Abstract
Several studies have suggested that the magnetic compass of birds is located only in the right eye. However, here we show that night-migrating garden warblers (Sylvia borin) are able to perform magnetic compass orientation with both eyes open, with only the left eye open and with only the right eye open. We did not observe any clear lateralization of magnetic compass orientation behaviour in this migratory songbird, and, therefore, it seems that the suggested all-or-none lateralization of magnetic compass orientation towards the right eye only cannot be generalized to all birds, and that the answer to the question of whether magnetic compass orientation in birds is lateralized is probably not as simple as suggested previously.
Keywords: magnetic sense, bird migration, lateralization, cluster N
1. Introduction
Night-migratory birds can use a magnetic compass to find their way during long-distance migration (e.g. Wiltschko & Wiltschko 1972, 1995, 2007; Cochran et al. 2004), and the majority of recent evidence indicates that the magnetic compass of night-migratory birds is based on light-dependent, radical-pair reactions in the birds’ eyes (Schulten et al. 1978; Wiltschko et al. 1993; Ritz et al. 2000, 2004, 2009; Muheim et al. 2002; Möller et al. 2004; Mouritsen et al. 2004, 2005; Wiltschko et al. 2005; Heyers et al. 2007; Liedvogel et al. 2007a,b; Wiltschko & Wiltschko 2007; Feenders et al. 2008; Zapka et al. 2009). In 2002, Wiltschko et al. published a very surprising paper suggesting that this light-dependent magnetic compass of night-migratory European robins (Erithacus rubecula) is very strongly lateralized. In fact, the data of Wiltschko et al. (2002) suggested that input from the left eye only cannot be used for magnetic compass orientation, whereas input through the right eye only was sufficient for perfectly oriented magnetic compass behaviour. Subsequently, Wiltschko et al. (2003) published similar results suggesting all-or-none right lateralization of magnetic compass orientation in the diurnally migrating songbird, the Australian silvereye, Zosterops lateralis.
The findings of Wiltschko et al. (2002, 2003) suggesting complete right lateralization of the magnetic compass in migratory songbirds are very surprising for several reasons. First, although lateralization is a common feature of the avian brain, so far no other sensory input or system has been shown to be lateralized in an all-or-none fashion (e.g. Güntürkün 1997, 2003; Rogers & Andrew 2002; Prior 2006). Usually, a preference towards one side or the other occurs. Such a non-exclusive preference was also found in studies of homing in pigeons with one eye occluded (Ulrich et al. 1999; Prior et al. 2004). Second, the distribution of candidate receptor molecules for magnetic compass information, neuronal activation patterns in the eye and neuronal connectivity between brain areas that are highly likely to be involved in detecting and processing the light-dependent magnetic information do not show evidence of strong lateralization (Mouritsen et al. 2004; Heyers et al. 2007; Liedvogel et al. 2007b; Zapka et al. 2009). The only significant lateralization, we have observed, relates to neuronal activity in cluster N, a light-dependent forebrain area in migratory birds (Mouritsen et al. 2005; Liedvogel et al. 2007b; Feenders et al. 2008), which has just been shown to be required for magnetic compass orientation in European robins (Zapka et al. 2009). Quantification of neuronal activity in cluster N of European robins revealed a significant dominance of the left eye and right brain hemisphere (Liedvogel et al. 2007b), i.e. in the opposite direction to that found by Wiltschko et al. (2002).
Owing to these apparently contradictory results, the aim of this study was to attempt to independently replicate the findings of Wiltschko et al. (2002, 2003) using garden warblers, Sylvia borin, a species that had not previously been tested for lateralization and that is known to have a cluster N that is highly active in both hemispheres during magnetic compass orientation (Mouritsen et al. 2005). We therefore tested the magnetic compass orientation capabilities of migratory garden warblers during autumn migration. The birds were fitted with light-tight, black, artificial leather hoods having openings in front of both eyes, an opening in front of the left eye only or an opening in front of the right eye only.
2. Material and Methods
2.1. Magnetic fields
Magnetic fields were produced with double-wrapped, three-dimensional Merritt four-coil systems (Kirschvink 1991) with average coil diameters of 2 m, and all experiments were performed within the central space of the coils where the heterogeneity was less than 1 per cent of the applied field. Before the beginning of each experiment, the actual magnetic field was measured in the centre and at the edges of the experimental volume within which the orientation cages were placed. Birds were tested in a natural magnetic field (NMF: MF strength = 49 000 ± 350 nT (s.d.), inclination = 67 ± 0.5° (s.d.), horizontal direction = 360 ± 0.1° (s.d.)) and in a field turned 120° counterclockwise (changed magnetic field (CMF: MF strength = 48 800 ± 470 nT (s.d.), inclination = 67 ± 0.7° (s.d.), horizontal direction = −120 ± 0.8° (s.d.)). Current flowed through the coils under all experimental conditions. To produce a CMF, the current ran through the two subsets of windings of the four-coil system in the same direction. Under the NMF condition, the coils were turned on, but the current ran through the two subsets of windings in opposite directions so that no significant changes (less than 10 nT) to the magnetic field were produced by the coils (Kirschvink 1991).
2.2. Test subjects
In our study, we tested 17 garden warblers caught at the Biological Station Rybachy, Russia, and transferred to Oldenburg, Germany, in spring 2007. The birds were housed indoors in individual cages in a windowless room under a light regime simulating the local photoperiod. The behavioural experiments were performed on the campus of the University of Oldenburg, Germany, during the autumn migratory season 2008. Before the behavioural experiments started, the birds were kept for one week in an aviary with access to celestial cues, allowing them the possibility of recalibrating their magnetic compass (Cochran et al. 2004; Muheim et al. 2006, 2008; Wiltschko et al. 2008). All animal procedures were approved by the Animal Care and Use Committees of the LAVES (Oldenburg, Germany).
2.3. Behavioural experiments
The orientation experiments were conducted in wooden huts. The walls of the huts were lined with aluminium shields, which act as Faraday cages and shield non-stationary electromagnetic disturbances by approximately two orders of magnitude. All technical supplies and equipment were also shielded by placing them in aluminium boxes to minimize electromagnetic disturbances.
One hour (±10 min) before sunset, the birds were placed outdoors in wooden transport cages that enabled them to see parts of the evening sky for 1 h to allow them the possibility of calibrating their magnetic compass from the local sunset sky (Cochran et al. 2004; Muheim et al. 2006, 2008; Wiltschko et al. 2008). Immediately thereafter, they were placed in modified aluminium Emlen funnels (35 cm diameter, 15 cm high, walls 45° inclined; Emlen & Emlen 1966), which were coated with thermal paper (Mouritsen et al. 2009) on which the birds left scratches as they moved. The overlap point of the paper (in N, S, E or W) was changed randomly between nights and huts. The birds were tested for 1 h under dim-light conditions (2.1 mW m−2) produced by incandescent bulbs. Nine birds were tested simultaneously in each hut, in either an NMF or a magnetic field shifted 120° counterclockwise (CMF). The birds were placed in a randomized funnel position each night and were put into the funnels from different directions, and we observed no systematic differences between the nine funnel positions or between the four huts. The birds were tested twice each night under the same magnetic field and hood condition. The second test on a given night started either 1.5 or 3 h after the lights in the bird rooms were switched off, and each bird was tested in a different hut from that in the first test. The orientation directions during the first and the second test were therefore entered into the calculation of the mean direction for that particular bird and condition. The magnetic field conditions tested in a given hut were switched every second night, and usually different magnetic fields were tested in different huts on any given night.
Before the eye-cover experiments started, we tested the birds without wearing the hoods to confirm that they were in migratory mood and to get a control direction. For the eye-cover experiments, we used the same procedures as in the control experiments, except that the birds were fitted with hoods just before the birds were placed outdoors in the transport cages. The hoods (less than 0.5 g) were made of light-tight, artificial leather with tightly fitted openings left for the beak and the neck. They looked similar to a falconer's hood for raptors or a Middle Ages executioner's hood. We tested the light-tightness of our hoods, and they reduced the ambient light intensity by at least five orders of magnitude. The hoods were slipped over the head and beak and then fixed around the neck by a string-pull system. We made sure that the eye(s) of the birds were placed in the middle of the (8 mm diameter) openings, exposing the right eye, the left eye or both eyes (controls) to light and visual input. The hoods were removed every night immediately after the behavioural tests were finished.
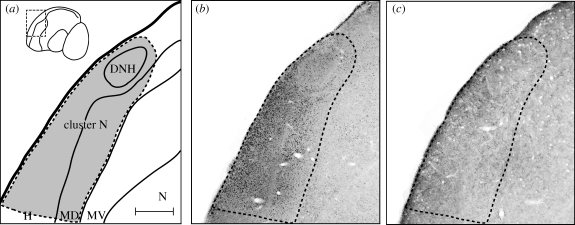
In the 2 years preceding the experiments, we tried to use the eye cap design of Wiltschko et al. (2002). However, garden warblers reacted with very strong stress behaviour to this design, and it was therefore not ethically acceptable to us to use the same design in this species. We therefore spent a lot of time developing eye caps or hoods that did not elicit stressful reactions in the experimental birds while simultaneously being completely light tight and easy to fix and remove from the bird's head. The hood design we used (see the description above) met all these criteria. Measurements of the light permeability of the hoods showed that, on a sunny day with an outdoors light intensity of 3.77 × 105 mW m−2, the light intensity under the closed side of the cap was 1.5 mW m−2. Transferred to the huts, where the light intensity was 2.1 mW m−2, it meant that the light intensity under the hoods during the experiments was less than 1 × 10−5 mW m−2. In addition to the light-tightness measurements of the hoods, we demonstrated that the hoods reduced the neuronal activity of cluster N to a background level in garden warblers (figure 1), which is in line with previous studies showing that the neuronal activation in cluster N of European robins fitted with hoods decreased to a background level (Mouritsen et al. 2005; Liedvogel et al. 2007b). Neuronal activation was measured by ZENK protein expression, which correlates with neuronal activity in most parts of the avian brain (e.g. Mello & Clayton 1995; Jarvis & Nottebohm 1997; Feenders et al. 2008).
Figure 1.
ZENK activation, which mirrors neuronal activation in cluster N, is reduced to a background level when garden warblers wore hoods covering both eyes. (a) Schematic drawing of cluster N (area within dashed line). (b,c) Photos of sagittal brain slices through the centre of cluster N stained against ZENK protein. (b) ZENK activation of cluster N in a garden warbler that had both eyes open. The small black dots are ZENK-positive neuron nuclei, i.e. these neurons were active when a garden warbler had both eyes open during the night under the dim-light conditions of our wooden testing huts. (c) ZENK activation of cluster N in a garden warbler that wore one of our hoods covering both eyes. Notice that the ZENK activation level and thus the number of active neurons are reduced to the background level. Consequently, our hoods are light-tight and seem to effectively block input to the brain area cluster N, which is required for magnetic compass orientation and which seems to be involved in the processing of magnetic compass information (Zapka et al. 2009). Scale bar in (a) (for a–c) = 400 µm. DNH, dorsal nucleus of the hyperpallium; H, hyperpallium; MD, mesopallium dorsale; MV, mesopallium ventrale; N, nidopallium.
2.4. Immunohistochemical detection of ZENK
We exposed two garden warblers, one wearing a hood covering both eyes and another bird wearing a hood with holes for both eyes, to the light regime used in the behavioural experiments for a period of 3 h. After 3 h, we sacrificed them by an intramuscular injection of an overdose of Narcoren (Merial, Hallbergmoos, Germany). Brain tissue was fixed by transcardial perfusion with 0.12 M phosphate-buffered saline (PBS) followed by 4 per cent paraformaldehyde (PFA) dissolved in PBS. Whole brains were dissected from the skull and post-fixed in PFA. After cryoprotection overnight in 30 per cent sucrose dissolved in PBS, tissue was cut in 40 µm sagittal sections.
Immunohistochemical detection of ZENK protein has been described previously (Heyers et al. 2007). In brief, series of brain slices were stained free-floating using a commercially available ABC-Kit (Vector ABC Elite Kit, Vector Laboratories, Burlingame, CA, USA). Slices were sequentially incubated with an antibody raised against ZENK (Santa Cruz, CA, USA), a biotinylated secondary antibody and avidin-coupled peroxidase complex. Peroxidase activity was detected using a 3′,3-diaminobenzidine reaction. To ensure comparability between series in each staining procedure, series from the two experimental birds were stained simultaneously. Stained sections were mounted on glass slides, dehydrated and coverslipped for microscopic analysis.
2.5. Orientation data analysis
Two researchers visually determined each bird's mean direction from the distribution of the scratches independently from each other. The human eye is very good at estimating the means of scratch patterns. This fast method is consistent between two independent observers to within 5–10° in 85–90% of the cases (Mouritsen 1998), and is therefore not less accurate than any attempt to count the scratches (this can also not be done precisely for birds showing medium to high activity). For extra quality control, we asked a third independent researcher to determine the mean direction, if the two first observers considered the scratches to be randomly distributed or if the two independently determined mean directions deviated by more than 30°. If this third individual determined a mean direction similar to one of the first two, and if the individual with the initially differing opinion also agreed with this direction, the mean of the two similar directions was recorded as the orientation result. If the three independent individuals could not agree on one mean direction, the bird's heading was defined as random and excluded from the analyses (10% of all control tests; 14% of all eye-cover tests). Birds with fewer than 35 scratches on the paper were considered inactive and also excluded from the analysis (25% of all control tests; 26% of all eye-cover tests). The average mean heading for each bird under each experimental condition was calculated. Based on these individual mean vectors, group mean vectors were calculated and the directional significance of the group mean vector was tested using the Rayleigh test.
3. Results
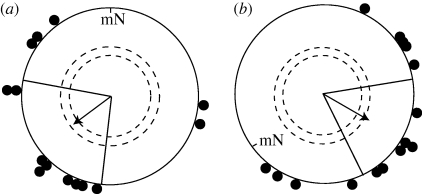
All birds were tested inside wooden huts, where no cues other than the geomagnetic field were available. In the control experiments without any hoods (figure 2), the birds oriented SW in the unchanged magnetic field (NMF; 233 ± 47° (95% confidence intervals), r = 0.4958, p = 0.05, n = 14) and ESE in the 120° turned magnetic field (CMF; 119 ± 38°, r = 0.5599, p = 0.01, n = 15).
Figure 2.
Control experiments showed that the birds were in migratory mood and able to perform magnetic compass orientation. (a) Our garden warblers oriented in their typical south-westerly migratory direction in autumn in the NMF when no hood was attached to their head. (b) The same birds’ orientation without hoods in a magnetic field turned 120° counterclockwise (CMF). mN, magnetic North. The arrows indicate the group mean vectors. The inner and outer dashed circles indicate the radius of the group mean vector needed for directional significance according to the Rayleigh test (p < 0.05 and p < 0.01, respectively). The radial lines flanking the group mean vector indicate the 95% confidence intervals for the group mean direction.
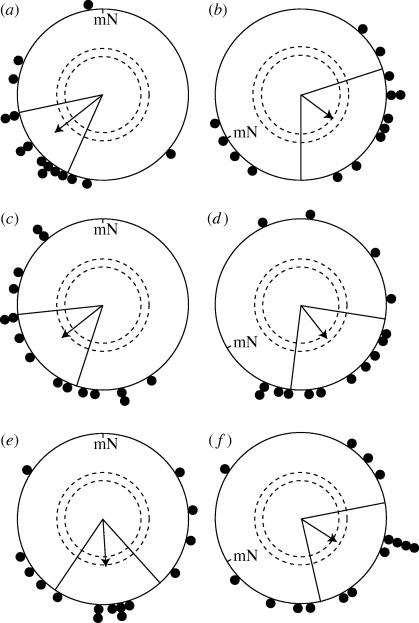
After the control experiments, we performed identical experiments with birds equipped with hoods, which enabled them to see with the left eye, the right eye or both eyes. Each bird was tested 9.4 ± 1.3 (s.d.) times per condition (six conditions). The results are shown in figure 3.
Figure 3.
Garden warblers wearing hoods can use their magnetic compass if light and/or visual input reaches any one eye. (a,b) The results from birds equipped with hoods with an 8 mm diameter hole in front of both eyes. (c,d) The results from birds equipped with hoods allowing light and visual input to reach only the left eye. (e,f) The results from birds equipped with hoods allowing light and visual input to reach only the right eye. The data in (a,c,e) were collected in an unchanged magnetic field (NMF). The data in (b,d,f) were collected in a magnetic field turned 120° counterclockwise (CMF). mN, magnetic North. For a description of the circular diagrams, see the legend to figure 2.
Birds equipped with hoods which made them able to see with both eyes oriented in their expected autumn migratory direction towards SW in the unchanged magnetic field (NMF: 231 ± 27°, r = 0.71, n = 15, p < 0.001; figure 3a). When the magnetic field was turned 120° counterclockwise, the birds with both eyes open oriented towards SE (CMF: 126 ± 54°, r = 0.46, n = 14, p < 0.05; figure 3b). The mean orientation in the CMF of the birds which had both eyes open differed significantly from the same birds’ orientation in the NMF (95% confidence intervals do not overlap) and was directed in the expected direction.
Birds equipped with hoods which enabled the birds to see only with their left eye oriented significantly towards SW when they experienced an unchanged magnetic field (NMF: 231 ± 33°, r = 0.61, n = 15, p = 0.002; figure 3c). When the magnetic field was turned 120° counterclockwise, the birds with the left eye open oriented significantly towards SE (CMF: 143 ± 43°, r = 0.51, n = 15, p = 0.02; figure 3d). The mean orientation in the 120° turned magnetic field of the birds which had only their left eye open differed significantly and in the expected direction from the same birds’ orientation in the NMF (95% confidence intervals do not overlap). Thus, garden warblers with only their left eye open could orient using their magnetic compass.
Birds equipped with hoods which enabled the birds to see only with their right eye oriented towards S when they experienced an unchanged magnetic field (NMF: 176 ± 38°, r = 0.55, n = 15, p < 0.01; figure 3e). This southern direction is not significantly different from the expected migratory direction towards approximately 205–215° (212° for Scandinavian birds (Mouritsen & Mouritsen 2000); 207° for birds with rings of Institut für Vogelforschung, Wilhelmshaven (F. Bairlein 2009, personal communication)). In the CMF, the same birds oriented towards SE (CMF: 123 ± 44°, r = 0.50, n = 15, p = 0.02; figure 3f), which is also not significantly different from the expected orientation in the CMF (212−120° = 92°). Even though the orientation of the birds which had their right eye open is not significantly different in the CMF and NMF (Watson test, W = 2.643, p = 0.27; and the 95% confidence intervals overlap), the tendencies observed suggest that the orientation of the right eye open birds was not different from the orientation of the both eyes open birds. Certainly, the orientation was significantly different from random under both magnetic field conditions and the mean directions were not significantly different from the directions expected given the position of magnetic North in the two test series. Therefore, the birds with their right eye open were also able to orient using their magnetic compass.
4. Discussion
Our results suggest that garden warblers are able to orient in their inherited mean autumn migratory direction towards SW using their magnetic compass when they have both eyes open, only their left eye open or only their right eye open. If there is any difference between the eyes, our results suggest a slight tendency towards a left eye preference.
The present results seem to contradict the findings of Wiltschko and Wiltschko and colleagues in European robins (Wiltschko et al. 2002) and Australian silvereyes, Z. lateralis (Wiltschko et al. 2003), where it was suggested that magnetic compass orientation functions with the right eye only, but not with the left eye. Wiltschko and Wiltschko and colleagues have therefore repeatedly stated that the magnetic compass of birds in general is exclusively located in the right eye (e.g. Wiltschko et al. 2002, 2003; Wiltschko & Wiltschko 2006, 2007). Recent experiments with domestic chicken, Gallus gallus, also indicated an apparent very strong right eye dominance in chicken magnetic compass responses (Rogers et al. 2008). However, in Rogers et al. (2008), the possibility was considered that hemispheric differences in higher level processing could be responsible for the results in chicken. Prior (2006) has also suggested a similar possibility in relation to the European robin results. Our data on garden warblers seem to agree with the view that general exclusivity of the right eye in magnetic compass orientation is a too simplified conclusion, since garden warblers could clearly orient in their autumn migratory direction in the geomagnetic field and they significantly turned their orientation in response to a 120° counterclockwise turn of the magnetic field using only their left eye.
Our results suggesting that garden warblers can use any one eye for magnetic orientation are in line with several molecular and neuroanatomical studies on magnetic compass sensing. It has been suggested that birds detect magnetic compass information through photochemical processes in their eyes (Schulten et al. 1978; Wiltschko et al. 1993; Ritz et al. 2000, 2004, 2009; Möller et al. 2004; Mouritsen et al. 2004, 2005; Wiltschko et al. 2005; Heyers et al. 2007; Liedvogel et al. 2007a; Maeda et al. 2008; Solov'yov & Schulten 2009), that cryptochrome molecules may be the primary sensory molecules behind this mechanism (Ritz et al. 2000; Möller et al. 2004; Mouritsen et al. 2004; Liedvogel et al. 2007a; Maeda et al. 2008), that cluster N is involved in the magnetic compass information processing circuit (Mouritsen et al. 2005; Liedvogel et al. 2007b; Feenders et al. 2008; Zapka et al. 2009) and that cluster N receives input from the eyes via the thalamofugal visual pathway (Heyers et al. 2007). In all of these molecular and physiological studies, which in several cases included data from European robins, no obvious lateralization was observed. In particular, cryptochromes are expressed in both eyes, and we did not find any obvious difference in cryptochrome expression or in neuronal activity and/or connectivity during a magnetic compass orientation task (Mouritsen et al. 2004; Heyers et al. 2007; Liedvogel et al. 2007b). Furthermore, the brain area cluster N is active in both hemispheres of the brain of European robins and garden warblers, and, when we tested European robins with eye covers, the activity in cluster N disappeared (Mouritsen et al. 2005). In a more detailed study on cluster N in European robins, we showed that neuronal activity in cluster N was in fact slightly lateralized with a small but significant dominance of the right hemisphere (Liedvogel et al. 2007b). Owing to the 99 per cent cross-over of fibres of the optic nerve in birds (Weidner et al. 1985), this suggests a slight dominance of the left eye, which is the opposite tendency to the one suggested by the Wiltschko et al. (2002, 2003) and Rogers et al. (2008) behavioural results. This lack of a strong lateralized dominance of either of the sides in cluster N expression is in line with our present behavioural results.
In figure 3e, it seems as though birds that had their right eye open oriented a bit more southerly than expected and than seen in the birds that had either both eyes open or only the left eye open (figure 3a,c). However, considering the spread of the data, it would be surprising if all three groups were oriented perfectly in the expected direction under both magnetic field conditions. We therefore believe that this tendency suggesting a more southerly orientation of the right eye open birds in the unchanged magnetic field is a coincidental statistical variation.
In conclusion, our data suggest that the idea that the magnetic compass of birds is located only in the right eye should not be generalized and that any putative lateralization of magnetic compass orientation is more complex than suggested by previous findings. Our data suggest that garden warblers, and potentially other night-migratory bird species, can extract magnetic compass information from both eyes; and from either single eye if they are forced to do so. We suggest that more studies, particularly independent studies involving the same species already tested with monocular occlusions, are needed before a serious attempt is made to understand the apparent species differences related to lateralization of magnetic compass orientation.
Acknowledgements
We thank Sonja Bem and Dmitry Kishkinev for their help in carrying out the experiments, Gesa Feenders for assistance in designing the hoods, Helmut Prior for constructive input to a previous version of this manuscript, the VW-Stiftung (Lichtenberg-Professur to H.M., ‘dynamics and adaptivity of neuronal systems’ stipend to D.H.) and the DFG (FOR 701 to H.M.) for financial support, X-CEN-TEC Wardenburg, Germany, for providing the cloth and the workshops of University of Oldenburg for building top-quality magnetic coil systems and electronic controls.
Authors contributions. H.M. and M.Z. designed the study. M.Z. designed the successful hood design with input from N.-L.S. C.M.H. supervised the orientation experiments. C.M.H. and S.K. performed the behavioural experiments and analysed the orientation results. D.H. and S.K. performed histological experiments and analyses. N.-L.S. suggested and made crucial improvements in the experimental set-up. H.M., M.Z. and C.M.H. wrote most of the paper.
Footnotes
One contribution to the Theme Supplement ‘Magnetoreception’.
References
- Cochran W. W., Mouritsen H., Wikelski M. 2004. Migrating songbirds recalibrate their magnetic compass daily from twilight cues. Science 304, 405–408. ( 10.1126/science.1095844) [DOI] [PubMed] [Google Scholar]
- Emlen S. T., Emlen J. T. 1966. A technique for recording migratory orientation of captive birds. Auk 83, 361–367. [Google Scholar]
- Feenders G., Liedvogel M., Rivas M., Zapka M., Horita H., Hara E., Wada K., Mouritsen H., Jarvis E. D. 2008. Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PLoS ONE 3, e1768 ( 10.1371/journal.pone.0001768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güntürkün O. 1997. Avian visual lateralization: a review. Neuroreport 8, R3–R11. [PubMed] [Google Scholar]
- Güntürkün O. 2003. Hemispheric asymmetry in the visual system of birds. In The asymmetrical brain (eds Hugdahl K., Davidson R. J.), pp. 3–36. Cambridge, MA: MIT Press. [Google Scholar]
- Heyers D., Manns M., Luksch H., Güntürkün O., Mouritsen H. 2007. A visual pathway links brain structures active during magnetic compass orientation in migratory birds. PLoS ONE 2, e937 ( 10.1371/journal.pone.0000937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis E. D., Nottebohm F. 1997. Motor-driven gene expression. Proc. Natl Acad. Sci. USA 94, 4097–4102. ( 10.1073/pnas.94.8.4097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschvink J. L. 1991. Uniform magnetic fields and double-wrapped coil systems: improved techniques for the design of bioelectromagnetic experiments. Bioelectromagnetics 13, 401–411. ( 10.1002/bem.2250130507) [DOI] [PubMed] [Google Scholar]
- Liedvogel M., Maeda K., Henbest K., Schleicher E., Simon T., Timmel C. R., Hore P. J., Mouritsen H. 2007a. Chemical magnetoreception: bird cryptochrome 1a is excited by blue light and forms long-lived radical-pairs. PLoS ONE 2, e1106 ( 10.1371/journal.pone.0001106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedvogel M., Feenders G., Wada K., Troje N. F., Jarvis E. D., Mouritsen H. 2007b. Lateralized activation of Cluster N in the brains of migratory songbirds. Eur. J. Neurosci. 25, 1166–1173. ( 10.1111/j.1460-9568.2007.05350.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K., Henbest K. B., Cintolesi F., Kuprov I., Rogers C. T., Lidell P. A., Gust D., Timmel C. R., Hore P. J. 2008. Chemical compass model of avian magnetoreception. Nature 453, 387–391. ( 10.1038/nature06834) [DOI] [PubMed] [Google Scholar]
- Mello C. V., Clayton D. F. 1995. Differential induction of the ZENK gene in the avian forebrain and song control-circuit after metrazole-induced depolarization. J. Neurobiol. 26, 145–161. ( 10.1002/neu.480260112) [DOI] [PubMed] [Google Scholar]
- Möller A., Sagasser S., Wiltschko W., Schierwater B. 2004. Retinal cryptochrome in a migratory passerine bird: a possible transducer for the avian magnetic compass. Naturwissenschaften 91, 585–588. ( 10.1007/s00114-004-0578-9) [DOI] [PubMed] [Google Scholar]
- Mouritsen H. 1998. Compasses and orientational strategies of night migrating passerines. PhD thesis, Institute of Biology, Odense University, Denmark. [Google Scholar]
- Mouritsen H., Mouritsen O. 2000. A mathematical expectation model for bird navigation based on the clock-and-compass strategy. J. Theor. Biol. 60, 283–291. [DOI] [PubMed] [Google Scholar]
- Mouritsen H., Janssen-Bienhold U., Liedvogel M., Feenders G., Stalleiken J., Dirks P., Weiler R. 2004. Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation. Proc. Natl Acad. Sci. USA 101, 14 294–14 299. ( 10.1073/pnas.0405968101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen H., Feenders G., Liedvogel M., Wada K., Jarvis E. D. 2005. Night-vision brain area in migratory songbirds. Proc. Natl Acad. Sci. USA 102, 8339–8344. ( 10.1073/pnas.0409575102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen H., Feenders G., Hegemann A., Liedvogel M. 2009. Thermal paper can replace typewriter correction paper in Emlen funnels. J. Ornithol. 150, 713–715. ( 10.1007/s10336-009-0421-3) [DOI] [Google Scholar]
- Muheim R., Backman J., Åkesson S. 2002. Magnetic compass orientation in European robins is dependent on both wavelength and intensity of light. J. Exp. Biol. 205, 3845–3856. [DOI] [PubMed] [Google Scholar]
- Muheim R., Phillips J. B., Åkesson S. 2006. Polarized light cues underlie compass calibration in migratory songbirds. Science 313, 837–839. ( 10.1126/science.1129709) [DOI] [PubMed] [Google Scholar]
- Muheim R., Åkesson S., Phillips J. B. 2008. Response to R. Wiltschko et al. (J. Ornithol.): Contradictory results on the role of polarized light compass calibration in migratory songbirds. J. Ornithol. 149, 659–662. ( 10.1007/s10336-008-0337-3) [DOI] [Google Scholar]
- Prior H. 2006. Lateralization of spatial orientation in birds. In Behavioural and morphological asymmetries in vertebrates (eds Malashichev Y., Deckel A. W.), pp. 75–85. Georgetown, TX: Landes-Bioscience. [Google Scholar]
- Prior H., Wiltschko R., Stapput K., Gunturkun O., Wiltschko W. 2004. Visual lateralization and homing in pigeons. Behav. Brain Res. 154, 301–310. ( 10.1016/j.bbr.2004.02.018) [DOI] [PubMed] [Google Scholar]
- Ritz T., Adem S., Schulten K. 2000. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718. ( 10.1016/S0006-3495(00)76629-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T., Thalau P., Phillips J. B., Wiltschko R., Wiltschko W. 2004. Resonance effects indicate a radical-pair mechanism for avian magnetic compass. Nature 429, 177–180. ( 10.1038/nature02534) [DOI] [PubMed] [Google Scholar]
- Ritz T., Wiltschko R., Hore P. J., Rogers C. T., Stapput K., Thalau P., Timmel C. R., Wiltschko W. 2009. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys. J. 96, 3451–3457. ( 10.1016/j.bpj.2008.11.072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L. J., Andrew R. J. 2002. Comparative vertebrate lateralization, p. 660 Cambridge, UK: Cambridge University Press. [Google Scholar]
- Rogers L. J., Munro U., Freire R., Wiltschko R., Wiltschko W. 2008. Lateralized response of chicks to magnetic cues. Behav. Brain Res. 186, 66–71. ( 10.1016/j.bbr.2007.07.029) [DOI] [PubMed] [Google Scholar]
- Schulten K., Swenberg C., Weller A. 1978. A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Z. Phys. Chem. NF 111, 1–5. [Google Scholar]
- Solov'yov I. A., Schulten K. 2009. Magnetoreception through cryptochrome may involve superoxide. Biophys. J. 96, 4804–4813. ( 10.1016/j.bpj.2009.03.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich C., Prior H., Duka T., Leshchins'ka I., Valenti P., Gunturkun O., Lipp H. P. 1999. Left-hemispheric superiority for visuospatial orientation in homing pigeons. Behav. Brain Res. 104, 169–178. ( 10.1016/S0166-4328(99)00062-5) [DOI] [PubMed] [Google Scholar]
- Weidner C., Reperant J., Miceli D., Haby M., Rio J. P. 1985. An anatomical study of ipsilateral retinal projections in the quail using radioautographic, horseradish peroxidase, fluorescence and degeneration techniques. Brain Res. 340, 99–108. ( 10.1016/0006-8993(85)90778-4) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 1972. Magnetic compass of European robins. Science 176, 62–64. ( 10.1126/science.176.4030.62) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 1995. Magnetic orientation in animals. Berlin, Germany: Springer. [Google Scholar]
- Wiltschko W., Wiltschko R. 2006. Magnetoreception. Bioessays 28, 157–168. ( 10.1002/bies.20363) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 2007. Magnetoreception in birds: two receptors for two different tasks. J. Ornithol. 148, S61–S76. [Google Scholar]
- Wiltschko R., Ritz T., Stapput K., Thalau P., Wiltschko W. 2005. Two different types of light-dependent responses to magnetic fields in birds. Curr. Biol. 15, 1518–1523. ( 10.1016/j.cub.2005.07.037) [DOI] [PubMed] [Google Scholar]
- Wiltschko R., Munro U., Ford H., Wiltschko W. 2008. Contradictory results on the role of polarized light compass calibration in migratory songbirds. J. Ornithol. 149, 607–614. ( 10.1007/s10336-008-0324-8) [DOI] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 1993. Red-light disrupts magnetic orientation of migratory birds. Nature 364, 525–527. ( 10.1038/364525a0) [DOI] [Google Scholar]
- Wiltschko W., Traudt J., Güntürkün O., Prior H., Wiltschko R. 2002. Lateralization of magnetic compass orientation in a migratory bird. Nature 419, 467–470. ( 10.1038/nature00958) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 2003. Lateralisation of magnetic compass orientation in silvereyes, Zosterops lateralis. Aust. J. Zool. 51, 1–6. [Google Scholar]
- Zapka M., et al. 2009. Visual but not trigeminal mediation of magnetic compass information in a migratory bird. Nature 461, 1274–1277. ( 10.1038/nature08528) [DOI] [PubMed] [Google Scholar]