Abstract
Neuropathic pain is relatively uncommon in children. Although some syndromes closely resemble those found in adults, the incidence and course of the condition can vary substantially in children, depending on developmental status and contextual factors. There are some neuropathic pain syndromes that are rare and relatively unique to the pediatric population. This article discusses the array of neuropathic pain conditions in children and available treatment strategies. Data are limited by small numbers and few randomized controlled trials. Research and clinical implications are discussed.
CNS = central nervous system; CRPS = complex regional pain syndrome; TENS = transcutaneous electronic nerve stimulation
Neuropathic pain is rarely studied systematically in infants, children, and adolescents. Perhaps a central reason for this is the fact that many of the most common neuropathic pain conditions seen in adults are rare in children. For example, among children with diabetes, complications do not progress to the point that neuropathy would be of concern. Likewise, conditions such as postherpetic neuralgia, trigeminal neuralgia, radiculopathies, and complications of stroke are of extremely low incidence in young patients. However, some neuropathic conditions are becoming increasingly recognized in children and adolescents, including complex regional pain syndromes (CRPSs) (principally type 1), phantom limb pain, spinal cord injury, trauma and postoperative neuropathic pain, autoimmune and degenerative neuropathies (eg, Guillain-Barré syndrome, Charcot-Marie-Tooth disease), and the effects of cancer disease processes and treatment. Finally, some neuropathic pain syndromes that are rare are relatively unique to the pediatric population, including toxic and metabolic neuropathies (eg, lead, mercury, alcohol, infection), hereditary neurodegenerative disorders (eg, Fabry disease), mitochondrial disorders, and primary erythromelalgia.
Neuropathic pain affects only a subgroup of adults with the same underlying lesion or disease, with incidences ranging from a small percentage with cortical stroke to more than half with spinal cord injury. This variability is attributed to individual differences in processes of degeneration and regeneration initiated by neural damage, often involving a reversal of cellular programs from the adult to an embryonic state. Throughout early development, and especially at the youngest ages, both the central nervous system (CNS) and peripheral nervous system are far more plastic than among adults. For example, after peripheral nerve injury, changes are likely in the CNS, reflecting neuronal plasticity and neuronal reorganization, all within the context of development. Thus, in comparing neuropathic pain in children vs adults, one can expect substantial differences in prevalence, presenting symptoms, course of disease or pain, especially with regard to chronicity, duration, and recurrences, and potential effect of different treatments.
Because of the relatively low incidence of this problem, data on the assessment and treatment of neuropathic pain in children are limited. Although recognition of neuropathic pain in children has increased during the past 20 years, good epidemiological studies are lacking to truly ascertain the incidence and correlates of neuropathic pain syndromes. Cases can be heterogeneous, making it difficult to articulate inclusion and exclusion criteria for research. In addition, the circumstances and context of the neuropathic pain may be of greater focus than the pain problem (ie, rarely is neuropathic pain the primary issue of concern in young patients). Longitudinal data for disease trajectories are extremely limited, including the course of neuropathic pain. Taking all these factors together, conducting epidemiological or well-controlled clinical trials of treatments in the pediatric population is exceptionally challenging.
A literature search was conducted using MEDLINE in June 2008, first entering the terms neuropathic pain and neuralgia with an age limitation of birth to 18 years. Subsequent analyses included terms for specific syndromes that may be considered neuropathic pain, including complex regional pain syndrome, reflex sympathetic dystrophy, and phantom limb and combining the term pain with other elements, such as Fabry disease, Charcot-Marie-Tooth disease, or Guillain-Barré syndrome, all with the same age limiters. Case reports, clinical series, and clinical trials were included in this review.
The Oxford Centre for Evidence-Based Medicine1 defined levels of evidence for evaluating outcomes. Ideally, the first level of evidence, randomized controlled clinical trials, should guide practice when possible. Because of the aforementioned limitations, most studies of neuropathic pain in children are principally descriptive, limited to case studies or clinical series (levels 4 and 5). It is hoped that, as awareness of these syndromes increases, there will be more collaborative endeavors among various sites so that studies of etiology and treatment can be powered to yield reliable results.
COMPLEX REGIONAL PAIN SYNDROMES
Formerly known as reflex sympathetic dystrophy, CRPS type 1 has been well described in the pediatric population. Formerly referred to as causalgia, CRPS type 2 is less common, with only sparse reports in the literature. Although causal factors for CRPS type 1 in both children and adults remain elusive,2 a handful of case studies and clinical series purport to identify coincident or correlational effects. Included among these are case studies of a 15-year-old girl whose symptoms were attributed to posttraumatic stress disorder,3 a 15-year-old girl whose symptoms were deemed to have arisen from Munchausen syndrome,4 an 11-year-old girl who developed symptoms after receiving a rubella vaccine,5 and a 10-year-old boy with evidence of a sympathetically maintained pain syndrome.6 An early report describing a clinical series of 18 patients aged 9 to 19 years described a number of features seen in the syndrome and specifically warns about attributing cause to psychiatric disorders, such as conversion disorder or malingering.7
A bit more helpful to understanding correlates is the handful of studies reporting on the prevalence of CRPS in children and adolescents. Wilder et al8 described a series of 70 patients evaluated at the Children's Hospital Boston, Boston, MA, during a 41-month period. Data showed a high female-to-male ratio (approximately 6:1) and that occurrences were more common in the lower extremities than upper extremities. Low et al,9 reporting on 20 patients, found an incidence in girls of 90%, that lower limbs were affected in 85% of patients, and that 80% of the time onset was precipitated by minor trauma. Murray et al10 found a history of trauma only 54% of the time among 46 patients.
Sethna et al11 performed standardized neurologic examinations and quantitative sensory testing on 42 patients aged 7 to 17 years with unilateral lower-extremity CRPS. Compared with a healthy control sample, most quantitative sensory testing parameters were unchanged except for cold and heat pain detection thresholds. Specifically, cold allodynia was observed, and a number of participants showed a combination of dynamic mechanical allodynia and hyperalgesia to pinprick. Tan et al12 conducted a medical record review of 78 children (≤16 years) and 951 adults with CRPS. The pediatric population was predominantly girls, with a median age of 13 years, consistent with the female predominance in adults. Compared with the adult sample, among children the skin temperature of the involved extremity at onset was cooler, the lower extremity was involved more frequently, and the neurologic and sympathetic symptoms were less pronounced.
Using functional magnetic resonance imaging, LeBel et al13 studied CNS activation in 12 patients, aged 9 to 18 years, with CRPS in the lower extremity. Participants underwent 2 functional magnetic resonance imaging sessions, once during an active period of pain and once after symptomatic recovery. Mechanical (brush) and thermal (cold) stimuli were applied to the affected region of the involved limb and the corresponding mirror region of the unaffected limb. Results showed that in the children with CRPS, stimuli that evoked mechanical or cold allodynia produced patterns of CNS activation similar to those reported in adults with CRPS because there were significant decreases in BOLD (blood oxygen level dependent) signal, suggesting pain-induced activation of endogenous pain modulatory systems. Cold- or brush-induced activations in regions such as the basal ganglia and parietal lobe may explain some CNS-related symptoms in CRPS, including movement disorders, hemineglect, and inattention. After resolution of CRPS, significant activation differences persisted despite nearly complete elimination of evoked pain. Although nonnoxious stimuli to the unaffected limb were perceived as equivalent in children during and after CRPS, the same stimulus produced different patterns of activation in the 2 states, suggesting that the CRPS brain responds differently to normal stimuli applied to unaffected regions and that significant changes occur in CNS circuitry in patients with CRPS.
Given the relative dearth of data on the causes of CRPS, it is not surprising that approaches to treatment have varied. In 1977, a case report described successful treatment with transcutaneous electronic nerve stimulation (TENS) in a 6-year-old girl with CRPS.14 This was followed by similar reports in a 3½-year-old boy15 and a clinical series of 9 girls and 1 boy between the ages of 8 and 18 years.16 In 1987, thermal biofeedback was reported to be helpful for a 12-year-old boy.17 In the aforementioned follow-up study by Wilder et al,8 57% of patients improved with conservative treatment alone, consisting of physical therapy, TENS, cognitive behavioral therapy, and tricyclic antidepressants, whereas 28 of 37 patients benefited from interventional sympathetic blocks. Unfortunately, 38 children continued to have pain or functional problems after treatment. Of importance, the treatment of CRPS in this population was not standardized by protocol, and therapies were not determined by strict criteria. Therefore, it is difficult to extrapolate any recommendations regarding the use of interventional or noninterventional treatment modalities or conclude which of many pharmacological agents is most effective in children.
Subsequent studies have shown the value of aggressive physical therapy without use of pharmacological agents or interventional nerve blocks. One of the first studies was an extended clinical series by Sherry et al18 that focused on 103 patients treated in a “reflex neurovascular clinic” during a nearly 13-year period. Standard diagnostic criteria were used, and treatment specifically eliminated the use of any medications. Exercise therapy that focused on aerobic exercise weight-bearing, functional activities, and hydrotherapy was at the core of intervention, administered 5 to 6 hours daily, in addition to evening and weekend regimens that ranged from 45 minutes to 3 hours. Although initially much of the work was performed in an inpatient setting, over time the focus was more on an outpatient basis. Complete resolution of pain and full function were restored in 92% of patients, and long-term data for 49 patients indicated lasting benefit for most patients. Recurrent episodes occurred 31% of the time but generally resolved with reinitiation of rehabilitative strategies. Although these data are impressive, more information is needed about how specific patients were selected or self-selected for completion of this treatment protocol before generalizability can be assessed.
Lee et al19 conducted a prospective, randomized, single-blind trial of 28 children aged 8 to 17 years who were diagnosed as having CRPS. Participants were randomly assigned to either (1) physical therapy once per week for 6 weeks or (2) physical therapy 3 times per week for 6 weeks; both groups received 6 sessions of cognitive-behavioral treatment. All 5 measures of pain and function improved significantly in both groups, and sustained benefits were evident in most patients at long-term follow-up. The number of participants in each group was too small to detect differences in treatment, and because there was no wait list or control group, one cannot determine whether these benefits of treatment were a result of placebo effects, the passage of time, or both. Recurrent episodes were reported in 50% of patients, with 10 patients eventually receiving sympathetic blockade.
A clinical series of 23 children used a multidisciplinary outpatient or inpatient approach that emphasized physical therapy (range of motion, function of affected limb, muscle strength, desensitization, balance, and proprioception) and counseling with reasonably good outcomes.20 Meier et al21 showed that aggressive physical therapy and cognitive behavioral interventions were effective in treating 20 children and adolescents for both pain and regional and systemic autonomic responses. Low et al9 described outcomes among 20 children diagnosed as having CRPS during a 4-year period. Treatment consisted of intensive physiotherapy and psychological therapy, although 70% received adjuvant medications (amitriptyline and/or gabapentin) for analgesia and to facilitate participation in physiotherapy. Although most children had complete resolution of symptoms with this treatment regimen (mean, 15.4 weeks; range, 3 days to 64 weeks), 40% required treatment as inpatients, and 20% had a relapse episode.
Strategies for using topical and regional anesthetic techniques have also been described. In 1994, EMLA was reported to have helped a 15-year-old boy.22 Intravenous regional anesthesia blocks (Bier blocks) with guanethidine and prilocaine paired with physical therapy were used in girls 10 and 13 years of age.23 In 2005, continuous peripheral nerve blocks provided at home were used in 13 children between 9 and 16 years of age who had intractable CRPS with substantial short-term benefit.24 A computed tomography—guided lumbar sympathetic block likewise was reported to be successful in a 13-year-old girl.25
A double-blind, placebo-controlled crossover trial was conducted with 23 patients 10 to 18 years of age who had unilateral lower limb CRPS.26 A catheter was placed along the lumbar sympathetic chain, and patients received intravenous lidocaine and lumbar sympathetic saline or lumbar sympathetic lidocaine and intravenous saline. Immediate short-term differences were noted as mean pain intensity of allodynia to brush, and pinprick temporal summation was reduced in the latter group, as well as reduction in pain intensity from pretreatment for allodynia to brush, pinprick, pin-prick temporal summation, and verbal pain scores. Findings led the authors to assert that a component of pain in CRPS may be mediated by abnormal sympathetic activity.
The diagnostic intravenous phentolamine test has been used as a predictor for therapeutic Bier block with guanethidine in adolescents,27 similar to its use for predicting sympathetic block efficacy in adults.28 Although attractive from a pharmacological perspective, the phentolamine test has not gained wide acceptance because of the cost of the drug, its commercial unavailability (in the United States), and the relatively low rate of sympathetically maintained pain in most cases of CRPS.
Pharmacological interventions have included gabapentin, first reported in 2 case studies of 9-year-old girls,29 dronabinol in a 15-year-old girl and a 14-year-old boy,30 and oxcarbazepine administered to a 12-year-old boy.31 Intravenous ketorolac and lidocaine were given to 11- and 15-year-old girls,32 pamidronate was infused into a 15-year-old girl and a 14-year-old boy,33 and anesthetic ketamine and midazolam were administered to a 17-year-old girl,34 all with reported success. Intrathecal preparations have also been reported, including high-dose ziconotide for a 17-year-old girl35 and a combination of ropivacaine and fentanyl for an 8-year-old girl.36 A combination of iloprost, physiotherapy, and psychological counseling was provided to 7 girls aged 6 to 11 years, with reported success.37 More invasive strategies have been reported, including a thorascopic sympathectomy performed on an 11-year-old girl38 and spinal stimulation for 7 girls between the ages of 11 and 14 years.39
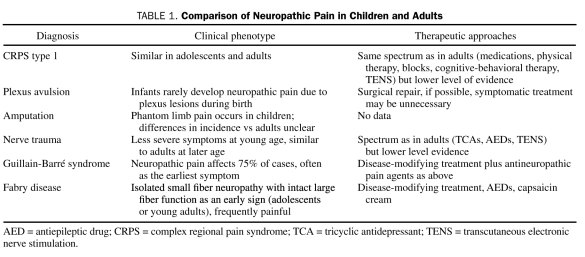
After reviewing the available literature on treatments of CRPS in children and adolescents, Wilder40 concluded that, although an array of treatments may have some benefit, the mainstay of treatment appears to be physical therapy involving desensitization, strengthening, and functional improvement. In summary, treatment of CRPS type 1 in children has been extrapolated from the treatment of CRPS type 1 in adults, with some low-level evidence for efficacy of physical therapy, TENS, cognitive-behavioral therapy, nerve blocks, tricyclic antidepressants, gabapentin, and some other drugs (Table 1).
TABLE 1.
Comparison of Neuropathic Pain in Children and Adults
PLEXUS AVULSION AND PHANTOM LIMB PAIN
On the basis of clinical anecdote, phantom limb pain is not unusual in children who have undergone amputations related to either neoplasm or trauma. Krane and Heller41 conducted a retrospective survey of 5- to 19-year-olds who had undergone limb amputation in the preceding 10 years because of congenital deformity, trauma or infection, or cancer. Phantom sensations were experienced in all patients, and most stated that they experienced phantom pain as well; 35% continued to have phantom pain at the time of data collection. Melzack et al42 reported that phantom limbs were experienced by 20% of those with congenital limb deficiencies and 50% of those who underwent amputation before the age of 6 years. Phantom pain was reported in 20% and 42% of these groups, respectively. Finally, Wilkins et al43 relied on diary data and noted recurrent episodes of phantom pain due to congenital limb deficiencies, surgery, and trauma, with an average intensity of 6.43 (on a 0- to 10-point scale).
Of interest, although brachial plexus injuries in adults frequently result in chronic pain, perinatal brachial plexus injuries usually do not produce chronic limb pain in children; at least it does not seem to produce pain at a later age at which children are able to report such pain. Using a range of noninvasive quantitative measures validated in adults, including mechanical, thermal, and vibration perception thresholds, Anand and Birch44 assessed sensory and cholinergic sympathetic function (sweating) in 24 patients between 3 and 23 years of age who had severe brachial plexus injury at birth. Recovery of function after spinal root avulsion was related to surgery, but differences from adults were striking, including excellent restoration of sensory function and evidence of exquisite CNS plasticity, even to the point of perfect localization of restored sensation in avulsed spinal root dermatomes, presumably routed via nerves that had been transferred from a distant spinal region. Sensory recovery exceeded motor or cholinergic sympathetic recovery; however, some patients who require surgical dissection of the plexus after obstetrical trauma may exhibit self-mutilation behavior in infancy.45
As sparse as reports on the epidemiology and etiology of phantom pain in the young may be, data on interventions are even more anecdotal and underwhelming. In 1995, intravenous ketamine was administered to a 17-year-old boy who had undergone amputation of the lower leg because of osteosarcoma of the tibia.46 Gabapentin was reported to be beneficial in a case series of 7 children and young adults with phantom pain.47
In summary, brachial plexus avulsions during birth have a lower risk of neuropathic pain than similar damage at a later age. Phantom limb pain after amputations occurs in children, but the incidence, prevention, and treatment efficacy are unclear (Table 1).
TRAUMA AND SURGERY
Trauma is a general category and may actually reflect an array of insults with implications for neuropathic pain. Atherton et al48 followed up 49 children who presented with distal upper limb nerve injury resulting from fracture, knife wounds, crush, or lacerations from broken glass at an average of 2.25 years after their injury. Patients younger than 5 years (n=15) did not report chronic neuropathic pain or allodynia. Patients with allodynia on sensory testing but no chronic neuropathic pain symptoms (n=8) were all older than 5 years. Chronic neuropathic pain (CRPS type 2) was found in 5 children, all of whom were older than 12 years at the time of injury. These authors reasonably concluded that “young children show better sensory recovery and are less likely to develop long-term chronic neuropathic pain than adults following nerve injury.” Indeed, this may partially or wholly explain why preadolescents with neuropathic pain are infrequent visitors to most pediatric pain clinics and why virtually all reported cases of CRPS in children are in those older than 8 years.
Additional case reports have highlighted some unusual bases for traumatic neuropathic pain, including a case of a 14-year-old boy who had been struck by a car and developed CRPS type 2 of the lower limb and who was treated with gabapentin and intensive physical therapy49 and an atypical trigeminal neuralgia related to tongue piercing in an 18-year-old woman.50 Lauder and White51 reported on a clinical series of 6 patients (11-17 years of age) with cerebral palsy who developed neuropathic pain after multilevel orthopedic surgery. Five of these patients improved markedly with interventions that included amitriptyline, gabapentin, and TENS as first-line treatments. One patient received additional mental health services, and one patient underwent a caudal epidural with bupivacaine and low-dose ketamine. Finally, a case report described use of ketamine to assist with pain and allodynia associated with an appendectomy wound in a 17-year-old girl.52
In conclusion, some evidence exists for a better prognosis of nerve injury—related neuropathic pain in young children than in older populations. Treatment approaches have been extrapolated with some success from adults (Table 1).
AUTOIMMUNE DISORDERS
Guillain-Barré syndrome is thought to occur when the body's immune system attacks native proteins in the peripheral nervous system. Korinthenberg et al53 prospectively followed up 95 children (median age, 6.2 years) with Guillain-Barré syndrome. Most often the first symptom was disturbance of gait or neuropathic pain, which progressed for a median of 7 days. Of note, 79% experienced neuropathic pain that was often severe. All but 8 children were treated with intravenous immunoglobulin, and improvement began approximately 2 weeks after the first symptom with a span of approximately 4 months for children to be symptom free; however, the role of intravenous immunoglobulin in healing of neuropathic pain is speculative, at best. At the end of the observation period (288 days), 75% of patients were free of symptoms, and 21% had residual symptoms that had no effect on daily functioning. Clearly, severe neuropathic pain should be recognized and treated in this patient group.
METABOLIC DISEASES
Fabry disease is an X-linked lysosomal disease caused by deficiency of α-galactosidase A. Hopkin et al54 evaluated signs and symptoms occurring during childhood and adolescence in 352 Fabry registry patients. At enrollment, 77% of male patients and 51% of female patients reported symptoms, with a median age of symptom onset of 6 and 9 years, respectively. Neuropathic pain was the most frequent symptom (59% of male patients; median age, 7 years; 41% of female patients; median age, 9 years). Using quantitative sensory testing in male patients with Fabry disease, Maag et al55 demonstrated a small-fiber sensory neuropathy that selectively affects C- and A-δ fibers. A comparison with somatosensory profiles of painful sensory neuropathies related to other causes showed that the Fabry disease profile is characterized by more severe impairment of thermal and preserved vibratory and mechanical discrimination. Laaksonen et al56 reported similar sensory profiles in heterozygous women. Treatment includes enzyme replacement as disease-modifying treatment (Table 1) and neuropathic pain medications, such as gabapentin,57,58 although the success of antineuropathic pain drugs in small-fiber neuropathies has not been impressive.
ADDITIONAL TREATMENT REPORTS
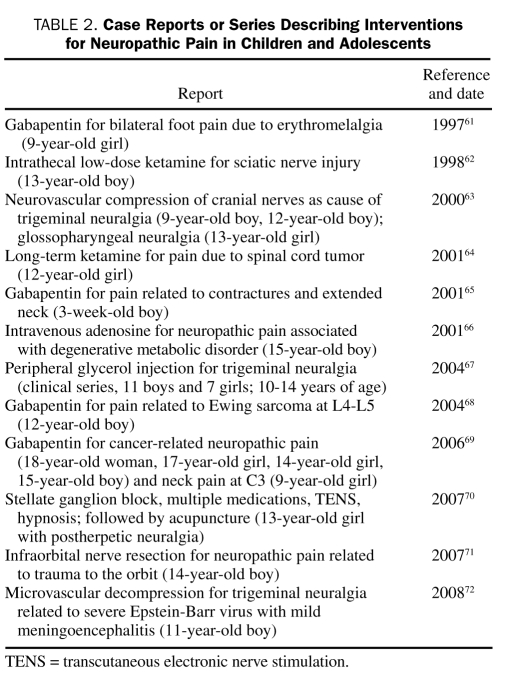
As previously mentioned, few controlled studies have been performed on interventions for neuropathic pain in children. Even the most commonly used first-line interventions,59 certain types of antidepressants and antiepileptic drugs, are almost exclusively prescribed on the basis of data from adults. In 2006, Golden et al60 published a review of nonepileptic uses of antiepileptic drugs in the pediatric population and found no published trials evaluating the safety or efficacy of antiepileptic drugs in children. During the past decade, reports on an array of presumed neuropathic pain problems in the young have been published, along with some relatively innovative approaches to management. These interventions, presented in chronological order, are outlined in Table 2.
TABLE 2.
Case Reports or Series Describing Interventions for Neuropathic Pain in Children and Adolescents
CLINICAL IMPLICATIONS
The paucity of research on neuropathic pain in children leaves us with many important and unanswered questions regarding clinical practice. Assessment of pain and sensory testing in children may be challenging, but appropriate tools have been developed and validated for pain other than that of neuropathic origin: recurrent or chronic musculoskeletal, abdominal, or headache pain.73-75 Validated indices of neuropathic pain in adults76-78 may be useful in children as well, but the developmental factors that are central to pain experience and expression in the young need to be considered.79,80
Many of the published studies on interventions for neuropathic pain in children are case reports or clinical series with few or no systematic controls. Thus, good evidence of efficacy is lacking, perhaps because placebo effects or merely passing of time may have led to similar outcomes. This may be of greater concern in children because clearly developmental processes strongly pull in the direction of normalcy.
In a parallel fashion, downward generalization of interventions used for neuropathic pain in adults may or may not be appropriate for children. Keeping in mind that medications with indications for the treatment of neuropathic pain were not studied in children or for pediatric problems as part of the review process for the Food and Drug Administration, data are lacking on the safety and efficacy of these drugs in children. Well-conducted pharmacological trials are needed to determine pharmacokinetic and pharmacodynamic properties of antiepileptic drugs and antidepressants in children. Clinical experience makes clear that children have different metabolic profiles than adults and demonstrate different adverse effects and manifestations of toxicity; thus, efficacy may be different as well.
RESEARCH IMPLICATIONS
In pursuing research of pharmacological and interventional strategies in children, a number of ethical and practical factors must be considered. For example, as previously discussed, many of these syndromes are rare in children and often present with heterogeneous symptom clusters. As a result, sufficiently powering a study with clear and meaningful inclusion criteria is difficult. Without sufficient power, the study lacks validity and is therefore unethical to pursue.
In addition, many analgesic trials are placebo-controlled studies. Federal law allows for placebo-controlled trials in children with strict limitations. Denying a child pain relief when alternatives are available presents greater than minimal risk with no direct benefit to the patient; this strategy can be considered ethically only if the test drug is presumed to have true equipoise with placebo.81 Thus, studies must be designed to meaningfully demonstrate the safety and efficacy of analgesic medications while still providing additional analgesia to ensure comfort to the degree possible. The need for different study designs than in adults is not specific for neuropathic pain in children but is shared with many other areas of pediatrics. Therefore, we hope that refinement of clinical research tools in pediatrics in general will also benefit this patient group.
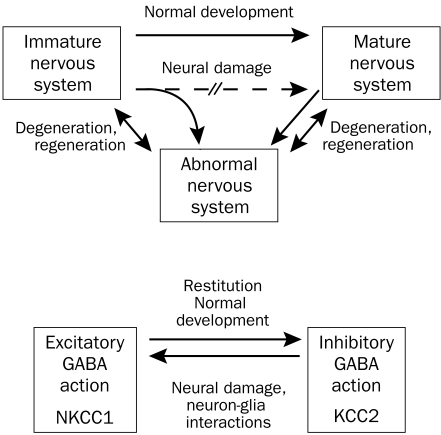
Through more systematic study of the natural history and physiology of pain resolution in children, valuable clues can be obtained about the mechanisms related to poor recovery in adults. This is related to the fact that neural damage often reverts cellular programs to an immature state, and therefore mechanisms of regeneration are partly similar to those of normal maturation (Figure, top). An example is illustrated in the Figure (bottom): in immature or damaged neurons, chloride uptake via the cotransporter NKCC1 shifts the chloride equilibrium potential to depolarized values, and hence the opening of chloride channels by γ-aminobutyric acid (GABA) may have excitatory effects.82 In contrast, healthy mature neurons express a potassium chloride cotransporter (KCC2) that helps maintain a low intracellular chloride concentration; in these neurons, GABA may produce its inhibitory effect via a chloride influx that hyperpolarizes the neurons.
FIGURE.
Top, Possible interactions of cellular mechanisms in neural damage and normal development. Bottom, Chloride equilibrium and γ-aminobutyric acid (GABA) actions in immature, damaged, and mature nervous system. KCC2 = potassium chloride cotransporter.
Finally, this review did not focus on the potential contribution of neuropathic mechanisms in common pain problems, such as chronic abdominal pain or headache. These syndromes may be associated with hyperalgesia and hence with central (or peripheral) sensitization and changes in descending control. Certainly these mechanisms will be important to address in treating such patients. Some medications that are efficacious in treating neuropathic pain may be active against chronic headache or recurrent abdominal pain if their mechanisms of action address those CNS mechanisms. If we maintain that neuropathic pain is defined as pain arising as a direct consequence of a lesion or disease affecting the somatosensory system,83 then central mechanisms that may underlie chronic headache and abdominal pain would not be included.
However, we recognize that this is an emerging field and that definitions of neuropathic pain certainly have not achieved universal consensus. The International Association for the Study of Pain84 defines neuropathic pain as “pain initiated or caused by a primary lesion or dysfunction in the nervous system.” If the focus is on dysfunction and not only identified lesions or diseases, our definitions may broaden. Although a comprehensive review of this debate is well beyond the scope of this article, a brief discussion of recent neuroimaging studies pertaining to headache will highlight some of the provocative issues.
The trigeminal brainstem nuclear complex, a rostral correlate of the dorsal horn of the spinal cord, shows neuronal excitation, best demonstrated in patients who have undergone noninvasive neuroimaging techniques, such as functional magnetic resonance imaging and cerebral near-infrared spectroscopy.85 Resulting cortical activity ultimately determines and modulates pain perception. In adult studies, a dynamic neural network is activated during the chronic pain state, including the thalamus, primary and secondary somatosensory cortices, anterior cingulate cortex, insula, nucleus accumbens, amygdala, ventral striatum, hippocampus, and cerebellum.86 Such a chronic pain signature has been noted in adults with interictal migraines,87 and magnetic resonance imaging spectroscopy shows biochemical differences in similar brain regions in patients with interictal migraine.88 There is also evidence of gray matter and cortical thickness changes with chronic symptoms.89,90 Investigation of chronic pain from both a developmental and a neuroplastic perspective may be possible because noninvasive neuroimaging methods are used in pediatric patients. In the pediatric population, the brain is undergoing rapid changes, is more plastic, and may have an increased ability to recover after injury, possibly leading to enhanced understanding of neuropathic treatment modalities.
CONCLUSION
Neuropathic pain conditions are relatively uncommon in children. In conditions typically associated with neuropathic pain in adults, such as diabetic neuropathy and postherpetic neuralgia, this may be due to a disease duration that is too short for the development of late complications; nevertheless, preventive measures would be indicated to lower the incidence of neuropathic pain persisting to adulthood. In conditions such as plexus avulsion and nerve trauma, some evidence shows that the higher plasticity of the young nervous system leads to a better restitution of function and lower incidence of pain than in adults. In other conditions, such as CRPS type 1, clinical phenotype and treatment efficacy in adolescents have been found to be similar to those in adults. Finally, some conditions lead to neuropathic pain syndromes specifically in pediatric and young adult populations; for Fabry disease, an efficacious disease-modifying treatment is available (enzyme replacement).
As with most pediatric disorders, few systematic studies have been performed on the nature, etiology, diagnosis, prognosis, and treatment of these conditions. Most of the literature consists of case reports or small clinical series with no controls and extremely limited follow-up. Because of developmental concerns, downward extension of assessment and intervention strategies used in adults with neuropathic pain are questionable and possibly dangerous. Through collaborative and carefully designed research studies, greater insights and more efficacious treatments may be identified for children with neuropathic pain. Multicenter randomized controlled trials based on research designs that address the major issues unique to pediatric patients are needed. This includes the fact that chronic pain is relatively rare in children, that there are major concerns with placebo controls, and that all these factors take place within the highly rich and variable context of synaptogenesis and growth of the central and peripheral nervous systems. Thus, suggested research designs may need to include crossover trials, multiple studies with sample sizes of one, enriched placebo study designs, and high- vs low-dosage studies.
REFERENCES
- 1. Centre for Evidence-based Medicine (CEBM) Oxford Centre for Evidence Based Medicine. Levels of Evidence (March 2009). CEBM Web site. http://www.cebm.net/index.aspx?o=1025 Accessed January 16, 2010
- 2. Littlejohn GO. Reflex sympathetic dystrophy in adolescents: lessons for adults. Arthritis Rheum. 2004;51(2):151-153 [DOI] [PubMed] [Google Scholar]
- 3. Lebovits AH, Yarmush J, Lefkowitz M. Reflex sympathetic dystrophy and posttraumatic stress disorder: multidisciplinary evaluation and treatment. Clin J Pain. 1990;6(2):153-157 [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez-Moreno J, Ruiz-Martin JM, Mateo-Soria L, Rozadilla A, Roig-Escofet D. Munchausen's syndrome simulating reflex sympathetic dystrophy. Ann Rheum Dis. 1990;49(12):1010-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Genc H, Karagoz A, Saracoglu M, Sert E, Erdem HR. Complex regional pain syndrome type-I after rubella vaccine. Eur J Pain. 2005;9(5):517-520 [DOI] [PubMed] [Google Scholar]
- 6. Agarwal V, Joseph B. Recurrent migratory sympathetically maintained pain syndrome in a child: a case report. J Pediatr Orthop B. 2006;15(1):73-74 [DOI] [PubMed] [Google Scholar]
- 7. Silber TJ, Majd M. Reflex sympathetic dystrophy syndrome in children and adolescents: report of 18 cases and review of the literature. Am J Dis Child. 1988;142(12):1325-1330 [DOI] [PubMed] [Google Scholar]
- 8. Wilder RT, Berde CB, Wolohan M, Vieyra MA, Masek BJ, Micheli LJ. Reflex sympathetic dystrophy in children: clinical characteristics and follow-up of seventy patients. J Bone Joint Surg Am. 1992;74(6):910-919 [PubMed] [Google Scholar]
- 9. Low AK, Ward K, Wines AP. Pediatric complex regional pain syndrome. J Pediatr Orthop. 2007;27(5):567-572 [DOI] [PubMed] [Google Scholar]
- 10. Murray CS, Cohen A, Perkins T, Davidson JE, Sills JA. Morbidity in reflex sympathetic dystrophy. Arch Dis Child. 2000;82(3):231-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sethna NF, Meier PM, Zurakowski D, Berde CB. Cutaneous sensory abnormalities in children and adolescents with complex regional pain syndromes. Pain. 2007;131(1-2):153-161 [DOI] [PubMed] [Google Scholar]
- 12. Tan EC, Zijlstra B, Essink ML, Goris RJ, Severijnen RS. Complex regional pain syndrome type I in children. Acta Paediatr. 2008;97(7):875-879 [DOI] [PubMed] [Google Scholar]
- 13. Lebel A, Becerra L, Wallin D, et al. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain. 2008;131(pt 7):1854-1879 [DOI] [PubMed] [Google Scholar]
- 14. Stilz RJ, Carron H, Sanders DB. Reflex sympathetic dystrophy in a 6-year-old: successful treatment by transcutaneous nerve stimulation. Anesth Analg. 1977;56(3):438-443 [DOI] [PubMed] [Google Scholar]
- 15. Richlin DM, Carron H, Rowlingson JC, Sussman MD, Baugher WH, Goldner RD. Reflex sympathetic dystrophy: successful treatment by transcutaneous nerve stimulation. J Pediatr. 1978;93(1):84-86 [DOI] [PubMed] [Google Scholar]
- 16. Kesler RW, Saulsbury FT, Miller LT, Rowlingson JC. Reflex sympathetic dystrophy in children: treatment with transcutaneous electric nerve stimulation. Pediatrics. 1988;82(5):728-732 [PubMed] [Google Scholar]
- 17. Barowsky EI, Zweig JB, Moskowitz J. Thermal biofeedback in the treatment of symptoms associated with reflex sympathetic dystrophy. J Child Neurol. 1987;2(3):229-232 [DOI] [PubMed] [Google Scholar]
- 18. Sherry DD, Wallace CA, Kelley C, Kidder M, Sapp L. Short- and long-term outcomes of children with complex regional pain syndrome type I treated with exercise therapy. Clin J Pain. 1999;15(3):218-223 [DOI] [PubMed] [Google Scholar]
- 19. Lee BH, Scharff L, Sethna NF, et al. Physical therapy and cognitive-behavioral treatment for complex regional pain syndromes. J Pediatr. 2002;141(1):135-140 [DOI] [PubMed] [Google Scholar]
- 20. Maillard SM, Davies K, Khubchandani R, Woo PM, Murray KJ. Reflex sympathetic dystrophy: a multidisciplinary approach. Arthritis Rheum. 2004;51(2):284-290 [DOI] [PubMed] [Google Scholar]
- 21. Meier PM, Alexander ME, Sethna NF, De Jong-De Vos Van Steenwijk CC, Zurakowski D, Berde CB. Complex regional pain syndromes in children and adolescents: regional and systemic signs and symptoms and hemodynamic response to tilt table testing. Clin J Pain. 2006;22(4):399-406 [DOI] [PubMed] [Google Scholar]
- 22. Rashiq S, Knight B, Ellsworth J. Treatment of reflex sympathetic dystrophy with EMLA cream. Reg Anesth. 1994;19(6):434-435 [PubMed] [Google Scholar]
- 23. di Vadi PP, Brill S, Jack T, Brown C, Edwards T. Intravenous regional blocks with guanethidine and prilocaine combined with physiotherapy: two children with complex regional pain syndrome, type 1. Eur J Anaesthesiol. 2002;19(5):384-386 [DOI] [PubMed] [Google Scholar]
- 24. Dadure C, Motais F, Ricard C, Raux O, Troncin R, Capdevila X. Continuous peripheral nerve blocks at home for treatment of recurrent complex regional pain syndrome I in children. Anesthesiology. 2005;102(2):387-391 [DOI] [PubMed] [Google Scholar]
- 25. Nordmann GR, Lauder GR, Grier DJ. Computed tomography guided lumbar sympathetic block for complex regional pain syndrome in a child: a case report and review. Eur J Pain. 2006;10(5):409-412 [DOI] [PubMed] [Google Scholar]
- 26. Meier PM, Zurakowski D, Berde CB, Sethna NF. Lumbar sympathetic blockade in children with complex regional pain syndromes: a double blind placebo-controlled crossover trial. Anesthesiology. 2009;111(2):372-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arner S. Intravenous phentolamine test: diagnostic and prognostic use in reflex sympathetic dystrophy. Pain. 1991;46(1):17-22 [DOI] [PubMed] [Google Scholar]
- 28. Raja SN, Treede RD, Davis KD, Campbell JN. Systemic alpha-adrenergic blockade with phentolamine: a diagnostic test for sympathetically maintained pain. Anesthesiology. 1991;74(4):691-698 [DOI] [PubMed] [Google Scholar]
- 29. Wheeler DS, Vaux KK, Tam DA. Use of gabapentin in the treatment of childhood reflex sympathetic dystrophy. Pediatr Neurol. 2000;22(3):220-221 [DOI] [PubMed] [Google Scholar]
- 30. Rudich Z, Stinson J, Jeavons M, Brown SC. Treatment of chronic intractable neuropathic pain with dronabinol: case report of two adolescents. Pain Res Manag. 2003;8(4):221-224 [DOI] [PubMed] [Google Scholar]
- 31. Lalwani K, Shoham A, Koh JL, McGraw T. Use of oxcarbazepine to treat a pediatric patient with resistant complex regional pain syndrome. J Pain. 2005;6(10):704-706 [DOI] [PubMed] [Google Scholar]
- 32. Suresh S, Wheeler M, Patel A. Case series: IV, regional anesthesia with ketorolac and lidocaine: is it effective for the management of complex regional pain syndrome 1 in children and adolescents? Anesth Analg. 2003;96(3):694-695 [DOI] [PubMed] [Google Scholar]
- 33. Brown SC, Jeavons M, Stinson J. Effectiveness of pamidronate for treating intractable chronic neuropathic pain: case report of two adolescents. Clin J Pain. 2005;21(6):549-552 [DOI] [PubMed] [Google Scholar]
- 34. Kiefer RT, Rohr P, Ploppa A, Altemeyer KH, Schwartzman RJ. Complete recovery from intractable complex regional pain syndrome, CRPS-type I, following anesthetic ketamine and midazolam. Pain Pract. 2007;7(2):147-150 [DOI] [PubMed] [Google Scholar]
- 35. Stanton-Hicks M, Kapural L. An effective treatment of severe complex regional pain syndrome type 1 in a child using high doses of intrathecal ziconotide. J Pain Symptom Manage. 2006;32(6):509-511 [DOI] [PubMed] [Google Scholar]
- 36. Farid IS, Heiner EJ. Intrathecal local anesthetic infusion as a treatment for complex regional pain syndrome in a child. Anesth Analg. 2007;104(5):1078-1080 [DOI] [PubMed] [Google Scholar]
- 37. Petje G, Radler C, Aigner N, Walik N, Kriegs G, Grill F. Treatment of reflex sympathetic dystrophy in children using a prostacyclin analog: preliminary results. Clin Orthop Relat Res. 2005;433:178-182 [DOI] [PubMed] [Google Scholar]
- 38. Honjyo K, Hamasaki Y, Kita M, Harano K, Totoki T, Miyazaki S. An 11-year-old girl with reflex sympathetic dystrophy successfully treated by thoracoscopic sympathectomy. Acta Paediatr. 1997;86(8):903-905 [DOI] [PubMed] [Google Scholar]
- 39. Olsson GL, Meyerson BA, Linderoth B. Spinal cord stimulation in adolescents with complex regional pain syndrome type I (CRPS-I). Eur J Pain. 2008;12(1):53-59 [DOI] [PubMed] [Google Scholar]
- 40. Wilder RT. Management of pediatric patients with complex regional pain syndrome. Clin J Pain. 2006;22(5):443-448 [DOI] [PubMed] [Google Scholar]
- 41. Krane EJ, Heller LB. The prevalence of phantom sensation and pain in pediatric amputees. J Pain Symptom Manage. 1995;10(1):21-29 [DOI] [PubMed] [Google Scholar]
- 42. Melzack R, Israel R, Lacroix R, Schultz G. Phantom limbs in people with congenital limb deficiency or amputation in early childhood. Brain. 1997;120(pt 9):1603-1620 [DOI] [PubMed] [Google Scholar]
- 43. Wilkins KL, McGrath PJ, Finley GA, Katz J. Phantom limb sensations and phantom limb pain in child and adolescent amputees. Pain. 1998;78(1):7-12 [DOI] [PubMed] [Google Scholar]
- 44. Anand P, Birch R. Restoration of sensory function and lack of long-term chronic pain syndromes after brachial plexus injury in human neonates. Brain. 2002;125(pt 1):113-122 [DOI] [PubMed] [Google Scholar]
- 45. McCann ME, Waters P, Goumnerova LC, Berde C. Self-mutilation in young children following brachial plexus birth injury. Pain. 2004;110(1-2):123-129 [DOI] [PubMed] [Google Scholar]
- 46. Knox DJ, McLeod BJ, Goucke CR. Acute phantom limb pain controlled by ketamine. Anaesth Intensive Care. 1995;23(5):620-622 [DOI] [PubMed] [Google Scholar]
- 47. Rusy LM, Troshynski TJ, Weisman SJ. Gabapentin in phantom limb pain management in children and young adults: report of seven cases. J Pain Symptom Manage. 2001;21(1):78-82 [DOI] [PubMed] [Google Scholar]
- 48. Atherton DD, Taherzadeh O, Elliot D, Anand P. Age-dependent development of chronic neuropathic pain, allodynia and sensory recovery after upper limb nerve injury in children. J Hand Surg Eur Vol. 2008;33(2):186-191 [DOI] [PubMed] [Google Scholar]
- 49. Saroyan JM, Winfree CJ, Schechter WS, Roye D, Gold AP. Sciatic neuropathy after lower-extremity trauma: successful treatment of an uncommon pain and disability syndrome in an adolescent. Am J Phys Med Rehabil. 2007;86(7):597-600 [DOI] [PubMed] [Google Scholar]
- 50. Gazzeri R, Mercuri S, Galarza M. Atypical trigeminal neuralgia associated with tongue piercing. JAMA. 2006;296(15):1840-1842 [DOI] [PubMed] [Google Scholar]
- 51. Lauder GR, White MC. Neuropathic pain following multilevel surgery in children with cerebral palsy: a case series and review. Paediatr Anaesth. 2005;15(5):412-420 [DOI] [PubMed] [Google Scholar]
- 52. Persson J, Axelsson G, Hallin RG, Gustafsson LL. Beneficial effects of ketamine in a chronic pain state with allodynia, possibly due to central sensitization. Pain. 1995;60(2):217-222 [DOI] [PubMed] [Google Scholar]
- 53. Korinthenberg R, Schessl J, Kirschner J. Clinical presentation and course of childhood Guillain-Barré syndrome: a prospective multicentre study. Neuropediatrics. 2007;38(1):10-17 [DOI] [PubMed] [Google Scholar]
- 54. Hopkin RJ, Bissler J, Banikazemi M, et al. Characterization of Fabry disease in 352 pediatric patients in the Fabry registry. Pediatr Res. 2008;64(5):550-555 [DOI] [PubMed] [Google Scholar]
- 55. Maag R, Binder A, Maier C, et al. Detection of a characteristic painful neuropathy in Fabry disease: a pilot study. Pain Med. 2008;9(8):1217-1223 [DOI] [PubMed] [Google Scholar]
- 56. Laaksonen SM, Roytta M, Jaaskelainen SK, Kantola I, Penttinen M, Falck B. Neuropathic symptoms and findings in women with Fabry disease. Clin Neurophysiol. 2008;119(6):1365-1372 [DOI] [PubMed] [Google Scholar]
- 57. Brady RO. Fabry's disease. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF, eds. Peripheral Neuropathy. 3rd ed. Philadelphia, PA: WB Saunders; 1993:1169-1178 [Google Scholar]
- 58. Ries M, Mengel E, Kutschke G, et al. Use of gabapentin to reduce chronic neuropathic pain in Fabry disease. J Inherit Metab Dis. 2003;26(4):413-414 [DOI] [PubMed] [Google Scholar]
- 59. Dworkin RH, O'Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237-251 [DOI] [PubMed] [Google Scholar]
- 60. Golden AS, Haut SR, Moshe SL. Nonepileptic uses of antiepileptic drugs in children and adolescents. Pediatr Neurol. 2006;34(6):421-432 [DOI] [PubMed] [Google Scholar]
- 61. McGraw T, Kosek P. Erythromelalgia pain managed with gabapentin. Anesthesiology. 1997;86(4):988-990 [DOI] [PubMed] [Google Scholar]
- 62. Takahashi H, Miyazaki M, Nanbu T, Yanagida H, Morita S. The NMDA-receptor antagonist ketamine abolishes neuropathic pain after epidural administration in a clinical case. Pain. 1998;75(2-3):391-394 [DOI] [PubMed] [Google Scholar]
- 63. Childs AM, Meaney JF, Ferrie CD, Holland PC. Neurovascular compression of the trigeminal and glossopharyngeal nerve: three case reports. Arch Dis Child. 2000;82(4):311-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Klepstad P, Borchgrevink P, Hval B, Flaat S, Kaasa S. Long-term treatment with ketamine in a 12-year-old girl with severe neuropathic pain caused by a cervical spinal tumor. J Pediatr Hematol Oncol. 2001;23(9):616-619 [DOI] [PubMed] [Google Scholar]
- 65. Behm MO, Kearns GL. Treatment of pain with gabapentin in a neonate. Pediatrics. 2001;108(2):482-484 [DOI] [PubMed] [Google Scholar]
- 66. Gyllenhammar E, Nordfors LO. Systemic adenosine infusions alleviated neuropathic pain. Pain. 2001;94(1):121-122 [DOI] [PubMed] [Google Scholar]
- 67. Yue WL. Peripheral glycerol injection for the relief of facial neuralgia in children. Int J Pediatr Otorhinolaryngol. 2004;68(1):37-41 [DOI] [PubMed] [Google Scholar]
- 68. Keskinbora K, Pekel AF, Aydinli I. The use of gabapentin in a 12-year-old boy with cancer pain [letter]. Acta Anaesthesiol Scand. 2004;48(5):663-664 [DOI] [PubMed] [Google Scholar]
- 69. Butkovic D, Toljan S, Mihovilovic-Novak B. Experience with gabapentin for neuropathic pain in adolescents: report of five cases. Paediatr Anaesth. 2006;16(3):325-329 [DOI] [PubMed] [Google Scholar]
- 70. Wang SM. An integrative approach for treating postherpetic neuralgia—a case report. Pain Pract. 2007;7(3):274-278 [DOI] [PubMed] [Google Scholar]
- 71. Watson CP, Stinson JN, Dostrovsky JO, Hawkins C, Rutka J, Forrest C. Nerve resection and re-location may relieve causalgia: a case report. Pain. 2007;132(1-2):211-217 [DOI] [PubMed] [Google Scholar]
- 72. Solth A, Veelken N, Gottschalk J, Goebell E, Pothmann R, Kremer P. Successful vascular decompression in an 11-year-old patient with trigeminal neuralgia. Childs Nerv Syst. 2008;24(6):763-766 [DOI] [PubMed] [Google Scholar]
- 73. Beggs S, Fitzgerald M. Development of peripheral and spinal nociceptive systems. In: Anand KJS, Stevens BJ, McGrath PJ, eds. Pain in Neonates and Infants. 3rd ed. Edinburgh, United Kingdom: Elsevier; 2007. [Google Scholar]
- 74. Craig KD, Korol CT. Developmental issues in understanding, assessing, and managing pediatric pain. In: Walco GA, Goldschneider KR, eds. Pain in Children: A Practical Guide for Primary Care. Totowa, NJ: Humana Press; 2008:9-20 [Google Scholar]
- 75. McGrath PJ, Walco GA, Turk DC, et al. PedIMMPACT. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9(9):771-783 [DOI] [PubMed] [Google Scholar]
- 76. Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92(1-2):147-157 [DOI] [PubMed] [Google Scholar]
- 77. Bennett MI, Attal N, Backonja MM, et al. Using screening tools to identify neuropathic pain. Pain. 2007;127(3):199-203 [DOI] [PubMed] [Google Scholar]
- 78. Rolke R, Magerl W, Campbell KA, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10(1):77-88 [DOI] [PubMed] [Google Scholar]
- 79. von Baeyer CL. Measurement and assessment of pediatric pain in primary care. In: Walco GA, Goldschneider KR, eds. Pain in Children: A Practical Guide for Primary Care. Totowa, NJ: Humana Press; 2008:21-27 [Google Scholar]
- 80. McGrath PA, Brown SC. Quantitative sensory testing in children: practical considerations for research and clinical practice. Pain. 2006;123(1-2):1-2 [DOI] [PubMed] [Google Scholar]
- 81. Miller FG, Wendler D, Wilfond B. When do the federal regulations allow placebo-controlled trials in children? J Pediatr. 2003;142(2):102-107 [DOI] [PubMed] [Google Scholar]
- 82. De Koninck Y. Altered chloride homeostasis in neurological disorders: a new target. Curr Opin Pharmacol. 2007;7(1):93-99 [DOI] [PubMed] [Google Scholar]
- 83. Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630-1635 [DOI] [PubMed] [Google Scholar]
- 84.International Association for the Study of Pain IASP pain terminology. IASP Web site. http://www.iasp-pain.org/AM/Template.cfm?Section=Pain_Definitions&Template=/CM/HTMLDisplay.cfm&ContentID=1728#Neuropathic. http://www.iasp-pain.org/AM/Template.cfm?Section=Pain_Definitions&Template=/CM/HTMLDisplay.cfm&ContentID=1728#Neuropathic Accessed January 15, 2010.
- 85. Sava S, Lebel AA, Leslie DS, et al. Challenges of functional imaging research of pain in children. Mol Pain. 2009. June 16;5:30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26(47):12165-12173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. May A, Ashburner J, Buchel C, et al. Correlation between structural and functional changes in brain in an idiopathic headache syndrome. Nat Med. 1999;5(7):836-838 [DOI] [PubMed] [Google Scholar]
- 88. Prescot A, Becerra L, Pendse G, et al. Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol Pain. 2009. June 30;5:34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rocca MA, Ceccarelli A, Falini A, et al. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37(7):1765-1770 [DOI] [PubMed] [Google Scholar]
- 90. Schmidt-Wilcke T, Leinisch E, Straube A, et al. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65(9):1483-1486 [DOI] [PubMed] [Google Scholar]