INTRODUCTION
This Product Profiler introduces health care professionals to Alpha1-Proteinase Inhibitor (Human), Zemaira®, a U.S. Food and Drug Administration-approved treatment indicated for chronic augmentation and maintenance therapy in individuals with alpha1-proteinase inhibitor (alpha1-PI) deficiency and clinical evidence of emphysema.
Zemaira® is a sterile, stable, lyophilized preparation of highly purified human alpha1-PI, also known as alpha1-antitrypsin (AAT), derived from human plasma. Clinical studies have shown Zemaira® to be a safe and effective treatment for alpha1-PI deficiency when administered once weekly at the recommended dose of 60 mg/kg body weight (CSL Behring 2007).
The following text presents a brief overview of alpha1-PI deficiency and current treatment options, followed by a review of the evidence-based literature supporting the FDA-approved indication for the administration of alpha1-proteinase inhibitor (Human).
DISEASE OVERVIEW
Alpha1 antitrypsin deficiency (AATD) is a relatively common but under-recognized genetic disorder in which the protease inhibitor, AAT, is appropriately produced in the liver but is not secreted into the serum, instead collecting in the endoplasmic reticulum of hepatocytes (ATS 2003, Perlmutter 1996). The main function of AAT circulating in the bloodstream is to protect normal body tissue from damage by nonspecific, neutrophil proteolytic enzymes, particularly neutrophil elastase, an enzyme that can attack lung elastin and compromise bronchial and alveolar wall integrity (ATS 2003, Mahadeva 2005, Ranes 2005). Interference with the protective activity of AAT predisposes patients to the development of lung damage and emphysema and, by its collecting in hepatic cells, liver damage (ATS 2003).
AATD was first described by Dr. Carl-Bertil Laurell and medical student Sten Eriksson at the University of Lund in Sweden when they observed the absence of the AAT protein band in serum electrophoresis gels from a series of samples (Eriksson 1964). Eriksson subsequently noted that three of the five affected patients developed emphysema in their 30s or 40s, including one individual with a family history of chronic obstructive pulmonary disease (COPD) (Eriksson 1964). Since the discovery of neutrophil elastase by Janoff and Schaefer in 1967 and its association with AAT — AAT serves as a protective inhibitor against host tissue degradation by neutrophil proteases — much has been learned about the biologic role of AAT in healthy lung function, and the genetics and clinical manifestations of AAT deficiency (Janoff 1967, Ranes 2005, Stoller 2005).
AATD has been estimated to affect approximately 80,000 to 100,000 individuals in the United States (Ranes 2005). Although AATD predominantly affects individuals of white European descent, there are reports of cases in nearly all countries and regions of the world, including sub-Saharan Africa, Asia, Australasia, and the Middle East as well as Europe and North America, and it has been observed in all racial and ethnic groups (Rachelefsky 2008, de Serres 2002). About 1% to 4.5% of all cases of emphysema are believed to be related to underlying AATD, and it has been suggested that if all 19.3 million patients with COPD in a hypothetical population were tested, as many as 1.8 million cases of AATD might be identified (ATS 2003, Rachelefsky 2008, de Serres 2006).
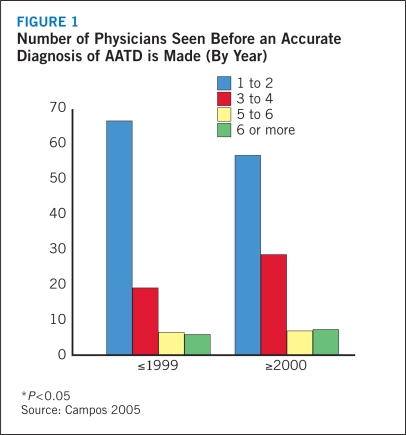
Yet even though organizations like the World Health Organization, the American Thoracic Society (ATS), and the European Respiratory Society have tried to increase awareness and promote early detection of AATD, the fact remains that AATD is largely misdiagnosed (ATS 2003, WHO 1997). In 2005, the diagnostic history over nearly four decades of patients enrolled in a not-for-profit health management company that functions to provide disease management services to patients with AATD was evaluated (Campos 2005). The investigators found that nearly two thirds of subjects visited one or two physicians to achieve a correct diagnosis, there was a substantial delay in correct diagnosis among a large portion of subjects, a high mean age at diagnosis, and a long symptomatic interval prior to diagnosis. Furthermore, the proportion of subjects diagnosed by the first or second physician has significantly decreased in recent years while the proportion who needed to see three to four physicians to obtain a diagnosis has increased (Figure 1).
FIGURE 1.
Number of Physicians Seen Before an Accurate Diagnosis of AATD is Made (By Year)
Despite evidence of improved diagnosis of symptomatic AATD in elderly individuals between 1990 and 2003, there either was no change or a small increase in the number of physicians visited and number of symptomatic years among younger patients aged 35 to 54 years (Campos 2005). Thus, the authors concluded that to better manage AATD and reduce the risk for associated lung disease and its complications, health care providers need to increase the use of genetic testing, screen all individuals with obstructive lung diseases, and increase awareness of AATD among patients and clinicians alike.
Still, fewer than 10% of individuals with AATD are diagnosed (Alpha-1 Association 2009). This may be due to the fact that despite being a disorder determined at birth, AATD symptoms often are absent or do not appear until at least young adulthood or middle age, even among individuals with severe deficiency. In addition, due to the variable phenotypic expression, clinicians may not think of AATD in the patient with respiratory and/or liver disease (Ranes 2005). A large number of individuals with AATD are likely misclassified as having bronchial asthma or smoking-related pulmonary disease (Kohnlein 2008). In fact, a correct diagnosis of AATD has been found to take a mean of seven years from first presentation, and 43% of patients see at least three physicians before diagnosis is made (Stoller 1994). In the same survey, 12% of subjects visited 6 to 10 physicians before reaching the AATD diagnosis.
This failure to improve early and accurate diagnosis is troubling because AATD can be a lethal disease. The yearly mortality from AATD is estimated at 3.5%, predominantly due to emphysema (72%) and cirrhosis of the liver (10%) (AATD Registry Study Group 1998, Larsson 1978). The accumulation of abnormal AAT in hepatocytes can cause cellular congestion, potentially leading to severe liver damage and organ failure (ATS 2003). The absence of AAT in blood and alveoli can result in progressive, severe, and potentially fatal emphysema (Rachelefsky 2008). Furthermore, mortality from respiratory disease has been shown to increase with advancing age, and among individuals with impaired lung function and smokers (AATD Registry Study Group 1998).
The recent availability of augmentation therapy with a replacement alpha1-PI has proven to be a safe and effective method to increase alpha1-PI levels, and thereby improve lung function, diminish progression of pulmonary disease, and extend survival in patients with AATD (Dirksen 2007, Rachelefsky 2008, Stocks 2006, Wencker 2001). Thus, treatment options are broadening and warrant proactive diagnosis aimed at early and optimal intervention. Future efforts, therefore, should focus on ensuring earlier diagnosis of AATD (Rachelefsky 2008). With early diagnosis, individuals with AATD can initiate preventive measures, such as smoking avoidance or limiting exposure to environmental airway irritants, in an effort to reduce the risk of emphysema. It also prompts entry into the health care system so that clinicians can monitor affected individuals to ensure timely recognition of symptoms and use of advanced therapies to prevent disease progression.
Particularly with the advent of effective augmentation therapy, early detection and proactive treatment is the key to improving the quality of life and survival of patients with AATD.
Genetics
AATD is an autosomal codominant disorder affecting the SERPINA1 gene, located on the long arm of chromosome 14 (Stoller 2005). SERPINA1 is highly pleomorphic, and there have been at least 30 genetic variants described that can lead to reduced serum levels of AAT (ATS 2003, Kohnlein 2008). The risk for lung and liver manifestations is determined by the extent of reduced AAT secretion or impaired function associated with the particular genetic defect.
The SERPINA1 alleles are identified by a letter code from A to Z, assigned according to the speed of migration of the molecule on an isoelectric pH gradient: the codes advance from A, which designates a highly anodal variant, to Z, defining variants of slower migration (ATS 2003, DeMeo 2004). The genetic variant is named according to the protein phenotype reflecting the codominant status. The most common allelic form associated with AATD involves the M alleles, and thus the homozygous form is termed PI*MM. PI*MM is considered a “normal variant” because it does not alter serum AAT levels or function (DeMeo 2004).
Disease expression is associated with null variants or deficiency genotypes that, in homozygous or heterozygous mutation combinations, impair AAT secretion and result in serum levels below a theoretical “protective threshold” of 11 μmol/L (ATS 2003). The most frequent mutation causing symptoms of severe AAT deficiency, responsible for an estimated 95% of symptomatic AATD, involves the Z allele (Stoller 2005). The homozygote PI*ZZ, highly prevalent in individuals of northern European heritage, generally is associated with serum concentrations of AAT about 15% of normal and carries significant risk for the development of pulmonary disease (ATS 2003). In comparison, the S variant, frequently associated with individuals of Mediterranean background, yields average serum concentrations 60% of normal among homozygous individuals resulting in a potentially less aggressive form of disease (ATS 2003). The null alleles, designated PI QOQO, induce the greatest AAT deficiency, generally producing concentrations <1% of normal (ATS 2003). Other frequent AAT allelic mutant genotypes include PI*SZ, PI*MS, and PI*MZ and all impair AAT secretion to some degree and confer increased risk for AATD-associated respiratory disease (ATS 2003).
In addition, AATD-associated mutant alleles can be classified into four groups defined by the level of serum AAT or functional defect (Stoller 2005). Normal alleles yield serum concentrations of approximately 20 to 53 μmol/L (Stoller 2005). Deficient alleles are associated with concentrations less than 20 μmol/L, although in some variants like Z, there also may be functional impairment of AAT (Stoller 2005). Null variants comprise that group of patients virtually absent of AAT due to transcriptional or translational errors that interfere with synthesis (Stoller 2005). Dysfunctional alleles are marked by abnormal function of AAT, leading to reduced binding to target tissue or other impairment of normal AAT activity (Stoller 2005). Table 1 lists the common allelic mutations of the AAT gene and the corresponding disease association.
TABLE 1.
Allelic Mutants of the SERPINA1 Gene and the Associated Disease Manifestations
| PI allele | Disease association |
|---|---|
| Normal alleles | |
| M (various subtypes) | Normal |
| Xchristchurch | Normal |
| Deficiency alleles | |
| S | Lung |
| Z* | Lung, liver |
| Mmalton | Lung, liver |
| Siiyama | Lung |
| Mheerlen | Lung |
| Mprocida | Lung |
| Mmineral springs* | Lung |
| Null alleles | |
| QOgranite falls | Lung |
| QOludwigshafen | Lung, liver |
| QOhongkong 1 | Lung |
| QOisola di procida | Lung |
| Dysfunctional alleles | |
| Pittsburgh | Bleeding diathesis |
| Mmineral springs* | Lung |
| Z* | Lung, liver |
Note that Mmineral springs and Z have dysfunctional characteristics described based on altered rates of association and inhibition of neutrophil elastase, as well as deficiency characteristics.
Source: DeMeo 2004
Pathophysiology
AAT is a member of the serine PI (serpin) superfamily of proteins (Lomas 2002). These compounds are key inhibitors of the activity of serine proteases, helping to maintain a desired balance between protease and antiprotease activity (Stoller 2005). Kinetic studies have suggested that neutrophil elastase, a 29-kD extracellular endopeptide released from neutrophils during inflammation that destroys antigen and host tissue alike, is the preferential target of AAT (ATS 2003).
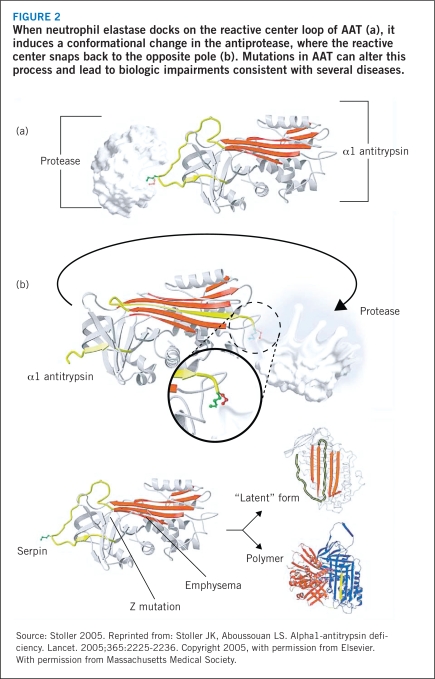
AAT is primarily produced in the liver and to a lesser degree in enterocytes and leukocytes, then released into the circulation during infection or inflammation. The function of AAT is to protect against the nonspecific activity of neutrophil elastase — the damage of tissue of the lower respiratory tract that occurs as neutrophil elastase carries out its primary function of fighting infection and inflammation (Stoller 2005). AAT exists in a high-energy, unstable state and, when its docking site, a methionine amino acid side-chain on the reactive center loop of AAT, binds to neutrophil elastase (Figure 2a), it induces a conformational change in the antiprotease (Carrell 2002, Stoller 2005). The cleaved reactive loop with protease in tow snaps back to the alternate pole of the molecule (Figure 2b), which distorts and inactivates the elastase (Huntington 2000, Stoller 2005). Both the protease and antiprotease are destroyed in this process.
FIGURE 2.
When neutrophil elastase docks on the reactive center loop of AAT (a), it induces a conformational change in the antiprotease, where the reactive center snaps back to the opposite pole (b). Mutations in AAT can alter this process and lead to biologic impairments consistent with several diseases.
The Z mutation of AAT, caused by substitution of lysine for glutamic acid at position 342 of the Z protein, leads to abnormal folding of the molecule that predisposes it to serial linking between reactive loops of nearby AAT molecules, resulting in irreversible polymerization (Stoller 2005). This polymerization of AAT within the hepatocyte impedes its secretion into the circulation, so that in homozygous Z mutant individuals, only about 15% of antitrypsin reaches the plasma (Lomas 2002, Stoller 2005). As a result of the decrease in AAT in the plasma, the destruction of lung tissue by neutrophil elastase can go unhindered (Janoff 1985, Lomas 2002, Stoller 2005). In the Z mutated phenotype, AAT activity is impaired: it has been shown to be about five times less effective as an inhibitor of neutrophil elastase than is the normal variant, or M form, of the protein (Ogushi 1987, Stoller 2005). Subjects in this state are at risk for accelerated lung breakdown and ultimately emphysema, a risk that can be further heightened in individuals who smoke or experience a lung infection (Stoller 2005).
The S mutation is characterized by substitution of glutamic acid for valine at position 264, a defect that impedes the formation of an integral salt bridge in the AAT molecule, making AAT susceptible to intracellular proteolysis (Ranes 2005). Like polymerized AAT, the degraded AAT is not released into the bloodstream, leading to mild reductions in plasma AAT. In addition, null mutations are due to deletions in the gene portion coding for AAT or to frameshift mutations that block messenger RNA production and impede protein translation (Ranes 2005).
Accumulation of mutated AAT within the endoplasmic reticulum of hepatocytes has been shown on electron microscopy and confirmed with periodic acid-Schiff stain (Stoller 2005). Although accumulation of AAT within the liver cells can lead to hepatic impairment, there is a wide inter-individual variation in expression of liver disease. Most patients, in fact, will not exhibit hepatic dysfunction, despite clear aggregation of AAT in the liver (Stoller 2005). Those phenotypes associated with uncontrolled intra-hepatic polymerization are responsible for the bulk of AATD-associated liver disease, a condition not driven by the “protective threshold” concept (Ranes 2005). In one clinical trial, the expression of clinical liver damage and cirrhosis was shown specifically to closely correlate with delayed degradation of the ZZ mutated-type protein within the hepatocyte (Stoller 2005, Wu 2002).
Clinical Features
The most frequent clinical outcomes of AATD are lung disease, liver disease, panniculitis, and C-ANCA-positive vasculitis (Ranes 2005).
Lung disease. The most common clinical manifestation of AATD is panacinar emphysema. AATD-associated emphysema often presents with an early onset (fourth or fifth decade of life) and symptoms including dyspnea, cough, phlegm production, asthma-like wheezing with upper respiratory tract infections, and clear evidence of pulmonary obstruction (McElvaney 1997, Ranes 2005). Like patients with AAT-replete COPD, airflow obstruction may be partially reversible, with forced expiratory volume in one second (FEV1) rising by as much as 12%, or 200 mL, on serial spirometries (Stoller 2005). Unlike AAT-replete disease, radiographic changes are disproportionately confined to the lung bases. In addition, in one series, three or more symptoms of asthma, such as wheezing, bronchodilator responsiveness, atopy, and increased serum IgE, were observed in 22% of patients with AATD, an overlap that can confuse correct diagnosis of AATD (ATS 2003).
Liver disease. Liver dysfunction affects approximately 10% to 34% of individuals with homozygous Z mutation AATD (Ranes 2005). The expression of clinically significant liver disease is generally confined to phenotypes marked by excessive intra-hepatic polymerization and rarely occurs in null or dysfunctional allelic forms (Ranes 2005). AATD-related liver disease manifests as hepatitis, cirrhosis, and/or hepatoma (Stoller 2005).
The likelihood of clinically evident liver disease increases with advancing age, and the biochemical and histopathologic findings of AATD, similar to those of alcoholic liver disease, may be confusing (Teckman 2006). The high association of liver disease with the homozygous Z variant of AATD has led to a recommendation by the combined ATS and European Respiratory Society for AATD testing in any case in which active liver disease remains unexplained after thorough evaluation, especially in the elderly (ATS 2003).
Panniculitis. Panniculitis, generally observed as tender, indurated cutaneous nodules on the trunk and proximal extremities, occurs in an estimated 1 in 1,000 individuals with AATD (Stoller 2005). The nodules are believed to result from unopposed proteolysis in the skin, and will drain an oily serosanguinous fluid similar to that seen in cellulitis. This symptom is most common in patients with ZZ, SZ, and SS phenotypes and was additionally reported in one subject with the MS phenotype but normal AAT serum levels (Loche 1999, Stoller 2005). Panniculitis generally responds extremely well to treatment with augmentation therapy, with inflamed skin and pain symptoms resolving rapidly with treatment (Chowdhury 2002, O’Riordan 1997, Stoller 2005).
C-ANCA-positive vasculitis. AATD has reportedly been observed in up to 17% of individuals with Wegener’s granulomatosis, or antiproteinase 3 antibody (C-ANCA)-positive vasculitis (Stoller 2005). Although the exact pathophysiology of this association is unclear the relationship is considered strong and established. Therefore, AATD testing is recommended for all adult patients with C-ANCA-positive vasculitis (ATS 2003).
Diagnosis
Laboratory tests. Evidence of a reduced or absent α1-globulin band on routine plasma protein electrophoresis should prompt both qualitative and quantitative testing for AATD (ATS 2003). Quantitative measures of AAT can be obtained via rocket immunoelectrophoresis, radial immunodiffusion, or nephelometry, with the threshold protective cut points at 11 μmol/L, 80 mg/dL, and 50 mg/dL, respectively (ATS 2003). As AAT is an acute phase reactant, it is important to remember that inflammatory conditions may augment AAT levels in Z heterozygotes, warranting reevaluation. Isoelectric focusing — or phenotyping — is the primary method for qualitative testing to identify the variants of AATD disease (e.g., Z or S forms) (ATS 2003). This approach requires specific expertise and therefore in most cases should be out-sourced to a reference laboratory (ATS 2003). It also can be supplemented by an immunoblot or immobilized pH gradient IEF gel. Basic molecular diagnosis can be performed with test kits available for S and Z allele mutation identification, but these often overestimate AAT concentration (ATS 2003). More specific genotyping, however, must be performed in the laboratory. Furthermore, failure to reveal a known mutation on qualitative testing may indicate a new variant rather than a negative test result, and will need to be further evaluated to establish if the subject indeed may have AATD (ATS 2003).
Prenatal genetic testing on samples from amniocentesis or chorionic villus sampling may be called for if there is a history of perinatal liver disease in a previous birth. However, cost often limits use of these techniques (ATS 2003). Postnatal testing when suspicion is high for AATD (e.g., neonatal hepatitis or strong family history) can be rapidly accomplished via DNA amplification using heel blood (ATS 2003).
Lung function tests. Spirometry is the most reliable and reproducible test of pulmonary function and therefore is the principle examination for following subjects with AATD (ATS 2003). Disease-specific indicators to watch for include a reduction in FEV1 and a normal or reduced forced vital capacity (FVC) (ATS 2003). A reduced FEV1/FVC ratio indicates impaired pulmonary elasticity secondary to emphysema and airway collapse (ATS 2003). Although the decreased lung volume that results is evident by concavity of the expiratory portion of the flow curve, a concurrent increase in pulmonary compliance leads to air trapping and an increase in residual volume and total lung capacity. This may be associated with elevations on plethysmography compared with traditional measures of indicator gas dilution (ATS 2003).
In addition, single-breath CO-diffusing capacity should be a supplemental measure to determine the overall severity of respiratory impairment, and arterial oxygen tension measures can reveal ventilation-perfusion disturbances (ATS 2003). Additional examinations that might further clarify the extent of physiologic compromise in patients with advanced disease include maximal inspiratory and expiratory mouth pressure (correlating with muscle activity of the thorax and diaphragm) and exercise testing to determine cardiopulmonary status (ATS 2003).
Radiologic evaluations. In advanced disease, X-ray or high-resolution computed tomography (CT) of the chest may be useful to characterize the extent and type of emphysema (ATS 2003). The X-ray can detect suggestive lung lesions or localized bullous disease. CT is a more sensitive diagnostic tool for emphysema than chest x-ray or pulmonary function tests (ATS 2003). In addition, ventilation-perfusion scanning may detect early pulmonary changes consistent with AATD (ATS 2003).