Abstract
Herpesvirions and varicella zoster virus (VZV) DNA were recently reported in all 15 cerebrospinal fluid (CSF) samples from patients with relapsing-remitting multiple sclerosis (MS) obtained within 1 week of exacerbation. Using identical electron microscopic and polymerase chain reaction techniques, including additional primer sets representing different regions of the VZV genome, we found no herpesvirions or VZV DNA in MS CSF or acute MS plaques. Although enzyme-linked immunosorbent assay analysis demonstrated a higher titer of VZV antibody in MS CSF than in inflammatory control samples, recombinant antibodies prepared from clonally expanded MS CSF plasma cells did not bind to VZV. VZV is not a disease-relevant antigen in MS.
The cause of multiple sclerosis (MS) is unknown. Epidemiological and genetic studies point to an environmental cause, a notion supported by increased concentrations of oligoclonal IgG in the brain and cerebrospinal fluid (CSF) of MS patients. Importantly, the oligoclonal IgG in other central nervous system diseases is antibody directed against the disease-causing agent (reviewed in Bennett and colleagues’1 article).
Recently, Sotelo and coauthors2 reported the detection of herpesvirions by electron microscopy (EM) in MS CSF and varicella zoster virus (VZV) DNA by polymerase chain reaction (PCR) amplification in CSF and peripheral blood mononuclear cells (PBMCs) of MS patients. Of particular interest was the detection of both VZ virions and VZV DNA in the CSF of each of 15 MS patients within 1 week of exacerbation.
To confirm these findings, we used EM to search for VZ virions and PCR to detect VZV DNA in CSF from five MS patients (four samples obtained within 8 days of exacerbation and one during remission) and three patients with a clinically isolated syndrome (CIS) at high risk for conversion to MS (oligoclonal bands and multiple white matter lesions). VZV PCR was performed in parallel on control CSF from patients with known and unknown chronic inflammatory central nervous system disease. Enzyme-linked immunosorbent assay (ELISA) and immunoblotting were used to compare binding of antibody from MS and control CSF to VZV. Finally, based on strategies and techniques in which recombinant antibodies (rAbs) prepared from clonally expanded plasma cells in subacute sclerosing panencephalitis brain3 and CSF4 successfully detected measles virus antigens, we used single-cell reverse transcriptase polymerase chain reaction (RT-PCR) to prepare rAbs from clonally expanded CD138+ plasma cells in six MS CSF samples.5 The rAbs were tested individually for binding to VZV-infected cells or cell lysates.
Subjects and Methods
Subjects and Cerebrospinal Fluid Collection
After local informed consent, CSF was collected from five patients with MS, three with CIS, and six patients with other inflammatory neurological diseases (OIND). Table 1 lists the laboratory findings for the patients. All MS patients had relapsing-remitting disease (RRMS). CSF from four of five MS patients was obtained within 8 days of exacerbation. All MS and CIS patients had oligoclonal bands and multiple white matter lesions on magnetic resonance imaging (MRI), although none of the CIS patients has experienced development of definite MS (a new attack or new white matter lesions) in up to 1 year of follow-up.
Table 1.
Clinical and Laboratory Features of Multiple Sclerosis Patients and Other Inflammatory Neurological Disease Control Subjects
| Patient No. | Diagnosis | Age (yr)a | Sex | CSF Cellsb | OCBs | White Matter Lesions |
|---|---|---|---|---|---|---|
| MS 1 | RRMS | 24 | F | 29 | + | + |
| MS 2 | RRMS | 30 | F | 10 | + | + |
| MS 3 | RRMS | 26 | F | 0 | + | + |
| MS 4 | RRMS | 35 | F | 32 | + | + |
| MS 08-1 (remission) | RRMS | 30 | F | 10 | + | + |
| CIS 1 | CIS | 29 | F | 6 | + | + |
| CIS 2 | CIS | 29 | F | 15 | + | + |
| CIS 3 | CIS | 28 | M | 9 | + | + |
| OIND 06-1 | SSPE | 12 | M | 7 | + | NA |
| OIND 08-1 | VZV vasculopathy | 78 | F | 14 | − | NA |
| OIND 08-5 | VZV radiculomyelitis | 78 | F | 14 | + | NA |
| OIND 04-5 | Cryptococcal meningitis | 57 | M | 42 | + | NA |
| OIND 05-1 | Neurosyphilis | 85 | M | 2 | + | NA |
| OIND 05-2 | Chronic meningitis | 31 | M | 30 | + | NA |
At time of lumbar puncture.
Cells per cubic millimeter.
CSF = cerebrospinal fluid; OCB = oligoclonal bands; MS = multiple sclerosis; RRMS = relapsing-remitting multiple sclerosis; CIS = clinically isolated syndrome; OIND = other inflammatory neurological disease; SSPE = subacute sclerosing panencephalitis; VZV = varicella zoster virus; NA = not applicable.
Electron Microscopy
Tissue culture fluid was collected from VZV-infected MeWo cells at the height of a cytopathic effect 3 days after infection. All CSF and tissue culture samples were stored at −80°C prior until examined. As Sotelo and coauthors2 described, CSF and VZV-infected tissue culture supernatants (0.6ml) were centrifuged at 2,800g for 40 minutes to remove cell debris. Supernatants were then centrifuged at 70,000g for 2 hours, and the pellet was resuspended in 65μl nuclease-free water. After ultracentrifugation, 3μl of the resuspended pellets from MS and CIS CSF and VZV-infected tissue culture supernatants were placed on 300-mesh Formvar-coated grids, negatively stained with 1% uranyl acetate and observed at 23,000× magnification with an FEI Tecnai G2 BioTWIN transmission electron microscope (FEI Company, Hillsboro, OR) for electron-dense viral particles.
Quantitative Real-time Polymerase Chain Reaction
A portion (2–5μl) of the resuspended ultracentrifuged pellet obtained earlier was analyzed by quantitative real-time PCR for the presence of VZV open reading frames (ORFs) 63, 21,6 and 312 using a 7500-Fast real-time PCR system (Applied Biosystems, Foster City, CA) equipped with fluorescence-based, simultaneous amplification and product detection. PCR assays were performed in 20μl volumes containing 2X TaqMan Universal PCR Master Mix (Perkin-Elmer, Norwalk, CT).7 Amplification conditions consisted of initial denaturation at 95°C for 10 minutes, followed by 40 two-step cycles of 15 seconds at 95°C and 1 minute at 60°C. Probe degradation-related fluorescence was recorded during the 60°C extension cycle and analyzed using the SDS program (Sequence Detection Software; Applied Biosystems). Standard curves were generated with serial dilutions of VZV DNA (0–106 copies) extracted from virus-infected cells.8 All samples were analyzed in duplicate. DNA extracted from tissue sections of frozen unfixed acute plaques from two MS brains was also analyzed by quantitative PCR.
Enzyme-Linked Immunosorbent Assay and Immunoblotting Assays
VZV-specific IgG activity was quantitated in 53 MS CSF samples and in 8 non-MS central nervous system inflammatory control CSFs (normalized to 10μg/ml) by ELISA as described elsewhere.9 Positive reactivity was scored as three times the mean of background binding by secondary antibody alone. Six MS CSFs and four OIND CSFs were also examined by immunoblotting for binding to VZV as described elsewhere.4,10
Recombinant Antibody Assays
Single-cell RT-PCR was used to prepare 43 rAbs from clonally expanded CD138+ plasma cells recovered as described elsewhere5 from the same six MS CSF samples used in immunoblotting. rAbs were individually tested for binding to VZV-infected and uninfected MeWo cell lysates by immunoblotting as described elsewhere.4 In addition, 40 of these rAbs were pooled into 9 groups (3–5 rAbs/group, 5μg/ml of each rAb) and used for immunostaining of VZV-infected MeWo cells as described elsewhere.4,10
Results
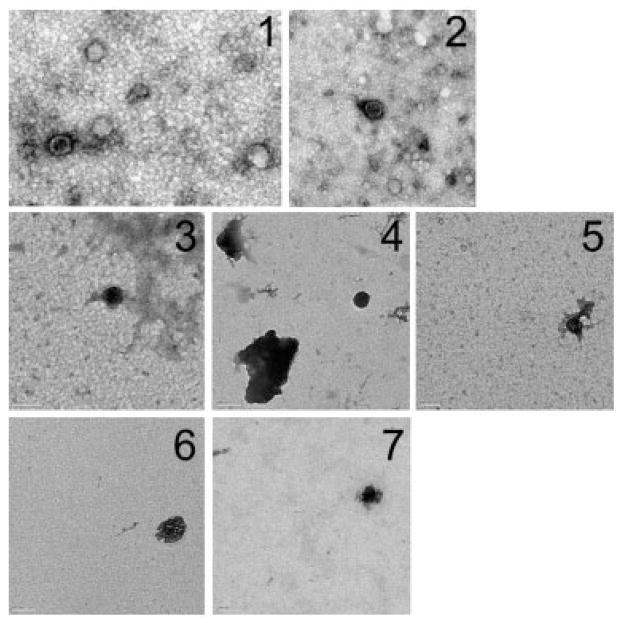
Transmission EM demonstrated herpesvirions in supernatants from VZV-infected cells in tissue culture (Fig 1, panels 1, 2), but not in the pelleted CSF from patients with MS (see Fig 1, panels 3, 4), CIS (see Fig 1, panels 5–7), or from one patient with VZV radiculomyelitis (data not shown).
Fig 1.
Analysis of human cerebrospinal fluid (CSF) and varicella zoster virus (VZV)-infected cells for VZ virions and DNA. Transmission electron microscopy was used to analyze ultracentrifuged pellets obtained from VZV-infected cells in tissue culture (1, 2) and in CSF from patients with multiple sclerosis (3, 4) and clinically isolated syndromes (CIS) (5–7). After ultracentrifugation, pellets were adsorbed onto 300-mesh grids, shadowed with 1% uranyl acetate, and visualized at 23,000× magnification. Note VZ virions in pellets of supernatants from VZV-infected cells in tissue culture (1, 2), but not after ultracentrifugation of CSFs from relapsing-remitting multiple sclerosis patients collected within 8 days of exacerbation (3, 4) or in CSF of patients during an acute CIS (5–7). Scale bar = 100nm.
Analysis of all ultracentrifuged pellets by quantitative PCR using primer sets from three regions of the VZV genome at a sensitivity of 400 copies (ORF 21), 40 copies (ORF 31), and 10 copies of DNA (ORF 63) readily demonstrated amplifiable VZV DNA in culture supernatants from VZV-infected MeWo cells and in CSF from the patient with VZV radiculomyelitis, but not in the CSF of any patients with MS, CIS, or OIND, or in acute MS brain plaques (Table 2). Trace-level amplification was detected in replicate samples of several CSF samples from patients with MS, patients with CIS, and two inflammatory control subjects with the most sensitive VZV ORF 63 primers, but these values were below the reliable lower limit of the standard curve for VZV. This “trace” amplification was recorded as less than 10 copies.
Table 2.
Quantitative Polymerase Chain Reaction Analysis of Varicella Zoster Virus DNA in Acute Multiple Sclerosis Plaques, in Cerebrospinal Fluid of Patients with Multiple Sclerosis, Clinically Isolated Syndrome, and Other Inflammatory Neurological Diseases, and in Ultracentrifuged Pellets Obtained from Varicella Zoster Virus–Infected Cells in Tissue Culture
| Sample | VZV 63,a copies/ml | VZV 21,b copies/ml | VZV 31,c copies/ml |
|---|---|---|---|
| MS 1 | 0 | 0 | 0 |
| MS 2 | Traced | 0 | 0 |
| MS 3 | 0 | 0 | 0 |
| MS 4 | 0 | 0 | 0 |
| MS 08-1 (remission) | 0 | 0 | 0 |
| CIS 1 | 0 | 0 | 0 |
| CIS 2 | Traced | 0 | 0 |
| CIS 3 | Traced | 0 | 0 |
| MS 95-A3 acute plaque | 0 | ND | ND |
| MS 03-A1 acute plaque | 0 | ND | ND |
| OIND 06-1 SSPE | 0 | 0 | 0 |
| OIND 08-1 VZV vasculopathy | 0 | 0 | 0 |
| OIND 08-5 VZV radiculomyelitis | 8,000 | 14,200 | 9,710 |
| OIND 04-5 cryptococcal meningitis | Traced | 0 | 0 |
| OIND 05-1 neurosyphilis | 0 | 0 | 0 |
| OIND 05-2 chronic meningitis | Traced | 0 | 0 |
| OIND VZV culture supernatant | 6.989 E6 | 18.7 E6 | 291,500 |
Open reading frames (ORF) 63 primers sensitive to detect 10 copies varicella zoster virus (VZV) DNA.
ORF 21 primers sensitive to detect 400 copies VZV DNA.
ORF 31 primers sensitive to detect 40 copies VZV DNA.
Trace: amplification at 40 cycles (between 1 and 10 copies) is below the lower limit of reliability for the standard curve with VZV ORF 63 primers.
MS = multiple sclerosis; CIS = clinically isolated syndrome; ND = not done; OIND = other inflammatory neurological disease; SSPE = subacute sclerosing panencephalitis.
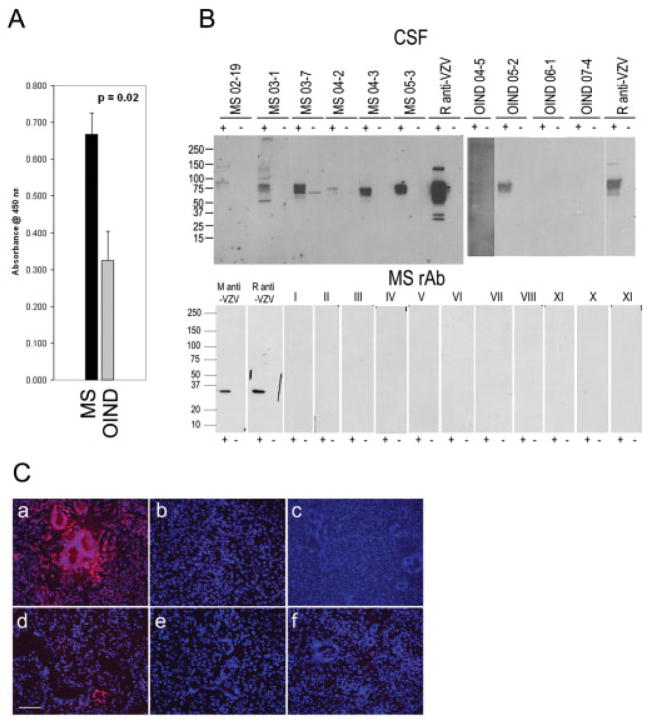
ELISA analysis showed reactivity in 43 of 53 MS CSF samples with VZV-infected MeWo cells, compared with 3 of 8 OIND CSF samples (Fig 2A). The average reactivity of MS CSFs to VZV lysates (0.668 ± 0.057 standard error of the mean) was significantly greater (p < 0.05, t test) than that of OIND CSFs (0.309 ± 0.082). Moreover, 6 of the 43 MS CSFs that were positive in ELISA were also used to immunoblot lysates of VZV-infected cells. All six showed reactivity to VZV compared with two of four OIND CSF samples (see Fig 2B).
Fig 2.
Analysis of human cerebrospinal fluid (CSF) and recombinant antibodies (rAbs) prepared from clonally expanded plasma cells in multiple sclerosis (MS) cerebrospinal fluid (CSF) for varicella zoster virus (VZV) reactivity. (A) Enzyme-linked immunosorbent assay (ELISA) analysis of human CSF binding to VZV. CSF samples from patients with relapsing-remitting multiple sclerosis (RRMS) and other inflammatory neurological diseases (OIND) were diluted to IgG concentrations of 10μg/ml and reacted with VZV-infected cell lysates. The eight OIND were VZV vasculopathy, cryptococcal meningitis, chronic meningitis, subacute sclerosing panencephalitis (SSPE) (2), paraneoplastic syndrome, neurosyphilis, and meningoencephalitis. Panel shows the average reactivity (± SEM) of MS CSF compared to average reactivity of OIND. (B) Immunoblotting of MS IgG with VZV. Lysates of VZV-infected (+) or uninfected (−) MeWo cells were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to nitro-cellulose membranes, and reacted with 5μg/ml IgG from MS CSF, rabbit anti-VZV, or OIND IgG. Positive control anti-VZV monoclonal antibody (M anti-VZV), polyclonal rabbit anti-VZV IgG antibody (R anti-VZV), and recombinant antibodies (rAbs) prepared from clonally expanded plasma cells in MS CSF (5μg/ml) were used to immunoblot VZV-infected and uninfected cell lysates. All rAbs were negative (lanes I-XI). Molecular size is given in kilodaltons. (C) Immunostaining. VZV-infected (a) and un-infected (b) MeWo cells were stained with rabbit anti-VZV IgG. The same MS CSF rAbs used in immunoblotting were pooled into nine groups of three to five rAbs (5μg/ml of each rAb) and used to stain VZV-infected MeWo cells. Representative staining is indicated in panels c through f. Note overall lack of reactivity of rAbs with VZV. Bar = 100μm.
Forty-three rAbs were prepared from the CSF of the six MS patients used in immunoblotting. None showed binding to VZV in ELISA (data not shown), and no rAbs showed reactivity with VZV-infected or uninfected cell lysates by immunoblotting (see Fig 2B). Of these 43 rAbs, 40 were pooled into groups of 3 to 5 rAbs each and used to immunostain VZV-infected cells. If positive staining occurred, the rAbs in that group were tested separately to determine specificity. Figure 2C shows representative staining by the rAbs. Only two rAbs showed positive staining, one of which stained occasional foci of cells (see Fig 2C, panel d) but not all VZV-infected multinucleated cells, and one rAb stained all cell nuclei (data not shown).
Discussion
The detection of VZV DNA in CSF and peripheral blood mononuclear cells of MS patients11–13 was recently reinforced by the reported presence of herpes virions and VZV DNA in CSF within 1 week of exacerbation and in peripheral blood mononuclear cells of RRMS patients.2 To reproduce these findings, we used EM and PCR, the same techniques that Sotelo and coauthors2 used, to analyze CSF from four patients with RRMS obtained within 8 days of exacerbation, CSF from one patient with RRMS obtained during remission, three CSFs from CIS patients at high-risk for development of MS, and six patients with OIND. Our PCR analysis included the VZV ORF 31 primers that Sotello and coauthors2 used, as well as primer sets from different regions of the viral genome corresponding to VZV ORFs 21 and 63.
No herpesvirions were detected in any CSF samples from patients with MS, CIS, or OIND, whereas viral particles were readily detectable in similarly prepared supernatants from cells productively infected with VZV in tissue culture. PCR analysis of CSF samples with three primer sets that amplified disparate regions of the VZV genome showed no VZV DNA in patients with MS, patients with CIS, and five OIND, whereas all three VZV DNA products were detected in culture supernatants from VZV-infected cells and in the CSF from the VZV radiculomyelitis patient. The lack of detection of VZ virions in the CSF of the patient with VZV radiculomyelitis is probably due to the low CSF viral titer 1 week after disease onset.
CSF IgG from both MS and OIND control patients reacted with VZV by ELISA and immunoblotting. This is not surprising because MS CSF contains antibodies against multiple ubiquitous viruses such as VZV, EBV, and rubella.14 None of these viral antibodies, however, corresponds to the oligoclonal IgG in MS CSF. In contrast, we showed that the oligoclonal IgG in CSF from a patient with VZV vasculopathy is VZV specific.15 Note that our 43 rAbs prepared from clonally expanded plasma cells in MS CSF did not bind to VZV-infected cells, yet are likely to represent the intrathecally synthesized oligoclonal IgG, based on a strong link between immunoglobulin variable region sequences and the corresponding peptide sequences generated from purified CSF oligoclonal bands.16 Furthermore, although immunological analyses demonstrated a significant difference in binding of MS CSF to VZV-infected cells compared with control CSF from OIND, this is not likely to reflect intrathecally synthesized antibody based on the fact that rAbs prepared from clonally expanded plasma cells in MS CSF did not bind to VZV. In contrast, rAbs prepared from clonally expanded plasma cells in subacute sclerosing panencephalitis brain and CSF are measles virus specific. Overall, we find no evidence to indicate that VZV is a relevant antigen in MS.
Acknowledgments
This work was supported the NIH (Public Health Service, NS041549, M.P.B.; NS32623, R.J.C, J.L.B., G.P.O., D.G.) and the National Multiple Sclerosis Society (RG3897, M.P.B.; RG3908, J.L.B.).
We thank D. Dill for technical assistance with transmission EM and the Rocky Mountain MS Center Tissue Bank for postmortem MS tissue. We also thank M. Hoffman for editorial review and C. Allen for preparing the manuscript.
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Bennett JL, Yu X, Gilden DH, et al. Infectious agents and multiple sclerosis. In: Raine CS, McFarland HF, Hohlfeld R, editors. Multiple sclerosis: a comprehensive text. New York: Saunders Elsevier; 2008. pp. 226–236. [Google Scholar]
- 2.Sotelo J, Martinez-Palomo A, Ordonez G, et al. Varicella-zoster virus in cerebrospinal fluid at relapses of multiple sclerosis. Ann Neurol. 2008;63:303–311. doi: 10.1002/ana.21316. [DOI] [PubMed] [Google Scholar]
- 3.Burgoon MP, Keays KM, Owens GP, et al. Laser-capture microdissection of plasma cells from subacute sclerosing panencephalitis brain reveals intrathecal disease-relevant antibodies. Proc Natl Acad Sci U S A. 2005;102:7245–7250. doi: 10.1073/pnas.0502323102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owens GP, Ritchie AM, Gilden DH, et al. Measles virus-specific plasma cells are prominent in subacute sclerosing panencephalitis CSF. Neurology. 2007;68:1815–1819. doi: 10.1212/01.wnl.0000262036.56594.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X, Gilden DH, Ritchie AM, et al. Specificity of recombinant antibodies generated from multiple sclerosis cerebrospinal fluid probed with a random peptide library. J Neuroimmunol. 2006;172:121–131. doi: 10.1016/j.jneuroim.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Cohrs RJ, Gilden DH. Prevalence and abundance of latently transcribed varicella-zoster virus genes in human ganglia. J Virol. 2007;81:2950–2956. doi: 10.1128/JVI.02745-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohrs RJ, Randall J, Smith J, et al. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol. 2000;74:11464–11471. doi: 10.1128/jvi.74.24.11464-11471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilden DH, Shtram Y, Friedmann A, et al. Extraction of cell-associated varicella-zoster virus DNA with Triton X-100-NaCl. J Virol Methods. 1982;4:263–275. doi: 10.1016/0166-0934(82)90073-8. [DOI] [PubMed] [Google Scholar]
- 9.Owens GP, Shearer AJ, Yu X, et al. Screening random peptide libraries with subacute sclerosing panencephalitis brain-derived recombinant antibodies identifies multiple epitopes in the C-terminal region of the measles virus nucleocapsid protein. J Virol. 2006;80:12121–12130. doi: 10.1128/JVI.01704-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgoon MP, Williamson RA, Owens GP, et al. Cloning the antibody response in humans with inflammatory CNS disease: isolation of measles virus-specific antibodies from phage display libraries of a subacute sclerosing panencephalitis brain. J Neuroimmunol. 1999;94:204–211. doi: 10.1016/s0165-5728(98)00243-4. [DOI] [PubMed] [Google Scholar]
- 11.Ordonez G, Pineda B, Garcia-Navarrete R, et al. Brief presence of varicella-zoster viral DNA in mononuclear cells during relapses of multiple sclerosis. Arch Neurol. 2004;61:529–532. doi: 10.1001/archneur.61.4.529. [DOI] [PubMed] [Google Scholar]
- 12.Mancuso R, Delbue S, Borghi E, et al. Increased prevalence of varicella zoster virus DNA in cerebrospinal fluid from patients with multiple sclerosis. J Med Virol. 2007;79:192–199. doi: 10.1002/jmv.20777. [DOI] [PubMed] [Google Scholar]
- 13.Sotelo J, Ordonez G, Pineda B. Varicella-zoster virus at relapses of multiple sclerosis. J Neurol. 2007;254:493–500. doi: 10.1007/s00415-006-0402-x. [DOI] [PubMed] [Google Scholar]
- 14.Forghani B, Cremer NE, Johnson KP, et al. Comprehensive viral immunology of multiple sclerosis. III. Analysis of CSF antibodies by radioimmunoassay. Arch Neurol. 1980;37:616–619. doi: 10.1001/archneur.1980.00500590040004. [DOI] [PubMed] [Google Scholar]
- 15.Burgoon MP, Hammack BN, Owens GP, et al. Oligoclonal immunoglobulins in cerebrospinal fluid during varicella zoster virus (VZV) vasculopathy are directed against VZV. Ann Neurol. 2003;54:459–463. doi: 10.1002/ana.10685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obermeier B, Mentele R, Malotka J, et al. Matching of the oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med. 2008;14:688–693. doi: 10.1038/nm1714. [DOI] [PubMed] [Google Scholar]