Abstract
This investigation examined Akt–FOXO3A signaling in young women (YW) and old women (OW) before and after 12 weeks of high-intensity resistance training. Muscle biopsies were taken from the vastus lateralis before and immediately after resistance exercise (RE) in the untrained and trained states. In response to RE in YW and OW, phospho Akt Thr308 increased in untrained and trained states, with no change on Ser473 site. FOXO3A–Ser253 site was dephosphorylated in untrained state among YW and OW, and nuclear phospho-FOXO3A increased mainly in YW in trained state. In the basal state, OW displayed lower cytosolic phospho-FOXO3A before training, higher total nuclear FOXO3A, and a trend for higher nuclear-to-cytosolic FOXO3A ratio versus YW after 12 weeks. Basal level MuRF-1 and myostatin mRNA decreased in YW, while OW increased myostatin mRNA after 12-weeks. These data suggest that FOXO3A signaling and FOXO3A-related target gene expression are altered in OW and may partially explain the attenuated training adaptations previously reported in these octogenarian women.
Keywords: Octogenarian, Plasticity, Forkhead, Proteolytic
AGING has deleterious effects upon skeletal muscle mass. As a result, both men and women more than the age of 65 years have a loss of muscle function, which contributes to a reduction in their activities of daily living, increased risk of injury from falling, and extended rehabilitation time (1,2). Understanding the mechanisms contributing to muscle wasting is important in an aging society (≥65 years), which is projected to nearly double by the year 2030 (3). Resistance exercise (RE) training has been used extensively by our laboratory and others (4–11) to attenuate age-related muscle atrophy, resulting in muscle size and strength improvements. Recently, we reported that skeletal muscle from independent-living octogenarian men and women (12,13) has limited capacity to adapt to a 12-week progressive resistance training (PRT) program, which is a cohort that demonstrates accelerated losses in skeletal muscle strength (14,15). Moreover, women who were aged 80 years or older represent approximately 70% of the population in this age group (16).
There are many factors that underpin age-related skeletal muscle loss. One area that has received considerable interest, as it relates to skeletal muscle mass and proteolysis, is the forkhead box O (FOXO) proteins. FOXOs are transcription factors within the forkhead family, which have a conserved DNA-binding domain, the “forkhead box” (17–19). Previous findings (20–22) show that FOXO3 (also known as FKHRL1) is associated with the ubiquitin–proteasome pathway and intracellular protein degradation by regulating the expression of the ubiquitin E3 ligase, atrogin-1, in models of muscle atrophy. FOXO proteins translocate in and out of the nucleus, depending on the prevailing stimulus, and when in the nucleus, FOXOs can bind to target DNA. However, addition of growth stimuli, such as insulin and insulin-like growth factor (IGF), results in the phosphorylation and translocation of FOXO outside the nucleus (19,23–25). This movement to the cytoplasm inhibits FOXOs ability to upregulate the proteolytic-related gene targets, inactivating FOXOs effects. The predominant pathway augmented by insulin and IGF is the Akt pathway (26–30). Upon activation, Akt moves into the nucleus and directly phosphorylates FOXO3A on the N-terminus at Ser253, causing the translocation of FOXO from the nucleus to the cytoplasm (19,31–33).
Numerous human studies (34–40), employing various models, training and nutritional states, and ages, have produced equivocal results as to the role of Akt signaling and exercise. However, few studies have examined FOXOs role in exercise models (37,41,42), and far less is known about FOXOs involvement in age-related sarcopenia (38,41,43). Understanding the mechanisms controlling skeletal muscle adaptation to high-intensity RE training would give insight to the age-related differences we have reported previously (6,12,13,44–46). Thus, these findings may provide possible targets for therapy and intervention during sarcopenia-related circumstances.
The objective of this investigation was to investigate the activation of the Akt–FOXO3A signaling pathway before and after a 12-week high-intensity PRT program in young (24 years) and very old (85 years) women. We recently published the single-muscle fiber and whole-muscle adaptations that occurred with the 12-week high-intensity PRT program in these young and old women (13). The young women increased whole-muscle strength (+36%) and size (+5%) and increased single-muscle fiber size (+28%), strength (+31%), and power (+28%) that were targeted to the fast-twitch muscle fibers. Conversely, the old women had a modest gain in muscle strength (+26%), with no hypertrophy or improvements in single-muscle fiber size and contractile function. The changes observed in the old women were in close agreement with an age-matched male population (12). We therefore hypothesized that 85-year-old women would have diminished resting and exercise-induced capacity to positively alter Akt signaling to FOXO3A and FOXO3A target genes, compared with young women, before and after high-intensity PRT.
EXPERIMENTAL PROCEDURES
Participants
Six nonexercising, healthy young women (YW; 24 ± 2 years, 67 ± 6 kg) and six old women (OW; 85 ± 1 years, 67 ± 3 kg) were recruited from the local community for this investigation. The older women underwent a physical examination, which included medical history, blood and urine chemistries, resting and exercising electrocardiogram, and blood pressure before participating in any resistance training. Participants were excluded if they had any acute or chronic illness; cardiac, pulmonary, liver, or kidney abnormalities; uncontrolled hypertension; insulin- or noninsulin-dependent diabetes; abnormal blood or urine chemistries; arthritis; a history of neuromuscular problems; or if they smoked tobacco. Additionally, these free-living women were not consuming any chronic medication (prescribed or over the counter). All participants were given oral and written information about the experimental procedures and potential risks before giving written consent. All procedures conformed to the standards set forth by the Declaration of Helsinki, and these procedures were approved by the Institutional Review Boards of Ball State University and Ball Memorial Hospital.
Experimental Design
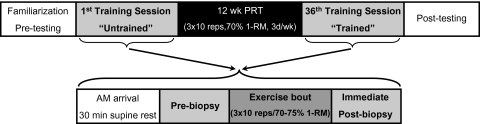
After an initial familiarization period, the women completed 12 weeks of high-intensity PRT for the knee extensors, with three training sessions per week for a total of 36 sessions. Each training session consisted of three sets of 10 repetitions at 70%–75% of one-repetition maximum (1-RM). Whole-thigh muscle size and strength (1-RM) were assessed before and after the training period (13). Skeletal muscle biopsies were obtained from the vastus lateralis before and immediately after an acute bout of RE and prior to (untrained) and following (trained) the PRT (see Figure 1).
Figure 1.
Study design schematic. 1-RM; one-repetition maximum; PRT = progressive resistance training.
PRT Program
Participants performed 12 weeks of a supervised PRT program designed to strengthen the quadriceps muscle group. Participants performed bilateral isotonic leg extensions on a seated device (Cybex Eagle, Medway, MA) 3 days per week on nonconsecutive days (36 total sessions). Participants performed three sets of 10 repetitions (3 × 10) at 70%–75% of their 1-RM with 2-minute rest between sets. The training was progressive in nature in that 1-RM was assessed every 2 weeks, and the weight was adjusted accordingly to maintain an intensity of 70%–75%. Each session was preceded by 10 minutes of warm-up (50–75 W) on a cycle ergometer (Monark 828E, Vansbro, Sweden). This entire protocol is identical to several previous investigations from our laboratory (6,12,13,44,46). All women completed every training session (n = 36) for 100% compliance to the training program.
Muscle Biopsy
Muscle biopsies (47) were obtained from the vastus lateralis under fasted conditions. Each muscle sample was processed, frozen in liquid nitrogen or placed in 0.5 mL of RNAlater (Ambion, Austin, TX), and stored at −20°C until RNA extraction.
Cytoplasmic and Nuclear Fractionation
Adapted from Rothermel and colleagues (48), the samples were homogenized in 10 volumes of 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS)-containing buffer (40 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [pH 7.5], 20 nM NaCl, 1 mM ethylenediaminetetraacetic acid, 10 mM pyrophosphate, 10 mM β-glycerolphosphate, 40 mM NaF, 1.5 mM sodium vanadate, 0.3% CHAPS, 0.1 mM phenylmethanesulphonylfluoride, 1 mM benzamidine, and 1 mM dithiothreitol and protease and phosphatase inhibitor mixture [Pierce, Rockford, IL]). The resulting homogenate was clarified by a 1,000g centrifugation for 3 minutes (at 4°C). The supernatant contained the cytoplasmic fraction. The pellet was washed with CHAPS buffer three times, followed by a 1,000g centrifugation for 3 minutes (at 4°C), then resuspended in 50 μL of lysis buffer, and 8.3 μL of 5 M NaCl was added to lyse the nuclei. This mixture was rotated at 4°C for 1 hour and then centrifuged at 12,578g for 15 minutes at 4°C. The supernatant contained the soluble nuclear fraction. An equal volume of 2× sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer was added to each fraction for Western analysis.
Western Analysis
Equal protein, as determined by protein concentration determination against bovine serum albumin standards, from samples was resolved by SDS-PAGE (BioRad, Hercules, CA). The proteins were transferred to polyvinylidene fluoride membranes, then incubated with primary antibodies against phospho Akt Thr308 and Ser473 and phospho-FOXO3A Ser253, followed by incubation in the appropriate horseradish peroxidase-conjugated secondary antibody. Protein immunoblots were visualized via enhanced chemiluminescence and captured by a camera-integrated software system and then quantified (Alpha Innotech Imaging System, Santa Clara, CA). The blots were stripped and reprobed with antibodies that recognize total forms of proteins examined. Purity of isolated fractions was verified by Western analysis for Histone H3, as a nuclear indicator, and β-tubulin, as a cytosolic indicator. All antibodies were purchased from Cell Signaling Technology (Beverly, MA).
Total RNA Extraction and RNA Quality Check
All the methods for RNA extraction and real-time reverse transcription–polymerase chain reaction (RT-PCR) have been described in detail previously by our laboratory (41,49). Total RNA was extracted in TRI reagent (Molecular Research Center, Cincinnati, OH). The quality and integrity of extracted total RNA were evaluated using an RNA 6000 Nano LabChip kit on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA).
RT and Real-Time PCR
Oligo (dT)–primed first-strand complementary DNA was synthesized using SuperScript II RT (Invitrogen, Carlsbad, CA) optimized for sensitive RT-PCR on low amounts of RNA. Quantification of messenger RNA (mRNA) levels (in duplicate) was performed in a 72-well Rotor-Gene 3000 Centrifugal Real-Time Cycler (Corbett Research, Mortlake, NSW, Australia). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene (HKG) for internal control after validation that GAPDH is not affected by age or exercise (50). All primers used in this study were mRNA specific (on different exons and/or crossing over an intron) and designed for gene expression real-time PCR analysis using Vector NTI Advance 9 software (Invitrogen). The primer sequences for atrogin-1, MuRF-1, FOXO3A, and myostatin have been reported previously by our laboratory (41,50). A melting curve analysis was generated at the end of each real-time PCR assay. A single melt peak was observed for each sample, validating that only one product was present.
Relative Quantification of Real-Time PCR Assay
The gene expression levels before and after 12 weeks of PRT were evaluated by a relative quantification method, as described by us previously (41,49). Each real-time PCR run contained samples (ran in duplicate) from both age groups (young and old) and both experimental conditions (pre- and post-PRT). In order to compare the relative gene expression between young and old women before and after 12 weeks of PRT, the 2−DCT method was used (51,52). As described by us previously (41), this method generates a value in arbitrary units of gene of interest (GOI) expression normalized to HKG expression .
Statistics
Protein content and activation state.—
In order to examine differences between young and old women in the basal state, an independent t test was used before and after the 12-week PRT protocol. To determine if any changes occurred in basal-level protein content and activation state after a bout of RE in the untrained and trained states, a two-way analysis of variance (ANOVA; Time × Age) was used to detect differences between young and old women.
Gene expression.—
For each GOI, the changes in basal-level relative mRNA expression over the 12-week period were compared between young and old women using a two-way ANOVA. In the presence of an interaction (Time × Age), paired Student's t tests were used as post hoc analysis. An independent t test was used to compare basal-level gene expression between young and old women after PRT. Values are means ± SE. Significance was set a priori at p < .05 for all analyses.
RESULTS
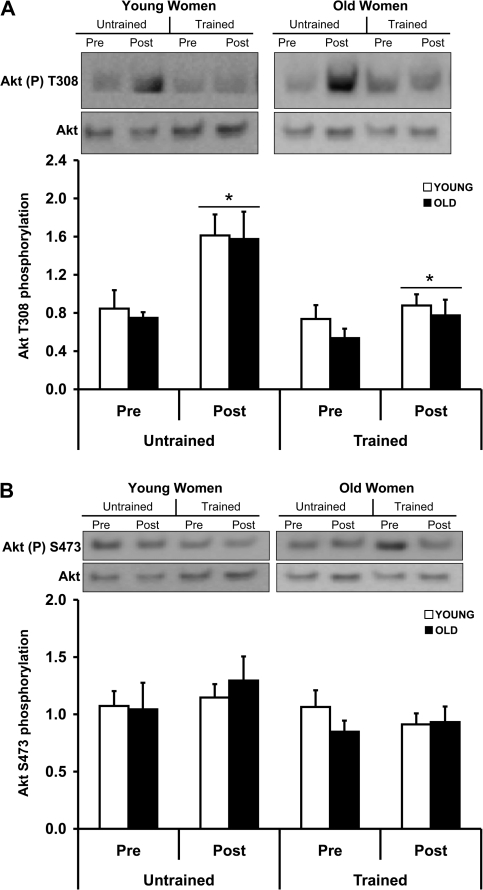
In an effort to determine factors contributing to the blunted response of 85-year-old female muscle to PRT (13), we examined components and targets of the Akt–FOXO3A signaling pathway. There were no age- or PRT-related differences in resting (Pre) cytosolic Akt Ser308 phosphorylation levels. Prior to (untrained) and following (trained) the PRT, phosphorylation of cytosolic Akt on Thr308 was increased (p < .05) immediately following a bout of RE in young and old women (Figure 2A). Phosphorylation of the Ser473 site on cytosolic Akt was not altered by age or exercise (Figure 2B). All phospho-Akt results were expressed relative to total Akt protein expression.
Figure 2.
A bout of resistance exercise (RE), before and after 12 weeks of progressive resistance training (PRT), increases Akt Threonine 308 phosphorylation from skeletal muscle of young and old women. Prior to (untrained) and following (trained) PRT, muscle biopsies were obtained from the vastus lateralis before (Pre) and immediately after (Post) a bout of RE under fasting conditions. Equal protein from cytosolic samples was analyzed for phospho Akt Thr308 (A), phospho-Akt Ser473 (B), and total Akt by Western blot analysis. Values are means ± SE. Representative Western blots are shown. *p < .05 from Pre. AU = arbitrary units.
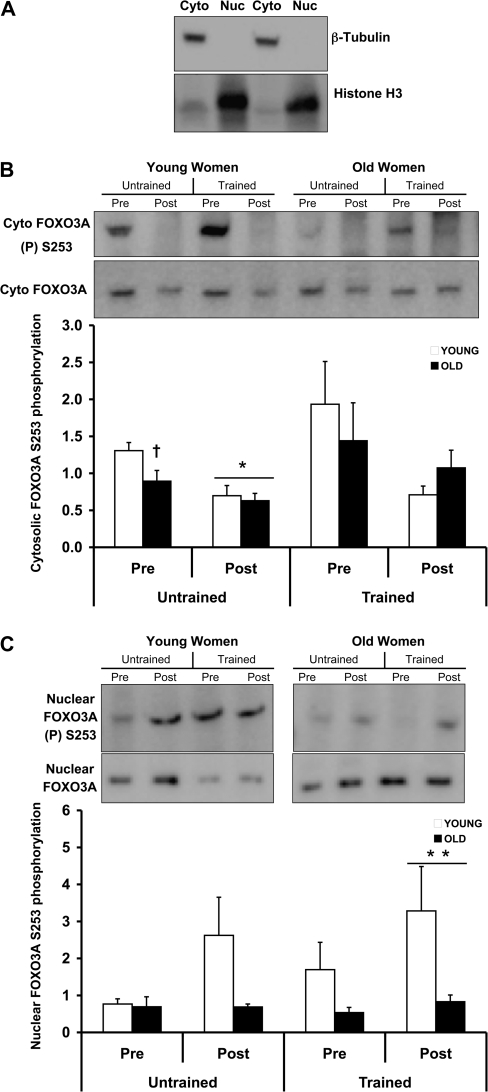
Next, we examined the activation status and the localization of the Akt substrate, FOXO3A. First, we verified that the fractions obtained from the sample preparation were cytosolic and nuclear by analyzing β-tubulin and Histone H3 protein expression from both fractions. β-Tubulin and Histone H3 are well-established markers of the cytosol and nucleus, respectively. Figure 3A shows that our extracts are enriched for cytosolic and nuclear fractions, as determined by Western blot analysis of β-tubulin and Histone H3. We observed that untrained preexercise levels of cytosolic FOXO3A phosphorylation on Ser253 were lower (p < .05) in the older women (Figure 3B) compared with the young women. However, both untrained young and old women displayed a similar Ser253 dephosphorylation of cytosolic FOXO3A (p < .05) after the exercise bout (Figure 3B). There was a main time effect (although driven by YW) in the activation of nuclear FOXO3A after RE in the trained state (Figure 3B). Consistent with these findings, the old women trended (Table 1; p = .06) toward a higher preexercise nuclear-to-cytosolic total FOXO3A protein expression ratio than the young women after PRT. Table 1 shows the basal (resting) levels of total FOXO3A (cytosolic and nuclear), which indicate age-related trends prior to PRT and age-related differences (p < .05) in total nuclear FOXO3A post-PRT.
Figure 3.
Age- and exercise-related alterations in cytosolic and nuclear FOXO3A before and after 12 weeks of progressive resistance training (PRT) from skeletal muscle of young and old women. Prior to (untrained) and following (trained) PRT, muscle biopsies were obtained from the vastus lateralis before (Pre) and immediately after (Post) a bout of resistance exercise under fasting conditions. Equal protein from cytosolic- and nuclear-enriched fractions was analyzed for (A) β-tubulin and Histone H3 protein expression, respectively, (B) cytosolic, and (C) nuclear phospho-FOXO3A Ser253 and total FOXO3A by Western blot analysis. Values are means ± SE. Representative Western blots are shown. *p < .05 from Pre, †p < .05 from young women Pre untrained, **p < .05 from Pre trained. AU = arbitrary units.
Table 1.
Basal Levels of Total Akt, Cytosolic (c) FOXO3A Protein, and Nuclear (n) FOXO3A Protein in Arbitrary Units per Microgram Loaded Protein in Young (24 years) and Old (85 years) Women Before (Baseline 1) and After (Baseline 2) 12 Weeks of Progressive Resistance Training
| Protein of Interest | Young Women | Old Women | p Value (Young vs Old) |
| Baseline 1—untrained | |||
| Akt | 33.2 ± 2.6 | 49.0 ± 4.1 | 0.009* |
| FOXO3A (c) | 38.1 ± 6.3 | 56.1 ± 5.7 | 0.06 |
| FOXO3A (n) | 48.1 ± 8.0 | 72.8 ± 15.0 | 0.17 |
| FOXO3A ratio (nuclear/cytosolic) | 1.45 ± 0.33 | 1.48 ± 0.48 | 0.97 |
| Baseline 2—trained | |||
| Akt | 41.6 ± 6.0 | 53.4 ± 2.7 | 0.10 |
| FOXO3A (c) | 34.9 ± 6.4 | 43.3 ± 3.3 | 0.27 |
| FOXO3A (n) | 33.4 ± 5.6 | 84.3 ± 11.0 | 0.004* |
| FOXO3A ratio (nuclear/cytosolic) | 1.06 ± 0.40 | 1.96 ± 0.21 | 0.06 |
p < .05 young versus old women.
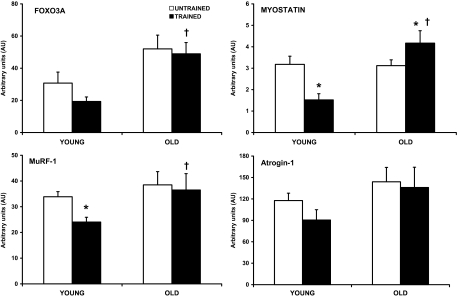
Finally, basal-level gene expression of FOXO3A, targets of FOXO3A, and proteolysis was also examined in young and old women before and after the 12-week PRT. The young women displayed downregulation of MuRF-1 (−29%, p < .05) and myostatin (−52%, p < .05) following the PRT (Figure 4). There was a trend (p = .08) for FOXO3A mRNA to decrease after PRT in the young women, consistent with the aforementioned gene changes. Conversely, the old women displayed no change in MuRF-1 or FOXO3A gene expression as a result of training. However, the old women did display elevated mRNA expression of the growth inhibitor, myostatin (+34%, p < .05), after the PRT. Neither the young nor the old women displayed a training-related change in atrogin-1. Consistent with our previously published data on basal-level gene expression (prior to PRT) in these women (41,50), at Baseline 2 (after 12-week PRT), the old women expressed higher (p < .05) mRNA levels of FOXO3A, MuRF-1, and myostatin, along with a tendency (p = .14) for atrogin-1, compared with the young women.
Figure 4.
Training-related alterations in the messenger RNA (mRNA) of FOXO3A and transcriptional targets of FOXO3A from skeletal muscle of young and old women. Muscle biopsies were obtained from the vastus lateralis before and after 12 weeks of progressive resistance training under fasting conditions. Samples were analyzed for relative mRNA expression of FOXO3A, MuRF-1, myostatin, and atrogin-1 by real time reverse transcription–polymerase chain reaction, then normalized to glyceraldehyde 3-phosphate dehydrogenase, and calculated using 2-DCT method. Values are means ± SE. *p < .05 from untrained, †p < .05 from trained young women.
DISCUSSION
We recently reported that the octogenarian women in the current study had limited plasticity at the whole-muscle and single-muscle fiber level compared with young women after 12 weeks of high-intensity PRT (13). This investigation sought to determine possible mechanisms that underlie these age-related skeletal muscle maladaptations to high-intensity RE training in the ninth decade of life (12,13). Given the propensity of aged skeletal muscle to be less sensitive to growth stimuli and more susceptible to proteolysis than younger counterparts (36,41,50,53,54), the Akt–FOXO3A pathway was examined. The main finding from this investigation was that FOXO3A signaling was differentially affected at rest and with RE compared with young women. Most notable was the finding that octogenarian women had high levels of nuclear FOXO3A and a limited ability to activate nuclear FOXO3A immediately following a RE bout after 12 weeks of high-intensity resistance training. This novel finding was independent of Akt signaling, which was similar between the young and the old women. These protein signaling data were supported by altered gene expression profiles, mainly proteolytic-related genes, at rest and in response to resistance training.
Cytoplasmic Akt
In the current investigation, we show an age-independent upregulation of Akt signaling immediately after a RE bout in an untrained state and a trained state. The activation of Ser308 on Akt we observed is likely due to contraction-mediated events because the women arrived at the laboratory the morning of the testing/biopsy following a 12-hour fast and performed three sets of 10 repetitions at 70%–75% of 1-RM on a knee extensor device. These data would suggest that young and old women were able to activate Akt to the same relative level, given a similar relative knee extensor exercise stimulus. Our previous data in young men (40) show that the same relative bout of RE to that performed in the current study causes minimal glycogen loss and disturbances to plasma insulin, glucose, and free fatty acid. This may be why previous exercise-mediated Akt activation results have been equivocal, owing differences to nutrient intake (ie, fasted or fed), exercise mode (ie, RE, treadmill, or cycle), exercise intensity (eg, 4–10 sets, 10 repetitions at 70%–80% of 1-RM, ∼40 to 100 contractions), Akt activation site measured, or a combination of these factors (34,36,39,40).
Adaptation of skeletal muscle to a PRT stimulus relies upon collective input from mechanical/contractile, hormonal, and nutrient cues. Akt (also called protein kinase B) has been well characterized as a prosurvival and progrowth protein for skeletal muscle (26–30,55). Current thought on Akt activation is that the phosphorylation of Akt on Ser473 is SIN1 dependent (in the SIN1-rictor-mTOR [TORC2] complex) and that PDK1 phosphorylation of Akt Thr308 in the T-loop is independent of prior Ser473 phosphorylation (56–58). Thus, activation of both sites is not required for full Akt function. Rictor knockout mouse embryonic fibroblasts have impaired serum-stimulated FOXO3A phosphorylation, showing the need of TORC2 complex formation for Akt phosphorylation of its substrates (56).
Nuclear and Cytoplasmic FOXO3A
Activated Akt phosphorylates FOXO3A on Ser253 (RXRXXS/T motif) and typically promotes the translocation of FOXO outside the nucleus and into the cytoplasm through the binding of 14-3-3 chaperone–binding proteins with FOXO proteins (19,23,24). Data from the current study showed that in the basal state, untrained 85-year-old women have lower cytosolic FOXO3A activation and higher levels of total nuclear FOXO3A and a trending higher nuclear:cytosolic FOXO3A protein ratio compared with young women after PRT. In response to RE, both young and old women showed a dephosphorylation of cytosolic FOXO3A in the untrained state and trained state (p = .09). The old women were unable to activate nuclear FOXO3A to the same relative level as the young women after RE. Collectively, these findings are noteworthy considering increased nuclear FOXO protein, or constitutively active FOXO has been shown to upregulate atrophy-related gene expression (22,25). Less is known about cytoplasmic FOXO function, but there may be phosphatases temporally acting upon Akt, 14-3-3, and/or FOXO3A (59–61). The importance of cytosolic FOXO3A phosphorylation, as it relates to skeletal muscle growth, remains unclear and warrants further investigation. Our current data support the contention that 80+ year-old skeletal muscles are limited in their capacity to fully adapt to a high-intensity progressive resistance-training stimulus (12,13).
There are limited data from human studies examining skeletal muscle FOXO. Chronic obstructive pulmonary disease patients (62) display significant skeletal muscle atrophy, associated with Akt-independent elevation of FOXO1, atrogin-1, and MuRF-1 expression. Conversely, Leger and colleagues (38) showed age-related (20- vs 70-year-old men) reductions in nuclear protein expression of FOXO1 and FOXO3. In a separate study Leger et al. (37), resistance trained adult (36 years) men for eight weeks and showed a reduction in nuclear FOXO1 protein expression, which was accompanied by a 10-fold increase in atrogin-1 mRNA and 10% increase in muscle mass. Collectively, the equivocal FOXO results point to possible differences in age, gender, training protocol, nutrient status, and/or FOXO protein isoform (1 vs 3).
Gene Expression
When skeletal muscle is undergoing atrophy, specific proteasomal–ubiquitin target genes, such as atrogin-1 and MuRF-1, are transcribed (20–22,26,27). In agreement, we have previously reported that the sarcopenic octogenarian women in the current study had higher mRNA levels of both FOXO3A and MuRF-1 compared with the young women before the onset of the PRT program (41). Others have reported similar increased proteolytic gene expression in aged rat muscle (63), spinal cord injury patients (36,64), and aged human muscle (65,66). The current study provides further insight into muscle biology with old age and shows that skeletal muscle from 85-year-old women has altered FOXO3A-related signaling, which may partially explain the age-related differences previously observed in proteolytic gene expression (41,63,65,66).
Interestingly, basal-level gene expression of MuRF-1 and myostatin decreased, whereas FOXO3A tended to decrease as a result of 12-week PRT in the young women. Perhaps even more interesting was the lack of change in proteolytic gene expression in the old women, with the exception of an increase in the growth repressor, myostatin mRNA, levels after the PRT. Collectively, the inability to decrease proteolytic gene expression, combined with increased mRNA levels of the growth inhibitor, myostatin, may have contributed to the lack of single-muscle fiber and whole-muscle hypertrophy previously reported in these octogenarian women (13). The older women had higher baseline levels of FOXO3A, MuRF-1, and myostatin, along with a trend for Atrogin-1 mRNA gene expression after PRT compared to the young trained women, which is in agreement with the age-related differences found for total nuclear FOXO3A levels after PRT. Collectively, these data warrant further investigation.
Limitations
The novel aspect of this investigation was the examination of Akt–FOXO3A signaling before and after a bout of RE in an untrained state and a trained state of independent-living octogenarian women. However, this novelty was also a limiting factor with respect to the population pool of relatively healthy, independent-living 85-year-old women. Therefore, acquiring skeletal muscle samples from octogenarian women for the current investigation is extremely limited and valuable. We also acknowledge the limitations with a single postexercise muscle biopsy that may not have captured additional aspects related to protein signaling. We based our postexercise muscle biopsy time point on our previous studies (40,42,50,51,67), which have been shown to be appropriate for exercise-induced signaling.
Summary
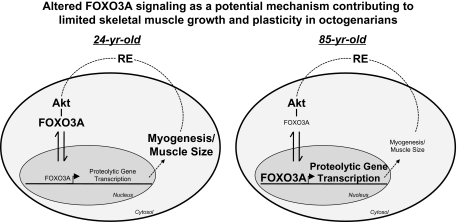
We show, for the first time, that the skeletal muscle of 85-year-old women has age-related alterations in FOXO3A signaling and FOXO3A-associated target gene expression when compared with young women. The age-dependent FOXO3A signaling did not appear to be related to Akt signaling and suggests that alternative pathway interactions led to the novel FOXO3A responses in the very old. These data provide initial insight that the limited hypertrophic response of skeletal muscle from octogenarians to PRT may be a result of lower FOXO3A phosphorylation and thus exaggerated nuclear FOXO3A levels (see Figure 5). Future experiments further investigating the unique FOXO3A signaling in aging muscle along with novel therapies to promote muscle health are warranted.
Figure 5.
Altered FOXO3A signaling as a potential mechanism contributing to limited skeletal muscle growth and plasticity in octogenarians. The current data show that 85-year-old skeletal muscle has elevated nuclear FOXO3A protein expression versus the young participants, which promotes proteolytic gene expression (eg, FOXO3A, MuRF-1, and atrogin-1) (41). Increased proteolytic activity may contribute to an inhibition of myogenesis and muscle size, which limits muscle plasticity and muscle function at the level of the whole-muscle and single-muscle fiber (13). Larger bold text within the cell cytosol and nuclear domains indicates more of an increase in protein expression and/or activation or a cellular process. Smaller unbold text (except domain designates) within the cell cytosol and nuclear domains indicates more of a decrease in protein expression and/or activation or a cellular process. RE = resistance exercise.
FUNDING
The study was supported by National Institutes of Health grant AG-18409 (to S.T.).
Acknowledgments
The authors would like to thank the participants for their dedication throughout the study. The authors would also like to thank Dr Bozena Jemiolo for her technical assistance with the real-time RT-PCR assays.
References
- 1.Nair KS. Aging muscle. Am J Clin Nutr. 2005;81:953–963. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- 2.Jorgensen SB, Treebak JT, Viollet B, et al. Role of ampkalpha2 in basal, training-, and aicar-induced glut4, hexokinase ii, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- 3.Boyle JP, Honeycutt AA, Venkat Narayan KM, et al. Projection of diabetes burden through 2050. Diabetes Care. 2001;24:1936–1940. doi: 10.2337/diacare.24.11.1936. [DOI] [PubMed] [Google Scholar]
- 4.Trappe S, Costill D, Thomas R. Effect of swim taper on whole muscle and single muscle fiber contractile properties. Med Sci Sports Exerc. 2001;32:48–56. [PubMed] [Google Scholar]
- 5.Fluckey JD, Pohnert SC, Boyd SG, Cortright RN, Trappe TA, Dohm GL. Insulin stimulation of muscle protein synthesis in obese zucker rats is not via a rapamycin-sensitive pathway. Am J Physiol Endocrinol Metab. 2000;279:E182–E187. doi: 10.1152/ajpendo.2000.279.1.E182. [DOI] [PubMed] [Google Scholar]
- 6.Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol. 2000;89:143–152. doi: 10.1152/jappl.2000.89.1.143. [DOI] [PubMed] [Google Scholar]
- 7.Sipila S, Elorinne M, Alen M, Suominen H, Kovanen V. Effects of strength and endurance training on muscle fibre characteristics in elderly women. Clin Physiol. 1997;17:459–474. doi: 10.1046/j.1365-2281.1997.05050.x. [DOI] [PubMed] [Google Scholar]
- 8.Morse CI, Thom JM, Mian OS, Muirhead A, Birch KM, Narici MV. Muscle strength, volume and activation following 12-month resistance training in 70-year-old males. Eur J Appl Physiol. 2005;95:197–204. doi: 10.1007/s00421-005-1342-3. [DOI] [PubMed] [Google Scholar]
- 9.Kalapotharakos V, Smilios I, Parlavatzas A, Tokmakidis SP. The effect of moderate resistance strength training and detraining on muscle strength and power in older men. J Geriatr Phys Ther. 2007;30:109–113. doi: 10.1519/00139143-200712000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians: effects on skeletal muscle. JAMA. 1990;263:3029–3034. [PubMed] [Google Scholar]
- 11.Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- 12.Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R273–R280. doi: 10.1152/ajpregu.00093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol. 2009;106:1611–1617. doi: 10.1152/japplphysiol.91587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aniansson A, Grimby G, Hedberg M. Compensatory muscle fiber hypertrophy in elderly men. J Appl Physiol. 1992;73:812–816. doi: 10.1152/jappl.1992.73.3.812. [DOI] [PubMed] [Google Scholar]
- 15.Danneskiold-Samsoe B, Kofod V, Munter J, Grimby G, Schnohr P, Jensen G. Muscle strength and functional capacity in 78-81-year-old men and women. Eur J Appl Physiol Occup Physiol. 1984;52:310–314. doi: 10.1007/BF01015216. [DOI] [PubMed] [Google Scholar]
- 16.Arany Z, He H, Lin J, et al. Transcriptional coactivator pgc-1alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Arden KC. Foxo animal models reveal a variety of diverse roles for foxo transcription factors. Oncogene. 2008;27:2345–2350. doi: 10.1038/onc.2008.27. [DOI] [PubMed] [Google Scholar]
- 18.Greer EL, Brunet A. Foxo transcription factors in ageing and cancer. Acta Physiol (Oxf) 2008;192:19–28. doi: 10.1111/j.1748-1716.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 19.Huang H, Tindall DJ. Dynamic foxo transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 20.Mammucari C, Milan G, Romanello V, et al. Foxo3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J, Brault JJ, Schild A, et al. Foxo3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of foxo in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 24.Jang SW, Yang SJ, Srinivasan S, Ye K. Akt phosphorylates msti and prevents its proteolytic activation, blocking foxo3 phosphorylation and nuclear translocation. J Biol Chem. 2007;282:30836–30844. doi: 10.1074/jbc.M704542200. [DOI] [PubMed] [Google Scholar]
- 25.Stitt TN, Drujan D, Clarke BA, et al. The igf-1/pi3k/akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting foxo transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 26.Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve. 2006;33:155–165. doi: 10.1002/mus.20442. [DOI] [PubMed] [Google Scholar]
- 27.Glass DJ. Molecular mechanisms modulating muscle mass. Trends Mol Med. 2003;9:344–350. doi: 10.1016/s1471-4914(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 28.Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003;5:87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- 29.Bodine SC, Stitt TN, Gonzalez M, et al. Akt/mtor pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 30.Rommel C, Bodine SC, Clarke BA, et al. Mediation of igf-1-induced skeletal myotube hypertrophy by pi(3)k/akt/mtor and pi(3)k/akt/gsk3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 31.Calnan DR, Brunet A. The foxo code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 32.Nakae J, Oki M, Cao Y. The foxo transcription factors and metabolic regulation. FEBS Lett. 2008;582:54–67. doi: 10.1016/j.febslet.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 33.Salih DA, Brunet A. Foxo transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mascher H, Tannerstedt J, Brink-Elfegoun T, Ekblom B, Gustafsson T, Blomstrand E. Repeated resistance exercise training induces different changes in mrna expression of mafbx and murf-1 in human skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E43–E51. doi: 10.1152/ajpendo.00504.2007. [DOI] [PubMed] [Google Scholar]
- 35.Dreyer HC, Drummond MJ, Pennings B, et al. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mtor signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–E400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drummond MJ, Miyazaki M, Dreyer HC, et al. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol. 2009;106(4):1403–1411. doi: 10.1152/japplphysiol.90842.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leger B, Cartoni R, Praz M, et al. Akt signalling through gsk-3beta, mtor and foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol. 2006;576:923–933. doi: 10.1113/jphysiol.2006.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in socs-3 and myostatin and a reduced efficiency of akt phosphorylation. Rejuvenation Res. 2008;11:163B–175B. doi: 10.1089/rej.2007.0588. [DOI] [PubMed] [Google Scholar]
- 39.Deldicque L, Atherton P, Patel R, et al. Decrease in akt/pkb signalling in human skeletal muscle by resistance exercise. Eur J Appl Physiol. 2008;104:57–65. doi: 10.1007/s00421-008-0786-7. [DOI] [PubMed] [Google Scholar]
- 40.Creer A, Gallagher P, Slivka D, Jemiolo B, Fink W, Trappe S. Influence of muscle glycogen availability on erk1/2 and akt signaling after resistance exercise in human skeletal muscle. J Appl Physiol. 2005;99:950–956. doi: 10.1152/japplphysiol.00110.2005. [DOI] [PubMed] [Google Scholar]
- 41.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci. 2007;62:1407–1412. doi: 10.1093/gerona/62.12.1407. [DOI] [PubMed] [Google Scholar]
- 42.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol. 2007;103:1744–1751. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 43.Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, Mori N. Effects of aging and caloric restriction on the gene expression of foxo1, 3, and 4 (fkhr, fkhrl1, and afx) in the rat skeletal muscles. Microsc Res Tech. 2002;59:331–334. doi: 10.1002/jemt.10213. [DOI] [PubMed] [Google Scholar]
- 44.Williamson DL, Godard MP, Porter D, Costill DL, Trappe SW. Progressive resistance training reduces myosin heavy chain co-expression in single muscle fibers from older men. J Appl Physiol. 2000;88:627–633. doi: 10.1152/jappl.2000.88.2.627. [DOI] [PubMed] [Google Scholar]
- 45.Williamson DL, Gallagher PM, Carroll CC, Raue U, Trappe SW. Reduction in hybrid single muscle fiber proportions with resistance training in humans. J Appl Physiol. 2001;91:1955–1961. doi: 10.1152/jappl.2001.91.5.1955. [DOI] [PubMed] [Google Scholar]
- 46.Trappe SW, Godard MP, Gallagher PM, Carroll CC, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol. 2001;281:C398–C406. doi: 10.1152/ajpcell.2001.281.2.C398. [DOI] [PubMed] [Google Scholar]
- 47.Bergstom J. Muscle electrolytes in man. Scand J Clin Lab Invest. 1962;68:1–110. [Google Scholar]
- 48.Rothermel B, Vega RB, Yang J, Wu H, Bassel-Duby R, Williams RS. A protein encoded within the down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem. 2000;275:8719–8725. doi: 10.1074/jbc.275.12.8719. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol. 2005;98:1745–1752. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]
- 50.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18-30 yr) and old (80-89 yr) women. J Appl Physiol. 2006;101:53–59. doi: 10.1152/japplphysiol.01616.2005. [DOI] [PubMed] [Google Scholar]
- 51.Schmittgen TD, Livak KJ. Analyzing real-time pcr data by the comparative c(t) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 52.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 53.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trappe T, Williams R, Carrithers J, et al. Influence of age and resistance exercise on human skeletal muscle proteolysis: A microdialysis approach. J Physiol. 2004;554:803–813. doi: 10.1113/jphysiol.2003.051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. 2004;15:1537–1545. doi: 10.1097/01.asn.0000127211.86206.e1. [DOI] [PubMed] [Google Scholar]
- 56.Guertin DA, Stevens DM, Thoreen CC, et al. Ablation in mice of the mtorc components raptor, rictor, or mlst8 reveals that mtorc2 is required for signaling to akt-foxo and pkcalpha, but not s6k1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Scheid MP, Marignani PA, Woodgett JR. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase b. Mol Cell Biol. 2002;22:6247–6260. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of akt/pkb by the rictor-mtor complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 59.Ni YG, Wang N, Cao DJ, et al. Foxo transcription factors activate akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci U S A. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schisler JC, Willis MS, Patterson C. You spin me round: mafbx/atrogin-1 feeds forward on foxo transcription factors (like a record) Cell Cycle. 2008;7:440–443. doi: 10.4161/cc.7.4.5451. [DOI] [PubMed] [Google Scholar]
- 61.Tremblay ML, Giguere V. Phosphatases at the heart of foxo metabolic control. Cell Metab. 2008;7:101–103. doi: 10.1016/j.cmet.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Doucet M, Russell AP, Leger B, et al. Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:261–269. doi: 10.1164/rccm.200605-704OC. [DOI] [PubMed] [Google Scholar]
- 63.Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B. Atrophy-related ubiquitin ligases, atrogin-1 and murf1 are up-regulated in aged rat tibialis anterior muscle. Mech Ageing Dev. 2006;127:794–801. doi: 10.1016/j.mad.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Urso ML, Chen YW, Scrimgeour AG, Lee PC, Lee KF, Clarkson PM. Alterations in mrna expression and protein products following spinal cord injury in humans. J Physiol. 2007;579:877–892. doi: 10.1113/jphysiol.2006.118042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Welle S, Brooks AI, Delehanty JM, Needler N, Thornton CA. Gene expression profile of aging in human muscle. Physiol Genomics. 2003;14:149–159. doi: 10.1152/physiolgenomics.00049.2003. [DOI] [PubMed] [Google Scholar]
- 66.Welle S, Bhatt K, Thornton CA. High-abundance mrnas in human muscle: comparison between young and old. J Appl Physiol. 2000;89:297–304. doi: 10.1152/jappl.2000.89.1.297. [DOI] [PubMed] [Google Scholar]
- 67.Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (mapk) pathway activation: effects of age and acute exercise on human skeletal muscle. J Appl Physiol. 2003;547:977–987. doi: 10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]