Abstract
Objective
Low childhood SES and a harsh early family environment have been linked with health disorders in adulthood. In this study we present a model to help explain these links and relate the model to blood pressure change over a ten-year period in the CARDIA sample.
Design
Participants (N =2738) completed measures of childhood family environment, parental education, health behavior, and adult negative emotionality.
Main Outcome Measures
These variables were used to predict initial systolic and diastolic blood pressure, as well as the rate of blood pressure change over ten years.
Results
Structural equation modeling indicated that family environment was related to negative emotions, which in turn predicted baseline diastolic and systolic blood pressure, as well as change in systolic blood pressure. Parental education directly predicted change in systolic blood pressure. Although African-American participants had higher systolic and diastolic blood pressure and steeper increases over time, multiple group comparisons indicated that the strength of most pathways was similar across race and gender.
Conclusion
Low childhood SES and harsh family environments help to explain variability in cardiovascular risk. Low SES predicted increased blood pressure over time directly, and also indirectly through associations with childhood family environment, negative emotionality, and health behavior.
Keywords: health, stress, family, SES, blood pressure, comorbidities, hypertension
Hypertension is a serious and prevalent medical problem, with one in three U.S. adults estimated to have high blood pressure (Fields et al., 2004). Hypertension is a primary risk factor for coronary artery disease, the major cause of death in the U.S., yet nearly 90 percent of hypertension is essential, that is, of unknown origin. Hypertension is especially prevalent in African-American communities, an association that is believed to result in part from stressors related to low socioeconomic status (SES) (Bell, Adair, & Popkin, 2004).
A propensity for high blood pressure can be evident at an early age. For example, Woodall and Matthews (1989) documented that boys (but not girls) from families characterized as unsupportive had stronger heart rate responses during a series of laboratory stressors, compared with boys from more supportive families. Matthews, Gump, Block, and Allen (1997) reported that high levels of background stress were associated with larger increases in diastolic blood pressure and total peripheral resistance during laboratory stressors in children aged 8 to 17. Matthews, Salomon, Brady, and Allen (2003) found that increases in blood pressure reactivity related to stress predicted future blood pressure rises in adolescence. As a group, these results suggest that early childhood environment likely contributes to high blood pressure Understanding how and why is an important research priority.
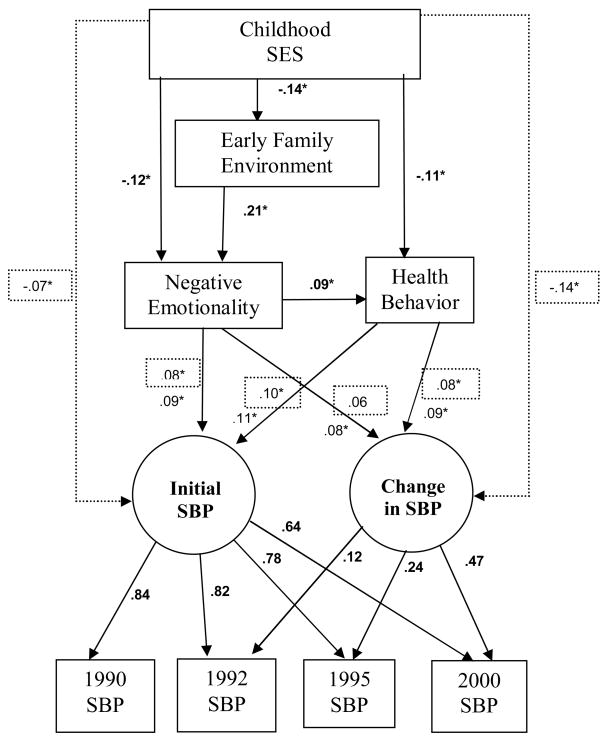
The goal of the present study was to test a model of pathways that may relate childhood socioeconomic status and family environment to blood pressure and blood pressure change over ten years in a large cohort study of African-American and White men and women (the Coronary Artery Risk Development in Young Adults (CARDIA) study). As presented in Figure 1, the model posits links among low childhood SES, an early family environment marked by harsh parenting, poor emotional functioning, and poor health behaviors as predictors of blood pressure. We describe it here in more detail.
Figure 1.
Sample standardized path coefficients for the model of SBP. Solid lines indicate the pathways included in the initial test of the model. The dotted lines and values in the dotted boxes indicate estimates when CSES is linked directly with blood pressure. Bold coefficients were identical for both models. A circle was used to represent the unmeasured (latent) initial SBP and nonlinear change variables. Consistent with SEM conventions, the arrows are shown as going from the latent constructs to boxes indicating measured variables in the model.
The first link in the model is childhood SES. Health-related risks of low SES begin in early childhood (Adler, Marmot, McEwen, & Stewart, 2000), and family environment is implicated in these processes (Repetti, Taylor, & Seeman, 2002). Considerable research links economic adversity in the family to poor or deteriorating quality of parenting, including higher levels of family conflict, a harsh restrictive parenting style, and chaotic or neglectful parenting (Emery & Laumann-Billings, 1998; McLoyd, 1998). Research also indicates that multiple early life environmental factors, including low socioeconomic status and a harsh family environment, can have a cumulative negative effect on children’s psychosocial functioning, health behavior, and physical health (Larson, Russ, Crall, & Halfon, 2008). Low SES is associated with elevated risk for hypertension (e.g., Karlamangla et al 2005; Lawlor & Smith, 2005), and this association may contribute to the SES gradient in coronary heart disease (Owen, Poulton, Hay, Mohamed-Ali, & Steptoe, 2003). Parental education is one indicator of childhood SES (Adler et al., 2000).
The next variable in the model, a harsh early family environment (FE), is temporally prior to the subsequent variables in the model and has been independently correlated with each of the subsequent steps. Early family environments marked by harsh or chaotic parenting are reliably associated with deficits in offspring emotion regulation skills, including both internalizing and externalizing behavior in children (Repetti et al., 2002). A risky family environment has been related to more negative emotions (Repetti et al., 2002); to poor health behavior (Kertesz et al., 2007); to preclinical risk factors for illness, including elevated autonomic and cortisol responses to stress (Luecken, Rodriguez, & Appelhans, 2005; Taylor, Lerner, Sage, Lehman, & Seeman, 2004), to major physical health disorders (Felitti et al., 1998); and indirectly to compromised metabolic functioning (Lehman, Taylor, Kiefe, & Seeman, 2005) and elevated C-reactive protein (Taylor, Lehman, Kiefe, & Seeman, 2006).
With respect to negative emotionality (NE), the next step in the model, indicators of chronic negative emotions, such as depression, anxiety, and hostility, have been tied to increased body mass index (BMI) (Pine, Goldstein, Wolk, & Weissman, 2001) to poor health behavior, (Caspi et al., 1997), and to hypertension (Davidson et al, 2000; Yan et al., 2003). Empirical and conceptual reviews suggest that negative emotions are linked to cardiovascular risk, and that examining NE as a construct, as opposed to merely one or more of its components, is appropriate, at least in prospective studies (Suls & Bunde, 2005). The fact that the behavioral and emotional consequences of growing up in low socioeconomic environment or in a harsh family environment are seen early in life, coupled with the relation of these variables to risks for health disorders later in life, makes them potentially useful in understanding the links between childhood SES, family environment, and blood pressure. Likewise, physical inactivity, risky health behaviors, and high BMI predict elevated blood pressure (Whelton, Chin, Xin, & He, 2002), and risk for mortality (Willcox et al., 2006). Because it is possible that health behaviors further contribute to the relation of childhood SES and family environment to elevated blood pressure they were assessed in the model.
We tested whether the proposed model (see Figure 1) would be substantiated in its entirety and hypothesized support for each of the proposed pathways by which emotional and behavioral risk factors may influence blood pressure. In addition, because CARDIA is a large sample, relatively evenly balanced between African-Americans and Whites and between men and women, the research afforded an opportunity to examine whether the model would be supported in each of the four race-sex subgroups, and whether the rate of longitudinal change in blood pressure was consistent across groups.
Method
Participants
The research made use of the CARDIA dataset, which tracks predictors of coronary artery disease as young people transition to adulthood. CARDIA is an ongoing epidemiologic study, in which African-American and White participants completed six assessments over 15 years, starting in 1985. Four assessments, 1990, 1992, 1995, and 2000, of blood pressure were used for this study. The 1990 assessment was selected as the baseline because that assessment included more indicators of negative emotions than in previous years. To be enrolled, participants must have self-identified as Black or White, had a permanent address in one of four urban areas (Birmingham, AL; Chicago, IL; Minneapolis, MN; or Oakland, CA), have been without symptoms of long-term disease or disability, and could not have been pregnant at the time of the assessment (see Friedman et al., 1988 for further detail).
In 2000 participants ranged in age from 33 to 45. Of the initial sample of 5,115 participants, 3,671 (74%) were examined in 2000. The sample for this study had complete data on all measures used in the analyses, including a report of childhood SES that was obtained during the initial 1985 assessment, indicators of negative emotionality and health behavior collected in 1990, the family environment measure taken in 2000, and blood pressure assessments in 1990, 1992, 1995, and 2000. Thirty-nine participants who were taking hypertensive medications in 1990 were excluded from the sample. Our sample therefore consisted of 2739 participants: 473 African-American men; 688 African-American women; 747 White men; 831 White women. Additional sample information, as well as means and standard deviations for the variables in the model, are found in Table A1 (online appendix).
Table A1.
Demographic, psychosocial, and physiological characteristics of participants (N = 2739).
| Variables in the model | Mean or Percent | sd | ||
|---|---|---|---|---|
| Mother’s education (years) | 13.17 | 2.23 | ||
| Father’s education (years) | 13.27 | 2.57 | ||
| Early Family Environment | 1.66 | .58 | ||
| Depression | 10.71 | 7.86 | ||
| Anxiety | 36.65 | 9.13 | ||
| Anger Expression | 6.75 | 1.56 | ||
| Hostility | 16.38 | 7.67 | ||
| Health Behavior Index | ||||
| Body Mass Index | 25.88 | 5.49 | ||
| Heavy Alcohol Consumption | 9.10% | N = 248 | ||
| Smoker | 14.6% | N = 401 | ||
| Blood Pressure | SBP | sd | DBP | sd |
| 1990 | 107.05 | 10.87 | 68.77 | 9.60 |
| 1992 | 107.93 | 11.53 | 68.73 | 9.73 |
| 1995 | 109.32 | 11.88 | 72.01 | 9.70 |
| 2000 | 112.47 | 14.33 | 73.86 | 11.18 |
| Sample descriptive information | Mean | sd | ||
| Age in 1990 | 30.09 | 3.59 | ||
| Years of Education | 14.68 | 2.36 | ||
| % income < $25,000 | 35.5% | |||
Procedure and Measures
For all CARDIA assessments, participants were asked not to exercise on the day of the exam, to fast for 12 hours, and to refrain from smoking for one half hour prior to the exam. The exams took approximately 3 hours, during which time participants completed informed consent, provided blood samples, blood pressure measurements, and anthropomorphic measurements. They also completed interviews and self-administered questionnaires.
Early Family Environment (FE)
Using a questionnaire adapted from Felitti et al. (1998), respondents rated aspects of their family environment on 4-point scales from one (rarely or none of the time) to 4 (most or all of the time). Items addressed whether the individual felt loved and cared for, was shown physical affection, was verbally abused, was physically abused, lived with a substance abuser, lived in a well-organized, well-managed household, and whether family members knew what the child was up to. Because individual items differed in their variability, each item was transformed into a z-score before a composite measure was formed. Cronbach’s alpha was .77, with higher values representing a riskier family environment. The FE assessment was included in 2000 through a special arrangement with the CARDIA coordinating center; no such measure was available from previous years.
Negative Emotionality (NE)
A composite measure of NE was formed using measures of depression, anxiety, anger expression (reverse coded), and hostility that were collected in 1990. A principal components analysis of these measures indicated that the four scales formed a single factor that explained 54.47% of the interitem variability, and that each of the measures contributed meaningfully to that factor. The NE variable was formed by computing standardized z-scores for each of the four summary measures described below and computing their mean (alpha = .70). For ease of interpretation, the NE scores were then transformed to T-scores (observed mean = 50, sd = 7.26). Depression was assessed using the 20-item Center for Epidemiologic Studies Depression Scale (Radloff, 1977). Cronbach’s alpha was .88. Anxiety was measured through the 20-item Spielberger Trait Inventory (Spielberger et al., 1980); Cronbach’s alpha was .90. Anger Expression was assessed through the Anger-In subscale of Spielberger et al.’ s (1985) measure of anger expression, with a Cronbach’s alpha for this three-item measure of .59. Hostility was measured using the 50-item Cook-Medley (1954) scale from the MMPI, alpha = .86.
Childhood Socioeconomic Status (CSES) was assessed at the baseline 1985 CARDIA. Participants indicated the highest level of education obtained by the participant’s parents or primary caregivers. The mean of standardized z-scores of the primary male and female caregivers’ levels of education (r = .56, p < .001) was used as a measure of childhood SES.
Health Behavior Index
To parsimoniously include available health behavior indicators that have been previously associated with cardiovascular health in CARDIA and elsewhere, we combined indicators of heavy alcohol consumption (Dyer et al., 1990), smoking, low physical activity (Jacobs et al., 1989), and high BMI into an index. As described below, participants could have between zero and four risk factors (M = 0.67, sd = .74). Alcohol use was included as part of CARDIA’s dietary survey. Consistent with Dyer et al. (1990), alcohol consumption for participants reporting alcohol use in the past year was converted to milliliters (M = 10.23, sd = 19.88), and one risk point was assigned to each participant whose self-reported typical daily alcohol consumption was 30 milliliters (approximately three drinks) or more. Next, those who reported smoking at least 5 cigarettes per week most weeks for the past three months were identified as current cigarette smokers and were assigned a point. Following Jacobs et al. (1989), total physical activity was assessed through “exercise units” that equate the frequency and physical intensity of different types of physical activities; those in the lowest quartile (those with values less than 165) were given one point. Finally, obese participants (those with BMI higher than 30) were assigned one risk factor.
Blood Pressure
For each of the four CARDIA assessments, certified technicians who were blind to the actual readings took three blood pressure measurements of systolic (SBP) and fifth phase diastolic (DBP) using a random zero sphygmomanometer. These measurements were taken after 5 minutes of rest at the start of the laboratory session. All readings were taken from the right arm, with a minimum of 30 seconds between each reading. The mean of the second and third readings was used. In 1990 blood pressure ranged from 75 to 178 SBP from and 11 to 107 DBP, and 184 of the participants with complete data for these analyses (6.8%) reported a diagnosis of hypertension. Although our sample excludes individuals taking hypertensive medications in 1990, 170 individuals (6%) who began using medications between 1990 and 2000 were included in the analysis. Table 1 presents the correlation matrix for the model variables.
Table 1.
Correlation matrix for entire sample (N = 2739).
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Childhood SES | |||||||||||||||
| 2. Early Family Env. | −.14* | ||||||||||||||
| 3. Depression | −.09* | .21* | |||||||||||||
| 4. Anxiety | −.06* | .25* | .72* | ||||||||||||
| 5. Anger Expression | .09* | −.02 | −.22* | −.20* | |||||||||||
| 6. Hostility | −.19* | .16* | .41* | .45* | −.22* | ||||||||||
| 7. Negative Emotions | −.15* | .22* | .81* | .82* | −.56* | .72* | |||||||||
| 8. Behavioral Risk | −.12* | .07* | .09* | .11* | −.02 | .09* | .11* | ||||||||
| 9. 1990 SBP | −.06* | −.02 | .03 | .03 | −.06* | .12* | .08* | .09* | |||||||
| 10. 1990 DBP | −.07* | −.01 | .02 | .02 | −.05* | .07* | .05* | .11* | .68* | ||||||
| 11. 1992 SBP | −.13* | −.01 | .05* | .03 | −.07* | .14* | .10* | .12* | .69* | .53* | |||||
| 12. 1992 DBP | −.11* | −.01 | .03 | .02 | −.07* | .09* | .07* | .12* | .53* | .63* | .69* | ||||
| 13. 1995 SBP | −.10* | −.00 | .04* | .03 | −.08* | .13* | .09* | .10* | .65* | .50* | .69* | .54* | |||
| 14. 1995 DBP | −.09* | −.01 | .03 | .04 | −.08* | .12* | .10* | .10* | .52* | .57* | .53* | .60* | .76* | ||
| 15. 2000 SBP | −.13* | .00 | .05* | .04* | −.08* | .14* | .11* | .12* | .54* | .44* | .59* | .49* | .63* | .53* | |
| 16. 2000 DBP | −.10* | .00 | .02 | .02 | −.08* | .09* | .07* | .10* | .45* | .51* | .46* | .53* | .49* | .56* | .76* |
Results
Statistical Analyses
Most of our analyses were conducted through a series of structural equation models tested using EQS 6.0. Systolic and diastolic blood pressure were modeled separately for all analyses. Data were not normally distributed, so robust standard errors were used for indices of model fit. Models were considered to be a good fit with the data if they had standardized discrepancies close to 0, and Root Mean-Square Error of Approximation (RMSEA) of .05 or less; Normed Fit Index (NFI) and Confirmatory Fit Index (CFI) estimates greater than .90. The Satorra-Bentler scaled chi-square is also reported, although nonsignificance of the value was not used as a criterion of model fit because of the large sample size.
The following steps were used to determine whether the model shown in Figure 1 explained initial variability and BP change over time. First, a series of latent growth models were used to capture change in BP between 1990 and 2000. These models drew on the four BP measures, with a goal of modeling individual differences in 1990 (baseline, for the purposes of this study) and differences in the rate of BP change over time. Second, change processes were incorporated into tests of the whole model (shown in Figure 1) and were used to examine initial differences in blood pressure, as well as blood pressure change between 1990 and 2000. The importance of individual model pathways was probed by testing a series of models excluding hypothesized pathways, and including in the final model those that were both theoretically and empirically supported. Third, this model was compared with an alternative model that gave negative emotionality (NE) priority. This model helps to guard against the possibility that NE affects retrospective reporting of childhood family environment and thus accounts for the downstream adverse effects on blood pressure. Fourth, group comparison analyses were used to test the model in the four race-sex subgroups in the CARDIA sample.
Longitudinal Change in SBP and DBP
A series of models tested change in SBP and DBP. Models were initially fitted using a latent variable with loadings for each year fixed to 1 (to capture between-person differences in 1990), and with a second latent variable included to capture linear change in BP (with weights of 0 in 1990, 2 in 1992, 5 in 1995, and 10 in 2000). Next, alternative models tested for autocorrelation among consecutive BP measures, for correlations between initial BP and the rate of linear change, for quadratic trends in the data, and for nonlinear patterns of change. Consistent with the recommendations of Duncan, Duncan, and Stoolmiller (1994), nonlinear trends were tested by allowing for the free estimation of two of the four blood pressure readings onto the latent factor representing longitudinal change. To aid in estimation, and in recognition of the limited change between 1990 and 1992 measures (see Figure 2), we assigned a fixed value of 0 to the 1990 measure, estimated the 1992 weight, assigned a value of 5 to the 1995 readings, and estimated the 2000 reading. See Table A2 on-line (at http://[fill in HP website]) for a summary of the growth models tested.
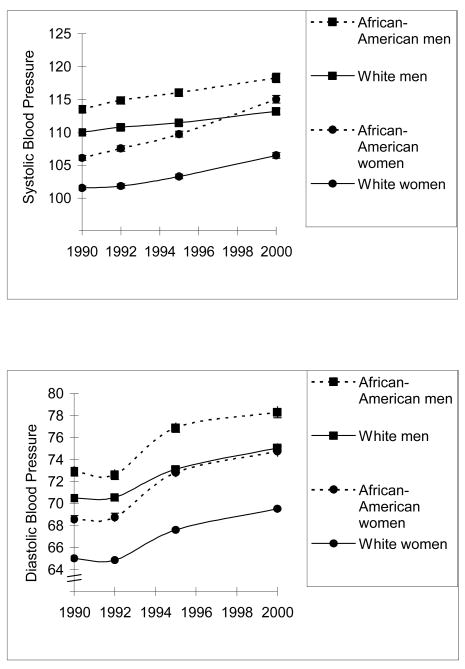
Figure 2.
Systolic blood pressure and diastolic blood pressure by year for each of the CARDIA race-sex subgroups and for the entire sample. Bars indicating one standard error are included, but are not always visible because the standard errors are small.
Table A2.
Models of 10-year longitudinal change in blood pressure.
| Model Tested | df | sig | NFI | CFI | RMSEA | Change | Change sig. | |
|---|---|---|---|---|---|---|---|---|
| SBP | ||||||||
| * Linear only | 13.91 | 6 | .031 | .997 | .998 | .022 | ---- | ---- |
| Linear with correlated growth and change disturbances | 13.71 | 5 | .018 | .997 | .998 | .025 | 0.01 | .937 |
| Linear with autocorrelation | 5.32 | 3 | .150 | 1.000 | 1.000 | .017 | 9.85 | .020 |
| Quadratic | 46.15 | 6 | <.001 | .992 | .994 | .049 | ---- | ---- |
| Non-linear growth | 9.18 | 4 | .057 | .997 | .998 | .022 | 3.07 | .220 |
| DBP | ||||||||
| * Linear only | 106.82 | 6 | <.001 | .995 | .996 | .078 | ---- | ---- |
| Linear with correlated growth and change disturbances | 106.84 | 5 | <.001 | .997 | .998 | .086 | ---- | ---- |
| Linear with autocorrelation | 96.82 | 3 | <.001 | .999 | 1.00 | .107 | ---- | ---- |
| Quadratic | 209.43 | 6 | <.001 | .994 | .995 | .111 | ---- | ---- |
| Non-linear growth | 10.16 | 4 | .037 | .997 | .997 | .024 | 112.16 | <.001 |
Indicates reference model for tests of chi-square change.
The initial model of linear change in SBP fit the data (χ2 (6) = 13.91, p = .031; NFI = .997; CFI = .998; RMSEA = .022). The estimated mean SBP in 1990 (after removing measurement error) was 106.91, while the average increase in SBP in one year was .53. Model fit was significantly improved when links reflecting autocorrelation among consecutive SBP measures (i.e., covariances between the error terms of consecutive years) were included (adjusted χ2Δ(3) = 9.85, p = .02). A third model suggested that individual differences in SBP in 1990 did not predict the rate of linear change (i.e., the disturbance terms between the two latent factors did not covary significantly), and a model testing for a quadratic trend did not fit the data. Finally, a model allowing for nonlinear change was a good fit with the data (χ2 (4) = 9.18, p = .057; NFI = .997; CFI = .998; RMSEA = .022). Although the nonlinear change model was not a significant improvement over the linear model (χ2Δ (2) = 3.07, p = .22) it was used to test the full risky families model. This choice was made for consistency of analytic approaches for SBP and DBP, and because predictions of nonlinear change are theoretically warranted.
The same set of growth models was used to estimate longitudinal change in DBP. The model of linear change (χ2 (6) = 106.82, p < .001; NFI = .99; CFI = .99; RMSEA = .08) was a moderately acceptable fit with the data. The linear model allowing for autocorrelation, the model using baseline to predict rate of change, and the quadratic growth models were all poor fits with the data. The nonlinear growth model was more consistent with the DBP data (χ2 (4) = 10.16, p = .037; NFI = .99; CFI = .99; RMSEA = .024), and was a significant improvement over the linear model (χ2Δ (2) = 112.16, p < .01). Using this model of longitudinal change in DBP, mean DBP in 1990 was estimated at 68.75, while the average increase in DBP in one year was .65. The nonlinear growth models were incorporated into tests of the entire risky families model.
Tests of the model
The model shown in Figure 1 was used to predict initial (1990) BP and change in BP. To probe the precise mechanisms by which the variables in our model were linked with blood pressure, analyses were first conducted without any direct links to or from childhood family environment to other model variables or between childhood SES and blood pressure. Hypothesized pathways linking family environment with other components of the model were then added; those with the largest standardized residuals were entered first. Tests of chi-square change were used to compare the each subsequent model. If the addition of a pathway was not statistically significant or did not produce a significant change in chi-square or in model fit, the simpler model was retained. Finally, the links between CSES and blood pressure were added as a separate step; the data were expected to fit the model both with and without that pathway.
A series of 6 models were fitted for SBP and for DBP. Using the criteria outlined above, a model (model C in Table 2) in which lower childhood SES predicted a harsher family environment, which in turn predicted negative emotions and blood pressure, proved to be the best model to capture the variance in these data. This model, shown in Figure 1, was a good fit with the data for systolic (χ2 (17) = 68.17, p < .001; NFI = .99; CFI = .99; RMSEA = .03) and diastolic blood pressure (χ2 (17) = 51.21, p < .001; NFI = .99; CFI = .99; RMSEA = .03). Childhood SES predicted NE and health behavior, which in turn predicted initial blood pressure, as well as change in systolic blood pressure. Note that the links between FE and health behavior and between FE and the latent blood pressure measures are not included in this model. As shown in Table 2, these pathways were tested, but were not significant and did not contribute to model fit.
Table 2.
Tests of the Model, with and without theorized pathways for family environment (FE) and for childhood SES.
| Model Tested | χ2 | df | sig | NFI | CFI | RMSEA | χ2 Change | Change sig. |
|---|---|---|---|---|---|---|---|---|
| SBP Growth Models | ||||||||
| A. All FE links excluded | 218.86 | 19 | <.001 | .953 | .956 | .062 | ||
| B. FE linked only with NE | 113.38 | 18 | <.001 | .976 | .979 | .044 | 103.12 | <.001 |
| *C. FE linked with NE and CSES | 68.17 | 17 | <.001 | .985 | .989 | .033 | 37.91 | <.001 |
| D. With direct FE link to health behavior | 65.27 | 16 | <.001 | .986 | .989 | .034 | 2.14 | .143 |
| E. With direct FE link to initial BP and BP change | 62.05 | 15 | <.001 | .987 | .990 | .034 | 3.97 | .137 |
| F. With direct CSES link to initial BP and BP change | 35.02 | 15 | <.001 | .993 | .996 | .025 | 23.77 | <.001 |
| DBP Growth Models | ||||||||
| A. All FE links excluded | 213.35 | 19 | <.001 | .948 | .951 | .061 | ||
| B. FE linked only with NE | 99.93 | 18 | <.001 | .975 | .979 | .041 | 127.01 | <.001 |
| *C. FE linked with NE and CSES | 51.21 | 17 | <.001 | .987 | .991 | .027 | 45.15 | <.001 |
| D. With direct FE link to health behavior | 48.45 | 16 | <.001 | .988 | .991 | .027 | 2.54 | .111 |
| E. With direct FE link to initial BP and BP change | 47.41 | 15 | <.001 | .988 | .991 | .028 | 3.37 | .185 |
| F. With direct CSES link to initial BP and BP change | 30.06 | 15 | .012 | .993 | .996 | .019 | 19.69 | <.001 |
Model C was used as the reference model for all subsequent tests of adjusted chi-square change. Change statistics for models B and C were calculated from models A and B, respectively. FE = Family Environment; NE = negative emotions; CSES is childhood socioeconomic status
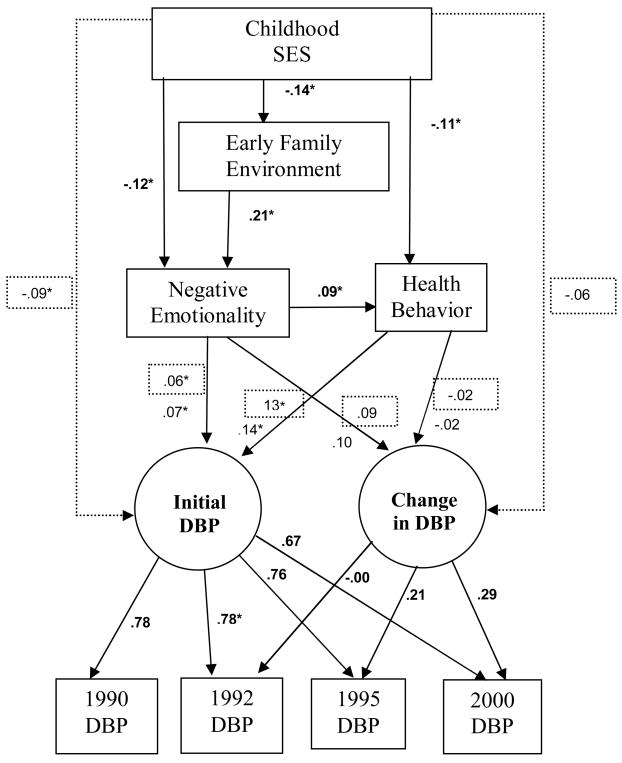
The next step tested the direct relationship between CSES and blood pressure. Standardized path coefficients for the SBP models both with and without direct links with childhood SES are shown in Figure 1, and standardized coefficients for the DBP models are shown in Figure 3. Unstandardized coefficients and robust standard errors for models with and without direct CSES links are summarized in Table 3. The addition of the theoretically warranted direct links between childhood SES and the latent SBP variables resulted in a statistically significant improvement in model fit (χ2Δ (2) = 23.77, p < .001) and a model that better fit the data (χ2 (15) = 35.02, p < .001; NFI = .99; CFI = .996; RMSEA = .03). Lower childhood SES predicted higher initial SBP and greater nonlinear change in SBP. When this link was included for the model of SBP change, most theoretically relevant pathways remained similar in magnitude, although the link between negative emotionality and change in SBP fell below statistical significance
Figure 3.
Whole sample standardized path coefficients for the model of nonlinear change in DBP. Solid lines indicate the pathways included in the initial test of the model. The dotted lines and the values in the dotted boxes indicate estimates when CSES was linked directly with blood pressure. Bold coefficients were identical for both models.
Table 3.
Path coefficients (with robust standard errors) for models of nonlinear change.
| MEAN AND COVARIANCE STRUCTURE (identical across models) | |
|---|---|
| Constant → Child SES | .07* (.02) |
| Constant → Family Environment | .01 (.01) |
| Constant → Negative Emotions | 50.09* (.14) |
| Constant → Health Behavior | .20 (.10) |
| Child SES → Family Environment | −.09* (.01) |
| Child SES → Negative Emotions | −.92* (.14) |
| Child SES → Health Behavior | −.09* (.02) |
| Family Environment → Negative Emotions | 2.33* (.23) |
| Negative Emotions → Health Behavior | .01* (.00) |
| LATENT VARIABLE MEAN AND COVARIANCE STRUCTURE (varies by model) | ||||
|---|---|---|---|---|
| Systolic Blood Pressure | Diastolic Blood Pressure | |||
| No Child SES | Child SES | No Child SES | Child SES | |
| Constant → Initial BP | 100.24*(1.31) | 100.97*(1.32) | 64.39* (1.12) | 65.10* (1.14) |
| Constant → BP Change | 0.11 (0.14) | .20 (0.14) | 0.38* (0.15) | 0.41* (0.15) |
| 1992 to BP Change | 2.24* (0.29) | 2.31* (0.29) | 0.03 (0.25) | −.02 (0.25) |
| 2000 to BP Change | 11.63* (0.85) | 11.58* (0.84) | 7.81* (0.37) | 7.83* (0.37) |
| Negative Emotions → Initial BP | 0.12* (0.03) | 0.10 (0.03) | 0.07* (0.02) | 0.06* (0.02) |
| Negative Emotions → BP Change | 0.01* (0.00) | 0.05 (0.00) | 0.01 (0.00) | 0.01 (0.00) |
| Health Behavior → Initial BP | 1.32* (0.27) | 1.22* (0.27) | 1.39* (0.22) | 1.29* (0.23) |
| Health Behavior → BP Change | 0.07* (0.03) | 0.06*(0.03) | −.01 (0.03) | −0.01 (0.03) |
| Child SES→ Initial BP | ---- | −0.71* (0.21) | ---- | −0.70* (0.18) |
| Child SES → BP Change | ---- | −0.09* (0.02) | ---- | −0.03 (0.02) |
Notes: Values are the unstandardized coefficients for each pathway, followed by robust standard errors. Each lower column summarizes a different analysis; two for systolic and two for diastolic BP, presented with and without the direct link between child SES and blood pressure. Values at the top of the table are consistent across tests. SES = Socioeconomic Status.
p < .05
For the diastolic blood pressure model that assumed full mediation of CSES by the variables in the model, all included pathways were statistically significant, with the exception of those used to test for change in DBP. Although neither health behavior nor NE predicted change in DBP, the negative emotions measure approached significance (p = .07). The model including direct links between childhood SES and DBP predicted initial DBP, but not DBP change. This model, χ2 (15) = 30.06, p < .012; NFI = .99; CFI = .996; RMSEA = .02, was a significant improvement over the fully mediated model (χ2Δ (2) = 19.69, p < .001).
An alternative model tested whether negative emotionality predicted the retrospective reports of family environment, which could then have downstream consequences for blood pressure. To do this, we repeated the growth model, but used negative emotions to predict family environment. These analyses indicated that for both SBP (χ2Δ (1) = 46.22, p < .001) and DBP (χ2Δ (1) = 43.16, p < .001), the alternate model was a poorer fit with the data than the hypothesized model.
Tests of the model in the race-sex subgroups
Two complimentary approaches were used to test race and sex differences in the model. First, a set of multiple group analyses were used to test the equality of the hypothesized covariance structure among the four race by sex subgroups. This approach allowed us to examine the strength of the associations in the model, but did not test mean differences. Second, dummy coded variables indicating race, sex, and a race by sex interaction term were used to test differences in latent initial BP and BP change. This approach tested the mean differences between men and women, and between African-American and white participants, as well as whether sex differences varied by race.
The initial multigroup test of covariance structure indicated that for both SBP (χ2 (60) = 112.28, p < .001; NFI = .98; CFI = .99; RMSEA = .02) and DBP (χ2 (60) = 76.77, p = .071; NFI = .98; CFI = .99; RMSEA = .01), the model fit the data after the race-sex groups were identified. Next, in separate tests for SBP and DBP, loadings on the growth term were constrained to be equal across groups. These pathways did not differ among the race-sex subgroups. Finally, an additional model constrained all pathways to be equal across the subgroups. The constrained model for both SBP (χ2 (99) = 172.90, p < .001; NFI = .96; CFI = .98; RMSEA = .02) and DBP (χ2 (99) = 134.90, p = .01; NFI = .97; CFI = .99; RMSEA = .01) fit the data, and most of the theoretically relevant pathways (e.g., those from CSES and from negative emotionality to initial BP and BP change) did not differ significantly among the groups. However, the link from negative emotions to health behavior was not significant for women, but for both African-American and White men, more negative emotions predicted riskier health behavior. In addition, the path from childhood SES to health behavior was not statistically significant for white men, but for all other groups, higher childhood SES predicted less risky health behavior.
Next, consistent with the patterns observable in Figure 2, differences in the SBP latent growth model suggested that race and sex were both significant predictors of initial SBP and of nonlinear SBP change. The direction of these differences indicated that both initial SBP and change in SBP was greater for men and for African-Americans. Furthermore, for nonlinear change only, this result was qualified by a race by sex interaction suggesting that the sex difference in the rate of change was smaller for African-American participants than for those who were white. This interaction is likely capturing the especially steep increase in SBP for African-American women between 1995 and 2000. A SBP model including main effects of both the sex and race predictors, and the sex by race interaction term for the nonlinear change variable, was an acceptable fit with the data, χ2 (13) = 124.64, p < .001; NFI = .97; CFI = .98, RMSEA = .06. For DBP, results indicated that race was a significant predictor of both initial DBP and change in DBP; African-Americans had higher initial DBP and also showed steeper increases over time. Although no other group differences were found in rates of change, baseline DBP was higher for men, and an interaction suggested that the sex differences in baseline DBP were greater for whites than for African-Americans. The model that used race, sex, and their interaction to predict initial DBP, and race to predict change in DBP, was an acceptable fit with the data, χ2 (15) = 146.78, p < .001; NFI = .96; CFI = .97; RMSEA = .06.
Discussion
We examined whether the model shown in Figure 1 explains change in systolic (SBP) and diastolic (DBP) blood pressure over a 10-year period in CARDIA, a large longitudinal investigation of risk factors for coronary artery disease in African-American and White men and women. Latent growth models of nonlinear change indicated that both systolic and diastolic blood pressure increased as participants aged to between 33 and 45 years old. Our conceptual model fit the data for both SBP and DBP. Consistent with previous research, low childhood SES (as operationalized through parent educational attainment) was associated with a harsh family environment, higher levels of negative emotionality (greater depression, anxiety, hostility, and less adaptive expression of anger), and poor health behavior. A harsh family environment was linked with elevated negative emotions. Participants with problematic emotional functioning and those who engaged in riskier health behavior demonstrated greater increases in SBP over time. However, the associations with negative emotionality dropped below statistical significance when the role of childhood socioeconomic status was considered. In addition, those from less educated families had higher baseline systolic and diastolic blood pressure and showed greater change in systolic blood pressure over time. Although none of the variables in the model were linked with change in diastolic blood pressure, those with more negative emotionality and with riskier health behavior did have higher baseline DBP.
As noted earlier, a propensity for high blood pressure has been documented among children who face adversity in early life. As an attempt to understand the mechanisms contributing to this relationship, this study examined the role of low childhood socioeconomic status and a harsh family environment in predicting poor health behavior and elevated levels of negative emotionality. Low childhood SES contributes to an increased likelihood of a harsh family environment marked by conflictual, cold, or neglectful family relationships and may aggravate autonomic responses by raising background stress (e.g., Matthews et al., 1997). In turn, both a low childhood SES and harsh family environment predict the likelihood of high levels of depressive symptomatology, hostility, anxiety and anger internalization. Although this research did not find a direct association between family environment and elevated blood pressure, the other relations outlined above are consistent with the model developed by Repetti et al. (2002), which argues that a harsh family environment exerts effects on adult health outcomes, in part, by compromising emotional and social functioning across the lifespan.
A previous study from our laboratory documented the applicability of the model to signs of HPA axis dysregulation, specifically, an elevated flat cortisol trajectory across laboratory stress tasks, and to heightened heart rate and blood pressure in a laboratory stress task (in men only) (Taylor et al., 2004). Results of our studies are consistent with McEwen and colleagues’ (Seeman, Singer, Rowe, Horwitz, & McEwen, 1997) allostatic load model of stress. The allostatic load model maintains that chronic or recurrent stress interacts with genetic or acquired risks, leading to cascading, potentially irreversible interactions between those predispositions and environmental factors; over time, these interactions and their accumulating effects can lead to large individual differences in susceptibility to stress, in biological markers of the cumulative effects of stress, and in stress-related physical and mental health disorders. Consistent with this model, using the CARDIA sample, Matthews et al. (2004) reported that blood pressure reactivity to psychological stressors predicted the development of hypertension. Although the present study does not directly test the allostatic load model, the evidence suggests that a chronically stressful early environment, particularly a low socioeconomic environment, can influence the development of elevated blood pressure and suggests that negative emotions and risky health behaviors may contribute to the accumulation of allostatic load as well. It is noteworthy that childhood socioeconomic status predicted not only resting blood pressure in young adulthood, but also longitudinal change in systolic blood pressure over ten—years above and beyond the baseline association. Such effects, some of which occur through negative emotions and health behavior, suggest that processes set in motion at a very early age may take a cumulative negative toll on body functions.
Significant differences in mean blood pressure emerged by race and sex, with African-Americans and men having higher blood pressure than the other groups, differences that were qualified by the greater similarity in the rate of change for African American women and men than for White women and men; the change in systolic blood pressure was especially steep for African American women, a pattern that has been previously documented in the CARDIA sample (Liu, Ruth, Flack, Jones-Webb, Burke, et. al., 1996). In spite of these group differences, increases in blood pressure were evident in all groups, and the pattern of associations among variables in the model was consistent across the four groups. For example, although the rate of change in systolic blood pressure was especially steep for African American women and was more gradual for White men, we found no evidence that the variables in the model were differentially related to rates of change in these groups. This suggests that, for blood pressure at least, the model may be applicable across levels of health risk.
Limitations
There are several additional limitations to the present findings. The model proposed in Figure 1 and tested here does not include factors such as variability in blood pressure due to genetic factors, or community-level influences on developmental or health outcomes. It is possible, for example, that genetic factors contribute both to negative emotions and to risks for elevated blood pressure. It is also possible that neighborhood factors correlated with parental education contribute to the association between childhood SES and blood pressure. In spite of the exclusion of these factors, the data indicate that early environment, negative emotions, and health behaviors, in conjunction, contribute to elevations in systolic blood pressure over time. This result is especially noteworthy because the sample is still relatively young. The long-term significance of these associations for health may become increasingly clear as the CARDIA sample ages.
The variables in the model were somewhat more effective in predicting systolic than diastolic blood pressure; reasons for this difference are not immediately apparent. However, it has been noted that systolic blood pressure is more significant for diagnosing hypertension than diastolic blood pressure (American Heart Association, 2003). The problem may have been statistical, as the models of DBP change did not capture the patterns as well as those for SBP. The trajectory for diastolic blood pressure should become more clear as the CARDIA sample ages. It should also be noted that, despite overall support for the model, some of the path coefficients are small. Because the sample is still relatively young, the majority of participants in this sample had blood pressure within normal limits at the end of our study, and so there was relatively little change to be predicted by the variables in the model. Nonetheless, the results add fuel to concerns that developmental trajectories marked by negative emotions and/or poor health behaviors contribute to accumulating health risks across the life span. As such, even relatively youthful samples may benefit from early interventions aimed at modifying these pathways.
A third limitation is that people who began taking hypertensive medication between 1990 and 2000 were kept in the sample; however, this limitation would probably work against support for the model rather than in its favor. A fourth limitation stems from our use of a short form of the family environment measure. This measure did not allow for the examination of specific types of family environments that may have the greatest effects on psychological and physical development. Fifth, negative emotionality was included as a composite variable in this study, and some specificity was lost through this approach. Indeed, the pattern of bivariate correlations in Table 2 indicates that in comparison to depression and anxiety, anger expression and hostility were more closely linked with blood pressure, a result consistent with Yan et al.’s (2003) examination of the development of hypertension in the CARDIA sample.
Sixth, our study was limited by the unavailability of some relevant measures at the best time of measurement. For example, because an expanded set of psychological measures was available in 1990 we chose that year as our baseline, thereby eliminating the 1985 and 1987 assessments from our analyses. Similarly, our measure of early family environment was collected in 2000. Reconstructions of family environments may include an emotional overlay, contributing to response bias that may influence relations among model variables. To assess this possibility, we evaluated the fit of an alternative model that gave negative emotions priority to see if it explained the reconstruction of childhood events. This model was an inferior fit to the data, as has been true in other empirical tests of this alternative model (Lehman et al., 2005; Taylor et al., 2006). Moreover, the instrument on which our assessment of family environment is based (Felitti et al., 1998) has demonstrated a dose-response relationship to a broad array of diagnosed health outcomes (e.g. cancer, CHD), and a response bias alone is highly unlikely to yield such effects.
Conclusions
This research relates low childhood SES to elevated blood pressure over time directly and via pathways implicating indirect associations through childhood family environment, negative emotionality, and health behaviors. Although the effects are modest, the model explained initial levels of both systolic and diastolic blood pressure and change in systolic blood pressure. The results underscore the importance of early experience in predicting morbidity in adulthood and extend these findings to include risks for hypertension. The results also point to psychosocial and biological mechanisms that may underlie the relation of SES to hypertension and thus may help to explain SES-related health disparities in hypertension and coronary artery disease. Finally, the results suggest the potential importance of intervening with those from low socioeconomic environments to help improve health behavior and promote healthy family environments, thereby potentially deterring the development of chronic negative emotional states that may have implications for long-term health.
Acknowledgments
Work on this manuscript was supported by contracts to University of Alabama at Birmingham, Coordinating Center, N01-HC-95095 University of Alabama at Birmingham, Field Center, N01-HC-48047 University of Minnesota, Field Center, N01-HC-48048 Northwestern University, Field Center, N01-HC-48049 Kaiser Foundation Research Institute, N01-HC-48050 University of California, Irvine, Echocardiography Reading Center, N01-HC-45134 Harbor-UCLA Research Education Institute, Computed Tomography Reading Center, and by grants N01-HC-05187 from the National Heart, Lung and Blood Institute, AG030309 from the National Institute of Aging, and a grant from the MacArthur Research Network on SES and Health and to CARDIA. The authors are grateful to the MacArthur Network on SES and Health for the inclusion of the Early Family Environment measure in the Year 15 CARDIA assessment; to Karen Matthews for her comments on an earlier draft; and to Claudio Barbaranelli for guidance with EQS syntax.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/hea
Contributor Information
Barbara J. Lehman, Department of Psychology, Western Washington University
Shelley E. Taylor, Department of Psychology, University of California, Los Angeles
Catarina I. Kiefe, Division of Preventive Medicine, University of Alabama at Birmingham and Birmingham Veterans Affairs Medical Center
Teresa E. Seeman, Department of Geriatrics, University of California, Los Angeles
References
- Adler N, Marmot M, McEwen B, Stewart J. Socioeconomic status and health in industrial nations: Social, psychological, and biological pathways. New York: New York Academy of Sciences; 2000. [PubMed] [Google Scholar]
- American Heart Association. Your High Blood Pressure Questions Answered - Top or Bottom? 2003 Retrieved September 30, 2005, from http://www.americanheart.org/presenter.jhtml?identifier=3027043.
- Bell AC, Adair LS, Popkin BM. Understanding the role of mediating risk factors and proxy effects in the association between socioeconomic status and untreated hypertension. Social Science and Medicine. 2004;59:275–283. doi: 10.1016/j.socscimed.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Caspi A, Begg D, Dickson N, Harrington H, Langley J, Moffitt TE, et al. Personality differences predict health-risk behaviors in young adulthood: Evidence from a longitudinal study. Journal of Personality and Social Psychology. 1997;73:1052–1063. doi: 10.1037//0022-3514.73.5.1052. [DOI] [PubMed] [Google Scholar]
- Cook W, Medley D. Proposed hostility and pharisaic-virtue scales for the MMPI. Journal of Applied Psychology. 1954;38:414–418. [Google Scholar]
- Davidson K, Jonas BS, Dixon KE, Markovitz JH. Do depression symptoms predict early hypertension incidence in young adults in the CARDIA study? Archives of Internal Medicine. 2000;160:1495–1500. doi: 10.1001/archinte.160.10.1495. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC, Stoolmiller M. Modeling developmental processes using latent growth structural equation methodology. Applied Psychological Measurement. 1994;18:343–354. [Google Scholar]
- Dyer AR, Cutter GR, Liu K, Armstrong MA, Friedman GD, Hughes GH, et al. Alcohol intake and blood pressure in young adults: The CARDIA study. Journal of Clinical Epidemiology. 1990;43:1–13. doi: 10.1016/0895-4356(90)90050-y. [DOI] [PubMed] [Google Scholar]
- Emery RE, Laumann-Billings L. An overview of the nature, causes, and consequences of abusive family relationships. American Psychologist. 1998;53:121–135. doi: 10.1037//0003-066x.53.2.121. [DOI] [PubMed] [Google Scholar]
- Felitti V, Anda R, Nordenberg D, Williamson D, Apitz A, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fields L, Burt V, Cutler J, Hughes J, Roccella E, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: A rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, et al. CARDIA: Study design, recruitment, and some characteristics of the examined subjects. Journal of Clinical Epidemiology. 1988;41:1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- Jacobs DR, Hahn L, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. Journal of Cardiopulmonary Rehabilitation. 1989;9:448–459. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Singer BH, Williams DR, Schwartz JE, Matthews KA, Kiefe CI, Seeman TE. Impact of socioeconomic status on longitudinal accumulation of cardiovascular risk in young adults: The CARDIA study. Social Science & Medicine. 2005;60:999–1015. doi: 10.1016/j.socscimed.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Kertesz SG, Pletcher MJ, Safford M, Halanych J, Kirk K, Schumacher J, et al. Illicit drug use in young adults and subsequent decline in general health: The coronary artery risk development in young adults (CARDIA) study. Drug and Alcohol Dependency. 2007;88:224–233. doi: 10.1016/j.drugalcdep.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson K, Russ SA, Crall JJ, Halfon N. Influence of multiple social risks on children’s health. Pediatrics. 2008;121:337–344. doi: 10.1542/peds.2007-0447. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Smith GD. Early life determinants of adult blood pressure. Current Opinion in Nephrology and Hypertension. 2005;14:259–264. doi: 10.1097/01.mnh.0000165893.13620.2b. [DOI] [PubMed] [Google Scholar]
- Lehman B, Taylor S, Kiefe C, Seeman T. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosomatic Medicine. 2005;67:846–854. doi: 10.1097/01.psy.0000188443.48405.eb. [DOI] [PubMed] [Google Scholar]
- Liu K, Ruth KJ, Flack JM, Jones-Webb R, Burke G, Savage PJ, Hulley SB. Blood pressure in young blacks and whites: Relevance of obesity and lifestyle factors in determining differences. Circulation. 1996;9(3):60–66. doi: 10.1161/01.cir.93.1.60. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Rodriguez BS, Appelhans BM. Cardiovascular stress responses in young adulthood associated with family of origin relationship experiences. Psychosomatic Medicine. 2005;67:514–521. doi: 10.1097/01.psy.0000160466.10397.18. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gump BB, Block DR, Allen MT. Does background stress heighten or dampen children’s cardiovascular responses to acute stress? Psychosomatic Medicine. 1997;59:488–496. doi: 10.1097/00006842-199709000-00005. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, et al. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Salomon K, Brady SS, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure in adolescence. Psychosomatic Medicine. 2003;65:410–415. doi: 10.1097/01.psy.0000057612.94797.5f. [DOI] [PubMed] [Google Scholar]
- McLoyd V. Socioeconomic disadvantage and child development. American Psychologist. 1998;53:185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Owen N, Poulton T, Hay FC, Mohamed-Ali V, Steptoe A. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain, Behavior, and Immunity. 2003;17:286–295. doi: 10.1016/s0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Pine D, Goldstein R, Wolk S, Weissman N. The association between childhood depression and adulthood body mass index. Pediatrics. 2001;107:1049–1056. doi: 10.1542/peds.107.5.1049. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation—allostatic load and its health consequences. MacArthur studies of successful aging. Archives of Internal Medicine. 1997;157:2259–2268. [PubMed] [Google Scholar]
- Spielberger CD, Johnson EH, Russell SF, Crane RJ, Jacobs GA, Worden TJ. The experience and expression of anger: construction and validation of an anger expression scale. In: Chesney MA, Rosenman RH, editors. Anger and Hostility in Cardiovascular and Behavioral Medicine. Washington DC: Hemisphere; 1985. [Google Scholar]
- Spielberger CD, Edwards CD, Lushene RE, Montuori J, Platzek D. STAIC Preliminary Manual. Palo Alto: Consulting Psychologists Press, Inc; 1980. [Google Scholar]
- Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: The problems and implications of overlapping affective dispositions. Psychological Bulletin. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the Coronary Artery Risk Development in Young Adults Study. Biological Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Taylor S, Lerner J, Sage R, Lehman B, Seeman T. Early environment, emotions, responses to stress, and health. Journal of Personality. 2004;72(Special Issue on Personality and Health):1365–1393. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Annals of Internal Medicine. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, He Q, Chen R, Yano K, Masaki KH, Grove JS, et al. Midlife risk factors and healthy survival in men. JAMA. 2006;296:2343–2350. doi: 10.1001/jama.296.19.2343. [DOI] [PubMed] [Google Scholar]
- Woodall KL, Matthews KA. Familial environment associated with Type A behaviors and psychophysiological responses to stress in children. Health Psychology. 1989;8:403–426. doi: 10.1037//0278-6133.8.4.403. [DOI] [PubMed] [Google Scholar]
- Yan LL, Liu K, Matthews KA, Daviglus ML, Ferguson TF, Kiefe CI. Psychosocial factors and risk of hypertension: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Journal of the American Medical Association. 2003;16:2138–2148. doi: 10.1001/jama.290.16.2138. [DOI] [PubMed] [Google Scholar]