Abstract
The Neuropathic Pain Special Interest Group of the International Association for the Study of Pain recently sponsored the development of evidence-based guidelines for the pharmacological treatment of neuropathic pain. Tricyclic antidepressants, dual reuptake inhibitors of serotonin and norepinephrine, calcium channel α2-δ ligands (ie, gabapentin and pregabalin), and topical lidocaine were recommended as first-line treatment options on the basis of the results of randomized clinical trials. Opioid analgesics and tramadol were recommended as second-line treatments that can be considered for first-line use in certain clinical circumstances. Results of several recent clinical trials have become available since the development of these guidelines. These studies have examined botulinum toxin, high-concentration capsaicin patch, lacosamide, selective serotonin reuptake inhibitors, and combination therapies in various neuropathic pain conditions. The increasing number of negative clinical trials of pharmacological treatments for neuropathic pain and ambiguities in the interpretation of these negative trials must also be considered in developing treatment guidelines. The objectives of the current article are to review the Neuropathic Pain Special Interest Group guidelines for the pharmacological management of neuropathic pain and to provide a brief overview of these recent studies.
DPN = diabetic peripheral neuropathy; HIV = human immunodeficiency virus; HRQoL = health-related quality of life; NeuPSIG = Neuropathic Pain Special Interest Group; NP = neuropathic pain; PHN = postherpetic neuralgia; RCT = randomized clinical trial; SSNRI = selective serotonin norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor; TCA = tricyclic antidepressant
Neuropathic pain (NP) has recently been redefined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system.”1 Several recent studies have shown that NP can adversely affect patients' overall health-related quality of life (HRQoL), including physical and emotional functioning,2-6 and that it is associated with substantial societal costs.6-11
Neuropathic pain is challenging to manage, and many patients have pain that is refractory to existing treatments. In randomized clinical trials (RCTs) that have examined pharmacotherapy, no more than half of patients experience clinically meaningful pain relief, which is almost always partial but not complete relief. In addition, patients frequently experience burdensome adverse effects and as a consequence are often unable to tolerate the treatment. Results of RCTs are consistent with several studies of NP in the community, which have also shown that patients continue to have, on average, pain of moderate severity despite taking prescribed medications for their pain.6
Because of limitations in the current treatment of patients with NP, the Neuropathic Pain Special Interest Group (NeuPSIG) of the International Association for the Study of Pain sponsored the development of evidence-based guidelines for the pharmacological treatment of NP that take into account clinical efficacy, adverse effects, effects on HRQoL, convenience, and costs.12 The objectives of the current article are to review these guidelines and to discuss results of recent studies that should be considered in the development of future pharmacological recommendations for NP. Although consensus guidelines for the pharmacological treatment of NP were also developed simultaneously by the European Federation of Neurological Societies13 and the Canadian Pain Society,14 it is beyond the scope of the current article to discuss these guidelines (a comparison of all 3 guidelines has been published15).
Several general considerations and limitations should be emphasized regarding pharmacological treatment recommendations for NP. First, despite the fact that many types of peripheral and central NP occur in clinical practice, most RCTs have examined patients with either postherpetic neuralgia (PHN) or painful diabetic peripheral neuropathy (DPN). Second, there are few head-to-head trials comparing different treatments and so direct comparisons of efficacy and tolerability are generally not possible. Indirect comparisons of different treatments are problematic because RCTs differ substantially in research design and outcomes reported. Many older RCTs used a crossover design, whereas newer medications have typically been studied using a parallel group research design. Outcome measures have also differed over time and across studies, with more recent RCTs assessing treatment response more comprehensively and including measures of HRQoL and patient global assessments of improvement and satisfaction, which were not collected in many older trials. Finally, treatment duration in RCTs of medications for NP has been relatively short, typically 3 months or less, which is in marked contrast to the chronic nature of most NP conditions and makes it impossible to extrapolate the results to long-term use.
The limitations of existing research constitute substantial challenges in developing treatment recommendations for NP. For example, the extent to which efficacy established in relatively short-term trials of PHN and painful DPN can be extrapolated to other conditions and to long-term use is unknown. In addition, appreciation of the considerable heterogeneity among different NP conditions is increasing, not only in responsiveness to different treatments but also in other factors, such as their patterns of signs and symptoms (ie, their “sensory phenotype”).16,17 Moreover, the lack of direct comparisons of different medications makes it difficult to contrast and rank medications on the basis of efficacy, safety, and tolerability. Therefore, the choice of medication in an individual patient with NP depends on a number of factors, including the potential for adverse effects, treatment of comorbidities (eg, depression, sleep disorders), drug interactions, risks of misuse and abuse, and cost.
GUIDELINES FOR THE PHARMACOLOGICAL MANAGEMENT OF NP
The NeuPSIG guidelines recommend medications as first-line treatment if efficacy in NP has been established in multiple RCTs (Oxford Centre for Evidence-based Medicine grade A recommendation18), and these results are consistent with the authors' clinical experience; as second-line if efficacy in NP has been established in multiple RCTs (grade A recommendation), but there were reservations about the use of the medication relative to the first-line medications based on the authors' clinical experience; and as third-line if only one RCT has shown efficacy in NP or if the results of 2 or more RCTs were inconsistent (grade B recommendation), but the authors thought that in selected circumstances the medication may be a reasonable treatment option.12 These consensus guidelines were not intended to apply to pediatric patients, patients with trigeminal neuralgia (for which separate treatment recommendations are available13,14,19,20), or conditions that are not clearly NP (eg, fibromyalgia and irritable bowel syndrome). They also do not take into account prescribing challenges faced in some developing and currency-restricted countries.
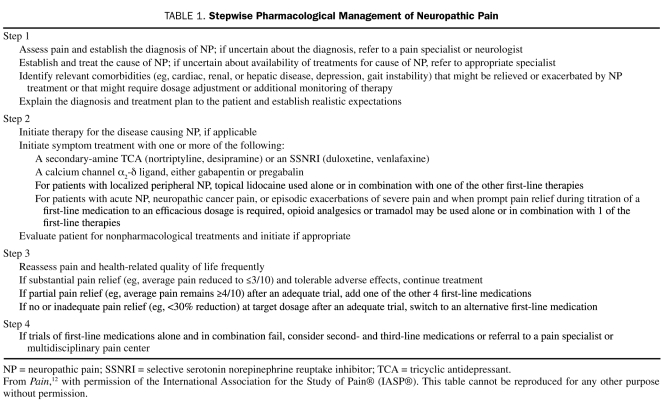
Because many patients treated with a single efficacious medication do not obtain satisfactory pain relief, the guidelines emphasize that patients may benefit from use of combinations of efficacious NP medications. A stepwise management strategy for patients with NP is presented in Table 1, and specific guidelines for use of the first- and second-line medications discussed in this article are provided in Table 2.
TABLE 1.
Stepwise Pharmacological Management of Neuropathic Pain
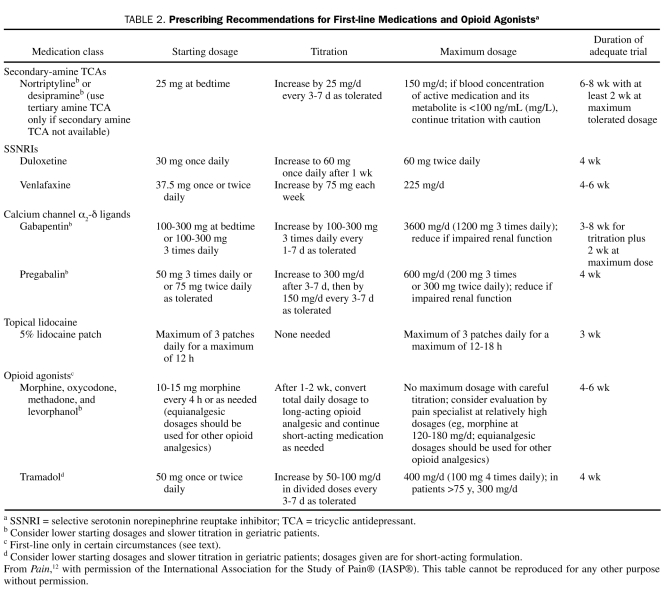
TABLE 2.
Prescribing Recommendations for First-line Medications and Opioid Agonistsa
First-Line Medications
Antidepressants With Both Norepinephrine and Serotonin Reuptake Inhibition. A large number of placebo-controlled RCTs have found tricyclic antidepressants (TCAs) to be efficacious for several different types of NP (see later section regarding negative trials in painful human immunodeficiency virus [HIV] and chemotherapy-associated peripheral neuropathy).21 In addition, TCAs are efficacious for the treatment of depression, a common comorbidity in patients with chronic pain, but their analgesic efficacy in NP has been established in nondepressed patients,22 which demonstrates that their beneficial effects in NP cannot be explained simply by their antidepressant effects. They are inexpensive and have the convenience of being administered once daily. However, anticholinergic adverse effects are common and include dry mouth, orthostatic hypotension, constipation, and urinary retention. These effects can be reduced by starting with low dosages administered at bedtime and with slow titration to higher dosages and also by using a secondary amine TCA (nortriptyline or desipramine).12,21 Cardiac toxicity is a concern, and the NeuPSIG guidelines recommend prescribing TCAs with caution in patients with ischemic cardiac disease or ventricular conduction abnormalities, limiting the dosages to less than 100 mg/d when possible, and obtaining a screening electrocardiogram for patients older than 40 years. It can take 6 to 8 weeks, including 2 weeks at the highest dosage tolerated, for an adequate trial of treatment with a TCA (Table 2).
Duloxetine and venlafaxine are selective serotonin norepinephrine reuptake inhibitors (SSNRIs) that have been studied in peripheral NP (a third SSNRI, milnacipran, has been studied only in fibromyalgia). Duloxetine has shown consistent efficacy in painful DPN,12 with effectiveness sustained for 1 year in an open-label trial.23 Unfortunately, duloxetine has not been studied in other types of NP, and so its efficacy in such conditions is unknown. Duloxetine has shown efficacy in the treatment of major depression and generalized anxiety disorder, and its dosing is simple, with 60 mg once daily appearing to be as effective as 60 mg twice daily. The most common adverse effect of duloxetine is nausea, which seems to be reduced by administering 30 mg once daily for 1 week before increasing to 60 mg once daily (Table 2). Duloxetine does not seem to produce clinically important electrocardiographic or blood pressure changes,24 and a recent review concluded that aminotransferase monitoring is unnecessary.25
Venlafaxine has shown efficacy in painful DPN and painful polyneuropathies of different origins but not in PHN.12 Typically, 2 to 4 weeks are required to titrate to an efficacious dosage (ie, 150-225 mg/d); venlafaxine is available in short- and long-acting preparations (Table 2). Cardiac conduction abnormalities have been reported in a small number of patients,26 and blood pressure increases can occur; therefore, venlafaxine should be prescribed with caution in patients with cardiac disease. In addition, venlafaxine should be tapered when treatment is being discontinued because a withdrawal syndrome has been described.27
Calcium Channel α2-δ Ligands (Gabapentin and Pregabalin). Gabapentin and pregabalin each bind to voltage-gated calcium channels at the α2-δ subunit and inhibit neurotransmitter release. They have shown efficacy vs placebo in several NP conditions.12,21 Although gabapentin and pregabalin have few drug interactions, both can produce dose-dependent dizziness and sedation, which can be reduced by starting with lower dosages and titrating cautiously. Both medications also require dosage reduction in patients with renal insufficiency, and dosage adjustments can be made in relation to creatinine clearance.
Gabapentin pharmacokinetics are nonlinear (due to saturable absorption), and dosing requires careful titration. Treatment should be initiated at low dosages with gradual increases until pain relief, dose-limiting adverse effects, or 3600 mg/d in 3 divided doses is reached (Table 2). An adequate trial of treatment with gabapentin can require 2 months or more.
The efficacy and tolerability of pregabalin seem to be similar to those of gabapentin; however, pregabalin has linear pharmacokinetics, and dosing is more straightforward. Most patients can start taking the drug at 150 mg/d in 2 or 3 divided doses, which is then titrated up to 300 mg/d after 1 or 2 weeks (Table 2). For patients who tolerate 300 mg/d but have inadequate pain relief, the dosage can be further titrated to 600 mg/d, but higher dosages are not consistently more effective than 300 mg/d and are associated with a greater rate of adverse effects. Pregabalin may provide analgesia more quickly than gabapentin because the initial dosage of 150 mg/d has been found to be efficacious in some trials and because the time required to titrate to a full dosage is less.28 In the United States, pregabalin is a Schedule V drug.
Topical Lidocaine. The 5% lidocaine patch has shown efficacy and excellent tolerability in RCTs involving patients with PHN and allodynia and in patients with allodynia due to different types of peripheral NP.12,21 As a topical treatment without substantial systemic absorption, the most common adverse effects are mild local reactions; the lack of systemic adverse effects and drug interactions can be particularly advantageous in older patients or patients with complex NP (Table 2). Lidocaine gel (5%), which is less expensive than the lidocaine patch, has also shown efficacy in patients with PHN and allodynia.12,21 Topical lidocaine is most appropriate in well-localized NP, and it is unlikely to be of benefit in patients with central NP; unfortunately, attempts to predict which patients are most likely to respond to treatment with topical lidocaine have been generally unsuccessful.29,30
Second-Line Medications That Are Appropriate for First-line Use in Certain Circumstances
Tramadol and opioid analgesics have shown efficacy in several high-quality RCTs involving patients with different types of NP. Nevertheless, as a result of concerns regarding their long-term safety relative to the first-line medications, the NeuPSIG guidelines recommend that tramadol and opioids should typically be reserved for patients who have not responded to first-line medications. However, these medications are recommended as first-line treatments for patients with acute NP, NP due to cancer, and episodic exacerbations of severe NP, as well as when titrating one of the first-line medications if prompt relief of pain is required.
Tramadol. Tramadol, which has shown efficacy in several NP conditions, is a weak opioid μ-receptor agonist that also inhibits reuptake of serotonin and norepinephrine. Like strong opioid analgesics, it provides relatively rapid pain relief, although it may be somewhat less efficacious than strong μ-agonists (eg, morphine and oxycodone).21 The risk of abuse with tramadol seems considerably less than that with opioid analgesics.12
The adverse effect profile of tramadol is similar to that of opioids, but tramadol also lowers the seizure threshold and can interact with certain medications (eg, SSNRIs and selective serotonin reuptake inhibitors [SSRIs]) to cause serotonin syndrome, a potentially fatal reaction. Although the risk of serotonin syndrome is important to consider, it appears to be relatively uncommon in clinical practice. Treatment with tramadol is typically started at 50 mg once or twice daily and then increased gradually as needed to a maximum of 400 mg/d; older patients and those with renal or hepatic dysfunction are more prone to drug accumulation and should be maintained with lower dosages (Table 2).
Opioid Analgesics. Several RCTs have shown that opioid analgesics provide greater pain relief than placebo in different types of NP,12,21,31 with analgesia at least as great as that found with TCAs and gabapentin.32 However, because of concerns regarding long-term safety, including risks of hypogonadism, immunologic changes, and opioid misuse or abuse, opioids are not recommended for routine first-line use and should generally be reserved for patients who do not respond to the first-line medications discussed herein.
Constipation, nausea, and sedation are the most common adverse effects of opioids. Initiating treatment with low dosages and titrating gradually can reduce nausea and sedation. However, constipation tends to be a chronic problem for patients taking opioids and should be monitored closely and treated with a bowel regimen. Various therapeutic strategies are emerging that have the potential to mitigate opioid-associated constipation that require further evaluation. All patients treated with long-term opioid therapy develop physical dependence; therefore, it is important to taper dosages gradually when treatment is being discontinued and to instruct patients not to abruptly stop taking their medication.
Patients with active or previous substance abuse (including alcoholism) and a family history of substance abuse are more likely to misuse and abuse opioids. This risk must be considered before treatment with an opioid analgesic is initiated; opioid-prescribing guidelines recommend using the lowest effective dosage and monitoring for signs of inappropriate use.33-35 Because the optimal opioid dosage varies substantially from patient to patient, patients must undergo individualized opioid titration, using dosages that have shown efficacy in NP trials and typically using extended-release formulations for long-term treatment (Table 2).
Third-line Medications
Several additional medications have shown efficacy for the treatment of NP in either a single RCT or inconsistently across multiple RCTs. The NeuPSIG guidelines recommend that these medications should generally be reserved for patients who cannot tolerate or who do not respond adequately to first- and second-line medications. These medications include certain antidepressant medications (eg, bupropion, citalopram, and paroxetine), certain antiepileptic medications (eg, carbamazepine, lamotrigine, oxcarbazepine, topiramate, and valproic acid), topical low-concentration capsaicin, dextromethorphan, memantine, and mexiletine.12
Central NP
Relatively few RCTs have been conducted in patients with NP caused by lesions in the central nervous system, and results of these trials and clinical experience suggest that such conditions may be relatively more refractory to treatment than peripheral NP.21,36 Efficacy has been shown for TCAs in central poststroke NP, calcium channel α2-δ ligands in spinal cord injury and central poststroke NP, and tramadol in spinal cord injury NP.12,21,37,38 Cannabinoids appear to be efficacious in multiple sclerosis—associated NP, but use of cannabinoids is limited by poor availability and concerns regarding risks of abuse and potential to precipitate psychosis, especially in high-risk individuals.12,39,40 Patients with central NP who do not respond adequately to these medications can be treated with the first- and second-line medications that have established efficacy in peripheral NP (except for topical lidocaine, which is not recommended for use in central NP).
RECENT CLINICAL TRIALS
In this section, we briefly discuss several recent RCTs that should be considered in future efforts to revise the treatment guidelines summarized herein. These studies do not represent a comprehensive update of recent NP trials but rather have been selected because they involve novel treatments or provocative issues.
Botulinum Toxin
Efficacy of botulinum toxin in the treatment of cervical dystonia and various types of spasticity is well established, but results of preclinical and human experimental studies41 have suggested that it might also have an analgesic effect in patients with NP. On the basis of this research and anecdotal observations, a double-blind trial was conducted in which 29 patients with PHN or posttraumatic or postoperative NP and mechanical allodynia were randomized to intradermal injection of botulinum toxin type A or matching placebo within the area of allodynia.42 Pain intensity during 24 weeks and brush-evoked allodynia at 4 and 12 weeks after treatment were significantly reduced in patients who received botulinum toxin vs placebo. In addition, a crossover trial of intradermal botulinum toxin injections into the feet of 20 patients with DPN showed significant pain reduction vs placebo during a 12-week period.43
However, in a randomized trial in which 117 patients with PHN received either botulinum toxin or placebo and were followed up for 12 weeks, the 2 treatment groups did not significantly differ (Susan Abu-Shakra, MD, written communication, October 14, 2009). It is difficult to interpret the conflicting findings of 2 small trials showing efficacy and an unpublished multicenter trial that failed to demonstrate efficacy. The mean dose of botulinum toxin per area of pain in the negative PHN trial was lower than the dose used in the positive PHN or posttraumatic or postoperative NP trial,42 and it is possible that this accounts for the different results. As discussed herein, the increasing number of negative trials of NP treatments will make it challenging to update guidelines for the pharmacological management of NP.
High-Concentration Capsaicin Patch
Topical low-concentration capsaicin is currently considered a third-line treatment of NP. A high-concentration capsaicin patch has been studied in multiple RCTs in patients with PHN and painful HIV neuropathy.44 Results of 2 phase 3 trials in PHN showed that a single application of the high-concentration patch vs a low-concentration control patch was efficacious in reducing pain from the second week after the capsaicin application throughout a subsequent 8-week period; this effect was also observed for 12 weeks in secondary analyses.45,46 Similarly, 1 of 2 RCTs in patients with painful HIV neuropathy showed significantly decreased pain continuing from the second week after treatment through the end of a 12-week period.47 However, in a second HIV neuropathy trial, the effects of high-concentration vs control patches did not significantly differ.44
Application of the high-concentration capsaicin patch in patients with PHN or painful HIV neuropathy was safe and well tolerated, and adverse events were limited to transient increases in pain associated with patch application and application site reactions (eg, erythema).44-47 Because a single treatment application may be associated with sustained reductions in pain that persists for 2 to 3 months, the high-concentration capsaicin patch has the potential to provide a novel addition to existing pharmacological treatments for NP, which are typically administered one or more times each day. However, the long-term benefits of this treatment are unknown, and the safety of repeated applications of high-concentration capsaicin must be carefully evaluated because skin biopsy studies have shown transient epidermal denervation by capsaicin48 that is paralleled by a functional loss, particularly of heat pain sensation.49
Lacosamide
Lacosamide is a new antiepileptic medication that has activity at voltage-gated sodium channels. In addition to epilepsy, lacosamide has been studied extensively in painful DPN. Evidence of the efficacy of lacosamide in patients with painful DPN has been provided by the results of a single phase 2 trial,50 3 parallel group phase 3 trials,51-53 and a randomized withdrawal trial conducted in patients who had been taking lacosamide on an open-label basis for at least 1 year.54 In one of the later trials, the statistical significance of the result was marginal (P=.0507),51 and a fourth phase 3 trial failed to show a statistically significant difference between lacosamide and placebo.55
Despite its approval for adjunctive treatment of partial-onset seizures, lacosamide was not approved for the treatment of painful DPN by either the Food and Drug Administration or the European Medicines Agency. It is unknown whether these negative decisions were based on inadequate evidence of efficacy—perhaps when a more conservative baseline observation carried forward strategy was used to impute missing data for individuals who withdrew from the trials56—or whether concerns about safety provide the explanation. Although the safety and tolerability of lacosamide appear generally comparable to other medications approved for NP, small dose-related increases in the PR interval have been associated with lacosamide treatment.55 It will be difficult to determine what recommendations should be made for lacosamide in future NP treatment guidelines without knowledge of the specific reasons for the 2 negative regulatory decisions regarding its approval for the treatment of painful DPN. In the United States, lacosamide is a Schedule V drug.
Selective Serotonin Reuptake Inhibitors
As previously mentioned, SSRIs have been considered third-line medications for patients with NP. This is because the evidence of the analgesic efficacy of this class of antidepressants has been inconsistent, with early crossover trials in patients with painful DPN showing modest beneficial effects for paroxetine58 and citalopram59 but no efficacy for fluoxetine60 compared with placebo. A recent crossover RCT in patients with various types of painful polyneuropathy, including painful DPN, found significantly greater pain relief with escitalopram compared with placebo, a benefit that appeared to be independent of antidepressant effects.61 However, the authors concluded that escitalopram “appears to have a clinically relevant effect in only few patients and … can probably not be recommended as first or second line treatment in neuropathic pain.”61(p281)
It is well known that within several years after their introduction, SSRIs began to replace TCAs in psychiatry as first-line medications for the treatment of depression. Undoubtedly, many reasons exist for this, including greater safety against overdose, the lack of a need for titration in many patients because the starting dosage can be the therapeutic dosage, and an adverse effect profile that was often preferable to that associated with TCAs. Many of these differences between SSRIs and TCAs can also be relevant in the treatment of NP. For this reason, results of the 3 positive trials in painful polyneuropathies provide an impetus not only for a careful reevaluation of the role of SSRIs in NP but also for well-designed RCTs of their efficacy in additional NP conditions, ideally in head-to-head trials directly comparing them with existing first-line treatments.
Combination Therapies
Most RCTs of treatments for NP have studied the effects of individual medications in specific conditions. However, as indicated earlier, no one medication is universally effective. Moreover, in most cases the medications we have discussed provide only partial pain relief, and adverse effects may limit dose escalation. Hence, in clinical practice, 2 or more medications are often used in combination to possibly achieve either an additive beneficial effect or a reduction in the adverse effects associated with the use of a single medication. Such a treatment paradigm makes intuitive sense, particularly if the medications act at different sites in pain signaling pathways or modulate different neurotransmitter systems. However, until recently little evidence was available to support the use of multiple medications in combination for the treatment of patients with NP.
In one of the first RCTs of combination therapy for NP, the combination of gabapentin and extended-release morphine titrated together required lower dosages of both medications and resulted in better pain relief than when either medication was administered alone in patients with PHN or painful DPN.62 However, results failed to show a beneficial effect of the combination with respect to medication-related adverse effects. Generally consistent results were obtained in a trial in which patients with painful DPN were randomized to receive either extended-release oxycodone or matching placebo in combination with existing gabapentin treatment63; however, results of a recent trial showed no additional benefit of a low dosage of 10 mg/d of oxycodone vs placebo when combined with pregabalin.64 In an open-label prospective cohort study of 403 patients with NP, the combination of extended-release oxycodone and pregabalin showed improved pain relief at lower dosages than either medication alone and was associated with improved HRQoL and better tolerability.65
Recent RCTs have examined the combination of nortriptyline and gabapentin,66 which was superior to either of these 2 medications administered alone, as well as the combinations of pregabalin and topical 5% lidocaine67 and sodium valproate and glyceryl trinitrate spray.68 Results of several trials that allowed patients to continue existing pharmacological treatments throughout double-blind evaluations of investigational medications provide additional evidence that combination therapies may have a role in the treatment of NP. For example, in several placebo-controlled trials of pregabalin in PHN, patients were allowed to continue treatment with opioids, TCAs, and other medications they were taking before inclusion in the study.69 The beneficial effects of pregabalin compared with placebo were comparable in patients who were and were not taking concomitant analgesics, suggesting that additional benefit was obtained when pregabalin was administered to patients already taking stable concomitant analgesics. A generally similar pattern of findings occurred in RCTs of high-concentration capsaicin discussed previously, in which patients were also allowed to continue their existing analgesics at stable dosages.44-47 However, a randomized crossover study of morphine, nortriptyline, and their combination in patients with lumbosacral radiculopathy failed to showed a beneficial effect of either the combination or the medications administered alone (although this might be a relatively refractory chronic pain condition).70 Additional studies are needed to develop guidelines for identifying specific combinations of medications and the patients who obtain the greatest benefits from rational polypharmacy.
NEGATIVE TRIALS OF PHARMACOLOGICAL TREATMENTS FOR NP AND THEIR INTERPRETATION
An increasing number of RCTs have failed to show significant differences in primary efficacy analyses comparing groups treated with a medication for NP and placebo, despite previous preclinical and clinical studies suggesting that efficacy would be expected.71-77 It is unclear whether these results reflect a true lack of efficacy in the specific conditions studied or whether other factors have accounted for the lack of success in demonstrating efficacy (eg, inadequate power to detect modest treatment benefits, excessive response rates in placebo groups, other methodological features of the trials).
Although most RCTs of pharmacological treatments for NP have examined either PHN or painful DPN, the extent to which results of RCTs in these 2 conditions apply to other types of NP is unknown. Moreover, most novel medications are validated in animal models of traumatic neuropathy, whereas the evidence of NP efficacy is based on PHN, painful DPN, and other peripheral neuropathies.78 Nevertheless, extrapolation of efficacy from first-line medications that have shown efficacy in one or more types of NP to other types of NP has seemed plausible, and medications that have shown efficacy in several different NP conditions may have the greatest probability of being efficacious in additional, as yet unstudied, conditions.12,79
The results of some negative trials, however, suggest that there may be types of NP that are less likely to respond to existing first-line treatments than PHN and painful DPN. The first indication of this was the publication in 1998 of 2 placebo-controlled RCTs in which amitriptyline failed to relieve pain in patients with painful HIV neuropathy,80,81 which has been followed by negative trials of topical lidocaine82 and pregabalin76 in HIV neuropathy. The results of a recent negative trial of memantine in HIV neuropathy83 are more difficult to interpret because memantine is considered a third-line NP treatment on the basis of inconsistent evidence of efficacy.12 Therefore, these negative results may reflect either minimal efficacy of memantine in NP or lack of efficacy in an NP condition (HIV neuropathy) that appears generally refractory to treatment (but that has been shown to respond to at least one treatment, high-concentration capsaicin).47
Chemotherapy-induced peripheral neuropathy may be another NP condition that is relatively refractory to existing first-line treatments. In 3 RCTs, there was no evidence of efficacy of nortriptyline,84 amitriptyline,85 or gabapentin.86 Finally, recent trials of nortriptyline and morphine and their combination70 and pregabalin87 and an equivocal trial of topiramate88 suggest that lumbosacral radiculopathy might be a third peripheral NP condition that is relatively refractory to existing first- and second-line medications. Interestingly, patients with failed back surgery syndrome, many of whom have lumbosacral radiculopathy, appear to respond to spinal cord stimulation,89 which suggests that this NP condition is not generally refractory to all treatment modalities.
Of course, these negative RCTs of HIV-asociated neuropathy, chemotherapy-induced neuropathy, and lumbosacral radiculopathy may have had methodological features that compromised their ability to demonstrate efficacy. However, the consistency of the negative results suggests that medications with established efficacy in the most prevalent painful polyneuropathy (painful DPN) might not have efficacy in other painful polyneuropathies, and, more generally, that it cannot be assumed that evidence of efficacy in one or two NP conditions can be extrapolated to others.
In addition to raising challenging questions about the extrapolation of efficacy, the negative results of recent RCTs make it difficult to determine the role of treatments in NP guidelines. Such recent developments as evidence of efficacy from several small RCTs combined with negative results from larger phase 3 trials, as well as positive and negative trials that have not provided an adequate basis for regulatory approval, are challenging to interpret and will require careful consideration in revising existing treatment guidelines.
Importantly, negative results of recent RCTs of NP and other chronic pain conditions have focused attention on the research methods used in chronic pain trials.90-94 It can be hoped that this increased attention to clinical trial research designs will lead to methodological innovations that improve assay sensitivity and reduce the number of negative trials of truly efficacious medications (ie, so-called failed trials).
CONCLUSION
Diverse pharmacological treatments of NP have become available, and interpreting the data on their efficacy and safety involves substantial complexities and ambiguities. In updating the NeuPSIG pharmacological guidelines for the management of NP, a multifactorial evaluation will be required that carefully considers the clinical importance of the improvements shown by patients and the benefits and risks of each treatment in view of the other available treatments for NP95-97 (Table 3). In the meantime, improved adherence to existing treatment guidelines is needed.6,12-14
TABLE 3.
Factors to Consider in Determining the Clinical Meaningfulness of Group Differences
To provide a superior evidence base for future treatment guidelines, additional RCTs must be conducted in which existing NP medications are directly compared with each other98-102 and administered in various combinations. In addition, increased efforts must be devoted to identifying new medications that show greater magnitudes of pain reduction, clinically meaningful pain relief in higher percentages of patients, better tolerability and safety, greater benefits on physical and emotional functioning, few or no drug interactions, and greater patient convenience and adherence. Future treatment guidelines will need to consider whether other approaches for the management of patients with NP (eg, physical therapy, spinal cord stimulation, and psychosocial interventions) should be used before, in combination with, or after pharmacological treatments.103 Traditional RCTs may ultimately not be the method of choice to answer all these questions104; alternative approaches should be developed and evaluated (eg, systematic comparative effectiveness studies of health care registry data).
It has become commonplace to conclude articles on the pharmacological treatment of NP by emphasizing either the public health benefits of identifying interventions that prevent NP or the importance of developing mechanism-based treatment approaches. One recent major advance is the widespread availability of a vaccine that halves the risk of herpes zoster in individuals older than 60 years and in so doing prevents PHN.105 Unfortunately, attention paid to developing preventive interventions for other NP conditions has been limited.106 In contrast, the prospects for developing a mechanism-based approach to the treatment of NP seem promising.107,108 Although important challenges remain,109 research groups in Germany,110,111 the United States,112 and elsewhere are now systematically identifying patterns of NP symptoms and signs that appear to correspond to underlying pathophysiologic mechanisms. Given the diverse mechanisms of action of the medications discussed in this article, these programs of research provide a basis for considerable optimism that future treatment guidelines for NP will be able to specify what medications are most effective for which types of patients.107,113
Acknowledgments
We thank Paul J. Lambiase for coordinating the meeting on which this supplement is based.
Footnotes
Support for the meeting on which this article is based and article preparation was provided by an unrestricted grant from Endo Pharmaceuticals to the University of Rochester Office of Continuing Professional Education, from which all authors received honoraria for their participation. Individual disclosures can be found on page S11.
REFERENCES
- 1. Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical research purposes. Neurology. 2008;70(18):1630-1635 [DOI] [PubMed] [Google Scholar]
- 2. Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain. 2005;6(6):356-363 [DOI] [PubMed] [Google Scholar]
- 3. Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B. Burden of illness in painful diabetic peripheral neuropathy: the patients' perspectives. J Pain. 2006;7(12):892-900 [DOI] [PubMed] [Google Scholar]
- 4. McDermott AM, Toelle TR, Rowbotham DJ, Schaefer CP, Dukes EM. The burden of neuropathic pain: results of a cross-sectional survey. Eur J Pain. 2006;10(2):127-135 [DOI] [PubMed] [Google Scholar]
- 5. Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007;68(15):1178-1182 [DOI] [PubMed] [Google Scholar]
- 6. O'Connor AB. Neuropathic pain: a review of the quality of life impact, costs, and cost-effectiveness of therapy. Pharmacoeconomics. 2009;27(2):95-112 [DOI] [PubMed] [Google Scholar]
- 7. Berger A, Dukes EM, Oster G. Clinical characteristics and economic costs of patients with painful neuropathic disorders. J Pain. 2004;5(3):143-149 [DOI] [PubMed] [Google Scholar]
- 8. Dworkin RH, White R, O'Connor AB, Baser O, Hawkins K. Health care costs of acute and chronic pain associated with a diagnosis of herpes zoster. J Am Geriatr Soc. 2007;55(8):1168-1175 [DOI] [PubMed] [Google Scholar]
- 9. Dworkin RH, Malone DC, Panarites CJ, Armstrong EP, Pham SV. Impact of postherpetic neuralgia and painful diabetic peripheral neuropathy on health care costs [published online ahead of print October 21, 2009]. J Pain. doi:10.1016/j.jpain. 2009.08.005 [DOI] [PubMed] [Google Scholar]
- 10. van Hoek AJ, Gay N, Melegaro A, Opstelten W, Edmunds WJ. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine. 2009;27(9):1454-1467 [DOI] [PubMed] [Google Scholar]
- 11. Donaldson Sir L. The 2008 report of the Chief Medical Officer:150 years of the Annual Report of the Chief Medical Officer: on the state of public health 2008. London, England: Department of Health, 2009. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/AnnualReports/DH_096206 Accessed January 12, 2010 [Google Scholar]
- 12. Dworkin RH, O'Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132(3):237-251 [DOI] [PubMed] [Google Scholar]
- 13. Attal N, Cruccu G, Haanpää M, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13(11):1153-1169 [DOI] [PubMed] [Google Scholar]
- 14. Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain—consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(10A):S22-S32 [DOI] [PubMed] [Google Scholar]
- 16. Attal N, Fermanian C, Fermanian J, Lanteri-Minet M, Alchaar H, Bouhassira D. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain. 2008;138(2):343-353 [DOI] [PubMed] [Google Scholar]
- 17. Baron R, Tölle TR, Gockel U, Brosz M, Freynhagen R. A cross sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: differences in demographic data and sensory systems. Pain. 2009;146(1-2):34-40 [DOI] [PubMed] [Google Scholar]
- 18. CEBM Centre for Evidence-based Medicine CEBM Web site. Levels of evidence and grades of recommendation. http://www.cebm.net/index.aspx?o=1025 Accessed January 12, 2010
- 19. Cruccu G, Gronseth G, Alksne J, et al. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008;15(10):1013-1028 [DOI] [PubMed] [Google Scholar]
- 20. Gronseth G, Cruccu G, Alksne J, et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008;71(15):1183-1190 [DOI] [PubMed] [Google Scholar]
- 21. Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118(3):289-305 [DOI] [PubMed] [Google Scholar]
- 22. Max MB, Culnane M, Schafer SC, et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology. 1987;37(4):589-596 [DOI] [PubMed] [Google Scholar]
- 23. Raskin J, Smith TR, Wong K, et al. Duloxetine versus routine care in the long-term management of diabetic peripheral neuropathic pain. J Palliat Med. 2006;9(1):29-40 [DOI] [PubMed] [Google Scholar]
- 24. Wernicke J, Lledó A, Raskin J, et al. An evaluation of the cardiovascular safety profile of duloxetine: findings from 42 placebo-controlled studies. Drug Saf. 2007;30(5):437-455 [DOI] [PubMed] [Google Scholar]
- 25. McIntyre RS, Panjwani ZD, Nguyen HT, et al. The hepatic safety profile of duloxetine: a review. Expert Opin Drug Metab Toxicol. 2008;4(3):281-285 [DOI] [PubMed] [Google Scholar]
- 26. Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697-706 [DOI] [PubMed] [Google Scholar]
- 27. Fava M, Mulroy R, Alpert J, Nierenberg AA, Rosenbaum JF. Emergence of adverse events following discontinuation of treatment with extended-release venlafaxine. Am J Psychiatry. 1997;154(12):1760-1762 [DOI] [PubMed] [Google Scholar]
- 28. Stacey BR, Barrett JA, Whalen E, Phillips KF, Rowbotham MC. Pregabalin for postherpetic neuralgia: placebo-controlled trial of fixed and flexible dosing regimens on allodynia and time to onset of pain relief. J Pain. 2008;9(11):1006-1017 [DOI] [PubMed] [Google Scholar]
- 29. Wasner G, Kleiner A, Binder A, Schattschneider J, Baron R. Postherpetic neuralgia: topical lidocaine is effective in nociceptor-deprived skin. J Neurol. 2005;252(6):677-686 [DOI] [PubMed] [Google Scholar]
- 30. Herrmann DN, Pannoni V, Barbano RL, Pennella-Vaughan J, Dworkin RH. Skin biopsy and quantitative sensory testing do not predict response to the lidocaine patch in painful neuropathies. Muscle Nerve. 2006;33(1):42-48 [DOI] [PubMed] [Google Scholar]
- 31. Wu CL, Agarwal S, Tella PK, et al. Morphine versus mexiletine for treatment of postamputation pain. Anesthesiology. 2008;109(2):289-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raja SN, Haythornthwaite JA, Pappagallo M, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002;59(7):1015-1021 [DOI] [PubMed] [Google Scholar]
- 33. British Pain Society Recommendations for the appropriate use of opioids for persistent non-cancer pain. London, England: British Pain Society, 2005. http://www.britishpainsociety.org/opioids_doc_2004.pdf Accessed January 12, 2010 [Google Scholar]
- 34. Højsted J, Sjögren P. Addiction to opioids in chronic pain patients: a literature review. Eur J Pain. 2007;11(5):490-518 [DOI] [PubMed] [Google Scholar]
- 35. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Katz J, Finnerup NB, Dworkin RH. Clinical trial outcome in neuropathic pain: relationship to study characteristics. Neurology. 2008;70(4):263-272 [DOI] [PubMed] [Google Scholar]
- 37. Vranken JH, Dijkgraaf MGW, Kruis MR, van der Vegt MH, Hollmann MW, Heesen M. Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain. 2008;136(1-2):150-157 [DOI] [PubMed] [Google Scholar]
- 38. Norrbrink C, Lundeborg T. Tramadol in neuropathic pain after spinal cord injury: a randomized, double-blind, placebo-controlled trial. Clin J Pain. 2009;25(3):177-184 [DOI] [PubMed] [Google Scholar]
- 39. O'Connor AB, Schwid SR, Markman J, Hermann D, Dworkin RH. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain. 2008;137(1):96-111 [DOI] [PubMed] [Google Scholar]
- 40. Rice ASC. Should cannabinoids be used as analgesics for neuropathic pain? Nat Clin Pract Neurol. 2008;4(12):654-655 [DOI] [PubMed] [Google Scholar]
- 41. Tugnoli V, Capone JG, Eleopra R, et al. Botulinum toxin type A reduces capsaicin-evoked pain and neurogenic vasodilatation in human skin. Pain. 2007;130(1-2):76-83 [DOI] [PubMed] [Google Scholar]
- 42. Ranoux D, Attal N, Morain F, Bouhassira D. Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann Neurol. 2008;64(3):274-284 [DOI] [PubMed] [Google Scholar]
- 43. Yuan RY, Sheu JJ, Yu JM, et al. Botulinum toxin for diabetic neuropathic pain: a randomized double-blind crossover trial. Neurology. 2009;72(17):1473-1478 [DOI] [PubMed] [Google Scholar]
- 44. European Medicines Agency CHMP assessment report for Qutenza. http://www.emea.europa.eu/humandocs/PDFs/EPAR/Qutenza/H-909-en6.pdf Accessed January 12, 2010
- 45. Backonja M, Wallace MS, Blonsky ER, et al. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol. 2008;7(12):1106-1112 [DOI] [PubMed] [Google Scholar]
- 46. Qutenza (NGX-4010) : Full prescribing information. http://www.neurogesx.com/ngx_4010 Accessed January 12, 2010
- 47. Simpson DM, Brown S, Tobias J. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70(24):2305-2313 [DOI] [PubMed] [Google Scholar]
- 48. Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127(pt 7):1606-1615 [DOI] [PubMed] [Google Scholar]
- 49. Magerl W, Fuchs PN, Meyer RA, Treede RD. Roles of capsaicin-in-sensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain. 2001;124(pt 9):1754-1764 [DOI] [PubMed] [Google Scholar]
- 50. Rauck RL, Shaibani A, Biton V, Simpson J, Koch B. Lacosamide in painful diabetic peripheral neuropathy: a phase 2 double-blind placebo-controlled study. Clin J Pain. 2007;23(2):150-158 [DOI] [PubMed] [Google Scholar]
- 51. Shaibani A, Fares S, Selam JL, et al. Lacosamide in painful diabetic neuropathy: an 18-week double-blind placebo-controlled trial. J Pain. 2009;10(8):818-828 [DOI] [PubMed] [Google Scholar]
- 52. Wymer JP, Simpson J, Sen D, Bongardt S. Efficacy and safety of lacosamide in diabetic neuropathic pain: an 18-week double-blind placebo-controlled trial of fixed-dose regimens. Clin J Pain. 2009;25(5):376-385 [DOI] [PubMed] [Google Scholar]
- 53. UCB, Inc. Clinical study summary (CSS) template, Study No. SP874. http://www.clinicalstudyresults.org/documents/company-study_8251_0.pdf Accessed January 12, 2010
- 54. Hidvégi T, Bretschneider B, Thierfelder S, Sommerville K, Bongardt S. Long-term efficacy of lacosamide in subjects with diabetic neuropathic pain: results of a double-blind, randomized withdrawal trial. Presented at: 27th annual meeting of the American Pain Society; Tampa, FL; May 8-10, 2008 [Google Scholar]
- 55. UCB, Inc. Clinical study summary (CSS) template, Study No. SP743. http://www.clinicalstudyresults.org/documents/company-study_8250_0.pdf Accessed January 12, 2010
- 56. O'Connor AB. The need for improved access to FDA reviews. JAMA. 2009;302(2):191-193 [DOI] [PubMed] [Google Scholar]
- 57. Shaibani A, Biton V, Rauck R, Koch B, Simpson J. Long-term oral lacosamide in painful diabetic neuropathy: a two-year open-label extension trial. Eur J Pain. 2009;13(5):458-463 [DOI] [PubMed] [Google Scholar]
- 58. Sindrup SH, Gram LF, Brøsen K, Eshøj O, Mogensen EF. The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain. 1990;42(2):135-144 [DOI] [PubMed] [Google Scholar]
- 59. Sindrup SH, Bjerre U, Dejgaard A, Brøsen K, Aaes-Jørgensen, Gram LF. The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy. Clin Pharmacol Ther. 1992;52(5):547-552 [DOI] [PubMed] [Google Scholar]
- 60. Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256 [DOI] [PubMed] [Google Scholar]
- 61. Otto M, Bach FW, Jensen TS, Brøsen K, Sindrup SH. Escitalopram in painful polyneuropathy: a randomized, placebo-controlled, cross-over trial. Pain. 2008;139(2):275-283 [DOI] [PubMed] [Google Scholar]
- 62. Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352(13):1324-1334 [DOI] [PubMed] [Google Scholar]
- 63. Hanna M, O'Brien C, Wilson MC. Prolonged-release oxycodone enhances the effects of existing gabapentin therapy in painful diabetic neuropathy patients. Eur J Pain. 2008;12(6):804-813 [DOI] [PubMed] [Google Scholar]
- 64. Zin CS, Nissen LM, O'Callaghan JP, Duffull SB, Smith MT, Moore BJ. A randomized controlled trial of oxycodone vs placebo in patients with postherpetic neuralgia and painful diabetic neuropathy treated with pregabalin [published online ahead of print December 2, 2009]. J Pain. doi:10.1016/j. jpain.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 65. Gatti A, Sabato AF, Occhioni R, Baldeschi GC, Reale C. Controlled-release oxycodone and pregabalin in the treatment of neuropathic pain: results of a multicenter Italian study. Eur Neurol. 2009;61(3):129-137 [DOI] [PubMed] [Google Scholar]
- 66. Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet. 2009;374(9697):1252-1261 [DOI] [PubMed] [Google Scholar]
- 67. Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. Efficacy and safety of combination therapy with 5% lidocaine medicated plaster and pregabalin in post-herpetic neuralgia and diabetic polyneuropathy. Curr Med Res Opin. 2009;25(7):1677-1687 [DOI] [PubMed] [Google Scholar]
- 68. Agrawal RP, Goswami J, Jain S, Kochar DK. Management of diabetic neuropathy by sodium valproate and glyceryl trinitrate spray: a prospective double-blind randomized placebo-controlled study. Diabetes Res Clin Pract. 2009;83(3):371-378 [DOI] [PubMed] [Google Scholar]
- 69. Dworkin RH, Thakur R, Griesing T, Sharma U, Young JP. Randomized clinical trials of pregabalin for neuropathic pain: methods, results, and implications. Progr Neurotherapeutics Neuropsychopharmacol. 2008;3(1):167-187 [Google Scholar]
- 70. Khoromi S, Cui L, Nackers L, Max MB. Morphine, nortriptyline and their combination vs. placebo in patients with chronic lumbar root pain. Pain. 2007;130(1-2):66-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Thienel U, Neto W, Schwabe SK, Vijapurkar U. Topiramate in painful diabetic polyneuropathy: findings from three double-blind placebo-controlled trials. Acta Neurol Scand. 2004;110(4):221-231 [DOI] [PubMed] [Google Scholar]
- 72. Beydoun A, Shaibani A, Hopwood M, Wan Y. Oxcarbazepine in painful diabetic neuropathy: results of a dose-ranging study. Acta Neurol Scand. 2006;113(6):395-404 [DOI] [PubMed] [Google Scholar]
- 73. Breuer B, Pappagallo M, Knotkova H, Guleyupoglu N, Wallenstein S, Portenoy RK. A randomized, double-blind, placebo-controlled, two-period, crossover, pilot trial of lamotrigine in patients with central pain due to multiple sclerosis. Clin Ther. 2007;29(9):2022-2030 [DOI] [PubMed] [Google Scholar]
- 74. Silver M, Blum D, Grainger J, Hammer AE, Quessy S. Double-blind, placebo-controlled trial of lamotrigine in combination with other medications for neuropathic pain. J Pain Symptom Manage. 2007;34(4):446-454 [DOI] [PubMed] [Google Scholar]
- 75. Vinik AI, Tuchman M, Safirstein B, et al. Lamotrigine for treatment of pain associated with diabetic neuropathy: results of two randomized double-blind, placebo-controlled studies. Pain. 2007;128(1-2):169-179 [DOI] [PubMed] [Google Scholar]
- 76. Simpson DM, Schifitto G, Clifford DB, et al. 1066 HIV Neuropathy Study Group Pregabalin in the treatment of painful HIV neuropathy: a randomized, double-blind, placebo-controlled trial. Poster presented at: 61st Annual Meeting of the American Academy of Neurology, Seattle, WA; April 29, 2009 [Google Scholar]
- 77. XenoPort, Inc. Phase II results for GSK1838262 (XP13512) reported for neuropathic pain associated with diabetic peripheral neuropathy. Santa Clara, CA: XenoPort, Inc; 2009. [Google Scholar]
- 78. Rice ASC, Cimino-Brown D, Eisenach JC, et al. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139(2):243-247 [DOI] [PubMed] [Google Scholar]
- 79. Hansson PT, Dickenson AH. Pharmacological treatment of peripheral neuropathic conditions based on shared commonalities despite multiple etiologies. Pain. 2005;113(3):251-254 [DOI] [PubMed] [Google Scholar]
- 80. Kieburtz K, Simpson D, Yiannoutsos C, et al. A randomized trial of amitriptyline and mexiletine for painful neuropathy in HIV infection. Neurology. 1998;51(6):1682-1688 [DOI] [PubMed] [Google Scholar]
- 81. Shlay JC, Chaloner K, Max MB, et al. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: a randomized controlled trial. JAMA. 1998;280(18):1590-1595 [DOI] [PubMed] [Google Scholar]
- 82. Estanislao L, Carter K, McArthur J, Olney R, Simpson D. A randomized controlled trial of 5% lidocaine gel for HIV-associated distal symmetric polyneuropathy. J Acquir Immune Defic Syndr. 2004;37(5):1584-1586 [DOI] [PubMed] [Google Scholar]
- 83. Schifitto G, Yiannoutsos CT, Simpson DM, et al. A placebo-controlled study of memantine for the treatment of human immunodeficiency virus-associated sensory neuropathy. J Neurovirol. 2006;12(4):328-331 [DOI] [PubMed] [Google Scholar]
- 84. Hammack JE, Michalak JC, Loprinzi CL, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain. 2002;98(1-2):195-203 [DOI] [PubMed] [Google Scholar]
- 85. Kautio AL, Haanpää M, Saarto T, Kalso E. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. J Pain Symptom Manage. 2008;35(1):31-39 [DOI] [PubMed] [Google Scholar]
- 86. Rao RD, Michalak JC, Sloan JA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer. 2007;110(9):2110-2118 [DOI] [PubMed] [Google Scholar]
- 87. Pfizer, Inc. PhRMA web synopsis (Protocol A0081007): a randomized placebo-controlled trial of the efficacy and safety of pregabalin in the treatment of subjects with neuropathic pain associated with lumbo-sacral radiculopathy. 2008. http://pdf.clinicalstudyresults.org/documents/company-study_4268_0.pdf Accessed January 12, 2010
- 88. Khoromi S, Patsalides A, Parada S, Salehi V, Meegan JM, Max MB. Topiramate in chronic lumbar radicular pain. J Pain. 2005;6(12):829-836 [DOI] [PubMed] [Google Scholar]
- 89. Cruccu G, Aziz TZ, Garcia-Larrea L, et al. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007;14(9):952-970 [DOI] [PubMed] [Google Scholar]
- 90. Raskin P, Donofrio PD, Rosenthal NR, et al. Topiramate vs placebo in painful diabetic neuropathy: analgesic and metabolic effects. Neurology. 2004;63(5):865-873 [DOI] [PubMed] [Google Scholar]
- 91. Dworkin RH, Katz J, Gitlin MJ. Placebo response in clinical trials of depression and its implications for research on chronic neuropathic pain. Neurology. 2005;65(12)(suppl 4):S7-S19 [DOI] [PubMed] [Google Scholar]
- 92. Katz N. Methodological issues in clinical trials of opioids for chronic pain. Neurology. 2005;65(12)(suppl 4):S32-S49 [DOI] [PubMed] [Google Scholar]
- 93. Quessy SN, Rowbotham MC. Placebo response in neuropathic pain trials. Pain. 2008;138(3):479-483 [DOI] [PubMed] [Google Scholar]
- 94. Katz N. Enriched enrollment randomized withdrawal trial designs for analgesics. Clin J Pain. 2009;25(9):797-807 [DOI] [PubMed] [Google Scholar]
- 95. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105-121 [DOI] [PubMed] [Google Scholar]
- 96. Guyatt GH, Juniper EF, Walter SD, Griffith LE, Goldstein RS. Interpreting treatment effects in randomised trials. BMJ. 1998;316(7132):690-693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146(3):238-244 [DOI] [PubMed] [Google Scholar]
- 98. Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic neuropathy pain. Arch Intern Med. 1999;159(16):1931-1937 [DOI] [PubMed] [Google Scholar]
- 99. Chandra K, Shafiq N, Pandhi P, Malhotra S. Gabapentin versus nortriptyline in post-herpetic neuralgia patients: a randomized, double-blind clinical trial—the GONIP trial. Int J Clin Pharmacol Ther. 2006;44(8):358-363 [DOI] [PubMed] [Google Scholar]
- 100. Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. Efficacy and safety of 5% lidocaine (lignocaine) medicated plaster in comparison with pregabalin in patients with postherpetic neuralgia and diabetic polyneuropathy: interim analysis from an open-label, two-stage adaptive, randomized, controlled trial. Clin Drug Investig. 2009;29(4):231-241 [DOI] [PubMed] [Google Scholar]
- 101. Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. 5% Lidocaine medicated plaster versus pregabalin in post-herpetic neuralgia and diabetic polyneuropathy: an open-label, non-inferiority two-stage RCT study. Curr Med Res Opin. 2009;25(7):1663-1676 [DOI] [PubMed] [Google Scholar]
- 102. Rintala DH, Holmes SA, Courtade D, Fiess RN, Tastard LV, Loubser PG. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Arch Phys Med Rehabil. 2007;88(12):1547-1560 [DOI] [PubMed] [Google Scholar]
- 103. Hansson PT, Attal N, Baron R, Cruccu G. Toward a definition of pharmacoresistant neuropathic pain. Eur J Pain. 2009;13(5):439-440 [DOI] [PubMed] [Google Scholar]
- 104. Jadad AR, Enkin MW. Randomised Controlled Trials: Questions, Answers and Musings. 2nd ed. London, England: Blackwell/BMJ Books; 2007. [Google Scholar]
- 105. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271-2284 [DOI] [PubMed] [Google Scholar]
- 106. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618-1625 [DOI] [PubMed] [Google Scholar]
- 107. Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-451 [DOI] [PubMed] [Google Scholar]
- 108. Rowbotham MC. Mechanisms of neuropathic pain and their implications for the design of clinical trials. Neurology. 2005;65(12)(suppl 4):S66-S73 [DOI] [PubMed] [Google Scholar]
- 109. Hansson P. Difficulties in stratifying neuropathic pain by mechanisms. Eur J Pain. 2003;7(4):353-357 [DOI] [PubMed] [Google Scholar]
- 110. Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231-243 [DOI] [PubMed] [Google Scholar]
- 111. Rolke R, Magerl W, Campbell KA, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10(1):77-88 [DOI] [PubMed] [Google Scholar]
- 112. Scholz J, Mannion RJ, Hord DE, et al. A novel tool for the assessment of pain: validation in low back pain. PLoS Med. 2009;6(4):e1000047 doi:10.1371/journal.pmed.1000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Turk DC. Customizing treatment for chronic pain patients: who, what and why? Clin J Pain. 1990;6(4):255-270 [DOI] [PubMed] [Google Scholar]