Abstract
Background
The mortality after hip fracture has remained high and stable the past 50 years despite improved surgical treatment. The aim of this study was to identify medications and medical factors associated with mortality after hip fracture.
Methods
This is a prospective observational study with median observation time of 21 months. Three hundred and sixty-four patients, mean age 83.4 years and 75.8% women, were enrolled. Information on comorbidity, medications, surgery, and clinical findings were collected at the time of fracture. Information on cause and time of death was obtained from the Norwegian Cause of Death Register.
Results
Six risk factors and one protective factor were identified by Cox proportional hazards model adjusted for propensity score: the use of diuretics (adjusted hazard ratio [HR] = 4.03, 95% confidence interval [CI] = 2.13–7.64), history of coronary heart disease (CHD) (HR = 2.61, CI = 1.37–4.98), male sex (HR = 2.32, CI = 1.27–4.24), Barthel Index ≤ 18/20 (HR = 2.48, CI = 1.23–5.01), heart rate > 100 on admission (HR = 2.47, CI = 1.18–5.14), body mass index ≤ 20 (HR = 1.94, CI = 1.13–3.34), and the use of statins (HR = 0.23, CI = 0.08–0.68). Patients using diuretics had increased risk of death from all causes, including death from CHD, chronic obstructive pulmonary disease, and falls or other accidents.
Conclusions
The use of diuretics is the strongest predictor of mortality, followed by CHD at the time of fracture, whereas the use of statins is associated with improved survival. Future research is needed to evaluate whether improved diagnosis and management of CHD and congestive heart failure among hip fracture patients would improve survival.
Keywords: Mortality, Hip fracture, Diuretics, Coronary heart disease
APPROXIMATELY 1.6 million hip fractures occur every year worldwide, and the incidence of hip fracture is increasing with increasing longevity (1–5). Hip fracture has been associated with increased morbidity and mortality; previous studies have reported that the risk of mortality is highest the first 6 months after the fracture, and after 1 year, the risk is reported to be in the range of 20%–25% (6–9). Despite improved surgical treatment, the mortality rates after hip fracture have remained high and stable over the past 50 years (3).
Patients admitted with a hip fracture are often frail and in need of medical evaluation and management. Knowledge of medical risk factors associated with mortality is necessary for the development of appropriate management strategies. Higher age and male sex are the most common risk factors identified across several studies (10–12). Other risk factors like time to surgery are inconsistently reported, and potentially important risk factors like use of medications, body mass index (BMI), and degree of cognitive impairment have been omitted in most studies (10,11,13,14). One study reported that patients with multiple comorbidities and clinically diagnosed postoperative complications, like chest infections and congestive heart failure (CHF), were at a higher risk of mortality (14). To our knowledge, no previous study has looked into medications as potential risk factors for mortality in this patient group.
Accordingly, the aims of this study were to identify common causes of death and to investigate whether medications and prefracture medical conditions predicted mortality after hip fracture when adjusting for potential confounders.
METHODS
Study Design
This is a prospective observational study of patients admitted to Oslo University Hospital, Ullevaal, and Diakonhjemmet Hospital in Oslo, Norway, from the 5th of September 2005 through 31st of December 2006. The patients were included on admission. Information on time and cause of death, in the period from the 5th of September 2005 through 31st of December 2007, was obtained from the Norwegian Cause of Death Register.
Participants
Patients were eligible for inclusion if they were acutely admitted for a hip fracture, 65 years of age or older, and able to speak Norwegian; had no severe aphasia, head injury, or terminal illness; were admitted for at least 24 hours; and had not been included in the same study for a previous hip fracture. A research team, two researchers and three study nurses, performed daily reviews of the patient registries of the orthopedic departments at Oslo University Hospital, Ullevaal, and Diakonhjemmet Hospital in Oslo to identify patients admitted with hip fracture (femoral neck fracture and intertrochanteric or subtrochanteric fracture). The patient registry was validated against the surgery records as well as the discharge diagnoses.
Cross-sectional data of the same cohort have previously been published (15). Of 575 consecutive patients admitted during the study period, 368 were initially enrolled, whereof 4 withdrew after enrollment. Failure to meet the eligibility criteria was the most common reason for not being enrolled (n = 111); including length of stay <24 hours (n = 85), too ill for approach or death (n = 13), did not speak Norwegian (n = 3), aphasia (n = 3), included earlier (n = 7) (15). In all, 364 patients were included; of whom, 255 signed their own consent form and 109 were enrolled based on presumed consent in combination with assent from next of kin. Mean age was 83.4 years; 276 (75.8%) patients were women. There were no statistically significant differences between patients enrolled (n = 364) and patients not enrolled (n = 211) with respect to the distribution of age (p = .63) or gender (p = .90). The 85 patients who were excluded due to length of stay <24 hours were more likely to be nursing home residents compared with those enrolled.
Measurements and Procedures
Potential risk factors for mortality were selected based on literature review and expert opinion. Risk factors were collected from medical records, anesthesiology records, information from the orthopedic unit staff, patient interview and observation, proxy interview and questionnaires, and supplemented by information from nursing home staff and home helpers when relevant.
Members of the research team performed all cognitive and functional assessments. The team followed standard procedures, had extensive training, and was not involved in the care of the enrolled patients. All patients were assessed daily (weekdays), through the fifth postoperative day or until discharge, for complications and other risk factors. Patients admitted on weekends were assessed by the regular staff and included retrospectively within 48 hours of admission. Biomedical factors included diagnoses on admission, medications on admission, the American Society of Anesthesiologists’ (ASA) score (16), type of fracture, BMI, and complications. ASA score was collected from the anesthesiology records, and type of fracture and operation were collected from the surgery records. Diagnoses and medications on admission were collected from previous medical records, information from patients and their general practitioner, and supplemented by information from relatives, nursing home staff, and home care staff. The body weight was measured using a chair scale on the first or second day postoperatively, and the height was estimated from length of the patients in supine position. Medications were classified according to the Anatomical Therapeutic Chemical classification system. Complications, diagnosed clinically by the regular staff, were broadly defined as any event occurring within 5 days of surgery requiring treatment measures that are not routinely applied postoperatively in hip fracture patients.
Process of care factors included delay to surgery and time in the operation theater. Clinical findings on admission and postoperatively were collected from medical records and included lowest mean arterial pressure (MAP = (systolic blood pressure + (2 × diastolic blood pressure))/3), heart rate, temperature, and cardiac rhythm as assessed by a standard 12-lead electrocardiogram. The regular staff performed the electrocardiograms on admission, and the cardiac rhythm was retrospectively evaluated by one of the researchers. If clinical findings were missing in the hospital records, a member of the research team performed the measurements. Surgical treatment variables, including type of surgery performed, type of anesthesia, and lowest MAP during surgery, were collected from the hospital records. Laboratory studies were measured according to standard hospital procedure.
Prefracture cognitive status was estimated by the Informant Questionnaire on Cognitive Decline in the Elderly Short Form (IQCODE-SF) (17,18), a 16-item instrument developed to acquire proxy information on the patient’s performance in daily tasks that require memory. IQCODE-SF was completed by a caregiver with regular face-to-face contact with the patient at least once in every 14 days. Prefracture functioning in activities of daily living (ADL) was determined by the Barthel Index scored by a close caregiver seeing the patient at least once in every 14 days. A score of 19 or 20 out of 20 was considered indicative of functional independence. For the IQCODE-SF as well as the Barthel Index, we asked the informant to describe the patient’s condition 14 days prior to the fracture in order to ascertain the patient’s function as far as possible in a stable clinical phase and not biased by a potential acute or subacute condition leading to the injury. Of 364 patients, 292 had a close caregiver willing to complete the IQCODE-SF and Barthel Index. We did not replace missing data, and the 72 patients without proxy information were hence excluded in the Cox proportional hazards model. There were no statistically significant differences between patients with missing data (n = 72) and patients not with missing data (n = 292) with respect to the distribution of age (p = .51), gender (p = .43), ASA score (p = .42), place of residence (p = .26), the use of diuretics (p = .27), the use of statins (p = .83), BMI < 20 (p = .37), or heart rate > 100 (p = .75).
Outcomes
The primary outcome was mortality, and the secondary outcome was cause of death. Date and cause of mortality was obtained from the Cause of Death Register. Median follow-up was 21.1 months in patients alive at the end of study (range 12.2–27.8 months).
Bias Reduction: Stratification by Propensity Score Quintiles
In view of the fact that this is an observational study and that patients are not randomly assigned to prescription of drugs, there are differences in observed covariates between the treatment and the control groups. In order to balance the covariates and minimize the risk of biased estimates, we stratified for the patients’ probability or propensity to receive diuretics in the final survival analysis. The propensity score (PS) for an individual is defined as the conditional probability of being treated given the individual’s covariates (19). We estimated the PS for diuretic therapy for each patient by multiple logistic regression analysis in which the receipt of diuretics was modeled using all baseline patient characteristics in Table 1. We also estimated the PS for statin therapy using the same baseline characteristics.
Table 1.
Characteristics of the Patients
| n = 364 | |
| Background characteristics | |
| Age at fracture (y), mean (SD) | 83.4 (6.8) |
| Male sex, n (%) | 88 (24.2) |
| Female sex, n (%) | 276 (75.8) |
| Living in nursing home, n (%) | 56 (15.4) |
| IQCODE*, mean (SD) | 3.74 (0.79)† |
| Cognitively impaired, IQCODE > 3.6, n (%) | 141 (42.6) |
| Barthel Index ≤ 18/20, n (%) | 140 (47.9)‡ |
| Body mass index ≤ 20, n (%) | 89 (27.1)§ |
| ASA group III, IV, or V, n (%) | 182 (50.0) |
| History of | |
| Hypertension, n (%) | 121 (33.2) |
| Congestive heart failure, n (%) | 39 (10.7) |
| Coronary heart disease (previous myocardial infarction, angina pectoris, or other ischemic heart disease), n (%) | 70 (19.2) |
| Previous stroke, n (%) | 64 (17.6) |
| Obstructive pulmonary disease, n (%) | 30 (8.2) |
| Use of | |
| Acetylsalicylic acid, n (%) | 116 (31.9) |
| Beta-blockers, n (%) | 84 (23.1) |
| Diuretics, n (%) | 109 (29.9) |
| Loop diuretics | 86 |
| Others | 23 |
| Statins, n (%) | 52 (14.3) |
| Simvastatin | 37 |
| Atorvastatin | 11 |
| Others | 4 |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor antagonist, n (%) | 91 (25) |
| Preoperative characteristics on admission | |
| Atrial fibrillation on admission, n (%) | 48 (14.0)¶ |
| Mean arterial pressure (mmHg), mean (SD) | 108.2 (17.1) |
| Heart rate > 100 beats per min, n (%) | 41 (11.3) |
| Body temperature >37.5°C, n (%) | 139 (38.2) |
| Time (h) from admission to surgery, mean (SD) | 23.4 (23.7) |
| Perioperative and postoperative characteristics | |
| Time (h) in operation theater, mean (SD) | 2.1 (1.0) |
| Type of operation, n (%) | |
| Hemiarthroplasty | 131 (36.1) |
| Arthroplasty | 4 (1.1) |
| Osteosynthesis | 228 (62.8) |
| Type of anesthesia, n (%) | |
| General | 20 (5.5) |
| Spinal | 337 (92.6) |
| Others | 7 (1.9) |
| Received blood transfusion during the stay, n (%) | 127/362 (64.9) |
Statistical Analyses
Survival was estimated by Kaplan–Meier plots and compared between groups by the log rank test. Cox proportional hazards model was used to assess the simultaneous effect of variables identified as risk factors (p < .05 in separate log rank tests), stratifying for PS quintiles for diuretic therapy. The assumption of proportionality was checked by visual inspection of log hazard plots.
If two variables demonstrated a correlation higher than 0.6, the one with weakest association with the outcome was omitted. Variable selection was done both by forward selection and by backward elimination, and the different models were compared with respect to p values of the different variables as well as the difference in log likelihood between models. For multilevel explanatory variables, an approximately linear relationship between the score levels and effect was assessed, and adjacent categories were collapsed as appropriate. Variables significantly associated with mortality in Cox regression survival analysis were finally checked for interactions (one at a time). Multicollinearity was checked by exploring the variance inflation factor. Any relationship between potential risk factors and use of diuretics was analyzed by the chi-square test.
To assess the robustness of our findings regarding the effects of diuretics and statins on mortality, we conducted sensitivity analyses adjusting for the probability of using diuretics and statins, respectively, using both the PS raw score and the stratification for PS quintiles in the model. Statistical calculations were performed using SPSS for windows, version 15 (SPSS, Inc., Chicago, IL).
Ethical Considerations
The study was undertaken in accordance with the Declaration of Helsinki and was approved by the Regional Committee for Ethics in Medical Research, the Data Inspectorate, as well as the Directorate of Health.
RESULTS
The univariate analyses were conducted with a total of 364 patients, and characteristics of the enrolled patients are shown in Table 1. The Cox model was conducted with a total of 292 patients. Ten of 364 patients (2.7%) died during the first month of follow-up, 16.2% (n = 59) had died at 6 months, 22.5% (n = 82) had died at 1 year, and 28.6% (n = 99) had died at the end of follow-up.
Of 36 potential risk factors with prevalence more than 5%, 21 variables were significantly associated with mortality in univariate analysis. The unadjusted hazard ratios (HRs) of the variables are shown in Table 2. Seven statistically significant factors increasing the risk of death after hip fracture were identified using the Cox model: use of diuretics, history of coronary heart disease (CHD) (previous myocardial infarction, angina pectoris, or other ischemic heart disease), prefracture functional impairment, prefracture cognitive decline, male sex, low BMI, and tachycardia (heart rate > 100) on admission. Both loop diuretics and a variable containing other diuretics were identified as risk factors for mortality using the Cox model, and the variables were therefore collapsed in the final model. Use of statins demonstrated a statistically significant positive association with survival. These eight factors remained statistically significant when adjusting for comorbidity diagnosed before the fracture like heart failure, hypertension, previous stroke, chronic obstructive pulmonary disease (COPD), age, the ASA score, nursing home residence, preoperative use of medications, laboratory studies on admission, clinical findings on admission, type of fracture, type of surgery, time to surgery, time in operation theater, and postoperative complications (CHF, chest infection, and urinary tract infections). The adjusted HR for each variable is shown in Table 3. Forward selection and backward elimination led to the same variables being included in the model. No statistically significant interactions were observed.
Table 2.
Univariate Analysis of All Variables and Risk of Mortality
| Unadjusted Hazard Ratio | p Value | |
| Background characteristics | ||
| Age at fracture (y) | 14.2 | <.001 |
| Male sex | 6.2 | .013 |
| Living in nursing home | 37.5 | <.001 |
| IQCODE score* | 38.8 | <.001 |
| Barthel Index ≤ 18/20 | 36.8 | <.001 |
| Body mass index ≤ 20 | 6.6 | .010 |
| ASA† group III, IV, or V | 34.4 | <.001 |
| History of | ||
| Hypertension | 3.9 | .049 |
| Congestive heart failure | 11.2 | .001 |
| Coronary heart disease (previous myocardial infarction, angina pectoris, or other ischemic heart disease) | 9.7 | .002 |
| Previous stroke | 2.3 | .127 |
| Obstructive pulmonary disease | 6.0 | .015 |
| Use of | ||
| Acetylsalicylic acid | 1.3 | .247 |
| Beta-blockers | 0.4 | .538 |
| Diuretics | 31.2 | <.001 |
| Statins | 0.5 | .045 |
| Angiotensin-converting enzyme inhibitor or angiotensin antagonist | 0.7 | .414 |
| Preoperative risk factors on admission | ||
| C-reactive protein > 10 mg/L | 6.7 | .010 |
| Anemia, hemoglobin <13.4 in male and <11.7 in female | 8.0 | .005 |
| White blood cells > 10 × 109/L | 0.246 | .620 |
| Serum Sodium <137 or >145 mmol/L | 1.8 | .178 |
| Serum Potassium <3.6 or >5.0 mmol/L | 0.1 | .820 |
| Serum Albumin <35 g/L | 6.8 | .009 |
| Serum Urea >8 mg/dL | 5.3 | .022 |
| Urea/creatinine ratio | 7.6 | .006 |
| Atrial fibrillation on admission | 4.1 | .042 |
| Mean arterial pressure (mmHg) | 0.05 | .822 |
| Heart rate > 100 beats per min | 4.5 | .033 |
| Body temperature >37.5°C | 1.7 | .193 |
| Time (h) from admission to surgery | 0.3 | .866 |
| Perioperative and postoperative risk factors | ||
| Time (h) in operation theater | 1.5 | .224 |
| Type of operation—hemiarthroplasty | 0.0 | .993 |
| Type of anesthesia | 3.0 | .082 |
| Lowest mean arterial pressure perioperatively (mmHg) | 1.9 | .166 |
| Received blood transfusion during the stay | 2.9 | .087 |
Table 3.
Final Cox Proportional Hazards Model
| Adjusted HR* | 95% CI | p Value | Adjusted HR† | 95% CI | p Value | |
| Use of diuretics | 3.35 | 2.06–5.47 | <.001 | 4.03 | 2.13–7.64 | <.001 |
| History of coronary heart disease (previous myocardial infarction, angina pectoris, or other ischemic heart disease) | 2.41 | 1.36–4.24 | .002 | 2.61 | 1.37–4.98 | .004 |
| Barthel Index ≤ 18/20 before fracture | 2.65 | 1.37–5.12 | .004 | 2.48 | 1.23–5.01 | .011 |
| Use of statins | 0.22 | 0.08–0.64 | .006 | 0.23 | 0.08–0.68 | .008 |
| Male sex | 1.98 | 1.15–3.42 | .014 | 1.53 | 1.27–4.24 | .006 |
| IQCODE‡ score | 1.53 | 1.04–2.24 | .029 | 1.44 | 0.95–2.20 | .086 |
| Heart rate > 100 beats per min at admittance | 2.13 | 1.05–4.31 | .035 | 2.47 | 1.18–5.14 | .016 |
| Body mass index ≤ 20 | 1.68 | 1.01–2.81 | .048 | 1.94 | 1.13–3.34 | .017 |
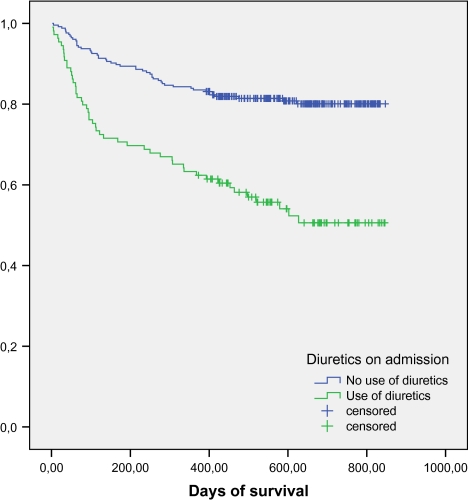
The proportion of deaths among patients using diuretics was significantly higher than those among patients not using diuretics: 29.4% (32/109) versus 10.6% (27/255) at 6 months, 36.7% (40/109) versus 16.5% (42/255) at 1 year, and 49.4% versus 19.9% at 2 years. The survival of patients using and those not using diuretics is shown in Figure 1. Among patients using diuretics on admission, 47.7% used diuretics alone; 38.6% used a beta-blocker, an angiotensin-converting enzyme inhibitor (ACEI), or an angiotensin II receptor blocker (ARB), and 16.5% used both a beta-blocker and an ACEI or ARB. 19.3% used a statin, and 37.6% used acetylsalicylic acid (aspirin). Patients using diuretics were more likely to suffer from derangements in potassium, urea, and urea/creatinine ratio on admission than those not using diuretics (Table 4).
Figure 1.
Comparison of survival patterns in patients using diuretics and those not using diuretics.
Table 4.
Univariate Analysis of the Relationship Between Patient Characteristics and the Use of Diuretics
| Use diuretics, n = 109 (29.9%) | No diuretics, n = 255 (70.1%) | p Value | |
| Cognitive impairment*, n (%) | 51† (53.7) | 90‡ (38.1) | .010 |
| Barthel Index ≤ 18/20, n (%) | 52§ (57.8) | 88 (43.6) | .025 |
| Age, mean (SD) | 85.0 (6.58) | 83.0 (6.85) | .016 |
| Male sex, n (%) | 21 (19.3) | 67 (26.3) | .153 |
| Body mass index ≤ 20, n (%) | 64 (27.6) | 25 (25.8) | .736 |
| Diagnosis on admission, n (%) | |||
| Coronary heart disease¶ | 35 (32.1) | 35 (13.7) | <.001 |
| Congestive heart failure | 34 (31.2) | 21 (8.2) | <.001 |
| Hypertension | 48 (44.0) | 73 (28.6) | .004 |
| Atrial fibrillation on admission, n (%) | 19# (18.3) | 29**(12.2) | .124 |
| Medications on admission, n (%) | |||
| Acetylsalicylic acid | 41 (37.6) | 75 (29.4) | .124 |
| Beta-blockers | 40 (36.7) | 44 (17.3) | <.001 |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor antagonist | 35 (32.1) | 56 (22.0) | .041 |
| Statins | 21 (19.3) | 31 (12.2) | .076 |
| ASA†† group III, IV, or V, n (%) | 73 (67.0) | 108 (42.5) | <.001 |
| Laboratory findings on admission, n (%) | |||
| Serum Sodium <137 or >145 mmol/L | 23 (21.1) | 59 (23.1) | .670 |
| Serum Potassium <3.6 or >5.0 mmol/L | 20 (18.3) | 19 (7.5) | .002 |
| Serum Urea >8 mg/dL | 69 (63.3) | 94 (36.9) | <.001 |
Patients using statins had increased survival compared with those not using statins. They were younger, more likely to be living in their own home, and more likely to suffer from established CHD than those not using statins. There were no differences in ASA score, dependency in ADL, or degree of cognitive impairment among patients using and those not using statins. 41.4% of the patients with established CHD used a statin. Use of statins remained statistically significantly associated with survival when adjusting for potential confounders.
Among patients with established CHD (n = 70), 58.6% (n = 41) received treatment with antiplatelet therapy, 44.3% (n = 31) received beta-blockers, 35.7% (n = 25) received ACEI or ARB, 41.4% (n = 29) received statins, and 50.0% (n = 35) were treated with diuretics.
The most common causes of death after hip fracture were cardiovascular diseases (17.2%), fall or accident (15.2%), COPD (13.1%), malignancy (12.1%), dementia (11.1%), and the hip fracture itself (11.1%). Patients using diuretics were more likely to die from COPD, CHD, and injury caused by falls or accidents than those not using diuretics (Table 5).
Table 5.
Causes of Death in Patients Using Diuretics and in Those Not Using Diuretics
| Causes of Death | Patients Using Diuretics, n = 50 | Patients Not Using Diuretics, n = 49 |
| Malignancy, n (%) | 4 (8.0) | 8 (16.3) |
| Dementia, n (%) | 5 (10.0) | 6 (12.2) |
| Cardiovascular disease, n (%) | 10 (20.0) | 7 (14.3) |
| Stroke, n (%) | 2 (4.0) | 4 (8.0) |
| Obstructive pulmonary disease, n (%) | 9 (18.0) | 4 (8.2) |
| Infections, n (%) | 4 (7.0) | 5 (10.2) |
| Fall or accident, n (%) | 9 (18.0) | 6 (12.2) |
| Others, n (%) | 4 (7.0) | 4 (8.0) |
| Death related to hip fracture, n (%) | 5 (10.0) | 6 (12.2) |
When stratifying for PS quintiles for diuretic therapy in the final Cox model, all factors except IQCODE score remained statistically significantly associated with mortality. The adjusted HR for each variable, both with and without adjusting for PS quintiles, is shown in Table 3. Sensitivity analyses, using both the PS raw score and the stratification on quintiles for diuretic therapy and statin therapy, respectively, were performed. The estimated effect of diuretics on mortality became stronger when adjusting for PS (unadjusted HR = 3.35, confidence interval [CI] = 1.9–5.6) versus adjusted for PS raw score (adjusted HR = 4.2, CI = 2.2–7.8) and stratifying for PS quintiles (HR = 4.0, CI = 2.13–7.64). The protective effect of statins became only slightly weaker when adjusting for the probability of using statins (HR = 0.22, CI = 0.08–0.64) versus adjusted for PS raw score (HR = 0.28, CI = 0.09–0.87) and stratifying for PS quintiles (HR = 0.25, CI = 0.08–0.79).
DISCUSSION
We have carried out a prospective study evaluating the association between mortality after hip fracture and medical conditions as well as medications. The proportion dying within 6 months was 16.2%; 22.5% were dead after 1 year and 28.6% were dead after 2 years of follow-up, and this accords well with previous studies on mortality after hip fracture (8–10).
Use of diuretics and presence of CHD on admission were the two most salient predictors of mortality, also when adjusting for potential confounders like CHF and hypertension and balancing for differences in covariates between the diuretic and the no-diuretic groups. Neither beta-blockers nor ACEI, ARB, or acetylsalicylic acid (aspirin) were associated with increased risk of mortality, indicating that the diuretics alone may predict mortality in this cohort of elderly, frail hip fracture patients. Assuming no hidden bias, the survival analysis provides strong evidence (HR = 4.0, p < .001) that treatment with diuretics decreases survival time. It is considered unlikely that any set of unknown confounders would be so strongly associated with both mortality and use of diuretics that they could completely outweigh this effect. Diuretics used by those without hypertension or CHF have previously been identified as a predictor of 5-year mortality in community-dwelling elderly African Americans (20), and a large observational study using PS methods reported chronic diuretic use to be associated with increased long-term mortality in patients with chronic heart failure (21). No randomized long-term study of the effect of diuretics on mortality in CHF patients has ever been conducted (22). Moreover, the use of loop diuretics increases urinary calcium secretion and has been associated with increased risk of hip fracture, increased rate of hipbone loss, and increased risk of implant failure after primary total hip arthroplasty (23–25). The medications have not previously been assessed as risk factors for mortality in hip fracture patients, and the finding needs to be evaluated by others.
29.9% of the patients (n = 109) used diuretics on admission, whereas 10.7% (n = 39) suffered from CHF. Only 31.2% of the patients using diuretics had an established diagnosis of CHF on admission, and 47.7% used diuretics as monotherapy. Patients using diuretics were more likely to suffer from derangement in potassium, increased level of urea, and increased urea/creatinine ratio, and they had an increased risk of death caused by falls or accidents. Moreover, the risk of death from COPD in patients using diuretics was almost equal to that from CHD. Diuretics cause dehydration, and theoretically, they may also enhance a potential hypokalemia caused by the beta2-agonists used in the management of COPD. The increased risk of death from COPD in patients using diuretics does also illustrate the difficulties in diagnosing the cause of dyspnea in old frail patients. Our findings are consistent with the hypothesis that drugs used for treatment of chronic pulmonary diseases, CHD, and CHF may be associated with fatal adverse drug events (26,27).
Use of statins was associated with improved survival, indicating a positive effect of the drug and hence supporting the hypothesis that statins are effective drugs in preventing mortality in elderly cardiovascular patients (28) as well as in surgical patients (29). In addition to their effects on lipids, statins have beneficial effects usually described under the term pleiotropic. The pleiotropic effects include anti-inflammatory properties, which may play a role in prevention of cardiovascular events as well as in modifying the inflammatory responses caused by the surgery and the hip fracture itself (29). We identified no differences regarding ASA score, cognitive impairment, or dependency in ADL in patients using and those not using statins. Statins as secondary prevention after myocardial infarction are reported to be underused in patients older than 80 years (30), which is consistent with our finding that patients using statins were younger than those not using statins. The effect of statins remained statistically significant also when adjusting for potential confounding factors like age, sex, and comorbidity and when stratifying for the probability of being treated with statins. We suggest therefore that the effect of statins on outcome after hip fracture needs to be further explored.
Cardiovascular diseases have previously been shown to be the most common comorbid condition in hip fracture patients (14). Our study identifies CHD as an independent risk factor of mortality and indicates that management of CHD and CHF in hip fracture patients does not accord with current guidelines (22,31,32). According to the guidelines of the American Cardiology Association, most patients with CHF should be treated with a combination of three types of drug: a diuretic, an ACEI or ARB, and a beta-blocker (22). Diuretics alone are unable to maintain the clinical stability over a long period of time and should not be used alone as treatment of CHF. Secondary prevention in patients with established CHD includes treatment with beta-blockers, ACEI or ARB, antiplatelet therapy, and lipid management (31,32). Beta-blockers and ACEI have been shown to lessen the symptoms and improve survival in patients with CHF and CHD. Improving the diagnosis and management of CHD and CHF might therefore have an impact on survival in hip fracture patients.
A BMI of 20 or less increases the risk of death, as stated in previous studies (33), including a recently published study identifying underweight patients to be at increased risk of developing an adverse cardiac event after hip fracture (34). Correspondingly, nutritional intervention has been reported to reduce postoperative complications and mortality after 4 months (35,36). Low body weight is associated with mortality also when adjusting for comorbidity (ASA score) and might be an indicator of physical frailty (frailty syndrome). Whether active measures to increase the body weight in frail elderly patients will improve their physical condition and hence improve survival in hip fracture patients remains to be investigated. Nutrition in the management of hip fracture patients constitutes an interesting area for further research. None of the surgery-related risk factors were associated with mortality in multivariable analysis.
The strengths of our study are the prospective design, the use of an official registry to assess date of death, the fact that all the patients were admitted acutely, the extent of risk factors evaluated, and the use of standardized validated diagnostic instruments for assessment of prefracture cognitive impairment and impairment of activities of daily living. The main limitations are the observational design, potentially causing limitations by biases related to unmeasured or hidden covariates, the relatively small sample size, the lack of evaluation of cardiac function (echocardiography), and the fact that 76 potentially eligible patients refused to participate in the study, thus possibly causing selection bias. Moreover, 72 patients did not have close relatives and were hence excluded from the Cox proportional hazards model due to missing data. The patients and their relatives were asked on admission to participate in a study including extensive cognitive testing. At this time, a majority of the patients were in pain and/or had received opioids. Frail patients and patients fearing the test results might have been more likely to decline enrollment.
In conclusion, use of diuretics, history of CHD, low BMI, functional impairment, tachycardia on admission, and male sex were found to be risk factors for death during the first 2 years after a hip fracture. Use of statins, on the other hand, was associated with increased survival. Our findings need to be confirmed by others, and improved diagnosis and management of CHD and CHF in elderly hip fracture patients might constitute avenues for future research. It remains to be investigated whether improving medical treatment with focus on CHD and congestive heart failure will improve survival in hip fracture patients.
FUNDING
The study was funded by South-Eastern Norway Regional Health Authority.
CONFLICT OF INTEREST
The financial disclosures of the authors are as follows: V.J. has given one lecture on delirium sponsored by Lundbeck. T.B.W. has given lectures on delirium and other geriatric issues sponsored by Roche, Pfizer, and Lundbeck. M.K., E.S., and K.E. have no conflict of interest.
The author contributions are as follows:
V.J. has the responsibility for content, conception and design, practical procedures, acquisition of data, supervision, analysis and interpretation of data, and drafting of the manuscript. M.K. and E.S. have the responsibility for content, conception and design, and analysis and interpretation of data. K.E. has the responsibility for content, conception and design, analysis and interpretation of data, and drafting of the manuscript. T.B.W. has the responsibility for content, conception and design, practical procedures, supervision, analysis and interpretation of data, and drafting of the manuscript. All authors have read and approved the final manuscript.
Acknowledgments
The authors would like to thank all the patients and the staff at the orthopedic departments at Oslo University Hospital, Ullevaal, and Diakonhjemmet Hospital. The authors would also like to thank Karen Bjøro, Anette Hylen Ranhoff, and Anne Garmark for cooperation and the study nurses Jorunn Scott, Elin Engh, and Ingrid Holmgren for data collection. The principal investigators designed this study. V.J. had access to all the data and takes full responsibility for the accuracy of data analysis.
References
- 1.Frihagen F, Nordsletten L, Madsen JE. Hemiarthroplasty or internal fixation for intracapsular displaced femoral neck fractures: randomised controlled trial. BMJ. 2007;335:1251–1254. doi: 10.1136/bmj.39399.456551.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lofthus CM, Osnes EK, Falch JA, et al. Epidemiology of hip fractures in Oslo, Norway. Bone. 2001;29:413–418. doi: 10.1016/s8756-3282(01)00603-2. [DOI] [PubMed] [Google Scholar]
- 3.Haleem S, Lutchman L, Mayahi R, et al. Mortality following hip fracture: trends and geographical variations over the last 40 years. Injury. 2008;39:1157–1163. doi: 10.1016/j.injury.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Giversen IM. Time trends of mortality after first hip fractures. Osteoporos Int. 2007;18:721–732. doi: 10.1007/s00198-006-0300-1. [DOI] [PubMed] [Google Scholar]
- 5.Schroder HM, Andreassen MD, Villadsen I, et al. Increasing age-specific incidence of hip fractures in a Danish municipality. Dan Med Bull. 1995;42:109–111. [PubMed] [Google Scholar]
- 6.Osnes EK, Lofthus CM, Meyer HE, et al. Consequences of hip fracture on activities of daily life and residential needs. Osteoporos Int. 2004;15:567–574. doi: 10.1007/s00198-003-1583-0. [DOI] [PubMed] [Google Scholar]
- 7.Robbins JA, Biggs ML, Cauley J. Adjusted mortality after hip fracture: from the cardiovascular health study. J Am Geriatr Soc. 2006;54:1885–1891. doi: 10.1111/j.1532-5415.2006.00985.x. [DOI] [PubMed] [Google Scholar]
- 8.Forsen L, Sogaard AJ, Meyer HE, et al. Survival after hip fracture: short- and long-term excess mortality according to age and gender. Osteoporos Int. 1999;10:73–78. doi: 10.1007/s001980050197. [DOI] [PubMed] [Google Scholar]
- 9.Center JR, Nguyen TV, Schneider D, et al. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–882. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 10.Schroder HM, Erlandsen M. Age and sex as determinants of mortality after hip fracture: 3,895 patients followed for 2.5-18.5 years. J Orthop Trauma. 1993;7:525–531. doi: 10.1097/00005131-199312000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kenzora JE, McCarthy RE, Lowell JD, et al. Hip fracture mortality. Relation to age, treatment, preoperative illness, time of surgery, and complications. Clin Orthop Relat Res. 1984;186:45–56. [PubMed] [Google Scholar]
- 12.Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307:1248–1250. doi: 10.1136/bmj.307.6914.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tosteson AN, Gottlieb DJ, Radley DC, et al. Excess mortality following hip fracture: the role of underlying health status. Osteoporos Int. 2007;18:1463–1472. doi: 10.1007/s00198-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roche JJ, Wenn RT, Sahota O, et al. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ. 2005;331:1374. doi: 10.1136/bmj.38643.663843.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juliebø V, Bjøro K, Krogseth M, Skovlund E, Ranhoff AH, Wyller TB. Risk factors for preoperative and postoperative delirium in elderly hip fracture patients. J Am Geriatr Soc. 2009;57:1354–1361. doi: 10.1111/j.1532-5415.2009.02377.x. [DOI] [PubMed] [Google Scholar]
- 16.American Society of Anesthesiologists. New classification of physical status. Anesthesiology. 1963;24:111. [Google Scholar]
- 17.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 18.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed A, Young JB, Love TE, Levesque R, Pitt B. A propensity-matched study of the effects of chronic diuretic therapy on mortality and hospitalization in older adults with heart failure. Int J Cardiol. 2008;125:246–253. doi: 10.1016/j.ijcard.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 21.D’Agostino RB., Jr Propensity scores in cardiovascular research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 22.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 23.Carbone LD, Johnson KC, Bush AJ, et al. Loop diuretic use and fracture in postmenopausal women: findings from the Women’s Health Initiative. Arch Intern Med. 2009;169:132–140. doi: 10.1001/archinternmed.2008.526. [DOI] [PubMed] [Google Scholar]
- 24.Rejnmark L, Vestergaard P, Mosekilde L. Fracture risk in patients treated with loop diuretics. J Intern Med. 2006;259:117–124. doi: 10.1111/j.1365-2796.2005.01585.x. [DOI] [PubMed] [Google Scholar]
- 25.Thillemann TM, Pedersen AB, Mehnert F, et al. Use of diuretics and risk of implant failure after primary total hip arthroplasty: a nationwide population-based study. Bone. 2009;45:499–504. doi: 10.1016/j.bone.2009.04.247. [DOI] [PubMed] [Google Scholar]
- 26.Ebbesen J, Buajordet I, Erikssen J, et al. Drug-related deaths in a department of internal medicine. Arch Intern Med. 2001;161:2317–2323. doi: 10.1001/archinte.161.19.2317. [DOI] [PubMed] [Google Scholar]
- 27.Buajordet I, Ebbesen J, Erikssen J, et al. Fatal adverse drug events: the paradox of drug treatment. J Intern Med. 2001;250:327–341. doi: 10.1046/j.1365-2796.2001.00892.x. [DOI] [PubMed] [Google Scholar]
- 28.Strandberg TE, Pitkala KH, Tilvis RS. Statin treatment is associated with clearly reduced mortality risk of cardiovascular patients aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2008;63:213–214. doi: 10.1093/gerona/63.2.213. [DOI] [PubMed] [Google Scholar]
- 29.Howard-Alpe G, Foex P, Biccard B. Cardiovascular protection by anti-inflammatory statin therapy. Best Pract Res Clin Anaesthesiol. 2008;22:111–133. doi: 10.1016/j.bpa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Kvan E, Pettersen KI, Landmark K, et al. Treatment with statins after acute myocardial infarction in patients >or=80 years: underuse despite general acceptance of drug therapy for secondary prevention. Pharmacoepidemiol Drug Saf. 2006;15:261–267. doi: 10.1002/pds.1172. [DOI] [PubMed] [Google Scholar]
- 31.Antman EM, Hand M, Armstrong PW, et al. 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 32.Fraker TD, Jr, Fihn SD Chronic Stable Angina Writing Committee. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 2007;50:2264–2274. doi: 10.1016/j.jacc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Meyer HE, Tverdal A, Falch JA. Body height, body mass index, and fatal hip fractures: 16 years’ follow-up of 674,000 Norwegian women and men. Epidemiology. 1995;6:299–305. [PubMed] [Google Scholar]
- 34.Batsis JA, Huddleston JM, Melton LJ, et al. Body mass index and risk of adverse cardiac events in elderly patients with hip fracture: a population-based study. J Am Geriatr Soc. 2009;57:419–426. doi: 10.1111/j.1532-5415.2008.02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan DG, Beck SJ, Hood K, Johansen A. Using dietetic assistants to improve the outcome of hip fracture: a randomised controlled trial of nutritional support in an acute trauma ward. Age Ageing. 2006;35:148–153. doi: 10.1093/ageing/afj011. [DOI] [PubMed] [Google Scholar]
- 36.Olofsson B, Stenvall M, Lundstrom M, et al. Malnutrition in hip fracture patients: an intervention study. J Clin Nurs. 2007;16:2027–2038. doi: 10.1111/j.1365-2702.2006.01864.x. [DOI] [PubMed] [Google Scholar]