Abstract
The catalytic turnover of cytochrome P450cam from Pseudomonas putida requires two auxiliary reduction partners, putidaredoxin (Pd) and putidaredoxin reductase (PdR). We report the functional expression in Escherichia coli of tricistronic constructs consisting of P450cam encoded by the first cistron and the auxiliary proteins, Pd and PdR by the second and the third. Transformed bacterial whole cells efficiently oxidized (1R)- (+)-camphor to 5-exo-hydroxycamphor and interestingly limonene to (−)-perillyl alcohol. These bioengineered E. coli cells possess a heterologous self-sufficient P450 catalytic system that may have advantages in terms of low cost and high yield for the production of fine chemicals.
Keywords: camphor, limonene, P450, tricistronic expression
Introduction
Cytochrome P450 (P450) enzymes constitute a superfamily of hemoproteins that catalyze mixed-function oxidation reactions in the metabolism of xenobiotic chemicals (Ortiz de Montellano 2005). P450 enzymes are found in nearly all life forms from bacteria to human (Guengerich 2001). P450cam (CYP101) was the first P450 for which an X-ray crystal structure was determined (Poulos et al. 1982). The function of P450cam in Pseudomonas putida is the oxidation of camphor to 5-exo-hydroxycamphor, which makes it possible for the bacteria to grow on camphor as a sole carbon source (Trudgill et al. 1966). P450cam requires two auxiliary proteins, the flavoprotein putidaredoxin reductase (PdR) and the iron-sulfur protein putidaredoxin (Pd) for catalytic turnover (Katagiri et al. 1968). As a prototypic model of P450 for most mechanistic and structural studies, the wealth of information available on P450cam makes it an attractive candidate for the development of a self-sufficient catalytic system (Sibbesen et al. 1996). In this study, we constructed a tricistronic expression system including P450cam, Pd, and PdR in Escherichia coli and demonstrated its catalytic activity towards camphor and limonene. This system may be applicable in the industrial production of fine chemicals.
Materials and methods
Materials
(1R)-(+)-camphor, L-limonene, perillyl alcohol, and nitroblue tetrazolium were purchased from Aldrich Chemical Co. (Milwaukee, WI). Other chemicals were of the highest grade commercially available. The plasmids containing cDNA clones for Pd and PdR were kindly provided by Prof. Julian A. Peterson (University of Texas Southwestern Medical Center, Dallas, TX).
Construction of tricistronic expreession vector
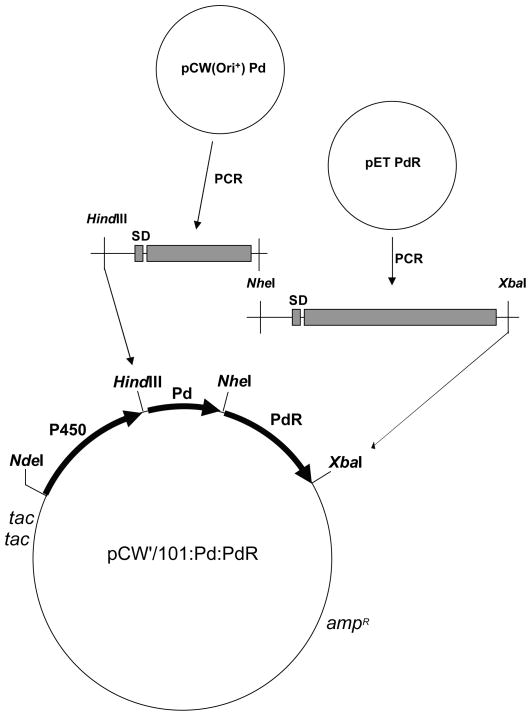
The open reading frames of Pd and PdR containing the Shine-Dalgarno (SD) ribosome binding sequences and HindIII, NheI and XbaI restriction enzyme sites were amplified using PCR as described in Fig. 1. The amplified PCR fragments were purified and cloned into the pCW(Ori+) P450cam vector containing HindIII and XbaI restriction enzyme sites at the C-terminus of the P450 open reading frame (Sibbesen et al. 1998). The ligation mixture was transformed into E. coli DH5α competent cells (Invitrogen, Carlsbad, CA) and then the plasmid DNA was purified using a Promega Wizard miniprep kit (Promega, Madison, WI). The recovered plasmid DNA was sequenced and verified for construction of the tricistronic system.
Fig. 1.
Generalized construction scheme for CYP101:Pd:PdR tricistronic expression plamids.
Tricistronic operon expression in E. coli
The constructed tricistronic DNA plasmid was transformed into E. coli DH5α and then plated on LB medium/ampicillin (100 μg/ml). The single colony was picked and transferred to single 5 ml of LB liquid medium/ampicillin. After overnight incubation at 37 °C, the starter culture was diluted into 1 liter of TB expression medium/ampicillin (100 μg/ml) containing1.0 mM IPTG, 0.5 mM δ-ALA, and 1.0 mM thiamine. The expression culture was grown at 37 °C for 3 h and then at 24 °C with shaking at 200 rpm for 24 h.
Enzyme assays
After the expression in TB medium, the E. coli cells were harvested and resuspended with M9 medium including 0.4% glucose as a carbon source. The reactions were performed at room temperature by addition of camphor or limonene (final 1 mM each) to the resuspended whole cells. The reaction products were analyzed by GC-MS after extraction with CH2Cl2.
Nitroblue tetrazolium (NBT) assays
The reductase activity of PdR in E. coli whole cells was analyzed using NBT assays with some modification. The increase in absorbance at 535 nm was determined after adding 1 mM NADH. Calculation of the specific activity for PdR assumed an absorption coefficient of 12.8 mM−1 cm−1 (Murphy et al. 1998).
GC-MS analyses of camphor and limonene and their metabolites
Analyses were performed on an Agilent 6850 gas chromatograph coupled to an Agilent 5973 Network Mass Selective Detector in the flame ionization mode. Helium was used as the carrier gas. The GC was equipped with an HP-5MS cross-linked 5% PH ME Siloxane capillary column (30 m, 0.25 mm i.d., 0.25 μm film thickness). The temperature program was 70 °C for 1 min and then 70–280 °C at 15 °C/min or 70 °C for 5 min, 70–160 °C at 5 °C/min, and then 160–280 °C at 20 °C/min. The temperatures were 250 °C for the injection port and 280 °C for the detector. A sample of 3 μl was injected in a 10:1 split ratio onto the column. Identification of camphor, 5-exo-hydroxycamphor, limonene, and (−)-perillyl alcohol was done by co-chromatography and mass spectrometric comparison with authentic standards.
Results
Tricistronic operon expression in E. coli
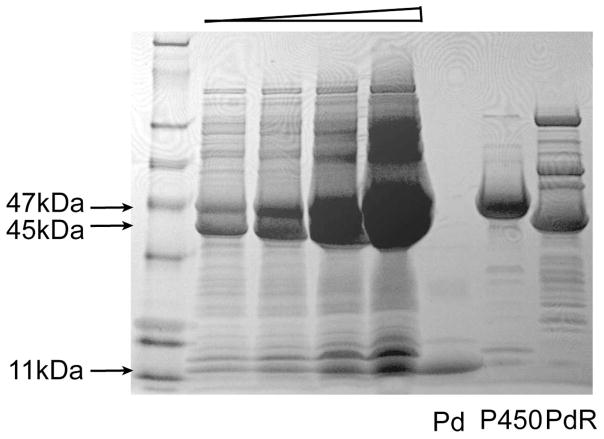
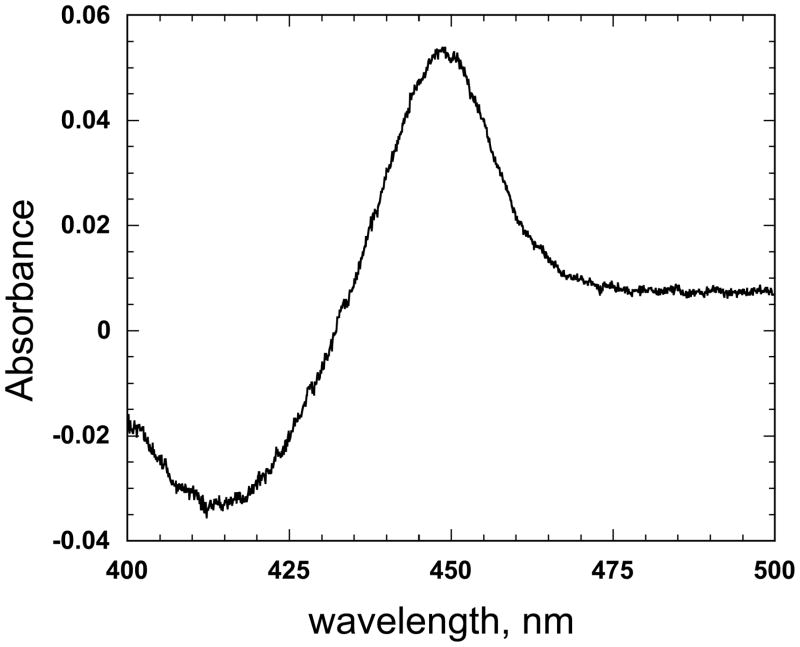
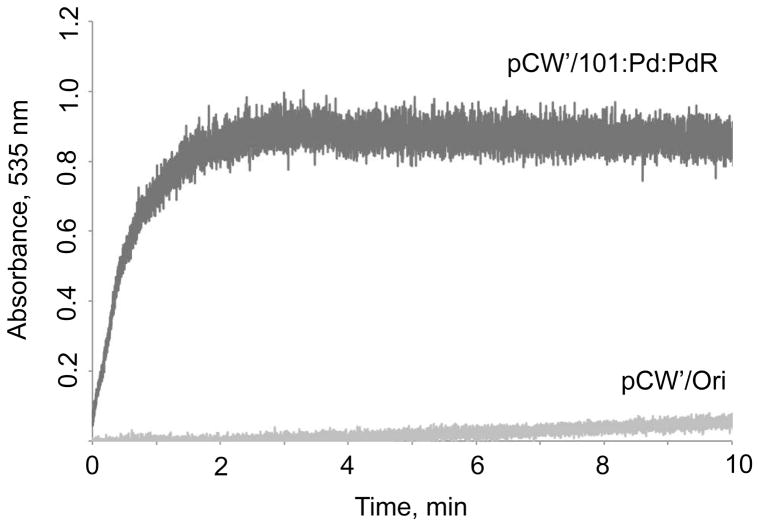
In order to establish that P450cam, Pd, and PdR were being overexpressed from the plasmid-borne operons, the soluble fraction was isolated and subjected to SDS-PAGE/Coomassie staining (Fig. 2). Bands with apparent molecular masses of ~47 kDa (P450), 45 kDa (PdR), and 11 kDa (Pd) were present. The identity of these proteins was confirmed by co-migration with previously purified proteins. The control experiment without IPTG induction showed that there was no expression of proteins with the corresponding sizes (data not shown). CO difference spectra were measured with concentrated whole cells (Fig. 3). The expression level was more than 500 nmol P450 holoenzyme per 1 liter culture. The reductase activity of PdR in the tricistronic system was verified by the reduction of nitroblue tetrazolium. A clear reduction activity in E. coli whole cells expressing three cistronic operons was observed but no color change in the control cells including pCW’(Ori+) vector (Fig. 4). The calculated reductase activity of PdR was about 30 nmol reduced NBT/min/ml whole cell culture. These results confirm the clear expression of functional PdR in the constructed system.
Fig. 2.
SDS-PAGE/Coomassie staining of soluble fraction. A molecular weight ladder is shown on the left, followed by four lanes with increasing concentrations of the expressed proteins, followed by lanes – as marked – for recombinant Pd, P450cam, and PdR.
Fig. 3.
Fe2+ vs Fe2+•CO difference spectrum of a CYP101:Pd:PdR tricistronic expression culture.
Fig. 4.
Activity measurement of PdR in CYP101:Pd:PdR tricistronic expression system. After 1 mM NADH was added to the sample cuvette, the absorbance at 533 nm was monitored until there was no further increase.
Camphor hydroxylation by E. coli whole cells expressing tricistronic operons
The camphor hydroxylation reaction products were analyzed by GC-MS after extraction with CH2Cl2. The 5-exo-hydroxycamphor was produced and identified by its molecular mass (m/z 168) (Supplementary Fig. 1). The 5-exo-hydroxycamphor was also produced in the reaction with the soluble fraction of the E. coli expression. These results indicate that the three proteins expressed in E. coli cells constitute a self-sufficient catalytic system.
L-Limonene hydroxylation by E. coli whole cells expressing tricistronic operons
The 7-hydroxylation of L-limonene by the tricistronic system was investigated for its bioengineering potential for the production of an anticancer compound (Reddy et al. 1997). The whole cell enzymatic reaction was performed as done for camphor hydroxylation. The production of (−)-perillyl alcohol was confirmed by cochromatography and mass spectrometric comparison of the metabolite with the authentic compound (Supplementary Fig. 2). No other metabolite was observed in the reaction. This result indicates that the 7-position is the major site of hydroxylation of L-limonene by P450cam and suggests that this system may be applicable to the large scale production of this metabolite.
Discussion
In this study, we used a tricistronic system that produces bacterial P450 and two auxiliary proteins from a single mRNA. The coding region for the three proteins was connected by the T7 gene leader including a Shine-Delgarno ribosomal binding sequence. The polycistronic expression approach has advantages including accommodation of the natural environment for the reconstitution of three proteins in prokaryotes and no need to purify the proteins for enzyme reactions (Parikh et al. 1997). Previous attempts to construct a self-sufficient E. coli catalytic system using a fusion protein showed that the order of the three proteins is important for optimal enzymatic efficiency regardless of the length of the linker peptides (Sibbesen et al. 1996).
One concern regarding biotransformation in living cells is transport of the substrate into the cell and the product out of the cell, and the availability of an energy source. Many P450 substrates are sufficiently hydrophobic to penetrate the polysaccharide E. coli cell wall. Camphor and limonene tested in this study successfully accessed the cytosolic compartment in which the three recombinant proteins were expressed. The P450cam reaction requires a coupled and stepwise supply of electrons from NADH (Paine et al. 2004). To circumvent the possibility of a limited NADH supply in the living cells, glucose was added as an energy source for the whole cell enzymatic reaction, as was previously shown with human CYP1A2 (Kim and Guengerich 2004).
Limonene, a monocyclic monoterpene, is present in orange peel and other plants and has been shown to have chemopreventive activities. The production of (−)-perillyl alcohol from limonene is of interest because of the limited availability of (−)-perillyl alcohol in nature and its proven anticarcinogenic properties in the treatment of breast, pancreatic, and colorectal cancer (van Beilen et al. 2005). Human CYP2C19 was able to oxidize L-limonene to perillyl alcohol (Miyazawa et al. 2002). Recently, the production of perillyl alcohol from limonene using an alkane hydroxylase from P. putida was reported (van Beilen et al. 2005). Although P450cam has traditionally been viewed as a camphor-specific enzyme, it metabolizes a diversity of compounds including limonene, as shown in this study, in a regio- and stereospecific manner. Therefore, the application of the tricistronic system in living cells for industrial production of (−)-perillyl alcohol in large-scale bioreactors is of potential interest.
Mutagenesis studies can provide information on the structure-function relationships of P450 proteins. However, it requires a high-throughput assay system including the complete reconstitution of P450, Pd, and PdR. Therefore, the development of this system presents the prospect of its use in mutagenesis studies of structure-function relationships, as well as its application in industrial bioreactions for the production of useful chemicals. Numerous orphan P450s are expressed in intriguing bacteria such as Mycobacterium tuberculosis and Steptomyces coelicolor (Lamb et al. 2006). However, it has been a challenge to establish a strategy for finding the substrates and metabolites of orphan P450s. Pd and PdR are known to be able to transfer electrons to many bacterial P450 enzymes (Paine et al. 2004). Tricistronic systems cloned with the orphan bacterial P450s could provide an alternative strategy to make the initial substrate screening in living organism possible.
Supplementary Material
Acknowledgments
This work was supported by grants from the U. S. Public Health Service (R01 GM25515) and by a Korea Research Foundation Grant funded by the Korean Government(MOEHRD, Basic Research Promotion Fund)(KRF-2007-331-E00025)
References
- Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- Katagiri M, Ganguli BN, Gunsalus IC. A soluble cytochrome P-450 functional in methylene hydroxylation. J Biol Chem. 1968;243:3543–3546. [PubMed] [Google Scholar]
- Kim D, Guengerich FP. Selection of human cytochrome P450 1A2 mutants with selectivity enhanced catalytic activity for heterocyclic amine N-hydroxylation. Biochemistry. 2004;43:981–988. doi: 10.1021/bi035593f. [DOI] [PubMed] [Google Scholar]
- Lamb DC, Guengerich FP, Kelly SL, Waterman MR. Exploiting Streptomyces coelicolor A3(2) P450s as a model for application in drug discovery. Expert Opin Drug Metab Toxicol. 2006;2:27–40. doi: 10.1517/17425255.2.1.27. [DOI] [PubMed] [Google Scholar]
- Miyazawa M, Shindo M, Shimada T. Metabolism of (+)- and (−)-limonenes to respective carveols and perillyl alcohols by CYP2C9 and CYP2C19 in human liver microsomes. Drug Metab Dispos. 2002;30:602–607. doi: 10.1124/dmd.30.5.602. [DOI] [PubMed] [Google Scholar]
- Murphy TM, Vu H, Nguyen T. The superoxide synthases of rose cells. Plant Physiol. 1998;117:1301–1305. doi: 10.1104/pp.117.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz de Montellano PR. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3. Plenum Press; New York: 2005. [Google Scholar]
- Paine MJI, Scrutton NS, Munro AW, Gutierrez A, Roberts GCK, Wolf CR. Electron Transfer Partners of Cytochrome P450. 3. Plenum Press; New York: 2004. [Google Scholar]
- Parikh A, Gillam EM, Guengerich FP. Drug metabolism by Escherichia coli expressing human cytochromes P450. Nat Biotechnol. 1997;15:784–788. doi: 10.1038/nbt0897-784. [DOI] [PubMed] [Google Scholar]
- Poulos TL, Perez M, Wagner GC. Preliminary crystallographic data on cytochrome P-450CAM. J Biol Chem. 1982;257:10427–10429. [PubMed] [Google Scholar]
- Reddy BS, Wang CX, Samaha H, Lubet R, Steele VE, Kelloff GJ, Rao CV. Chemoprevention of colon carcinogenesis by dietary perillyl alcohol. Cancer Res. 1997;57:420–425. [PubMed] [Google Scholar]
- Sibbesen O, De Voss JJ, Montellano PR. Putidaredoxin reductase-putidaredoxin-cytochrome p450cam triple fusion protein. Construction of a self-sufficient Escherichia coli catalytic system. J Biol Chem. 1996;271:22462–22469. doi: 10.1074/jbc.271.37.22462. [DOI] [PubMed] [Google Scholar]
- Sibbesen O, Zhang Z, Ortiz de Montellano PR. Cytochrome P450cam substrate specificity: relationship between structure and catalytic oxidation of alkylbenzenes. Arch Biochem Biophys. 1998;353:285–296. doi: 10.1006/abbi.1998.0632. [DOI] [PubMed] [Google Scholar]
- Trudgill PW, DuBus R, Gunsalus IC. Mixed function oxidation. V. Flavin interaction with a reduced diphosphopyridine nucleotide dehydrogenase, one of the enzymes participating in camphor lactonization. J Biol Chem. 1966;241:1194–1205. [PubMed] [Google Scholar]
- van Beilen JB, Holtackers R, Luscher D, Bauer U, Witholt B, Duetz WA. Biocatalytic production of perillyl alcohol from limonene by using a novel Mycobacterium sp. cytochrome P450 alkane hydroxylase expressed in Pseudomonas putida. Appl Environ Microbiol. 2005;71:1737–1744. doi: 10.1128/AEM.71.4.1737-1744.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.