Abstract
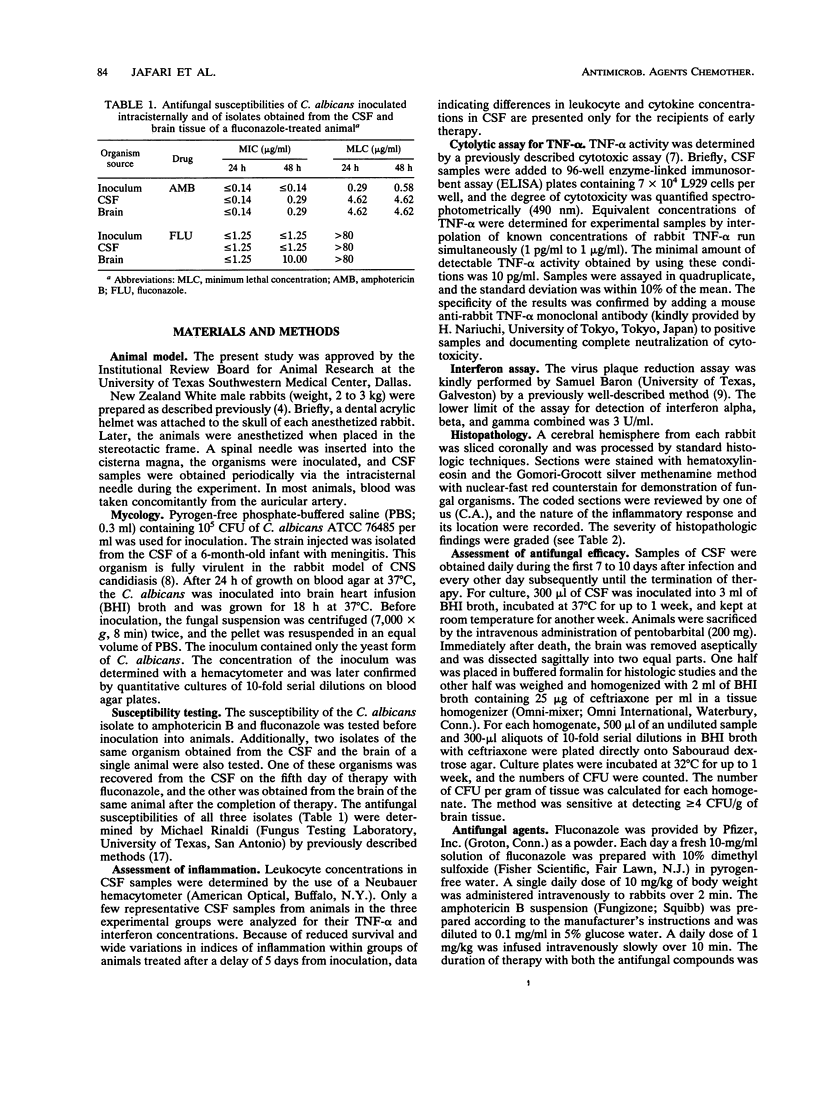
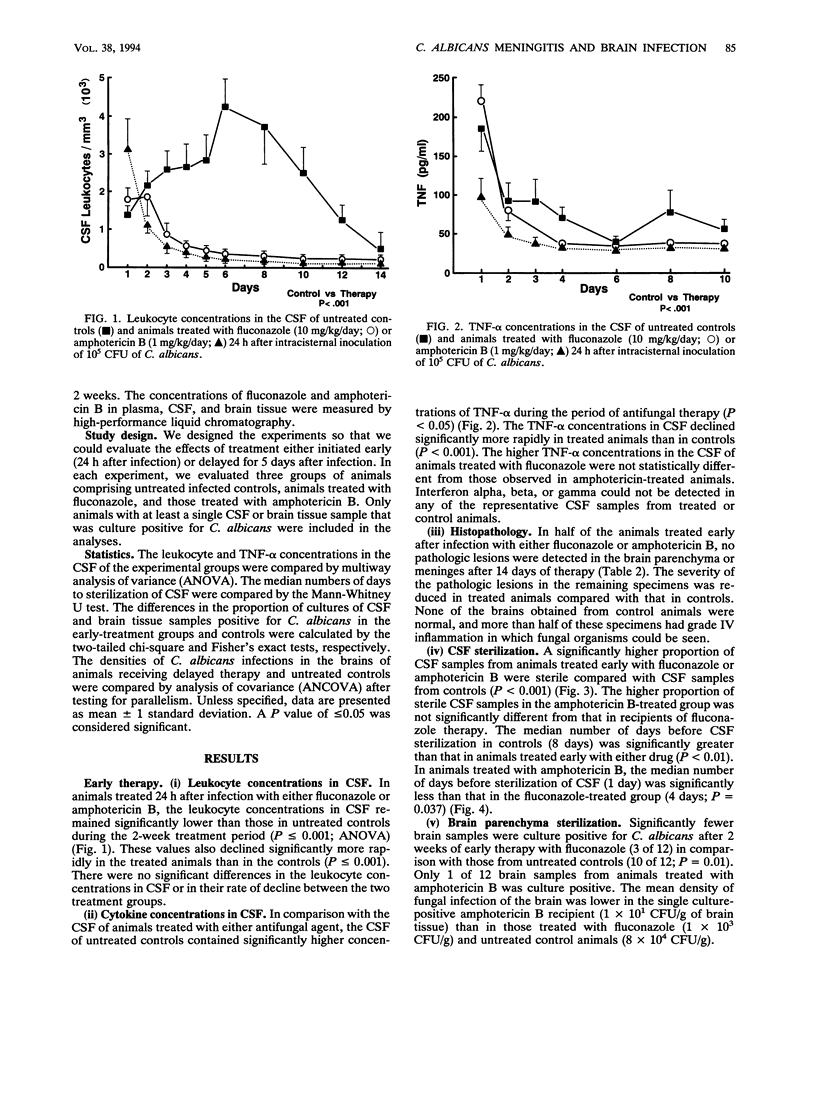
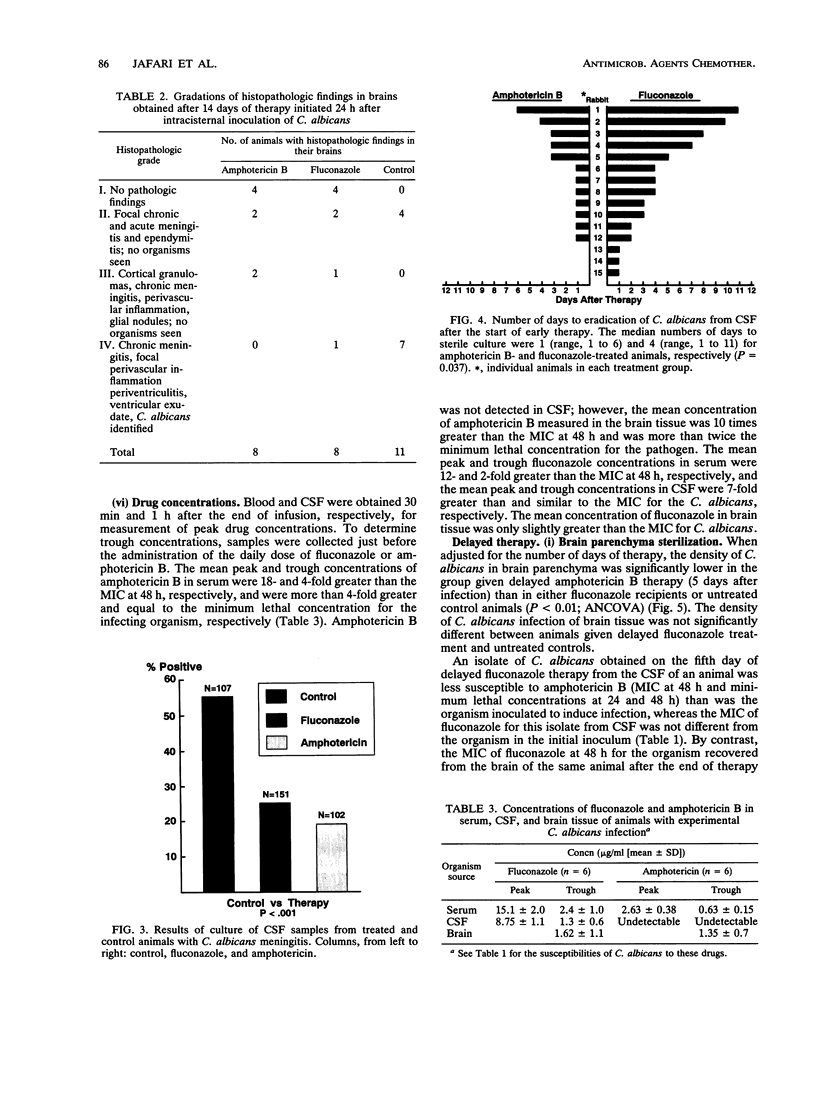
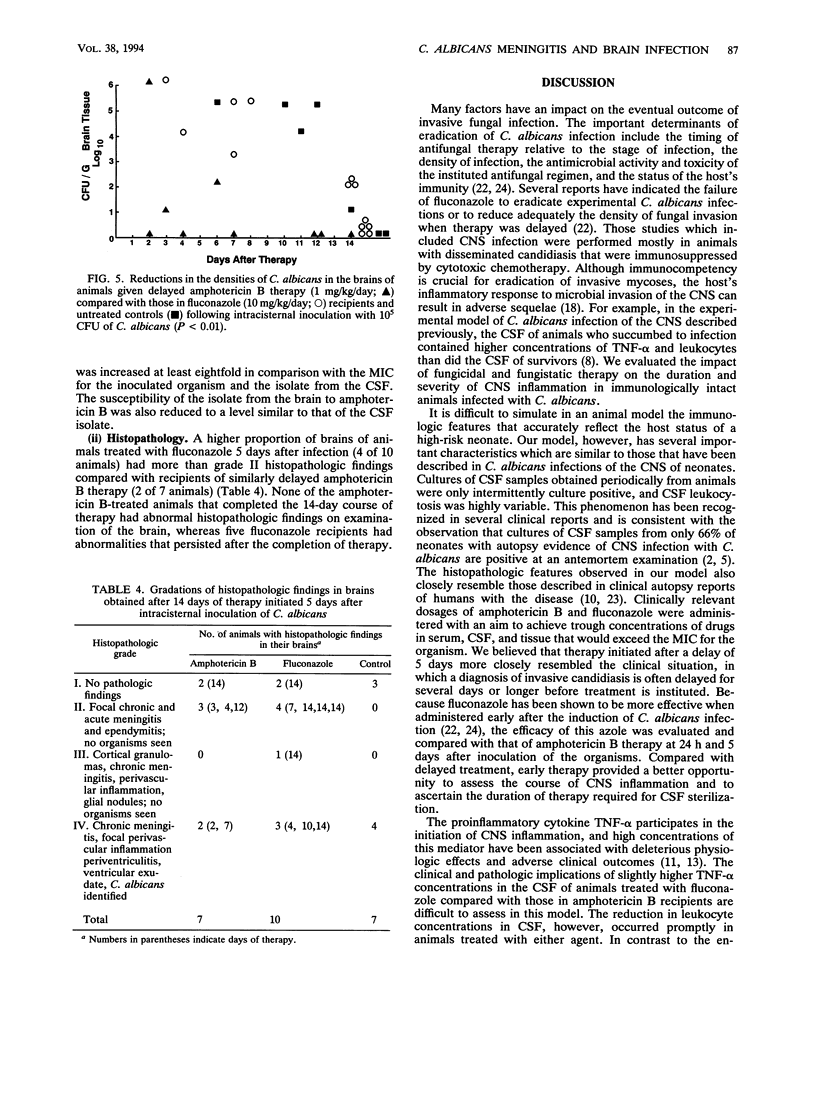
To assess the effects of antifungal therapy on the course of Candida albicans central nervous system infection and inflammation, we inoculated intracisternally 10(5) CFU of C. albicans into rabbits. Fluconazole (10 mg/kg of body weight) or amphotericin B (1 mg/kg) was infused intravenously daily for 14 days. Treatment was initiated 24 h or 5 days after infection. Cerebrospinal fluid (CSF) was repeatedly obtained to culture the organisms, assess the level of inflammation, and measure drug concentrations. Brain tissue was obtained at the end of therapy for culture, drug concentration determinations, and histopathology. The median number of days of treatment required to sterilize CSF cultures was 4 days for fluconazole therapy and 1 day for amphotericin B therapy (P = 0.037). There was a significant reduction in tumor necrosis factor alpha and leukocyte concentrations in the CSF of animals treated early versus those in untreated control animals (P < 0.05 and P < 0.001, respectively; analysis of variance). Compared with treated animals, a higher proportion of cultured CSF samples from untreated animals were positive for Candida (P < 0.001). A cultured brain sample from 1 of the 12 animals treated early with amphotericin B was positive for C. albicans (P < 0.01 versus controls); cultures of brain samples from 3 of 12 animals treated early with fluconazole were positive, whereas cultures of brain samples from 10 of 12 controls were positive (P < 0.05). The mean density of C. albicans was lower in the single culture-positive amphotericin B recipient (1 x 10(1) CFU/g of brain tissue) than in those treated with fluconazole (1 x 10(3) CFU/g) and in controls (8 x 10(4) CFU/g). In animals treated late, the density of C. albicans in the brain in relation to the number of days of therapy was significantly lower in amphotericin B recipients than in those treated with fluconazole (P < 0.01) and untreated controls (P < 0.01; analysis of covariance). By histopathology, a larger proportion of untreated animals compared with those treated early demonstrated features of severe infection such as perivasculitis, ventriculitis, and evidence of fungal organisms. Compared with amphotericin B-treated rabbits, those given fluconazole had a trend toward more severe pathologic lesions. Reduced susceptibility to both fluconazole and amphotericin B was observed in the C. albicans organisms isolated from the brain of one fluconazole-treated animal. These data suggest that amphotericin B is the preferred treatment for C. albicans infections of the central nervous system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt C. A., Walsh T. J., McCully C. L., Balis F. M., Pizzo P. A., Poplack D. G. Fluconazole penetration into cerebrospinal fluid: implications for treating fungal infections of the central nervous system. J Infect Dis. 1988 Jan;157(1):178–180. doi: 10.1093/infdis/157.1.178. [DOI] [PubMed] [Google Scholar]
- Baley J. E., Kliegman R. M., Fanaroff A. A. Disseminated fungal infections in very low-birth-weight infants: clinical manifestations and epidemiology. Pediatrics. 1984 Feb;73(2):144–152. [PubMed] [Google Scholar]
- Butler K. M., Baker C. J. Candida: an increasingly important pathogen in the nursery. Pediatr Clin North Am. 1988 Jun;35(3):543–563. doi: 10.1016/s0031-3955(16)36471-9. [DOI] [PubMed] [Google Scholar]
- Dacey R. G., Sande M. A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974 Oct;6(4):437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faix R. G. Systemic Candida infections in infants in intensive care nurseries: high incidence of central nervous system involvement. J Pediatr. 1984 Oct;105(4):616–622. doi: 10.1016/s0022-3476(84)80433-3. [DOI] [PubMed] [Google Scholar]
- Filler S. G., Crislip M. A., Mayer C. L., Edwards J. E., Jr Comparison of fluconazole and amphotericin B for treatment of disseminated candidiasis and endophthalmitis in rabbits. Antimicrob Agents Chemother. 1991 Feb;35(2):288–292. doi: 10.1128/aac.35.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Jafari H. S., Sáez-Llorens X., Grimprel E., Argyle J. C., Olsen K. D., McCracken G. H., Jr Characteristics of experimental Candida albicans infection of the central nervous system in rabbits. J Infect Dis. 1991 Aug;164(2):389–395. doi: 10.1093/infdis/164.2.389. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Hickey W. F., Morris J. H., Loscalzo J. Candidal infection in the central nervous system. Am J Med. 1984 Jan;76(1):101–108. doi: 10.1016/0002-9343(84)90757-5. [DOI] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Mustafa M. M., Ramilo O., Olsen K. D., Risser R. C. Cerebrospinal fluid interleukin 1-beta and tumor necrosis factor concentrations and outcome from neonatal gram-negative enteric bacillary meningitis. Pediatr Infect Dis J. 1989 Mar;8(3):155–159. [PubMed] [Google Scholar]
- Mustafa M. M., Ramilo O., Mertsola J., Risser R. C., Beutler B., Hansen E. J., McCracken G. H., Jr Modulation of inflammation and cachectin activity in relation to treatment of experimental Hemophilus influenzae type b meningitis. J Infect Dis. 1989 Nov;160(5):818–825. doi: 10.1093/infdis/160.5.818. [DOI] [PubMed] [Google Scholar]
- Mustafa M. M., Ramilo O., Olsen K. D., Franklin P. S., Hansen E. J., Beutler B., McCracken G. H., Jr Tumor necrosis factor in mediating experimental Haemophilus influenzae type B meningitis. J Clin Invest. 1989 Oct;84(4):1253–1259. doi: 10.1172/JCI114292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Durack D. T. Penetration of imidazoles and triazoles into cerebrospinal fluid of rabbits. J Antimicrob Chemother. 1985 Jul;16(1):81–86. doi: 10.1093/jac/16.1.81. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Savani D. V., Durack D. T. Comparison of itraconazole and fluconazole in treatment of cryptococcal meningitis and candida pyelonephritis in rabbits. Antimicrob Agents Chemother. 1986 Apr;29(4):579–583. doi: 10.1128/aac.29.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou M. A., Rogers T. R. Interactions in vitro between polyenes and imidazoles against yeasts. J Antimicrob Chemother. 1991 Apr;27(4):491–506. doi: 10.1093/jac/27.4.491. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., Suter S., Gianella-Borradori A., Pfenninger J., Auckenthaler R., Bernath O., Cheseaux J. J., Wedgwood J. A comparison of ceftriaxone and cefuroxime for the treatment of bacterial meningitis in children. N Engl J Med. 1990 Jan 18;322(3):141–147. doi: 10.1056/NEJM199001183220301. [DOI] [PubMed] [Google Scholar]
- Sáez-Llorens X., Ramilo O., Mustafa M. M., Mertsola J., McCracken G. H., Jr Molecular pathophysiology of bacterial meningitis: current concepts and therapeutic implications. J Pediatr. 1990 May;116(5):671–684. doi: 10.1016/s0022-3476(05)82647-2. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Cenci E., Puliti M., Blasi E., Puccetti P., Cassone A., Bistoni F. Protective immunity induced by low-virulence Candida albicans: cytokine production in the development of the anti-infectious state. Cell Immunol. 1989 Dec;124(2):334–344. doi: 10.1016/0008-8749(89)90135-4. [DOI] [PubMed] [Google Scholar]
- Walsh T. J., Aoki S., Mechinaud F., Bacher J., Lee J., Rubin M., Pizzo P. A. Effects of preventive, early, and late antifungal chemotherapy with fluconazole in different granulocytopenic models of experimental disseminated candidiasis. J Infect Dis. 1990 Apr;161(4):755–760. doi: 10.1093/infdis/161.4.755. [DOI] [PubMed] [Google Scholar]
- Walsh T. J., Hier D. B., Caplan L. R. Fungal infections of the central nervous system: comparative analysis of risk factors and clinical signs in 57 patients. Neurology. 1985 Nov;35(11):1654–1657. doi: 10.1212/wnl.35.11.1654. [DOI] [PubMed] [Google Scholar]
- Walsh T. J., Lee J. W., Lecciones J., Kelly P., Peter J., Thomas V., Bacher J., Pizzo P. A. SCH-39304 in prevention and treatment of disseminated candidiasis in persistently granulocytopenic rabbits. Antimicrob Agents Chemother. 1990 Aug;34(8):1560–1564. doi: 10.1128/aac.34.8.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Etten E. W., van de Rhee N. E., van Kampen K. M., Bakker-Woudenberg I. A. Effects of amphotericin B and fluconazole on the extracellular and intracellular growth of Candida albicans. Antimicrob Agents Chemother. 1991 Nov;35(11):2275–2281. doi: 10.1128/aac.35.11.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]



