Abstract
The augmented Berlin-Frankfurt-Münster (aBFM) regimen has demonstrated improved outcomes in children with acute lymphomblastic leukemia (ALL), but efficacy in adults is unknown. In this retrospective study, we evaluated clinical outcomes in 29 adult ALL patients (ages 19–70) treated with standard BFM (sBFM) or dose-intensive aBFM. Patients were stratified into risk groups based on age, cytogenetic abnormalities, peripheral leukocytosis, and response to induction chemotherapy. Intermediate-risk patients less than 50 years old and all high-risk patients were assigned to aBFM. Complete remission after induction therapy was achieved in 93% of patients. Fifteen patients completed a full course of BFM chemotherapy, with 7 discontinuing due to relapse, 3 due to toxicity, 2 due to transplantation, and 2 toxic deaths. Five year event-free survival was 45% (95% CI 30–67%), with 39% and 50% rates of EFS observed in the aBFM and sBFM subgroups at 5 years, respectively. Overall survival at 5 years was 62% (95% CI 46–82%), with 61% and 62% in the aBFM and sBFM subgroups alive at 5 years, respectively. Two toxic deaths were observed, and infections and neuropathy were the most common toxicities. Standard and augmented BFM have efficacy and toxicity comparable with other adult ALL regimens.
Keywords: acute lymphoblastic leukemia, acute lymphocytic leukemia, Berlin-Frankfurt-Münster chemotherapy
INTRODUCTION
Over the last 40 years, multiple effective chemotherapy regimens have been developed to successfully treat acute lymphoblastic leukemia (ALL). Advances in the field of ALL treatment have primarily been demonstrated in the pediatric and adolescent populations, with a more limited progress reported in adults. Generally, children with ALL are better able to tolerate chemotherapy and experience improved outcomes compared with adults treated with similar chemotherapy programs.[1–3] The Berlin-Frankfurt-Münster (BFM) chemotherapy regimen has proven efficacious in pediatric and young adult ALL patients,[4–7] and the more dose-intensive augmented BFM chemotherapy regimen has shown improvements in overall and event-free survival even among children and adolescents at higher risk for relapse.[8,9] Based on these results, we adopted the BFM regimen for the treatment of adults with newly-diagnosed ALL.
METHODS
Patients
Approval by the University of Wisconsin Institutional Review Board was obtained for this retrospective analysis. Patients who received treatment with standard BFM (sBFM) or augmented BFM (aBFM) chemotherapy for ALL from 1987 to 2003 were identified retrospectively. Patients presenting with lymphomatous features such as an isolated mediastinal mass or a solitary peripheral mass were excluded. The diagnosis of ALL was confirmed by bone marrow biopsy on all patients, and cytogenetic analysis was available on most patients. Routine testing for specific cytogenetic abnormalities by FISH or PCR analysis was not obtained on all subjects.
Treatment
Previous data in ALL have suggested multiple prognostic factors influencing the risk of relapse.[10–14] Beginning in 1987, the standard practice at our institution was to stratify adult patients with newly-diagnosed ALL into risk groups based on several established prognostic factors including age, peripheral white blood cell count, cytogenetic characteristics, and response to induction therapy. After completing induction chemotherapy with the BFM regimen, patients underwent a bone marrow biopsy to confirm remission followed by ongoing treatment with either augmented or standard BFM chemotherapy based on their age and risk category. Stratification into risk groups based on these prognostic factors is described in Table 1. Low-risk patients received the sBFM regimen, intermediate-risk patients received aBFM if <50 years old and sBFM if ≥50 years old, and high-risk patients received aBFM. Prophylactic cranial irradiation was omitted in patients without central nervous system involvement after 1993.
Table 1.
Stratification of ALL risk groups.
| Adverse prognostic characteristics | ||
|---|---|---|
| ||
| Low risk ALL | Intermediate risk ALL | High risk ALL |
| No adverse prognostic characteristics |
Does not meet criteria for low risk ALL |
Does not meet criteria for low or intermediate risk ALL |
| AND | OR | |
| ≥ 1 adverse prognostic characteristic |
Presence of any adverse cytogenetics |
|
| BUT | OR | |
| No adverse cytogenetics | Day 28 bone marrow biopsy with persistent leukemia |
|
| Treatment by risk group | ||
| Standard BFM regimen | Age <50 years: Augmented BFM regimen |
Augmented BFM regimen |
| Age ≥50 years: Standard BFM regimen |
||
Patients were treated with the standard or augmented BFM regimen as described by Nachman et al.[9] The chemotherapy regimens for aBFM and sBFM are shown in Table 2, highlighting the differences between the treatment schedules. The aBFM regimen includes a second interim maintenance phase and intensification phase, as well as additional vincristine and L-asparaginase during the consolidation and reconsolidation phases. In the first year post-induction, patients undergoing aBFM receive a significantly increased dose-intensity of multiple chemotherapy agents, most notably of vincristine, L-asparaginase, and corticosteroids.
Table 2.
Augmented and standard BFM chemotherapy regimens.
| Induction phase (5 weeks): • VCR† 1.5 mg/m2/day IV D0,7,14,21 • PDN 60 mg/m2/day PO D0–27 • ASP 6000 units/m2/day IM thrice weekly for 9 doses starting week 1 • Daunomycin 25 mg/m2/day IV D0,7,14,21 • ARA-C 70 mg IT D0 • MTX 12 mg IT D14 | |
| Stratify into risk groups | |
| Standard BFM | Augmented BFM |
Consolidation (5 weeks):
|
Consolidation (9 weeks): |
Interim maintenance (8 weeks):
|
Interim maintenance 1 (8 weeks):
|
|
Delayed intensification – Part 1 (Reinduction, 4 weeks):
|
Delayed intensification – Part 1 (Reinduction, 4 weeks):
|
|
Delayed intensification – Part 2 (Reconsolidation, 3 weeks):
|
Delayed intensification – Part 2 (Reconsolidation, 4 weeks):
|
Maintenance (12 weeks):
|
Interim maintenance 2 (8 weeks): Same as interim maintenance 1 with addition of:
|
|
Delayed intensification – Part 2 (same as for delayed intensification part 1) | |
|
Maintenance (12 weeks): Same regimen as maintenance in standard BFM except prednisone dose is 60 mg/m2/day PO D0–4,28–32,56–60 | |
| Maintenance repeated for 6–8 cycles | |
Vincristine dose maximized at 2 mg.
Radiotherapy for CNS prophylaxis (1800 cGy in 10 fractions) delivered during the first 2 weeks of consolidation therapy. Patients with CNS involvement at diagnosis received 2400 cGy to the cranial midplane in 12 fractions and 600 cGy to the spinal cord in 3 fractions.
Abbreviations: ARA-C = cytarabine, ASP = L-asparaginase, CTX = cyclophosphamide, DEX = dexamethasone, DXR = doxorubicin, 6-MP = 6-mercaptopurine, MTX = methotrexate, PDN = prednisone, 6-TP = 6-thioguanine, VCR = vincristine. IT = intrathecal, IM = intramuscular, IV = intravenous, PO=orally, SQ=subcutaneous.
All patients underwent bone marrow aspiration and biopsy on day 28 of treatment to assess response to induction therapy. Patients received anti-infective prophylaxis with acyclovir, fluconazole, and trimethoprim/sulfamethoxazole. Patients did not receive routine growth factor support during chemotherapy.
Dose modifications
Dose modifications of vincristine were instituted primarily for neuropathy and severe liver dysfunction. Methotrexate dose modifications were made for renal failure, acute cerebral dysfunction (i.e., motor paresis, seizures, cognitive dysfunction, behavioral abnormalities), or the presence of effusions. Any symptoms of acute cerebral dysfunction thought to possibly be attributable to intrathecal methotrexate resulted in discontinuation and substitution with intrathecal cytarabine.
Daunorubicin and 6-mercaptopurine doses were reduced for events of liver dysfunction. L-asparaginase was discontinued for clinically significant liver toxicity or events of pancreatitis, thromboembolism, or bleeding complications. In general, no dose modifications were made for thrombocytopenia unless platelet counts could not be supported with intermittent platelet transfusions. Similarly, dose modifications were not made for neutropenia unless these occurred in the setting of severe recurrent infections or fever.
Statistical methods
The primary endpoints were event-free and overall survival among adult ALL patients treated with augmented or standard BFM chemotherapy. Event-free survival (EFS) was defined from the day of starting BFM chemotherapy until discontinuation of BFM chemotherapy due to relapsed leukemia, toxicity, or death from any cause. Patients undergoing transplantation in first complete remission were censored on the date of transplantation. Overall survival (OS) was defined from the day of starting BFM chemotherapy until death from any cause, and surviving patients were censored on the day of last contact. Estimates of EFS and OS were performed with the Kaplan-Meier method, and comparisons were made between the two regimens using the log-rank test.[15,16] The secondary endpoint was toxicity associated with BFM chemotherapy, with toxicity data summarized descriptively with the number of incidents and frequency.
RESULTS
Patients
Twenty-nine adult patients with newly-diagnosed ALL are included in this analysis (Table 3). Based upon the risk stratification by age and disease status, 13 patients were treated with aBFM chemotherapy and 16 patients were treated with sBFM chemotherapy. One patient with low-risk ALL received aBFM. The group consisted of 15 women and 14 men. The median age overall was 38 (range 19–72), with median ages of 37 and 45 in the subgroups treated with aBFM and sBFM, respectively. Three patients in the aBFM group had evidence of translocation 9;22, and 1 patient in the sBFM group had translocation 4;11. Only 1 patient had documented central nervous system involvement with positive spinal fluid cytology, and an additional 3 patients received prophylactic cranial irradiation. None of the patients presented with bulky mediastinal masses (>10 cm), and only 4 patients presented with significant lymphadenopathy (>2 cm). Six patients had splenomegaly with or without concurrent hepatomegaly at diagnosis. The majority of cases were B cell in origin (72%), with 8 cases of T cell ALL (5 and 3 cases in the aBFM and sBFM groups, respectively).
Table 3.
Baseline patient characteristics.
| Total (n=29) |
Augmented BFM (n=13) |
Standard BFM (n=16) |
|
|---|---|---|---|
| Age (years) | |||
| Median (range) | 37 (19–55) | 45 (21–72) | |
| <30 | 10 (34%) | 5 (38%) | 5 (31%) |
| 30–49 | 10 (34%) | 7 (54%) | 3 (19%) |
| ≥50 | 9 (31%) | 1 (8%) | 8 (50%) |
| Sex | |||
| Women | 15 (52%) | 4 (31%) | 11 (69%) |
| Men | 14 (48%) | 9 (69%) | 5 (31%) |
| Cytogenetics | |||
| Normal | 12 (41%) | 5 (38%) | 7 (44%) |
| Adverse or complex† | 9 (31%) | 6 (46%) | 3 (19%) |
| Translocation (9;22) | 3 (10%) | 3 (23%) | 0 (0%) |
| Clinical presentation | |||
| Hepatomegaly/ splenomegaly |
6 (21%) | 3 (23%) | 3 (19%) |
| Lymphadenopathy | 3 (10%) | 0 (0%) | 3 (19%) |
| Mediastinopathy | 3 (10%) | 2 (15%) | 1 (6%) |
| CSF cytology positive | 1 (3%) | 1 (8%) | 0 (0%) |
| WBC >30,000 | 11 (38%) | 7 (54%) | 4 (25%) |
| Elevated LDH | 23 (79%) | 12 (92%) | 11 (69%) |
| Histology | |||
| Pre-B cell | 19 (66%) | 8 (62%) | 11 (69%) |
| B-cell | 2 (7%) | 0 (0%) | 2 (13%) |
| T-cell | 8 (28%) | 5 (38%) | 3 (19%) |
| Treatment | |||
| Prophylactic cranial irradiation |
4 (14%)‡ | 3 (23%) | 1 (6%) |
Adverse cytogenetics include t(9;22), t(1;19), and t(4;11); complex cytogenetics are defined as >2 cytogenetic abnormalities.
One patient with documented positive CSF cytology; the remaining patients received prophylactic cranial irradiation (total dose of 1800 cGy).
Responses
A complete response, as defined by a morphologically normal marrow with <5% blasts, was achieved in 27 patients at the end of induction chemotherapy, for an overall CR rate of 93% (95% CI 77.2%–99.2%). One patient with high-risk ALL failed to achieve a remission with induction chemotherapy, and ultimately required 2 additional attempts at re-induction before achieving remission. One 70 year-old patient with standard-risk ALL died during induction therapy from complications of neutropenic sepsis.
Events and survival
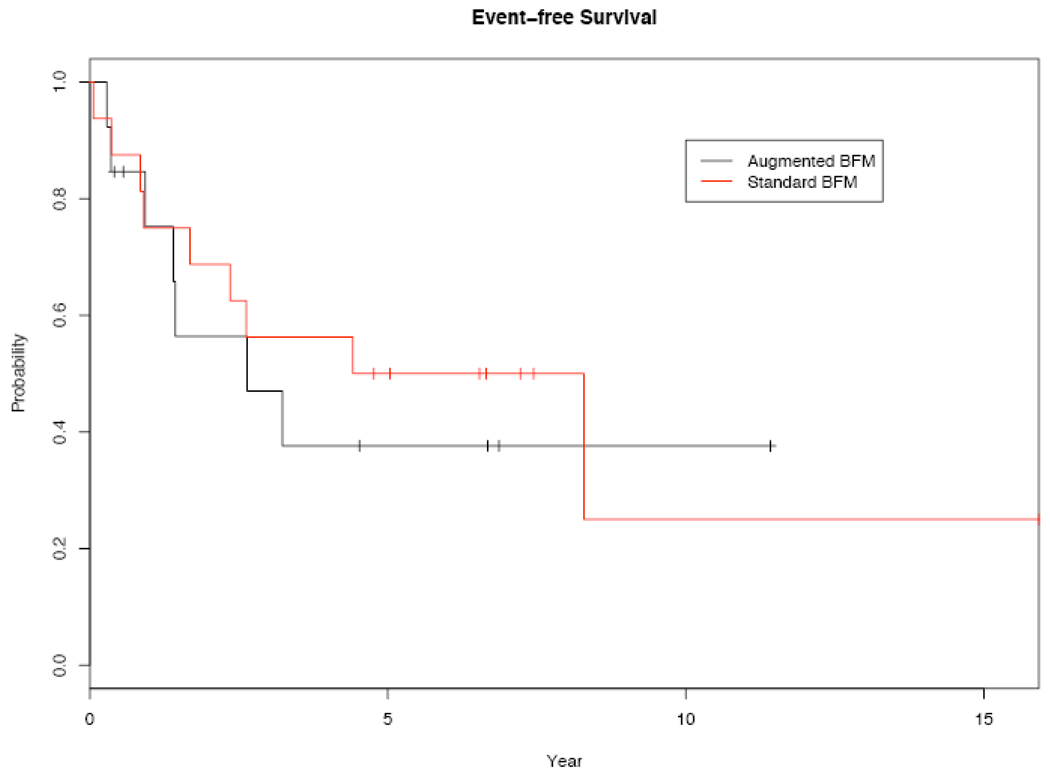
As of May 1, 2007, 16 events have been observed after a median follow-up of 6.7 years. These events consisted of 13 relapses and 3 events of early discontinuation of BFM chemotherapy related to toxicity (including 2 toxic deaths). Of the 13 relapses, 7 occurred during therapy while 6 occurred after the completion of therapy (Table 4). Two central nervous system relapses were observed, 1 as the initial presentation of relapse and 1 as occult central nervous system involvement. Due to physician preference, 3 patients within the aBFM group proceeded to stem cell transplantation (2 autologous and 1 allogeneic) in first complete remission. One of these patients had experienced excessive toxicity and early discontinuation of aBFM as an event, and the remaining 2 patients were considered censored at the time of transplant. Three and 5 year event-free survivals were 56% and 50% in the sBFM group and 47% and 39% in the aBFM group, respectively. Kaplan-Meier estimates of EFS are shown in Figure 1, and comparison of the sBFM and aBFM subgroups by log-rank analysis shows no statistically significant differences (p=0.694).
Table 4.
Outcomes with BFM regimen.
| Combined groups (n=29) |
Augmented BFM (n=13) |
Standard BFM (n=16) |
|
|---|---|---|---|
| Treatment tolerability | |||
| Completed full course of BFM† |
15 (52%) | 5 (38%) | 10 (63%) |
| Discontinued BFM early (without relapse) |
6 (21%) | 3 (23%) | 3 (19%) |
| Discontinued BFM early (with relapsed or refractory disease) |
7 (24%) | 5 (38%) | 3 (19%) |
| Treatment-related mortality |
2 (7%) | 0 (0%) | 2 (13%) |
| Responses to induction therapy‡ | |||
| Refractory disease | 1 (4%) | 1 (8%) | 0 (0%) |
| Complete remission | 27 (93%) | 12 (92%) | 15 (94%) |
| Relapses | |||
| Relapsed disease | 13 (45%) | 6 (46%) | 7 (44%) |
| Relapse during BFM | 7 (24%) | 4 (31%) | 3 (19%) |
| Relapse after BFM | 6 (21%) | 2 (15%) | 4 (25%) |
| CNS relapses* | 2 (7%) | 0 (0%) | 2 (13%) |
Includes patients completing all phases of BFM including ≥6 cycles of maintenance chemotherapy.
One patient in the standard BFM group died from toxicity during induction and is considered a non-responder.
Nine of 13 relapses with documented lumbar punctures at relapse.
Figure 1.
Kaplan-Meier plots of event-free survival (n=29).
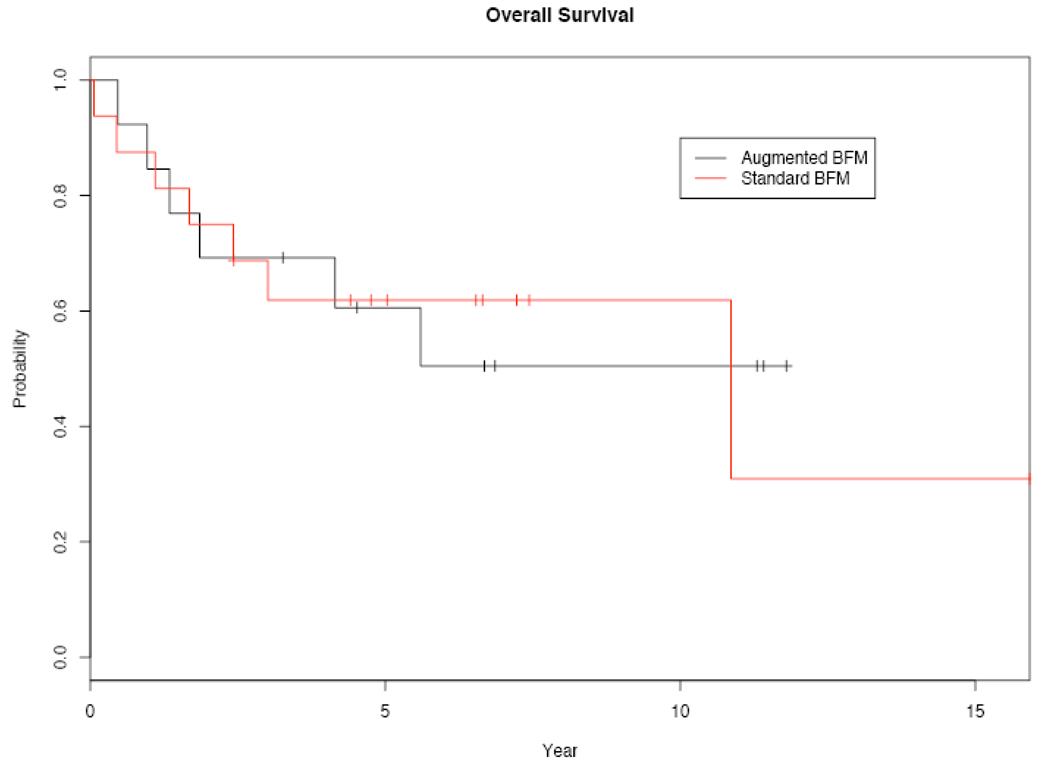
Thirteen deaths have been observed, with 9 deaths directly related to relapsed, progressive leukemia. Two patients died from toxicity of BFM therapy without evidence of relapse, and 2 additional patients died from complications following allogeneic transplant without evidence of relapse. Overall survivals at 3 and 5 years were 69% and 62% in the sBFM group and 69% and 61% in the aBFM group, respectively. Kaplain-Meier estimates of OS are shown in Figure 2, and comparison of the subgroups by log-rank analysis again shows no statistically significant differences (p=0.948).
Figure 2.
Kaplan-Meier plots of overall survival (n=29).
Toxicity
All patients receiving induction therapy with BFM are included in the analysis of toxicity (Table 5). Infectious complications and sensory neuropathy were the most commonly experienced toxicities. Events of grade ≥3 neutropenic fever and neutropenic infections were comparable in number between the augmented and standard BFM groups, but more patients in the sBFM completed a full course of therapy and were evaluable for toxicity for a longer period. Two deaths related to infectious complications were observed, both in the sBFM group.
Table 5.
Toxicity events from BFM chemotherapy.
| Augmented BFM (n=13) |
Standard BFM (n=16) |
|||||
|---|---|---|---|---|---|---|
| Event | Gr2 | Gr3 | Gr4 (5) | Gr2 | Gr3 | Gr4 (5) |
| Infectious events | ||||||
| Neutropenic fever | 0 | 6 | 0 | 1 | 13 | 0 |
| Infection with neutropenia† | 0 | 7 | 3 | 0 | 3 | 1 (1) |
| Infection without neutropenia or with unknown ANC |
2 | 2 | 0 | 9 | 11 | 1 (1) |
| Neurologic events | ||||||
| Sensory neuropathy | 9 | 0 | 0 | 13 | 1 | 0 |
| Motor neuropathy | 2 | 1 | 0 | 0 | 0 | 0 |
| Encephalopathy and confusion | 0 | 2 | 0 | 3 | 0 | 0 |
| Hepatic/Gastrointestinal events | ||||||
| Constipation/ileus | 2 | 2 | 0 | 3 | 3 | 0 |
| Nausea | 7 | 4 | 0 | 6 | 1 | 0 |
| Pancreatitis | 0 | 0 | 0 | 0 | 1 (0) | |
| Liver dysfunction/Hepatitis‡ | 1 | 1 | 0 | 3 | 2 | 0 |
| Serum total bilirubin | 5 | 2 | 1 | 1 | 2 | 2 |
| Serum transaminases | 15 | 2 | 0 | 18 | 9 | 0 |
| Hematologic events | ||||||
| Thromboembolic events | 0 | 1 | 1 | 2 | 4 | 0 |
| Hemorrhage | 0 | 1 | 0 | 0 | 1 | 0 |
| DIC | 0 | 0 | 0 | 0 | 1 | 0 |
| Pulmonary events | ||||||
| Pneumonitis | 0 | 0 | 0 | 0 | 1 | 0 |
| Renal events | ||||||
| Renal failure | 0 | 0 | 0 | 0 | 1 | 1 (0) |
| Musculoskeletal events | ||||||
| Avascular necrosis | 1 | 0 | 0 | 0 | 1 | 0 |
| Weakness | 0 | 0 | 0 | 0 | 3 | 0 |
Grade 3 or 4 neutropenia as defined by CTCAE, v 2.0.
Peripheral neuropathy was common in both groups, with grade ≥2 sensory neuropathy experienced by 9 (69%) patients in the aBFM group and 14 (88%) patients in the sBFM group. Almost all patients experienced abnormalities in liver function tests (LFTs), primarily related to methotrexate, L-asparaginase, and/or 6-mercaptopurine. Only 3 patients in the aBFM group and 2 patients in the sBFM group did not experience any elevation in LFTs. Elevations in LFTs were moderate, but only 7 (24%) patients developed clinical signs or symptoms of liver dysfunction associated with LFT abnormalities. The induction phase of chemotherapy was the most common period during which grade ≥2 elevations of serum LFTs were observed. Within the sBFM group, the first event of elevated LFTs was during induction chemotherapy for 9 patients; thereafter, 2 patients were first observed to experience elevated LFTs during consolidation and/or delayed intensification, and only 3 patients first experienced elevated LFTs during the maintenance phase of chemotherapy. Within the aBFM group, the first event of elevated LFTs was observed in 8 patients during induction. An additional 2 patients in the aBFM group experienced the first event of elevated LFTs during consolidation and/or delayed intensification, and all patients experiencing elevated LFTs during maintenance had events during previous phases of treatment.
One patient in the sBFM group never received L-asparaginase due to toxicity concerns. None of the patients developed anaphylactic reactions to E. coli L-asparaginse. Venous thromboembolic complications occurred in 6 patients, with all but 1 event related to indwelling venous catheters. Avascular necrosis of the femoral heads was observed in 2 patients.
The full chemotherapy course was completed in 15 patients, 5 (38%) in the aBFM group and 10 (63%) in the sBFM group (Table 4). The reasons for early discontinuation in the aBFM group were relapsed or refractory disease (n=5), excessive toxicity from chemotherapy (n=1), and transplantation in first remission without intolerance to aBFM (n=2). The reasons for early discontinuation among 6 patients in the sBFM group were relapsed disease (n=3) and excessive toxicity from chemotherapy (n=3).
DISCUSSION
The BFM chemotherapy regimen has been a standard treatment for pediatric ALL, with promising outcomes in terms of remission and long-term survival.[4–9] In an attempt to improve upon these results with standard BFM chemotherapy, the Children’s Cancer Group (CCG) developed a more dose-intensive version of BFM chemotherapy, termed augmented BFM, which ultimately demonstrated improved survival among high-risk pediatric patients with a slow early response to induction therapy.[8] The results with aBFM suggested that application of this more dose-intensive regimen may overcome the effects of several adverse prognostic factors, and subsequently a randomized study of standard and augmented BFM in high-risk pediatric ALL confirmed a significant improvement in 5 year EFS (75% vs. 55%, p<.001) and OS (78% vs. 67%, p=.02) with aBFM compared with sBFM.[9] However, it is unknown whether these same benefits may be extrapolated to adult populations. Similarly, limited data are available in terms of the tolerability and efficacy of BFM chemotherapy in adult ALL.
Multiple other complex chemotherapy regimens have been reported in the treatment of adult ALL, with response rates of approximately 80–90% and long-term disease-free survival of 30–40%.[12–14,17–25] In our small single-institution experience with BFM chemotherapy in adults, the rate of complete remission exceeding 90% with induction therapy is excellent and comparable to remission rates reported with other adult ALL chemotherapy regimens. Similarly, the observed rates of long-term EFS (45% at 5 years) and OS (62% at 5 years) compare favorably to outcomes reported with other ALL regimens that incorporate similar chemotherapy agents during the induction, consolidation, and maintenance phases.[12–14,17–19]
Interestingly, overall and event-free survival at 3 and 5 years did not differ between the groups stratified by risk to receive either aBFM or sBFM, suggesting that use of a more dose-intensive chemotherapy approach with aBFM may improve outcomes in the setting of adverse prognostic indicators in adult ALL. Although our data support this possibility, the small sample size limits such a definitive conclusion. However, multiple other reports have demonstrated consistent and significant disparity between outcomes in standard-risk and high-risk ALL using criteria for risk stratification very similar to those applied in our report. For example, results of the French LALA-87 trial found that adult patients with high-risk ALL treated with chemotherapy alone had an overall 10-year survival of <20% versus 40% in a comparison group with standard-risk disease.[26] A follow-up study (LALA-94) utilized a complex stratification by disease risk and availability of a sibling donor to randomize patients to chemotherapy or autologous or allogeneic transplantation. The high-risk group (n=59) assigned to chemotherapy alone contained patients with adverse prognostic features similar to the high-risk group reported in our study. The high-risk group had very poor outcomes, with 3- and 5-year overall survival rates of 35% and 21%, respectively. In comparison, patients with standard-risk ALL had 3- and 5-year survival rates of 45% and 43%, respectively.[27]
Although aBFM was effective in our high risk population, there clearly remains room for improvement. A recently published randomized clinical trial indicates that selected patients have superior outcomes when offered allogeneic stem cell transplantation (alloSCT) in first CR.[28,29] Aside from patients with Philadelphia chromosome positive ALL, it is presently unclear exactly which adult ALL patients should be offered alloSCT in first CR. A major challenge at present is to appropriately risk stratify patients so that those in need of alloSCT receive it, while those who can be cured with intensive chemotherapy are spared the transplant-related risks. Optimizing the frontline approach is particularly critical given the extremely poor outcomes reported in relapsed adult ALL.[28]
In addition, several retrospective reviews suggest improved survival for older adolescents and young adults treated on pediatric chemotherapy protocols.[30–34] For example, a comparison of outcomes among adolescents treated in the French pediatric ALL protocol FRALLE-93 (n=77) and the adult ALL protocol LALA-94 (n=100) found that 5 year EFS significant favored the patients in the pediatric protocol (67% versus 41%, p<.0001).[31] Similarly, outcomes among young adolescents aged 15–18 treated on the pediatric Dutch Childhood Oncology Group (DCOG) or adult Dutch-Belgian Hemato-Oncology Cooperative Study Group (HOVON) showed a 5 year EFS of 69% on the DCOG protocols versus 34% on the adult HOVON protocols (p=.0001).[32] Finally, a large retrospective review comparing outcomes of 321 adolescent and young adults ages 16–20 treated on Cancer and Leukemia Group B (CALGB) and CCG studies from 1988–2001 found that despite identical complete remission rates of 90% between the pediatric and adult protocols, 7 year rates of EFS and OS significantly favored patients treated on the CCG protocols (EFS 64% versus 34%, OS 67% versus 46%). Further analysis by age found very poor outcomes for 18–20 year olds, with 7 year EFS of only 29% observed in the CALGB studes.[30] Based on these compelling data, protocols such as the Children’s Oncology Group AALL0232 are allowing accrual of high-risk young adult patients up to age 30.
BFM chemotherapy was feasible in this adult ALL population, and the majority of patients were able to tolerate the full protocol treatment. Although 2 toxic deaths were observed with sBFM, this is comparable to toxicities experienced in adult ALL populations with other regimens and is not unexpected given the older age of the patients in this group.[12–14,17–19] Both the augmented and standard regimens demonstrated significant peripheral neuropathy and myelosuppression with frequent infectious complications, and the numbers of serious events were relatively similar between the groups. Ultimately, our data support that both sBFM and aBFM are feasible chemotherapy regimens for adult patients with ALL, with efficacy that appears comparable to outcomes observed with other regimens used in adult ALL.
ACKNOWLEDGMENTS
Grant support
The biostatistical work was supported in part from a P30 CA14520 grant and a T32 GM074904 training grant.
Footnotes
Conflict of interest
The authors have no conflicts of interest to report.
REFERENCES
- 1.Copelan EA, McGuire EA. The biology and treatment of acute lymphoblastic leukemia in adults. Blood. 1995;85:1151–1168. [PubMed] [Google Scholar]
- 2.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 3.Laport GF, Larson RA. Treatment of adult acute lymphoblastic leukemia. Semin Oncol. 1997;24:70–82. [PubMed] [Google Scholar]
- 4.Riehm H, Gadner H, Henze G, Kornhuber B, Langermann HJ, Müller-Weihrich S, et al. Acute lymphoblastic leukemia: treatment results in three BFM studies (1970–81) In: Murphy SB, Gilbert JR, editors. Leukemia Research: Advances in Cell Biology and Treatment. New York: Elsevier Science Publishing; 1983. pp. 251–263. [Google Scholar]
- 5.Riehm H, Langermann HJ, Gadner H, Odenwald E, Henze G. The Berlin childhood acute lymphoblastic leukemia therapy study, 1970–1976. Am J Ped Hematol Oncol. 1980;2:299–306. [Google Scholar]
- 6.Gaynon PS, Steinherz PG, Bleyer WA, Albin AR, Albo VC, Finkelstein JZ, et al. Intensive therapy for children with acute lymphoblastic leukaemia and unfavourable presenting features: Early conclusions of study CCG-106 by the Children’s Cancer Study Group. Lancet. 1988;22:921–924. doi: 10.1016/s0140-6736(88)92596-2. [DOI] [PubMed] [Google Scholar]
- 7.Henze G, Langermann HJ, Bramswig J, Breu H, Gadner H, Schellong G, et al. The BFM 76/79 acute lymphoblastic leukemia therapy study. Klinische Padiatrie. 1981;193:145–154. doi: 10.1055/s-2008-1034450. [DOI] [PubMed] [Google Scholar]
- 8.Nachman J, Sather HN, Gaynon PS, Lukens JN, Wolff L, Trigg ME. Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: a report from the Children’s Cancer Group. J Clin Oncol. 1997;15:2222–2230. doi: 10.1200/JCO.1997.15.6.2222. [DOI] [PubMed] [Google Scholar]
- 9.Nachman JB, Sather HN, Sensel MG, Trigg ME, Cherlow JM, Lukens JN, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 10.Hoelzer D, Thiel E, Loffler H, Bodenstein H, Plaumann L, Buchner T, et al. Intensified therapy in acute lymphoblastic and acute undifferentiated leukemia in adults. Blood. 1984;64:38–44. [PubMed] [Google Scholar]
- 11.Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–3767. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 12.Hoelzer D, Thiel E, Löffler H, Büchner T, Ganser A, Heil G, et al. Prognostic factors in a multicentric study for treatment of acute lymphoblastic leukemia in adults. Blood. 1988;71:123–131. [PubMed] [Google Scholar]
- 13.Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: Cancer and Leukemia Group B Study 8811. Blood. 1995;85:2025–2037. [PubMed] [Google Scholar]
- 14.Gaynor J, Chapman D, Little C, McKenzie S, Miller W, Andreeff M, et al. A cause-specific hazard rate analysis of prognostic factors among 199 adults with acute lymphoblastic leukemia: the Memorial Hospital experience since 1969. J Clin Oncol. 1988;6:1014–1030. doi: 10.1200/JCO.1988.6.6.1014. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- 16.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;50:163–170. [PubMed] [Google Scholar]
- 17.Annino L, Vegna ML, Camera A, Specchia G, Visani G, Fioritoni G, et al. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002;99:863–871. doi: 10.1182/blood.v99.3.863. [DOI] [PubMed] [Google Scholar]
- 18.Linker CA, Levitt LJ, O’Donnell M, Forman SJ, Ries CA. Treatment of adult acute lymphoblastic leukemia with intensive cyclical chemotherapy: a follow-up report. Blood. 1991;78:2814–2822. [PubMed] [Google Scholar]
- 19.Hussein KK, Dahlberg S, Head D, Waddell CC, Dabich L, Weick JK, et al. Treatment of acute lymphoblastic leukemia in adults with intensive induction, consolidation, and maintenance chemotherapy. Blood. 1989;73:57–63. [PubMed] [Google Scholar]
- 20.Schauer P, Arlin ZA, Mertelsmann R, Cirrincione C, Friedman A, Gee TS, et al. Treatment of acute lymphoblastic leukemia in adults: results of the L-10 and L-10M protocols. J Clin Oncol. 1983;1:462–470. doi: 10.1200/JCO.1983.1.8.462. [DOI] [PubMed] [Google Scholar]
- 21.Taylor PR, Reid MM, Bown N, Hamilton PJ, Proctor SJ. Acute lymphoblastic leukemia in patients aged 60 years and over: a population-based study of incidence and outcome. Blood. 1992;80:1813–1817. [PubMed] [Google Scholar]
- 22.Chessells JM, Hall E, Prentice HG, Durrant J, Bailey CC, Richards SM. The impact of age on outcome in lymphoblastic leukaemia; MRC UKALL X and XA compared: a report from the MRC Paediatric and Adult Working Parties. Leukemia. 1998;12:463–473. doi: 10.1038/sj.leu.2400959. [DOI] [PubMed] [Google Scholar]
- 23.Gokbuget N, Hoelzer D, Arnold R, Böhme A, Bartram CR, Freund M, et al. Treatment of adult ALL according to protocols of the German Multicenter Study Group for Adult ALL (GMALL) Hematol Oncol Clin North Am. 2000;14:1307–1325. doi: 10.1016/s0889-8588(05)70188-x. [DOI] [PubMed] [Google Scholar]
- 24.Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;13:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 25.Linker C, Damon L, Ries C, Navarro W. Intensified and shortened cyclical chemotherapy for adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20:2464–2471. doi: 10.1200/JCO.2002.07.116. [DOI] [PubMed] [Google Scholar]
- 26.Thiebaut A, Vernant JP, Degos L, Huguet FR, Reiffers J, Sebban C, et al. Adult acute lymphocytic leukemia study testing chemotherapy and autologous and allogeneic transplantation: a follow-up report of the French protocol LALA 87. Hematol Oncol Clin N Amer. 2000;14:1353–1365. doi: 10.1016/s0889-8588(05)70190-8. [DOI] [PubMed] [Google Scholar]
- 27.Thomas X, Boiron J-M, Huguet F, Dombret H, Bradstock K, Vey N, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;20:4075–4086. doi: 10.1200/JCO.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 28.Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL): an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 29.Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UK ALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 30.Stock W, La M, Sanford B, Bloomfield CD, Vardiman JW, Gaynon P, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112:1646–1654. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boissel N, Auclerc MF, Lhéritier V, Perel Y, Thomas X, Leblanc T, et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol. 2003;21:774–780. doi: 10.1200/JCO.2003.02.053. [DOI] [PubMed] [Google Scholar]
- 32.De Bont JM, van der Holt B, Dekker AW, van der Does-van den Berg A, Sonneveld P, Pieters R. Significant difference in outcomes for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands. Leukemia. 2004;18:2032–2053. doi: 10.1038/sj.leu.2403538. [DOI] [PubMed] [Google Scholar]
- 33.Nachman J. Clinical characteristics, biological features and outcome for young adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2005;130:166–173. doi: 10.1111/j.1365-2141.2005.05544.x. [DOI] [PubMed] [Google Scholar]
- 34.Vitale A, Guarini A, Chiaretti S, Foá R. The changing scene of adult acute lymphoblastic leukemia. Curr Opin Oncol. 2006;18:652–659. doi: 10.1097/01.cco.0000245317.82391.1b. [DOI] [PubMed] [Google Scholar]