Abstract
Ames dwarf (Prop1df, df/df) mice lack growth hormone (GH), prolactin, and thyrotropin and live remarkably longer than their normal siblings. Significance of reduced activity of the somatotropic and thyroid axes during development and adulthood on longevity are unknown. Because enhanced insulin sensitivity and reduced insulin levels are among likely mechanisms responsible for increased longevity in these mutants, we compared the effects of GH and thyroxine (T4) replacement on various parameters related to insulin signaling in young and old male df/df mice. The results suggest that altered plasma adiponectin and insulin-like growth factor-1 (IGF-1) and hepatic IGF-1, insulin receptor (IR), IR substrate-1, peroxisome proliferator–activated receptor (PPAR) γ, and PPARγ coactivator-1 α may contribute to increased insulin sensitivity in Ames dwarfs. The stimulatory effect of GH and T4 treatment on plasma insulin and inhibitory effect on expression of hepatic glucose transporter-2 were greater in old than in young dwarfs. These results indicate that GH and T4 treatment has differential impact on insulin signaling during development and adulthood.
Keywords: Ames dwarf, Aging, Insulin, Growth hormone
THE majority of spontaneous or experimentally induced mutations that extend the longevity of mice alter the function of the somatotropic axis, growth hormone (GH), and insulin-like growth factor-1 (IGF-1). The Ames dwarf, a spontaneous mutation resulting in a dwarf phenotype, was the first mutant mouse reported to have extended life span (1). Ames dwarf mice have a mutation in the transcription factor Prop1df, which causes hypofunctional development of the anterior pituitary and results in primary endocrine deficiencies of GH, thyroid-stimulating hormone (TSH), and prolactin (PRL) (2–4). As a result of these primary hormonal deficits, several secondary characteristics are observed in Ames dwarf mice. GH deficiency is the major cause of reduced hepatic production and circulating levels of IGF-1, diminished growth and adult body size, delayed puberty, and decreased circulating insulin and glucose levels (5). Primary TSH deficiency causes severe hypothyroidism, which results in decreased body temperature in Ames dwarf mice (6,7). PRL deficiency leads to female sterility because PRL is required for luteal function, implantation, and maintenance of pregnancy in mice (3). Altered regulation of carbohydrate metabolism, gene expression profiles, and body composition in Ames dwarfs have also been reported (6,8).
The actions of GH in the liver stimulate production of IGF-1, which is the main mediator of GH actions on growth and development. Reduced GH and IGF-1 signaling affects insulin sensitivity through multiple mechanisms. Although GH does not bind to the insulin receptor (IR), several signaling events are shared between GH and IGF-1 and insulin. The GH and IGF-1 system has complex feedback mechanisms that, in general, promote insulin resistance (9). Deficiency of GH is associated with increased insulin sensitivity, decreased insulin secretion, and reduced glucose levels, which are determinants of extended longevity (10–13). Enhanced insulin sensitivity was observed in Ames dwarf mice compared with normal mice as indicated by greater glucose clearance during insulin tolerance tests (14,15). Moreover, insulin produces greater upregulation of the early steps in insulin signaling, which include increased expression of IR, IR substrate (IRS)-1, and IRS-2, in the liver of Ames dwarf as compared with normal mice (14,16,17). Moreover, in these animals, the expression of genes related to insulin sensitivity is altered in the liver, one of the main target organs of insulin. Increased expression of peroxisome proliferator–activated receptor (PPAR) γ, a major regulator of insulin and glucose metabolism, was detected in middle-aged Ames dwarfs compared with normal mice of the same age (17). PPARγ is a target receptor for thiazolidinediones, which are used as insulin sensitizers to treat patients with type 2 diabetes. The transcriptional coactivator for PPARγ known as PPARγ receptor–coactivator-1α (PGC-1α) is a key mediator of the effects of energy intake on metabolism and an activator of gluconeogenesis. Elevated levels of PGC-1α and genes involved in fatty acid oxidation and gluconeogenesis were detected in the liver of Ames dwarf mice (18). Increasing evidence implicates the involvement of PCG-1α in regulating life span (19).
The somatotropic axis and the thyroid axis (TSH–thyroid hormones) are interdependent and critical in regulating aging. However, it is not known which stage(s) of life history is/are critical for the actions of GH and IGF-1 on aging and longevity. In this study, we examined the age-related effects of GH and T4 treatment on insulin signaling. The results suggest that rapid growth during the pubertal period may be an important stage for the beneficial actions of the somatotropic axis, whereas increased GH and IGF-1 in middle age may have detrimental effects on longevity.
METHODS
Experimental Overview
Animals were entered into the study at 2 weeks of age (young group) or at 16–18 months of age (old group). Because Ames dwarf mice live longer than their normal siblings, the latter group is considered old only for normal mice, but all animals in the 16- to 18-month-old group from now on will be referred to as “old”. Young and old male Ames dwarfs were treated with GH and thyroxine (T4) or with saline vehicle for 6 weeks. No injections were administered to normal animals. After 6 weeks, insulin stimulation was performed, and whole blood and liver tissue were collected. Real-time polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA) were performed to assess the effects on insulin signaling.
Animals
Male Ames dwarf mice and their normal littermates were produced in our breeding colony by mating homozygous (df/df) males with heterozygous (N/df) females. Animals were housed under controlled temperature and light conditions (20–23°C, 12-hour light and 12-hour dark cycle) and were provided ad libitum with nutritionally balanced diet (Rodent Laboratory Chow 5001: 23.4% protein, 4.5% fat, 5.8% crude fiber; LabDiet PMI Feeds, Inc., St Louis, MO).
GH and T4 Replacement Therapy
Recombinant porcine GH (Alpharma, Victoria, Australia) was dissolved in 0.1 M NaHCO3 solution (pH ∼8.3) and adjusted to pH 7.8. GH was administered by subcutaneous injections (3 μg/g body weight; 21 μg/50 μL dose) twice daily and once daily double dose on Saturday and Sunday following our previous protocol (20). T4 (l-thyroxine; Sigma, St Louis, MO) in 0.9% saline solution, pH 7.8, was administered by subcutaneous injections (0.1 μg/g body weight; 0.7 μg/50 μL dose) three times per week (Monday, Wednesday, and Friday). Control groups of young and old male Ames dwarf mice were injected with 0.9% saline daily. Normal age-matched animals were not given any injections. Body weight was measured at the beginning and end and once a week during the treatment period.
Insulin Stimulation and Tissue Collection
After 6 weeks of the study, mice were fasted overnight for the insulin stimulation procedure. Mice were anesthetized using a mixture of ketamine (65 mg/kg) and xylosine (4.1 mg/kg) administered by intraperitoneal injection. Blood was collected by cardiac puncture. Porcine insulin (Sigma) at the dose of 10 IU/kg body weight was injected through the vena cava. After 60 seconds, the liver was collected, snap frozen on dry ice, and stored at −80°C. Whole blood was centrifuged in order to isolate the plasma supernatant, which was stored at −80°C. Animals were killed by cervical dislocation.
RNA Extraction and Complementary DNA Synthesis
Approximately 150 mg of liver tissue was homogenized in 250 μL cold phosphate-buffered saline solution. RNA was extracted using phenol and chloroform by standard technique (21). RNA quantification was performed by running total RNA on 1.5% agarose gel. Genomic DNA was removed using DNase I (Promega, Madison, WI). Synthesis of complementary DNA (cDNA) from 1 μg total messenger RNA (mRNA) was performed using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) according to the maufacturer’s protocol. Conditions of 25°C for 5 minutes, 42°C for 30 minutes, and 85°C for 5 minutes were used in a thermocycler (Eppendorf North America, Hamburg, Germany).
Real-Time PCR
cDNA was amplified using iQ SYBR Green PCR Supermix (Bio-Rad) and specific forward and reverse primers (Table 1). A Bio-Rad iCycler was used for denaturation step at 95°C for 2 minutes, followed by 45 cycles of 95°C denaturation for 15 seconds, 62°C annealing for 30 seconds, and 72°C extension for 30 seconds. Fluorescence was read at the end of the 72°C extension. A melting curve was calculated for each reaction to evaluate any nonspecific products. The data were analyzed using the Bio-rad iQ5 software, version 2.0.
Table 1.
Sequences of Primers for Real-Time Polymerase Chain Reaction
| Gene | Accession Number | Primer Sequences (forward and reverse) |
| β2-Microglobulin | NM_009735 | F: 5′-AAGTATACTCACGCCACCCA-3′ |
| R: 5′-CAGGCGTATGTATCAGTCTC-3′ | ||
| Insulin receptor | NM_010568 | F: 5′-CTTGGACAACCAGAACCTGA-3′ |
| R: 5′-CCTTAGTTCCGGAGACTTCT-3′ | ||
| IGF-1 | NM_010512 | F: 5′-CTGAGCTGGTGGATGCTCTT-3′ |
| R: 5′-CACTCATCCACAATGCCTGT-3′ | ||
| IRS-1 | NM_010570 | F: 5′-AGCCCAAAAGCCCAGGAGAATA-3′ |
| R: 5′-TTCCGAGCCAGTCTCTTCTCTA-3′ | ||
| PPARγ | NM_011146 | F: 5′-GTCAGTACTGTCGGTTTCAG-3′ |
| R: 5′-CAGATCAGCAGACTCTGGGT-3′ | ||
| PGC1-α | NM_008904 | F: 5′-TACGCAGGTCGAACGAAACT-3′ |
| R: 3′-ACTTGCTCTTGGTGGAAGGA-3′ | ||
| GLUT2 | NM_012879 | F: 5′-GGTCAGAACTACCTCTCTTTG-3′ |
| R: 5′-GTGTGTGTGGAATTGTCCTC-3′ |
Note: GLUT2 = glucose transporter 2; IGF-1 = insulin-like growth factor-1; IRS-1 = insulin receptor substrate-1α; PGC-1α = PPARγ coactivator-1α; PPAR = peroxisome proliferator–activated receptor.
All real-time PCR reactions were repeated and values were averaged. β2-Microglobulin was used as a housekeeping gene after previous validation. Relative expression was calculated using the equation 2A − B/2C − D (A = cycle threshold [Ct] number of the gene of interest in the first control sample, B = Ct number of the gene of interest in each sample, C = Ct number of the housekeeping gene in the first control sample, and D = Ct number of the housekeeping gene in each sample). Relative expression of the first control sample was expressed as 1, and the relative expression of all other samples was calculated using this equation. The results of the old normal group were averaged, and all other results were divided by this average to obtain the fold change of expression of the genes of interest compared with this control group.
Protein Extraction
Total protein was extracted from the liver using tissue protein extraction reagent (T-PER; Pierce, Rockford, IL) with 0.5% protease inhibitor cocktails. One microliter of T-PER cocktail was added to 1 g of liver tissue, which was then homogenized and centrifuged. The supernatant was collected and quantified for total protein using bicinchoninic acid protein assay kit (Pierce).
Enzyme-Linked Immunosorbent Assay
Plasma insulin levels were determined using Ultra Sensitive Rat Insulin ELISA Kit (Crystal Chem Inc., Downers Grove, IL). Plasma IGF-1 levels were determined using Rat/Mouse IGF-1 ELISA Kit (Immunodiagnostic Systems, Adelaide, Australia). Plasma adiponectin levels were determined using Mouse Adiponectin ELISA Kit (Linco Research, St Charles, MO). IR and IRS-1 quantification of proteins using ELISA was performed according to manufacturer’s instructions (Biosource, Camarillo, CA). Samples were compared with a standard curve and read at 450 nm absorbance using spectrophotometer plate reader (μQuant; Bio-Tek Instruments, Winooski, VT; KCjunior software, version 1.17).
Statistical Analysis
All results are reported as mean ± SEM. Analysis of variance was performed using SPSS version 16.0 (SPSS Inc., Chicago, IL). Pairwise comparisons were performed using Bonferroni corrections. Values of p < .05 are considered significant. All figures were made using Excel or Prism 4.02 (GraphPad Software, San Diego, CA).
RESULTS
Effects of GH and T4 Replacement Therapy on Body Weight
The body weight of saline-treated young dwarf mice did not increase significantly from their weight at the beginning of treatment. At Week 3, GH and T4-treated young dwarf mice showed increased body weight (p < .001) compared with saline-treated young dwarfs. By the end of treatment, GH and T4-treated young dwarfs reached 84% of the weight of age-matched normal mice (Figure 1A). Old dwarf mice treated with GH and T4 experienced much smaller increase in body weight that became statistically significant at Week 4 until the end of treatment (p < .001). The body weight of saline-injected old dwarf mice did not significantly increase. The old normal mice weighed more than old dwarf mice, but similarly, did not experience changes in body weight during the 6-week period of monitoring (Figure 1B).
Figure 1.
Body weight of Ames dwarf mice during 6 weeks of growth hormone (GH)/thyroxine (T4) treatment. (A) Ames dwarf (df) 2-week-old mice were treated with GH and T4 injections (young-df GH and T4, n = 12) or saline (young-df saline, n = 12). No injections were administered to normal (N) siblings (young-N, n = 13). (B) Ames df 16- to 18-month old mice were treated with GH and T4 (old-df GH and T4, n = 10) or saline injections (old-df saline, n = 10). No injections were administered to N siblings (old-N, n = 10). Each time point represents the average weight of each group.
Effects of GH and T4 Replacement on Plasma Insulin, Adiponectin, and IGF-1 Levels
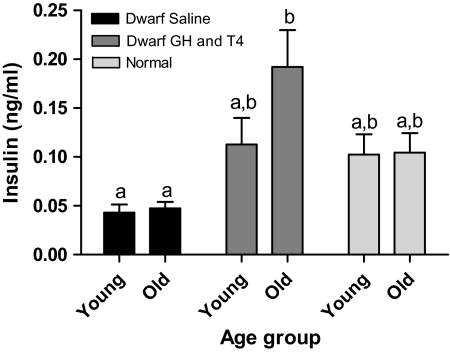
Saline-treated Ames dwarfs had extremely low plasma insulin levels, and they maintained low insulin levels into old age. GH and T4 treatment appeared to increase insulin levels in young dwarfs and significantly increased insulin levels in old dwarfs (p < .005) when compared with age-matched saline-treated dwarf mice (Figure 2). Plasma insulin levels in GH and T4-treated dwarfs were not significantly different from the levels measured in age-matched normal animals.
Figure 2.
Plasma insulin of Ames dwarf (df) mice after growth hormone (GH)/thyroxine (T4) treatment. Groups that do not share a superscript are significantly different (p < .05). Young-df saline, n = 12; old-df saline, n = 8; young-df GH and T4, n = 12; old-df GH and T4, n = 10; young-N, n = 12; and old-N, n = 10.
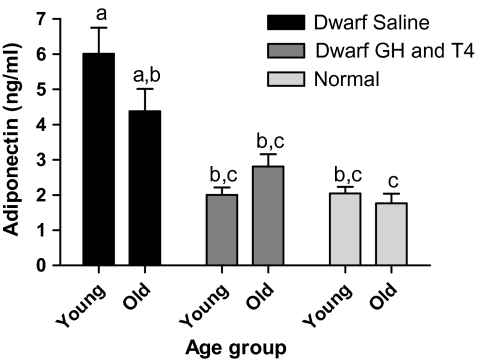
Saline-treated young dwarf mice had elevated plasma adiponectin levels compared with age-matched normal animals (p < .001). Adiponectin levels tended to decline with increased age in saline-treated dwarfs but remained higher than adiponectin levels in age-matched normal animals (p < .01). GH and T4 treatment decreased plasma adiponectin in young and old dwarfs, compared with young saline-treated dwarfs (p < .001), to the levels of normal mice (Figure 3).
Figure 3.
Plasma adiponectin of Ames dwarf (df) mice after growth hormone (GH)/thyroxine (T4) treatment. Groups that do not share a superscript are significantly different (p < .05). Young-df saline, n = 12; old-df saline, n = 8; young-df GH and T4, n = 12; old-df GH and T4, n = 10; young-N, n = 12; and old-N, n = 10.
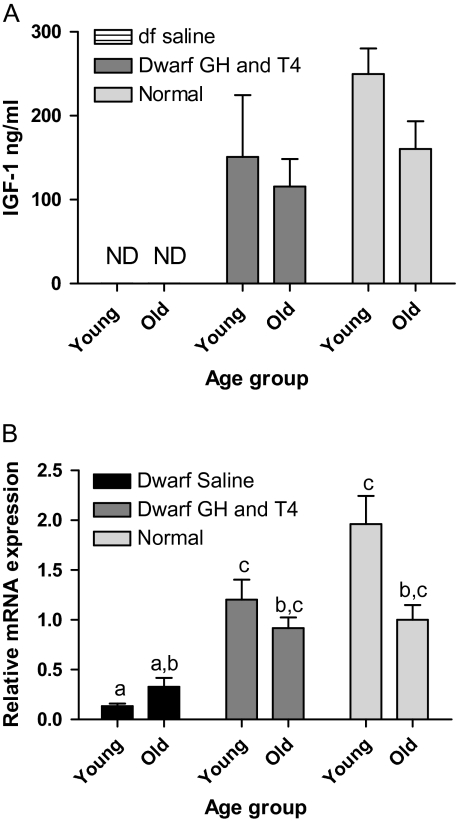
Plasma IGF-1 levels were extremely low and often undetectable by ELISA in saline-treated dwarf mice and therefore are not shown on the graph. GH and T4 treatment elevated plasma IGF-1 levels in dwarfs to become detectable, although only in a few animals explains the low n values (Figure 4A). Saline-treated young dwarf mice had decreased IGF-1 mRNA levels compared with age-matched normal animals (p < .001). GH and T4 treatment increased IGF-1 mRNA, compared with young saline-treated dwarfs (p < .005) and numerically compared with old saline-treated dwarfs, to reach levels not different from those measured in normal mice (Figure 4B).
Figure 4.
Insulin-like growth factor-1 (IGF-1) in Ames dwarf (df) mice after growth hormone (GH)/thyroxine (T4) treatment. (A) Plasma IGF-1 in young-df GH and T4, n = 3; old-df GH and T4, n = 5; young-N, n = 11; and old-N, n = 8. Nondetectable levels in df-saline and few became detectable in df-GH and T4, resulting in low n values. (B) Hepatic IGF-1 messenger RNA (mRNA) in young-df saline, n = 12; old-df saline, n = 10; young-df GH and T4, n = 12; old-df GH and T4, n = 10; young-N, n = 13; and old-N, n = 10. Groups that do not share a superscript are significantly different (p < .05).
Effects of GH and T4 Replacement on Hepatic mRNA and Protein Levels of Insulin Signal Transducers
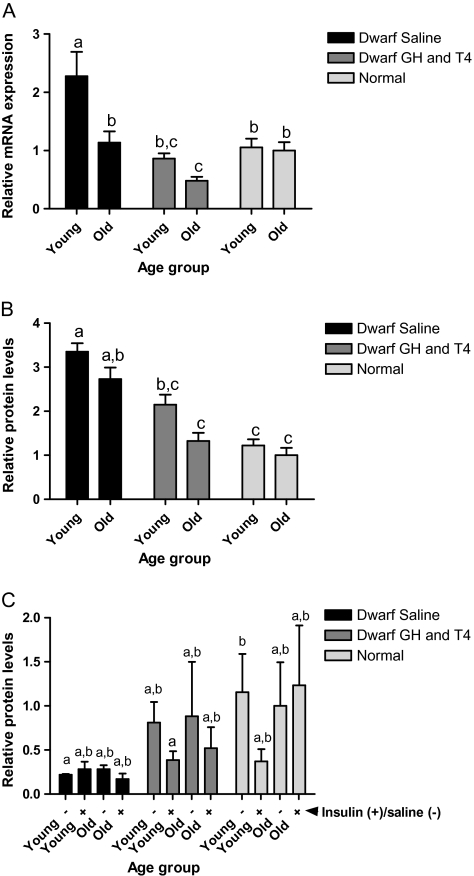
IR mRNA in the liver was higher in young saline-treated dwarfs than in age-matched normal animals (p < .005). IR mRNA levels declined with age in saline-treated dwarfs and with GH and T4 treatment to similar levels as normal animals (p < .05; Figure 5A). Total IR protein showed trends similar to IR mRNA. Young saline-treated dwarfs had higher IR protein than age-matched normal animals (p < .001). IR protein declined with age in saline-treated dwarfs (p < .01) but levels were still greater than young normal animals (p < .01). GH and T4 treatment decreased IR protein in young (p < .001) and old (p < .01) dwarfs, reaching levels similar to those observed in normal mice (Figure 5B). Phosphorylation of IR at tyrosine 1158 (IR pY1158) is important in the activation of insulin signal transduction. Insulin-stimulated IR pY1158 was markedly elevated in young saline-treated dwarfs when compared with old saline-treated dwarfs, GH and T4-treated dwarfs, and normal animals (p < .001; Figure 5C).
Figure 5.
Insulin receptor (IR) in Ames dwarf (df) mice after growth hormone (GH)/thyroxine (T4) treatment. (A) Hepatic IR messenger RNA (mRNA) in young-df saline, n = 12; old-df saline, n = 10; young-df GH and T4, n = 12; old-df GH and T4, n = 10; young-N, n = 13; and old-N n = 10. (B) Hepatic IR total protein in young-df saline, n = 11; old-df saline, n = 9; young-df GH and T4, n = 12; old-df GH and T4, n = 10; young-N, n = 13; and old-N, n = 10. (C) Hepatic phosphotyrosine 1158 IR in young-df saline (−), n = 5; young-df saline (+), n = 5; old-df saline (−), n = 5; old-df saline (+), n = 4; young-df GH and T4 (−), n = 6; young-df GH and T4 (+), n = 6; old-df GH and T4 (−), n = 5; old-df GH and T4 (+), n = 5; young-N (−), n = 7; young-N (+), n = 6; old-N (−), n = 5; old-N (+), n = 5, where (+) are animals stimulated with insulin and (−) saline control. Groups that do not share a superscript are significantly different (p < .05).
The levels of IRS-1 mRNA in the liver were higher in young saline-treated dwarfs than in age-matched normal animals (p < .01). IRS-1 mRNA decreased with age in saline-treated dwarfs and with GH and T4 treatment (p < .01) to similar levels as normal animals (Figure 6A). Saline-treated dwarfs had higher IRS-1 protein levels than normal animals (p < .001), and these levels were maintained in old age (p < .001). GH and T4 treatment decreased IRS-1 protein in young (p < .005) and old (p < .001) dwarfs compared with age-matched saline-treated dwarfs, reaching levels similar to those observed in normal mice (Figure 6B). Phosphorylation of IRS-1 on serine 307 (IRS-1 pS307) is an important mechanism to attenuate insulin signaling. There were no significant differences detected in the amount of IRS-1 pS307 due to genotype, age, or GH and T4 treatment (data not shown). However, the ratio of IRS-1 pS307 to total IRS-1 protein was greatly reduced in saline-treated dwarfs compared with normal mice. GH and T4 treatment of dwarfs tended to increase the ratio of IRS-1 pS307 to total IRS-1 protein, toward the levels measured in normal animals (Figure 6C).
Figure 6.
Insulin receptor substrate (IRS)-1 in Ames dwarf (df) mice after growth hormone (GH)/thyroxine (T4) treatment. (A) Hepatic IRS-1 messenger RNA (mRNA) in young-df saline, n = 12; old-df saline, n = 10; young-df GH and T4, n = 12; old-df GH and T4, n = 10; young-N, n = 13; and old-N, n = 10. (B) Hepatic IRS-1 total protein in young-df saline, n = 11; old-df saline, n = 9; young-df GH and T4, n = 12; old-df GH and T4, n = 10; young-N, n = 13; and old-N, n = 9. (C) Ratio of hepatic phosphoserine 307 IRS-1 to total IRS-1 protein in young-df saline (−), n = 5; young-df saline (+), n = 5; old-df saline (−), n = 5; old-df saline (+); n = 4, young-df GH and T4 (−), n = 4; young-df GH and T4 (+), n = 4; old-df GH and T4 (−), n = 3; old-df GH and T4 (+), n = 2; young-N (−), n = 4; young-N (+), n = 4; old-N (−), n = 4; and old-N (+), n = 4. Groups that do not share a superscript are significantly different (p < .05).
Effects of GH and T4 Replacement on Expression of Genes Related to Insulin Signaling
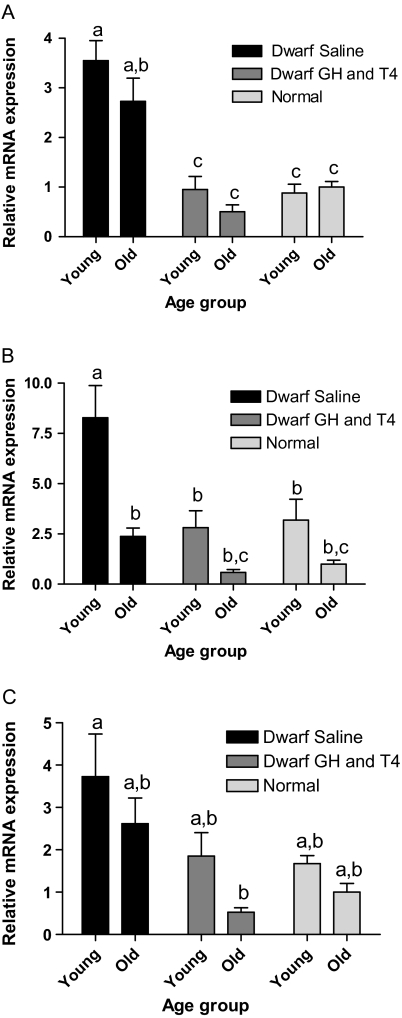
PPARγ mRNA levels in the liver were increased in young saline-treated dwarfs compared with age-matched normal animals (p < .001). Although PPARγ mRNA appeared to decline in old age, levels remained higher than young and old normal animals (p < .005). GH and T4 treatment decreased PPARγ in both age groups to levels comparable with normal animals (Figure 7A). Hepatic PGC-1α mRNA levels were higher in saline-treated young dwarfs than in age-matched normal animals (p < .01). PGC-1α mRNA decreased with age in saline-treated dwarfs to the levels observed in normal animals. GH and T4 treatment decreased PGC-1α mRNA in young and apparently also in old dwarfs, reaching levels comparable with normal animals (Figure 7B). Glucose transporter-2 (GLUT2) mRNA levels in the liver of young dwarfs were numerically higher in saline-treated dwarfs than in age-matched normal animals and appeared to decline with GH and T4 treatment. GLUT2 mRNA in GH and T4-treated old dwarfs was extremely low or undetectable by real-time PCR, so levels may have been below those observed in old normal animals (Figure 7C).
Figure 7.
Peroxisome proliferator–activated receptor (PPAR) γ in Ames dwarf (df) mice after growth hormone (GH)/thyroxine (T4) treatment. (A) Hepatic PPARγ messenger RNA (mRNA) in young-df saline, n = 12; old-df saline, n = 10; young-df GH and T4, n = 12; old-df GH and T4, n = 10; young-N, n = 13; and old-N n = 10. (B) Hepatic PGC-1α mRNA in young-df saline, n = 12; old-df saline, n = 10; young-df GH and T4, n = 12; old-df GH and T4, n = 7; young-N, n = 12; and old-N, n = 9. (C) Hepatic glucose transporter-2 mRNA in young-df saline, n = 12; old-df saline, n = 9; young-df GH and T4, n = 10; old-df GH and T4, n = 5; young-N, n = 12; and old-N, n = 8. Nondetectable levels in old-df GH and T4 resulted in low n values. Groups that do not share a superscript are significantly different (p < .05).
DISCUSSION
The enhanced insulin sensitivity and reduced plasma insulin levels observed in Ames dwarf mice are among likely mechanisms involved in the remarkable longevity of these mutants. Reduced activity of the somatotropic and thyroid axes due to deficiencies in GH and thyrotropin in these mutants is associated with a multitude of phenotypic effects that include increased insulin sensitivity and extended life span. We have recently analyzed the suspected cause–effect relationship between insulin signaling and longevity in mice (22). Naturally, other mechanistic links between GH deficiency, hypothyroidism, and aging may contribute to longevity of Ames dwarf mice. However, it is not known at which stages of life the actions of the somatotropic and thyroid axes are critical in regulating aging. The objective of the present study was to compare the effects of GH and T4 replacement therapy on important elements of the insulin signaling pathway in Ames dwarf mice of young age, during the prepubertal and peripubertal period of rapid growth and old adult age.
Our laboratory has shown that GH and T4 replacement therapy in Ames dwarfs increased body weight to more closely approach normal body weight than was achieved with injections of GH or T4 alone (J. Panici unpublished data, 2007). Vergara and colleagues (23) studied the effects of GH and T4 replacement in young Snell dwarf mice, which have the same hormone deficiencies as Ames dwarf mice. Injections of GH and T4 for 11 weeks beginning at 4 weeks of age increased growth without decreasing life extension or reducing resistance to age-related disease; however, administration of T4 in food until the end of life did decrease life span. This suggests that hormone replacement only during the prepubertal and peripubertal period of rapid growth can increase body size without diminishing longevity.
Because it has not been determined to what extent the effects of GH and T4 replacement in Ames dwarf mice may be age dependent, young and old (middle-aged) Ames dwarfs were subjected to the GH and T4 treatment protocol as described in the Methods section. Young dwarf mice were administered GH and T4 injections from 2 to 8 weeks of age because this developmental stage is a critical period of growth to reach adult body size. Administration of GH and T4 and the resulting increase in IGF-1 levels were undoubtedly responsible for the observed rapid growth of young animals. In contrast, old dwarfs administered the same doses (per gram body weight) of GH and T4 experienced small increases in body weight. The old group was 16–18 months of age, long after somatic growth has reached plateau, and perhaps, this is why the response to GH and T4 treatment was reduced compared with the young group.
Plasma insulin levels increase to compensate for decreased insulin responsiveness of target tissues, such as the liver and muscle. In contrast, Ames dwarf mice have decreased circulating insulin levels and enhanced insulin sensitivity (5,14,15). Hypoinsulinemia in Ames dwarfs is presumably due to reduced number of large islets in the pancreas (24). Our data show that saline-treated Ames dwarfs had extremely low plasma insulin levels and maintained low insulin levels into old age. GH and T4 treatment appeared to increase insulin levels further in old dwarfs than in young dwarfs. This suggests that GH and T4 treatment in older animals may have detrimental effects, such as inducing insulin resistance.
The response of target tissues to insulin is influenced by the cytokine adiponectin, which is secreted by adipocytes. Adiponectin is an insulin-sensitizing hormone that also regulates metabolism of lipids and glucose (25). Increased adiponectin levels are associated with enhanced insulin sensitivity, and adiponectin levels are elevated in Ames dwarf mice compared with normal mice (26). We have shown that GH and T4 treatment decreased plasma adiponectin in dwarf mice to the levels observed in normal mice. Decreased adiponectin may be implicated as an important factor contributing to the reduced insulin responsiveness.
Upregulation of the early steps in insulin signaling, which include increased expression of IR, IRS-1, and IRS-2, was previously observed in the liver of Ames dwarf mice and likely accounts for the increased response to insulin (14,16,17). In contrast to our previous findings in 4- to 5-month-old females (14), insulin-induced phosphorylation (activation) of IR was significantly greater in young male Ames dwarf mice than in normal animals of the same age (Figure 5C). This response to acute insulin stimulation was severely attenuated in old Ames dwarf mice, and no significant differences in the levels of tyrosine 1158 phosphorylated IR were detected between insulin- and vehicle-injected old dwarfs or normal animals of either age. Additional studies will be necessary to relate these age-related changes to the action of physiological levels of insulin in intact animals. Our data show that GH and T4 treatment decreased IR and IRS-1 mRNA and protein in dwarf mice to levels of normal animals. GH and T4 treatment also decreased insulin-stimulated phosphorylation of tyrosine 1158 of IR (IR pY1158), a cytoplasmic site important in the activation of insulin signal transduction. Transgenic mice overexpressing GH had chronic activation of IR/IRS-1/Phosphatidylinositol 3-Kinase in the liver and a reduction in the degree of insulin-induced activation compared with normal mice (27). A similar mechanism in which insulin stimulation does not alter the degree of IR pY1158 phosphorylation may be occurring in GH and T4-treated dwarf mice.
Serine 307 phosphorylation of IRS-1 (IRS-1 pS307) plays an important role in attenuation of insulin signaling (28). GH treatment is known to upregulate IRS-1 pS307 and cause insulin resistance, which can be reversed by inhibition of serine phosphorylation of IRS-1 using aspirin treatment (29). The mechanism of this regulation is not well known. However, GH activates phosphorylation of mammalian target of rapamycin (mTOR), which is linked to development of insulin resistance through its ability to regulate IRS-1 pY307 phosphorylation (30–33). mTOR signaling is reduced in Ames dwarf as compared with normal mice (34). We have shown that GH and T4 treatment decreased IRS-1 mRNA and protein in dwarf mice, and the ratio of IRS-1 pS307 to total IRS-1 protein appeared to increase, indicating attenuation of the insulin signal compared with saline-treated dwarfs, and consistent with normalization of insulin resistance by GH and T4 replacement therapy in Ames dwarf mice.
PPARs are ligand-activated transcription factors in the nuclear receptors superfamily. The levels of PPARs have been reported to decline with age (35). PPARγ is highly expressed in adipose tissue and is a major regulator of insulin and glucose metabolism. In previous studies, increased mRNA and protein levels of PPARγ were detected in the liver of 18-month-old Ames dwarfs compared with normal mice (17) and are suspected of playing a role in the increased insulin sensitivity of Ames dwarf mice. In the present study, we have shown that GH and T4 treatment decreased PPARγ in both age groups to levels comparable with normal animals.
PGC-1α is a PPAR transcriptional coactivator, and there is increasing evidence for its involvement in longevity. PGC-1α mRNA and protein levels were also increased in the liver of long-lived GH-resistant mice (36). Our data show that GH and T4 treatment decreased PGC-1α mRNA from the increased levels of saline-treated dwarfs to the levels of normal animals.
Insulin-induced glucose uptake by hepatocytes and pancreatic beta cells involves expression of GLUT2, a carrier of glucose with higher capacity but relatively lower affinity than other members of the glucose transporter family. Levels of GLUT2 mRNA appeared to decline to normal levels in young GH and T4-treated dwarfs, but this decline was more pronounced in old GH and T4-treated dwarfs, suggesting reduced glucose uptake due to insulin resistance.
Thus, it can be concluded that in comparison with middle-aged animals, treatment of juvenile Ames dwarfs with GH and T4 produced much greater stimulation of somatic growth and less pronounced attenuation of the enhanced insulin sensitivity. In both young and old dwarfs, GH and T4 treatment altered important steps in the insulin signaling pathway and “the rescue” of wild-type phenotype was achieved in young and old GH and T4-treated Ames dwarf mice. It remains to be determined how these findings relate to the potential impact of hormone replacement therapy on the longevity of these long-lived mutants or to the physiological role of the somatotropic and thyroid axes at different stages of life history in the control of aging and longevity.
FUNDING
This study was supported by National Institute on Aging, AG 19899 and U19 AG023122, The Ellison Medical Foundation, Central Research Committee of Southern Illinois University School of Medicine, Southern Illinois University Excellence in Academic Medicine Award.
Acknowledgments
We thank Steve Sandstrom for editorial assistance.
References
- 1.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- 2.Sornson MW, Wu W, Dasen JS, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- 3.Bartke A. Influence of luteotrophin on fertility of dwarf mice. J Reprod Fertil. 1965;10:93–103. doi: 10.1530/jrf.0.0100093. [DOI] [PubMed] [Google Scholar]
- 4.Bartke A. Prolactin-deficient mice. In: Alexander NJ, editor. Animal Models for Research on Contraception and Fertility. Hagerstown, MD: Harper & Row; 1979. pp. 360–365. [Google Scholar]
- 5.Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- 6.Bartke A, Coschigano K, Kopchick J, et al. Genes that prolong life: relationships of growth hormone and growth to aging and lifespan. J Gerontol A Biol Sci Med Sci. 2001;56:B340–B349. doi: 10.1093/gerona/56.8.b340. [DOI] [PubMed] [Google Scholar]
- 7.Hunter WS, Croson WB, Bartke A, Gentry MV, Meliska CJ. Low body temperature in long-lived Ames dwarf mice at rest and during stress. Physiol Behav. 1999;67:433–437. doi: 10.1016/s0031-9384(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 8.Bartke A. Delayed aging in Ames dwarf mice. Relationships to endocrine function and body size. In: Hekimi S, editor. The Molecular Genetics of Aging. Vol. 29. Berlin, Heidelberg, Germany: Springer-Verlag; 2000. pp. 181–202. [DOI] [PubMed] [Google Scholar]
- 9.Dominici FP, Argentino DP, Munoz MC, Miquet JG, Sotelo AI, Turyn D. Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Horm IGF Res. 2005;15:324–336. doi: 10.1016/j.ghir.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Bougneres PF, Artavia-Loria E, Ferre P, Chaussain JL, Job LC. Effect of hypopituitarism and growth hormone replacement therapy on the production and utilization of glucose in childhood. J Clin Endocrinol Metab. 1985;61:1152–1157. doi: 10.1210/jcem-61-6-1152. [DOI] [PubMed] [Google Scholar]
- 11.Daugaard JR, Lausten JL, Hanten BS, Richter EA. Insulin action in growth hormone-deficient and age-matched control rats: effect of growth hormone treatment. J Endocrinol. 1999;160:127–135. doi: 10.1677/joe.0.1600127. [DOI] [PubMed] [Google Scholar]
- 12.Borg KE, Brown-Borg HM, Bartke A. Assessment of the primary adrenal cortical and pancreatic hormone basal levels in relation to plasma glucose and age in the unstressed Ames dwarf mouse. Proc Soc Exp Biol Med. 1995;210:126–133. doi: 10.3181/00379727-210-43931. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh CC, DeFord JH, Flurkey K, Harrison DE, Papaconstantinou J. Effects of the Pit1 mutation on the insulin signaling pathway: implications on the longevity of the long-lived Snell dwarf mouse. Mech Ageing Dev. 2002;123:1245–1255. doi: 10.1016/s0047-6374(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 14.Dominici FP, Hauck SJ, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. J Endocrinol. 2002;173:81–94. doi: 10.1677/joe.0.1730081. [DOI] [PubMed] [Google Scholar]
- 15.Liu JL, Coschigano KT, Robertson K, et al. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287:E405–E413. doi: 10.1152/ajpendo.00423.2003. [DOI] [PubMed] [Google Scholar]
- 16.Dominici FP, Arostegui Diaz G, Bartke A, Kopchick JJ, Turyn D. Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice. J Endocrinol. 2000;166:579–590. doi: 10.1677/joe.0.1660579. [DOI] [PubMed] [Google Scholar]
- 17.Masternak MM, Al-Regaiey K, Bonkowski MS, et al. Divergent effects of caloric restriction on gene expression in normal and long-lived mice. J Gerontol A Biol Sci Med Sci. 2004;59A:784–788. doi: 10.1093/gerona/59.8.b784. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchiya T, Dhahbi JM, Cui X, Mote PL, Bartke A, Spindler SR. Additive regulation of hepatic gene expression by dwarfism and caloric restriction. Physiol Genomics. 2004;17:307–315. doi: 10.1152/physiolgenomics.00039.2004. [DOI] [PubMed] [Google Scholar]
- 19.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 20.Masternak MM, Panici JA, Wang F, Wang Z, Spong A. The effects of growth hormone (GH) treatment on GH and insulin/IGF-1 signaling in long-lived Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2010;65(1):24–30. doi: 10.1093/gerona/glp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 22.Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009;64(5):516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated Snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A Biol Sci Med Sci. 2004;59A:1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons JA, Bartke A, Sorenson RL. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology. 1995;136:2013–2021. doi: 10.1210/endo.136.5.7720649. [DOI] [PubMed] [Google Scholar]
- 25.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J Gerontol A Biol Sci Med Sci. 2005;61A:323–331. doi: 10.1093/gerona/61.4.323. [DOI] [PubMed] [Google Scholar]
- 27.Dominici FP, Cifone D, Bartke A, Turyn D. Loss of sensitivity to insulin at early events of the insulin signaling pathway in the liver of growth hormone-transgenic mice. J Endocrinol. 1999;161:383–392. doi: 10.1677/joe.0.1610383. [DOI] [PubMed] [Google Scholar]
- 28.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 29.Prattali RR, Barreiro GC, Caliseo CT, et al. Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in growth hormone treated animals. FEBS Lett. 2005;579(14):3152–3158. doi: 10.1016/j.febslet.2005.04.075. [DOI] [PubMed] [Google Scholar]
- 30.Berg CE, Lavan BE, Rondinone CM. Rapamycin partially prevents insulin resistance induced by chronic insulin treatment. Biochem Biophys Res Commun. 2002;293(3):1021–1027. doi: 10.1016/S0006-291X(02)00333-9. [DOI] [PubMed] [Google Scholar]
- 31.Gual P, Gremeaux T, Gonzalez T, Le Marchand-Brustel Y, Tanti JF. MAP kinases and mTOR mediate insulin-induced phosphorylation of insulin receptor substrate-1 on serine residues 307, 612 and 632. Diabetologia. 2003;46(11):1532–1542. doi: 10.1007/s00125-003-1223-4. [DOI] [PubMed] [Google Scholar]
- 32.Pederson TM, Kramer DL, Rondinone CM. Serine/threonine phosphorylation of IRS-1 triggers its degradation: possible regulation by tyrosine phosphorylation. Diabetes. 2001;50(1):24–31. doi: 10.2337/diabetes.50.1.24. [DOI] [PubMed] [Google Scholar]
- 33.Sun XJ, Goldberg JL, Qiao LY, Mitchell JJ. Insulin-induced insulin receptor substrate-1 degradation is mediated by the proteasome degradation pathway. Diabetes. 1999;48(7):1359–1364. doi: 10.2337/diabetes.48.7.1359. [DOI] [PubMed] [Google Scholar]
- 34.Sharp ZD, Bartke A. Evidence for down-regulation of phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory signaling pathways in Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2005;60(3):293–300. doi: 10.1093/gerona/60.3.293. [DOI] [PubMed] [Google Scholar]
- 35.Masternak MM, Bartke A. PPARs in calorie restricted and genetically long-lived mice. PPAR Res. 2007;2007:28436. doi: 10.1155/2007/28436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]