Abstract
Cryptochromes have been suggested to be the primary magnetoreceptor molecules underlying light-dependent magnetic compass detection in migratory birds. Here we review and evaluate (i) what is known about these candidate magnetoreceptor molecules, (ii) what characteristics cryptochrome molecules must fulfil to possibly underlie light-dependent, radical pair based magnetoreception, (iii) what evidence supports the involvement of cryptochromes in magnetoreception, and (iv) what needs to be addressed in future research. The review focuses primarily on our knowledge of cryptochromes in the context of magnetoreception.
Keywords: orientation and navigation, magnetic compass, magnetic sense, bird migration, night-migratory birds
1. Introduction
A wealth of behavioural data indicate that the magnetic compass of migratory birds is light-dependent and that the eye is somehow involved in sensing the reference compass direction provided by the geomagnetic field (see box 1). Theoretical considerations have suggested that cryptochromes are likely to be the primary sensory molecules in this light-dependent magnetodetection mechanism, which has been suggested to be radical pair based. The radical pair model with a focus on the physical chemistry of the primary reception mechanism has recently been reviewed in detail by Rodgers & Hore (2009). Here, we aim to review (i) what is generally known about different types of cryptochromes in different taxa, (ii) what is currently known about the biochemical and physical properties of the photolyase/cryptochrome family, (iii) what functional roles of photolyases/cryptochromes have been documented or assumed, and finally (iv) what we still need to know about cryptochromes in order to determine whether cryptochromes act as magnetoreceptor in birds.
Box 1. Behavioural evidence—the magnetic compass in birds.
— In all bird species studied so far, the magnetic compass was shown to be an inclination compass (Wiltschko & Wiltschko 1972). An inclination compass has been documented for short- and long-distance migrants, birds of both hemispheres, and night, twilight and day migrants (Wiltschko & Wiltschko 1996).
— The birds' magnetic compass seems to be narrowly tuned to the total intensity of the ambient magnetic field (Wiltschko & Wiltschko 1972, 1978, 2001), but adaptable to changes in field strength exceeding those experienced during natural migration (Wiltschko & Wiltschko 1972; Wiltschko et al. 2006).
— Migratory birds seem to use head scans to detect the reference direction provided by the Earth's magnetic field during magnetic orientation (Mouritsen et al. 2004b).
— Experiments on free flying Catharus thrushes suggest that, in free flight, the magnetic compass is calibrated daily by sunset cues during twilight (Cochran et al. 2004).
— Magnetic orientation performance under low-intensity monochromatic light is wavelength-dependent: birds orient well under blue, turquoise and green, but are challenged under yellow or red light (Wiltschko et al. 1993, 2001; Wiltschko & Wiltschko 1995a, 1999, 2001, 2002; Munro et al. 1997; Rappl et al. 2000; Muheim et al. 2002).
— Experiments testing birds under higher intensities or a mixture of monochromatic light colours as well as experiments where birds had time to adapt to yellow or red light suggest that a rather complex relationship between receptors is involved in magnetoreception, since birds in some cases appear to show oriented responses that were not in line with the expected migratory direction and in some cases bipolar responses (Wiltschko et al. 2000, 2003, 2004a,b).
— Experiments with oscillating magnetic fields in the low radio-frequency range have been shown to disrupt magnetic orientation behaviour of migratory birds (Ritz et al. 2004, 2009; Thalau et al. 2005). These results provide the strongest, albeit indirect, evidence that the biophysical mechanism underlying the magnetic compass of birds involves a radical pair reaction.
— The pineal gland of birds is at least not crucial for magnetic compass orientation: birds with their pineal gland removed are still able to use their magnetic compass to orient when receiving injections of melatonin (Schneider et al. 1994).
— It is important to keep in mind that the magnetic compass interacts with celestial compass cues and that the magnetic compass is not essential for orientation. Birds are still able to orient if celestial but no magnetic cues are available (e.g. Mouritsen 1998).
2. Light-dependent radical pair based magnetoreception
In 1978, Schulten proposed that magnetic compass sensing might have spin chemical origins (Schulten et al. 1978; Schulten 1982). He suggested that the yield of a biochemical reaction proceeding via a radical pair might be sensitive to the orientation of an external magnetic field. Earth-strength magnetic fields influence correlated spin states of paired radicals because of anisotropy in the hyperfine interactions (i.e. magnetic coupling between an (unpaired) electron spin and a nuclear spin, e.g. 1H, 14N; Schulten 1982; Ritz et al. 2000; Weaver et al. 2000). Electron spins are not strongly coupled to the thermal bath (i.e. translational, rotational and vibrational motions of molecules) and therefore represent one of only a few molecular features that might plausibly be influenced by the Earth's magnetic field (Edmonds 2001). The suggested mechanism involves a light-induced electron transfer between two molecules. This electron transfer must result in the generation of a radical pair intermediate that can either exist in a singlet or a triplet excited state (the state depends on the spin correlation of the unpaired electrons being either antiparallel (↑↓) in the singlet state or parallel (↑↑) in the case of a triplet excited state). In a radical pair mechanism the magnetic field can alter the rates and product yields of these two naturally interconverting radical intermediate states depending on the orientation of the radical within the field and thus influence the proportion of biochemically different singlet or triplet end products (Ritz et al. 2000).
Both theoretical calculations (Eichwald & Walleczek 1996; Ritz et al. 2000) and in vitro experiments (Harkins & Grissom 1994, 1995; Brocklehourst 2002; Timmel & Henbest 2004; Henbest et al. 2008; Maeda et al. 2008) showed that the ratio between singlet and triplet products from radical pair reactions can be modulated by an Earth-strength magnetic field, thereby potentially providing the basis for a magnetic compass. The effect does not necessarily affect differences in singlet–triplet yields, a simple ‘delay’ in the reaction kinetics (i.e. a variation of the lifetime of the radical pair intermediate) would be sufficient to specifically alter the reaction depending on the ambient magnetic field. This proposal has been revived by Ritz et al. (2000), who proposed that the retina with its almost perfect half-ball shape is well suited as an ordered structure, and that the radical pair intermediate is involved in some kind of visual reception system. According to this theory, the reaction yield anisotropy of the receptor radical pair governs the directional response. For further details on the radical-based magnetoreception hypothesis, see Ritz et al. (2010).
3. Required characteristics of the putative primary magnetic sensory molecules
For a spin chemical receptor mechanism located in the bird's eye to detect the direction of magnetic fields as weak as that of the Earth's, several stringent conditions must be met (Grissom 1995; Adair 2000; Ritz et al. 2000; Weaver et al. 2000; reviewed in detail in Rodgers & Hore 2009): (i) any suggested candidate molecule must exist in the retina of migratory birds, (ii) the cells containing the candidate molecule have to be active at night, when the bird performs magnetic orientation, (iii) motion of the radical pair forming molecule has to be restricted, i.e. the molecule must somehow be at least partly fixed in the cell, (iv) the molecule has to be excited by light, (v) the absorption spectrum has to match the range that has been shown to elicit magnetic orientation performance in behavioural experiments (Wiltschko et al. 1993, 2000, 2001, 2003, 2004a,b; Wiltschko & Wiltschko 1995a, 1999, 2001, 2002; Munro et al. 1997; Rappl et al. 2000; Muheim et al. 2002), (vi) the initial electron transfer must not randomize the original parallel or opposite spin relationship of the two electrons, i.e. conservation of spin angular momentum is crucial, which is not true for all electron transfer processes but is often the case when the transfer is induced by photo-excitation (Ritz et al. 2000), (vii) the half-life of the light-excited radical pair intermediates including the spin correlation of the two electrons has to persist long enough (more than 1 µs) to allow the Earth's magnetic field to modulate the singlet triplet interconversion of the radical pair, (viii) because the influence of the Earth's magnetic field on the putative chemical processes is rather weak, the effect must presumably be integrated over a large area, and (ix) a suitably sensitive transduction of the changing product yields is necessary (e.g. one reaction product might be a neurotransmitter, the other might not; or one end-product interfering with the sensitivity of a visual receptor, the other not).
Considering that the putative light sensitive magnetoreceptor should be located in the eye, the visual system might be used for transduction. This allows for the distinct possibility that birds may ‘see’ the Earth's magnetic field (Ritz et al. 2000; Mouritsen et al. 2005; Heyers et al. 2007; Liedvogel et al. 2007a; Zapka et al. 2009).
4. Cryptochrome
In their key theoretical paper, Ritz et al. (2000) suggested that the blue-light receptor cryptochrome would be a promising candidate for a photo-magnetoreceptor for the following reasons: cryptochromes which have been discovered in plants, birds and other animals are the only known class of photoreceptor molecules found in vertebrates that have been shown to be able to form a radical pair upon photoexcitation, at least in some organisms (e.g. Liedvogel et al. 2007a; Biskup et al. 2009).
Cryptochromes were first discovered as blue-light and ultraviolet (UV-A) photoreceptors in plants (Ahmad & Cashmore 1993). Cryptochromes share moderate sequence (but significant structural) similarity to photolyases, which are flavin containing proteins that catalyse the repair of UV light-damaged DNA via electron transfer mechanisms, but cryptochromes do not have photolyase activity (Hsu et al. 1996; Öztürk et al. 2007). It is widely accepted that cryptochromes are probably the evolutionary descendents of DNA photolyases (Cashmore et al. 1999).
The first cryptochrome encoding gene to be identified was Arabidopsis HY4 (Ahmad & Cashmore 1993). The gene product (a protein of 681 amino acids) was named Cry1 from ‘cryptochrome’ previously proposed for ‘cryptic’ blue-light receptor (Gressel 1979). Cryptochromes have now been found in other plants (e.g. Small et al. 1995; Cashmore 1997; Imaizumi et al. 2000, 2002; Xu et al. 2009) and in various animal lineages, including cubozoa (Brinkman & Burnell 2007), insects (e.g. Todo et al. 1996; Emery et al. 1998; Egan et al. 1999; Rosato et al. 2001; Zhu et al. 2005, 2008), fish (e.g. Kobayashi et al. 2000; Levy et al. 2007), amphibians (e.g. Zhu & Green 2001), birds (e.g. Bailey et al. 2002; Mouritsen et al. 2004a; Möller et al. 2004; Helfer et al. 2006) and mammals (e.g. Hsu et al. 1996; Todo et al. 1996; Kobayashi et al. 1998; Griffin et al. 1999; Kume et al. 1999; van der Horst et al. 1999).
Cryptochromes are implicated in multiple blue-light-dependent signalling pathways in plants and animals (extensively reviewed in Cashmore et al. 1999; Deisenhofer 2000; Sancar 2000, 2003, 2004; Ahmad 2003; Bouly et al. 2003; Lin & Todo 2005). In plants, cryptochromes control different aspects of growth and development, i.e. involvement in de-etiolation responses such as inhibition of hypocotyl growth (Ahmad & Cashmore 1993; Lin et al. 1995, 1998; Lin 2002), anthocyanin accumulation (Ahmad et al. 1995) in leaf and cotyledon expansion (Cashmore et al. 1999; Lin 2002; Lin & Shalitin 2003), transitions to flowering (El-Din El-Assal et al. 2003) or regulation of blue-light regulated genes (Jiao et al. 2003).
In animals, cryptochromes have been shown to play a direct role in circadian rhythms as components of the circadian pacemakers (Miyamoto & Sancar 1998; van der Horst et al. 1999; Sancar 2000, 2004; Shearman et al. 2000; Zhu et al. 2008). In Drosophila, cryptochromes seem to be more indirectly involved by feeding light information into the circadian clock (Stanewsky 2002; Busza et al. 2004) and recent experiments have suggested a cryptochrome-dependent magnetic sensitivity of the Drosophila circadian clock (Yoshii et al. 2009), which may stimulate future research in the field of light-dependent magnetoreception. In monarch butterflies Danaus plexippus vertebrate-like cryptochrome (Cry2; in addition to Drosophila-like cryptochrome (Cry1) that probably functions as a blue-light photoreceptor for circadian clock entrainment) was suggested to function as a core clock element. In the monarch butterfly's brain, Cry2 may even have an additional role as an output that regulates circadian activity in the central complex (a topographically ordered neuropil structure in the centre of the insect brain), the putative location of the sun compass (Zhu et al. 2008). In migratory birds, cryptochrome is mainly discussed as putative receptor molecule for light-dependent magnetic compass orientation. Most research in the field of migratory bird orientation is carried out on the two model organisms European robin Erithacus rubecula and garden warbler Sylvia borin (figure 1a). Today four different members of the cryptochrome family have been identified in migratory birds (Möller et al. 2004; Mouritsen et al. 2004a; see paragraph on the identification of cryptochromes in the retina of migratory birds), but the functional role of migratory bird cryptochromes still remains to be determined with any certainty. A further type of cryptochrome, distinct from ‘classic’ plant (Arabidopsis HY4) or animal (Drosophila or Homo sapiens) cryptochromes, though more closely resembling the latter (Brudler et al. 2003), was first identified in cyanobacteria Synechocystis sp. (Hitomi et al. 2000). On the basis of sequence alignment these DASH-cryptochromes (‘DASH’ to indicate the relationship with cryptochromes found in Drosophila, Arabidopsis, Synechocystis and Homo) were grouped together with the animal cryptochromes and (6-4) photolyases, but the perception of this class of proteins is not well established yet, and is currently undergoing a shift, as recent data show that DASH-cryptochromes are single strand DNA photolyases (Kleine et al. 2003; Selby & Sancar 2006; Pokorny et al. 2008). Recent results indicate that DASH-cryptochromes could work as transcriptional regulators (Brudler et al. 2003; Daiyasu et al. 2004), DNA repair enzymes for single-stranded DNA (Selby & Sancar 2006), and they have further been suggested to participate in circadian input pathways (Facella et al. 2006; Brunelle et al. 2007).
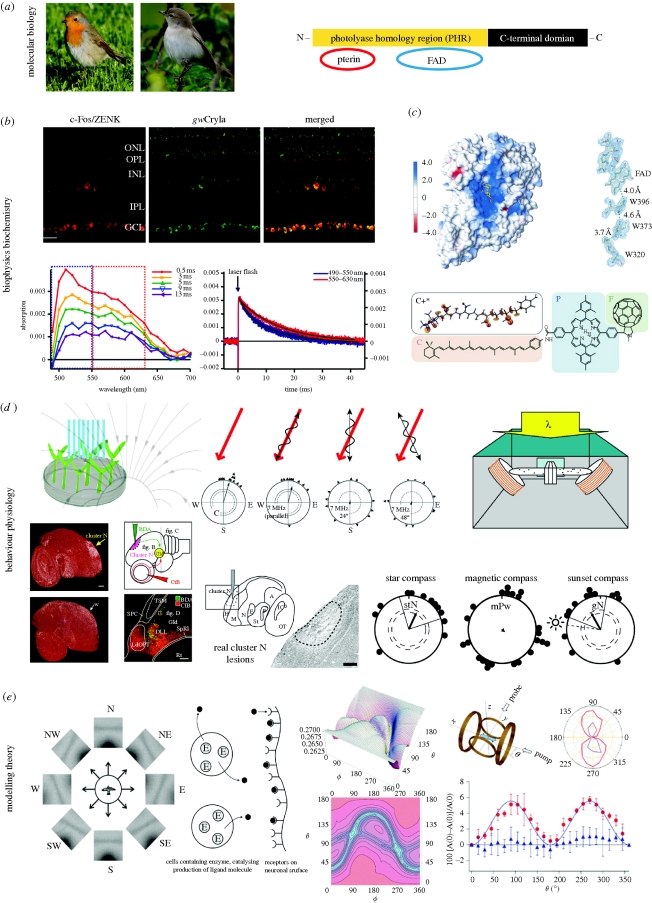
Figure 1.
Key evidence suggesting that cryptochromes could function as magnetoreceptors. (a) Photos (©Henrik Mouritsen) show the two migratory model bird species from which, so far, four different members of the cryptochrome multigene family have been detected in the retina (Möller et al. 2004; Mouritsen et al. 2004a; and box 2 of this paper). The schematic illustration indicates the structure of a typical cryptochrome protein (redrawn after Lin & Todo 2005). (b) Cryptochrome has been shown to be expressed in retinal cells that are neuronally active when the birds perform magnetic orientation. The upper panel shows immunohistochemistry staining of a cross section of a garden warbler retina, indicating from left: the expression pattern of neuronal activity marker protiens (c-Fos/ZENK, see main text for details on the use of immediate early genes as neuronal activity markers) in red, the cryptochrome protein expression pattern (which probably included signal from several cryptochromes and not just from gwCry1a as originally thought) in green, and the merged picture which shows colocalization (yellow) of both proteins (reprinted from Mouritsen et al. (2004a). Copyright (2004) National Academy of Sciences, USA). The two graphs show the transient absorption spectra (bottom left) and absorption time profiles (right) of garden warbler cryptochrome 1a. The radical decay rates (blue and red curve, bottom right) indicate that garden warbler cryptochromes form long lived radical species (half-life approx. 10 ms) (reprinted from Liedvogel et al. (2007a)). (c) The first cryptochrome structure presented was cryptochrome-DASH of Synechocystis sp. (reprinted from Brudler et al. (2003). Copyright (2003) with permission from Elsevier (copyright-hyperlink for version published electronically: http://www.cell.com/molecular-cell)); the conserved electron transfer chain is shown at the right. The structure of a migratory bird (or any other animal) cryptochrome remains to be solved. The lower part of (c) shows the structure of the carotenoid–porphyrin–fullerene model system that was designed to demonstrate the feasibility of chemical magnetoreception (reprinted from Maeda et al. (2008). Copyright (2008) with permission from Nature (copyright-hyperlink for version published electronically: http://www.nature.com)). (d) Top left: observations of light-dependent, cryptochrome mediated magnetic field effects on plant growth have been claimed (Ahmad et al. 2007), but unfortunately these findings could not be replicated in a blinded study (Harris et al. 2009; figure redrawn after Ahmad et al. 2007). Top centre: further evidence supporting a radical pair based magnetic compass comes from the observation that weak radiofrequency magnetic fields seem to disrupt the ability of birds to orient by the Earth's magnetic field (Ritz et al. 2004, 2009, 2010; Thalau et al. 2005). The red arrow indicates the orientation of the Earth's magnetic field. The wavy lines indicate the orientation of the oscillating field applied. The circular diagrams show the magnetic orientation of European robins in the magnetic field scenario indicated above the diagram. Each triangle at the circle periphery indicates the mean orientation of an individual bird based on three tests under the given magnetic condition. Arrows indicate the group mean vectors. Inner and outer dashed circles indicate the radii of the group mean vectors needed for directional significance according to the Rayleigh test (inner, p < 0.05; outer, p < 0.01) (redrawn after Ritz et al. (2004). Copyright (2004) with permission from Nature (copyright-hyperlink for version published electronically: http://www.nature.com)). Top right: conditioning experiments on Drosophila fruit flies (setup shown) have recently suggested that a cryptochrome-based magnetosensitive system may also exist in this animal (reprinted from Gegear et al. (2008). Copyright (2008) with permission from Nature (copyright-hyperlink for version published electronically: http://www.nature.com)). Bottom left: in migratory birds, a forebrain area (cluster N) has been identified, which shows light-dependent, movement-independent, neuronal activation at night time when migratory birds performed magnetic orientation under dim light (sagittal brain section through centre of cluster N showing neuronal activation pattern as indicated by ZENK mRNA expression (white signal) at night in a migratory (upper photo) and non-migratory bird (lower photo); Mouritsen et al. 2005; Liedvogel et al. 2007b; Feenders et al. 2008; reprinted from Mouritsen et al. (2005). Copyright (2005) National Academy of Sciences, USA). Bottom centre: a tracing study documented a functional connection between the retinal ganglion cells and cluster N via the thalamofugal visual pathway (schematic drawing). The photo shows that in the visual thalamus, red axons projecting from the eye meet with green backfilled neurons projecting from the thalamus to cluster N (reprinted from Heyers et al. (2007)). Bottom right: the photo shows an example part of a sagitally cut brain section through the centre of cluster N from a cluster N lesioned European robin, stained with a neuroanatomical marker. Notice that the tissue where cluster N should be located is destroyed due to the lesion. The circular diagrams show that cluster N lesioned birds tested in a planetarium simulating the local starry sky (stN, star north) still orient towards the typical north-northeast spring migratory direction, that birds with cluster N lesions could not orient using their magnetic compass (mPw, magnetic poleward), and that birds with cluster N lesions could also orient during sunset, presumably using their sun compass (gN, geographical north). Radial lines indicate the 95% confidence intervals (figure redrawn after Zapka et al. (2009). Copyright (2009) with permission from Nature (copyright-hyperlink for version published electronically: http://www.nature.com)). (e) Theoretical work has shown that, in principle, a radical pair based mechanism could form the basis of magnetic compass orientation (e.g. Ritz et al. 2000; Weaver et al. 2000; Cintolesi et al. 2003; Solov'yov et al. 2007). Left: theoretical visual modulation patterns by the Earth's magnetic field for a bird flying parallel to the horizon looking towards different directions, assuming radical pair receptors oriented perpendicularly to the retina's surface in the bird's eye (reprinted from Ritz et al. (2000). Copyright (2000), with permission from Elsevier (copyright-hyperlink for version published electronically: http://www.cell.com/biophysj)). Centre left: a model for an enzyme (E)-catalysed magnetic field sensitive radical pair reaction. The catalytic reaction produces a ligand (black circles) that leaves the cell and binds to a receptor attached to neural tissue. Subsequent signal transduction depends on the equilibrium between free and unbound ligand (reprinted from Weaver et al. (2000). Copyright (2000) with permission from Nature (copyright-hyperlink for version published electronically: http://www.nature.com)). Centre right: theoretical calculations of radical pair reaction product yields: the picture shows a simulation of the orientation dependence of singlet recombination probability for a flavin–tryptophan radical pair under Earth-strength magnetic fields (B0 = 50 µT, the lifetime of the radical pair is 5µs); orientation dependence is shown both as a three-dimensional representation (top) and as a contour plot (bottom). The direction of the applied magnetic field with respect to the radical pair is defined in terms of the polar angles ϕ and θ (reprinted from Cintolesi et al. (2003). Copyright (2003) with permission from Elsevier (copyright-hyperlink for version published electronically: http://www.elsevier.com/locate/chemphys)). Right: illustration of the chemical compass model (also see c, bottom) providing the proof of principle that a light-induced, radical pair process in a chemical molecule can be sensitive to Earth-strength magnetic fields (Maeda et al. 2008). Right, top left: the experimental setup used to measure the anisotropy of the magnetic field effect: the direction of the applied magnetic field is generated by two orthogonal pairs of Helmholtz coils; the sample alignment along the x-axis is shown by the blue arrow; ϕ is the angle between the magnetic field vector and the x-axis; continuous probe light and pump laser pulses spread along the x and y axes. Top right: a polar plot of the anisotropy of the magnetic field effect as a function of ϕ is plotted for an aligned sample (purple), and (red) by photoselection (i.e. orientation-selective excitation of chromophores by photon absorption). Right, bottom: absorption data from the photoselection measurements (red dots) are plotted as a function of the direction of the applied magnetic field ϕ. As a control, the blue dots confirm that no angular dependence is present in a scenario when the polarization axis of the probe light is vertical (i.e. along z-axis; reprinted from Maeda et al. (2008). Copyright (2008) with permission from Nature (copyright-hyperlink for version published electronically: http://www.nature.com)).
5. Structural characteristics of cryprochromes
The state of the art on structural biology of photolyases and cryptochromes has recently been reviewed by Müller & Carell (2009), and will therefore not be covered in detail here. In brief, several structures of photolyases (Park et al. 1995; Tamada et al. 1997; Komori et al. 2001; Kort et al. 2004; Mees et al. 2004; Klar et al. 2006; Fujihashi et al. 2007; Maul et al. 2008; Glas et al. 2009; Hitomi et al. 2009) and cryptochromes (Brudler et al. 2003; Brautigam et al. 2004; Huang et al. 2006; Klar et al. 2007) are available today, but the structure of an animal cryptochrome remains to be solved (functional and structural data available for different photolyases/cryptochromes in the context of chemical magnetoreception are summarized in table 1). The defining characteristics of cryptochromes are N-terminal domains with marked sequence similarity to DNA photolyases (Deisenhofer 2000; Brautigam et al. 2004). Within the amino-terminal photolyase homology region (PHR), cryptochromes and photolyases have similar three-dimensional structures, characterized by an N-terminal α/β domain and a C-terminal α-helical domain. A further defining characteristic of both plant and animal cryptochromes are C-terminal extensions of varying size, not present in photolyases (see figure 1a for a schematic sketch of cryptochrome domains). This extension is longer in most plant cryptochromes than in animal cryptochromes and is generally less conserved than the PHR region. The C-terminal domain is likely to be essential for a number of cryptochrome-specific functions, i.e. in Arabidopsis Cry1 it mediates the signalling mechanism of constitutive blue-light response (Yang et al. 2000). Cry-DASH proteins/ssDNA–photolyases lack this domain (Lin & Shalitin 2003).
Table 1.
Functional and structural evidence on photolyase/cryptochrome proteins. The table lists all photolyase and cryptochrome proteins from different species where functional information with respect to a putative role of cryptochromes as magnetoreceptor and/or structural information are available. Experimental data in the context of magnetosensitive responses and/or the detection of radical pair reactions both in vitro and in vivo are indicated. CPD = cyclobutane pyrimidine dimer; PHR = photolyase homology region.
| species | protein | structure resolved? | radical pair mechanism/magnetic field effect? | reference |
|---|---|---|---|---|
| Anacystis nidulans | AnCPD-PHR | + | Tamada et al. (1997) | |
| Mees et al. (2004) | ||||
| Kort et al. (2004) | ||||
| Arabidopsis thaliana | At(6-4)-PHR | + | Hitomi et al. (2009) | |
| AtCry1 | + | physiological cry-mediated magnetosensitive response (500 µT) | Giovani et al. (2003) Brautigam et al. (2004) Ahmad et al. (2007) | |
| Harris et al. (2009) | ||||
| AtCry2 | Banerjee et al. (2007) | |||
| AtCry3 | + | Song et al. (2006) | ||
| Huang et al. (2006) | ||||
| Klar et al. (2007) | ||||
| Drosophila melanogaster | d(6-4)-PHR | + | Maul et al. (2008) Glas et al. (2009) | |
| dCry | behavioural cry-based magnetosensitive response (500 µT) | Berndt et al. (2007) Gegear et al. (2008) | ||
| Escherichia coli | EcCPD-PHR | + | + | Li et al. (1991) |
| (39 mT) | Park et al. (1995) | |||
| Aubert et al. (2000) | ||||
| Henbest et al. (2008) | ||||
| Sulfolobus tokodaii | StCPD-PHR | + | Fujihashi et al. (2007) | |
| Synechocystis sp. | Cry DASH | + | Brudler et al. (2003) | |
| Thermus thermophilus | TtCPD-PHR | + | Komori et al. (2001) | |
| Klar et al. (2006) | ||||
| Xenopus laevis | XlCry-DASH | Biskup et al. (2009) | ||
| Sylvia borin | gwCry1a | radical pair formation (transient absorption) | Liedvogel et al. (2007a) | |
| model chemical compass | carotenoid–porphyrin–fullerene model system | + (≤50 µT) | Maeda et al. (2008) |
The protein structure of cryptochromes includes two non-covalently bound cofactors: flavin adenine dinucleotide (FAD), which is essential for catalysis, and a second light-harvesting chromophore. Due to their sequence- and structural similarities to photolyases, the second chromophore of cryptochromes is assumed to be either an 8-hydroxy-5-deazariboflavin (8-HDF) or a 5,10-methenyltetrahydrofolate (MTHF; Malhotra et al. 1995; Hsu et al. 1996). In photolyases, the role of the second chromophore as an antenna, harvesting light to initiate the photoreaction process is well established (e.g. Klar et al. 2007). Because there is spectral overlap between the absorption spectra of the flavin and antenna molecule fluorescence in this two-chromophore system, the antenna molecule can transfer excitatory energy to the catalytic cofactor (Jorns et al. 1990; Park et al. 1995). This Förster-type energy transfer occurs from the short-wavelength absorbing donor (the antenna molecule) to the long-wavelength absorbing catalytic cofactor. The energy transfer efficiency depends on the distance between donor and acceptor molecule and has been shown to be very high (70–100%; Payne & Sancar 1990; Kim et al. 1992) in photolyases. For Arabidopsis Cry3 (DASH type), it was calculated to be between 78 and 87 per cent (Song et al. 2006). The FAD-access cavity of the helical domain is the catalytic site of photolyases and is also predicted to be important in the mechanism of cryptochrome functions (for a review see Lin & Todo 2005). Electron transfer between the FAD cofactor and the cryptochrome molecule is an essential part of the suggested radical pair mechanism (Ritz et al. 2000). Even though cryptochromes bind similar cofactors to photolyases, they lack DNA repair activity (Lin et al. 1995; Malhotra et al. 1995) suggesting evolution of novel functions of the cryptochromes' role in signalling.
Despite high topological similarities and apparent functional analogy, plant and animal cryptochromes seem to have evolved independently from different ancestral photolyases, with animal cryptochromes more similar to type 6-4 photolyases and plant cryptochromes sharing greater sequence similarity with type I microbial photolyases (Kanai et al. 1997).
6. Experimental evidence supporting the involvement of cryptochromes in magnetic sensing
6.1. Identification of cryptochromes in the retina of migratory birds
Explanation and deeper understanding of the previously obtained behavioural results (see box 1) supporting an involvement of a light-induced radical pair magnetoreception mechanism inevitably require the presence of a radical pair forming photopigment in the eye of the migratory bird (prerequisite (i)). Therefore, the experimental demonstration of the presence of cryptochromes in the retina of night-migratory songbirds (Möller et al. 2004; Mouritsen et al. 2004a) was the key prerequisite and set the scene for all subsequent experiments. Today, four members of the cryptochrome family have been identified in migratory songbirds: Cry1a and Cry1b, Cry2 (Möller et al. 2004; Mouritsen et al. 2004a; Liedvogel et al. 2007a) and Cry4 (new sequence information presented here; see box 2 and figure 2).
Box 2. Identification and cloning of a partial coding sequence of Cry4, a new potentially magnetosensitive cryptochrome candidate from the migratory garden warbler [GenBank accession no. GQ896539].
We identified a new member of the cryptochrome multigene family, which is expressed in the retina of garden warblers. This is the fourth type of cryptochrome molecule to be found in migratory birds; Cry1a, Cry1b and Cry2 have previously been identified in European robins (Möller et al. 2004) and garden warblers (Mouritsen et al. 2004a).
Methods
RNA isolation and cDNA synthesis from garden warbler retina was carried out according to Mouritsen et al. (2004a). To detect the additional and so far unknown Cry4 of the cryptochrome multigene family in garden warbler, we designed degenerate primers from publicly available sequence information of cryptochromes from several taxa using a cloning strategy similar to the one described earlier for identification of garden warbler Cry1a, Cry1b and Cry2 (Mouritsen et al. 2004a). Forward primer (0.8 µl), 5′-AGCACGTCGACGAACCCCATCTGCATCCA-3′ and reverse primer (0.8 µl) 5′-ACGAAGAATTCGACGAAAGCCACATCCAG-3′ were used for polymerase chain reaction (PCR) amplification with conditions described in Mouritsen et al. (2004a); primer sequences were derived from a sequence alignment of cryptochrome 2 cDNAs from mouse [NM009963], human [NM021117], chicken [AY034433]. The same primer combination was used for detection of partially coding sequence for Cry2 [AY739908] (Mouritsen et al. 2004a). The additional amplification of Cry4 sequence reported here can be explained by cross-priming of the oligonucleotide pair at sequences conserved between Cry 2 and Cry 4. The resulting 237 bp long PCR product was cloned into pTBlue-3 by means of the Perfectly Blunt cloning kit (Novagen) and transformed into NovaBlue cells. Plasmid purification and sequencing was carried out as described in Mouritsen et al. (2004a). DNA similarities and identity scores were analysed by WU-BLAST2 and FASTA algorithms.
Results
The amplified partially coding sequence (see figure 2) was confirmed as being derived from garden warbler cryptochrome 4 (gwCry4 [GQ896539]). WU-BLAST2 and FASTA algorithms reveal closest homology with three other avian cryptochrome 4 sequences published: the partial coding sequence of garden warbler Cry4 reveals 95% sequence identity score with cryptochrome 4 from the house sparrow Passer domesticus [AY494987] and the predicted Cry4 sequence of zebra finch Taeniopygia guttata [XM_002198497], both belonging to the songbirds as does the garden warbler, and 89% identity with the established sequences of chicken [AY102068]. In comparison, the identity scores of the newly amplified cryptochrome fragment with garden warbler Cry1a [AJ632120], Cry1b [DQ838738] and Cry2 [AY739908] are 76% (in all cases) and 75%, respectively, for both Cry1a [AY585716] and Cry1b [AY585717] from European robins, which confirms the identity of the amplified fragment being cryptochrome 4 (gwCry4). This is the fourth type of cryptochrome molecule identified for the migratory garden warbler.
Figure 2.
Partial sequence alignment of Cry4 partially coding DNA sequence of the migratory garden warbler Sylvia borin [GQ896539], house sparrow Passer domesticus [AY494987], chicken Gallus gallus [AY102068] and zebra finch Taeniopygia guttata [XM_002198497]. The alignment shows that Cry 4 sequence is highly homologous across species, a common feature of members of the cryptochrome/photolyase family.
6.2. Location of cryptochromes within the retina of birds
Immunohistochemical stainings have shown that garden warbler Cry1 (gwCry1) is predominantly expressed in the cytosol of ganglion cells (the retinal cell class which communicates information from the eye to the brain), large displaced ganglion cells (retinal ganglion cells that are not located in the ganglion cell layer but in the inner half of the so-called inner nuclear layer; in pigeons, the displaced ganglion cells have been shown to project preferentially to the nucleus of the basal optic root, a part of the accessory optic system; Karten & Finger 1976; Karten & Fite 1977), and in photoreceptor cells (Mouritsen et al. 2004a). From the cell-anatomical point of view, the membrane structures of the photoreceptor cell outer segment discs and associated structures or the inner segments, which each provide a oriented cylindrical membrane would provide ideal substrates for the highly oriented ensemble of molecules required for a radical pair based magnetoreception mechanism. Therefore, we consider the cryptochromes located in the photoreceptor cells to be the most probable magnetoreceptor candidates. Another interesting finding is the high cytosolic/membrane immunoreactivity of gwCry1 in ganglion cells and photoreceptor cells (Mouritsen et al. 2004a). This rather hints towards a putative role of these cryptochromes as magnetic compass detectors than towards a function as a control element of the internal circadian clock, since the cryptochromes would have to get to the nucleus in order to perform a regulatory clock function.
Also, the cryptochrome expression levels significantly differed between migratory and non-migratory birds at night (Fu et al. 2002; Haque et al. 2002; Mouritsen et al. 2004a,b): during the day, high levels of cryptochrome are found both in migratory and non-migratory species (Mouritsen et al. 2004a,b). During night time, cryptochrome levels in non-migratory zebra finches drop to background expression levels, whereas cryptochrome expression levels in migratory garden warblers at night are as high as or even higher than during the day (Mouritsen et al. 2004a). This finding of high cryptochrome expression during the night time seems to be a special adaptation in migratory birds.
To be potentially relevant for magnetoreception, it is important to show that at least some cryptochrome containing retinal cells are active at night when the garden warblers perform magnetic orientation (prerequisite (ii)). This could be demonstrated by mapping the neuronal activity pattern of retinal cells using neuronal activity marker genes ZENK (acronym for the gene known in other species as zif-268, egr-1, NGF-IA and krox-2) and c-Fos (Mello et al. 1992; Jarvis & Nottebohm 1997; Mouritsen et al. 2004a), both of which are known to show vision-dependent expression in the retina of many animals (Dragunow & Faull 1989; Yoshida et al. 1993; Araki & Hamassaki-Britto 1998; Fisher et al. 1999) including chicken (Fisher et al. 1999), and their expression requires neuronal activity (Worley et al. 1991; Chaudhuri et al. 1995; see figure 1b, upper panel).
In magnetic orientation experiments on garden warblers during night time, both activity marker genes co-localized with cryptochromes in most ganglion cells and large displaced ganglion cells, which are the cell types sending visual information from the eye to the brain. This diagnostic tool for visualizing cells' activity status does not work in retinal photoreceptor cells as they do not communicate via axon potentials, but it is probable that at least some photoreceptor cells are active when the ganglion cells are highly active.
Cryptochromes could be directly linked to the visual pathway and there is growing evidence showing that cryptochrome expressing retinal cells and ‘cluster N’, a vision-dependent forebrain region that shows high levels of neuronal activation during night time when birds perform magnetic orientation under dim light (Mouritsen et al. 2005; Feenders et al. 2008), are connected via the thalamofugal pathway, which is one of the three well-known visual pathways in birds (Heyers et al. 2007). Cluster N shows characteristic light-dependent, movement-independent neuronal activation at night time in migratory birds (Mouritsen et al. 2005; Liedvogel et al. 2007b; Feenders et al. 2008), and lesioning of cluster N disrupts the magnetic compass orientation capabilities of migratory European robins (Zapka et al. 2009). The robins with lesioned cluster N could still perform star compass orientation and sun compass orientation showing that cluster N is required for magnetic compass orientation only (Zapka et al. 2009). Since cluster N is part of the visual system of birds, these findings strongly support the idea of a light-dependent, vision-mediated magnetoreception and is in line with an involvement of a photoreceptor molecule like cryptochrome in magnetic compass information detection (figure 1d).
6.3. Biophysical characterization of bird cryptochromes and related molecules
To investigate the putative involvement of cryptochrome in a light-mediated magnetic compass based on a radical pair reaction, the protein chemistry and biophysical properties of cryptochromes must be deciphered in order to understand the potential and suitability of cryptochrome as a magnetic field detector. Do cryptochromes form spin-correlated radical pairs with coherent singlet–triplet mixing that can be influenced by the Earth's magnetic field? Experiments focusing on these questions are based on chemical and biophysical characterization of recombinantly expressed proteins from various species. In photolyases, the light-induced reduction of the FAD cofactor (photoreactivation process) triggers subsequent electron transfer along a cascade of three uniformly conserved tryptophans followed by proton uptake (Aubert et al. 2000). This motif is conserved in all structurally characterized photolyases/cryptochromes and it is probable that this or a similar electron transfer also takes place in animal cryptochromes (see below), but this still needs to be experimentally validated. Compared with photolyases, we know relatively little about the photoreaction process in cryptochromes, particularly animal cryptochromes.
An alignment of Cry1a and Cry1b (Cry1a,b are alternative splicing products; Möller et al. 2004) of garden warbler and European robin with published sequences of the photolyase/cryptochrome family shows that the three tryptophane residues (Trp382, Trp359 and Trp306 in Escherichia coli photolyase) involved in the electron transfer pathway for photoreduction in E. coli photolyase (EcPL; Aubert et al. 2000) and Arabidopsis Cry1 (AtCry1; Giovani et al. 2003; Zeugner et al. 2005) are also conserved in migratory bird cryptochromes (Liedvogel et al. 2007a; Solov'yov et al. 2007). However, at the current stage we cannot say anything about their potential function as an electron donor in a radical pair reaction in migratory bird cryptochromes. In Drosophila cryptochrome (dCry), the conserved tryptophane residues do not appear to act as electron donors (Froy et al. 2002; Song et al. 2007; Hoang et al. 2008; Öztürk et al. 2008).
One apparent difference between photolyases and cryptochromes is the redox form of their signalling state. Whereas in photolyases the excited state of the flavin chromophore is the fully reduced state (FADH−), both in vitro and in vivo studies of cryptochrome flavoproteins suggest that absorption of blue light (prerequisite (v), but note the limitations of this result as outlined below) leads to formation of protein bound flavosemiquinone radical (FAD•/FADH•; Giovani et al. 2003; Banerjee et al. 2007; Berndt et al. 2007; Liedvogel et al. 2007a). In analogy to photolyases (Aubert et al. 2000), it is currently assumed that in this reaction, photoexcited FAD (in its fully oxidized ground state) gets reduced to the flavosemiquinon radical via intraprotein electron transfer along a sequence of three conserved tryptophan residues (figure 1c). Each of these three radical pair states that are formed during the photoreduction process might interact with the Earth's magnetic field, but recent results along the tryptophan triad in E. coli photolyases suggest that the radical pair intermediate interacting with the magnetic field is only the radical species between the terminal tryptophan and the flavin cofactor (Lukacs et al. 2008). In both Arabidopsis and the migratory garden warbler (figure 1b) half-life times of the cryptochrome radical intermediate were optically determined and characterized to be on the time scale of several milliseconds (prerequisite (vii), but note current limitations outlined in the following; Giovani et al. 2003; Bouly et al. 2007; Liedvogel et al. 2007a). Life times of these orders of magnitude could be well suited of allow for a radical pair based magnetoreception mechanism, but it is important to realize that the radical pairs do not only have to be long lived, but the spin-correlation has also to persist for at least approximately 1 µs to allow for any magnetic field effect. This issue can be addressed by time-resolved electron paramagnetic resonance (EPR) spectroscopy and measurements of the magnetic field-sensitivity of the radical pair lifetime. Using this approach, direct formation of radical pair intermediates was recently demonstrated for Xenopus laevis Cry DASH (XlCry-DASH); these data further suggest radical pair formation from a singlet state precursor (Biskup et al. 2009). Similar observations of long-lived (more than 10 µs) radical species have been reported for photolyases (Gindt et al. 1999; Weber et al. 2002; reviewed in Weber 2005).
Another key prerequisite for cryptochrome-based magnetoreception is that the rate and yield of the radical pair process must depend on the orientation of the protein in the Earth's magnetic field. Magnetic field effects on the photochemical yield of a flavin–tryptophan radical pair have recently been demonstrated in E. coli photolyase (Henbest et al. 2008). This experiment provides the proof of principle that photolyases (and due to the close relatedness probably cryptochromes as well) possess the magnetic and kinetic properties that are essential to function as a magnetoreceptor. To date, garden warbler cryptochrome is the only migratory bird cryptochrome that has been preliminarily characterized (Liedvogel et al. 2007a). In summary, spectral analyses on recombinantly expressed and purified garden warbler cryptochrome 1a could show that cryptochrome 1a of migratory garden warblers are excited by light (prerequisite (iv)) and that their absorption spectrum has a range that partly corresponds to the wavelength range known to elicit magnetic orientation performance in behavioural experiments (prerequisite (v)), but note the limitations of this result in the context of cryptochrome as a potential magnetoreceptor, as birds are also able to perform magnetic compass orientation under green light (centred around 565 nm), where the flavin cofactor of the cryptochromes does not absorb in vitro. Furthermore, garden warbler cryptochrome has been shown to form radical pair intermediates with millisecond lifetimes upon photoexcitation in the blue spectral range. The crucial model assumption (prerequisite (vii)) that garden warbler cryptochromes form long lived (approx. 10 ms) radical pair intermediates, and thus have the potential to be differentially affected depending on the direction of Earth-strength magnetic fields, could be optically (but not yet by means of EPR, see above) validated (Liedvogel et al. 2007a). Together these findings provide experimental evidence that cryptochromes possess all so far testable properties needed to act as a magnetic compass (Liedvogel et al. 2007a; Henbest et al. 2008; Biskup et al. 2009).
Further support for the possibility that a photochemical reaction can act as a magnetic compass sensor comes from a study on a carotenoid–porphyrin–fullerene molecule, which has been selected as a ‘model chemical compass’ (Maeda et al. 2008; see figure 1c for model compounds). The data obtained from this model chemical compass provide the general proof of principle that a radical pair based magnetic compass is feasible (figure 1e). The model chemical compass allowed a detectable response in Earth-strength magnetic fields (approx. 50 µT) at least at −20°C and below. The experimental data also provide insight into structural features and kinetics in vitro (i.e. anisotropical response to an external magnetic field and favourable asymmetric distribution of hyperfine interactions) that are required for a magnetoreceptor to detect directional information from the Earth's magnetic field, which is a crucial requirement to putatively be able to work as a magnetic compass. Efforts to further optimize the model chemical compass to work at physiological temperatures are on the way (Rodgers & Hore 2009).
6.4. Magnetic responses in the model plant Arabidopsis thaliana
Experiments on A. thaliana have suggested that magnetic intensity affects cryptochrome-dependent growth responses (figure 1d; Ahmad et al. 2007). But these reported cryptochrome-mediated magnetic field effects on plant growth (Ahmad et al. 2007) could not be replicated in an independent study (Harris et al. 2009). These findings would be very important, if they turn out to exist and be independently replicable, since even though magnetic responses do not seem biologically relevant for the plant, they would show in principle that biological tissue is sensitive to the magnetic field responses that are linked to cryptochrome-dependent signalling pathways and could thus confirm the ability of cryptochrome to mediate magnetic field responses. The claimed magnetosensitive responses can best be explained by the radical pair model, as Arabidopsis cryptochromes form radical pairs after photoexcitation (Giovani et al. 2003; Bouly et al. 2007) and these experiments might reflect common physical properties of photoexcited cryptochromes in both plants and animals. Assuming that the reported effects are real, one could further speculate that the magnetic field sensitivity of many organisms was not necessarily a huge step in evolution. Magnetic modulations of cryptochrome responses are likely to be an intrinsic property of the molecule, which was present by chance and might then have been selectively refined in birds to allow for vision-dependent magnetoreception/magnetic compass orientation. However, in the light of the fact that the effect found in the non-blinded experiments of Ahmad et al. (2007) could not be independently replicated in the seemingly more carefully controlled and blinded experiments of Harris et al. (2009), we want to emphasize here that any interpretation of these experiments should be done with great caution.
7. Conclusions and future research
Recent progress achieved through interdisciplinary studies of cryptochrome molecules and by studies on the magnetic compass mechanisms of migratory birds has provided a lot of correlative evidence supporting the role of one or more cryptochromes in magnetic compass information detection. Nevertheless, there is still no conclusive evidence demonstrating that cryptochromes are the primary sensory molecule underlying light-dependent magnetic compass orientation in birds. Many open questions remain (many of which are summarized in Mouritsen & Ritz (2005) and Rodgers & Hore (2009)).
For instance, it is essential that the motion of the radical pair forming molecule is restricted (prerequisite (iii)); otherwise no molecule can function as a primary magnetic compass detector providing accurate directional information. In photoreceptor cells, the highly oriented membrane structures would provide a well-suited substrate to allow for fixed orientations of cryptochromes. Other candidate anchor structures in other cell types are cytoskeleton proteins and cytosolicly embedded membranes. However, at the current stage, the anchoring, spatial organization and exact position of cryptochromes within the cells have still not been demonstrated. Since cryptochrome is a globular protein, it cannot be directly embedded into any membrane itself, but would have to interact either directly or indirectly with a membrane protein. To investigate whether cryptochromes are motion-restricted in the retinal cells of any bird, further experiments determining the localization of the protein on an ultramicroscopic scale and protein–protein interaction studies are necessary.
As the primary magnetoreceptor molecule underlying light-dependent magnetoreception remains unknown, any cryptochrome or any combination of several members of this multigene family are equally probable candidates. But how many different members of the cryptochrome multigene family are expressed in the retina of migratory birds? What are their spectral sensitivities and can they explain the results of orientation experiments under various wavelengths? What are the signalling pathways and the three-dimensional crystal structure of migratory bird cryptochromes? Future experiments on identification, characterization and subsequent recombinant expression of all members of the cryptochrome multigene family expressed in the retina of migratory birds are necessary. In addition to extending the set of candidate molecules within species, we also need to investigate cryptochromes from additional bird species, including day-, night- and non-migratory birds.
Even though the molecular reaction mechanism for most photolyases is now well established, the mechanism of the closely related magnetic compass receptor candidate cryptochrome remains unclear. Understanding structure and function of animal cryptochromes in general and cryptochromes of migratory birds in particular in greater depth requires further investigation, such as crystallization studies and experiments focusing on understanding the catalytic reaction mechanism, signalling pathways and the role of the putative electron donor sites in the electron transfer pathway, e.g. by site directed mutagenesis of the putatively involved Trp-donor sites. As suggested in Maeda et al. (2008), spectroscopic measurements similar to those carried out on the model chemical compass should be transferred to experiments on isolated cryptochromes. These in vitro experiments will finally allow testing for the existence of magnetic field effects on cryptochrome radical pair reactions, one key untested requirement for potential cryptochrome function as primary receptor molecule in a magnetic compass.
Even if we answer all these questions, we are likely to still be stuck with important, but inherently correlative, indirect evidence. So, how could we conclusively demonstrate that cryptochromes are the primary sensory molecules underlying light-dependent magnetic sensing in an animal?
There are at least two obvious and very direct approaches, which could conclusively implicate cryptochromes in magnetodetection. One such approach would involve electrophysiological recordings from cryptochrome expressing cells in the retina and the second approach would need to combine a robust and easily replicable behavioural essay or conditioning paradigm with genetic manipulation of the tested animals.
Both of these approaches are extremely challenging in practice. Electrophysiological recording of neuronal responses to changes in the magnetic field has been hampered by a large number of irreproducible claims, probably because interferences between the magnetic stimuli and the recording equipment have been interpreted as real neural responses to magnetic fields. The second approach is hampered by the difficulty of finding a truly robust behavioural paradigm in a genetic model animal such as Drosophila, the laboratory mouse Mus musculus or the zebrafish Danius rerio. Magnetic conditioning of birds to magnetic stimuli has proven incredibly difficult to replicate (Wiltschko & Wiltschko 1995b; in the laboratory of H.M., we have tried to replicate several conditioning paradigms which appeared straightforward according to the literature, including the chicken paradigm of Freire et al. (2005), but with no success). The only reasonably robust behavioural paradigm, where the biological relevance of the response is fully understood, is the magnetic compass response based on migratory restlessness behaviour in night-migratory birds in orientation cages such as Emlen funnels (Emlen & Emlen 1966). Here, the challenge is rather on the genetics side, since all migratory bird species are notoriously difficult to breed in captivity and, so far, no single transgenic (migratory) bird has been produced. Various temporary genetic interference techniques may be feasible in principle, but they would certainly also be very challenging to conduct in an extreme non-standard animal like a wild-caught migratory bird.
To sum up, much correlative evidence supports the hypothesis that cryptochromes may function as the primary magnetoreceptor molecule in a light-dependent magnetic compass, but it will be very challenging to provide a direct demonstration for this hypothesis. Considering the difficulty of independent replication in the past, any direct demonstration should only be generally accepted after it has been independently replicated using double-blind approaches.
Footnotes
One contribution to a Theme Supplement ‘Magnetoreception’.
References
- Adair R. K. 2000. Static and low-frequency magnetic field effects: health risks and therapies. Rep. Prog. Phys. 63, 415–454. ( 10.1088/0034-4885/63/3/204) [DOI] [Google Scholar]
- Ahmad M. 2003. Cryptochromes and flavoprotein blue-light photoreceptors. In Handbook of photochemistry and photobiology (ed. Nalwa H. S.), p. 4 San Diego, CA: Academic Press. [Google Scholar]
- Ahmad M., Cashmore A. R. 1993. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366, 162–166. ( 10.1038/366162a0) [DOI] [PubMed] [Google Scholar]
- Ahmad M., Lin C., Cashmore A. R. 1995. Mutations throughout an Arabidopsis blue-light photoreceptor impair blue light-responsive anthocyanin accumulation and inhibition of hypocotyls elongation. Plant J. 8, 653–658. ( 10.1046/j.1365-313X.1995.08050653.x) [DOI] [PubMed] [Google Scholar]
- Ahmad M., Galland P., Ritz T., Wiltschko R., Wiltschko W. 2007. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta 225, 615–624. ( 10.1007/s00425-006-0383-0) [DOI] [PubMed] [Google Scholar]
- Araki C. M., Hamassaki-Britto D. E. 1998. Motion-sensitive neurons in the chick retina: a study using Fos immunohistochemistry. Brain. Res. 794, 333–337. [DOI] [PubMed] [Google Scholar]
- Aubert C., Vos M. H., Mathis P., Eker A. P., Brettel K. 2000. Intraprotein radical transfer during photoactivation of DNA photolyase. Nature 405, 586–590. ( 10.1038/35038135) [DOI] [PubMed] [Google Scholar]
- Bailey M. J., Chong N. W., Xiong J., Cassone V. M. 2002. Chickens' Cry2: molecular analysis of an avian cryptochrome in retinal and pineal photoreceptors. FEBS Lett. 513, 169–174. ( 10.1016/S0014-5793(02)02276-7) [DOI] [PubMed] [Google Scholar]
- Banerjee R., Schleicher E., Maier S., Viana R. M., Pokorny R., Ahmad M., Bittl R., Batschauer A. 2007. The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. J. Biol. Chem. 282, 14 916–14 922. ( 10.1074/jbc.M700616200) [DOI] [PubMed] [Google Scholar]
- Berndt A., Kottke T., Breitkreuz H., Dvorsky R., Hennig S., Alexander M., Wolf E. 2007. A novel photoreaction mechanism for the circadian blue light photoreceptor Drosophila cryptochrome. J. Biol. Chem. 282, 13 011–13 021. ( 10.1074/jbc.M608872200) [DOI] [PubMed] [Google Scholar]
- Biskup T., Schleicher E., Okafuji A., Link G., Hitomi K., Getzoff E. D., Weber S. 2009. Direct observation of a photoinduced radical pair in a cryptochrome blue-light photoreceptor. Angew. Chem. Int. Ed. 48, 404–407. ( 10.1002/anie.200803102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouly J. P., Giovani B., Djamei A., Mueller M., Zeugner A., Dudkin E. A., Batschauer A., Ahmad M. 2003. Novel ATP-binding and autophosphorylation activity associated with Arabidopsis and human cryptochrome-1. Eur. J. Biochem. 270, 2921–2928. ( 10.1046/j.1432-1033.2003.03691.x) [DOI] [PubMed] [Google Scholar]
- Bouly J.-P., et al. 2007. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem. 282, 9383–9391. ( 10.1074/jbc.M609842200) [DOI] [PubMed] [Google Scholar]
- Brautigam C. A., Smith B. S., Ma Z., Palnitkar M., Tomchick D. R., Machius M., Deisenhofer J. 2004. Structure of the photolyase-like domain of cryptochrome 1 from Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 101, 12 142–12 147. ( 10.1073/pnas.0404851101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman D., Burnell J. 2007. Identification, cloning and sequencing of two major venom proteins from the box jellyfish Chironex fleckeri. Toxicon 50, 850–860. ( 10.1016/j.toxicon.2007.06.016) [DOI] [PubMed] [Google Scholar]
- Brocklehourst B. 2002. Magnetic fields and radical reactions: recent developments and their role in nature. Chem. Soc. Rev. 31, 301–311. ( 10.1039/b107250c) [DOI] [PubMed] [Google Scholar]
- Brudler R., et al. 2003. Identification of a new cryptochrome class. Structure, function, and evolution. Mol. Cell 11, 59–67. ( 10.1016/S1097-2765(03)00008-X) [DOI] [PubMed] [Google Scholar]
- Brunelle S. A., Hazard E. S., Sotka E. E., van Dolah F. M. 2007. Characterization of a dinoflagellate cryptochrome blue-light receptor with a possible role in circadian control of the cell cycle. J. Phycol. 43, 509–518. ( 10.1111/j.1529-8817.2007.00339.x) [DOI] [Google Scholar]
- Busza A., Emery-Le M., Rosbash M., Emery P. 2004. Roles of the two Drosophila cryptochrome structural domains in circadian photoreception. Science 204, 1503–1506. ( 10.1126/science.1096973) [DOI] [PubMed] [Google Scholar]
- Cashmore A. R. 1997. The cryptochrome family of photoreceptors. Plant Cell Environ. 20, 764–767. ( 10.1111/j.1365-3040.1997.tb00648.x) [DOI] [Google Scholar]
- Cashmore A. R., Jarillo J. A., Wu Y.-J., Liu D. 1999. Cryptochromes: blue light receptors for plants and animals. Science 284, 760–765. ( 10.1126/science.284.5415.760) [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., Matsubara J. A., Cynader M. S. 1995. Neuronal activity in primate visual cortex assessed by immunostaining for the transcription factor Zif 268. Vis. Neurosci. 12, 35–50. [DOI] [PubMed] [Google Scholar]
- Cintolesi F., Ritz T., Kay C. W. M., Timmel C. R., Hore P. J. 2003. Anisotropic recombination of an immobilized photoinduced radical pair in a 50-µT magnetic field: a model avian photomagnetoreceptor. Chem. Phys. 294, 385–399. ( 10.1016/S0301-0104(03)00320-3) [DOI] [Google Scholar]
- Cochran B., Mouritsen H., Wikelski M. 2004. Free-flying songbirds recalibrate their magnetic compass daily from sunset cues. Science 304, 405–408. ( 10.1126/science.1095844) [DOI] [PubMed] [Google Scholar]
- Daiyasu H., Ishikawa T., Kuma K., Iwai S., Todo T., Toh H. 2004. Identification of cryptochrome DASH from vertebrates. Genes Cells 9, 479–495. ( 10.1111/j.1365-9597.2004.00738.x) [DOI] [PubMed] [Google Scholar]
- Deisenhofer J. 2000. DNA photolyases and cryptochromes. Mutat. Res. DNA Repair 460, 143–149. ( 10.1016/S0921-8777(00)00023-9) [DOI] [PubMed] [Google Scholar]
- Dragunow M., Faull R. 1989. The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Meth. 29, 261–265. [DOI] [PubMed] [Google Scholar]
- Edmonds D. 2001. Electricity and magnetism in biological systems. Oxford, UK: Oxford University Press. [Google Scholar]
- Egan E. S., Franklin T. S., Hilderbrand-Chae M. J., McNeil G. P., Roberts M. A., Schroeder A. J., Zhang X., Jackson F. R. 1999. An extraretinally expressed insect cryptochrome with similarity to the blue light photoreceptors of mammals and plants. J. Neurosci. 19, 3665–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichwald C., Walleczek J. 1996. Model for magnetic field effects on radical pair recombination in enzyme kinetics. Biophys. J. 71, 623–631. ( 10.1016/S0301-4622(98)00180-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Din El-Assal S., Alonso-Blanco C., Peeters A. J. M., Wagemaker C., Weller J. L., Koornneef M. 2003. The role of cryptochrome 2 in flowering in Arabidopsis. Plant Physiol. 133, 1504–1516. ( 10.1104/pp.103.029819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P., So W. V., Kaneko M., Hall J. C., Rosbash M. 1998. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95, 669–679. ( 10.1016/S0092-8674(00)81637-2) [DOI] [PubMed] [Google Scholar]
- Emlen S. T., Emlen J. T. 1966. A technique for recording migratory orientation of captive birds. Auk 83, 361–367. [Google Scholar]
- Facella P., Lopez L., Chiappetta A., Bitonti M. B., Giuliano G., Perrotta G. 2006. CRY-DASH gene expression is under the control of the circadian clock machinery in tomato. FEBS Lett. 580, 4618–4624. ( 10.1016/j.febslet.2006.07.044) [DOI] [PubMed] [Google Scholar]
- Feenders G., Liedvogel M., Rivas M., Zapka M., Horita H., Hara E., Wada K., Mouritsen H., Jarvis E. D. 2008. Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PLoS ONE 3, e1768 ( 10.1371/journal.pone.0001768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A. J., McGuire J. J., Schaeffel F., Stell W. K. 1999. Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat. Neurosci. 2, 706–712. ( 10.1038/11167) [DOI] [PubMed] [Google Scholar]
- Freire R., Munro U., Rogers L. J., Wiltschko R., Wiltschko W. 2005. Chickens orient using a magnetic compass. Curr. Biol. 15, R620–R621. ( 10.1016/j.cub.2005.08.017) [DOI] [PubMed] [Google Scholar]
- Froy D., Chang D. C., Reppert S. M. 2002. Redox potential: differential roles in dCRY and mCRY1 functions. Curr. Biol. 12, 147–152. ( 10.1016/S0960-9822(01)00656-X) [DOI] [PubMed] [Google Scholar]
- Fu Z. W., Inaba M., Noguchi T., Kato H. 2002. Molecular cloning and circadian regulation of cryptochrome genes in Japanese quail (Coturnix coturnix japonica). J. Biol. Rhythms 17, 14–27. ( 10.1177/074873002129002302) [DOI] [PubMed] [Google Scholar]
- Fujihashi M., Numoto N., Kobayashi Y., Mizushima A., Tsujimura M., Nakamura A., Kawarabayasi Y., Miki K. 2007. Crystal structure of archaeal photolyase from Sulfolobus tokodaii with two FAD molecules: implication of a novel light-harvesting cofactor. J. Mol. Biol. 365, 903–910. ( 10.1016/j.jmb.2006.10.012) [DOI] [PubMed] [Google Scholar]
- Gegear R. J., Casselman A., Waddell S., Reppert S. 2008. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature 454, 1014–1018. ( 10.1038/nature07183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindt Y. M., Vollenbroek E., Westphal K., Sackett H., Sancar A., Babcock G. T. 1999. Origin of the transient electron paramagnetic resonance signals in DNA photolyase. Biochemistry 38, 3857–3866. ( 10.1021/bi981191+) [DOI] [PubMed] [Google Scholar]
- Giovani B., Byrdin M., Ahmad M., Brettel K. 2003. Light-induced electron transfer in a cryptochrome blue-light photoreceptor. Nat. Struct. Biol. 10, 489–490. ( 10.1038/nsb933) [DOI] [PubMed] [Google Scholar]
- Glas A. F., Maul M. J., Cryle M. J., Barends T. R., Schneider S., Kaya E., Schlichting I., Carell T. 2009. The archeal cofactor F0 is a light harvesting antenna chromophore in eucaryotes. Proc. Natl Acad. Sci. USA 106, 11 540–11 545. ( 10.1073/pnas.0812665106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressel J. 1979. Blue light photoreception. Photochem. Photobiol. 30, 749–754. ( 10.1101/sqb.2000.65.157) [DOI] [Google Scholar]
- Griffin E. A., Staknis D., Weitz C. J. 1999. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286, 768–771. ( 10.1126/science.286.5440.768) [DOI] [PubMed] [Google Scholar]
- Grissom C. B. 1995. Magnetic field effects in biology: a survey of possible mechanisms with emphasis on radical pair recombination. Chem. Rev. 95, 3–24. ( 10.1021/cr00033a001) [DOI] [Google Scholar]
- Haque R., Chaurasia S. S., Wessel J. H., Iuvone P. M. 2002. Dual regulation of chryptochrome I mRNA expression in chicken retina by light and circadian oscillators. Neuroreport 13, 2247–2251. [DOI] [PubMed] [Google Scholar]
- Harkins T. T., Grissom C. B. 1994. Magnetic field effects on B12 ethanolamine ammonia lyase: evidence for a radical mechanism. Science 263, 958–960. ( 10.1126/science.8310292) [DOI] [PubMed] [Google Scholar]
- Harkins T. T., Grissom C. B. 1995. The magnetic field dependent step in B12 ethanolamine ammonia lyase is radical-pair recombination. J. Am. Chem. Soc. 117, 566–567. ( 10.1021/ja00106a079) [DOI] [Google Scholar]
- Harris S. R., Henbest K. B., Maeda K., Pannell J. R., Timmel C. R., Hore P. J., Okamoto H. 2009. Effect of magnetic fields on cryptochrome-dependent responses in Arabidopsis thaliana. J. R. Soc. Interface 6, 1193–1205. ( 10.1098/rsif.2008.0519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer G., Fidler A. E., Vallone D., Foulkes N. S., Brandstaetter R. 2006. Molecular analysis of clock gene expression in the avian brain. Chronobiol. Int. 23, 113–127. ( 10.1080/07420520500521871) [DOI] [PubMed] [Google Scholar]
- Henbest K. B., Maeda K., Hore P. J., Joshi M., Bacher A., Bittl R., Weber S., Timmel C. R., Schleicher E. 2008. Magnetic-field effect on the photoactivation reaction of Escherichia coli DNA photolyase. Proc. Natl Acad. Sci. USA 38, 14 395–14 399. ( 10.1073/pnas.0803620105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyers D., Manns M., Luksch H., Güntürkün O., Mouritsen H. 2007. A visual pathway links brain structures active during magnetic compass orientation in migratory birds. PLoS ONE 2, e937 ( 10.1371/journal.pone.0000937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi K., Okamoto K., Daiyasu H., Miyashita H., Iwai S., Toh H., Ishiura M., Todo T. 2000. Bacterial cryptochrome and photolyase: characterization of two photolyase-like genes of Synechocystis sp. PCC6803. Nucl. Acid Res. 28, 2353–2362. ( 10.1093/nar/28.12.2353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi K., et al. 2009. Functional motifs in the (6–4) photolyase crystal structure make a comparative framework for DNA repair photolyases and clock cryptochromes. Proc. Natl Acad. Sci. USA 106, 6962–6967. ( 10.1073/pnas.0809180106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang N., et al. 2008. Human and Drosophila cryptochromes are light activated by flavin photoreduction in living cells. PLoS Biol. 6, e160 (doi:10.1371%2Fjournal.pbio.0060160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu D. S., Zhao X., Zhao S., Kazantsev A., Wang R. P., Todo T., Wei Y. F., Sancar A. 1996. Putative human blue-light photoreceptors hCRY1and hCRY2 are flavoproteins. Biochemistry 35, 13 871–13 877. ( 10.1021/bi962209o) [DOI] [PubMed] [Google Scholar]
- Huang Y., Baxter R., Smith B. S., Partch C. L., Colbert C. L., Deisenhofer J. 2006. Crystal structure of cryptochrome 3 from Arabidopsis thaliana and its implications for photolyase activity. Proc. Natl Acad. Sci. USA 103, 17 701–17 706. ( 10.1073/pnas.0608554103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T., Kanegae T., Wada M. 2000. Cryptochrome nucleocytoplasmic distribution and gene expression are regulated by light quality in the fern Adiantum capillus-veneris. Plant Cell 12, 81–96. ( 10.2307/3871031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T., Kadota A., Hasebe M., Wada M. 2002. Cryptochrome light signals control development to suppress auxin sensitivity in the moss Physcomitrella patens. Plant Cell 14, 373–386. ( 10.1105/tpc.010388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis E. D., Nottebohm F. 1997. Motor-driven gene expression. Proc. Natl Acad. Sci. USA 94, 4097–4102. ( 10.1073/pnas.94.8.4097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., et al. 2003. A genome-wide analysis of blue-light regulation of Arabidopsis transcription factor gene expression during seedling development. Plant Physiol. 133, 1480–1493. ( 10.1104/pp.103.029439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorns M. S., Wang B. Y., Jordan S. P., Chanderkar L. P. 1990. Chromophore function and interaction in Escherichia coli DNA photolyase: reconstitution of the apoenzyme with pterin and/or flavin derivatives. Biochemistry 29, 552–561. ( 10.1021/bi00454a032) [DOI] [PubMed] [Google Scholar]
- Kanai S., Kikuno R., Toh H., Ryo H., Todo T. 1997. Molecular evolution of the photolyase-blue-light photoreceptor family. J. Mol. Evol. 45, 535–548. ( 10.1007/PL00006258) [DOI] [PubMed] [Google Scholar]
- Karten H. J., Finger T. E. 1976. A direct thalamocerebellar pathway in pigeon and catfish. Brain Res. 102, 335–338. [DOI] [PubMed] [Google Scholar]
- Karten H. J., Fite K. V. 1977. Specific projection of displaced retinal ganglion cells upon the accessory optic system in the pigeon (Columbia livia). Proc. Natl Acad. Sci. USA 74, 1753–1756. ( 10.1073/pnas.94.8.4097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. T., Heelis P. F., Sancar A. 1992. Energy transfer (deazaflavin.fwdarw. FADH2) and electron transfer (FADH2.fwdarw. T.ltbbrac.rtbbrac.T) kinetics in Anacystis nidulans photolyase. Biochemistry 31, 11 244–11 248. ( 10.1021/bi00160a040) [DOI] [PubMed] [Google Scholar]
- Klar T., Kaiser G., Hennecke U., Carell T., Batschauer A., Essen L. O. 2006. Natural and non-natural antenna chromophores in the DNA photolyase from Thermus thermophilus. Chembiochem 7, 1798–1806. ( 10.1002/cbic.200600206) [DOI] [PubMed] [Google Scholar]
- Klar T., Pokorny R., Moldt J., Batschauer A., Essen L. O. 2007. Cryptochrome 3 from Arabidopsis thaliana: structural and functional analysis of its complex with a folate light antenna. J. Mol. Biol. 366, 954–964. ( 10.1016/j.jmb.2006.11.066) [DOI] [PubMed] [Google Scholar]
- Kleine T., Lockhart P., Batschauer A. 2003. An Arabidopsis protein closely related to Synechocystis cryptochrome is targeted to organelles. Plant J. 35, 93–103. ( 10.1046/j.1365-313X.2003.01787.x) [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Kanno S., Smit B., van der Horst G. T., Takao M., Yasui A. 1998. Characterization of photolyase/blue-light receptor homologs in mouse and human cells. Nucl. Acids Res. 26, 5086–5092. ( 10.1093/nar/26.22.5086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., et al. 2000. Molecular analysis of zebrafish photolyase/cryptochrome family: two types of cryptochromes present in zebrafish. Genes Cells 5, 725–738. ( 10.1046/j.1365-2443.2000.00364.x) [DOI] [PubMed] [Google Scholar]
- Komori H., Masui R., Kuramitsu S., Yokoyama S., Shibata T., Inoue Y., Miki K. 2001. Crystal structure of thermostable DNA photolyase: pyrimidine–dimer recognition mechanism. Proc. Natl Acad. Sci. USA 98, 13 560–13 565. ( 10.1073/pnas.241371398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort R., Komori H., Adachi S., Miki K., Eker A. 2004. DNA apophotolyase from Anacystis nidulans: 1.8A structure, 8-HDF reconstitution and X-ray-induced FAD reduction. Acta Crystallogr. D Biol. Crystallogr. 60, 1205–1213. ( 10.1107/S0907444904009321) [DOI] [PubMed] [Google Scholar]
- Kume K., Zylka M. J., Sriram S., Shearman L. P., Weaver D. R., Jin X., Maywood E. S., Hastings M. H., Reppert S. M. 1999. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98, 193–205. ( 10.1016/S0092-8674(00)81014-4) [DOI] [PubMed] [Google Scholar]
- Levy O., Appelbaum L., Leggat W., Gothlif Y., Hayward D. C., Miller D. J., Hoegh-Guldberg O. 2007. Light-responsive cryptochromes from a simple multicellular animal, the coral Acropora millepora. Science 318, 467–470. ( 10.1126/science.1145432) [DOI] [PubMed] [Google Scholar]
- Li Y. F., Heelis P. F., Sancar A. 1991. Active site of DNA photolyase: tryptophan-306 is the intrinsic hydrogen atom donor essential for flavin radical photoreduction and DNA repair in vitro. Biochemistry 30, 6322–6329. ( 10.1021/bi00239a034) [DOI] [PubMed] [Google Scholar]
- Liedvogel M., Maeda K., Henbest K., Schleicher E., Simon T., Timmel C. R., Hore P. J., Mouritsen H. 2007a. Chemical magnetoreception: bird cryptochrome 1a is excited by blue light and forms long-lived radical-pairs. PLoS ONE 2, e1106 ( 10.1371/journal.pone.0001106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedvogel M., Feenders G., Wada K., Troje N. F., Jarvis E. D., Mouritsen H. 2007b. Lateralized activation of cluster N in the brains of migratory songbirds. Eur. J. Neurosci. 25, 1166–1173. ( 10.1111/j.1460-9568.2007.05350.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. 2002. Blue light receptors and signal transduction. Plant Cell 14, S207–S225. ( 10.1105/tpc.000646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Shalitin D. 2003. Cryptochrome structure and signal transduction. Annu. Rev. Plant Biol. 54, 469–496. ( 10.1146/annurev.arplant.54.110901.160901) [DOI] [PubMed] [Google Scholar]
- Lin C., Todo T. 2005. Protein family review: the cryptochromes. Gen. Biol. 6, 220.1–220.9. ( 10.1186/gb-2005-6-5-220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. T., Robertson D. E., Ahmad M., Raibekas A. A., Jorns M. S., Dutton P. L., Cashmore A. R. 1995. Association of flavin adenine-dinucleotide with the Arabidopsis blue-light receptor Cry1. Science 269, 968–970. ( 10.1126/science.7638620) [DOI] [PubMed] [Google Scholar]
- Lin C. T., Yang H. Y., Guo H. W., Mockler T., Chen J., Cashmore A. R. 1998. Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc. Natl Acad. Sci. USA 95, 2686–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs A., Eker A. P. M., Byrdin M., Brettel K., Vos M. H. 2008. Electron hopping through the 15 Å triple tryptophan molecular wire in DNA photolyase occurs within 30 ps. J. Am. Chem. Soc. 130, 14 394–14 395. ( 10.1021/ja805261m) [DOI] [PubMed] [Google Scholar]
- Maeda K., Henbest K., Cintolesi F., Kuprov I., Rodgers D. T., Liddell P. A., Gust D., Timmel C. R., Hore P. J. 2008. Chemical compass model of avian magnetoreception. Nature 453, 387–390. ( 10.1038/nature06834) [DOI] [PubMed] [Google Scholar]
- Malhotra K., Kim S. T., Batschauer A., Dawut L., Sancar A. 1995. Putative blue-light photoreceptors from Arabidopsis thaliana and Sinapis alba with a high degree of sequence homoloy to DNA photolyase contain the 2 photolyase cofactors but lack DNA-repair activity. Biochemistry 34, 6892–6899. ( 10.1021/bi00020a037) [DOI] [PubMed] [Google Scholar]
- Maul M. J., Barends T. R., Glas A. F., Cryle M. J., Domratcheva T., Schneider S., Schlichting I., Carell T. 2008. Crystal structure and mechanism of a DNA (6–4) photolyase. Angew. Chem. Int. Ed. Engl. 47, 10 076–10 080. ( 10.1002/anie.200804268) [DOI] [PubMed] [Google Scholar]
- Mees A., Klar T., Gnau P., Hennecke U., Eker A. P., Carell T., Essen L. O. 2004. Crystal structure of a photolyase bound to a CPD-like DNA lesion after in situ repair. Science 306, 1789–1793. ( 10.1126/science.1101598) [DOI] [PubMed] [Google Scholar]
- Mello C. V., Vicario D. S., Clayton D. F. 1992. Song presentation induces gene-expression in the songbird forebrain. Proc. Natl Acad. Sci. USA 89, 6818–6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Sancar A. 1998. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc. Natl Acad. Sci. USA 95, 6097–6102. ( 10.1073/pnas.95.11.6097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller A., Sagasser S., Wiltschko W., Schierwater B. 2004. Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenchaften 91, 585–588. ( 10.1007/s00114-004-0578-9) [DOI] [PubMed] [Google Scholar]
- Mouritsen H. 1998. Redstarts, Phoenicurus phoenicurus, can orient in a true-zero magnetic field. Anim. Behav. 55, 1311–1324. ( 10.1006/anbe.1997.0696) [DOI] [PubMed] [Google Scholar]
- Mouritsen H., Ritz T. 2005. Magnetoreception and its use in bird navigation. Curr. Opin. Neurobiol. 15, 406–414. ( 10.1016/j.conb.2005.06.003) [DOI] [PubMed] [Google Scholar]
- Mouritsen H., Janssen-Bienhold U., Liedvogel M., Feenders G., Stalleicken J., Dirks P., Weiler R. 2004a. Cryptochrome and activity markers co-localize in bird retina during magnetic orientation. Proc. Natl Acad. Sci. USA 101, 14 294–14 299. ( 10.1073/pnas.0405968101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen H., Feenders G., Liedvogel M., Kropp W. 2004b. Migratory birds use head scans to detect the direction of the earth's magnetic field. Curr. Biol. 14, 1946–1949. ( 10.1016/j.cub.2004.10.025) [DOI] [PubMed] [Google Scholar]
- Mouritsen H., Feenders G., Liedvogel M., Wada K., Jarvis E. D. 2005. Night-vision brain area in migratory songbirds. Proc. Natl Acad. Sci. USA 102, 8339–8344. ( 10.1073/pnas.0409575102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muheim R., Backman J., Åkesson S. 2002. Magnetic compass orientation in European robins is dependent on both wavelength and intensity of light. J. Exp. Biol. 205, 3845–3856. [DOI] [PubMed] [Google Scholar]
- Müller M., Carell T. 2009. Structural biology of DNA photolyases and cryptochromes. Curr. Opin. Struct. Biol. 19, 277–285. ( 10.1016/j.sbi.2009.05.003) [DOI] [PubMed] [Google Scholar]
- Munro U., Munro J. A., Philips J. B., Wiltschko W. 1997. Effect of wavelength of light and pulse magnetisation on different magnetoreception systems in a migratory bird. Aust. J. Zool. 45, 189–198. ( 10.1071/ZO96066) [DOI] [Google Scholar]
- Öztürk N., Song S. H., Özgür S., Selby C. P., Morrison L., Partch C., Zhong C., Sancar A. 2007. Structure and function of animal cryptochromes. Cold Spring Harb. Symp. Quant. Biol. 72, 119–131. ( 10.1101/sqb.2007.72.015) [DOI] [PubMed] [Google Scholar]
- Öztürk N., Song S. H., Selby C. P., Sancar A. 2008. Animal type 1 cryptochromes: analysis of the redox state of the flavin cofactor by site-directed mutagenesis. J. Biol. Chem. 283, 3256–3263. ( 10.1074/jbc.M708612200) [DOI] [PubMed] [Google Scholar]
- Park H. W., Kim S. T., Sancar A., Deisenhofer J. 1995. Crystal structure of DNA photolyase from Escherichia coli. Science 268, 1866–1872. ( 10.1126/science.7604260) [DOI] [PubMed] [Google Scholar]
- Payne G., Sancar A. 1990. Absolute action spectrum of E-FADH2 and E-FADH2-MTHF forms of Escherichia coli DNA photolyase. Biochemistry 29, 7715–7727. ( 10.1021/bi00485a021) [DOI] [PubMed] [Google Scholar]
- Pokorny R., Klar T., Hennecke U., Carell T., Batschauer A., Essen L. O. 2008. Recognition and repair of UV lesions in loop structures of duplex DNA by DASH-type cryptochrome. Proc. Natl Acad. Sci. USA 105, 21 023–21 027. ( 10.1073/pnas.0805830106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappl R., Wiltschko R., Weindler P., Berthold P., Wiltschko W. 2000. Orientation behavior of garden warblers (Sylvia borin) under monochromatic light of various wavelengths. Auk 117, 256–260. ( 10.1642/0004-8038(2000)117[0256:OBOGWS]2.0.CO;2) [DOI] [Google Scholar]
- Ritz T., Adem S., Schulten K. 2000. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718. ( 10.1016/S0006-3495(00)76629-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T., Thalau P., Phillips J. B., Wiltschko R., Wiltschko W. 2004. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 429, 177–180. ( 10.1038/nature02534) [DOI] [PubMed] [Google Scholar]
- Ritz T., Wiltschko W., Hore P. J., Rodgers C. T., Stapput K., Thalau P., Timmel C. R., Wiltschko W. 2009. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys. J. 96, 3451–3457. ( 10.1016/j.bpj.2008.11.072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T., Ahmad M., Mouritsen H., Wiltschko W., Wiltschko R. 2010 Photoreceptor-based magnetoreception: optimal design of receptor molecules, cells, and neuronal processing. J. R. Soc. Interface. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers C. T., Hore P. 2009. Chemical magnetoreception in birds: the radical pair mechanism. Proc. Natl Acad. Sci. USA 106, 353–360. ( 10.1073/pnas.0711968106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosato E., Codd V., Mazzotta G., Piccin A., Zordan M., Costa R., Kyriacou C. P. 2001. Light-dependent interaction between Drosophila CRY and the clock protein PER mediated by the carboxy terminus of CRY. Curr. Biol. 11, 909–917. ( 10.1016/S0960-9822(01)00259-7) [DOI] [PubMed] [Google Scholar]
- Sancar A. 2000. Cryptochrome: the second photoactive pigment in the eye and its role in circadian photoreception. Annu. Rev. Biochem. 69, 31–67. ( 10.1146/annurev.biochem.69.1.31) [DOI] [PubMed] [Google Scholar]
- Sancar A. 2003. Structure and function of DNA photolyases and cryptochrome blue-light photoreceptors. Chem. Rev. 103, 2203–2237. ( 10.1021/cr0204348) [DOI] [PubMed] [Google Scholar]
- Sancar A. 2004. Regulation of the mammalian circadian clock by cryptochromes. J. Biol. Chem. 279, 34 079–34 082. ( 10.1074/jbc.R400016200) [DOI] [PubMed] [Google Scholar]
- Schneider T., Thalau H. P., Semm P., Wiltschko W. 1994. Melatonin is crucial for the migratory orientation of pied flycatchers (Ficedula hypoleuca pallas). J. Exp. Biol. 194, 255–262. [DOI] [PubMed] [Google Scholar]
- Schulten K. 1982. In Festkörperprobleme (ed. Treusch J.), Advances in Solid State Physics, vol. 22, pp. 61–83. Braunschweig, Germany: Vieweg. [Google Scholar]
- Schulten K., Swenberg C., Weller A. 1978. A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Z. Phys. Chem. NF 111, 1–5. [Google Scholar]
- Selby C. P., Sancar A. 2006. A cryptochrome/photolyase class of enzymes with single-stranded DNA-specific photolyase activity. Proc. Natl Acad. Sci. USA 103, 17 696–17 700. ( 10.1073/pnas.0607993103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman L. P., et al. 2000. Interacting molecular loops in the mammalian circadian clock. Science 288, 1013–1019. ( 10.1126/science.288.5468.1013) [DOI] [PubMed] [Google Scholar]
- Small D. G., Min B., Lefebvre P. A. 1995. Characterization of a Chlamydomonas reinhardtii gene encoding a protein of the DNA photolyase/blue light photoreceptor family. Plant Mol. Biol. 28, 443–454. ( 10.1007/BF00020393) [DOI] [PubMed] [Google Scholar]
- Solov'yov I. A., Chandler D. E., Schulten K. 2007. Magnetic field effects in Arabidopsis thaliana cryptochrome 1. Biophys. J. 92, 2711–2726. ( 10.1529/biophysj.106.097139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.-H., Dick B., Penzkofer A., Pokorny R., Batschauer A., Essen O. 2006. Absorption and fluorescence spectroscopic characterization of cryptochrome 3 from Arabidopsis thaliana. J. Photochem. Photobiol. B 85, 1–16. ( 10.1016/j.jphotobiol.2006.03.007) [DOI] [PubMed] [Google Scholar]
- Song S.-H., Öztürk N., Denaro T. R., Arat Ö., Kao Y. T., Zhu H., Zhong D., Reppert S. M., Sancar A. 2007. Formation and function of flavin anion radical in cryptochrome 1 blue-light photoreceptor of monarch butterfly. J. Biol. Chem. 282, 17 608–17 612. ( 10.1074/jbc.M702874200) [DOI] [PubMed] [Google Scholar]
- Stanewsky R. 2002. Clock mechanisms in Drosophila. Cell Tissue Res. 309, 11–26. ( 10.1007/s00441-002-0569-0) [DOI] [PubMed] [Google Scholar]
- Tamada T., Kitadokoro K., Higuchi Y., Inaka K., Yasui A., de Ruiter P. E., Eker A. P., Miki K. 1997. Crystal structure of DNA photolyase from Anacystis nidulans. Nat. Struct. Biol. 4, 887–891. ( 10.1038/nsb1197-887) [DOI] [PubMed] [Google Scholar]
- Thalau P., Ritz T., Stapput K., Wiltschko R., Wiltschko W. 2005. Magnetic compass orientation of migratory birds in the presence of a 1.315 MHz oscillating field. Naturwissenschaften 92, 86–90. ( 10.1007/s00114-004-0595-8) [DOI] [PubMed] [Google Scholar]
- Timmel C. R., Henbest K. 2004. A study of spin chemistry in weak magnetic fields. Phil. Trans. R. Soc. Lond. A 362, 2573–2589. ( 10.1098/rsta.2004.1459) [DOI] [PubMed] [Google Scholar]
- Todo T., Ryo H., Yamamoto K., Toh H., Inui T., Ayaki H., Nomura T., Ikenaga M. 1996. Similarity among the Drosophila (6-4) photolyase, a human photolyase homolog, and the DNA photolyase-blue-light photoreceptor family. Science 272, 109–112. ( 10.1126/science.272.5258.109) [DOI] [PubMed] [Google Scholar]
- van der Horst G. T. J., et al. 1999. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630. ( 10.1038/19323) [DOI] [PubMed] [Google Scholar]
- Weaver J. C., Vaughan T. E., Astumian R. D. 2000. Biological sensing of small field differences by magnetically sensitive chemical reactions. Nature 405, 707–709. ( 10.1038/35015128) [DOI] [PubMed] [Google Scholar]
- Weber S. 2005. Light-driven enzymatic catalysis of DNA repair: a review of recent biophysical studies on photolyase. Biochim. Biophys. Acta 1707, 1–23. ( 10.1016/j.bbabio.2004.02.010) [DOI] [PubMed] [Google Scholar]
- Weber S., Kay C. W. M., Mögling H., Möbius K., Hitomi K., Todo T. 2002. Photoactivation of the flavin cofactor in Xenopus laevis (6–4)photolyase: observation of a transient tyrosyl radical by time-resolved electron paramagnetic resonance. Proc. Natl Acad. Sci. USA 99, 1319–1322. ( 10.1073/pnas.032469399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 1972. Magnetic compass of European robins. Science 176, 62–64. ( 10.1126/science.176.4030.62) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 1978. Further analysis of the magnetic compass of migratory birds. In Animal migration, navigation and homing (eds Schmidt-König K., Keeton W. T.), pp. 302–310. Berlin, Germany: Springer. [Google Scholar]
- Wiltschko W., Wiltschko R. 1995a. Migratory orientation of European robins is affected by the wavelength of light as well as by a magnetic pulse. J. Comp. Physiol. 177, 363–369. ( 10.1007/BF00192425) [DOI] [Google Scholar]
- Wiltschko R., Wiltschko W. 1995b. Magnetic orientation in animals. Berlin, Germany: Springer. [Google Scholar]
- Wiltschko W., Wiltschko R. 1996. Magnetic orientation in birds. J. Exp. Biol. 199, 29–38. [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 1999. The effect of yellow and blue light on magnetic compass orientation in European robins, Erithacus rubecula. J. Comp. Physiol. 184, 295–299. ( 10.1007/s003590050327) [DOI] [Google Scholar]
- Wiltschko W., Wiltschko R. 2001. Light-dependent magnetoreception in birds: the behaviour of European robins (Erithacus rubecula) under monochromatic light of various wavelengths and intensities. J. Exp. Biol. 204, 3295–3302. [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 2002. Magnetic compass orientation and its physiological basis. Naturwissenschaften 89, 445–452. ( 10.1007/s00114-002-0356-5) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 1993. Red light disrupts magnetic orientation of migratory birds. Nature 364, 525–527. ( 10.1038/364525a0) [DOI] [Google Scholar]
- Wiltschko W., Wiltschko R., Munro U. 2000. Light-dependent magnetoreception in birds: does directional information change with light intensity? Naturwissenschaften 87, 36–40. ( 10.1007/s001140050006) [DOI] [PubMed] [Google Scholar]
- Wiltschko R., Gesson M., Wiltschko W. 2001. Magnetic compass orientation of European robins under 565 nm green light. Naturwissenschaften 88, 387–390. ( 10.1007/s001140100248) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 2003. Magnetic orientation in birds: non-compass responses under monochromatic light of increased intensity. Proc. R. Soc. Lond. B 270, 2133–2140. ( 10.1098/rspb.2003.2476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltschko W., Gesson M., Stapput K., Wiltschko R. 2004a. Light-dependent magnetoreception in birds: interaction of at least two different receptors. Naturwissenschaften 91, 130–134. ( 10.1007/s00114-003-0500-x) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Möller A., Gesson M., Noll C., Wiltschko R. 2004b. Light-dependent magnetoreception in birds: analysis of the behaviour under red light after pre-exposure to red light. J. Exp. Biol. 207, 1193–1202. ( 10.1242/jeb.00873) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Stapput K., Thalau P., Wiltschko R. 2006. Avian magnetic compass: fast adjustment to intensities outside the normal functional window. Naturwissenschaften 93, 300–304. ( 10.1007/s00114-006-0102-5) [DOI] [PubMed] [Google Scholar]
- Worley P. F., Christy B. A., Nakabeppy Y., Bhat R. V., Cole A. J., Baraban J. M. 1991. Constitutive expression of zif268 in neocortex is regulated by synaptic activity. Proc. Natl Acad. Sci. USA 88, 5106–5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Xiang Y., Zhu H., Xu H., Zhang Z., Zhang C., Zhang L., Ma Z. 2009. Wheat cryptochromes: subcellular localization and involvement in photomorphogenesis and osmotic stress responses. Plant Physiol. 149, 760–774. ( 10.1104/pp.108.132217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Q., Wu Y. J., Tang R. H., Liu D., Liu Y., Cashmore A. R. 2000. The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell 103, 815–827. ( 10.1016/S0092-8674(00)00184-7) [DOI] [PubMed] [Google Scholar]
- Yoshida K., Kawamura K., Imaki J. 1993. Differential expression of c-fos mRNA in rat retinal cell: regulation by light/dark cycle. Neuron 10, 1049–1054. [DOI] [PubMed] [Google Scholar]
- Yoshii T., Ahmad M., Helfrich-Föster C. 2009. Cryptochrome mediates light-dependent magnetosensitivity of Drosophila's circadian clock. PLoS Biol. 7, e1000086 ( 10.1371/journal.pbio.1000086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapka M., et al. 2009. Visual but not trigeminal mediation of magnetic compass information in a migratory bird. Nature 461, 1274–1277. ( 10.1038/nature08528) [DOI] [PubMed] [Google Scholar]
- Zeugner A., Byrdin M., Bouly J. P., Bakrim N., Giovani B., Brettel K., Ahmad M. 2005. Light-induced electron transfer in Arabidopsis cryptochrome-1 correlates with in vivo function. J. Biol. Chem. 280, 19 437–19 440. ( 10.1074/jbc.C500077200) [DOI] [PubMed] [Google Scholar]
- Zhu H., Green C. B. 2001. A putative flavin electron transport pathway is differentially utilized in Xenopus CRY1 and CRY2. Curr. Biol. 11, 1945–1949. ( 10.1016/S0960-9822(01)00601-7) [DOI] [PubMed] [Google Scholar]
- Zhu H., Yuan Q., Briscoe A. D., Froy O., Casselman A., Reppert S. M. 2005. The two CRYs of the butterfly. Curr. Biol. 15, R953–R954. ( 10.1016/j.cub.2005.11.030) [DOI] [PubMed] [Google Scholar]
- Zhu H., Sauman I., Yuan Q., Casselman A., Emery-Le M., Emery P., Reppert S. M. 2008. Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. PLoS Biol. 6, e4 ( 10.1371/journal.pbio.0060004) [DOI] [PMC free article] [PubMed] [Google Scholar]