Abstract
Purpose
Children and adolescents with malignant astrocytomas recurring after initial treatment have a dismal prognosis, with only rare patients surviving one year beyond recurrence. The purpose of this study was to attempt to improve their survival.
Methods
Twenty-seven children and adolescents with malignant astrocytomas (17 glioblastoma multiforme and 10 anaplastic astrocytoma) following initial tumor progression, received myeloablative chemotherapy followed by autologous marrow rescue with one of three thiotepa and etoposide-based chemotherapy regimens, administered alone (n=11) or combined with carmustine (n=5) or carboplatin (n=11). Time to progression and death following myeloablative chemotherapy for these patients was compared non-randomly with outcome of a contemporaneously treated cohort of similar patients who received only conventional chemotherapy following initial tumor progression. The two cohorts were compared for age, histology, prior therapies, extent of surgical resection at progression and time from initial diagnosis to progression.
Results
Five of 27 children (two with glioblastoma multiforme and three with anaplastic astrocytoma) survive event-free from 8.3 to 13.3 years (median of 11.1 years) following myeloablative chemotherapy. Of 56 children with recurrent malignant astrocytoma who received conventional chemotherapy following initial progression, no patient survives. Differences in distributions of survival were not significant when stratified by surgical debulking (p=0.39). However, for patients who were surgically debulked, the survival distributions are significantly different (p=0.017).
Conclusions
Myeloablative chemotherapy with autologous marrow rescue can produce durable remissions in children and young adults with recurrent malignant gliomas, in the setting of minimal residual tumor burden achieved surgically.
Keywords: Myeloablative chemotherapy, autologous bone marrow rescue, recurrent malignant astrocytoma
INTRODUCTION
The prognosis for children and adolescents with newly diagnosed malignant astrocytomas is poor despite surgical extirpation, irradiation and conventional chemotherapy. Patients with glioblastoma multiforme (GBM) usually develop disease progression within 12 months of diagnosis and die shortly thereafter [1]. The time to progression as well as the proportion of patients who survive is somewhat more favorable for patients with anaplastic astrocytoma (AA) [2]. In an effort to improve the outcome for patients with recurrent malignant astrocytomas, myeloablative chemotherapy with autologous bone marrow rescue (ABMR) has been evaluated in several studies, the majority using carmustine alone, with generally disappointing results [3,4,5,6]. Children with recurrent brain tumors, including malignant astrocytomas, have been shown to radiographically respond to myeloablative regimens of thioTEPA and etoposide with or without the addition of carmustine, followed by marrow rescue [7,8,9,10]. We now report upon our initial cohort of 27 consecutive patients less than 21 years of age with malignant astrocytomas treated with myeloablative chemotherapy following initial tumor progression. Within an attempt to put these results into context of outcome in similar patients, the survival of patients in this review was non-randomly compared to the outcome of patients enrolled on a contemporaneous Children's Cancer Group (CCG) malignant glioma study (CCG-945), who were treated with “conventional” chemotherapy at relapse [1].
METHODS
Myeloablative Chemotherapy Regimens
Twenty-seven patients aged 5 months - 21 years (median of 10.3 years) at date of treatment, received marrow rescue following myeloablative chemotherapy with one of three regimens: thioTEPA (900 mg/m2 over three days) with etoposide (750 or 1500 mg/m2 over three days) either alone (n=11), or preceded by either carmustine (600 mg/m2 over 3 days), (n=5), or carboplatin (1500 mg/m2 over three days or dosed daily by the Calvert formula [11] with an area under the curve of 7 mg/ml/min/day) (n=11). Previously cryopreserved (n=26) or refrigerated (n=1) autologous bone marrow was infused approximately 72 hours following completion of the chemotherapy. A single patient received irradiation following treatment prior to documented tumor progression.
Conventional Chemotherapy Strategies
In the comparison group, of all patients less than 21 years of age enrolled on CCG-945 who relapsed, 60 patients received conventional chemotherapy regimens at disease progression, including: ifosfamide-containing regimens plus etoposide (n=17), etoposide plus mannitol (n=13), the “8-in-1" regimen (n=12), thioTEPA (n=7), carboplatin-containing regimens (n=9), nitrosourea-containing regimens (n=2), 5-fluorouracil/leucovorin (n=2), beta-interferon (n=1), and unspecified regimens (n=7).
Patient Characteristics
The characteristics of the patients undergoing either myeloablative or conventional chemotherapy following initial tumor progression are summarized in Tables I, II and III. All patients had radiographic documentation of recurrence. Pathological documentation of recurrence was not mandated, but was undertaken in 26 of 27 (96%) patients undergoing myeloablative chemotherapy; all patients had recurrent tumor confirmed as malignant astrocytoma, centrally reviewed by one of three neuropathologists in 26 of 27 cases. Eligibility for myeloablative chemotherapy required documentation of adequate organ function, as well as adequate hematological parameters.
Table 1.
Patient characteristics at initial diagnosis
| Standard-dose Chemotherapy |
Myeloablative Chemotherapy |
P-value | |
|---|---|---|---|
| Numbers of patients | 56 | 27 | |
| Characteristics | |||
| Median age in years (range) | 11.1 (0.1–19.3) | 8.5 (0.2–20.9) | .25 |
| Age ≤ 3 years | 7 (12%) | 6 (22%) | .33 |
| Females | 27 (48%) | 12 (44%) | .82 |
| Spinal Cord Primaries | 2 (4%) | 3 (8%) | >0.3 |
| Neuropathology | 0.25 | ||
| Glioblastoma Multiforme | 27 (48%) | 17 (63%) | |
| Anaplastic Astrocytoma | 29 (52%) | 10 (37%) |
Table 2.
Initial treatment received by the patients in both groups
| Standard-dose Chemotherapy |
Myeloablative Chemotherapy |
P-value | |
|---|---|---|---|
| Numbers of patients | 56 | 27 | |
| Treatment modality | |||
| Chemotherapy | 56 (100%) | 22 (81%) | 0.003 |
| Platinum compounds | 30 (53%) | 17 (63%) | 0.48 |
| Nitrosoureas | 56 (100%) | 20 (74%) | 0.0002 |
| Procarbazine | 30 (53%) | 11 (41%) | 0.35 |
| Cyclophosphamide | 0 (0%) | 3 (11%) | 0.032 |
| Etoposide | 0 (0%) | 5 (18%) | 0.003 |
| Irradiation Only | 0 (0%) | 3 (11%) | 0.032 |
Table 3.
Patient characteristics at initial progression
| Standard-dose Chemotherapy |
Myeloablative Chemotherapy |
||
|---|---|---|---|
| Numbers of patients | 56 | 27 | |
| Characteristics | P-value | ||
| Median age in years (range) | 12.0 (0.4–22.1) | 10.3 (0.4–21.1) | .35 |
| Interval from diagnosis to progression in months (range) | 9.7 (0.7–65.1) | 6.8 (1.1–65.2) | .77 |
| Irradiation/re-irradiation on progression | 9 (16%) | *1 (4%) | 0.16 |
| Standard-dose chemotherapy | 56 (100%) | *2 (8%) | <0.0001 |
| Surgical debulking of tumor | .003 | ||
| Debulked** | 6 (11%) | 11 (41%) | |
| Non-debulked*** | 49 | 16 |
Prior to undergoing myeloablative chemotherapy;
Extent of resection was not available for one patient;
<3 cm tumor diameter for the myeloablative and gross total resection or at least 90% (≥90%) resection for the chemo control group.
A significantly smaller proportion of the CCG-945 study patients had pathological confirmation of relapse. Four of the 60 patients on the CCG-945 study who received conventional chemotherapy at initial progression had pathological confirmation of low-grade glioma at the time of progression, and have been excluded from this present analysis. Ten of the 27 patients were included in prior preliminary publications of toxicity and response [7,8,9,10].
Response Criteria
Radiographic responses to treatment were analyzed between four and eight weeks following myeloablative chemotherapy. A complete response (CR) indicated disappearance of all measurable disease on magnetic resonance or computed tomography imaging (with and without contrast); a partial response (PR), reduction of at least 50% in tumor size (assessed as the product of the two longest tumor diameters); a minor response (MR), reduction of between 25% and 50% in tumor size; stable disease (SD), less than 25% decrease in tumor size and less than 25% increase in tumor size; progressive disease (PD), greater than 25% increase in tumor size. The designation ‘continuing complete response (CCR)’ indicated that no evidence of residual disease was identified radiographically at the time of study entry, with no evidence of disease progression at the time of follow-up evaluation.
Biostatistics
Survival distributions were estimated by the Kaplan-Meier method and tests for equality by stratified Mantel-Haenszel or stratified exact log rank statistics [12,13]. Time at risk for both survival and event-free survival (EFS) was calculated from the date of recurrence for the conventional chemotherapy group and the date of myeloablative chemotherapy for the ABMR group. In estimating event -free survival (EFS), toxic deaths, tumor progression or death from other causes, whichever developed first, were identified as events. Tumor recurrence or progression was determined radiographically. Cox Proportional Hazards models [14] were used to estimate the hazard ratio and associated 95% confidence intervals. The hazard ratio reported is the risk of death of the conventional chemotherapy group relative to the myeloablative chemotherapy patients. The data in this report reflect follow-up information updated in 2003. The significance levels (based on Fisher's exact or Wilcoxon rank-sum tests) in Tables I, II and III are not adjusted for multiple comparisons and are provided for descriptive purposes. All p-values are for two-sided tests. While this comparison suffers from the many known weaknesses of non-random historical controls and the potential biases of getting patients to transplantation, we have conservatively identified a control group for assessing outcome. Only those patients with initially confirmed malignant astrocytoma who received conventional chemotherapy following progression on CCG-945 were included.
Informed Consent for Myeloablative Chemotherapy Regimens
Informed consent was obtained for all patients prior to undergoing myeloablative chemotherapy. The options of conventional chemotherapy, surgery, irradiation, or no further therapy were particularly emphasized. Informed consent was obtained for patients treated on CCG-945 according to institutional guidelines.
RESULTS
Response to Myeloablative Chemotherapy
Five of the treated patients had no radiographically evident residual tumor, and are therefore not evaluable for assessment of response to therapy; however, they are evaluable for assessment of toxicity and tumor progression. Out of these five patients, one patient demonstrated PD 15 days following marrow rescue and four patients are CCRs. One patient is inevaluable for tumor response, having received focal re-irradiation in the immediate post-treatment period. Of the 21 patients with radiographically evident residual tumor prior to starting myeloablative chemotherapy, there were four CRs (19%; 95% CI (0.06 to 0.37)), one PR and 10 SD. One patient with residual disease demonstrated radiographic tumor progression 25 days following autologous marrow transplant. The remaining five patients with residual tumor died of toxicity. The numbers of patients with responses were evenly distributed between the three regimens.
Toxicity of Myeloablative Chemotherapy
Overall toxic mortality of the regimens was 19% (five of 27 patients; 95% CI (0.06–0.38)), toxic deaths occurring between one and 68 days (median of 17 days) following marrow rescue. A single toxic death (9%) occurred amongst the 11 patients treated with the thioTEPA/etoposide regimen. There were two toxic deaths amongst the five patients treated with the carmustine-containing regimen, due to acute renal failure with or without neurological deterioration. There were two toxic deaths (9%) amongst 11 patients treated with the carboplatin-containing regimen. The grade III/IV acute toxicity profile is similar to the previously published results on a partial cohort of patients by Gururangan et al [9]. Of the patients surviving beyond the first two months following treatment, there has been no patient with significant organ function sequelae, no patients lost their hematopoietic grafts post-treatment or developed a second malignancy. Occasional patients previously treated either with cisplatin or irradiation to the auditory apparatus developed mild to moderate sensorineural hearing loss.
Comparison of Characteristics of Patients Receiving Myeloablative and Conventional Chemotherapy
Table I shows the two groups to have similar distributions of ages at initial diagnosis, of patients less than three years of age at initial diagnosis, of sex ratios, and of patients with primary spinal cord primary tumors. There is a higher frequency of GBM in the myeloablative treated group than in the conventionally treated group; however, this did not reach statistical significance (p=0.25). The conventionally treated group had a higher prior exposure to chemotherapy, specifically to nitrosoureas. Three patients undergoing myeloablative chemotherapy had received prior irradiation without chemotherapy as compared to none of the patients in the “conventional” chemotherapy group (Table II). The two groups have similar distributions of age and of time from diagnosis to initial progression. Sixteen percent of patients receiving conventional treatment at initial progression underwent irradiation in addition to chemotherapy, while three patients receiving myeloablative chemotherapy at initial progression underwent irradiation (two prior to and one following treatment). The percentage of patients undergoing surgical debulking was significantly different between the two treatment groups (p=0.003); 41% underwent debulking in the ABMR group compared with 11% in the conventional chemotherapy group (Table III).
Event-free Survival and Survival
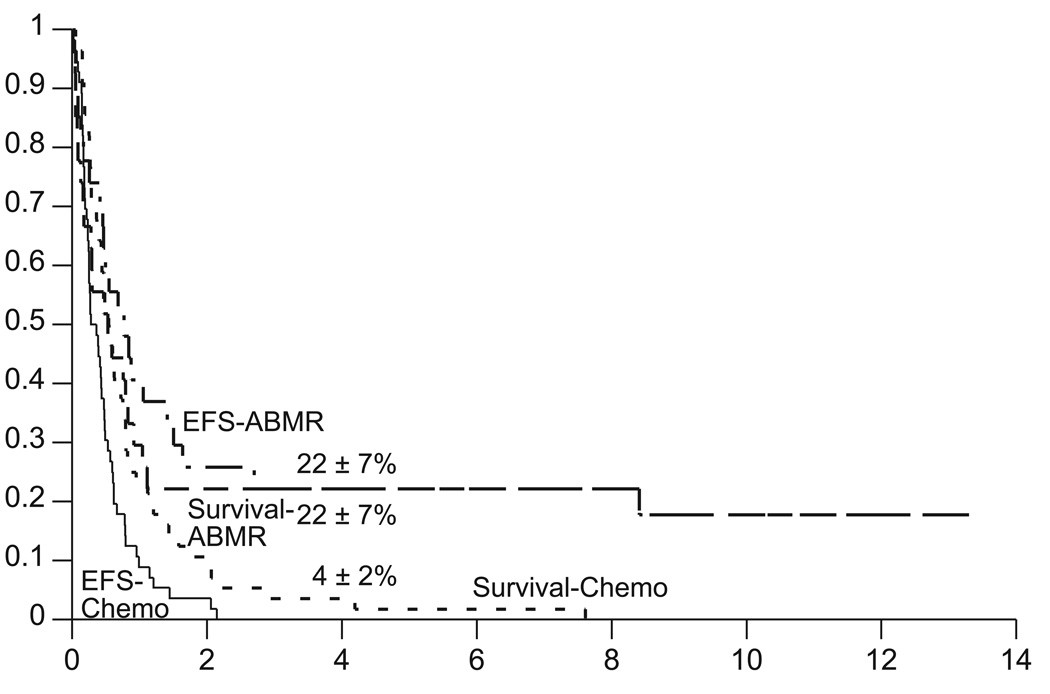
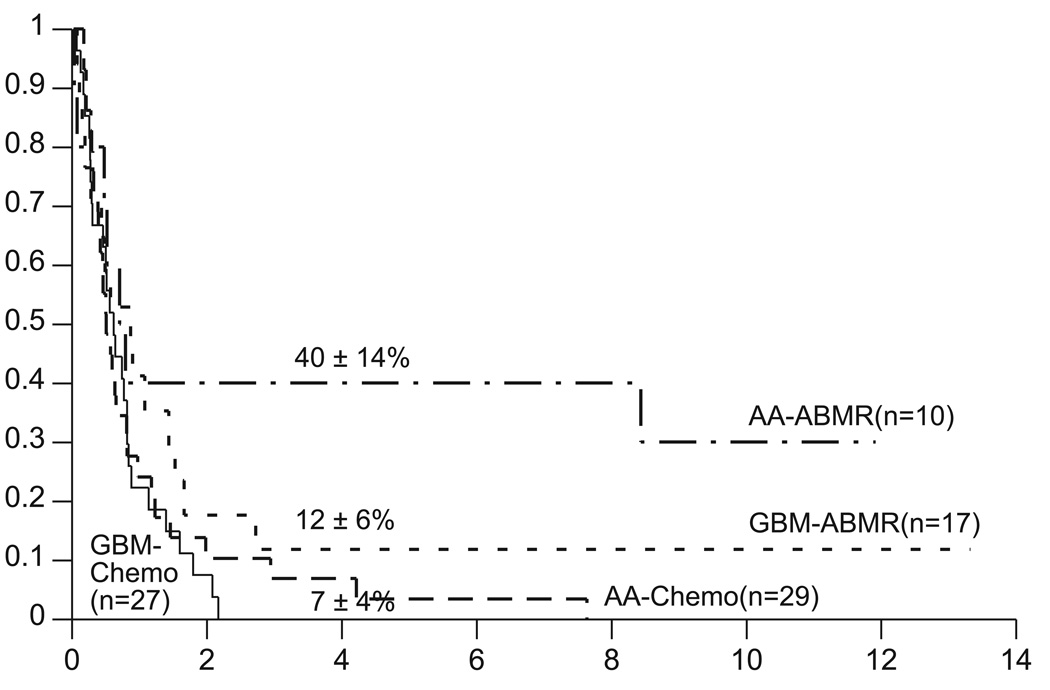
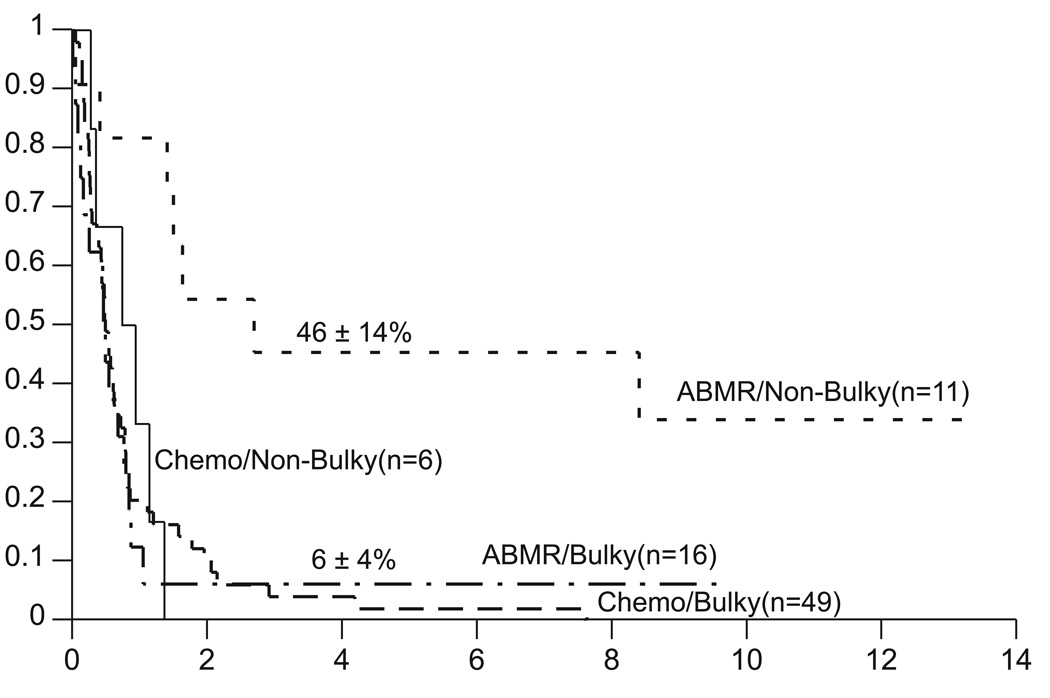
Of the 27 patients who received myeloablative chemotherapy, five died of toxicity and 17 developed disease progression. The Kaplan-Meier estimates of event-free survival (EFS) and overall survival (OS) for the myeloablative chemotherapy with ABMR and “conventional” chemotherapy control groups are shown in Figure 1. Based on these data, all subsequent comparisons of the two treatment groups are based on the primary outcome of survival. Conclusions would remain the same if EFS were the outcome of interest (data not shown). Differences in distributions of survival are significant at the p=0.010 levels when stratified for pathology (Fig. 2). For patients with GBM the survival distributions are not significantly different [p=0.16, hazard ratio=1.6 (95% confidence interval .83 – 3.1)], with four-year estimates of 12±6% with myeloablative chemotherapy and 0% with conventional chemotherapy. For patients with AA the survival distributions are significantly different [p=0.017, hazard ratio=2.7 (95% confidence interval 1.1 – 6.6)], with four-year estimates of 40±14% with myeloablative chemotherapy and 7±4% with conventional chemotherapy. Differences in distributions of survival are not significant when stratified by surgical debulking (p=0.39). However for patients who were surgically debulked, the survival distributions are significantly different (p=0.017), with four-year estimates of 46±14% with myeloablative chemotherapy and 0% with conventional chemotherapy. Two of the five survivors were infants and three survivors are more than three years of age.
Fig. 1.
Event-free survival and survival distributions for patients with recurrent malignant astrocytomas treated with either conventional chemotherapy (Chemo, n=56) or myeloablative chemotherapy with autologous bone marrow rescue (ABMR, n=27). Chemo versus ABMR unstratified comparison of event-free survival: p=0.014 [hazard ratio=1.9 (95% confidence interval 1.1–3.2)]. Chemo versus ABMR unstratified comparison of survival: p=0.018 [hazard ratio=1.9 (95% confidence interval 1.1–3.1)]
Fig. 2.
Survival distributions by histology for patients with recurrent malignant astrocytomas treated with either conventional chemotherapy (Chemo) or with myeloablative chemotherapy with autologous bone marrow rescue (ABMR). Chemo versus ABMR comparison stratified by histology: p=0.010.
Variables predictive of Survival Following Myeloablative Chemotherapy
Eleven patients survived longer than one year and five remain alive at a median of 11.1 years after transplant (range: 8.3 – 13.3 years). The single characteristic that most strongly distinguishes the surviving patients is minimal residual disease (MRD) status, as defined by less than 3 cm tumor diameter at the time of myeloablative chemotherapy (Fig. 3, unadjusted p=0.003). For patients with non-bulky tumor, the survival at four years is 46±14% versus 6±4% for those patients with bulky disease. Other potential prognostic factors were evaluated univariately and after stratifying for MRD. Patient age at diagnosis (p=0.46), age under three years at relapse (p=0.59), pathology (p=0.29), prior nitrosourea exposure (p=0.97), platinum exposure (p=0.20) and exposure to irradiation prior to undergoing myeloablative chemotherapy (p=0.11) did not impact upon survival following myeloablative chemotherapy.
Fig. 3.
Survival distributions by surgical debulking for patients with recurrent malignant astrocytomas treated with either conventional chemotherapy (Chemo) or with myeloablative chemotherapy with autologous bone marrow rescue (ABMR). Chemo versus ABMR comparison stratified by surgical debulking: p=0.39. Chemo/Non-Bulky versus ABMR/Non-Bulky unstratified exact comparison: p=.017 [hazard ratio=9.1 (95% confidence interval 1.7–47.2)].
DISCUSSION
Several therapeutic trials, in both adults and children with recurrent malignant astrocytoma, have demonstrated that the prognosis for such patients, irrespective of the therapeutic modality under evaluation, remains dismal [1–8, 15]. Agents such as carboplatin, cisplatin, ifosfamide, thiotepa, idarubicin, etoposide, topotecan, irinotecan and temozolomide have shown minimal activity with ≤10% of children demonstrating objective responses [16–24]. Although combination chemotherapy has been slightly more effective in treating recurrent malignant gliomas in children, few studies if any have shown long-term disease control [25,26,27]. Because of this failure of “conventional” chemotherapy, the ability of myeloablative chemotherapy in both adults and children has been explored with variable results.
Several studies dating back to the early 1980s, using high-dose carmustine alone followed by marrow rescue, failed to impact favorably upon survival, both in recurrent and newly-diagnosed patients with malignant astrocytoma [3,4,5,6]. The myeloablative regimen used in this study has evolved in a stepwise fashion. In 1986, a pilot study of thioTEPA and etoposide in recurrent malignant brain tumors was initiated. Subsequently, carmustine was added at a dose of 600 mg/m2 and resulted in a 38% toxic mortality in recurrent patients [10]. Due to this toxicity, a third regimen was developed, substituting carboplatin for carmustine [16,28,29]. With the implementation of the Calvert formula for basing carboplatin dosage on renal and non-renal clearance rather than body surface area or body weight, the use of hematopoietic growth factors in the post-treatment period, interventional means to prevent and ameliorate veno-occlusive disease, the recognition of importance of early harvesting of bone marrow prior to exposure of myelotoxic chemotherapy, and the replacement of bone marrow by leukapheresed peripheral blood stem cells, there has been a seeming decrease in toxic mortality in the carboplatin-containing regimen [11,30,31,32]. The number of responses was similar with each regimen. However, a definitive conclusion about the efficacy of each regimen cannot be drawn due to the small number of patients.
Even with these advances, in this series there were five toxic deaths, including three in 22 patients treated with non-carmustine-containing regimens. However, the major positive finding of this study is that in comparison to other published studies utilizing either conventional or higher-dose chemotherapy regimens, five of 27 children have experienced long term event-free survival, at a median of 11 years from progression, including two with GBM. Only one of the long-term survivors also received additional radiotherapy.
Overall patients treated with non-myeloablative retrieval therapies, including 16% who received additional radiotherapy had a survival rate of 23±5% at one year, as compared to 41±9% for those treated with high-dose chemotherapy and ABMR. These results compare favorably to a group of contemporarily treated patients entered on the CCG randomized clinical trial performed between 1985 and 1992. Comparing the two groups of patients is difficult and there are likely inherent biases in patient selection that cannot be overcome, even with careful retrospective analysis. Patients with GBM were, if anything, more likely to be referred for myeloablative therapy than those with anaplastic tumors. The apparent major difference in the two groups is that those undergoing myeloablative chemotherapy were more likely to undergo a major surgical debulking than those who went on to receive “conventional” chemotherapy with or without further radiotherapy. Despite all these issues, the fact remains that over 20% of children with malignant astrocytoma undergoing myeloablative therapy at the time of recurrence are long-term survivors compared to none who failed initial treatment and were subsequently treated with “conventional” chemotherapeutic strategies.
It is difficult to ascertain the relative contributions of myeloablative chemotherapy and surgical re-resection towards the salvage rate observed in this study. Re-resection of malignant glioma alone at recurrence, while of some palliative benefit, has never been associated with improved cure rate. Future studies should seek to determine the potential role of focused re-irradiation as an adjunct to myeloablative chemotherapy, particularly in patients unable to achieve minimal tumor burden surgically. Additionally, the effectiveness of single versus tandem sequential myeloablative chemotherapy courses can be evaluated. . This current report demonstrates that with aggressive therapy some children and adolescents can have prolonged survival following relapsed malignant astrocytoma, and a nihilistic approach in such patients will retard progress. If confirmed in prospective clinical trials, this approach could then be further investigated for the treatment of children with newly diagnosed malignant astrocytoma as well.
Supplementary Material
Acknowledgements
Drs. Charles August (formerly of Children's Hospital of Philadelphia), Bruce Bostrom (Minneapolis Children's Hospital, MN), Sal Bertolone (Korsair Children's Hospital, Louisville, KY), Lennie Sender (formerly of Korsair Children’s Hospital, Louisville, KY), Peter Coccia (University of Nebraska, Omaha, NE) and Sarah Strandjord (formerly of University of Nebraska, Omaha, NE), and Michael Stevens (formerly of Birmingham Children's Hospital, England) for their participation in the myeloablative chemotherapy pilot studies at their specified institutions. We would also like to acknowledge Dr. Lucy Rorke (Children's Hospital of Philadelphia) for neuropathological review, Dr. Robert de La Paz (formerly of Memorial Sloan-Kettering Cancer Center) for neuroradiological review, and Hao Li, MS (St. Jude Children's Research Hospital), for assistance with statistical analyses.
Contributing Children's Cancer Group Investigators, Institutions and Grant Numbers are given in the Appendix. Grant support from the Division of Cancer Treatment, National Cancer Institute, National Institutes of Health, and Department of Health and Human Services. JMB and DW are supported by grant number CA 21765 and by the American Lebanese Syrian Associated Charities.
REFERENCES
- 1.Finlay JL, Boyett JM, Yates AJ, et al. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. J Clin Oncol. 1995;13:112–123. doi: 10.1200/JCO.1995.13.1.112. [DOI] [PubMed] [Google Scholar]
- 2.Levin VA, Silver P, Hannigan J, et al. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990;18:321–324. doi: 10.1016/0360-3016(90)90096-3. [DOI] [PubMed] [Google Scholar]
- 3.Hochberg FH, Parker LM, Takvorian T, et al. High-dose BCNU with autologous bone marrow rescue for recurrent glioblastoma multiforme. J Neurosurg. 1981;54:455–460. doi: 10.3171/jns.1981.54.4.0455. [DOI] [PubMed] [Google Scholar]
- 4.Mbidde EK, Selby PJ, Perren TJ, et al. High dose BCNU chemotherapy with autologous bone marrow transplantation and full dose radiotherapy for grade IV astrocytoma. Br J Cancer. 1998;58:779–782. doi: 10.1038/bjc.1988.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson DB, Thompson JM, Corwin JA, et al. Prolongation of survival for high-grade malignant gliomas with adjuvant high-dose BCNU and autologous bone marrow transplantation. J Clin Oncol. 1987;5:783–789. doi: 10.1200/JCO.1987.5.5.783. [DOI] [PubMed] [Google Scholar]
- 6.Phillips GL, Wolff SN, Fay JW, et al. Intensive 1,3-bis 2- chlorethyl-1 nitrosourea (BCNU) monochemotherapy and autologous marrow transplantation for malignant glioma. J Clin Oncol. 1986;4:639–645. doi: 10.1200/JCO.1986.4.5.639. [DOI] [PubMed] [Google Scholar]
- 7.Finlay JL, August C, Parker R, et al. High-dose multi-agent chemotherapy followed by bone marrow “rescue” for malignant astrocytomas of childhood and adolescence. J Neurooncol. 1990;9:239–248. doi: 10.1007/BF02341155. [DOI] [PubMed] [Google Scholar]
- 8.Finlay JL, Goldman S, Wong MC, et al. Pilot study of high-dose thiotepa and etoposide with autologous bone marrow rescue in children and young adults with recurrent CNS tumors. J Clin Oncol. 1996;14:2495–2503. doi: 10.1200/JCO.1996.14.9.2495. [DOI] [PubMed] [Google Scholar]
- 9.Gurarangan S, Boyett J, Yates A, et al. Outcome following high-dose chemotherapy with autologous marrow rescue for young children with recurrent malignant brain tumors without prior irradiation. J Clin Oncol. 1998;19:2486–2493. doi: 10.1200/JCO.1998.16.7.2486. [DOI] [PubMed] [Google Scholar]
- 10.Papadakis V, Dunkel IJ, Kramer E, et al. High dose Carmustine, Thiotepa and Etoposide followed by autologous bone marrow rescue for the treatment of high risk central nervous system tumors. Bone Marrow Transplant. 2000;26:153–160. doi: 10.1038/sj.bmt.1702475. [DOI] [PubMed] [Google Scholar]
- 11.Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]; Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1996;50:163–170. [PubMed] [Google Scholar]
- 13.StatXact-Turbo & LogXact: Statistical Software for Exact Nonparametric Inference, CITEL Software Corporation, 675 Massachusetts Avenue. Cambridge, MA: 02139. [Google Scholar]
- 14.Cox DR. Regression models and life tables (with discussion) J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 15.Sposto R, Ertel IJ, Jenkin RDT, et al. The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial. J Neurooncol. 1989;7:165–177. doi: 10.1007/BF00165101. [DOI] [PubMed] [Google Scholar]
- 16.Gaynon PS, Ettinger LJ, Baum ES, et al. Carboplatin in childhood rain tumors. A Children’s Cancer Group Phase II trial. Cancer. 1990;66:2465–2469. doi: 10.1002/1097-0142(19901215)66:12<2465::aid-cncr2820661204>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 17.Bertolone SJ, Baum ES, Krivit W, et al. A phase II study of cisplatin therapy in recurrent childhood brain tumors. A report from the Children’s Cancer Study Group. J Neurooncol. 1989;7:5–11. doi: 10.1007/BF00149372. [DOI] [PubMed] [Google Scholar]
- 18.Chastagner P, Sommelet-Olive D, Kalifa C, et al. Phase II study of ifofsamide in childhood brain tumors: a report by the French Society of Pediatric Oncology (SFOP) Med Pediatr Oncol. 1993;21:49–53. doi: 10.1002/mpo.2950210110. [DOI] [PubMed] [Google Scholar]
- 19.Heideman RL, Packer RJ, Reaman GH, et al. A phase II evaluation of thiotepa in pediatric central nervous system malignancies. Cancer. 1993;72:271–275. doi: 10.1002/1097-0142(19930701)72:1<271::aid-cncr2820720147>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 20.Arndt CA, Krailo MD, Steinherz L, et al. A Phase II clinical trial of idarubicin administered to children with relapsed brain tumors. Cancer. 1998;83:813–816. doi: 10.1002/(sici)1097-0142(19980815)83:4<813::aid-cncr27>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Tirelli U, D’Incalci M, Canetta R, et al. Etoposide (VP-16-213) in malignant brain tumors: a phase II study. J Clin Oncol. 1984;2:432–437. doi: 10.1200/JCO.1984.2.5.432. [DOI] [PubMed] [Google Scholar]
- 22.Kadota RP, Stewart CF, Horn M, et al. Topotecan for the treatment of recurrent or progressive central nervous system tumors - a Pediatric Oncology Group phase II study. J Neurooncol. 1999;43:43–47. doi: 10.1023/a:1006294102611. [DOI] [PubMed] [Google Scholar]
- 23.Blaney S, Berg SL, Pratt C, et al. A phase I study of irinotecan in pediatric patients: a Pediatric Oncology Group study. Clin Cancer Res. 2001;7:32–37. [PubMed] [Google Scholar]
- 24.Ruggiero A, Cefalo G, Garre ML, et al. Phase II trial of temozolomide in children with recurrent high-grade glioma. J Neurooncol. 2006;77:89–94. doi: 10.1007/s11060-005-9011-2. [DOI] [PubMed] [Google Scholar]
- 25.Pendergrass TW, Milstein JM, Geyer JR, et al. Eight drugs in one day chemotherapy for brain tumors: experience in 107 children and rationale for preradiation chemotherapy. J Clin Oncol. 1987;5:1221–1231. doi: 10.1200/JCO.1987.5.8.1221. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe K, Kanaya H, Fujiyama Y, Kim P. Combination chemotherapy using carboplatin (JM-8) and etoposide (JET therapy) for recurrent malignant gliomas: a phase II study. Acta Neurochir (Wien) 2002;144:1265–1270. doi: 10.1007/s00701-002-1023-5. [DOI] [PubMed] [Google Scholar]
- 27.Huncharek M, Wheeler L, McGarry R, Geschwind JF. Chemotherapy response rates in recurrent/progressive pediatric glioma; results of a systematic review. Anticancer Res. 1999;19:3569–3574. [PubMed] [Google Scholar]
- 28.Warnick RE, Prados MD, Mack EE, et al. A phase II study of intravenous carboplatin for the treatment of recurrent gliomas. J Neurooncol. 1994;19:69–74. doi: 10.1007/BF01051050. [DOI] [PubMed] [Google Scholar]
- 29.Poisson M, Pereon Y, Chiras J, et al. Treatment of recurrent malignant supratentorial gliomas with carboplatin (CBDCA) J Neurooncol. 1991;10:139–144. doi: 10.1007/BF00146875. [DOI] [PubMed] [Google Scholar]
- 30.George D, Ginsberg J, Weingast R, et al. Multiorgan system failure syndrome in patients undergoing myeloablative chemotherapy and autologous bone marrow rescue for malignant brain tumors. Exp Hematol. 1994;22:379. (abstr 109) [Google Scholar]
- 31.Faulkner LB, Lindsley KL, Kher U, et al. High-dose chemotherapy with autologous marrow rescue for malignant brain tumors: analysis of the impact of prior chemotherapy and craniospinal irradiation on hematopoietic recovery. Bone Marrow Transplant. 1996;17:389–394. [PubMed] [Google Scholar]
- 32.Gardner S, Dunkel I, Bayer L, et al. Autologous stem cell rescue using peripheral blood stem cells versus bone marrow in patients with recurrent malignant brain tumors. Exp Hematol. 1996;24:1043. (abstr 130) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.