Abstract
Human embryonic stem cells (hESC) are capable of give rise to all cell types in the human body during the normal course of development. Therefore, these cells hold a great promise in regenerative cell replacement based therapeutical approaches. However, some controversy exists in literature concerning the ultimate fate of hESC after exposure to genotoxic agents, in particular, regarding the effect of DNA damaging insults on pluripotency of hESC. To comprehensively address this issue, we performed an analysis of the expression of marker genes, associated with pluripotent state of hESC, such as Oct-4, Nanog, Sox-2, SSEA-4, TERT, TRA-1-60 and TRA-1-81 up to 65 hrs after exposure to ionizing radiation (IR) using flow cytometry, immunocytochemistry and quantitative real-time polymerase chain reaction techniques. We show that irradiation with relatively low doses of gamma-radiation (0.2 Gy and 1 Gy) does not lead to loss of expression of the pluripotency-associated markers in the surviving hESC. While changes in the levels of expression of some of the pluripotency markers were observed at different time points after IR exposure, these alterations were not persistent, and, in most cases, the expression of the pluripotency-associated markers remained significantly higher than that observed in fully differentiated human fibroblasts, and in hESCs differentiated into definitive endodermal lineage. Our data suggest that exposure of hESC to relatively low doses of IR as a model genotoxic agent does not significantly affect pluripotency of the surviving fraction of hESC.
Keywords: human embryonic stem cells, genotoxic agent, pluripotency marker, ionizing radiation, directed differentiation
1. Introduction
Human embryonic stem cells (hESCs) possess the capacity to differentiate into all cell types in the body (pluripotency) and, as such, can serve as a valuable model of embryonic development. Human ESCs are an ultimate source of differentiated cells that may be used in cell-based substitutive therapy (Liew et al., 2005). To fully benefit from the regenerative potential of hESCs in clinical settings one has to anticipate problems inherent to the unique biological characteristics of ES cells. The key properties of ES cells under normal conditions are their ability to self-renew and to maintain pluripotency. However, published data concerning the ultimate fate of ES cells after exposure to genotoxic stress are somewhat contradictory. On the one hand, both murine, non-human primate and human ES cells were shown to be hypersensitive to DNA damaging agents and respond by undergoing apoptosis and/or differentiation (Aladjem et al., 1998; Hong and Stambrook, 2004; Lin et al., 2005; Qin et al., 2007). It is also known that the developing human embryo is considered to be among the most vulnerable to genotoxic agent exposures (McCollough et al., 2007). On the other hand, a more recent study suggests that hESC maintain pluripotency for at least 24 hours after 2 Gy of IR exposure (Momcilovic et al., 2009). Hence, how DNA damaging agents, for instance, IR exposure with relatively low doses, might affect the pluripotency state of hESCs remains to be addressed.
The key regulators of pluripotency are transcription factors Oct-4, Nanog and Sox-2; they are found to be expressed in undifferentiated stem cells (Matin et al., 2004; Boyer et al., 2005; Hyslop et al., 2005). Together with these factors comprising the core of the transcription regulatory circuitry underlying undifferentiated state of stem cells, hESCs can be characterized by the expression of SSEA-4, TRA-1-60, TRA-1-81 and TERT (Ginis et al., 2004; Fong et al., 2009). In order to shed light on how genotoxic stress such as IR affects the pluripotent state of hESC in culture, in this study we comprehensively characterized the expression of these markers after IR exposures of hESC using three independent methodologies. In addition, in this study we cultivated hESC using feeder free conditions to avoid potential effects of MEFs on the measurements of expression of pluripotency markers.
2. Materials and methods
2.1. Cell Lines and Cell Culture
Initially hESCs (H9 cell line, WiCell, Madison, WI, passage 35 - 40) were maintained on a feeder layer of irradiated MEFs using medium consisting of 80% Knockout Dulbecco’s modified Eagle’s medium (KO-DMEM, Invitrogen, Carlsbad, CA) supplemented with 15% Fetal bovine serum (Invitrogen), 5% Knockout serum replacement (KSR, Invitrogen), 0.1 mM 2-mercaptoethanol (Sigma, St.Louis, MO), 1% non-essential amino acids, 2 mM L-Alanyl-L-glutamine and 4 ng/ml basic fibroblast growth factor (bFGF, Invitrogen). Cell cultures were passaged using Collagenase IV (Invitrogen) every 6-7 days, only phenotypically uniform hESC colonies were collected. Subcequently, hESCs were transferred to feeder-independent culture conditions, using BD Matrigel hESC-qualified Matrix (BD Biosciences, San Jose, CA), and grown in mTeSR-1 (Stemcell Technologies, Vancouver, Canada) at 37°C and 5% CO2. Cell cultures were maintained and expanded following manufacturer’s protocol. The medium was changed every day.
BJ and IMR-90 normal human diploid fibroblast (ATCC, Manassas, VA) were grown in Earle’s modified Eagle’s medium (EMEM) supplemented with 10% fetal bovine serum, non-essential amino acids, 1 mM sodium pyruvate and 2 mM L-glutamine (Invitrogen) at 37°C and 5% CO2 and passaged every 5–7 days using 0.5% Trypsin-EDTA.
Exposure of cells to ionizing radiation was accomplished as follows: cultured cells were divided into three groups and were exposed either to 0.2 Gy or 1Gy of 60Co gamma-radiation using Eldorado 8 60Co teletherapy unit (MDS Nordion, Ottawa, Ontario, Canada, formerly Atomic Energy of Canada, Ltd.; dose rate about 1 Gy/min), or, alternatively, were sham-irradiated. Cells then were returned to CO2 incubator and collected at 17 h, 41 h and 65 h post-irradiation for analysis. These time points correspond to approximately 1, 2 and 3 average duplication time for H9 hESC line (Becker et al., 2006).
2.2. Directed differentiation of hESC into definitive endoderm
H9 hESCs were seeded onto 6-well plates covered with BD Matrigel hESC-qualified Matrix (BD Biosciences) at 105 cells per well. Then, the cells were maintained in mTeSR1 medium at 5% CO2 and 37°C for two days with medium changed every day. Starting from day three cells in culture were maintained in differentiation medium (DMEM/F12 supplemented with 20% KSR, 100 ng/ml Activin A, 4 ng/ml bFGF and 20μM LY294002), which was changed every day (McLean et al., 2007). After four days of differentiation cells were trypsinized and collected for further studies.
2.3. Immunocytochemistry
For immunohistochemistry cells were grown on glass-bottom LabTek® two-well Chamber Slide™ (BD Biosciences) in the feeder-free conditions described above. The cell cultures were fixed with 4% paraformaldehyde for 10 minutes, and then permeabilized with 0.1% Triton-X-100 in phosphate-buffered saline (PBS) for 5 minutes. Primary antibodies were applied for 1-2 hours (overnight at 4°C for cleaved caspase 3), and appropriately coupled Alexa Fluor secondary antibodies (Invitrogen) were used for single or double labeling for 1 hour. All secondary antibodies were tested for nonspecific immunoreactivity. The following primary antibodies were used: Oct-4, SSEA4, TRA-1-81, Nestin, and Sox7 (Santa Cruz Biotechnology, Santa Cruz, CA), cleaved caspase 3 (Cell Signaling Technology, Danvers, MA), TERT and Brachyury (Abcam, Cambridge, MA). DAPI stain was used to identify the nuclei. After mounting in antifade media (VectaShield, Vector Laboratories, Inc., Burlingame, CA), the samples were examined by Axioplan Zeiss epifluorescent microscope (Carl Zeiss, Thornwood, NY). The camera exposure time and microscope settings were kept constant across all corresponding samples.
2.4. Cell viability and Flow cytometry
At the indicated time points, flasks containing hESCs were rinsed with PBS supplemented with 0.5% bovine serum albumin (BSA, Sigma) to remove detached cells. Then remaining cells, that we call surviving fraction, were collected by treatment with Trypsin-EDTA for 3 min at 37C, and washed three times with PBS buffer supplemented with 0.5% BSA. Before third wash cell pellet was resuspended in 1ml of the same buffer, and 50 ul aliquot was taken into Trypan Blue exclusion assay. Cell count was performed using hemacytometer for each aliquot immediately after addition of equal volume of Trypan Blue.
To assess the viability of hESC in colonies after IR exposures, cells were incubated at 37 °C for 1 hr with Hoechst 33342 (8 μg/ml; Molecular Probes, Eugene, OR) and propidium iodide (PI, 20 μg/ml; Sigma, St.Louis, MO). Hoechst 33342 is known to stain the nuclei of both live and dying cells whereas PI penetrates the cell membrane of only dying/dead cells. Cell colonies were visualized using an inverted fluorescence microscope (Axiovert 200M, Thornwood, NY) equipped with a fluorescent light source.
For flow cytometry experiments, each sample was diluted with PBS/0.5% BSA buffer yielding total 3 × 105 cells. For analysis of the expression level of cell surface antigens, cells were incubated either with SSEA-4-phycoerithrin (PE)-conjugated antibody (R&D Systems, Minneapolis, MN), or with TRA-1-60 antibody (Santa Cruz Biotechnology) and, after two washes with PBS/0.5% BSA buffer, with secondary FITC-conjugated antibody (Vector Laboratories, Inc.). Prior to staining, cells were blocked with human IgG for 15 min at room temperature. For intracellular staining with Oct-4-PE and Sox2-PE conjugated antibody (R&D Systems), the cells were fixed with 4% paraformaldehyde in PBS for 10 min, washed two times with PBS and permeabilized with 0.1% saponin (Sigma) in PBS prior to incubation with antibodies. All antibody incubations were performed according to manufacturer’s instructions. Isotype controls were included for each antibody staining.
After staining, cells were washed and resuspended in PBS. Fluorescence activated cell sorting (FACS) analysis was performed on a FACScalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA) utilizing an emission wavelength of 488 nm and a 525 nm excitation detector. Cell Quest Pro software was used for both data acquisition and analysis to produce histogram plots and median peak values. As a control for nonspecific binding for each conjugated antibody we used the same IgG subclass with the same fluorochrome conjugation and for non-conjugated antibody - the same IgG subclass conjugated to fluorochrome. A total of 10,000 events were acquired for each analysis.
2.5. Quantitative RT-PCR
Cells were trypsinized, washed two times with PBS and finally suspended in PBS with a concentration 10,000 cells per μl. cDNA was synthesized using SuperScript III CellsDirect cDNA System (Invitrogen) according to manufacturer’s recommendations. Quantitative real-time PCR (qPCR) was performed on iCycler iQ instrument (Bio-Rad, Hercules, CA) using SYBR GreenER qPCR SuperMix for iCycler (Invitrogen). Primers were purchased from Qiagen (Quantitech Primer Assays, Valencia, CA). PCR protocol consisted of 50°C for 2 min, 95°C for 10 min, followed by 50 cycles (95°C – 15 seconds, 55°C – 30 seconds, 72°C – 30 seconds) according to Quantitech Primer Assay recommendations. Ct (cycle threshold) values were obtained for each sample, averaged over triplicates in two biological replicates and normalized to beta-actin, according to formula E=2(Ct[beta-actin]-Ct[studied gene]), where E is a normalized expression of studied gene, Ct[beta-actin] and Ct[studied gene] are Ct values of beta-actin and studied gene in corresponding samples. Data are presented as mean plus/minus standard error. Differences were considered statistically significant at p value less than 0.05.
3. Results
3.1. Analysis of cell viability
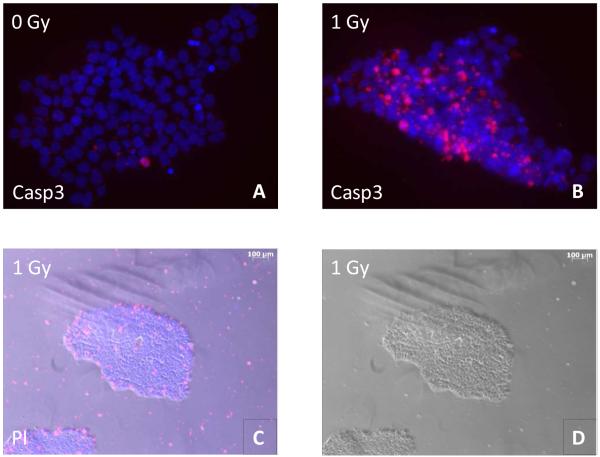
To examine the viability of hESC in culture after IR exposure, we stained the cells with vital dye assessing the percentage of cells in a population that excludes the dye indicative of cell survival. Only the colony-forming cells that remained attached were counted in this assay. The results of this count are shown in Table 1. The total number of the attached cells decreases at all time points roughly proportional to the received dose of IR. This decrease was most likely due to apoptosis that was observed at the early time points after irradiation by staining cell colonies with cleaved caspase 3 antibody, an early marker for apoptosis (Figure 1, A and B). We followed the irradiated cells for up to 65 hrs that correspond to approximately four cycles of cell division; after that sham-irradiated control cells became overgrown and thus required to be split. The sham-irradiated cells demonstrated viability in the range 87% - 94% up to 65 hrs of post-exposure incubation. The percentage of viable cells among the attached hESCs did not change after exposure to both doses of IR with exception of 65 hrs post 1 Gy irradiation when viability dropped to 72.1%. The surviving cells formed colonies characteristic of normal hESCs (Figure 1, D). Staining with Hoechst 33342 and propidium iodide revealed that cells permeable to the latter die that are presumably dead were localized mostly at the periphery of the colonies or were detached (Figure 1, C) Therefore, we conclude that despite dose-dependent cell death the majority of the remaining colony-forming attached hESCs that we used in our subsequent assays were viable, at least in terms of integrity of their cytoplasmic membrane.
Table 1.
H9 hESC post-IR cell count and viability
| 0 Gy | 0.2 Gy | 1 Gy | ||||
|---|---|---|---|---|---|---|
| Viability | Cell count | Viability | Cell count | Viability | Cell count | |
| 17h | 94% | 122×104 | 89.7% | 78 ×104 | 87.1% | 31 ×104 |
| 41h | 86% | 144×104 | 93.1% | 116×104 | 95.2% | 21×104 |
| 65h | 87% | 336×104 | 89.3% | 225×104 | 72.1% | 44 ×104 |
FIGURE 1.
Apoptosis induction assay. The cells were grown as described in Materials and Methods section, then either irradiated with 1 Gy IR or sham-irradiated and allowed to incubate for either 6 hrs before staining with cleaved caspase 3 antibody (Casp3) (A, B), or for 65 hrs before staining with Hoechst 33342 and propidium iodide (PI) (C.D). A,B: merged images of a hESC colony are shown (blue – DAPI, red – cleaved caspase 3), magnification 40x. C,D: C - merged image of hESC colonies is shown (blue – Hoechst 33342, red – propidium iodide); D – bright field image of the same colony, magnification 5x.
3.2. Immunocytochemical analysis of pluripotency and early differentiation markers
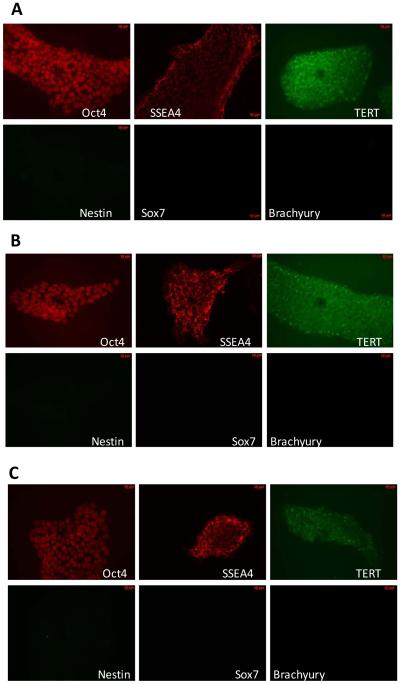
To qualitatively assess the expression and intracellular distribution of established markers of pluripotency, we performed immunocytochemical analysis of cultured H9 cells following irradiation with 0.2 Gy and 1 Gy doses up to 65 hrs post-IR (Figure 2). There was no detectable expression of Oct-4, SSEA-4, TERT, TRA-1-81 in primary human BJ fibroblasts (data not shown). However, in H9 cells Oct-4 showed intranuclear pattern of staining, TERT (catalytic subunit of telomerase) demonstrated intracellular localization and SSEA-4 localized to cell surface. Compared to sham-irradiated controls (Figure 2, A), neither 0.2 Gy nor 1 Gy irradiation led to any noticeable changes in expression and/or intracellular distribution of these markers of pluripotency (Figure 2, B, C). To further characterize the response of H9 hESCs to IR, we examined the expression of markers of early differentiation, such as, Nestin (ectodermal lineage), Brachyury (indicative of mesoendoderm differentiation) and Sox7 (endoderm) after IR exposure. We found no up-regulation of these gene products up to 65 hr post-IR, suggesting the continued undifferentiated state of cells, as judged by immunocytochemistry.
FIGURE 2.
Immunocytochemical analyses of IR exposure effects on pluripotency in cultured H9 hESC. A. Mock-irradiation, 65 hrs. B. Irradiation with 0.2 Gy dose, 65 hrs post-IR. C. Irradiation with 1 Gy dose, 65 hrs post-IR. Shown are representative images depicting the expression patterns of corresponding proteins described in the material and methods, bar code equals to 10 μm.
3.3. Analysis of pluripotency markers with Flow cytometry
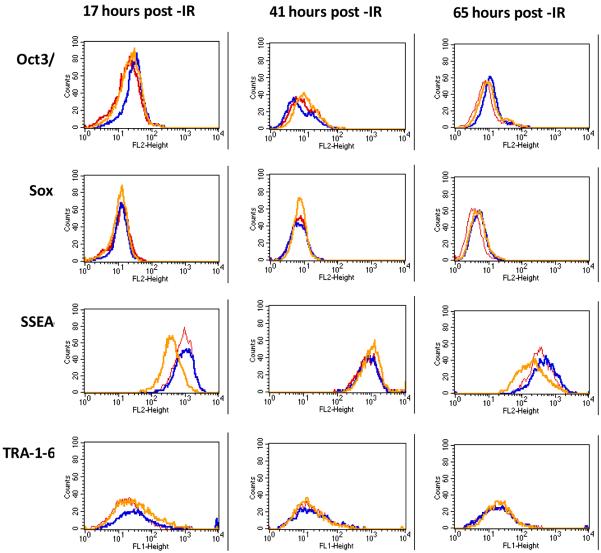
We used flow cytometry to quantitatively follow the expression level of both intracellular and cell surface antigens known to be associated with plurupotent state of stem cells. Histogram plots derived from flow cytometry experiments using cells stained with indicated antibodies are shown in Figure 3, while the median peak values obtained from these histograms are presented in Table 2. For Oct-4, we observed a slight increase in the expression level of this transcription factor after exposure of hESCs to 0.2 Gy at 17 hrs post-IR. After 41 hrs a slight increase in Oct-4 expression was observed only in 1 Gy irradiated sample, while expression of Oct-4 in 0.2 Gy irradiated sample was even slightly lower that that in the sham control. After 65 hrs post-IR expression of Oct-4 remained somewhat elevated for both 0.2 Gy and 1 Gy samples (Figure 3, top row). The expression of Sox-2, which is another transcription factor essential for maintenance of pluripotency in stem cells, was not changed at 17 and 41 hrs, however a slight increase was observed at 65 hrs after IR exposure (Figure 3, second row). The expression of cell surface glycosphingolipid antigens associated with pluripotency in stem cells revealed more complicated pattern after IR exposure. Expression of cell surface glycosphingolipid SSEA-4 after 0.2 Gy irradiation of H9 cells was not significantly changed up to 65 hrs compared to sham-irradiated control cells (Figure 3, third row). In contrast, exposure of hESCs to 1 Gy dose of IR led to 2.4-fold decrease in the expression level of SSEA-4 at 17 hrs post-IR, followed by a transient increase at 41 hr time point and return to a lower than in sham-irradiated cells level by 65 hrs post-IR. We investigated the pattern of expression of yet another cell surface hESC pluripotency marker, namely TRA-1-60 (Figure 3, bottom row). In general, expression of this marker varied significantly within hESC population. We observed a 1.5-1.6 fold increase following 0.2 Gy and 1 Gy IR exposures only at the earliest post–IR time point studied (17 hrs). Later, expression of TRA-1-60 returned to the level close to that of sham-irradiated hESCs.
FIGURE. 3.
Histograms showing expression of markers of pluripotency following IR exposures of cultured H9 hESC determined in Flow cytometry experiments (0 Gy - red, 0.2 Gy - blue, 1 Gy - yellow).
Table 2.
Expression of pluripotency markers from flow cytometry experiments. Numbers represent median peak values derived from histograms shown in Figure 3
| 17 hrs post IR | 41 hrs post IR | 65 hrs post IR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 Gy | 0.2 Gy | 1 Gy | 0 Gy | 0.2 Gy | 1 Gy | 0 Gy | 0.2 Gy | 1 Gy | |
| Oct-4 | 18.9 | 28.6 | 22.3 | 8.4 | 6.2 | 10.2 | 7.6 | 10.8 | 9.2 |
| Sox2 | 11.9 | 11.4 | 11.1 | 6.7 | 6.5 | 7.2 | 3.5 | 4.7 | 4.6 |
| SSEA4 | 858 | 882 | 359 | 711 | 757 | 922 | 334 | 441 | 183 |
| TRA-1-60 | 17.3 | 27.4 | 25.3 | 14.1 | 15.1 | 12.6 | 17.6 | 17.3 | 19.3 |
3.4. Analysis of expression of pluripotency markers with qRT-PCR
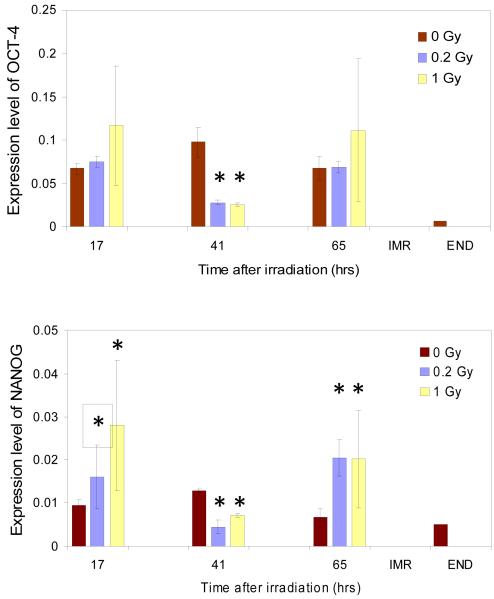
To further validate our findings, we quantitatively assessed the changes in gene expression for established markers of pluripotency state in hESCs with qRT-PCR experiments (Figure 4). For two genes, Oct-4 and Nanog, which constitute the core of transcriptional machinery governing the pluripotency of hESCs, no statistically significant changes were observed for sham-irradiated H9 cells over the entire course of our studies; the slight increase in both Oct-4 and Nanog expression at 41 hours after irradiation was not statistically significant and apparently consistent with natural variation in the expression level of these genes in H9 cells in culture. In contrast, after irradiation of hESCs with 1.0 Gy expression of both Oct-4 and Nanog became somewhat increased at 17 hrs, and then went down at 41 hrs post-irradiation after both doses of IR. However, at 65 hrs after irradiation expression of both markers returned to the level (Oct-4, 0.2 Gy) or even remained elevated compared to the level of expression in the sham irradiated control. To further validate the results of qRT-PCR assay, we set up two independent control experiments. First, as a negative control, we examined the level of expression of Oct-4 and Nanog genes in IMR-90 human primary diploid fibroblast representing differentiated cells of human fetal lung. Second, we employed a scheme of directed differentiation of pluripotent H9 cells into definitive endoderm. In the case of IMR-90 cells, the levels of expression of Oct-4 and Nanog genes were undetectable with our assay. In the case of the cells differentiated into endoderm the level of expression of Oct-4 was considerably lower while the level of expression of Nanog was comparable with that of non-differentiated cultured H9 cells (Figure 4).
FIGURE. 4.
Expression of pluripotency markers mRNA following IR exposures of cultured H9 hESC (0 Gy - red, 0.2 Gy - blue, 1 Gy - yellow) measured by qPCR. Expression levels are relative to the expression of beta-actin calculated as described in the Materials and Methods. Statistically significant differences in the expression levels of irradiated samples versus unirradiated controls are marked with stars. Differences were considered statistically significant at p value less than 0.05
4. Discussion
IR exposures with 0.2 Gy and 1.0 Gy led to an increase of expression an apoptotic marker and a decrease in cell count that was roughly proportional to the received radiation dose within hESCs colonies. After detachment and removal of the apoptotic cells, the surviving fraction of the hESCs formed characteristic colonies and remained viable in terms of maintaining integrity their plasma membrane. These observations are in agreement with published reports where other authors demonstrated massive apoptosis in hESCs irradiated IR with doses of equal or more than 2 Gy or with UV light (Qin et al., 2007; Filion et al., 2009; Momcilovic et al., 2009). However, in the above studies hESCs were exposed to considerably higher doses of gamma (2 Gy and 5 Gy), or a lethal dose of UV irradiation; and the analyses of cell responses were carried out within 24 hrs post irradiation on the mixture of dying and/or dead and surviving cells. To the best of our knowledge, no studies of hESCs responses to IR following exposures with relatively low doses (below 1 Gy) have been reported in the literature. Low-dose irradiation is more relevant in research and/or clinical settings where computer tomography (CT), positron emission tomography (PET), single photon emission computer tomography (SPECT) and other IR imaging techniques may be used to assess the success of human stem cell based substitutive therapy. The findings in our study suggest that low-dose IR exposures may not adversely affect the potential of hESC to survive and retain pluripotency.
It is generally appreciated that transcriptional control contributes to the maintenance of pluripotency, and three transcription factors, including the homeodomain proteins Oct-4 and Nanog, and the SRY-related HMG box containing protein Sox-2, have been involved in the maintenance of pluripotent hESCs (Matin et al., 2004; Boyer et al., 2005; Hyslop et al., 2005; Chambers and Tomlinson, 2009). Interestingly, it has been shown that Oct-4 must be present at appropriate levels to maintain pluripotency, because even a twofold increase in expression causes differentiation into primitive endoderm and mesoderm, whereas loss of Oct-4 induces the formation of trophectoderm accompanied by a loss of pluripotency (Niwa et al., 2000). In our experiments, the levels of Oct-4 mRNA expression were on level or slightly elevated at 17 and 65 hrs post-IR and significantly lower at 41 hrs as compared with sham-irradiated controls (Figure 4). However, this variation in the mRNA levels did not translate to sizeable changes in the expression of Oct-4 protein (Figure 3) suggesting that the cell cultures retained the undifferentiated state associated with the Oct-4 expression.
Expression of the homeodomain protein Nanog has been shown to be absent from differentiated cells (Chambers et al., 2003). Nanog is considered not only to maintain pluripotency, but also to inhibit the transition of undifferentiated hES cells to primitive endoderm (Zhou et al., 2007). Our data indicate that the expression level of Nanog mRNA after some decrease at 41 hrs remained elevated at 65 hrs post-IR following irradiation with both 0.2 Gy and 1 Gy dose as compared with that in sham-irradiated controls. It worth mention here that, as it was observed before (Momcilovic et al., 2009), decrease in mRNA levels of Oct-4 and Nanog did not translate to immediate changes in protein levels of these transcription factors.
It has been shown that downregulation/knockdown of Sox-2 is associated with differentiation/trophectoderm development in human ESCs (Fong et al., 2008). Our findings show that expression of Sox-2 remains at approximately the same level after both mock- and IR exposures up to 65 hrs post-IR. These three transcription factors have been demonstrated to co-occupy a significant portion of target genes (Boyer et al., 2005; Kim et al., 2008). In human hESCs, the network governed by Oct-4, Nanog and Sox-2 activate or suppress gene transcription, including many key genes that are transcriptional regulators involved in lineage specification during development (Boyer et al., 2005; Kim et al., 2008).
The cell surface glycosphingolipids SSEA-4, TRA-1-60 and TRA-1-81 have been shown to be present on hESCs and human embryonic carcinoma cells and are decreased in abundance upon cell differentiation (Thomson et al., 1998; Reubinoff et al., 2000). We showed modest up-regulation after 0.2 Gy and down-regulation after 1 Gy IR of SSEA-4 and essentially no changes in expression of TRA-1-81 (Figure. 2) and TRA-1-60 (Figure 3) at the latest time point studied (65 hrs) following IR exposures, implying that H9 hESCs retain high level of expression of these markers following IR. It is noteworthy that contradictory data are available for SSEA-4 in the literature; some showing SSEA-4 to be non-essential for hESCs pluripotency (Brimble et al., 2007). Therefore, the biological importance of down-regulation of SSEA-4 observed in our studies at 17 hrs following 1 Gy IR exposures of hESCs is unclear. Based on the totality of the obtained data we conclude that pluripotency of surviving hESCs is not considerably affected by IR exposures up to 1 Gy dose at least until 65 hours post-irradiation.
Activation of p53 involved substantial reduction in expression of both Nanog (on the second day after treatment) and Oct-4 (on the third day) concomitant with the increase in expression of markers of early differentiation (Maimets et al., 2008). It has been established that murine as well as human ES cells reduce the expression of Nanog and undergo differentiation in response to DNA damage (Lin et al., 2005; Qin et al., 2007). Previous studies showed that the mRNA levels for Oct-4 and Nanog decreased relative to non-irradiated cells six hours following 2 Gy irradiation (Filion et al., 2009) consistent to what has been demonstrated in murine and human ES cells (Lin et al., 2005; Qin et al., 2007). Interestingly, mRNA levels returned to those observed in sham-irradiated cells by 24 hours post 2 Gy exposure and there was no decrease in the protein level of Oct-4 and Nanog over the 24 hour period following IR exposure, suggesting that human ES cells remained pluripotent (Momcilovic et al., 2009). This observation is in agreement with our findings we report in this study. However, we extended this observation and present the results here that hESCs retain the expression of pluripotency markers at least up to 65 hrs post 0.2 Gy or 1 Gy IR exposures. To the best of our knowledge, no previous reports were published examining the pluripotency of hESCs following relatively low, clinically relevant doses of IR up to a timepoint corresponding to about four population doublings in hESCs (Becker et al., 2006).
It has been shown recently that hESC in culture may undergo transient cell cycle arrest following IR exposure (Momcilovic et al., 2009). Interestingly, Oct-4 has been reported to sustain proliferation of ESC by repressing p21, and, as such, be involved in cell cycle control of ESC (Lee et al., 2009). However, markers of pluripotency (Oct-4, Nanog, tumor rejection antigens etc.) are not known to be expressed only at specific cell cycle stages in hESC; therefore the relevance of cell cycle arrest on maintenance of pluripotency of hESC after IR is not directly evident.
One of the intriguing aspects of our results pertains to an apparent temporal change/fluctuation of the pluripotency marker expression levels at early time-points following IR exposures. This phenomenon has been previously observed (Momcilovic et al., 2009). It could be explained by the fact that epigenetics of pluripotent hESCs is distinct from that of somatic cells. It has been shown that the chromatin in ESCs exists in a much more open, “breathable” state in contrast to fully differentiated somatic cells (Meshorer et al., 2006); and promoters of many genes, including developmentally important genes, bear “bivalent marks” such as activating marks for transcription associated with methylation of histone3, lysine 4 (H3K4) and, at the same time, repression marks connected with methylation of histone3, lysine 27 (H3K27) (Bernstein et al., 2006). As such, the expression of genes can be modulated and fine-tuned very quickly in response to internal/external cues, such as IR. Alternatively it is possible that temporal changes/fluctuation of the pluripotency marker expression levels observed in our studies at early time points following IR exposures reflect the stochastic noise/heterogeneity inherent to hESCs cultures (Hough et al., 2009) and exacerbated by influence of genotoxic agent. This may also explain the temporal differences in the shapes of curves depicting the distribution pattern for expression of pluripotency markers on our flow cytometry data graphs (Figure. 3).
5. Conclusions
We believe our report is the first study to systematically examine the influence of IR exposure on pluripotency in hESCs. We found that the pluripotency of surviving hESCs, judged by the markers of undifferentiated state, is not affected by IR exposures in doses up to 1 Gy. These findings could provide an important foundation for the design and implementation of stem cell monitoring techniques in research settings. Our experimental data could perhaps also help to overcome one of the key uncertainties for clinical testing in patients participating in the translation of hESC based therapy from experiment to clinical practice.
Acknowledgements
We thank W. Degraff for his invaluable help with cell culture γ-irradiation. This research was supported by the Intramural Research Program of the NIH, Clinical Center.
Abbreviations
- hESC
human embryonic stem cells
- ES cells
embryonic stem cells
- IR
ionizing radiation
- qRT-PCR
quantitative real-time polymerase chain reaction
- EMEM
Earle’s modified Eagle’s medium
- DMEM
Dulbecco’s modified Eagle’s medium
- MEF
murine embryonic fibroblasts
- KSR
Knockout serum replacement
- bFGF
basic fibroblast growth factor
- PBS
phosphate-buffered saline
- BSA
bovine serum albumin
- PI
propidium iodide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aladjem MI, Spike BT, Rodewald LW, Hope TJ, Klemm M, Jaenisch R, Wahl GM. ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr Biol. 1998;8:145–55. doi: 10.1016/s0960-9822(98)70061-2. [DOI] [PubMed] [Google Scholar]
- Becker KA, Ghule PN, Therrien JA, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J Cell Physiol. 2006;209:883–93. doi: 10.1002/jcp.20776. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimble SN, Sherrer ES, Uhl EW, Wang E, Kelly S, Merrill AH, Jr., Robins AJ, Schulz TC. The cell surface glycosphingolipids SSEA-3 and SSEA-4 are not essential for human ESC pluripotency. Stem Cells. 2007;25:54–62. doi: 10.1634/stemcells.2006-0232. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–55. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chambers I, Tomlinson SR. The transcriptional foundation of pluripotency. Development. 2009;136:2311–22. doi: 10.1242/dev.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filion TM, Qiao M, Ghule PN, Mandeville M, van Wijnen AJ, Stein JL, Lian JB, Altieri DC, Stein GS. Survival responses of human embryonic stem cells to DNA damage. J Cell Physiol. 2009;220:586–92. doi: 10.1002/jcp.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong CY, Peh GS, Gauthaman K, Bongso A. Separation of SSEA-4 and TRA-1-60 labelled undifferentiated human embryonic stem cells from a heterogeneous cell population using magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS) Stem Cell Rev Rep. 2009;5:72–80. doi: 10.1007/s12015-009-9054-4. [DOI] [PubMed] [Google Scholar]
- Fong H, Hohenstein KA, Donovan PJ. Regulation of self-renewal and pluripotency by Sox2 in human embryonic stem cells. Stem Cells. 2008;26:1931–8. doi: 10.1634/stemcells.2007-1002. [DOI] [PubMed] [Google Scholar]
- Ginis I, Luo Y, Miura T, Thies S, Brandenberger R, Gerecht-Nir S, Amit M, Hoke A, Carpenter MK, Itskovitz-Eldor J, Rao MS. Differences between human and mouse embryonic stem cells. Dev Biol. 2004;269:360–80. doi: 10.1016/j.ydbio.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Hong Y, Stambrook PJ. Restoration of an absent G1 arrest and protection from apoptosis in embryonic stem cells after ionizing radiation. Proc Natl Acad Sci U S A. 2004;101:14443–8. doi: 10.1073/pnas.0401346101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough SR, Laslett AL, Grimmond SB, Kolle G, Pera MF. A continuum of cell states spans pluripotency and lineage commitment in human embryonic stem cells. PLoS One. 2009;4:e7708. doi: 10.1371/journal.pone.0007708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop L, Stojkovic M, Armstrong L, Walter T, Stojkovic P, Przyborski S, Herbert M, Murdoch A, Strachan T, Lako M. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005;23:1035–43. doi: 10.1634/stemcells.2005-0080. [DOI] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–61. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Go Y, Kang I, Han YM, Kim J. Oct-4 controls cell cycle progression of embryonic stem cells. Biochem J. doi: 10.1042/BJ20091439. doi:10.1042/BJ20091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew CG, Moore H, Ruban L, Shah N, Cosgrove K, Dunne M, Andrews P. Human embryonic stem cells: possibilities for human cell transplantation. Ann Med. 2005;37:521–32. doi: 10.1080/07853890500379463. [DOI] [PubMed] [Google Scholar]
- Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–71. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]
- Maimets T, Neganova I, Armstrong L, Lako M. Activation of p53 by nutlin leads to rapid differentiation of human embryonic stem cells. Oncogene. 2008;27:5277–87. doi: 10.1038/onc.2008.166. [DOI] [PubMed] [Google Scholar]
- Matin MM, Walsh JR, Gokhale PJ, Draper JS, Bahrami AR, Morton I, Moore HD, Andrews PW. Specific knockdown of Oct4 and beta2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells. 2004;22:659–68. doi: 10.1634/stemcells.22-5-659. [DOI] [PubMed] [Google Scholar]
- McCollough CH, Schueler BA, Atwell TD, Braun NN, Regner DM, Brown DL, LeRoy AJ. Radiation exposure and pregnancy: when should we be concerned? Radiographics. 2007;27:909–17. doi: 10.1148/rg.274065149. discussion 917-8. [DOI] [PubMed] [Google Scholar]
- McLean AB, D’Amour KA, Jones KL, Krishnamoorthy M, Kulik MJ, Reynolds DM, Sheppard AM, Liu H, Xu Y, Baetge EE, Dalton S. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- Meshorer E, Yellajoshula D, George E, Scambler PJ, Brown DT, Misteli T. Hyperdynamic plasticity of chromatin proteins in pluripotent embryonic stem cells. Dev Cell. 2006;10:105–16. doi: 10.1016/j.devcel.2005.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momcilovic O, Choi S, Varum S, Bakkenist C, Schatten G, Navara C. Ionizing radiation induces ataxia telangiectasia mutated-dependent checkpoint signaling and G(2) but not G(1) cell cycle arrest in pluripotent human embryonic stem cells. Stem Cells. 2009;27:1822–35. doi: 10.1002/stem.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–6. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Qin H, Yu T, Qing T, Liu Y, Zhao Y, Cai J, Li J, Song Z, Qu X, Zhou P, Wu J, Ding M, Deng H. Regulation of apoptosis and differentiation by p53 in human embryonic stem cells. J Biol Chem. 2007;282:5842–52. doi: 10.1074/jbc.M610464200. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Chipperfield H, Melton DA, Wong WH. A gene regulatory network in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:16438–43. doi: 10.1073/pnas.0701014104. [DOI] [PMC free article] [PubMed] [Google Scholar]