the Animal Navigation Group of the Royal Institute of Navigation (RIN). The triennial RIN meeting is probably the most important venue for the magnetoreception community as a whole.
The radical-pair/cryptochrome hypothesis has propelled a great deal of research activities and half a dozen of the papers are directly concerned with this topic. Ritz et al. (2010) set the scene with their perspective article on photoreceptor-based magnetoreception. They approach from theoretical considerations the pivotal question of what generic chemical structure the compounds in a radical pair would ideally have in order to be sensitive to Earth-strength magnetic fields under physiological conditions and, more specifically, to be sensitive to the spatial orientation of the magnetic field lines. They then consider potential consequences of such optimally devised radical pairs on oxidative stress levels in cells and suggest possible transduction pathways and neural processing strategies for magnetic stimuli detected by photoreceptors. Liedvogel & Mouritsen (2010) summarize what is known and what is not known about cryptochrome in the context of magnetoreception and review all current experimental data that furnish correlative evidence in support of the radical-pair/cryptochrome hypothesis. They point out the challenges yet to be overcome in providing direct evidence for the involvement of cryptochrome in magnetic orientation and how these may be tackled in future studies.
As cryptochrome can be activated by short-wavelength light, a number of behavioural studies have been conducted to systematically investigate magnetic orientation responses under various light conditions. Wiltschko et al. (2010) survey the influence of mono- and bi-chromatic light of different wavelengths and intensity on magnetic orientation in migratory birds. The authors present a useful classification to juxtapose the characteristics of the two essentially different types of observed magnetic orientation behaviour, a normal inclination compass response and a fixed direction response, and discuss these in terms of the radical-pair and magnetite hypothesis, respectively. Kirschvink et al. (2010) depict an alternative model that may explain the light sensitivity of magnetic orientation responses in birds by a non-light-dependent, magnetite-based magnetoreception mechanism that secondarily interacts with other light-dependent processes. They make recommendations for fully blinding protocols in behavioural and physiological experiments so as to eliminate potential confounding by subtle differences between test and control conditions and propose fluorescence studies to find out whether or not cryptochrome in the avian retina is suitably anchored to be able to mediate a magnetic compass response.
The enormous number of behavioural studies stands in stark contrast to the few physiological attempts targeted at identifying neuronal responses to magnetic field stimuli. In their perspective article, Cadiou & McNaughton (2010) review structural, physiological and behavioural evidence for trigeminally mediated magnetoreception in birds and propose a number of powerful physiological techniques to directly observe responses of candidate magnetoreceptors to magnetic and mechanical stimuli. Implementing these tools should allow one to test and refine theoretical models that predict the magnetite clusters in the nerve terminals of the pigeon beak to respond to a magnetic stimulus by some displacement that in turn is to be transduced into a receptor potential by way of mechanosensitive elements elastically coupled to the magnetic structures, or, as Cadiou & McNaughton (2010) propose, by way of a secondary messenger that triggers a cascade of other processes that amplify the primary response. The authors also give an overview of various mechanosensitive ion channels and chemical compounds to inhibit these.
Besides birds, honey bees were among the first animals for which effects of magnetic fields on orientation were demonstrated. Wajnberg et al. (2010) review magnetoreception studies in a polyphyletic group of eusocial insects, such as ants, some bees and wasps, as well as termites. After giving an overview of magnetic alignment behaviour as well as untrained and trained orientation responses in these insects, the authors focus on the detection of iron-bearing minerals in body parts or tissue samples, based on magnetometry, electron paramagnetic resonance/ferromagnetic resonance absorption and electron microscopical techniques.
A number of research papers complete this Theme Supplement. The first two papers are concerned with the question of whether or not magnetic field perception in birds might be lateralized with respect to vision-mediated magnetic information. For this purpose, Hein et al. (2010) studied magnetic orientation performance in migratory songbirds with one or the other eye open. They also employ immunohistochemical methods to test for hemispherical differences in neuronal activity. Wilzcek et al. (2010) designed magnetic conditioning experiments with homing pigeons to find out whether lateralization of learned components in the orientation behaviour favours lateralization towards the left brain hemisphere.
Phillips et al. (2010) revisited light-dependent magnetic orientation in amphibians and insects and explore the possibility that the magnetic field may have antagonistic effects on photo-signalling pathways. They examine whether this phenomenon reflects light-sensitive magnetoreception processes in photoreceptors such as cryptochrome and link differences between light-absorption spectra of insect and vertebrate cryptochromes to differences in magnetic orientation between these two animal groups.
As mentioned above, a key requirement for a radical-pair-based magnetic compass is that the host proteins do not float about freely in the receptor cell, but are anchored to a larger structure that restricts their rotational motion and acts as a frame of reference for orientation-sensitive radical-pair reactions. It is not known yet how strictly this requirement would have to be implemented in an operable magnetoreceptor cell, but the following two theoretical papers provide important constraints. Lau et al. (2010) explored the tolerable amount of restricted rotational motion and molecular disorder for a model radical-pair compass to be viable. Hill & Ritz (2010) modelled the effect of molecular disorder on the performance of a radical-pair compass and estimate the minimum number of radical pairs required in a receptor cell to achieve a certain resolution in terms of directional and intensity variations of the magnetic field.
The last paper (Winklhofer & Kirschvink 2010) is concerned with the magnetite hypothesis and focused on magnetic torque transducer models, where elongated magnetic structures like a chain of magnetosomes respond to a magnetic field by restricted rotational motion about an elastic pivot. The authors theoretically analyse interactions of magnetosomes with cytoskeletal filaments and thermal fluctuations to derive constraints for possible transduction mechanisms involving mechanosensory elements such as ion channels.
We hope that this Theme Supplement conveys a sense of excitement about new developments in this research area that naturally crosses the divide between biological and physical sciences and thus is within the very scope of the Journal of the Royal Society Interface. The editors, in particularly Dr Tim Holt, are gratefully acknowledged for commissioning this Theme Supplement and their efficient handling of the manuscripts. Special thanks goes to all colleagues for their contributions.
Footnotes
See http://jupiter.ethz.ch/~cfinlay/gufm1.html for secular variation and animations showing the temporal evolution of the historical field between 1590 and 1990.
One contribution to a Theme Supplement ‘Magnetoreception’.
References
- Åkesson S., Morin J., Muheim R., Ottosson U. 2001. Avian orientation at steep angles of inclination: experiments with migratory white-crowned sparrows at the magnetic North Pole. Proc. R. Soc. Lond. B 268, 1907–1913. ( 10.1098/rspb.2001.1736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadiou H., McNaughton P. A. 2010. Avian magnetite-based magnetoreception: a physiologist's perspective. J. R. Soc. Interface 7 ( 10.1098/rsif.2009.0423.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran W. W., Mouritsen H., Wikelski M. 2004. Migrating songbirds recalibrate their magnetic compass daily from twilight cues. Science 304, 405–408. ( 10.1126/science.1095844) [DOI] [PubMed] [Google Scholar]
- Diebel C. E., Proksch R., Green C. R., Neilson P., Walker M. M. 2000. Magnetite defines a vertebrate magnetoreceptor. Nature 406, 299–302. ( 10.1038/35018561) [DOI] [PubMed] [Google Scholar]
- Evans D. A. D. 2006. Proterozoic low orbital obliquity and axial-dipolar geomagnetic field from evaporite palaeolatitudes. Nature 444, 51–55. ( 10.1038/nature05203) [DOI] [PubMed] [Google Scholar]
- Fleissner G., Holtkamp-Rotzler E., Hanzlik M., Winklhofer M., Petersen N., Wiltschko W. 2003. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J. Comp. Neurol. 458, 350–360. ( 10.1002/cne.10579) [DOI] [PubMed] [Google Scholar]
- Frankel R. B., Blakemore R. P., Wolfe R. S. 1979. Magnetite in freshwater magnetotactic bacteria. Science 203, 1355–1356. ( 10.1126/science.203.4387.1355) [DOI] [PubMed] [Google Scholar]
- Hanzlik M., Heunemann C., Holtkamp-Rotzler E., Winklhofer M., Petersen N., Fleissner G. 2000. Superparamagnetic magnetite in the upper beak tissue of homing pigeons. Biometals 13, 325–331. ( 10.1023/A:1009214526685) [DOI] [PubMed] [Google Scholar]
- Hein C. M., Zapka M., Heyers D., Kutzschbach S., Schneider N. L., Mouritsen H. 2010. Night migratory garden warblers can orient with their magnetic compass using the left, the right, or both eyes. J. R. Soc. Interface 7 ( 10.1098/rsif.2009.0376.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E., Ritz T. Can disordered radical-pair systems provide a basis for a magnetic compass in animals? J. R. Soc. Interface. 2010;7 doi: 10.1098/rsif.2009.0378.focus. ( ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschvink J. L., Jones D. S., McFadden B. J. 1985. Magnetite biomineralization and magnetoreception in organisms: a new biomagnetism. Topics in geobiology New York, NY: Plenum Press. [Google Scholar]
- Kirschvink J. L., Winklhofer M., Walker M. M. 2010. Biophysics of magnetic orientation: strengthening the interface between theory and experimental design. J. R. Soc. Interface 7 ( 10.1098/rsif.2009.0491.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J. C. S., Wagner-Rundell N., Rodgers C. T., Green N. J. B., Hore P. J. 2010. Effects of disorder and motion in a radical pair magnetoreceptor. J. R. Soc. Interface 7 ( 10.1098/rsif.2009.0399.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedvogel M., Mouritsen H. Cryptochromes—a potential magnetoreceptor: what do we know and what do we want to know? J. R. Soc. Interface. 2010;7 doi: 10.1098/rsif.2009.0411.focus. ( ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. B., Jorge P. E., Muheim R. 2010. Light-dependent magnetic compass orientation in amphibians and insects: candidate receptors and candidate molecular mechanism. J. R. Soc. Interface 7 ( 10.1098/rsif.2009.0459.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T., Adem S., Schulten K. 2000. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718. ( 10.1038/37057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T., Ahmad M., Mouritsen H., Wiltschko R., Wiltschko W. 2010. Photoreceptor-based magnetoreception: optimal design of receptor molecules, cells, and neuronal processing. J. R. Soc. Interface 7 ( 10.1098/rsif.2009.0456.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajnberg E., Acosta-Avalos D., Alves C. O., de Oliveira J. F., Srygley R. B., Esquivel D. M. S. 2010. Magnetoreception on eusocial insects: a review. J. R. Soc. Interface 7 ( 10.1098/rsif.2009.0382.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. M., Diebel C. E., Haugh C. V., Pankhurst P. M., Montgomery J. C., Green C. R. 1997. Structure and function of the vertebrate magnetic sense. Nature 390, 371–376. ( 10.1038/37057) [DOI] [PubMed] [Google Scholar]
- Weaver J., Vaughan T., Astumian R. 2000. Biological sensing of small field differences by magnetically sensitive chemical reactions. Nature 405, 707–709. ( 10.1038/35015128) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 1972. Magnetic compass of European robins. Science 176, 62–64. ( 10.1126/science.176.4030.62) [DOI] [PubMed] [Google Scholar]
- Wiltschko R., Stapput K., Thalau P., Wiltschko W. 2010. Directional orientation of birds by the magnetic field under different light conditions. J. R. Soc. Interface 7 ( 10.1098/rsif.2009.0367.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilzcek C., Wiltschko W., Güntürkün O., Wiltschko R., Prior H. 2010. Lateralization of magnetic compass orientation in pigeons. J. R. Soc. Interface 7 ( 10.1098/rsif.2009.0436.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklhofer, Kirschvink 2010. A quantitative assessment of magnetite-based torque transducers for magnetoreception. J. R. Soc. Interface 7 ( 10.1098/rsif.2009.0435.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]