Abstract
Objective
The occurrence of a seizure after the arterial switch operation is associated with a worse long-term neurodevelopmental outcome. The significance of seizures after neonatal and infant repair of other congenital heart defects is not known.
Methods
A recent study at our institution demonstrated seizures documented by 48-hour electroencephalographic monitoring in 20 (11%) of 178 neonates and infants after surgery for complex congenital heart defects, including hypoplastic left heart syndrome or variants. The developmental outcomes of this cohort were evaluated at 1 year of age by using the Bayley Scales of Infant Development II, which yields 2 scores: the Mental Developmental Index and the Psychomotor Developmental Index.
Results
Developmental evaluations were performed in 114 (70%) of 164 survivors, including 36 with hypoplastic left heart syndrome. Postoperative electroencephalographic seizures had occurred in 15 (13%) of 114 of the entire group and in 8 (22%) of 36 of those with hypoplastic left heart syndrome. For the entire cohort, the Mental Developmental Index was 92.3 ± 13.5, and the Psychomotor Developmental Index was 79.9 ± 18.8 for patients without seizures, compared with 90.3 ± 10.7 and 74.4 ± 19.3 for those with seizures (both P > .5). For the hypoplastic left heart syndrome subgroup, the Mental Developmental Index was 92.3 ± 14.9, and the Psychomotor Developmental Index was 74.8 ± 19.3 for patients with seizures, compared with 91.9 ± 12.4 and 73.9 ± 18.3 for those without seizures (both P > .5). A frontal onset of seizures was predictive of a lower score on the Psychomotor Developmental Index, but not on the Mental Developmental Index.
Conclusions
The occurrence of a seizure after cardiac operation is a marker of central nervous system injury. However, in this cohort of neonates and infants with complex congenital heart defects, the occurrence of a seizure was not predictive of a worse developmental outcome at 1 year of age as assessed by the Bayley Scales of Infant Development II.
The occurrence of a seizure in the early postoperative period after repair or palliation of congenital heart defects (CHD) is a marker for a central nervous system (CNS) injury and has been associated with adverse neurodevelopmental sequelae.1–3 The research standard for detection and quantification of postoperative seizures remains continuous electroencephalographic (EEG) monitoring. In the Boston Circulatory Arrest Study conducted between 1988 and 1992, continuous EEG monitoring in the first 48 hours after the arterial switch operation for transposition of the great arteries (TGA), with or without a ventricular septal defect (VSD), demonstrated seizures in 27 (20%) of 136 neonates and infants.1 Follow-up evaluation of the patients in the Circulatory Arrest Study demonstrated that the occurrence of a postoperative EEG seizure was associated with a worse neurodevelopmental outcome at 1 and 4 years of age.2,3 A recent study from our institution demonstrated that the frequency of postoperative EEG seizures was less than reported in previous studies.1,4,5 EEG seizures were identified in 11.2% of a heterogeneous cohort of neonates and infants with complex CHDs, including hypoplastic left heart syndrome (HLHS). An increasing duration of deep hypothermia and circulatory arrest (DHCA) was identified as a predictor of seizures. However, the incidence of seizures in children with a limited duration of DHCA was similar to that in infants who underwent continuous cardiopulmonary bypass (CPB) alone. This study was undertaken to evaluate the effect of postoperative EEG seizures on neurodevelopmental outcome at 1 year of age in this cohort.
Patients and Methods
Sample
A subgroup of children enrolled in a prospective study evaluating polymorphisms of apolipoprotein E (APOE) as a risk factor for neurodevelopmental dysfunction also underwent continuous video-EEG monitoring in the early postoperative period.6 Patients 6 months of age or younger who underwent CPB with or without DHCA for repair of CHD were eligible. Exclusion criteria included (1) multiple congenital anomalies, (2) a recognizable genetic or phenotypic syndrome other than chromosome 22q11 microdeletions, and (3) a language other than English spoken in the home. The study was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia. Informed consent was obtained from the parent or guardian.
Operative Management
Operations were performed by 5 cardiac surgeons with a dedicated team of cardiac anesthesiologists. Alpha-stat blood gas management was used. Pump flow rates were not standardized for this study. DHCA was used at the surgeon’s discretion. Before DHCA, patients underwent core cooling with topical hypothermia of the head to a nasopharyngeal temperature of 18°C. Modified ultrafiltration was performed in all patients.
Video-EEG Examination
Details of the video-EEG evaluation have been previously published.5,7 Video-EEGs were recorded on 1 of 3 identical dedicated portable Telefactor Millenium Beehive machines (Conshohocken, Pa), which capture time-synchronized video images and digital EEG data. A brief 15-minute preoperative baseline study was recorded. Recording was reinitiated after surgery immediately after admission to the cardiac intensive care unit. Video-EEGs were recorded continuously for the first 48 hours after surgery. Studies were terminated only for early death or at parental request. Each record was visually reviewed in its entirety every 24 hours by the recording EEG technologist and independently by a pediatric neurologist. The number of seizures during the study period was recorded. In addition, the sites of origin of the seizures were recorded and classified as frontal or nonfrontal. After confirmation of the presence of seizures, the attending physician was informed of the occurrence of a seizure. All treatment decisions, including the institution of antiepileptic drug therapy, were made at the discretion of the attending physician.
Data Collection
Preoperative factors including gestational age, birth head circumference, birth weight, Apgar scores, and preoperative intubation were obtained from birth and hospital records. Weight, age at operation, and type of operation were recorded along with perfusion data, including CPB time, aortic crossclamp time, and duration of DHCA. Total support time was calculated as CPB time plus DHCA time. Total DHCA time was calculated as the sum of the duration of each episode of DHCA.
One-Year Neurodevelopmental Examination
Children were scheduled for their 1-year follow-up visit as they approached 1 year of age. A 2-week window was established so that children were seen at 12 months plus or minus 2 weeks. Children born before 37 weeks of gestation were seen at their corrected age. The developmental assessment was performed first, followed by a medical history and physical and neurologic examinations. The developmental assessment included the Bayley Scales of Infant Development II, which yields scores on 2 indices: the Psychomotor Development Index (PDI) and the Mental Developmental Index (MDI). The medical evaluation included an interim health history and physical examination. The neurologic examination included neuromuscular status, reflexes, and bulbar function. Growth measurements, including weight, length, and head circumference, were obtained. Patients were also evaluated by a genetic dysmorphologist. Chromosomal analysis and testing for microdeletions of chromosome 22q11 were performed as indicated. Recognition of neonatal dysmorphic features may be difficult; therefore, some patients were enrolled in whom the diagnosis of a genetic syndrome was not made until the 1-year evaluation. Results were classified as no definite genetic syndrome or chromosomal abnormality (normal), a definite genetic syndrome or chromosomal abnormality (abnormal), or a suspected genetic syndrome or abnormality (suspect).
Data Analysis and Statistical Methods
Patients were coded according to a previously described classification that incorporates cardiac anatomy and perioperative physiology and that has been shown to be predictive of perioperative mortality.8 Class I indicates 2 ventricles with no aortic arch obstruction, class II indicates 2 ventricles with aortic arch obstruction, class III indicates a single ventricle with no arch obstruction, and class IV indicates a single ventricle with arch obstruction. Patients with tetralogy of Fallot and TGA are class I, whereas patients with HLHS and variants are class IV. Data are presented as median (range).
Tests for differences in the demographic characteristics of children who did return for their 1-year follow-up versus those who did not were conducted by using the Student t test for qualitative predictors and χ2 or Fisher exact tests for qualitative predictors for the entire cohort and for the subgroup of patients with HLHS or variants (class IV). The χ2 test was used to test the effects of seizure status on neuromuscular outcome unless cell sizes were less than 5 in a 2 × 2 table; then the Fisher exact test was used. To determine whether the number of seizures was predictive of worse outcome, analysis of variance was used to compare data for significant effects of number of seizures on MDI and PDI. The Student t test was used to test the effects of seizure location (frontal vs nonfrontal) on MDI and PDI, and the χ2 test or Fisher exact test was used for neuromuscular outcome. All tests were 2-sided.
Results
Study Population
Between September 16, 2001, and April 2, 2003, 238 eligible infants underwent cardiac operation. Of these, 209 (88%) enrolled in the study of APOE genotype. Continuous postoperative EEG monitoring was performed in 183 (88%). All patients enrolled in the APOE study were approached for enrollment in the EEG study. Reasons for not undergoing EEG monitoring included parental refusal and unavailability of an EEG monitor.
Complete 48-hour EEG studies were obtained in 178 patients (97%). There were 5 hospital deaths (5/178; 3%) and 9 additional deaths (9/173; 12%) before 1 year of age. Of the 164 patients alive at 1 year of age, 114 (70%) returned for evaluation. Characteristics of patients who returned and those who were alive at 1 year and did not return are shown in Table 1. The only significant difference between the 2 groups was that patients with seizures were more likely to return for evaluation.
TABLE 1.
Characteristics of patients who returned and those who were alive at 1 year and did not return
| Variable | Returned for 1-y evaluation (n = 114) | Alive, but did not return (n = 50) | P value |
|---|---|---|---|
| Sex | |||
| Male | 64 (56%) | 32 (64%) | .35 |
| Female | 50 (44%) | 18 (36%) | |
| Gestational age (wk) | 39 (29–41) | 39 (28–41) | .80 |
| Birth weight (g) | 3182 (956–4460) | 2984 (1335–3955) | .09 |
| Apgar score at 5 min | 9 (6–9) | 9 (4–9) | .28 |
| Diagnostic class | |||
| I | 54 (47%) | 26 (52) | |
| II | 16 (14%) | 5 (10%) | .88 |
| III | 8 (7%) | 4 (8%) | |
| IV | 36 (32%) | 15 (30%) | |
| Age at first operation (d) | 8.5 (1–180) | 9 (1–188) | |
| Aged ≤30 d at first operation | 67 (59%) | 32 (64%) | .26 |
| Number of operations with CPB during first year | 1 (1–4) | 1 (1–3) | .169 |
| Number of episodes of DHCA during first year | 76/114 (67%) | 30/50 (60%) | |
| 1 | 49 (64%) | .10 | |
| 2 | 23 (30%) | 14 (47%) | |
| 3 | 4 (6%) | 16 (53%) | |
| Total support during first year (CPB + DHCA) (min) | 78.5 (18–183) | 80 (28–191) | .491 |
| Total DHCA during first year (min) | 45.5 (1–97) | 61.5 (23–134) | .88 |
| EEG seizures | 15/114 (13%) | 1/50 (2%) | .02 |
CPB, Cardiopulmonary bypass; DHCA, deep hypothermia and circulatory arrest; EEG, electroencephalogram.
The study population included 7 patients with TGA, 16 patients with tetralogy of Fallot, 23 patients with VSD (with or without coarctation), 36 patients with HLHS or variants, 7 patients with other forms of functional single ventricle, and 25 patients with a variety of other 2-ventricle cardiac defects. Postoperative seizures had been identified in 15 (13%) of the 144 patients, including 8 (22%) of the 36 patients with HLHS or variants. Seizures had not been identified in any of the patients with TGA or VSD and had been identified in only 1 of the patients with tetralogy of Fallot. Seizures had occurred in 2 of 7 patients with other forms of functional single ventricle. The mean number of seizures in the 48-hour study period was 79 ± 83, with a median of 52 and a range of 1 to 217. Frontal seizures occurred in 10 (67%) of 15 patients. All patients were treated with phenobarbital after identification of seizure activity. In addition, 3 patients received benzodiazepine therapy.
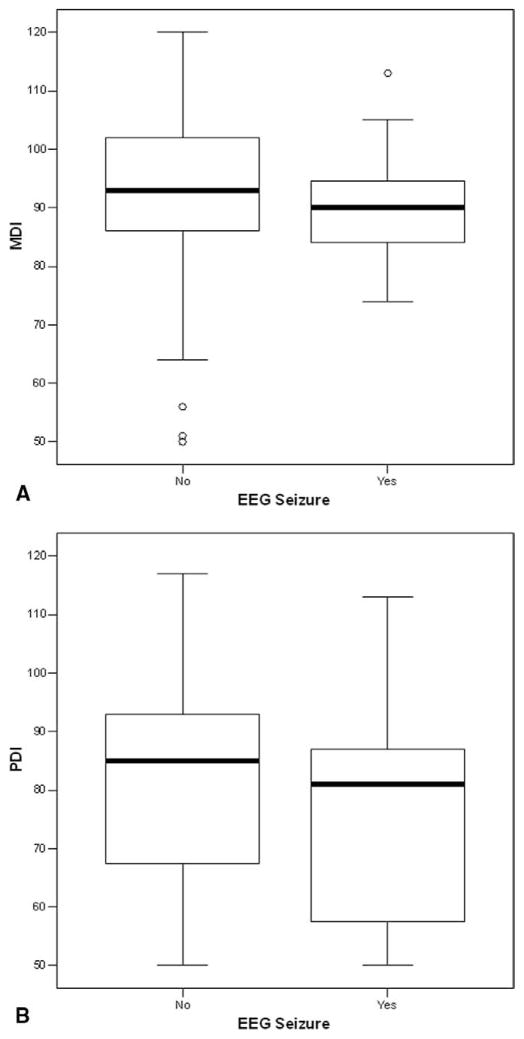
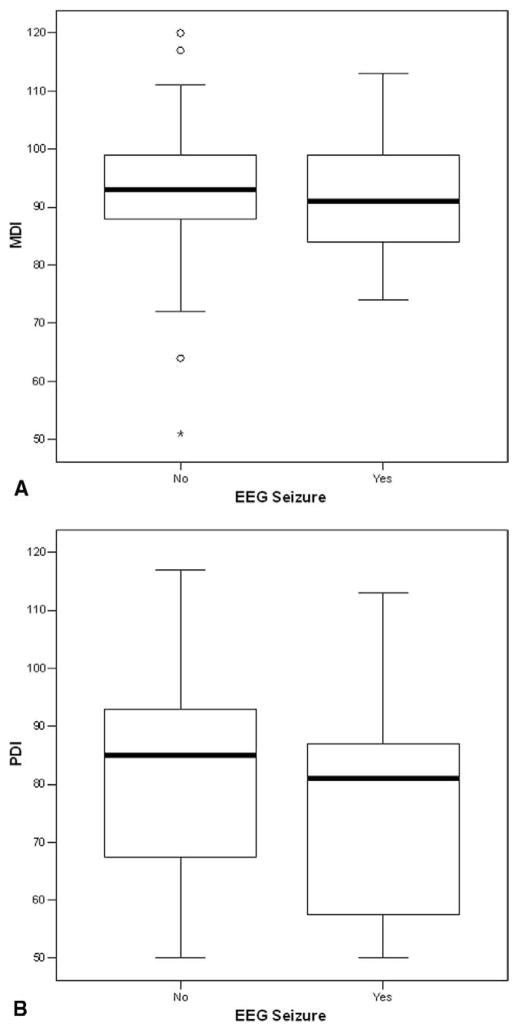
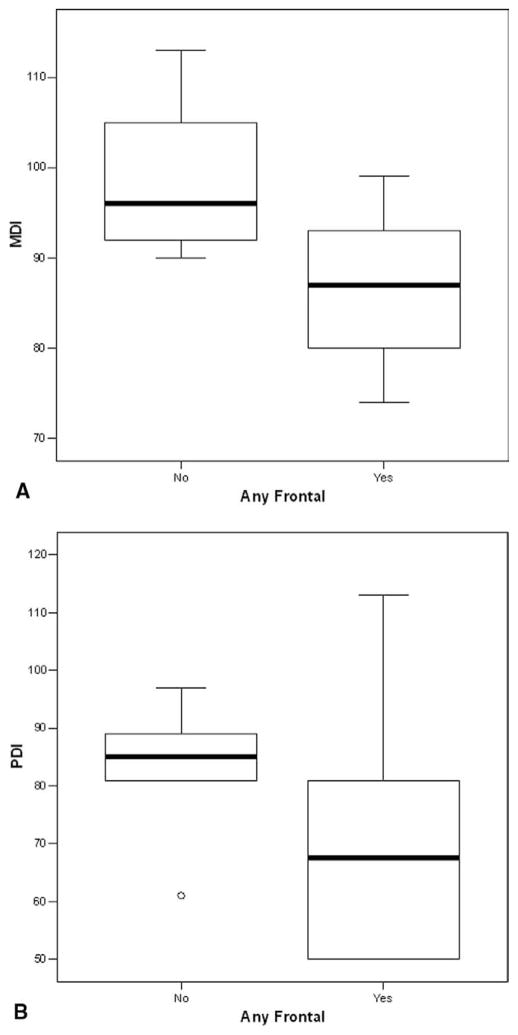
At the 1-year evaluation, the neuromuscular examination results were abnormal or suspect in 52 (46%) of 114 patients. Abnormal or suspect examination results occurred in 41 (41%) of 99 patients without seizures, compared with 11 (73%) of 15 patients with postoperative seizures (P = .027). For the entire cohort, the MDI was 92.0 ± 13.1, and the PDI was 79.2 ± 18.9. For patients without seizures, the MDI was 92.3 ± 13.5, compared with 90.3 ± 10.7 for patients with seizures (P > .5; Figure 1, A). Similarly, the PDI was 79.9 ± 18.8 for patients without seizures, compared with 74.4 ± 19.3 for those with seizures (P > .5; Figure 1, B). Within the subgroups of patients with HLHS or variants, the findings were similar. The MDI was 92.3 ± 14.9, and the PDI was 74.8 ± 19.3 for patients without seizures, compared with 91.9 ± 12.4 and 73.9 ± 18.3, respectively, for those with seizures (both P > .5; Figure 2). The number of seizures was not predictive of either the MDI or the PDI. However, frontal-onset seizures were predictive of a lower score on the MDI (P = .03), but not on the PDI (P = .2), compared with non–frontal-onset seizures (Figure 3). The frontal onset of a seizure was not associated with increased abnormalities on the neuromuscular examination (P > .4).
Figure 1.
A, Box plot of MDI scores for all patients (n = 114), stratified by the occurrence of a seizure. B, Box plot of PDI scores for all patients (n = 114), stratified by the occurrence of a seizure. Within a box, the solid bar represents the median value, the upper boundary of the closed box represents the 75th percentile, and the lower boundary represents the 25th percentile. The vertical lines extend to the 10th and 90th percentiles, with outliers plotted as circles.
Figure 2.
A, Box plot of MDI scores for patients with HLHS or variants (n = 36), stratified by the occurrence of a seizure. B, Box plot of PDI scores for patients with HLHS or variants (n = 36), stratified by the occurrence of a seizure.
Figure 3.
A, Box plot of MDI scores for patients with seizures (n = 15), stratified by frontal or nonfrontal onset of the seizure. B, Box plot of PDI scores for patients with seizures (n = 15), stratified by frontal or nonfrontal onset of the seizure.
Discussion
The occurrence of a seizure in the postoperative period after a neonatal infant heart operation is a marker of CNS injury.1–4 Recent studies at our institution and others have shown that the incidence of seizures in the early postoperative period, as documented by continuous video-EEG monitoring, is less than reported in previous eras.1,5,7 Previous studies demonstrated that the occurrence of a postoperative seizure was a predictor of subsequent abnormal neurodevelopmental development.2,3,9 In this study, evaluation of children with complex CHDs at 1 year of age after neonatal and infant heart operations suggested that the adverse effect of a postoperative seizure may be less than previously reported. For the entire cohort, there was approximately a 2-point mean decrease in the MDI and a 5-point mean decrease in the PDI, and these decreases were not statistically significant. However, the occurrence of a seizure was associated with a significantly increased incidence of abnormal or suspect neuromuscular examination results.
In the Boston Circulatory Arrest Study, postoperative EEG seizures occurred in 20% of the patients. Evaluation of the cohort at 1 year of age showed that the occurrence of an EEG seizure predicted a lower PDI score (with a mean decrease of 13 points).2,9 Although the MDI scores of children with EEG seizures were lower than those of children without seizures, the differences were not statistically significant. A longer total duration of seizures and seizures with a frontal onset were associated with a trend toward a worse PDI score.2,9 Subsequent evaluation of this cohort at 4 years of age demonstrated that the occurrence of an EEG seizure increased the risk of a lower IQ score and increased the risk of abnormalities on neurologic examination.3 It is interesting to note that the occurrence of a postoperative seizure was not associated with worse performance on tests of visual and spatial skills.10
There are important differences between this study and the Boston Circulatory Arrest Study. The Boston Circulatory Arrest Study evaluated only patients with TGA with or without a ventricular VSD. The current study enrolled children with a variety of complex CHDs, including functional single ventricle and HLHS. Children in the Boston Circulatory Arrest Study underwent operation between 1988 and 1992, whereas the children in this study underwent operation between 2001 and 2003. In the interval between the studies, there have been significant changes in prenatal diagnosis; preoperative management; intraoperative management, including the management of bypass and deep hypothermic circulatory arrest; and postoperative care. The Boston Circulatory Arrest Study was a prospective randomized trial that evaluated techniques of CPB and neurodevelopmental outcomes. The current study was subgroup of a prospective observational study that evaluated the effects of APOE polymorphisms on neurodevelopmental outcome. In this study, bypass strategies were at the discretion of the surgeon. It is important to note that investigators in the Boston Circulatory Arrest Study were blinded to the outcomes of the video-EEG and that the children with EEG seizures were not treated with antiepileptic medications (personal communication, Gil Wernovsky, Children’s Hospital of Philadelphia, 2005 and Jane Newburger, Children’s Hospital Boston, 2005). In the current study, because of the previous finding that the occurrence of a seizure was a risk factor for worse outcome, the physicians caring for the child were notified if video-EEG seizures were identified, and treatment was instituted at their discretion. All of the children in this study in whom a seizure was identified received antiepileptic medications, and this may have decreased the number of seizures and the duration of seizure activity.
The magnitude of the adverse effect of a postoperative seizure in this study was less than that described in the Boston Circulatory Arrest Study. However, as in that study, frontal seizures were associated with a worse outcome compared with nonfrontal seizures. Several factors may have lessened the effect of seizures. Improvements in preoperative and postoperative care may ameliorate the adverse effects of a seizure on the CNS. The patient population in this study was heterogeneous and included patients with complex forms of single ventricle. Many patients required more than 1 operation before 1 year of age. Factors other than seizures may impair neurodevelopmental outcomes in these patients and confound determination of the effects of a seizure. Finally, patients in this study were treated with antiepileptic medications, which may terminate the seizures and lessen secondary CNS injury.
The long-term neurodevelopmental sequelae of postoperative seizures are likely secondary in large part to the underlying CNS injury provoking them. However, recent research in pertinent newborn animal models demonstrates that recurrent seizures induced by proconvulsant drugs that do not otherwise injure the brain may have long-term undesirable effects on brain structure, learning, and susceptibility to spontaneous seizures.11–15 Similarly, in other animal models, neonatal seizures create long-term changes in the profile of γ-aminobutyric acid A subunit composition, the brain’s principal inhibitory neurotransmitter receptor site.16,17 In addition, phenobarbital administration may be neuroprotective, apart from its antiseizure effects.18 Although the major force behind the ultimate neurodevelopmental abnormalities after postoperative seizures may largely be their underlying cause, the magnitude of the contribution of the seizures themselves to the final extent of disability is not known in human neonates.
There are several limitations to this study. Although this cohort was one of the largest to undergo postoperative EEG monitoring and subsequent neurodevelopmental evaluation, the statistical power to discern differences in outcome was limited because of the small number of patients in whom seizures occurred. One-year evaluation was performed in only 70% of the cohort; however, all but 1 of the surviving seizure patients returned. The Bayley scales provide an excellent assessment of status at 1 year, but their predictive value for neurodevelopmental status at an older age is limited. Children who are at risk for a poor long-term outcome may not be identified on testing at 1 year of age.19 More detailed testing of this cohort at an older age may demonstrate differences between the groups. Finally, this was not a treatment trial. Patients were treated with antiepileptic medication after identification of a seizure, but treatment was not standardized, and there was no control group. Therefore, it is not possible to determine conclusively whether treatment affected the neurodevelopmental outcome.
The occurrence of a postoperative seizure is a marker of CNS injury. However, the magnitude of the adverse effect of a seizure on subsequent neurodevelopmental outcome may be less than previously reported.2,9 In this study, postoperative seizures were not predictive of a worse outcome at 1 year of age as assessed by the Bayley Scales of Infant Development. However, postoperative seizures were associated with an increase in abnormal findings on the neuromuscular examination. Among the patients with seizures, frontal onset of a seizure was associated with a worse outcome. Treatment with antiepileptic medication may improve outcome; however, further studies are necessary. Evaluation at an older age is needed, and the cohort is currently undergoing a detailed developmental assessment at 4 years of age.
Acknowledgments
Supported by a grant from the Fannie E. Rippel Foundation, an American Heart Association National Grant-in-Aid (9950480N), and grant HL071834 from the National Institutes of Health.
Abbreviations and Acronyms
- APOE
apolipoprotein E
- CHD
congenital heart defect
- CNS
central nervous system
- CPB
cardiopulmonary bypass
- DHCA
deep hypothermia and circulatory arrest
- EEG
electroencephalogram
- HLHS
hypoplastic left heart syndrome
- MDI
Mental Developmental Index
- PDI
Psychomotor Development Index
- TGA
transposition of the great arteries
- VSD
ventricular septal defect
Footnotes
Supplemental material is available online.
Read at the Eighty-fifth Annual Meeting of The American Association for Thoracic Surgery, San Francisco, Calif, April 10–13, 2005.
References
- 1.Newburger JW, Jonas RA, Wernovsky G, Wypij D, Hickey PR, Karl CK, et al. Comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993;329:1057–64. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 2.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KCK, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332:549–5. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger DC, Wypij D, Kuban KC, Rappaport LA, Hickey PR, Wernovsky G, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100:526–32. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- 4.Clancy RR, McGaurn SA, Wernovsky G, Gaynor JW, Spray TL, Norwood WI, et al. Risk of seizures in survivors of newborn heart surgery using deep hypothermic circulatory arrest. Pediatrics. 2003;111:592–601. doi: 10.1542/peds.111.3.592. [DOI] [PubMed] [Google Scholar]
- 5.Gaynor JW, Nicolson SC, Jarvik GP, Wernovsky G, Montenegro LM, Burnham NB, et al. Increasing duration of deep hypothermic circulatory arrest is associated with an increased incidence of postoperative electroencephalographic seizures. J Thorac Cardiovasc Surg. 2005;130:1278–86. doi: 10.1016/j.jtcvs.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaynor JW, Gerdes M, Zachai EH, Bernbaum J, Wernovsky G, Clancy RR, et al. Apolipoprotein E genotype and neurodevelopmental sequelae of infant cardiac surgery. J Thorac Cardiovasc Surg. 2003;126:1736–45. doi: 10.1016/s0022-5223(03)01188-7. [DOI] [PubMed] [Google Scholar]
- 7.Clancy RR, Sharif U, Ichord R, Spray TL, Nicolson S, Tabbutt S, et al. Electrographic neonatal seizures after infant heart surgery. Epilepsia. 2005;46:84–90. doi: 10.1111/j.0013-9580.2005.22504.x. [DOI] [PubMed] [Google Scholar]
- 8.Clancy RR, McGaurn SA, Wernovsky G, Spray TL, Norwood WI, Jacobs ML, et al. Preoperative risk-of-death prediction model in heart surgery with deep hypothermic circulatory arrest in the neonate. J Thorac Cardiovasc Surg. 2000;119:347–57. doi: 10.1016/S0022-5223(00)70191-7. [DOI] [PubMed] [Google Scholar]
- 9.Rappaport LA, Wypij D, Bellinger DC, Helmers SL, Holmes GL, Barnes PD, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation. 1998;97:773–9. doi: 10.1161/01.cir.97.8.773. [DOI] [PubMed] [Google Scholar]
- 10.Bellinger DC, Bernstein JH, Kirkwood MW, Rappaport LA, New-burger J. Visual-spatial skills in children after open-heart surgery. J Dev Behav Pediatr. 2003;24:169–79. doi: 10.1097/00004703-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Schmid R, Tandon P, Stafstrom CE, Holmes GL. Effects of neonatal seizures on subsequent seizure-induced brain injury. Neurology. 1999;53:1754–61. doi: 10.1212/wnl.53.8.1754. [DOI] [PubMed] [Google Scholar]
- 12.McCabe BK, Silveira DC, Cilio MR, Cha BH, Liu X, Sogawa Y, et al. Reduced neurogenesis after neonatal seizures. J Neurosci. 2001;21:2094–103. doi: 10.1523/JNEUROSCI.21-06-02094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol. 1998;44:845–57. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 14.Wasterlain CG. Neonatal seizures and brain growth. Neuropadiatrie. 1978;9:213–28. doi: 10.1055/s-0028-1091482. [DOI] [PubMed] [Google Scholar]
- 15.Yager JY, Armstrong EA, Miyashita H, Wirrell EC. Prolonged neonatal seizures exacerbate hypoxic-ischemic brain damage: correlation with cerebral energy metabolism and excitatory amino acid release. Dev Neurosci. 2002;24:367–81. doi: 10.1159/000069049. [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, Hsu FC, Raol YH, Coulter DA, Brooks-Kayal AR. Selective alterations of GABA A receptor subunit expression and function in hippocampal dentate granule cells after seizures in the developing brain. Epilepsia. 2001;42(suppl 7):224. [Google Scholar]
- 17.Zhang G, Raol YH, Brooks-Kayal AR. Selective alteration of excitatory and inhibitory receptors and transporters in hippocampal dentate granule cells after seizures in the developing brain. Epilepsia. 2002;43(suppl 7):27. [Google Scholar]
- 18.Hall RT, Hall FK, Daily DK. High-dose phenobarbital therapy in term newborn infants with severe perinatal asphyxia: a randomized, prospective study with three-year follow-up. J Pediatr. 1998;132:345–8. doi: 10.1016/s0022-3476(98)70458-5. [DOI] [PubMed] [Google Scholar]
- 19.McGrath E, Wypij D, Rappaport LA, Newburger JW, Bellinger DC. Prediction of IQ and achievement at age 8 years from neurodevelopmental status at age 1 year in children with D-transposition of the great arteries. Pediatrics. 2004;114:e572–6. doi: 10.1542/peds.2003-0983-L. [DOI] [PubMed] [Google Scholar]