Abstract
It is now well established that animals use the Earth's magnetic field to perform long-distance migration and other navigational tasks. However, the transduction mechanisms that allow the conversion of magnetic field variations into an electric signal by specialized sensory cells remain largely unknown. Among the species that have been shown to sense Earth-strength magnetic fields, birds have been a model of choice since behavioural tests show that their direction-finding abilities are strongly influenced by magnetic fields. Magnetite, a ferromagnetic mineral, has been found in a wide range of organisms, from bacteria to vertebrates. In birds, both superparamagnetic (SPM) and single-domain magnetite have been found to be associated with the trigeminal nerve. Electrophysiological recordings from cells in the trigeminal ganglion have shown an increase in action potential firing in response to magnetic field changes. More recently, histological evidence has demonstrated the presence of SPM magnetite in the subcutis of the pigeon's upper beak. The aims of the present review are to review the evidence for a magnetite-based mechanism in birds and to introduce physiological concepts in order to refine the proposed models.
Keywords: magnetoreception, birds, magnetite, trigeminal nerve, mechanosensitive ion channels
1. Introduction
Many bird species undertake long-distance migrations to allow them to use available resources for feeding and breeding. A record is the migration of the arctic tern, which flies each year from its breeding colony in the Arctic to the Antarctic, a 19 000 km each-way journey, with the pay-off that the tern sees two summers each year and ensures a continuous food supply. Another type of movement common in birds is dispersal, where birds move away from their hatching place. Finally, birds accomplish daily or more long-term journeys in order to gather the food they need. In addition to these natural journeys, some birds have been bred by humans to act as messengers. Swallows were used by the Romans to announce the results of chariot races. Homing pigeons, the direct descendants of the rock dove, have been used since the days of ancient Egypt to carry messages and are well known for accurate navigation over long distances and in poor weather conditions where many of the more obvious navigational cues are lacking.
Birds rely on a wide range of cues in order to navigate successfully. Cues used for these steps include visual landmarks (Biro et al. 2007); the direction of Sun, Moon and stars, and polarized light, which can indicate the Sun's position even in heavy cloud (Muheim et al. 2006); olfactory cues (Wallraff 2004; Nevitt et al. 2008); and auditory cues (Hagstrum 2000). It has, by now, been well established that birds also rely heavily on navigational cues provided by the Earth's magnetic field (the geomagnetic field (GMF); see Able 1994). The GMF is a direct consequence of convection currents in the Earth's liquid metallic iron core, which generate electric currents and, in turn, produce a magnetic field. To a good approximation, the surface field would result from a magnetic dipole located in the Earth's centre with its south pole pointing towards geomagnetic north. The inclination angle formed between the magnetic field lines and the Earth's surface depends on the latitude—field lines are parallel to the surface of the globe at the equator but are vertical at the poles and the angle of inclination can therefore be used as a guide to latitude. Another important parameter for animal navigation is the magnetic field intensity, which is weakest near the equator (around 30 µT) and strongest at the poles (approx. 60 µT). In addition, local variations of magnetic field angle and intensity exist owing to the presence of ferromagnetic mineral deposits within the crust. In summary, the GMF can be defined by three parameters, direction, inclination and intensity. Each of these parameters independently forms a magnetic landscape that could, in principle, be used by animals for both long- and short-distance migrations.
The idea that birds can use the GMF probably dates back to the early use of the GMF for human navigation, but was first articulated in the second half of the nineteenth century (Von Middendorf 1859). Many experiments have been carried out on pigeons and their homing behaviour in field trials. Vigiuer (1882) proposed that pigeons use a navigational map that includes magnetic parameters. It was later shown that pigeons can home without visual cues (Walcott & Schmidt-Koenig 1973; Schmidt-Koenig & Walcott 1978) and that the orientation behaviour of pigeons was altered by the application of a magnetic field (Walcott & Green 1974). The idea of magnetic sensation in birds was experimentally confirmed by Wiltschko & Merkel (1966), who demonstrated that captive European robins in the migratory season attempt to escape confinement, in the absence of any other directional cues, in a direction dictated by the ambient magnetic field. Since then, a large amount of evidence has been produced to support the existence of a magnetic sense in many bird species that helps the animal to head in a particular direction during migration and establishes a magnetic map that is based on the detection of small changes in magnetic intensity (see Able 1994; Wiltschko & Wiltschko 2002, 2005; Mouritsen & Ritz 2005).
2. Possible transduction mechanisms to explain magnetoreception
In order to detect a sensory stimulus, a sensory neuron must transform the chemical or physical energy of the stimulus into an electrical signal that can be transmitted to, and processed by, the brain. In the simplest case, the stimulus can be directly detected by an ion channel (e.g. in the case of thermal sensation; see Dhaka et al. 2006). More commonly, the stimulus is detected by a specialized detection mechanism—a G-protein-coupled receptor in the case of vision or olfaction, or the hair cell cilia in the case of hearing—and is then transmitted to the ion channel that modulates the cell membrane potential. It has been shown that ion channel activity can be directly modulated by high-intensity magnetic fields through an action on membrane phospholipids (Petrov & Martinac 2007) but the field intensities required are far above the Earth-strength magnetic fields whose detection must underlie navigation. During the past 40 years, researchers have proposed a number of theories to explain the ability of living organisms to detect Earth-strength magnetic fields, but only two are backed by experimental evidence and are currently in contention: light-dependent and magnetite-based magnetoreception. This review mainly considers evidence for the second, but we will first give a brief overview of the light-based mechanism.
2.1. Light-dependent magnetosensation
One of the first indications that birds may use light-based magnetoreception was provided by a work on pigeons (Wiltschko & Wiltschko 1981). European robins can orient well in blue or green light but are disorientated in red or yellow-orange light (Wiltschko & Wiltschko 1995, 1999, 2001; Wiltschko et al. 2001). Similar results were found for Australian silvereyes (Wiltschko et al. 2000). These results suggest that illumination with blue/green light is necessary for the birds to extract information from the GMF. More recent work suggested that this is a highly lateralized process, and that only the right eye of the European robin is involved in the detection (Wiltschko et al. 2002a).
A theory that may explain these results is the radical pair mechanism involving electron transfer from a donor to an acceptor molecule (Schulten & Weller 1978; Ritz et al. 2000). Cryptochrome has been suggested as a possible molecular candidate (Ritz et al. 2000; Mouritsen et al. 2004) and has been recently shown to generate free radicals (Liedvogel et al. 2007). In addition, it was also shown that migratory birds displayed co-localization of cryptochrome and neuronal activity during migration (Mouritsen et al. 2004). In a recent behavioural assay for magnetosensitivity, wild-type flies show significant naive and trained responses to a magnetic field under full-spectrum light (approx. 300–700 nm) but do not respond to the field when wavelengths in the Cry-sensitive, ultraviolet-A/blue-light part of the spectrum (less than 420 nm) were blocked. Notably, Cry-deficient flies do not show either naive or trained responses to a magnetic field under full-spectrum light (Gegear et al. 2008). For reviews of the evidence supporting the light-dependent theory of magnetosensation, the interested reader is referred to a recent review (Wiltschko & Wiltschko 2005) and articles on cryptochrome-based magnetosensation by Liedvogel & Mouritsen (2010) and by Ritz et al. (2010).
2.2. Magnetite-based magnetosensation in birds
Magnetite (Fe3O4 or iron (II, III) oxide) is the most common magnetic mineral found on Earth. Of all natural iron oxides, it has the strongest magnetism. Until the late 1960s, magnetite was thought to be a mineral found in purely inorganic contexts, but in 1967, Lowenstam (1967) demonstrated that the tongue of the chiton, a polyplacophoran marine mollusc, contains magnetite in order to help this animal graze on the biofilm present on the surfaces of rocks. This major discovery was followed by observations by Blakemore that certain types of mud bacteria produce chains of nanometre-sized magnetite crystals (Blakemore 1975; Frankel et al. 1979) surrounded by a membrane (Gorby et al. 1988). The magnetite crystals have a well-defined shape and size and their formation is under tight biological control (for a detailed review see Komeili 2007; Faivre & Schuler 2008). These observations led researchers including Kirschvink & Gould (1981) to propose that magnetite may mediate magnetoreception in animals. Since then, magnetite has been described in a large number of animals.
The ability of magnetite to retain a permanent magnetic field depends on the crystal size. When the magnetite crystal length is below approximately 50 nm, the predominant force acting on an individual crystal is thermal agitation, which constantly flips the direction of magnetization of the crystal. The consequence is that the Earth's magnetic field cannot exert a magnetic torque on an individual crystal smaller than 50 nm or so. Such crystals are referred to as superparamagnetic (SPM). As opposed to a single SPM particle, an assemblage of SPM particles can be magnetized in an external magnetic field, but loses its magnetization as soon as the magnetic field is turned off. Magnetite crystals larger than 50 nm, on the other hand, retain their magnetization and are called magnetic single-domain particles. Still larger crystals of magnetite (larger than about 100 nm) are too large to host a single magnetic domain and consist of multiple domains in which a number of single domains each of approximately 50 nm diameter coexist. Initially, only single-domain crystals were thought to provide optimal detection of Earth-strength magnetic fields; however, models based on SPM particles have been generated and tested (see below).
2.2.1. Single-domain magnetite
Walcott et al. (1979) located an area of the pigeon head containing magnetic material between the skull and the dura. Electron microscopy revealed the presence of small electron-dense objects of 80–150 nm in length. The tissue contains nerve fibres and connective tissue. The magnetic remanence of the material disappeared at 575°C indicating the presence of magnetite. Several authors subsequently reported the presence of single-domain magnetite in birds, mainly based on magnetic measurements (Beason & Nichols 1984; Beason & Brennan 1986; Edwards et al. 1992). There have been no subsequent reports of single-domain magnetite in birds. Pulse-remagnetization experiments carried out in the silvereye (Wiltschko et al. 2002b) have argued against the possibility of a freely rotating chain of single-domain magnetite crystals as had been proposed for fish (Walker 2008). So far, direct evidence of single-domain magnetite in vertebrates only exists for the fish species Scombridœ (Walker et al. 1984) and Salmonidœ (Mann et al. 1988; Walker et al. 1988, 1997).
2.2.2. Superparamagnetic magnetite
Small crystals of magnetite of size less than 50 nm, too small to form single domains, were initially described in the skin of the upper beak of the homing pigeon, as marked at site 1 in figure 2a (Hanzlik et al. 2000). Strong Prussian blue staining revealed the presence of iron between fat cells (figure 1a) denoting the presence of clusters 1–3 µm in size of extremely fine-grained magnetite (1–5 nm; see Winklhofer et al. 2001). Magnetic measurements confirmed the presence of SPM iron, which cannot retain a remanent magnetization at room temperature, since a remanence imparted at 10 K largely decayed when the temperature was brought to 200 K. This result was confirmed by Tian and co-workers (2007) in a recent study where they showed that the beak from female pigeons displayed a higher concentration of magnetite. On the basis of initial electron micrographs of an SPM cluster in the pigeon beak, Shcherbakov & Winklhofer (1999) proposed a model in which SPM particles are suspended in a biological liquid and therefore behave like a ferrofluid. According to the model, the ambient magnetic field is converted into a deformation of the cluster, which could produce deformation of the plasma membrane and therefore trigger mechanosensitive ion channel openings. This magnetic-field-induced deformation model applies to an individual cluster. A refined model proposed by the same group (Davila et al. 2003) is based on magnetic interactions among SPM clusters, which were found to occur in groups of 10–20 clusters.
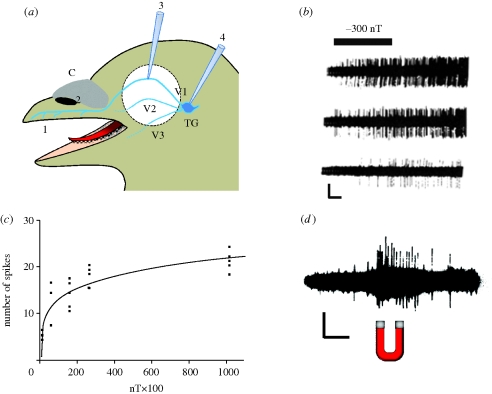
Figure 2.
Trigeminal magnetosensation in birds. (a) Schematic representation of a bird head with the trigeminal system. 1, Site for the SPM magnetite found by the Fleissner group (figure 1); 2, site for iron accumulation found by Williams & Wild (2001) linked to trigeminal nerve ending in the pigeon (Columbia livia) and in the zebrafinch (Taeniopygia guttata); 3, electrophysiological recording from the trigeminal nerve in the bobolink by Beason & Semm (1987); 4, electrophysiological recordings from trigeminal neurons by Semm & Beason (1990) in the bobolink; TG, trigeminal ganglion; C, cere; V1, ophthalmic branch of trigeminal nerve; V2, maxillary branch; V3, mandibular branch. Figure modified from Fleissner et al. (2003). (b) Extracellular recordings obtained from cells within the trigeminal ganglion of the bobolink showing an increased activity in the presence of a magnetic stimulus (solid bar), which shows a decrease in the vertical component of the GMF. Note that these are three successive responses and that they decrease in frequency. Horizontal scale bar 1 s, vertical 2 mV. (c) Stimulus-response curve obtained with the same conditions as in (b). (d) Application of a strong magnet close to the preparation triggers the same response as described in (b). Scale bar: horizontal 50 ms, vertical 2 mV. (b–d) are taken from Semm & Beason (1990) with permission of Elsevier.
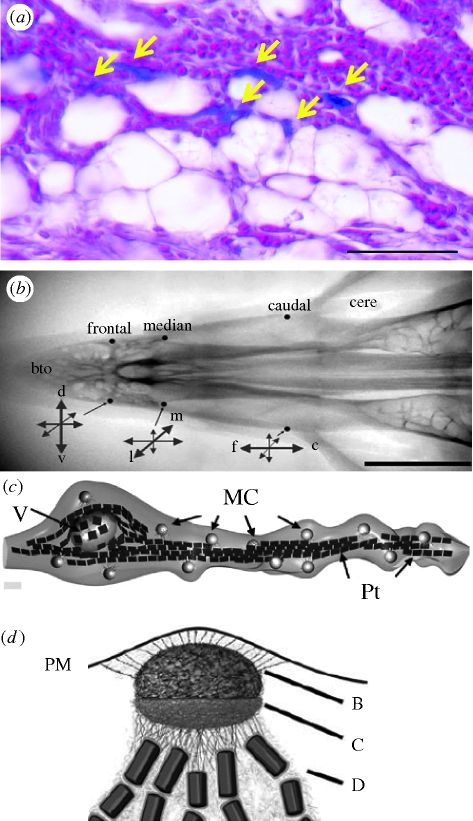
Figure 1.
Presence of SPM magnetite in the upper skin of the beak of the homing pigeon (Columbia livia). (a) Prussian blue staining showing iron deposits (yellow arrows) between fat cells. Scale bar: 50 µm. (b) View of the beak showing where magnetite was found by the Fleissner group. Three pairs of spots were found with different alignments. The caudal nerve terminals were mostly aligned in a longitudinal pattern, whereas the median ones where set transversally. The frontal magnetite-containing endings were aligned vertically. Scale bar: 0.5 cm. (c) Three-dimensional reconstruction of a nerve ending showing the SPM magnetite clusters (MC) connected by iron platelets (Pt). Note the presence of a giant vacuole (V) on the left of the panel. (d) Close-up diagram of magnetite clusters. Note that the cluster (SPM) is connected to the plasma membrane (PM) through filaments (B). The cluster is resting in a fibrous basket (C) and is connected to the iron platelets (D). Illustrations are taken from Fleissner et al. (2007).
Further investigations carried out by the Fleissner group used an antibody directed against avian neurofilaments to demonstrate that SPM clusters were within nerve terminals (Fleissner et al. 2003). These nerve endings were found in six spots (three each side) along the rim of the beak and each was aligned differently with respect to the beak axis, as shown in figure 1b (Fleissner et al. 2007). Reconstruction of the nerve terminals by the use of electron microscopy showed a rather complex system. The SPM magnetite is contained within a fibrous basket (figure 1d) and seems to communicate with the plasma membrane via thin filaments. On the other side of the cluster, the fibrous basket is bound to iron-rich platelets, which run through the axonal process joining clusters as shown in figure 1c. Another conspicuous feature is a vesicle that is surrounded by the platelets. The platelets were first proposed to be amorphous and made of iron and phosphorus. In a new study, the Fleissner group demonstrated that the iron in these platelets is ferric (FeIII). Using microsynchrotron X-ray near-edge absorption spectroscopy, Fleissner et al. (2007) showed that the X-ray absorption spectrum of the dendrite can be explained by a combination of 10–20% magnetite and 80–90% maghemite, a ferromagnetic oxide that is chemically and structurally closely related to magnetite, except that all iron in maghemite is ferric. They propose a novel concept for SPM-based magnetoreception, which is built on the assumption that the platelets each represent single crystalline domains of maghemite and that the maghemite platelets would concentrate magnetic field lines onto the SPM cluster. Deformation of the membrane owing to the pull of the SPM clusters on linkers (figure 1d) would then open mechanosensitive ion channels. This model was later given a theoretical basis by Solov'yov & Greiner (2007). Recently, the Fleissner group extended their observations to other species such as the domestic chicken, the European robin and the garden warbler (Stahl et al. 2007).
In a critical comment, Winklhofer & Kirschvink (2008) questioned the mineralogical interpretation given in Fleissner et al. (2007). First, the window selected for the measurements does not allow discrimination between maghemite and other possible ferric iron compounds. Second and more importantly, the crystalline nature of the platelets postulated in Fleissner et al. (2007) contradicts the previous electron diffraction results reported in Fleissner et al. (2003).
Nevertheless, and even if questions remain about the components of this putative magnetosensitive structures, tremendous progress has been achieved by the Fleissner group in understanding biogenic magnetite-containing structures in the beak of the pigeon. These structures possess several attractive characteristics to be one of the sites for magnetoreception: (i) the magnetite-containing compartments are of neural origin; (ii) iron deposits are spatially organized both at the beak and at the cellular level, which rules out the possibility of random contamination, and both maghemite platelets and SPM clusters are embedded in filaments of organic origin; (iii) the SPM clusters are located close to and tethered to the plasma membrane, which could link them to mechanosensation and ion channel transduction (see below); and (iv) the localization is consistent with the physiological work carried out by Semm and Beason on the trigeminal system of the bobolink (Beason & Semm 1987; Semm & Beason 1990) and the behavioural experiments carried out by Mora et al. (2004).
2.2.3. Other possible sites
In addition to single-domain magnetite and SPM magnetite, other locations in birds could play a role in iron-based magnetoreception. Williams & Wild (2001) found an area behind the cere heavily stained with Prussian blue and innervated by the trigeminal nerve. Contrary to the structures described earlier, the Prussian blue seems to be located within non-neural cells in contact with the trigeminal nerve. However, the nature of these iron deposits and the ultrastructure of the cells remain to be investigated. Another interesting finding is the presence of magnetic material in the cochlea of birds (Harada et al. 2001; Harada 2008; Zhao et al. 2009). These particles can be moved when a magnetic field is applied (Harada et al. 2001) and are present in the otoconia, which line the otolithic membrane. A change in magnetic field could therefore cause a change in the orientation of hair cell cilia and subsequently the opening of mechanosensitive ion channels. Magnetic material has also been discovered in the neck musculature of the pigeon and the white-crowned sparrow (Presti & Pettigrew 1980).
2.2.4. Physiological evidence for a magnetite-based receptor in birds
To the best of our knowledge, the most convincing physiological work regarding magnetite-based receptors in birds has been performed by Beason & Semm (1987) on the bobolink (Dolichonyx oryzivorus). Initially, extracellular recordings were carried out mainly on the ophthalmic branch of the trigeminal nerve, corresponding to site number 3 in figure 2a. Three types of electrical changes in response to alteration of either the horizontal or vertical components of the GMF could be observed: (i) a rapid and complete inhibition of existing action potential discharge, (ii) a progressive increase in discharge, and (iii) a slow decrease in discharge. The fibres could only be re-stimulated successfully 4–5 min after the previous response suggesting the presence of a refractory period. More importantly, the latency was short suggesting that the transduction mechanism was taking place within the fibres. Because recordings from nerve fibres were limited by potential mechanical artefacts owing to preparation movements, they then decided to carry out recordings from the neuronal soma within the trigeminal ganglion itself (Semm & Beason 1990; site number 4 in figure 2a). Although the number of responsive units was lower, they were able to observe acceleration of the spontaneous activity in response to either a decrease (figure 2b) or an increase in the vertical component of the ambient magnetic field. Cells were shown to be responsive to as little as 200 nT and the number of spikes showed a plateau between 20 and 100 µT, as shown in figure 2c. The latency of the response was longer compared with the previous work (4 s in this case). However, the cells could be re-stimulated within a period of 10–20 s. Because trigeminal neurons are sensitive to heat (Liu & Simon 2000), the responses observed could be due to neurons activated by heat generated by the coil system. In order to rule out such an effect, the authors moved a permanent magnet near the preparation and were able to observe similar effects (figure 2d). Another interesting feature is that the cells could also be activated by a sine wave and apparently locked on to one phase of the wave cycle but not to its anti-phase. As Kirschvink and co-workers (2001) pointed out, this behaviour is similar to that observed by Walker et al. (1997), who reported that units in the ophthalmic branch of the trigeminal nerve of the rainbow trout responded to either the onsets or offsets of step changes in magnetic intensity, but not to both.
From these experiments, it can be concluded that there is in the trigeminal nerve of birds a magnetic sensing system independent of the visual system. The responses show the characteristics of true physiological responses, such as the appearance of a plateau as stimulus intensity is increased (figure 2c). However, it has to be stressed that these pioneering studies have never been reproduced (see Ritz et al. 2002). One of the possible explanations could be that the positions of the somas of neurons within the trigeminal ganglion were not investigated and mapped in detail.
2.2.5. Behavioural experiments supporting a magnetite-based mechanism
The pigeon is well known for its homing feats and for this reason has been extensively used to investigate the effects of magnetic fields on navigational behaviour. Pioneering work carried out by Keeton (1971) showed that pigeons carrying magnets were disoriented when flying in overcast conditions (Wiltschko et al. 1981). Similar results were obtained by Walcott & Green (1974) with pigeons carrying a coil system. However, experiments implemented in natural conditions are not always consistent owing to the number of different navigational cues that pigeons can rely upon (Walcott 1996). Several attempts have therefore been made to investigate pigeon magnetosensation in controlled experimental conditions. In 1977, Bookman used a flight tunnel in which the magnetic field could be controlled. Using food for conditioning, pigeons were shown to be able to discriminate between magnetic fields of 50 µT and 2 µT (Bookman 1977). Some of the best experimental evidence for trigeminally mediated magnetoreception in pigeons has been provided by Walker and co-workers (Mora et al. 2004). In an elegant set of conditioning experiments, they were able to train pigeons to recognize a magnetic anomaly of 189 µT located at the centre of the experimental tunnel, which the birds could discriminate with a sensitivity significantly greater than 50 per cent. Their ability to perform the task was impaired by small neodymium iron boron magnets attached to the cere. The anaesthetic lignocaine applied in the nasal cavity completely suppressed the bird's ability to detect the anomaly, as did section of the ophthalmic branch of the trigeminal nerve at the level of the orbits. Birds that had their olfactory nerve sectioned did not show any change in their behaviour except when their trigeminal nerve was subsequently sectioned. These results confirmed (i) that the pigeons were able to detect the magnetic anomaly at levels significantly above chance and (ii) that the trigeminal nerve was critical for this task.
Confirmation that pigeons use magnetic field information in natural conditions was obtained by the same group (Dennis et al. 2007). Birds were fitted with a miniature global positioning device, which when recovered could allow the flight trajectory of the pigeons to be analysed. One of the findings of the analysis was that following release the birds flew in box-like patterns following lines of magnetic field intensity. One of the possible interpretations is that the birds are sampling the local GMF in order to build a navigational map. Additional evidence that pigeons detect magnetic field intensities is that they are able to use a magnetic anomaly to locate food (Thalau et al. 2007). However, in another set of experiments, Gagliardo et al. (2006) found that trigeminally sectioned pigeons were almost unaffected in their homing behaviour.
Magnetic pulse experiments provided further support for the idea that birds use magnetite to sense Earth-strength magnetic fields. A strong pulse will alter the arrangement of single-domain magnetite crystals but will not affect a photopigment-based mechanism. A short pulse of 500 mT applied for 2 ms can also disrupt a chain of SPM droplets (Davila et al. 2005). A pulse of similar value was able to disorient silvereyes (Zosterops lateralis), a Southern Hemisphere migratory bird (Munro et al. 1997; Wiltschko et al. 1998). Only adults appeared to be affected by the pulse, perhaps because juvenile birds rely on an innate mechanism for their heading whereas adults have constructed a navigational map that they have previously built by sampling magnetic field intensities during their journeys. Pulse effects last only a few days (Beason et al. 1997), perhaps because this is the time for magnetosensitive tissue damaged by the pulse to be repaired or to regenerate (Wiltschko et al. 2007). The source of the damage could be either a dislocation of the SPM magnetite clusters, or possibly overstimulation of the receptors by the intense pulse magnetic field, which renders the transduction mechanism non-functional for a time.
An elegant experiment carried out on the silvereye gives some clues about the area implicated in magnetoreception (Wiltschko et al. 2009). Birds were not disoriented by a pulse when xylocaine, a local anaesthetic, was gently applied to the skin of the upper beak, which suggests a possible involvement of the structures described by the Fleissner group (see earlier). Another pulse-type test consists of applying a biasing field and then another set of brief pulses: parallel and anti-parallel. If magnetite crystals are free to rotate then the parallel pulse will have no effect but the anti-parallel pulse will reorient the magnetic moment in the opposite direction. This approach is well established in bacteria and has been successful in bats (Holland et al. 2008), but in the silvereye it disorients the animals but does not have the expected effect as there was no difference between parallel and anti-parallel pulses (Wiltschko et al. 2002a). This experiment indicates either that single-domain magnetite crystals are not free to move, or that SPM particles are instead involved.
3. Mechanosensitive ion channels
As detailed earlier, a possible model based on current evidence is that SPM magnetite clusters present in the upper beak of the homing pigeon are tethered to mechanosensitive ion channels situated in the plasma membranes of trigeminal nerve terminals. The observation of filaments linking the SPM clusters and the plasma membrane supports this assumption. Mechanosensitive ion channels are present in all living organisms, but despite intensive work their identities remain open to debate, especially in higher organisms. We present below a brief review of the known properties of mechanosensitive ion channels, focusing mainly on what is currently understood about mechanosensory processes in sensory neurons, with the rationale that the same processes may apply in magnetite-based mechanotransduction.
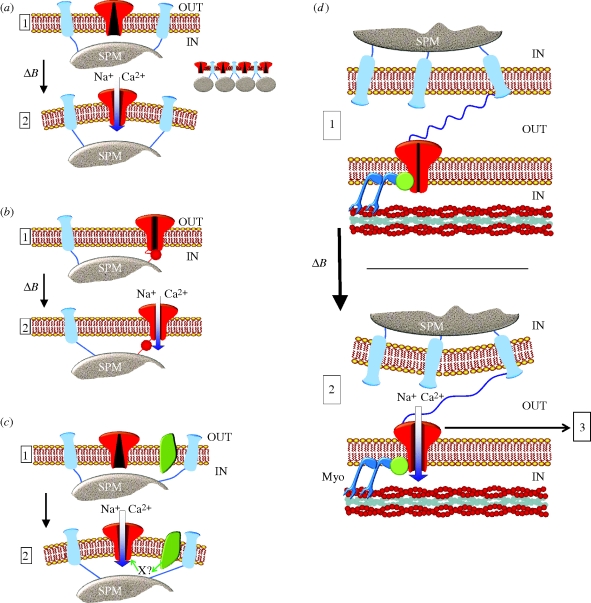
Figure 3 presents four possible scenarios. In the first model, presented in figure 3a, magnetite exerts a pull directly on the membrane through the filaments described by Fleissner and co-workers (2003). Mechanosensitive ion channels then open in response to membrane stress, allowing positively charged ions (e.g. Na+, Ca2+) to flow into the cell causing membrane depolarization. There are many possible candidates for an ion channel activated by membrane deformation rather than by some more direct connection. Ion channels that are directly gated by membrane stress are abundant in bacteria and archaea, where they open in response to a decrease in osmolarity and therefore help to maintain cellular integrity. An example is MscL from Escherichia coli discovered by Martinac et al. (1987) and cloned and sequenced subsequently by the same team (Sukharev et al. 1994). MscL analogues have also been discovered in plants but not to date in animals (Martinac & Kloda 2003). In animals, an important class of mechanosensitive ion channels is formed by TREK-1/2 and TRAAK, which belong to the two-pore-domain potassium channel family (K2P) (Dedman et al. 2009). TREK-1, the best studied, is expressed in the brain (Hervieu et al. 2001) and in sensory neurons (Alloui et al. 2006; Honore 2007). Another important class of mechanosensitive ion channel is formed by the large transient receptor potential (TRP) ion channel superfamily. The prototype TRP channel was discovered in Drosophila where TRPL mediates the response to light (Hardie & Minke 1992). TRP channels are cation channels mediating a wide range of sensory processes and possessing characteristic multiple ankyrin motifs in the N-terminal cytoplasmic and consist of 33 isoforms spread over seven subfamilies—TRPC, TRPA, TRPV, TRPM, TRPP, TRPML and TRPN. Among the TRP channel family, TRPV4 is the most studied mechanosensitive channel and is expressed in trigeminal neurons (Chen et al. 2009). A homologue of TRPV4 was found in Caenorhabditis elegans, where Osm-9 mutants displayed a lack of osmotic avoidance behaviour (Colbert et al. 1997). Interestingly, and although they only share 24 per cent homology, the osmotic avoidance behaviour could be restored by transfecting the mutant with TRPV4 (Liedtke et al. 2003). Another interesting mechanosensitive TRP is TRPC1. Martinac's group (Maroto et al. 2005) found the presence of an ionic current in response to suction when a membrane fraction of Xenopus laevis oocytes was reconstituted into giant proteoliposomes. The observed activity could be traced back to TRPC1. This ion channel is present in sensory neurons (Elg et al. 2007). However, further studies of TRPC1 are limited by the fact that it cannot be expressed in heterologous system (Gottlieb et al. 2008).
Figure 3.
Proposed scenarios for mechanically based magnetoreception. (a) Mechanosensitive ion channels are located in the membrane but do not have a physical link with the magnetite. Magnetite field changes (ΔB) trigger movement of a SPM magnetite cluster or a chain of clusters (right panel), which in turn induces deformation in the plasma membrane. This latter event induces the opening of a non-selective mechanosensitive ion channel. (b) Magnetite is physically connected to the ion channel. At rest, the ion channel is blocked. Change in the magnetite field (ΔB) relieves the blockade allowing sodium and calcium entry into the cells. (c) Magnetite movement releases a second messenger (X), either directly or through the creation of tension in the membrane. The messenger binds to the ion channel and opens it. (d) Mechanism based on auditory hair cell mechanotransduction. In this model, a cell containing magnetite is linked to a neighbouring nerve terminal through a ‘tip link’ (blue filament). The ion channel is linked via a molecular motor (Myo) to actin filaments (red). Movements in the magnetite-containing compartment stretch the link and open the ion channel. After channel opening, a molecular motor could reposition the ion channel reducing the tension in the link and allowing the system to be stimulated again (step 3).
A second possible model for magnetite-based mechanosensation postulates an intracellular connection from magnetite movement to an ion channel, either via a mechanical link (figure 3b) or via an intracellular messenger (figure 3c). A direct mechanical connection is well known in the case of the touch mechanoreceptor complex in C. elegans. By a screen of mutant worms insensitive to touch, Chalfie & Sulston (1981) identified a group of genes dubbed MEC. It was later found that MEC-2-4-6 and 10 formed an ion channel while MEC-1, 5 and 9 link the cuticule externally to the ion channel and MEC-2 connects the channel to cytoskeletal elements represented by MEC-1, 2 and 7 (see review by Drew & Wood 2005). Ion channels homologous to MEC-4 have been cloned and are expressed in vertebrate sensory neurons (Waldmann et al. 1997). These ion channels, known as acid-sensing ion channels (ASICs), are gated by a drop in extracellular pH and are involved in acid-mediated pain (Jones et al. 2004), but they have also been proposed to act as mechanosensors (Price et al. 2001; Page et al. 2004). Interestingly, ASIC3 co-immunoprecipitates with SPL3 (stomatin-like protein 3), a mammalian homologue of MEC-2 and mice with a SPL3 mutation show a strong reduction in touch sensation (Wetzel et al. 2007). These results indicate that a mechanosensitive complex similar to that found in the nematode may also exist in sensory neurons of upper vertebrates.
A second messenger rather than a direct mechanical link is a third possibility. In this model, presented in figure 3c, movement of magnetite results in the production of a small molecule, X, which in turn activates the transducer ion channel. In olfactory neurons, mechanical stimulation enhances the production of cAMP and triggers the opening of cyclic nucleotide-gated channels, which increases odour sensitivity (Grosmaitre et al. 2007). Another possible messenger candidate is PIP2, which has been shown to modulate GIRK (G-protein-activated inwardly rectifying potassium channel) in another mechanically activated system (Zhang et al. 2004).
A final possibility, analogous to the mechanotransduction processes present in auditory hair cells, postulates a connection between magnetite-containing cells and neurons expressing mechanically gated ion channels (figure 3d). Hair cells are highly specialized neuronal cells present in the inner ear of vertebrates and in the lateral line of fish. Mechanically gated ion channels are connected to adjacent cilia via an extracellular tip link (Pickles et al. 1984) made of cadherin 23 and protocadherin 15 (Kazmierczak et al. 2007). Deflections of the adjacent cilia increase tension in the tip link, which in turn opens the ion channel, allowing entry of cations (in this case K+ and Ca2+). An adaptation mechanism allows the system to be re-stimulated. Part of this system encompasses a molecular motor (Myosin1C) that helps to close the ion channel by returning tension in the tip link to its resting level (see Gillespie & Corey 1997 for review). The identity of the ion channel itself remains open to debate. P2X and ENaC ion channels have been proposed (Strassmaier & Gillespie 2002), but more recent evidence favours members of the TRP ion channel superfamily (Corey 2006). As depicted in figure 3d, the hair cell mechanism could be adapted to magnetoreception. In this model, the magnetite is located in a nerve terminal, which is connected to a neighbouring cell via a ‘tip link’. Movement of the first compartment due to magnetic field changes pulls the link, which in turn opens the ion channel. A molecular motor would also be an interesting mechanism to consider for magnetoreception (step 3 in figure 3d). This mechanism could reset the alignment of the nerve terminals and since the GMF is always present, it would allow the system to be very sensitive to changes of magnetic field intensity.
The earlier mentioned review of our knowledge of mechanically activated ion channels explores possible mechanisms by which a mechanically mediated magnetoreception could transduce the mechanical energy resulting from magnetite movements into changes in cell membrane potentials. Ion channels of the TRP superfamily may be involved, as these channels are known to be mechanosensitive, and several members are closely involved with sensory transduction in other contexts. It may also be of interest to investigate a possible role for other mechanosensitive ion channels such as SPL3 or TREK.
4. Physiological tools to approach magnetite-based magnetoreception in birds
As stressed by Němec and co-workers (2005), compared with the vast amount of behavioural data, only a few studies have tackled magnetoreception using neurobiological and physiological techniques. The past 10 years have seen an increase in our knowledge of magnetite-containing structures in birds owing, in particular, to the meticulous work of the Fleissner group. However, the central question that remains is the involvement of this neuronal tissue in magnetosensation. Implementing physiological methods should enable us to solve the cellular basis of magnetite-based magnetoreception. Techniques developed in the field of somatosensation, such as the skin nerve preparation (Reeh 1986), could be adapted to the pigeon's beak. This preparation has proven useful to investigate the functional properties of afferent fibres and the regulation of their electrical properties by various pharmacological agents (for a recent methodological paper see Zimmermann et al. 2009). Other methods such as detection of stimulus-evoked expression of inducible transcription factor (Němec et al. 2005) should also be used extensively for trigeminal-mediated magnetoreception in birds. We also envision that a combination of nerve-tracing experiments and tissue/cell culture approaches should provide material for further electrophysiological and/or calcium-imaging experiments.
Once a technique is established to record consistent magnetic responses from magnetite-containing fibres/cells, a set of experiments should be designed to investigate whether the magnetite response is indeed mechanically mediated, in the sense that applying a mechanical stimulus to the preparation mimics the effect of magnetic field changes. Several methods exist to apply a mechanical stimulus to a cell. A popular method in the field of prokaryotic mechanosensitive ion channels consists of applying a negative pressure to the spheroplast or liposomes through a patch-clamp pipette (see Perozo 2006; Martinac et al. 2008 for reviews). This method has been used for a variety of eukaryotic cells such as Xenopus oocytes (Maroto et al. 2005) and rat dorsal root ganglion (DRG) neurons (Su et al. 2000), but one could argue that the technique may disrupt the delicate arrangement of the intracellular magnetosensory structure. A more convenient way to mechanically stimulate a cell consists of applying a glass pipette using a piezoelectric system. This approach has been used successfully on isolated DRG neurons (Drew et al. 2002; Di Castro et al. 2006). Alternatively, membrane deformation can be induced by application of a hypo-osmotic solution, which swells the cell, or by perfusing the cell with conical amphipathic molecules, which insert into the cell membrane to cause a change of curvature of the membrane (Markin & Martinac 1991). Trinitrophenol or picric acid induces the activation of a bacterial mechanosensitive channel (Martinac et al. 1990) and TRPA1 (Hill & Schaefer 2007), whereas chlorpromazine inhibits TREK (Miller et al. 2003). These compounds are of particular relevance when the ion channels are intrinsically gated by membrane stress (figure 3a). Other compounds are also useful tools to study mechanosensitive ion channels: gadolinium, a lanthanide, and some aminoglycoside antibiotics such as neomycin and streptomycin are potent blockers of some mechanically activated ion channels (Hamill & McBride 1993). Cytoskeletal inhibitors can also be of assistance particularly when the ion channels are linked to the cytoskeleton (cases presented in figure 3b,d). Ultimately, a tip-link model could be tested (figure 3d) using calcium chelators and lanthanum ions, which have been shown to disrupt the link (Assad et al. 1991; Kachar et al. 2000).
5. Conclusion
A body of evidence suggests that birds use magnetite associated with the trigeminal nerve (ophthalmic branch) to detect magnetic fields, in accordance with the results found in other vertebrates such as the rainbow trout (Walker et al. 1997) and mole rat (Wegner et al. 2006). However, three major differences exist between birds and fish regarding the putative magnetoreceptor cells: (i) in birds, magnetite is located in nerve terminals, whereas in the fish it appears to be located in the cell soma; (ii) birds appear to use SPM magnetite, while fish use single-domain magnetite; and (iii) the magnetoreceptors are in the beak of birds but in the olfactory mucosa in fish.
A few important questions remain. The first, of course, is to collect direct observations of magnetic sensing by the SPM magnetite clusters present in the skin of the upper beak of birds using physiological methods. Secondly, it would be of vital importance to generalize observations to a wide range of bird species and to other vertebrate species. Finally, we need to understand how organisms integrate information from light and magnetite-based magnetoreception.
Acknowledgements
The authors are grateful to Dr Pavel Němec and to Dr Michael Winklhofer for fruitful discussion concerning magnetoreception and their constructive suggestions about the manuscript. We thank Mrs Nur Airina Muhamad for proofreading the manuscript. The authors are also immensely grateful to Mr Peter Bircham for his insight into avian ecology and zoology.
Footnotes
One contribution to a Theme Supplement ‘Magnetoreception’.
References
- Able K. P. 1994. Magnetic orientation and magnetoreception in birds. Prog. Neurobiol. 42, 449–473. ( 10.1016/0301-0082(94)90047-7) [DOI] [PubMed] [Google Scholar]
- Alloui A., et al. 2006. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 25, 2368–2376. ( 10.1038/sj.emboj.7601116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad J. A., Shepherd G. M., Corey D. P. 1991. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7, 985–994. ( 10.1016/0896-6273(91)90343-X) [DOI] [PubMed] [Google Scholar]
- Beason R. C., Brennan W. J. 1986. Natural and induced magnetization in the bobolink, Dolichonyx oryzivorus (Aves: Icteridae). J. Exp. Biol. 125, 49–56. [Google Scholar]
- Beason R. C., Nichols J. E. 1984. Magnetic orientation and magnetically sensitive material in a transequatorial migratory bird. Nature 309, 151–153. ( 10.1038/309151a0) [DOI] [Google Scholar]
- Beason R. C., Semm P. 1987. Magnetic responses of the trigeminal nerve system of the bobolink (Dolichonyx oryzivorus). Neurosci. Lett. 80, 229–234. ( 10.1016/0304-3940(87)90659-8) [DOI] [PubMed] [Google Scholar]
- Beason R. C., Wiltschko R., Wiltschko W. 1997. Pigeon homing: effects of magnetic pulses on initial orientation. Auk 114, 405–415. [Google Scholar]
- Biro D., Freeman R., Meade J., Roberts S., Guilford T. 2007. Pigeons combine compass and landmark guidance in familiar route navigation. Proc. Natl Acad. Sci. USA 104, 7471–7476. ( 10.1073/pnas.0701575104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore R. 1975. Magnetotactic bacteria. Science 190, 377–379. ( 10.1126/science.170679) [DOI] [PubMed] [Google Scholar]
- Bookman M. A. 1977. Sensitivity of the homing pigeon to an earth-strength magnetic field. Nature 267, 340–342. ( 10.1038/267340a0) [DOI] [PubMed] [Google Scholar]
- Chalfie M., Sulston J. 1981. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 82, 358–370. ( 10.1016/0012-1606(81)90459-0) [DOI] [PubMed] [Google Scholar]
- Chen L., Liu C., Liu L. 2009. Osmolality-induced tuning of action potentials in trigeminal ganglion neurons. Neurosci. Lett. 452, 79–83. ( 10.1016/j.neulet.2009.01.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert H. A., Smith T. L., Bargmann C. I. 1997. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 17, 8259–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. P. 2006. What is the hair cell transduction channel? J. Physiol. 576, 23–28. ( 10.1113/jphysiol.2006.116582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila A. F., Fleissner G., Winklhofer M., Petersen N. 2003. A new model for a magnetoreceptor in homing pigeons based on interacting clusters of superparamagnetic magnetite. Phys. Chem. Earth 28, 647–652. [Google Scholar]
- Davila A. F., Winklhofer M., Shcherbakov V. P., Petersen N. 2005. Magnetic pulse affects a putative magnetoreceptor mechanism. Biophys. J. 89, 56–63. ( 10.1529/biophysj.104.049346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedman A., Sharif-Naeini R., Folgering J. H., Duprat F., Patel A., Honore E. 2009. The mechano-gated K(2P) channel TREK-1. Eur. Biophys. J. 38, 293–303. ( 10.1007/s00249-008-0318-8) [DOI] [PubMed] [Google Scholar]
- Dennis T. E., Rayner M. J., Walker M. M. 2007. Evidence that pigeons orient to geomagnetic intensity during homing. Proc. Biol. Sci. 274, 1153–1158. ( 10.1098/rspb.2007.3768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A., Viswanath V., Patapoutian A. 2006. TRP ion channels and temperature sensation. Annu. Rev. Neurosci. 29, 135–161. ( 10.1146/annurev.neuro.29.051605.112958) [DOI] [PubMed] [Google Scholar]
- Di Castro A., Drew L. J., Wood J. N., Cesare P. 2006. Modulation of sensory neuron mechanotransduction by PKC- and nerve growth factor-dependent pathways. Proc. Natl Acad. Sci. USA. 103, 4699–4704. ( 10.1073/pnas.0508005103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew L. J., Wood J. N. 2005. Worm sensation! Mol. Pain 1, 8 ( 10.1186/1744-8069-1-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew L. J., Wood J. N., Cesare P. 2002. Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J. Neurosci. 22, 228RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards H. H., Schnell G. D., DuBois R. L., Hutchison V. H. 1992. Natural and induced remnant magnetism in birds. Auk 109, 43–56. [Google Scholar]
- Elg S., Marmigere F., Mattsson J. P., Ernfors P. 2007. Cellular subtype distribution and developmental regulation of TRPC channel members in the mouse dorsal root ganglion. J. Comp. Neurol. 503, 35–46. ( 10.1002/cne.21351) [DOI] [PubMed] [Google Scholar]
- Faivre D., Schuler D. 2008. Magnetotactic bacteria and magnetosomes. Chem. Rev. 108, 4875–4898. ( 10.1021/cr078258w) [DOI] [PubMed] [Google Scholar]
- Fleissner G., Holtkamp-Rotzler E., Hanzlik M., Winklhofer M., Fleissner G., Petersen N. 2003. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J. Comp. Neurol. 458, 350–360. ( 10.1002/cne.10579) [DOI] [PubMed] [Google Scholar]
- Fleissner G., Stahl B., Thalau P., Falkenberg G., Fleissner G. 2007. A novel concept of Fe-mineral-based magnetoreception: histological and physicochemical data from the upper beak of homing pigeons. Naturwissenschaften 94, 631–642. ( 10.1007/s00114-007-0236-0) [DOI] [PubMed] [Google Scholar]
- Frankel R. B., Blakemore R. P., Wolfe R. S. 1979. Magnetite in freshwater magnetotactic bacteria. Science 203, 1355–1356. ( 10.1126/science.203.4387.1355) [DOI] [PubMed] [Google Scholar]
- Gagliardo A., Ioale P., Savini M., Wild J. M. 2006. Having the nerve to home: trigeminal magnetoreceptor versus olfactory mediation of homing in pigeons. J. Exp. Biol. 209, 2888–2892. ( 10.1242/jeb.02313) [DOI] [PubMed] [Google Scholar]
- Gegear R. J., Casselman A., Waddell S., Reppert S. M. 2008. Cryptochrome mediates light-dependent magnetosensitivity in Drosophila. Nature 454, 1014–1018. ( 10.1038/nature07183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie P. G., Corey D. P. 1997. Myosin and adaptation by hair cells. Neuron 19, 955–958. ( 10.1016/S0896-6273(00)80387-6) [DOI] [PubMed] [Google Scholar]
- Gorby Y. A., Beveridge T. J., Blakemore R. P. 1988. Characterization of the bacterial magnetosome membrane. J. Bacteriol. 170, 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb P., et al. 2008. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Arch. 455, 1097–1103. ( 10.1007/s00424-007-0359-3) [DOI] [PubMed] [Google Scholar]
- Grosmaitre X., Santarelli L. C., Tan J., Luo M., Ma M. 2007. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat. Neurosci. 10, 348–354. ( 10.1038/nn1856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrum J. T. 2000. Infrasound and the avian navigational map. J. Exp. Biol. 203, 1103–1111. [DOI] [PubMed] [Google Scholar]
- Hamill O., McBride D. 1993. Molecular clues to mechanosensitivity. Biophys. J. 65, 17–18. ( 10.1016/S0006-3495(93)81028-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzlik M., Heunemann C., Holtkamp-Rotzler E., Winklhofer M., Petersen N., Fleissner G. 2000. Superparamagnetic magnetite in the upper beak tissue of homing pigeons. Biometals 13, 325–331. ( 10.1023/A:1009214526685) [DOI] [PubMed] [Google Scholar]
- Harada Y. 2008. The relation between the migration function of birds and fishes and their lagenal function. Acta Otolaryngol. 128, 432–439. ( 10.1080/00016480701724920) [DOI] [PubMed] [Google Scholar]
- Harada Y., Taniguchi M., Namatame H., Iida A. 2001. Magnetic materials in otoliths of bird and fish lagena and their function. Acta Otolaryngol. 121, 590–595. ( 10.1080/000164801316878872) [DOI] [PubMed] [Google Scholar]
- Hardie R. C., Minke B. 1992. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 8, 643–651. ( 10.1016/0896-6273(92)90086-S) [DOI] [PubMed] [Google Scholar]
- Hervieu G. J., Cluderay J. E., Gray C. W., Green P. J., Ranson J. L., Randall A. D., Meadows H. J. 2001. Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience 103, 899–919. ( 10.1016/S0306-4522(01)00030-6) [DOI] [PubMed] [Google Scholar]
- Hill K., Schaefer M. 2007. TRPA1 is differentially modulated by the amphipathic molecules trinitrophenol and chlorpromazine. J. Biol. Chem. 282, 7145–7153. ( 10.1074/jbc.M609600200) [DOI] [PubMed] [Google Scholar]
- Holland R. A., Kirschvink J. L., Doak T. G., Wikelski M. 2008. Bats use magnetite to detect the earth's magnetic field. PLoS One 3, e1676 ( 10.1371/journal.pone.0001676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore E. 2007. The neuronal background K2P channels: focus on TREK1. Nat. Rev. Neurosci. 8, 251–261. ( 10.1038/nrn2117) [DOI] [PubMed] [Google Scholar]
- Jones N. G., Slater R., Cadiou H., McNaughton P. A., McMahon S. B. 2004. Acid-induced pain and its modulation in humans. J. Neurosci. 24, 10 974–10 979. ( 10.1523/JNEUROSCI.2619-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B., Parakkal M., Kurc M., Zhao Y., Gillespie P. G. 2000. High-resolution structure of hair-cell tip links. Proc. Natl Acad. Sci. USA 97, 13 336–13 341. ( 10.1073/pnas.97.24.13336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P., Sakaguchi H., Tokita J., Wilson-Kubalek E. M., Milligan R. A., Muller U., Kachar B. 2007. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449, 87–91. ( 10.1038/nature06091) [DOI] [PubMed] [Google Scholar]
- Keeton W. T. 1971. Magnets interfere with pigeon homing. Proc. Natl Acad. Sci. USA 68, 102–106. ( 10.1073/pnas.68.1.102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschvink J. L., Gould J. L. 1981. Biogenic magnetite as a basis for magnetic field detection in animals. Biosystems 13, 181–201. ( 10.1016/0303-2647(81)90060-5) [DOI] [PubMed] [Google Scholar]
- Kirschvink J. L., Walker M. M., Diebel C. E. 2001. Magnetite-based magnetoreception. Curr. Opin. Neurobiol. 11, 462–467. ( 10.1016/S0959-4388(00)00235-X) [DOI] [PubMed] [Google Scholar]
- Komeili A. 2007. Molecular mechanisms of magnetosome formation. Annu. Rev. Biochem. 76, 351–366. ( 10.1146/annurev.biochem.74.082803.133444) [DOI] [PubMed] [Google Scholar]
- Liedtke W., Tobin D. M., Bargmann C. I., Friedman J. M. 2003. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 100(Suppl. 2), 14 531–14 536. ( 10.1073/pnas.2235619100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedvogel M., Mouritsen H. 2010. Cryptochromes—a potential magnetoreceptor: what do we know and what do we want to know? J. R. Soc. Interface. ( 10.1098/rsif.2009.0411.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedvogel M., Maeda K., Henbest K., Schleicher E., Simon T., Timmel C. R., Hore P. J., Mouritsen H. 2007. Chemical magnetoreception: bird cryptochrome 1a is excited by blue light and forms long-lived radical-pairs. PLoS One 2, e1106 ( 10.1371/journal.pone.0001106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Simon S. A. 2000. Capsaicin, acid and heat-evoked currents in rat trigeminal ganglion neurons: relationship to functional VR1 receptors. Physiol. Behav. 69, 363–378. ( 10.1016/S0031-9384(00)00209-2) [DOI] [PubMed] [Google Scholar]
- Lowenstam H. A. 1967. Lepidocrocite, an apatite mineral, and magnetite in teeth of chitons (Polyplacophora). Science 156, 1373–1375. ( 10.1126/science.156.3780.1373) [DOI] [PubMed] [Google Scholar]
- Mann S., Sparks N. H., Walker M. M., Kirschvink J. L. 1988. Ultrastructure, morphology and organization of biogenic magnetite from sockeye salmon, Oncorhynchus nerka: implications for magnetoreception. J. Exp. Biol. 140, 35–49. [DOI] [PubMed] [Google Scholar]
- Markin V. S., Martinac B. 1991. Mechanosensitive ion channels as reporters of bilayer expansion. A theoretical model. Biophys. J. 60, 1120–1127. ( 10.1016/S0006-3495(91)82147-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroto R., Raso A., Wood T. G., Kurosky A., Martinac B., Hamill O. P. 2005. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat. Cell Biol. 7, 179–185. ( 10.1038/ncb1218) [DOI] [PubMed] [Google Scholar]
- Martinac B., Kloda A. 2003. Evolutionary origins of mechanosensitive ion channels. Prog. Biophys. Mol. Biol. 82, 11–24. ( 10.1016/S0079-6107(03)00002-6) [DOI] [PubMed] [Google Scholar]
- Martinac B., Buechner M., Delcour A. H., Adler J., Kung C. 1987. Pressure-sensitive ion channel in Escherichia coli. Proc. Natl Acad. Sci. USA 84, 2297–2301. ( 10.1073/pnas.84.8.2297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B., Adler J., Kung C. 1990. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 348, 261–263. ( 10.1038/348261a0) [DOI] [PubMed] [Google Scholar]
- Martinac B., Saimi Y., Kung C. 2008. Ion channels in microbes. Physiol. Rev. 88, 1449–1490. ( 10.1152/physrev.00005.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P., Kemp P. J., Lewis A., Chapman C. G., Meadows H. J., Peers C. 2003. Acute hypoxia occludes hTREK-1 modulation: re-evaluation of the potential role of tandem P domain K+ channels in central neuroprotection. J. Physiol. 548, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora C. V., Davison M., Wild J. M., Walker M. M. 2004. Magnetoreception and its trigeminal mediation in the homing pigeon. Nature 432, 508–511. ( 10.1038/nature03077) [DOI] [PubMed] [Google Scholar]
- Mouritsen H., Ritz T. 2005. Magnetoreception and its use in bird navigation. Curr. Opin. Neurobiol. 15, 406–414. ( 10.1016/j.conb.2005.06.003) [DOI] [PubMed] [Google Scholar]
- Mouritsen H., Janssen-Bienhold U., Liedvogel M., Feenders G., Stalleicken J., Dirks P., Weiler R. 2004. Cryptochromes and neuronal-activity markers colocalize in the retina of migratory birds during magnetic orientation. Proc. Natl Acad. Sci. USA 101, 14 294–14 299. ( 10.1073/pnas.0405968101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muheim R., Phillips J. B., Akesson S. 2006. Polarized light cues underlie compass calibration in migratory songbirds. Science 313, 837–839. ( 10.1126/science.1129709) [DOI] [PubMed] [Google Scholar]
- Munro U., Munro J. A., Phillips J. B., Wiltschko W. 1997. Effect of wavelength of light and pulse magnetisation on different magnetoreception systems in a migratory bird. Aust. J. Zool. 45, 189–198. ( 10.1071/ZO96066) [DOI] [Google Scholar]
- Němec P., Burda H., Oelschlager H. H. 2005. Towards the neural basis of magnetoreception: a neuroanatomical approach. Naturwissenschaften 92, 151–157. ( 10.1007/s00114-005-0612-6) [DOI] [PubMed] [Google Scholar]
- Nevitt G. A., Losekoot M., Weimerskirch H. 2008. Evidence for olfactory search in wandering albatross, Diomedea exulans. Proc. Natl Acad. Sci. USA 105, 4576–4581. ( 10.1073/pnas.0709047105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page A. J., et al. 2004. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology 127, 1739–1747. ( 10.1053/j.gastro.2004.08.061) [DOI] [PubMed] [Google Scholar]
- Perozo E. 2006. Gating prokaryotic mechanosensitive channels. Nat. Rev. Mol. Cell Biol. 7, 109–119. ( 10.1038/nrm1833) [DOI] [PubMed] [Google Scholar]
- Petrov E., Martinac B. 2007. Modulation of channel activity and gadolinium block of MscL by static magnetic fields. Eur. Biophys. J. 36, 95–105. ( 10.1007/s00249-006-0109-z) [DOI] [PubMed] [Google Scholar]
- Pickles J. O., Comis S. D., Osborne M. P. 1984. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res. 15, 103–112. ( 10.1016/0378-5955(84)90041-8) [DOI] [PubMed] [Google Scholar]
- Presti D., Pettigrew J. D. 1980. Ferromagnetic coupling to muscle receptors as a basis for geomagnetic field sensitivity in animals. Nature 285, 99–101. ( 10.1038/285099a0) [DOI] [PubMed] [Google Scholar]
- Price M. P., et al. 2001. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32, 1071–1083. ( 10.1016/S0896-6273(01)00547-5) [DOI] [PubMed] [Google Scholar]
- Reeh P. W. 1986. Sensory receptors in mammalian skin in an in vitro preparation. Neurosci. Lett. 66, 141–146. ( 10.1016/0304-3940(86)90180-1) [DOI] [PubMed] [Google Scholar]
- Ritz T., Adem S., Schulten K. 2000. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718. ( 10.1016/S0006-3495(00)76629-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T., Dommer D. H., Phillips J. B. 2002. Shedding light on vertebrate magnetoreception. Neuron 34, 503–506. ( 10.1016/S0896-6273(02)00707-9) [DOI] [PubMed] [Google Scholar]
- Ritz T., Ahmad M., Mouritsen H., Wiltschko R., Wiltschko W. 2010. Photoreceptor-based magnetoreception: optimal design of receptor molecules, cells, and neuronal processing. J. R. Soc. Interface. ( 10.1098/rsif.2009.0456.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Koenig K., Walcott C. 1978. Tracks of homing pigeons wearing frosted lenses. Anim. Behav. 26, 480–486. ( 10.1016/0003-3472(78)90065-9) [DOI] [Google Scholar]
- Schulten K., Weller A. 1978. Exploring fast electron transfer processes by magnetic fields. Biophys. J. 24, 295–305. ( 10.1016/S0006-3495(78)85378-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semm P., Beason R. C. 1990. Responses to small magnetic variations by the trigeminal system of the bobolink. Brain Res. Bull. 25, 735–740. ( 10.1016/0361-9230(90)90051-Z) [DOI] [PubMed] [Google Scholar]
- Shcherbakov V. P., Winklhofer M. 1999. The osmotic magnetometer: a new model for magnetite-based magnetoreceptors in animals. Eur. Biophys. J. 28, 380–392. ( 10.1007/s002490050222) [DOI] [Google Scholar]
- Solov'yov I. A., Greiner W. 2007. Theoretical analysis of an iron mineral-based magnetoreceptor model in birds. Biophys. J. 93, 1493–1509. ( 10.1529/biophysj.107.105098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl B., Fleissner G., Fleissner G., Falkenberg G. 2007. Cross-species unveiling of a putative avian magnetoreceptor. HASYLAB Annual Report 2006, DESY, Hamburg, Germany, pp. 1269–1270. [Google Scholar]
- Strassmaier M., Gillespie P. G. 2002. The hair cell's transduction channel. Curr. Opin. Neurobiol. 12, 380–386. ( 10.1016/S0959-4388(02)00344-6) [DOI] [PubMed] [Google Scholar]
- Su X., Wachtel R. E., Gebhart G. F. 2000. Mechanosensitive potassium channels in rat colon sensory neurons. J. Neurophysiol. 84, 836–843. [DOI] [PubMed] [Google Scholar]
- Sukharev S. I., Blount P., Martinac B., Blattner F. R., Kung C. 1994. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature 368, 265–268. ( 10.1038/368265a0) [DOI] [PubMed] [Google Scholar]
- Thalau P., Holtkamp-Rotzler E., Fleissner G., Wiltschko W. 2007. Homing pigeons (Columba livia f. domestica) can use magnetic cues for locating food. Naturwissenschaften 94, 813–819. ( 10.1007/s00114-007-0259-6) [DOI] [PubMed] [Google Scholar]
- Tian L., Xiao B., Lin W., Zhang S., Zhu R., Pan Y. 2007. Testing for the presence of magnetite in the upper-beak skin of homing pigeons. Biometals 20, 197–203. ( 10.1007/s10534-006-9027-x) [DOI] [PubMed] [Google Scholar]
- Viguier C. 1882. Le sens de l'orientation et ses organes chez les animaux et chez l'homme. Rev. Phil. France Étranger 14, 1–36. [Google Scholar]
- Von Middendorf A. 1859. Die Isepipetsen Russlands, Grundlagen zur Erforschung der Zugzeiten und Zugrichtungen der Vogel Russlands. Mem. Acad. Sci. St. Petersbourg 8, 1–143. [Google Scholar]
- Walcott C. 1996. Pigeon homing: observations, experiments and confusions. J. Exp. Biol. 199, 21–27. [DOI] [PubMed] [Google Scholar]
- Walcott C., Green R. P. 1974. Orientation of homing pigeons altered by a change in the direction of an applied magnetic field. Science 184, 180–182. ( 10.1126/science.184.4133.180) [DOI] [PubMed] [Google Scholar]
- Walcott C., Schmidt-Koenig K. 1973. The effect on pigeon homing of anesthesia during displacement. Auk 90, 281–286. [Google Scholar]
- Walcott C., Gould J. L., Kirschvink J. L. 1979. Pigeons have magnets. Science 205, 1027–1029. ( 10.1126/science.472725) [DOI] [PubMed] [Google Scholar]
- Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M. 1997. A proton-gated cation channel involved in acid-sensing. Nature 386, 173–177. ( 10.1038/386173a0) [DOI] [PubMed] [Google Scholar]
- Walker M. M. 2008. A model for encoding of magnetic field intensity by magnetite-based magnetoreceptor cells. J. Theor. Biol. 250, 85–91. ( 10.1016/j.jtbi.2007.09.030) [DOI] [PubMed] [Google Scholar]
- Walker M. M., Kirschvink J. L., Chang S. B., Dizon A. E. 1984. A candidate magnetic sense organ in the yellowfin tuna, Thunnus albacares. Science 224, 751–753. ( 10.1126/science.224.4650.751) [DOI] [PubMed] [Google Scholar]
- Walker M. M., Quinn T. P., Kirschvink J. L., Groot C. 1988. Production of single-domain magnetite throughout life by sockeye salmon, Oncorhynchus nerka. J. Exp. Biol. 140, 51–63. [DOI] [PubMed] [Google Scholar]
- Walker M. M., Diebel C. E., Haugh C. V., Pankhurst P. M., Montgomery J. C., Green C. R. 1997. Structure and function of the vertebrate magnetic sense. Nature 390, 371–376. ( 10.1038/37057) [DOI] [PubMed] [Google Scholar]
- Wallraff H. G. 2004. Avian olfactory navigation: its empirical foundation and conceptual state. Anim. Behav. 67, 189–204. ( 10.1016/j.anbehav.2003.06.007) [DOI] [Google Scholar]
- Wegner R. E., Begall S., Burda H. 2006. Magnetic compass in the cornea: local anaesthesia impairs orientation in a mammal. J. Exp. Biol. 209, 4747–4750. ( 10.1242/jeb.02573) [DOI] [PubMed] [Google Scholar]
- Wetzel C., et al. 2007. A stomatin-domain protein essential for touch sensation in the mouse. Nature 445, 206–209. ( 10.1038/nature05394) [DOI] [PubMed] [Google Scholar]
- Williams M. N., Wild J. M. 2001. Trigeminally innervated iron-containing structures in the beak of homing pigeons, and other birds. Brain Res. 889, 243–246. ( 10.1016/S0006-8993(00)03114-0) [DOI] [PubMed] [Google Scholar]
- Wiltschko R., Nohr D., Wiltschko W. 1981. Pigeons with a deficient sun compass use the magnetic compass. Science 214, 343–345. ( 10.1126/science.7280697) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Merkel F. W. 1966. Orientierung zugunruhiger Rotkehlchen im statischen Magnetfeld. Verh. Dtsch. Zool. Ges. 59, 362–367. [Google Scholar]
- Wiltschko W., Wiltschko R. 1981. Disorientation of inexperienced young pigeons after transportation in total darkness. Nature 291, 433–434. ( 10.1038/291433a0) [DOI] [Google Scholar]
- Wiltschko W., Wiltschko R. 1995. Migratory orientation of European robins is affected by the wavelength of light as well as by magnetic pulse. J. Comp. Physiol. A 177, 363–369. ( 10.1007/BF00192425) [DOI] [Google Scholar]
- Wiltschko W., Wiltschko R. 1999. The effect of yellow and blue light on magnetic compass orientation in European robins, Erithacus rubecula. J. Comp. Physiol. A 184, 295–299. ( 10.1007/s003590050327) [DOI] [Google Scholar]
- Wiltschko W., Wiltschko R. 2001. Light-dependent magnetoreception in birds: the behaviour of European robins, Erithacus rubecula, under monochromatic light of various wavelengths and intensities. J. Exp. Biol. 204, 3295–3302. [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 2002. Magnetic compass orientation in birds and its physiological basis. Naturwissenschaften 89, 445–452. ( 10.1007/s00114-002-0356-5) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R. 2005. Magnetic orientation and magnetoreception in birds and other animals. J. Comp. Physiol. A 191, 675–693. ( 10.1007/s00359-005-0627-7) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 1998. Effect of a magnetic pulse on the orientation of silvereyes, Zosterops l. lateralis, during spring migration. J. Exp. Biol. 201, 3257–3261. [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Wiltschko R., Munro U. 2000. Light-dependent magnetoreception in birds: the effect of intensity of 565-nm green light. Naturwissenschaften 87, 366–369. ( 10.1007/s001140050742) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Gesson M., Wiltschko R. 2001. Magnetic compass orientation of European robins under 565 nm green light. Naturwissenschaften 88, 387–390. ( 10.1007/s001140100248) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Traudt J., Gunturkun O., Prior H., Wiltschko R. 2002a. Lateralization of magnetic compass orientation in a migratory bird. Nature 419, 467–470. ( 10.1038/nature00958) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Wiltschko R., Kirschvink J. L. 2002b. Magnetite-based magnetoreception in birds: the effect of a biasing field and a pulse on migratory behavior. J. Exp. Biol. 205, 3031–3037. [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Ford H., Munro U., Winklhofer M., Wiltschko R. 2007. Magnetite-based magnetoreception: the effect of repeated pulsing on the orientation of migratory birds. J. Comp. Physiol. A 193, 515–522. ( 10.1007/s00359-006-0207-5) [DOI] [PubMed] [Google Scholar]
- Wiltschko W., Munro U., Ford H., Wiltschko R. 2009. Avian orientation: the pulse effect is mediated by the magnetite receptors in the upper beak. Proc. Biol. Sci. 276, 2227–2232. ( 10.1098/rspb.2009.0050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklhofer M., Kirschvink J. L. Does avian magnetoreception rely on both magnetite and maghemite? 2008 (http://arxiv.org/abs/0805.2249. ) [Google Scholar]
- Winklhofer M., Holtkamp-Rotzler E., Hanzlik M., Fleissner G., Petersen N. 2001. Clusters of superparamagnetic magnetite particles in the upper-beak skin of homing pigeons: evidence of a magnetoreceptor? Eur. J. Mineral. 13, 659–669. ( 10.1127/0935-1221/2001/0013-0659) [DOI] [Google Scholar]
- Zhang L., Lee J. K., John S. A., Uozumi N., Kodama I. 2004. Mechanosensitivity of GIRK channels is mediated by protein kinase C-dependent channel-phosphatidylinositol 4,5-bisphosphate interaction. J. Biol. Chem. 279, 7037–7047. ( 10.1074/jbc.M307323200) [DOI] [PubMed] [Google Scholar]
- Zhao Y., Huang Y. N., Shi L., Chen L. 2009. Analysis of magnetic elements in otoliths of the macula lagena in homing pigeons with inductively coupled plasma mass spectrometry. Neurosci. Bull. 25, 101–108. ( 10.1007/s12264-009-0311-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K., Hein A., Hager U., Kaczmarek J. S., Turnquist B. P., Clapham D. E., Reeh P. W. 2009. Phenotyping sensory nerve endings in vitro in the mouse. Nat. Protoc. 4, 174–196. ( 10.1038/nprot.2008.223) [DOI] [PMC free article] [PubMed] [Google Scholar]