Abstract
Non-Hodgkin’s lymphoma (NHL) is the most common hematological malignancy in adults, with B-cell lymphomas accounting for 85% of all NHLs. The most substantial advancement in the treatment of B-cell malignancies, since the advent of combination chemotherapy, has been the addition of the monoclonal anti-CD20 antibody rituximab (Rituxan). Since its initially reported single-agent activity in indolent lymphomas in 1997, the role of rituximab has expanded to cover both indolent and aggressive lymphomas.
This article focuses on the impact of rituximab on the treatment, survival, and long-term outcomes of patients with indolent and aggressive lymphomas over the past two decades.
Keywords: rituximab, non-Hodgkin’s lymphoma
INTRODUCTION
Non-Hodgkin’s lymphoma (NHL) is the most common adult hematological cancer. In 2009, almost 66,000 cases were anticipated in the U.S. alone.1 The incidence of NHL in the U.S. over the previous 15 years has increased by approximately 4% annually, despite the decline in age-adjusted incidence rates for all cancers combined.
NHL encompasses a heterogeneous group of lymphomas that have been classified in various ways. In 1995, the World Health Organization developed a classification that included a combination of morphology, immunotyping, genetic features, and clinical syndromes. The goal was to define disease entities of B cells, T cells, and natural killer (NK) cells that pathologists could recognize and that had clinical relevance. The lymphomas were further subdivided into categories based on their clinical behavior (indolent, aggressive, or highly aggressive).2 More recent updates of this classification have clarified some less common entities but have left the overall schema intact.3
B-cell lymphomas account for about 85% of all NHL diagnoses.4 Although many subtypes of NHL exist clinically, most are grouped as either indolent (characterized by a prolonged median survival but generally considered incurable) or aggressive (characterized by rapid growth but with the potential for cure). Because patients with indolent lymphoma eventually die with this disease if they do not die of intercurrent illness, new treatments are needed to prolong survival, with the ultimate goal to provide cure. For patients with aggressive lymphoma, unmet needs include higher initial cure rates, improved salvage chemotherapy options, and less toxic therapies for old and frail patients.
Conventional methods of treatment, including chemotherapy and radiation, are associated with toxicity and lack specific antitumor-targeted activity. Cell–surface proteins, such as CD19, CD20, and CD22, are highly expressed on B-cell lymphomas and represent key potential targets for treatment.
Antibody therapy directed against CD20 has had the most important clinical impact to date. CD20 is thought to be involved in the regulation of intracellular calcium, cell cycle, and apoptosis. CD20 is not shed, modulated, or internalized significantly upon antibody binding, thus making it an ideal target for passive immunotherapy.5
Over the past two decades, significant progress has been made in the development of new therapies for B-cell lymphoma. Perhaps the most important advance is the addition of rituximab (Rituxan, Genentech/Biogen Idec), which the FDA approved for use in the U.S. in 1997. Rituximab is a chimeric (mouse and human) monoclonal antibody directed against the B-cell antigen CD20. It depletes B cells by several mechanisms, including direct antibody-dependent cellular cytotoxicity (ADCC), complement-mediated cell death, and signaling apoptosis.6–11
Phase 1 trials of two doses of rituximab (500 mg/m2 and 375 mg/m2 for four weeks) showed clinical responses with no dose-limiting toxicity.12 The weekly 375-mg/m2 dose, given for four weeks, was selected for further phase 2 evaluation and is currently the standard single-agent dose and schedule. Since this first reported activity, the role of rituximab has expanded to include both indolent and aggressive lymphomas.
This article addresses the effect of rituximab on survival and long-term outcomes in patients with NHL.
TREATMENT
Indolent, Low-Grade Lymphomas
Unlike aggressive lymphomas, indolent B-cell lymphomas are not considered curable with conventional therapies. Many patients are observed for prolonged periods without requiring treatment.13 In one study, more than 50% of the patients remained untreated for a median period of almost six years after diagnosis.14 Treatment goals focus on maintaining good quality of life with minimal symptoms. The indications for treatment include the presence of B symptoms (fevers, night sweats, and weight loss), compromise of normal organ function, bulky disease, or the presence of cytopenias resulting from marrow involvement. Transformation to an aggressive histological pattern warrants treatment for the aggressive component.
Although many active therapies are available for indolent NHL, patients ultimately die of this disease, which is incurable. Additional therapeutic options with improved efficacy and reduced toxicity are still needed for patients with indolent NHL. In light of this unmet need, the FDA’s approval of rituximab for the treatment of relapsed or refractory CD20-positive (CD20+) NHL in 1997 was an important clinical advance. The approval was based on the pivotal trial reported by McLaughlin et al., in which single-agent rituximab brought about significant response rates in heavily pretreated patients with indolent lymphoma.15
Initial Therapy for Indolent (Follicular) Lymphoma
Rituximab as first-line therapy has been widely studied in patients with indolent lymphomas, both as a single agent and in combination with conventional chemotherapy (Table 1). Witzig et al. evaluated the use of single-agent rituximab, 375 mg/m2 weekly for four doses, as an initial therapy for patients with stage III or IV grade 1 follicular lymphoma (FL). In this small phase 2 trial of only 37 patients, the reported objective response rate (ORR) was 72% and the complete remission rate (CRR) was 36%.16 Similarly, a phase 2 study by Hainsworth et al., which evaluated initial therapy in patients with indolent lymphomas, showed response rates in the range of 50%.17
Table 1.
Randomized Trials Using Rituximab in First-Line and Relapsed Settings in Indolent Lymphomas
| Study | Regimen | Efficacy |
|---|---|---|
| Newly diagnosed follicular lymphoma | ||
| Marcus, 200819 | R-CVP vs. CVP | ORR: 81% vs. 57%* Median TTP: 34 months vs. 15 months* |
| Herold, 200720 | R-MCP vs. MCP | ORR: 92% vs. 75%* Median PFS (follow-up, 47 months): NR vs. 28.8 months* |
| Hiddemann, 200521 | R-CHOP vs. CHOP | ORR: 96% vs. 90%* OS (2 years): 95% vs. 90% |
| Salles, 200822 | R-CHVP + IFN vs. CHVP + IFN | EFS (5 years): 53% vs. 37%* OS (5 years): 84% vs. 79% |
| Relapsed/refractory disease | ||
| Van Oers, 200626 | R-CHOP vs. CHOP | ORR: 85% vs. 72%* Median PFS: 33 months vs. 20 months* |
| Forstpointner, 200427 | R-FCM vs. FCM | ORR: 79% vs. 58%* Median PFS: 16 months vs. 10 months* OS (2 years): 90% vs. 70% |
Values were found to be statistically significant with P ≤ 0.05.
CHOP = cyclophosphamide, doxorubicin, vincristine, prednisone; CHVP = cyclophosphamide, doxorubicin, etoposide, prednisolone; CVP = cyclophosphamide, vincristine, prednisone; EFS = event-free survival; FCM = fludarabine, cyclophosphamide, mitoxantrone; IFN = interferon; MCP = mitoxantrone, chlorambucil, prednisolone; NR = not reached; ORR = overall response rate; OS = overall survival; PFS = progression-free survival; TTP = time to progression.
Using rituximab as a first-line therapy in patients with low-tumor-burden, indolent NHL, Colombat et al. reported an ORR of 73%.18 Long-term follow-up results of the completed randomized phase 3 Rituximab Extended Schedule Or Re-treatment Trial (RESORT, ECOG 4402 [Eastern Cooperative Oncology Group]) are still pending. In this randomized study, patients received four weekly rituximab treatments. Retreatment is then given as a single dose of rituximab every three months or upon disease progression with four weekly doses. The aim of the study is to define the benefit of maintenance therapy or re-treatment with rituximab (in terms of time to requiring a therapy other than rituximab) when progressive disease is documented.
The benefit of adding rituximab to combination chemotherapy during the initial treatment of FL has been documented in multiple clinical trials over the past decade. The phase 3 trial by Marcus et al. compared cyclophosphamide, vincristine, and prednisone (CVP), with and without rituximab, in 318 previously untreated patients with stage III and IV CD20+ FL.19 The addition of rituximab to CVP (R-CVP) significantly improved time to disease progression (34 months with R-CVP vs. 15 months with CVP, respectively; P < 0.0001) and duration of response (38 months vs. 14 months, respectively; P < 0.0001). Disease-free survival was 21 months with CVP but has not yet been determined in the group receiving R-CVP.
The East German Study Group evaluated the combination of rituximab with mitoxantrone, chlorambucil, and prednisone (MCP), followed by maintenance interferon in treatment-naive patients with stage III/IV CD20+ FL.20 The ORR was 92% with rituximab and 75% with chemotherapy alone (P = 0.0009).
Rituximab was also tested in combination with cyclophosphamide, hydroxydaunorubicin (doxorubicin), Oncovin (vincristine), and prednisone (R-CHOP) as first-line therapy in 428 patients with FL in a randomized phase 3 study with three years of follow-up.21 This combination showed a significant prolongation of time to treatment failure (P < 0.001) and prolonged duration of remission (P = 0.001) with the addition of rituximab. A higher ORR was observed in the group receiving R-CHOP (96%), compared with CHOP alone (90%) (P = 0.011). Even with a short follow-up, overall survival rates improved in the group receiving chemotherapy and rituximab (P = 0.016).
Similar results were seen in the GELA–GOELAMS FL 2000 trial (Groupe d’Etude des Lymphomes de l’Adulte/Groupe Ouest Est des Leucémies et Autres Maladies du Sang). This study was designed to examine the combination of rituximab with cyclophosphamide, hydroxydaunorubicin (doxorubicin), etoposide (VP-16), and prednisolone (CHVP) plus interferon-2 .22 A significant improvement in event-free survival at five years was noted for the rituximab patients (37% vs. 53%, respectively; P = 0.0004).
A meta-analysis of seven randomized controlled trials assessed the value of adding rituximab to conventional chemotherapy for 1,943 patients with FL, mantle-cell lymphoma, and other indolent lymphomas.23 This analysis demonstrated improved overall survival with the combination, as follows:
hazard ratio (HR) for mortality, 0.65
95% confidence interval (CI), 0.51–0.79
disease control (HR for the disease event, 0.62; 95% CI, 0.55–0.71)
response rates (relative risk for response 1.21; 91% CI, 1.16–1.27)
Specifically in FL, overall survival was better with rituximab plus chemotherapy (HR for mortality, 0.60; 95% CI, 0.37–0.98).23 The study authors concluded that the combination of rituximab and chemotherapy for patients with indolent lymphomas was superior to chemotherapy alone with respect to overall survival, disease-free survival, and response rates.23
Relapsed/Refractory Indolent Non-Hodgkin’s Lymphoma
The pivotal trial upon which the initial approval of rituximab was based showed the drug’s efficacy as a single agent in relapsed/refractory indolent NHL.15 Re-treatment with rituximab alone in 57 patients with low-grade FL who had previously responded to single-agent rituximab yielded a response rate of 40% and a similar duration of response, indicating sensitivity to re-treatment with the same agent.24
Davis et al. studied the use of single-agent rituximab in patients with bulky lesions (larger than 10 cm) and relapsed NHL.25 Patients receiving rituximab 375 mg/m2 weekly for four doses had an ORR of 43%. Among patients with a partial response, lesion size decreased by 76%.
The addition of rituximab to standard chemotherapy was found to be beneficial in the treatment of FL patients with relapsed/refractory NHL (see Table 1). An international trial by van Oers et al. evaluated the combination of six cycles of CHOP with rituximab 375 mg/m2 given intravenously on day 1 of each cycle, compared with chemotherapy alone in 465 patients with advanced disease.26 The ORR was higher with the addition of rituximab (85% with R-CHOP vs. 72% with CHOP alone; P < 0.001), and the median progression-free survival rate was also significantly improved in the rituximab group (33.1 vs. 20 months; P < 0.001). The addition of rituximab to the combination of fludarabine, cyclophosphamide, and mitoxantrone (FCM) in a similar group of patients also showed superior responses.27
Rituximab with bendamustine (Treanda, Cephalon) was studied in a phase 2 trial in patients with relapsed disease. This combination was found to be very effective, with an ORR of 92%.28
Maintenance Therapy for Follicular Lymphoma
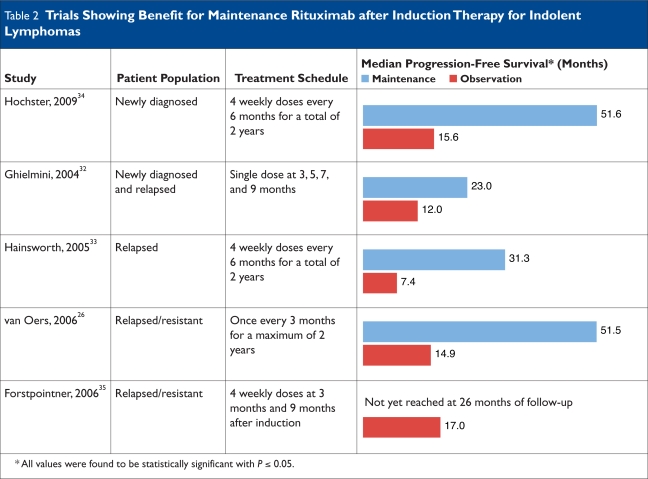
Some authors consider rituximab to be an ideal medication to use as maintenance therapy for an incurable disease such as FL because of its minimal toxicity and long half-life, which obviates the need for frequent administration.29 The use of rituximab as maintenance therapy after induction treatment has been the subject of several studies (Table 2) and is being evaluated by two large phase 3 trials: Primary Rituximab and Maintenance (PRIMA) and RESORT.30,31
Table 2.
Trials Showing Benefit for Maintenance Rituximab after Induction Therapy for Indolent Lymphomas
In the PRIMA trial, patients with previously untreated FL requiring therapy received a rituximab–chemotherapy regimen designated by the participating center as R-CHOP, R-CVP, or R-FCM. Responding patients were then randomly assigned to receive observation or scheduled re-treatment with rituximab as a single dose every eight weeks for two years. The RESORT trial was designed for asymptomatic patients with low-tumor-burden, indolent NHL (as discussed in detail on page 149).
The Swiss Group for Clinical Cancer Research (SAKK) evaluated maintenance rituximab following induction with rituximab monotherapy in a phase 3 trial in patients with newly diagnosed NHL and in previously treated patients with FL.32 In this study, the maintenance schedule consisted of four infusions at two-month intervals. Event-free survival was significantly longer among patients who received this maintenance schedule.
A phase 2 trial by the Minnie Pearl Cancer Research Network compared maintenance rituximab (four weekly doses repeated every six months for two years) with re-treatment using rituximab upon disease progression.33 The study showed significant prolongation of progression-free survival in the maintenance therapy group (31.3 vs. 7.4 months, respectively; P = 0.007), although no difference in overall survival or duration benefit from rituximab was observed between the two cohorts.
ECOG 1496 was a study that compared the use of maintenance rituximab with observation after induction with a non-rituximab chemotherapy regimen (CVP) in 282 patients with newly diagnosed FL.34 Improvement in progression-free survival at three years (68% with rituximab vs. 33% with CVP; P < 0.001) and overall survival at three years (91% vs. 86%, respectively; P = 0.08) were noted in the maintenance arm. In the relapsed setting, the prolonged use of rituximab was found to be beneficial with improved progression-free survival when it was used after CHOP or R-CHOP and after treatment with R-FCM.26,35
Although significant evidence exists for the improved progression-free survival with the use of maintenance therapy for FL, the benefit in terms of overall survival is still controversial. Moreover, because different dosing schedules were used in these studies, no data are available for the optimal dosing schedule of maintenance therapy and the recommended duration of this treatment.
Rituximab plus Chemotherapy: Effect on Survival In Follicular Lymphoma
There is no doubt that the clinical development of rituximab has been a significant breakthrough in the field of indolent lymphomas. However, its effect on overall survival in this group of patients is still open to debate.
An analysis of survival in patients 15 years of age and older with NHL diagnosed between 1990 and 2004, using data from the Surveillance, Epidemiology and End Results (SEER) program, revealed a markedly improved outcome for patients with NHL in recent years. This finding may be related, in part, to the addition of rituximab.36
A large retrospective analysis by Swenson et al. was conducted to examine survival rates of 14,564 patients with FL diagnosed between 1978 and 1999 in the U.S.37 Improvement in survival was noted over the past 25 years, and a reduction in the relative risk of death by 1.8% per year was observed from 1983 to1999.
In a second analysis, the Southwest Oncology Group (SWOG) looked at the survival of patients with FL on three large randomized clinical trials between 1974 and 2000.38 Overall survival rates improved over this period of time. The greatest improvement was observed with the most recent treatment approach consisting of CHOP with an anti-CD20 monoclonal antibody.
The study by Marcus et al., published in 2008, showed improved survival rates among untreated patients receiving CVP plus rituximab when compared with CVP alone (four-year survival, 83% vs. 77%, respectively; P = 0.029).19 Other studies, including a Cochrane meta-analysis, have shown similar trends toward improved survival.23,26,34
These observations can be attributed to multiple factors, including improved supportive care measures, enhancements in education of physicians and patients, and better treatments of relapsed and transformed cases.39 Despite these uncertainties regarding its effect on overall survival, it is clear that rituximab has substantially advanced the treatment of indolent lymphomas in the last decade.
Diffuse Large B-Cell Lymphoma
As the most common high-grade form of NHL, diffuse large B-cell lymphoma (DLBCL) accounts for more than 30% of new diagnoses. The median age of presentation is 60 years. Unlike indolent lymphomas, DLBCL is an aggressive lymphoma; if it is untreated, survival can be measured in months. More than 70% of patients with DLBCL present at an advanced stage, and systemic chemotherapy is the foundation of treatment. Since its development in the 1970s, CHOP has been the mainstay of treatment for this group of patients.
A milestone phase 3 trial found that complex regimens that included the addition of other chemotherapy agents to CHOP did not demonstrate any significant difference in overall survival, disease-free survival, or remission rates over CHOP.40–43 Moreover, CHOP was associated with significantly less toxicity and cost.
Based on these results, CHOP remained the gold standard of therapy for DLBCL. Nonetheless, long-term remission occurred in only about 45% of patients, so that more than half of patients relapsed with the best therapy possible in the early 1990s. A relatively small percentage of relapsed DLBCL patients (25%–50%) might have been “salvaged” with high-dose chemotherapy and stem-cell support, yet many patients were not even eligible for such therapy.
Thus, in the early 1990s, the addition of more chemotherapy drugs into complex regimens had not improved results with CHOP, and there was a sense that future improvements in therapy would not come from additional “standard” drugs. While rituximab was approved for treatment of low-grade lymphoma in 1997, several trials combining rituximab with CHOP (R-CHOP) for aggressive lymphomas began prior to that time. Because rituximab-related toxicities were not overlapping with those of CHOP, both CHOP and rituximab could be administered at full doses. Results from large international, randomized trials have demonstrated the significant benefits of the addition of rituximab to standard chemotherapy for DLBCL. These trials are summarized next.
Previously Untreated Diffuse Large B-Cell Lymphoma
Based on the efficacy of rituximab in low-grade lymphomas, Vose et al. conducted a phase 2 study of rituximab with CHOP chemotherapy in 33 previously untreated patients with advanced-stage, aggressive B-cell lymphoma.44 Rituximab at a dose of 375 mg/m2 was administered on day 1 of each of six cycles of CHOP. The ORR was 94%; 61% of patients had complete responses (CRs), and 33% had partial responses (PRs). This was the first report that demonstrated an improved efficacy of the combination without worsening toxicity.
GELA investigators randomized previously untreated elderly patients (60–80 years of age) to eight cycles of CHOP alone (197 patients) or eight cycles of R-CHOP given on day 1 of each cycle (202 patients).45 The rate of CRs was significantly higher in the rituximab group (76% vs. 63% receiving CHOP alone, P = 0.005). Sixty percent of patients exhibited features of poor risk, with age-adjusted International Prognostic Index (aaIPI) scores of 2 to 3. With a median follow-up of two years, event-free survival rates (57% vs. 38%; P < 0.001) and overall survival rates (70% vs. 57%; P = 0.007) were significantly higher with rituximab (Table 3). Furthermore, toxicity was not greater with the addition of rituximab.
Table 3.
Trials Using Rituximab for Diffuse Large B-Cell Lymphomas in the First-Line Setting
| Study | Patient Population | Regimen | Overall Survival | Progression-Free Survival |
|---|---|---|---|---|
| Coiffier, 200245 | (n = 399) Previously untreated Age 60–80 years |
R-CHOP vs. CHOP | 70% vs. 57% | 57% vs. 38% |
| Pfreundschuh, 200649 | (n = 824) Previously untreated Age 18–60 years |
R-CHOP-like chemotherapy vs. CHOP-like chemotherapy | 93% vs. 84% (P = 0.0001) | 79% vs. 59% (P < 0.0001) |
| Habermann, 200648 | (n = 632) Previously untreated Age > 60 years |
R-CHOP vs. CHOP | Not reached | 53% vs. 46% (P = 0.04) |
A long-term analysis at seven years has confirmed the benefit of the addition of rituximab.46 Event-free survival (42% with R-CHOP vs. 25%; P < 0.0001), progression-free survival (52% vs. 29%, respectively; P < 0.0001) and disease-free survival (66% vs. 42% respectively, P = 0.0001) were all statistically better for patients treated with combination therapy.
A retrospective analysis of the GELA trial suggested that R-CHOP increased overall survival preferentially in bcl-2–positive patients compared with CHOP alone.47 These data suggested that rituximab may overcome chemotherapy resistance associated with bcl-2 in patients with DLBCL. However, other retrospective analyses have led to conflicting results on whether the benefit of R-CHOP is primarily or only observed in bcl-2 expressing DLBCL.
Habermann et al. randomly assigned patients older than 60 years of age to receive CHOP or R-CHOP, with a second random assignment to maintenance rituximab therapy or observation in responders (see Table 3).48 This study demonstrated the benefit of the addition of rituximab to CHOP using a modified schedule of rituximab administration. Three-year failure-free survival rates were 53% and 46% (P = 0.04). Failure-free survival was higher for patients who received maintenance therapy with rituximab after CHOP but not for patients who received R-CHOP initially.
The trials described above established R-CHOP as standard first-line therapy for elderly patients with DLBCL. With respect to younger patients, the MabThera (rituximab) International Trial (MInT) confirmed the benefit of adding rituximab to standard chemotherapy in 824 patients (18 to 60 years of age) with only zero (0) to one risk factor, as assessed by the IPI (see Table 3).49 Patients with stage II to IV or stage I disease with bulky lymphadenopathy were randomly assigned to six cycles of CHOP-like chemotherapy with or without the addition of rituximab. Radiation therapy was subsequently administered to initial sites of bulky disease. Three-year event-free survival rates (79% vs. 59%; P < 0.0001) and overall survival rates (93% vs. 84%; P = 0.00001) were both significantly higher for patients treated with the addition of rituximab. There were no additional major adverse effects.
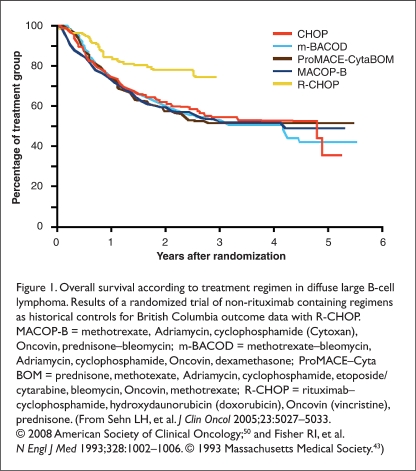
Sehn et al. compared outcomes during a three-year period; 18 months pre- and post-inclusion of rituximab in standard treatment protocols guided care for patients with newly diagnosed advanced-stage DLBCL in British Columbia.50 All age and risk factor groups were included. Adding rituximab resulted in dramatic improvement in both progression-free survival and overall survival (Figure 1). These studies have indicated significant benefit for the addition of rituximab to chemotherapy for the treatment of DLBCL in a wide range of patient ages and risk categories. Although adding other cytotoxic chemotherapy agents to CHOP failed to improve outcomes, R-CHOP is now the gold standard for treating DLBCL in all subgroups.43
Figure 1.
Overall survival according to treatment regimen in diffuse large B-cell lymphoma. Results of a randomized trial of non-rituximab containing regimens as historical controls for British Columbia outcome data with R-CHOP. MACOP-B = methotrexate, Adriamycin, cyclophosphamide (Cytoxan), Oncovin, prednisone–bleomycin; m-BACOD = methotrexate–bleomycin, Adriamycin, cyclophosphamide, Oncovin, dexamethasone; ProMACE–Cyta BOM = prednisone, methotexate, Adriamycin, cyclophosphamide, etoposide/cytarabine, bleomycin, Oncovin, methotrexate; R-CHOP = rituximab–cyclophosphamide, hydroxydaunorubicin (doxorubicin), Oncovin (vincristine), prednisone. (From Sehn LH, et al. J Clin Oncol 2005;23:5027–5033. © 2008 American Society of Clinical Oncology;50 and Fisher RI, et al. N Engl J Med 1993;328:1002–1006. © 1993 Massachusetts Medical Society.43)
Relapsed/Refractory Diffuse Large B-Cell Lymphoma
Coiffier et al. conducted a randomized phase 2 trial to evaluate the efficacy and tolerability of rituximab in patients with relapsed/refractory DLBCL, mantle-cell lymphoma, or other intermediate-grade or high-grade B-cell lymphomas and previously untreated patients older than 60 years of age.51 Fifty-four patients received eight weekly infusions of rituximab 375 mg/m2 in arm A or one infusion of 375 mg/m2, followed by seven weekly infusions of 500 mg/m2 in arm B. A total of five complete responses and 12 partial responses were observed among the 54 enrolled patients, with no difference between the two doses. The ORR was 31%. An analysis of prognostic factors showed that response rates were lower in patients with refractory disease, in patients with lymphoma not classified as DLBCL, and patients with a tumor larger than 5 cm in diameter. Single-agent rituximab is active in aggressive NHL but not as active as in indolent NHL. This finding led to the use of combinations of rituximab plus chemotherapy in such patients.
The combination of rituximab, ifosfamide, carboplatin, and etoposide (R-ICE) was evaluated in relapsed DLBCL for cytoreduction prior to autologous hematopoietic stem cell transplantation (HSCT).52 Thirty-six eligible patients received rituximab plus ICE (RICE), and 34 patients received all three planned cycles. The CR rate was 53%, significantly better than the 27% CR rate (P = 0.01) achieved among 147 similar consecutive historical control patients with DLBCL treated with ICE; the PR rate was 25%. Progression-free survival in patients who underwent transplantation after RICE was marginally better than for 95 consecutive historical controls who underwent transplantation after ICE alone, but the results did not reach statistical significance (54% with RICE vs. 43% with ICE alone at two years, respectively; P = 0.25). Preliminary results of the CORAL study (Collaborative Trial in Relapsed Aggressive Lymphoma) demonstrated a decreased response to rituximab in the salvage setting of patients previously treated with rituximab-containing regimens.53
In a phase 2 study, rituximab was evaluated in addition to etoposide, prednisone, Oncovin (vincristine), doxorubicin, and cyclophosphamide (EPOCH) in patients with relapsed or refractory aggressive NHL.54 The ORR of 68% included 28% of patients in complete remission. At three years, event-free survival and overall survival rates were 28% and 38%, respectively.
Few studies have explored the use of rituximab as an adjunct to autologous HSCT after high-dose chemotherapy in patients with relapsed DLBCL.55–57 These studies have reported positive results with rituximab in this setting. Larger, randomized trials are needed to establish a definitive role for rituximab in these patients. While overall the data on rituximab efficacy in aggressive NHL is not as strong in relapsed patients as for initial R-CHOP, rituximab is active and additional confirmatory studies are needed in various relapsed settings.
Maintenance Therapy for Diffuse Large B-Cell Lymphoma
Unlike the situation with indolent lymphomas, there is no apparent benefit to maintenance rituximab in DLBCL. In an ECOG trial, responding patients were randomly assigned to receive maintenance rituximab or to observation alone.48 Two-year failure-free survival was 76% for maintenance therapy and 61% for observation alone, but these figures were confounded depending on whether rituximab was used initially. No significant differences in survival were seen when rituximab was included either as maintenance or as induction therapy. Failure-free survival was prolonged with maintenance therapy after CHOP but not after R-CHOP. This study confirmed the role of R-CHOP as standard first-line therapy in older DLBCL patients, with maintenance therapy to be used only for patients not previously treated with rituximab.
ADVERSE EFFECTS
Rituximab is usually well tolerated, and toxicities are generally mild.12,24,58 Common side effects include pruritus, nausea, vomiting, dizziness, headaches, fevers, and rigors. A major concern is the potential for an infusion-related reaction, such as rigors, chills, anaphylactic reactions potentially leading to myocardial infarction and cardiogenic shock. These reactions occur most commonly during the first administration of rituximab. Although infusion reactions are rarely fatal, predisposing cardiac conditions can increase the risk of death. Pre medication with acetaminophen and antihistamines is recommended prior to infusion. Reactions usually abate if the infusion is discontinued and can then be restarted at a slower rate. The benefit of premedication with glucocorticoids is not entirely clear, but they are useful if a reaction occurs. Mucocutaneous reactions, including Stevens–Johnson syndrome, have also been reported within one to 13 weeks following rituximab exposure.
Tumor lysis syndrome has also occurred in patients with bulky lymphoma. Hepatitis B reactivation with fulminant hepatitis, hepatic failure, and death have been reported in patients with previous hepatitis B infection who have been treated with rituximab. Consultation with a hepatologist and administration of antiviral therapy should be considered if hepatitis B antigen is detectable. The risk of reactivation of hepatitis C is not well defined. The use of live vaccines, including those against herpes zoster, is not recommended during rituximab therapy secondary to the risk of causing an active infection. Rituximab-treated patients are also at risk for other viral infections, including cytomegalovirus, herpes simplex, parvovirus B19, and West Nile virus.
Late-onset neutropenia has been described as a possible complication of adding rituximab to chemotherapy.59 In a retrospective review, patients who received chemotherapy plus rituximab for CD20+, B-cell NHL had a higher rate of late-onset neutropenia compared with historical controls receiving chemotherapy alone.
A study published in 2009 reported 57 cases of progressive multifocal leukoencephalopathy (PML) following the administration of rituximab, usually with additional therapy, in HIV-negative patients.60 PML, a viral infection that affects the white matter of the brain, is usually fatal. This cohort of patients was treated with a median of six doses of rituximab. The median time from last rituximab dose to PML diagnosis was 5.5 months, and median survival after the diagnosis of PML was two months. In accordance with these data, the FDA issued a boxed (black-box) warning.
Reversible posterior leukoencephalopathy (RPLE), a subacute neurological syndrome manifested as headaches, cortical blindness, and seizures with a characteristic appearance on magnetic resonance imaging (MRI), has also been described in rare cases.61,62 It is not clear whether these events are directly related to rituximab, because most of these patients have received multiple therapies, but RPLE has also been reported after other antibody and small-molecule therapeutics. Cardiac arrhythmias, renal toxicity, and bowel obstruction with perforation have also been reported.57
Rituximab induces B-cell depletion, which may compromise the immune system; however, recovery of the normal B-cell population usually occurs six to nine months after discontinuation of therapy.15 Despite this depletion, rituximab has not been definitively shown to cause a significant decrease in circulating immunoglobulin levels, although this may occur with more prolonged maintenance strategies. Stable immunoglobulin levels are likely to reflect that plasma cells are long-lived and do not express CD20.
In a prospective study, van der Kolk et al. investigated the effect of rituximab on the humoral immune response to two primary antigens and two recall antigens.63 After rituximab treatment, the humoral immune response to the recall antigens was significantly decreased when compared with the response before treatment.
FUTURE DIRECTIONS
Attempts to improve upon rituximab have focused on antibody engineering, including humanized instead of chimeric antibodies, stronger binding affinity for CD20, or enhancing effector functions such as antibody-dependent, cell-mediated cytotoxicity (ADCC) or complement activation. Ofatumumab (Arzerra, GlaxoSmithKline) is a humanized monoclonal anti-CD20 antibody that targets a small loop epitope of CD20. Compared with rituximab, in the laboratory it delivers stronger complement-dependent cytotoxicity, even in lymphoma cells with low expression of CD20. Approved by the FDA in October 2009 for the treatment of fludarabine and alemtuzumab–refractory chronic lymphocytic leukemia (CLL), the drug also showed activity in relapsed/refractory FL.64,65
Additional humanized antibodies under development include some with enhanced ADCC, stronger binding to low-affinity polymorphisms of FcgRIII, or targeting other epitopes on the CD20 molecule. Whether these agents are more effective, less immunogenic, or faster to infuse with fewer infusion reactions resulting may be difficult to determine.
Other proteins on the surface of B cells are also potential antibody targets. CD22 has a pattern of expression similar to that of CD20 on normal and malignant B lymphocytes, and it is targeted by epratuzumab (UCB/Immunomedics).66,67 Because CD22 is internalized upon antibody binding, it might be better suited for delivering toxins inside CD22+ cells. Examples of this approach include inotuzumab ozogamicin (CMC-544, Wyeth), an anti-CD22 immunoconjugate with the antitumor antibiotic calicheamicin, and CAT-3888 (Cambridge Antibody Technology), formerly called BL22, which uses a Pseudomonas exotoxin fragment.68,69
CONCLUSION
Rituximab (Rituxan) has changed the treatment paradigms and outcomes for all CD20+ NHL and represents arguably the most noteworthy advance in lymphoma treatment over the past decade. In patients with NHL, the addition of rituximab to standard treatment significantly enhanced response to therapy and overall outcomes. Rituximab is currently approved for treatment of relapsed and refractory indolent lymphomas as single-agent therapy and as initial therapy in combination with standard chemotherapy regimens. In patients with DLBCL, it is approved for use as initial therapy with CHOP or other anthracycline-based chemotherapy. The drug was also recently approved for use with chemotherapy in previously treated and untreated patients with CLL.
Benefits have been sustained among all age groups, and the drug has been safe and well tolerated in elderly patients as well. Overall survival of patients with NHL has improved over the last two decades. While some of this improvement may stem from earlier or more precise diagnosis and better supportive care, the results of many trials reviewed in this article indicate significant improvement in outcomes with the addition of rituximab to the therapeutic armamentarium.
Despite these advances, questions remain, mainly in the field of indolent lymphomas. More research is under way to establish the optimal schedule, timing, and duration for maintenance rituximab. Reports of clinical trials demonstrating longer follow-up of indolent lymphoma are eagerly awaited in an attempt to clarify the effect of rituximab on overall survival.
Rituximab represents a paradigm shift in treatment of B-cell NHL; it marks the beginning of a new age of targeted therapies in oncology, being the first approved therapeutic monoclonal antibody for cancer. In the years to come, we anticipate more clinical trials combining rituximab with targeted treatments that might further improve outcomes while minimizing toxicity.
Footnotes
Disclosure.The authors are solely responsible for the development and writing of the manuscript. Support for third-party editorial assistance for this manuscript was provided by Genentech, Inc.
REFERENCES
- 1.Jemal A, Siegel R, Ward E. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Rudiger T, Muller-Hermelink HK. WHO classification of malignant lymphomas. Radiologe. 2002;42(12):936–942. doi: 10.1007/s00117-002-0832-0. [DOI] [PubMed] [Google Scholar]
- 3.Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization Classification of Neoplastic Diseases of the Hematopoietic and Lymphoid Tissues. Report of the Clinical Advisory Committee meeting. Airlie House, Virginia, November 1997; Ann Oncol; 1999. pp. 1419–1432. [DOI] [PubMed] [Google Scholar]
- 4.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: Clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16(8):2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 5.Tedder TF, Engel P. CD20: A regulator of cell–cycle progression of B lymphocytes. Immunol Today. 1994;15(9):450–454. doi: 10.1016/0167-5699(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 6.Silverman GJ, Weisman S. Rituximab therapy and autoimmune disorders: Prospects for anti-B cell therapy. Arthritis Rheum. 2003;48(6):1484–1492. doi: 10.1002/art.10947. [DOI] [PubMed] [Google Scholar]
- 7.Shan D, Ledbetter JA, Press OW. Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. Blood. 1998;91(5):1644–1652. [PubMed] [Google Scholar]
- 8.Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83(2):435–445. [PubMed] [Google Scholar]
- 9.Golay J, Zaffaroni L, Vaccari T, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95(12):3900–3908. [PubMed] [Google Scholar]
- 10.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 11.Alas S, Bonavida B. Rituximab inactivates signal transducer and activation of transcription 3 (STAT3) activity in B–non-Hodgkin’s lymphoma through inhibition of the interleukin 10 autocrine/paracrine loop and results in down-regulation of Bcl-2 and sensitization to cytotoxic drugs. Cancer Res. 2001;61(13):5137–5144. [PubMed] [Google Scholar]
- 12.Maloney DG, Liles TM, Czerwinski DK, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (Idec-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84(8):2457–2466. [PubMed] [Google Scholar]
- 13.Horning SJ, Rosenberg SA. The natural history of initially untreated low-grade non-Hodgkin’s lymphomas. N Engl J Med. 1984;311(23):1471–1475. doi: 10.1056/NEJM198412063112303. [DOI] [PubMed] [Google Scholar]
- 14.Advani R, Rosenberg SA, Horning SJ. Stage I and II follicular non-Hodgkin’s lymphoma: Long-term follow-up of no initial therapy. J Clin Oncol. 2004;22(8):1454–1459. doi: 10.1200/JCO.2004.10.086. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 16.Witzig TE, Vukov AM, Habermann TM, et al. Rituximab therapy for patients with newly diagnosed, advanced-stage, follicular grade I non-Hodgkin’s lymphoma: A phase II trial in the North Central Cancer Treatment Group. J Clin Oncol. 2005;23(6):1103–1108. doi: 10.1200/JCO.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 17.Hainsworth JD, Burris HA, III, Morrissey LH, et al. Rituximab monoclonal antibody as initial systemic therapy for patients with low-grade non-Hodgkin lymphoma. Blood. 2000;95(10):3052–3056. [PubMed] [Google Scholar]
- 18.Colombat P, Salles G, Brousse N, et al. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: Clinical and molecular evaluation. Blood. 2001;97(1):101–106. doi: 10.1182/blood.v97.1.101. [DOI] [PubMed] [Google Scholar]
- 19.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 20.Herold M, Haas A, Srocket S, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: An East German Study Group Hematology and Oncology Study. J Clin Oncol. 2007;25(15):1986–1992. doi: 10.1200/JCO.2006.06.4618. [DOI] [PubMed] [Google Scholar]
- 21.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 22.Salles G, Mounier N, de Guibert S, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: Results of the GELA–GOELAMS FL 2000 study. Blood. 2008;112(13):4824–4831. doi: 10.1182/blood-2008-04-153189. [DOI] [PubMed] [Google Scholar]
- 23.Schulz H, Bohlius JF, Trelle S, et al. Immunochemotherapy with rituximab and overall survival in patients with indolent or mantle cell lymphoma: A systematic review and meta-analysis. J Natl Cancer Inst. 2007;99(9):706–714. doi: 10.1093/jnci/djk152. [DOI] [PubMed] [Google Scholar]
- 24.Davis TA, Grillo-López AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: Safety and efficacy of re-treatment. J Clin Oncol. 2000;18(17):3135–3143. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 25.Davis TA, White CA, Grillo-López AJ, et al. Single-agent monoclonal antibody efficacy in bulky non-Hodgkin’s lymphoma: Results of a phase II trial of rituximab. J Clin Oncol. 1999;17(6):1851–1857. doi: 10.1200/JCO.1999.17.6.1851. [DOI] [PubMed] [Google Scholar]
- 26.van Oers MH, Klasa R, Marcus RE, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab during induction: Results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108(10):3295–3301. doi: 10.1182/blood-2006-05-021113. [DOI] [PubMed] [Google Scholar]
- 27.Forstpointner R, Dreyling M, Rapp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104(10):3064–3071. doi: 10.1182/blood-2004-04-1323. [DOI] [PubMed] [Google Scholar]
- 28.Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(27):4473–4479. doi: 10.1200/JCO.2008.17.0001. [DOI] [PubMed] [Google Scholar]
- 29.van Oers MH. Rituximab maintenance therapy: A step forward in follicular lymphoma. Haematologica. 2007;92(6):826–833. doi: 10.3324/haematol.10894. [DOI] [PubMed] [Google Scholar]
- 30.Williams ME. ECOG 4402: Randomized phase III trial comparing two different rituximab dosing regimens for patients with low tumor burden indolent non-Hodgkin’s lymphoma. Curr Hematol Rep. 2004;3(6):395–396. [PubMed] [Google Scholar]
- 31.Groupe d’Etude des Lymphomes de L’Adulte: Primary rituximab and maintenance: ClinicalTrials.gov identifier: NCT00140582.
- 32.Ghielmini M, Schmitz SFH, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly × 4 schedule. Blood. 2004;103(12):4416–4423. doi: 10.1182/blood-2003-10-3411. [DOI] [PubMed] [Google Scholar]
- 33.Hainsworth JD, Litchy S, Shaffer DW, et al. Maximizing therapeutic benefit of rituximab: Maintenance therapy versus retreatment at progression in patients with indolent non-Hodgkin’s lymphoma. A randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2005;23(6):1088–1095. doi: 10.1200/JCO.2005.12.191. [DOI] [PubMed] [Google Scholar]
- 34.Hochster H, Weller E, Gascoyne RD, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: Results of the randomized phase III ECOG 1496 Study. J Clin Oncol. 2009;27(10):1607–1614. doi: 10.1200/JCO.2008.17.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forstpointner R, Unterhalt M, Dreyling M, et al. Maintenance therapy with rituximab leads to a significant prolongation of response duration after salvage therapy with a combination of rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) in patients with recurring and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group (GLSG) Blood. 2006;108(13):4003–4008. doi: 10.1182/blood-2006-04-016725. [DOI] [PubMed] [Google Scholar]
- 36.Pulte D, Gondos A, Brenner H. Ongoing improvement in outcomes for patients diagnosed as having non-Hodgkin lymphoma from the 1990s to the early 21st century. Arch Intern Med. 2008;168(5):469–476. doi: 10.1001/archinternmed.2007.125. [DOI] [PubMed] [Google Scholar]
- 37.Swenson WT, Wooldridge JE, Lynch CF, et al. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol. 2005;23(22):5019–5026. doi: 10.1200/JCO.2005.04.503. [DOI] [PubMed] [Google Scholar]
- 38.Fisher RI, LeBlanc M, Press OW, et al. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23(33):8447–8452. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 39.Horning SJ. Follicular lymphoma, survival, and rituximab: Is it time to declare victory? J Clin Oncol. 2008;26(28):4537–4538. doi: 10.1200/JCO.2008.16.1398. [DOI] [PubMed] [Google Scholar]
- 40.Gaynor ER, Unger JM, Miller TP, et al. Infusional CHOP chemotherapy (CVAD) with or without chemosensitizers offers no advantage over standard CHOP therapy in the treatment of lymphoma: A Southwest Oncology Group Study. J Clin Oncol. 2001;19(3):750–755. doi: 10.1200/JCO.2001.19.3.750. [DOI] [PubMed] [Google Scholar]
- 41.Meyer RM, Quirt IC, Skillings JR, et al. Escalated as compared with standard doses of doxorubicin in BACOP therapy for patients with non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(24):1770–1776. doi: 10.1056/NEJM199312093292404. [DOI] [PubMed] [Google Scholar]
- 42.Sertoli MR, Santini G, Chisesi T, et al. MACOP-B versus ProMACE–MOPP in the treatment of advanced diffuse non-Hodgkin’s lymphoma: Results of a prospective randomized trial by the non-Hodgkin’s Lymphoma Cooperative Study Group. J Clin Oncol. 1994;12(7):1366–1374. doi: 10.1200/JCO.1994.12.7.1366. [DOI] [PubMed] [Google Scholar]
- 43.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328(14):1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 44.Vose JM, Link BK, Grossbard ML, et al. Phase II study of rituximab in combination with CHOP chemotherapy in patients with previously untreated, aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2001;19(2):389–397. doi: 10.1200/JCO.2001.19.2.389. [DOI] [PubMed] [Google Scholar]
- 45.Coiffier B, Lepage E, Brier J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 46.Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: A study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23(18):4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 47.Mounier N, Briere J, Gisselbrecht C, et al. Rituximab plus CHOP (R-CHOP) in the treatment of elderly patients with diffuse large B-cell lymphoma (DLBCL) overcomes bcl2-associated chemotherapy resistance (Abstract) Blood. 2002;100(Suppl 1):161a, 603. doi: 10.1182/blood-2002-11-3442. [DOI] [PubMed] [Google Scholar]
- 48.Habermann TM, Weller EA, Morrison VA, et al. Rituximab–CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 49.Pfreundschuh M, Trümper L, Österborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7(5):379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 50.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 51.Coiffier B, Haioun C, Ketterer N, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: A multicenter phase II study. Blood. 1998;92(6):1927–1932. [PubMed] [Google Scholar]
- 52.Kewalramani T, Zelenetz AD, Nimer SD, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103(10):3684–3688. doi: 10.1182/blood-2003-11-3911. [DOI] [PubMed] [Google Scholar]
- 53.Gisselbrecht C, Glass B, Mounie N. R-ICE versus R-DHAP in relapsed patients with CD20 diffuse large B-cell lymphoma (DLBCL) followed by autologous stem cell transplantation: CORAL study (Abstract 8509) Clin Oncol. 2009;27(Suppl):15s. [Google Scholar]
- 54.Jermann M, Jost LM, Taverna C, et al. Rituximab–EPOCH, an effective salvage therapy for relapsed, refractory or transformed B-cell lymphomas: Results of a phase II study. Ann Oncol. 2004;15(3):511–516. doi: 10.1093/annonc/mdh093. [DOI] [PubMed] [Google Scholar]
- 55.Tarella C, Zanni M, Magni M, et al. Rituximab improves the efficacy of high-dose chemotherapy with autograft for high-risk follicular and diffuse large B-cell lymphoma: A multicenter Gruppo Italiano Terapie Innnovative nei linfomi survey. J Clin Oncol. 2008;26(19):3166–3175. doi: 10.1200/JCO.2007.14.4204. [DOI] [PubMed] [Google Scholar]
- 56.Khouri IF, Lee M-S, Saliba RM, et al. Nonablative allogeneic stem cell transplantation for chronic lymphocytic leukemia: Impact of rituximab on immunomodulation and survival. Exp Hematol. 2004;32(1):28–35. doi: 10.1016/j.exphem.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 57.Horwitz SM, Horning SJ. Rituximab in stem cell transplantation for aggressive lymphoma. Curr Hematol Rep. 2004;3(4):227–229. [PubMed] [Google Scholar]
- 58.Physicians’ Desk Reference. 63rd ed. Montvale, NJ: Thomson Reuters; 2009. [Google Scholar]
- 59.Nitta E, Izutsu K, Sato T, et al. A high incidence of late-onset neutropenia following rituximab-containing chemotherapy as a primary treatment of CD20-positive B-cell lymphoma: A single-institution study. Ann Oncol. 2007;18(2):364–369. doi: 10.1093/annonc/mdl393. [DOI] [PubMed] [Google Scholar]
- 60.Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: A report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113(20):4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haefner MD, Siciliano RD, Widmer LA, et al. Reversible posterior leukoencephalopathy syndrome after treatment of diffuse large B-cell lymphoma. Onkologie. 2007;30(3):138–140. doi: 10.1159/000098706. [DOI] [PubMed] [Google Scholar]
- 62.Vaughn C, Zhang L, Schiff D. Reversible posterior leukoencephalopathy syndrome in cancer. Curr Oncol Rep. 2008;10(1):86–91. doi: 10.1007/s11912-008-0013-z. [DOI] [PubMed] [Google Scholar]
- 63.van der Kolk LE, Baars JW, Prins MH, van Oers MHJ. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. 2002;100(6):2257–2259. [PubMed] [Google Scholar]
- 64.Osterborg A, Kipps T, Mayer J. Ofatumumab (HuMaz-CD20), a novel CD20 monoclonal antibody, is an active treatment for patients with CLL refractory to both fludarabine and alemtuzumab or bulky fludarabine-refractory disease: Results from the planned interim analysis of an international pivotal trial (Abstract 328) Blood. 2008;112 [Google Scholar]
- 65.Hagenbeek A, Gadeberg O, Johnson P, et al. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: Results of a phase 1/2 trial. Blood. 2008;111(12):5486–5495. doi: 10.1182/blood-2007-10-117671. [DOI] [PubMed] [Google Scholar]
- 66.Leonard JP, Coleman M, Ketas JC, et al. Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2003;21(16):3051–3059. doi: 10.1200/JCO.2003.01.082. [DOI] [PubMed] [Google Scholar]
- 67.Leonard JP, Schuster SJ, Emmanouilides C, et al. Durable complete responses from therapy with combined epratuzumab and rituximab: Final results from an international multicenter, phase 2 study in recurrent, indolent, non-Hodgkin lymphoma. Cancer. 2008;113(10):2714–2723. doi: 10.1002/cncr.23890. [DOI] [PubMed] [Google Scholar]
- 68.DiJoseph JF, Dougher MM, Kalyandrug LB, et al. Antitumor efficacy of a combination of CMC-544 (inotuzumab ozogamicin), a CD22-targeted cytotoxic immunoconjugate of calicheamicin, and rituximab against non-Hodgkin’s B-cell lymphoma. Clin Cancer Res. 2006;12(1):242–249. doi: 10.1158/1078-0432.CCR-05-1905. [DOI] [PubMed] [Google Scholar]
- 69.Kreitman RJ, Stetler-Stevenson M, Margulies I, et al. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol. 2009;27(18):2983–2990. doi: 10.1200/JCO.2008.20.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]