Abstract
Activation of AMP-activated protein kinase (AMPK) by exercise induces several cellular processes in muscle. Exercise activation of AMPK is unaffected in lean (BMI ~25 kg/m2) subjects with type 2 diabetes. However, most type 2 diabetic subjects are obese (BMI >30 kg/m2), and exercise stimulation of AMPK is blunted in obese rodents. We examined whether obese type 2 diabetic subjects have impaired exercise stimulation of AMPK, at different signaling levels, spanning from the upstream kinase, LKB1, to the putative AMPK targets, AS160 and peroxisome proliferator–activated receptor coactivator (PGC)-1α, involved in glucose transport regulation and mitochondrial biogenesis, respectively. Twelve type 2 diabetic, eight obese, and eight lean subjects exercised on a cycle ergometer for 40 min. Muscle biopsies were done before, during, and after exercise. Subjects underwent this protocol on two occasions, at low (50% VO2max) and moderate (70% VO2max) intensities, with a 4–6 week interval. Exercise had no effect on LKB1 activity. Exercise had a time- and intensity-dependent effect to increase AMPK activity and AS160 phosphorylation. Obese and type 2 diabetic subjects had attenuated exercise-stimulated AMPK activity and AS160 phosphorylation. Type 2 diabetic subjects had reduced basal PGC-1 gene expression but normal exercise-induced increases in PGC-1 expression. Our findings suggest that obese type 2 diabetic subjects may need to exercise at higher intensity to stimulate the AMPK-AS160 axis to the same level as lean subjects.
Exercise is a fundamental aspect of type 2 diabetes prevention and treatment. Accumulating evidence indicates that the enzyme AMP-activated protein kinase (AMPK), which is stimulated upon increases in AMP/ATP, plays an important role in mediating several cellular and metabolic processes during exercise. For example, activation of AMPK is thought to mediate, at least partially, the increases in skeletal muscle fatty acid oxidation (1,2) and glucose transport (3–5) that occur during acute exercise. The stimulatory effect on fatty acid oxidation results from the phosphorylation and inhibition of acetyl CoA carboxylase (ACC) by AMPK (1,2). While the role of AMPK and ACC on exercise-induced fat oxidation is somewhat well defined, the signaling mechanism, downstream of AMPK, which regulates muscle glucose transport, is unclear. The Akt substrate AS160 is a novel Rab GTPase that is phosphorylated by Akt upon insulin stimulation (6). In adipocytes (6,7) and L6 muscle cells (8), AS160 plays a key role in insulin-stimulated GLUT4 exocytosis. It was recently reported that two AMPK-activating stimuli, muscle contraction and the AMP-mimetic compound 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR), increase AS160 phosphorylation in muscle (9–12). This suggests that AS160 is involved in the mechanism by which AMPK stimulates glucose transport.
In addition to its role on contraction-induced muscle glucose transport and fatty acid oxidation with a single bout of exercise, it has also been hypothesized that the repetitive increases in muscle AMPK activity that occur during physical training with each exercise bout lead to increased mitochondrial biogenesis and function (13,14). Increases in mitochondrial number/function with physical training are thought to occur, in part, through a mechanism that involves AMPK-mediated peroxisome proliferator–activated receptor coactivator-1 (PGC-1) gene expression (15). PGC-1 is a transcriptional coactivator that functions as a master coordinator of mitochondrial biogenesis through its interaction with transcription factors, such as nuclear respiratory factor (NRF)-1 (rev. in 16). Collectively, AMPK activation contributes to the beneficial effects of exercise on glucose and lipid metabolism by acutely increasing muscle glucose disposal and fatty acid oxidation and, chronically, by enhancing mitochondrial number and function.
Because AMPK is an important target for the treatment of insulin resistance and type 2 diabetes (17), a central issue in the AMPK field has been to determine whether subjects with type 2 diabetes have normal AMPK signaling. Studies done in subjects with type 2 diabetes and BMI ranging from 26 to 29 kg/m2 have not shown abnormalities in muscle AMPK protein content or activity (18,19). In many countries, however, the majority of subjects with type 2 diabetes are significantly obese and have a BMI ≥30 kg/m2. Moreover, several studies indicate that obese insulin-resistant rodents have abnormal AMPK signaling (14,20,21). Thus, our goal was to determine whether type 2 diabetic subjects with moderate-to-severe obesity (BMI > 30 kg/m2) have impaired AMPK signaling. For this purpose, we examined the effect of exercise on the AMPK pathway at different signaling levels. We first examined whether acute exercise stimulates the major upstream AMPK kinase in muscle, LKB1 (22). We then determined if obese type 2 diabetic subjects have impaired exercise stimulation of AMPK, its substrate ACC, and the putative AMPK target, AS160. Finally, we evaluated the effects of exercise on PGC-1 and NRF-1 gene expression in obese subjects with type 2 diabetes. Based on previous investigations (23–25), we hypothesized that exercise would have a time- and intensity-dependent effect to stimulate AMPK signaling in lean, obese, and type 2 diabetic subjects. However, based on studies indicating attenuated AMPK activity in insulin-resistant rodents (14,20,21), we also hypothesized that obese type 2 diabetic subjects would have reduced stimulation of the AMPK system by exercise.
RESEARCH DESIGN AND METHODS
We studied 12 obese subjects with type 2 diabetes, 8 obese nondiabetic subjects, and 8 lean nondiabetic subjects. All subjects were sedentary (zero or one exercise bout per week) and had poor fitness levels. Each subject underwent a medical history, physical examination, screening laboratory tests, and a 75-g oral glucose tolerance test (OGTT). Three type 2 diabetic subjects were taking glipizide, which was withdrawn 3 days before the OGTT and 1 day before the exercise studies. Nine type 2 diabetic subjects were treated with diet. Lean and obese control subjects did not have a family history of type 2 diabetes and were normal glucose tolerant. Other than glipizide, no subject was taking any medication known to affect glucose metabolism. The study was approved by the institutional review board of the University of Texas Health Science Center at San Antonio, and all subjects gave written consent.
Insulin sensitivity index
Using the plasma glucose and insulin concentrations obtained during the OGTT, insulin sensitivity was calculated using the Matsuda index, which strongly (r = 0.73, P < 0.0001) correlates with whole-body glucose disposal measured with the insulin clamp (26).
Exercise testing
VO2max was determined using a cycle ergometer and a Metabolic Measurement System (Sensormedics, Savi Park, CA). Subjects warmed-up and performed exercise in a ramped fashion increasing at a rate of 8–10 W/min to exhaustion and until at least two of the following criteria for a valid test were obtained: a leveling of VO2, respiratory exchange ratio >1.1, and a maximal heart rate within 15 beats of age-predicted maximal heart rate.
Exercise protocols and muscle biopsies
All the subjects underwent two separate acute exercise protocols on different days with a 4–6 week interval, 1 day at low (50% VO2max) and 1 at moderate (70% VO2max) intensity. Subjects were first randomly assigned to exercise at either low or moderate intensity. Within 7–10 days after the baseline VO2max measurement, subjects returned to the Clinical Research Center at 8 a.m. after an overnight fast to undergo the first exercise protocol. Subjects refrained from any exercise, other than habitual walking, for 48 h before the exercise experiments. Subjects rested for 30 min in the supine position, followed by a basal vastus lateralis muscle biopsy obtained under sterile conditions. The muscle was frozen in liquid nitrogen within 3 s after the biopsy. Subjects then exercised on a cycle ergometer for a total of 40 min as follows: after 6 min, exercise was stopped, the subjects briefly (~30 s) rested on a bed while local anesthesia with 1% lidocaine was given, and exercise was continued for an additional 4 min. After a total of 10 min of exercise, subjects were placed on the bed and a second muscle biopsy was obtained (10-min biopsy). Muscle tissue was frozen 60–90 s after exercise cessation (local regulations did not allow us to perform the biopsy on the bicycle, a common practice to preserve postexercise energy state). Exercise proceeded for 26 min, anesthesia was rapidly given, and subjects exercised for an additional 4 min, followed by a third muscle biopsy (40-min biopsy). After the cessation of exercise, the subjects rested in a bed for 150 min, followed by a final muscle biopsy. Each biopsy site was separated by at least 5 cm. Four to six weeks after the first exercise protocol, the subjects returned to the Clinical Research Center to undergo the same experiment but at different exercise intensity. If they first exercised at low intensity, on this occasion subjects would exercise at a moderate intensity and vice versa.
Laboratory analyses
Plasma insulin (Diagnostic Products, Los Angeles, CA) and adiponectin (Linco Research, St. Charles, MO) were measured by radioimmunoassay, glucose by the oxidase method and using a Beckman analyzer, and A1C using a DCA2000 analyzer (Bayer, Tarrytown, NY). Free fatty acid (FFA) concentrations were determined using a colorimetric method (Wako, Richmond, VA). Plasma leptin and interleukin (IL)-6 concentrations were measured using enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN).
Glycogen and nucleotides
To measure glycogen, samples weighing 5–10 mg were hydrolyzed in 20 μl of 2N HCl at 100°C for 2 h followed by neutralization with 20 μl 2N NaOH, and glycogen content was measured using a Beckman analyzer (oxidase method). During preliminary experiments, we validated the use of this method comparing it with measurements obtained using a hexokinase reagent from Sigma (r = 0.97, P < 0.0001). For measurements of basal ATP and free AMP, muscle samples were homogenized in 30% perchloric acid and neutralized with 2 mol/l KHCO3. A 20 μl sample was analyzed by high-pressure liquid chromatography using an LC-18 column, and measurements of ATP, creatine, and creatine phosphate were obtained as described (27). Muscle lactate concentrations were measured using a commercial kit (Biovision, Mountain View, CA) and used to calculate pH (28). Free AMP was calculated using the ATP, creatine, creatine phosphate, and pH measurements based on the creatine kinase and adenylate kinase reactions (29).
AMPK activity
AMPKα1, AMPKα2, and total AMPK activities were measured after immuonoprecipitating 200 μg protein (18) using antibodies against AMPKα1 (Upstate, Lake Placid, NY), AMPKα2 (18), or AMPKα1/2 (30).
Western blotting
Immunoblotting was performed as described (5) using antibodies against phospho–AMPK-Thr172, phospho–ACC-Ser79, phospho-Akt substrate (PAS), phospho–Akt-Ser473, phospho–Akt-Thr308, and AMPKα1/2 from Cell Signaling (Beverly, MA); AMPKβ1/β2 and AS160 from Upstate; Akt from Santa Cruz Biotechnology (Santa Cruz, CA); AMPKα1 (18); AMPKα2 (18); AMPKγ3 (14); LKB1 (31); and MO25α (22). ACC was detected using streptavidin (Pierce, Rockford, IL). For comparisons of basal protein content and phosphorylation between groups, an equal number of samples from each group were distributed in two gels, and a control sample was used to normalize the data. To assess the effect of exercise, all the samples from each subject were loaded in one gel, and the same internal control was present in all gels to normalize the data.
LKB1 activity
LKB1 activity was measured after immunoprecipitating 500 μg protein with 5 μg LKB1 antibody and using the LKBtide peptide, as previously described (32).
Quantitative RT-PCR
Tissue was homogenized in RNAStat solution (Tel-Test, Friendswood, TX) and total RNA purified with RNeasy and DNase I (Qiagen, Chatsworth, CA). An Agilent Bioanalyzer was used to confirm that all samples had a A260/A280 range of 1.8–2.1. PGC-1 and NRF-1 gene expression were determined using one-step quantitative RT-PCR on an ABI-Prism-7900HT System (Applied Biosystems, Foster City, CA) using TaqMan RT-PCR Master-Mix Reagents and Assay-on-Demand primer pairs/probes (Hs00173304_ml for PGC-1 and Hs00602161_ml for NRF-1), as previously described (33). The quantity of the mRNA for each gene of interest was normalized to that of 18S RNA using the 2−ΔΔCT method (34).
Statistical analysis
Data are expressed as means ± SE. Comparison of baseline data between groups was done using one-way ANOVA. The interaction of exercise and the different groups were analyzed using two-way repeated-measures ANOVA, followed by Fisher’s analysis.
RESULTS
There were no significant differences in age, VO2max, and workload between the type 2 diabetic, obese, and lean subjects (Table 1). The type 2 diabetic and obese groups were matched for BMI, and these subjects had higher BMI than lean subjects. Compared with the lean group, obese and type 2 diabetic subjects had lower adiponectin and higher leptin concentrations in plasma. Subjects with type 2 diabetes had higher fasting plasma glucose, A1C, FFA, and IL-6 concentrations than obese and lean subjects, Type 2 diabetic and obese subjects were more insulin resistant than lean subjects based on the lower Matsuda index.
TABLE 1.
Clinical and laboratory characteristics
| Lean | Obese | Type 2diabetes | |
|---|---|---|---|
| n | 8 | 8 | 12 |
| Sex (male/female) | 5/3 | 3/5 | 4/8 |
| Age (years) | 45 ± 3 | 44 ± 4 | 53 ± 3 |
| BMI (kg/m2) | 25.5 ± 1.0 | 30.5 ± 1.0* | 31.5 ± 1.2* |
| Glucose (mmol/l) | 5.3 ± 0.2 | 5.6 ± 0.1 | 7.7 ± 0.7*† |
| A1C (%) | 5.0 ± 0.1 | 4.9 ± 0.2 | 6.3 ± 0.3*† |
| Insulin (pmol/l) | 44 ± 3 | 126 ± 19* | 85 ± 13*† |
| FFA (μmol/l) | 430 ± 40 | 510 ± 50 | 700 ± 40*† |
| Insulin sensitivity index | 6.5 ± 0.9 | 3.1 ± 0.5* | 3.4 ± 0.8* |
| Adiponectin (μg/ml) | 22.4 ± 3.0 | 15.4 ± 2.7* | 14.8 ± 2.8* |
| Leptin (ng/ml) | 8.6 ± 2.4 | 19.6 ± 3.0* | 20.0 ± 3.2* |
| IL-6 (pg/ml) | 1.2 ± 0.2 | 1.9 ± 0.5 | 2.4 ± 0.4* |
| VO2max (ml · kg−1 · min−1) | 21.1 ± 2.3 | 20.8 ± 2.6 | 16.4 ± 0.4 |
| Work | |||
| Low intensity (W) | 54 ± 5 | 53 ± 4 | 46 ± 2 |
| Moderate intensity (W) | 79 ± 7 | 77 ± 6 | 67 ± 3 |
Data are mean ± SE.
P < 0.05 vs. lean;
P < 0.05 vs. obese.
Effect of exercise on plasma glucose and muscle glycogen concentrations
In type 2 diabetic subjects, low- and moderate-intensity exercise decreased plasma glucose concentrations by 0.6 ± 0.3 (P = 0.05) and 1.3 ± 0.3 mmol/l (P = 0.001), respectively. Exercise did not decrease glucose concentrations in the lean and obese nondiabetic groups. Basal muscle glycogen content was not different between groups (88 ± 4, 101 ± 6, and 88 ± 7 nmol/mg muscle in lean, obese, and type 2 diabetic subjects, respectively). Forty minutes of moderate- but not low-intensity exercise reduced glycogen content by 35, 53, and 56% in the lean, obese, and type 2 diabetic subjects, respectively (P < 0.05 pre- vs. postexercise in all groups, P = NS between groups), suggesting similar muscle fiber recruitment between groups.
Nucleotide content in muscle
There were no differences in basal ATP (4.5 ± 0.6, 3.8 ± 0.4, and 4.4 ± 1.3 nmol/mg muscle in lean, obese, and type 2 diabetes groups, respectively) and calculated free AMP (0.39 ± 0.15, 0.23 ± 0.07, and 0.28 ± 0.08 nmol/mg in lean, obese, and type 2 diabetes groups, respectively) content in muscle between groups. Baseline creatine phosphate, creatine, and lactate content were similar in all groups (data not shown). We did not observe increases in the free AMP/ATP ratio with exercise in any group. Because muscle energetics are restored within 60 s after exercise (35), our measurements, however, do not accurately reflect postexercise nucleotide levels because the tissue was frozen 60–90 s after exercise.
AMPK, ACC, Akt, and AS160 muscle content and phosphorylation
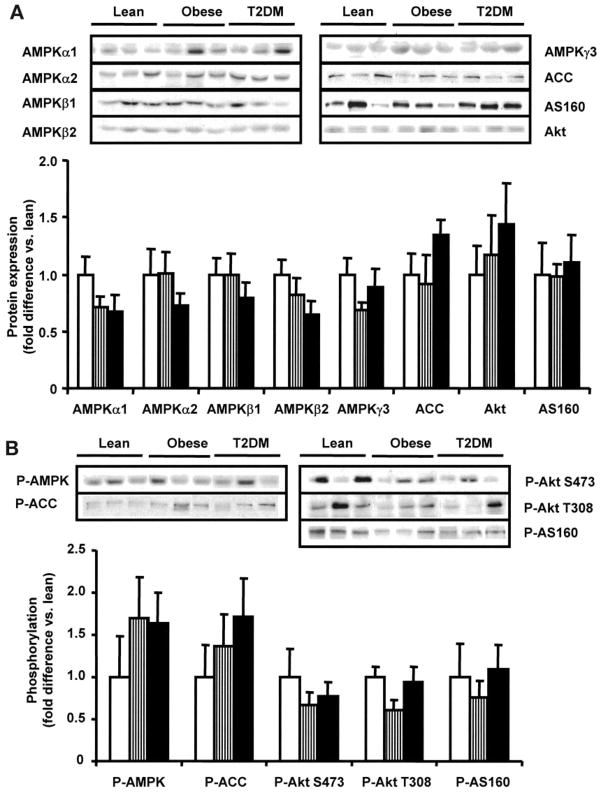
The muscle protein content of the AMPK subunits α1, α2, β1, β2, and γ3 and of ACC, Akt, and AS160 was similar between the lean, obese, and type 2 diabetes groups (Fig. 1A). There were no statistically significant differences in basal AMPK, ACC, Akt, or AS160 phosphorylation between groups (Fig. 1B).
FIG. 1.
Basal AMPK, ACC, AS160, and Akt. AMPK subunit, ACC, AS160, and Akt protein content (A) and phosphorylation (B) were measured in 8 lean (□), 8 obese (▥), and 12 type 2 diabetic (T2DM) (■) subjects. Data are means ± SE. Blots are shown for three subjects/group.
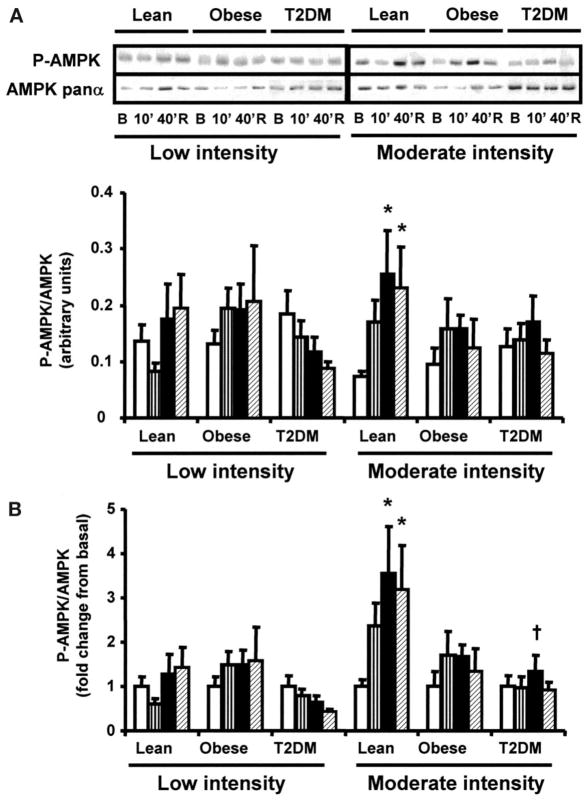
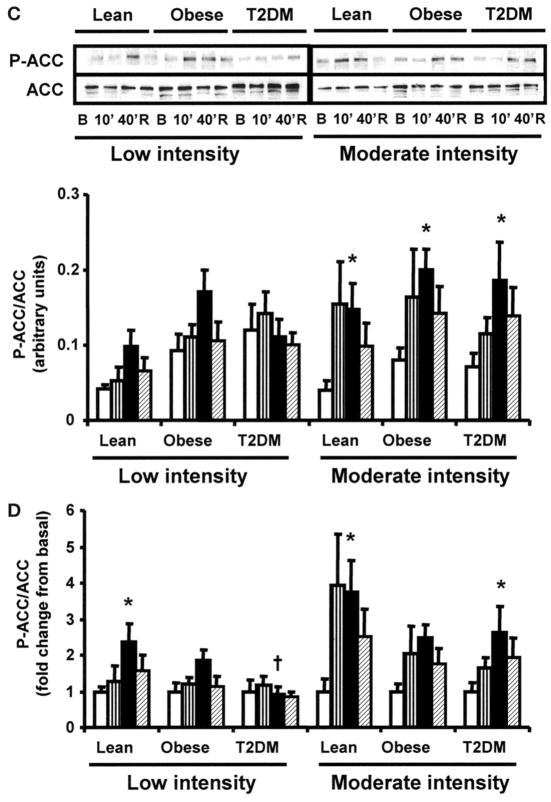
Time course and dose response of AMPK and ACC phosphorylation during exercise
AMPK and ACC phosphorylation were measured before exercise, at 10 and 40 min of exercise, and 150 min postexercise. Low-intensity exercise for 40 min did not increase AMPK phosphorylation in any group (Fig. 2A and B). Forty minutes of moderate-intensity exercise increased AMPK phosphorylation by 3.5-fold over basal (P < 0.05) in lean subjects (Fig. 2A and B), and this effect was sustained in the postexercise period. AMPK phosphorylation only tended to increase after 40 min of moderate-intensity exercise in the obese and type 2 diabetes groups, resulting in lower exercise-stimulated AMPK phosphorylation compared with the lean subjects (P < 0.05 lean vs. type 2 diabetes). Low-intensity exercise increased ACC phosphorylation in the lean subjects only (Fig. 2D). Moderate-intensity exercise for 40 min significantly increased ACC phosphorylation in the lean, obese, and type 2 diabetic subjects (Fig. 2C), although the fold increases in ACC phosphorylation tended to be smaller in the obese and type 2 diabetes groups (Fig. 2D). After the 150-min recovery period, ACC phosphorylation was no longer significantly elevated, although it still tended to be higher than baseline.
FIG. 2.
Effect of exercise on AMPK and ACC phosphorylation. Biopsies were done at basal (□), after 10 (▥) and 40 min (■) of exercise, and 150 min postexercise (▨). Data are means ± SE in 8 lean, 8 obese, and 12 type 2 diabetic (T2DM) subjects. Data are expressed as arbitrary units (A and C) and as fold change (B and D). *P < 0.05 vs. basal of respective group; †P < 0.05 vs. lean group at 40 min. Blots are shown for one subject/group. B, basal; R, rest postexercise.
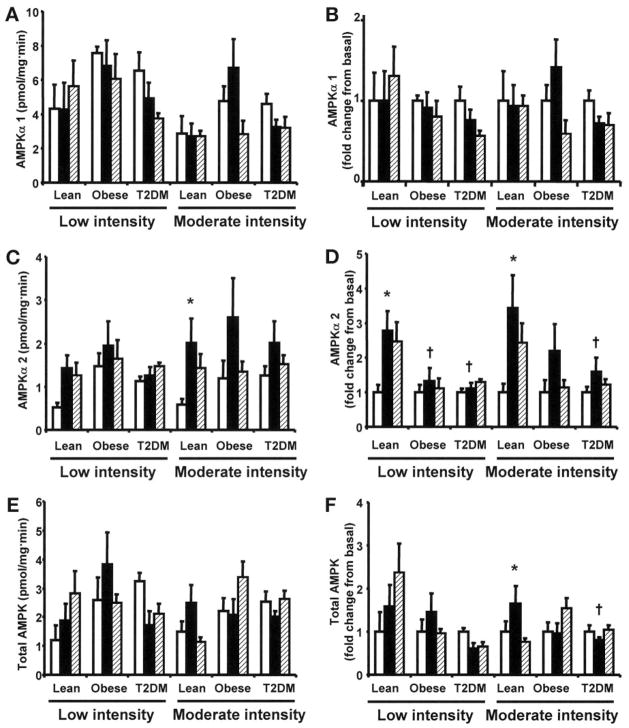
Effect of exercise on AMPK activity
To compare the effect of exercise on isoform-specific AMPK activity between groups, AMPKα1, AMPKα2, and total (pan α) activities were measured before exercise, at 40 min of exercise, and 150 min postexercise. Overall, there was a tendency for higher basal AMPKα1, AMPKα2, and total AMPK activities in the obese and type 2 diabetes groups compared with lean subjects (Fig. 3A, C, and E). Neither low-nor moderate-intensity exercise increased AMPKα1 activity (Fig. 3A and B). After 40 min of low-intensity exercise, AMPKα2 activity increased significantly in the lean group (P < 0.05) but not in the obese and type 2 diabetes groups (Fig. 3D). Consistent with the measurements of AMPK phosphorylation, 40 min of moderate-intensity exercise significantly increased AMPKα2 activity by 3.4-fold (P < 0.05) in the lean group (Fig. 3D). In contrast, moderate-intensity exercise did not significantly increase AMPKα2 activity in the obese and type 2 diabetes groups. Total AMPK activity did not increase with low-intensity exercise in any group (Fig. 3E and F). In line with the effects on AMPKα2 activity, moderate-intensity exercise increased total AMPK activity in the lean group by 1.7-fold (P < 0.05) but had no effect in the obese and type 2 diabetes groups. The lower activation of total AMPK versus AMPKα2 in the lean group likely reflects some “dilution” of the exercise effect because AMPKα1-containing complexes were not stimulated by exercise.
FIG. 3.
AMPK activity. AMPKα1, AMPKα2, and total AMPK activities were measured before (□), after 40 min of exercise (■), and 150 min postexercise (▨). Data are means ± SE. Data are expressed as kinase activity (A, C, and E) and as fold change (B, D, and F). n = 6–12 in each time point (samples were not available for all the assays). *P < 0.05 vs. basal of respective group; †P < 0.05 vs. lean group in respective time point. T2DM, type 2 diabetes.
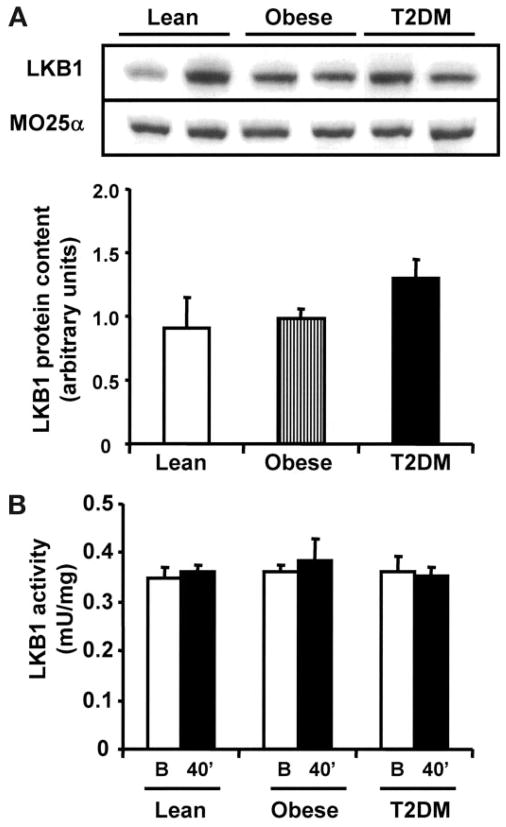
LKB1 protein content and effect of exercise on LKB1 activity
We measured baseline content of the AMPK kinase, LKB1, and its accessory subunit MO25α, and there were no differences between groups (Fig. 4A). Basal LKB1 activity was similar in lean, obese, and type 2 diabetic subjects (Fig. 4B). Consistent with findings in rodents (36), acute exercise did not alter muscle LKB1 activity in humans, supporting the notion that LKB1 may be a constitutively active enzyme.
FIG. 4.
LKB1 expression and activity. Equal amounts of protein (40 μg) were used for blotting of LKB1 and MO25α (A). Blots are shown for two subjects per group. LKB1 activity was measured as described in research design and methods (B). B, basal; T2DM, type 2 diabetes.
Effect of exercise on Akt and AS160 phosphorylation
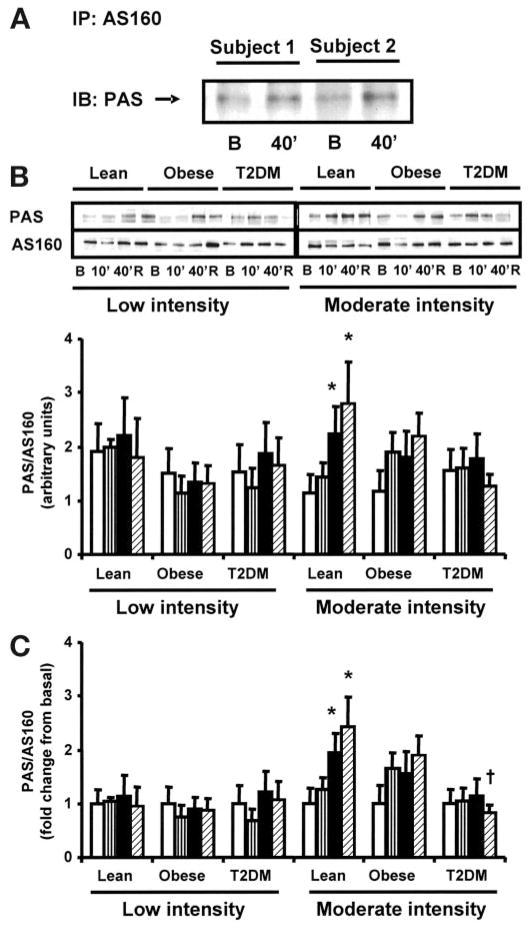
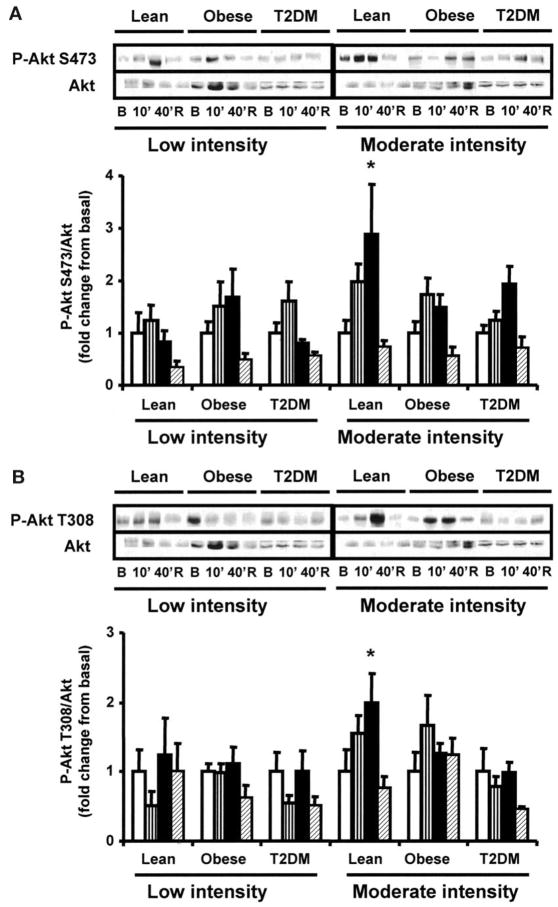
Recent studies have shown that Akt and AS160 can be activated in muscle by contractile activity (9–12,37,38). To determine the effect of exercise on AS160 and Akt in insulin-resistant muscle, we measured AS160 (using the PAS antibody) and Akt (Ser473 and Thr308) phosphorylation in muscle from control and type 2 diabetic subjects before exercise, at 10 and 40 min of exercise, and 150 min postexercise. During preliminary experiments, we confirmed that the 160-kDa band was AS160 by immunoprecipitating basal and exercise-stimulated samples with AS160 and detected them using PAS antibody (Fig. 5A). Low-intensity exercise did not significantly increase AS160 phosphorylation in any group (Fig. 5B and C). In the lean group, moderate-intensity exercise caused a time-dependent increase in AS160 phosphorylation, which was maximal at 150 min postexercise, but this effect was blunted in obese and type 2 diabetic subjects (Fig. 5B and C), resulting in lower exercise-induced AS160 phosphorylation in type 2 diabetic versus lean subjects (P < 0.05). Low-intensity exercise had no effect on Akt-Ser473 and Thr308 phosphorylation in lean, obese, and type 2 diabetic subjects (Fig. 6A and B). In the lean group, Akt Ser473 and Thr308 phosphorylation tended to increase within 10 min of moderate-intensity exercise; by 40 min, Akt-Ser473 and Thr308 phosphorylation were significantly elevated (P < 0.05), whereas moderate exercise did not significantly increase Akt-Ser473 or Thr308 phosphorylation in the obese and type 2 diabetic subjects (Fig. 6A and B). After 150 min of rest, Akt-Ser473 and Thr308 phosphorylarion had fully returned to basal levels in the lean group.
FIG. 5.
AS160 phosphorylation. Immunoblots are shown for two subjects in the basal state and after 40 min of exercise, after immunoprecipitation with AS160 and probing with PAS antibody (A). Biopsies were done at basal (□), after 10 (▥) and 40 min (■) of exercise, and 150 min postexercise (▨). Data are means ± SE in 8 lean, 8 obese, and 12 type 2 diabetic (T2DM) subjects. Data are expressed as arbitrary units (B) and as fold change (C). *P < 0.05 vs. basal of respective group; †P < 0.05 vs. lean at 150 min postexercise. Blots are shown for one subject/group. B, basal; R, rest postexercise.
FIG. 6.
Akt phosphorylation. Biopsies were done at basal (□), after 10 (▥) and 40 min (■) of exercise, and 150 min postexercise (▨). Akt-Ser473 (A) and Thr308 (B) were measured as described in research design and methods. Data are means ± SE in 8 lean, 8 obese, and 12 type 2 diabetic (T2DM) subjects. *P < 0.05 vs. basal of respective group. Blots are shown for one subject/group. B, basal; R, rest postexercise.
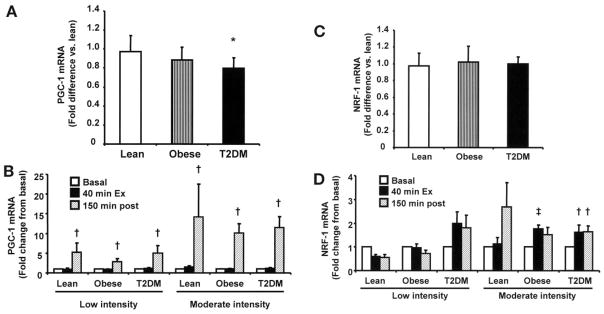
Effect of acute exercise on PGC-1 and NRF-1 gene expression
As shown in Fig. 7A, basal PGC-1 expression was lower in the type 2 diabetes group. Both low- and moderate-intensity exercise acutely stimulated PGC-1 gene expression, which peaked after the 150-min rest period, and this effect was similar in the three groups (Fig. 7B). There were no significant differences in basal NRF-1 expression between groups (Fig. 7C). Moderate exercise increased NRF-1 expression in the three groups (Fig. 7D), although statistical significance was not achieved in the lean group (P = 0.1), probably due to the lesser number of subjects studied, compared with the type 2 diabetes group.
FIG. 7.
PGC-1 and NRF-1 gene expression. Basal PGC-1α (A) and NRF-1 (C) gene expression. Effect of exercise on PGC-1 (B) and NRF-1 (D) gene expression. Gene expression was measured at baseline, after 40 min of exercise, and 150 min postexercise. Data are means ± SE in 8 lean, 8 obese, and 12 type 2 diabetic (T2DM) subjects. *P < 0.05 vs. type 2 diabetes group; †P < 0.05 vs. basal of respective group; ‡P = 0.05 vs. basal in obese group.
DISCUSSION
The main finding of this study is that obese and type 2 diabetic subjects had attenuated exercise-induced increases in AMPKα2 activity, total AMPK activity, and AMPK phosphorylation (Figs. 2 and 3). Obese and type 2 diabetic subjects also had attenuated increases in the phosphorylation of the AMPK substrate ACC (Fig. 2D) and the putative AMPK target AS160 (Fig. 5C). What is the cause of the reduced response in the obese and type 2 diabetic subjects? Some evidence suggests that AMPK activity is regulated by muscle glycogen content by interacting with the β subunit of AMPK (39). Elevations in glycogen content are associated with low AMPK activity at rest and during exercise, whereas low glycogen content is associated with elevated AMPK activity (40–43). Yet, in the present study, basal glycogen content was not different between groups, and exercise caused similar degrees of glycogen consumption. Glucose is another nutrient that could have potentially affected the stimulation of AMPK in the type 2 diabetes group. Akerstrom et al. (44) reported that ingestion of a carbohydrate-containing drink, which caused a slight increase in plasma glucose levels, attenuated exercise-induced AMPK activation; yet, this finding was not confirmed by other investigators (45). We previously reported that lean type 2 diabetic subjects with elevated fasting plasma glucose concentrations achieve normal AMPK stimulation with exercise (18). Moreover, in the current study, both the obese and type 2 diabetes groups displayed lower exercise responses; thus, it is unlikely that hyperglycemia is the sole cause for the reduced stimulation of AMPK. Obese and type 2 diabetic subjects had higher fasting insulin levels compared with the lean group, reflecting their insulin-resistant state, and in liver cells, insulin inhibits AMPK (46). However, we previously did not observe reduced exercise-stimulated AMPK activity in lean type 2 diabetic subjects with high insulin levels (18), arguing against a suppressive effect of physiologic insulin levels on AMPK in vivo. Thus, there does not appear to be a single explanation for the suppressed stimulation of AMPK observed in the obese and type 2 diabetes groups. Even though there were no statistical differences in VO2max between the three groups (Table 1), some type 2 diabetic subjects with the lowest VO2max within their group exercised at lower workloads than most lean subjects and could have contributed to the attenuation in AMPK activity after exercise (47). Yet, the lower stimulation of AMPK was also observed in the obese group, which clearly had similar VO2max compared with the lean subjects. Thus, it is unlikely that differences in workload are the only reason for the blunted response to exercise.
Roepstorff et al. (48) recently reported lower exercise activation of AMPK in female subjects compared with male subjects. In the current study, there were more female subjects in the obese and type 2 diabetes groups (Table 1). Subgroup analysis suggested that female subjects indeed had lower AMPK activation, although sex differences could not be conclusively determined due to the low number of subjects upon subdividing the groups. Yet, the attenuated exercise effect on AMPK was still observed within both the obese and type 2 diabetes groups, regardless of sex (data not shown). In the study by Roepstorff et al. (48), the reduced AMPK stimulation observed in the female subjects was associated with smaller increases in free AMP/ATP. Presumably, a higher proportion of type 1 muscle fibers and more capillarization in female subjects (which would help preserve energy balance) could have been responsible for these sex differences (48). In the current study, we did not examine muscle fiber distribution or muscle capillarization because muscle tissue was no longer available for analysis.
An intriguing observation from the current study is the apparent elevation in basal AMPK signaling in the obese and type 2 diabetic subjects, evidenced by a trend for higher AMPK activity (α2, α1, and total) (Fig. 3). While statistical differences were not observed upon analysis using two-way ANOVA, the obese and type 2 diabetes groups did appear to have higher basal AMPKα1 and α2 activities if compared with the lean group using Student’s t test (P < 0.05 lean vs. obese and P < 0.05 lean vs. type 2 diabetes). These findings are in line with the study from Christopher et al. (49) who found that alloxan-induced diabetes in dogs caused an increase in basal AMPKα1 and α2 activities and attenuated exercise-stimulated AMPK activity. Certainly, our current findings argue against a suppressive effect of FFA, insulin, or glucose on basal AMPK activity. Differences in basal glycogen or nucleotide content were not observed between groups. Nonetheless, we cannot entirely rule out subtle differences in muscle energetics between groups in view that investigations done in the heart using 31P magnetic resonance spectroscopy indicate that even minimal changes in free AMP, which are likely not detectable through biochemical tissue analysis using high-pressure liquid chromatography, can affect AMPK activity (29). Adiponectin stimulates muscle AMPK (50,51); yet, as expected, obese and type 2 diabetic subjects had lower adiponectin plasma concentrations (Table 1). The obese subjects did, however, have higher plasma leptin concentrations, and in type 2 diabetic subjects, both leptin and IL-6 were higher than the lean group (Table 1). Leptin (52) and IL-6 (53,54) stimulate the AMPK axis in muscle from rodents and humans and could have contributed to the apparent elevation in basal AMPK activity in the obese and type 2 diabetes groups.
Moderate-intensity exercise decreased mean plasma glucose levels in the type 2 diabetic subjects by 1.3 mmol/l. It is generally accepted that reductions in glycemia with acute exercise result from increased muscle glucose disposal (rev. in 55). The minimal stimulation of AMPK during moderate-intensity exercise in the type 2 diabetes group (Fig. 3) indicates that significant activation of this enzyme is not essential for acute exercise to reduce plasma glucose levels and, possibly, to enhance muscle glucose disposal. Consistent with this notion, other groups found dissociation between glucose disposal and AMPK activity during low-intensity exercise (42,56). Even though we cannot rule out small transient AMPK stimulation (note the increases in ACC phosphorylation), this finding is consistent with the hypothesis that AMPK activation contributes to contraction-stimulated glucose transport, but other redundant pathways function in parallel to increase glucose transport (4,57,58).
Until recently, the pathway downstream of AMPK, which mediates glucose transport, has been largely unknown. AS160 is a novel Rab GTPase-activating protein that contains six putative Akt target sites, and insulin stimulation of adipocytes increases phosphorylation of five of these sites (6). AS160 plays a key role in insulin-mediated GLUT4 translocation both in adipocytes (7,59) and in L6 myoblasts (8). In muscle, not only insulin but also contraction (11,12) and AICAR (12) increase AS160 phosphorylation. Moreover, in a cell-free assay, heterotrimeric AMPK complexes phosphorylate AS160 (10), although the specific sites phosphorylated by AMPK are yet to be defined. These exciting findings suggest that AMPK-induced increases in muscle glucose transport may be mediated through AS160 and that this protein may be a point of convergence linking insulin- and contraction-stimulated glucose transport. In the present study, exercise increased AS160 phosphorylation in an intensity-dependent manner, and the highest degree of phosphorylation was observed at the later time points. However, it is not possible from these data to determine whether Akt or AMPK had a predominant effect to phosphorylate AS160 because exercise started to stimulate both kinases within 10 min. Furthermore, both exercise-induced phosphorylation of AMPK and Akt were blunted in the obese and type 2 diabetic subjects; thus, impaired AMPK- and/or Akt-induced phosphorylation of AS160 could have occurred. Recent studies in transgenic and knockout mice indicate that insulin-stimulated AS160 phosphorylation is mediated by Akt, while AICAR exerts its effect on AS160 through AMPK (9,10). Interestingly, ablation of AMPK, but not of Akt, activity causes a partial reduction in contraction-stimulated AS160 phosphorylation, suggesting a more predominant role for AMPK. Based on these important animal experiments, we speculate that impaired AMPK activation is responsible, at least in part, for the attenuation in AS160 stimulation observed in the obese and type 2 diabetes groups. As specific AS160 antibodies for all the different phosphorylation sites become available, the basis for the attenuated AS160 phosphorylation observed with the PAS antibody will be better understood. In a previous study, Kennedy et al. (60) found that acute exercise lead to a similar increase in plasma membrane GLUT4 content in muscle from moderately obese (BMI 27 kg/m2) subjects with type 2 diabetes versus lean control subjects (relative to baseline GLUT4 plasma membrane content), although there was a trend for lower absolute postexercise plasma membrane GLUT4 content. In view of our finding of attenuated exercise-induced AS160 phosphorylation in more obese (BMI ~30 kg/m2) subjects with type 2 diabetes, it will be important to examine whether these subjects have similar exercise-stimulated GLUT4 translocation as lean control subjects and how this relates to muscle glucose disposal and AS160 phosphorylation.
The recent generation of muscle-specific LKB1 knockout mice provided genetic evidence that LKB1 is the major upstream kinase of AMPK in response to contraction (22). We previously found that insulin-resistant Zucker rats have lower LKB1 muscle protein content compared with lean littermates (14), associated with abnormal AMPK–PGC-1 regulation. In contrast, we did not observe differences in LKB1 content and activity in the basal and exercise states among lean, obese, and type 2 diabetic subjects. This discrepancy could be related to the differences in experimental models (i.e., species). It was reported that acute exercise leads to increases in total AMPK kinase activity (61). Thus, the dissociation between LKB1 and AMP kinase activity could also be explained by the activation of one or more AMPKKs, other than LKB1, in response to exercise.
Subjects with type 2 diabetes had reduced PGC-1 gene expression. Importantly, acute exercise increased PGC-1 gene expression, and this effect was similar in the type 2 diabetes, obese, and lean groups, underscoring the importance of exercise to reverse cellular defects. Of note, PGC-1 expression increased normally in the type 2 diabetic subjects during low-intensity exercise (Fig. 7B), even in the absence of significant AMPK stimulation. Jorgensen et al. (62) previously showed that exercise-induced PGC-1 gene expression in mouse muscle is not altered by ablation of either AMPKα1 or α2. Thus, AMPK likely contributes to exercise-induced increases in PGC-1 expression; nonetheless, in some situations, activation of this kinase may not be necessary to stimulate PGC-1 expression. Importantly, exercise appears to have a dual effect on NRF-1 because it not only increased the gene expression of PGC-1, which stimulates mitochondrial function and biogenesis, in part, by binding to and coactivating the transcription function of NRF-1 (16), but exercise also increased the gene expression of this transcription factor.
In summary, we found that exercise increases AMPK activity and the phosphorylation of ACC and AS160 in an intensity- and time-dependent manner. Obese diabetic and nondiabetic subjects have attenuated stimulation of the AMPK-AS160 axis; thus, they may need to exercise at a higher intensity to stimulate this pathway to the same level as lean subjects. Finally, PGC-1 and NRF-1 gene expression increases normally with exercise in type 2 diabetes, and this effect does not appear to require significant AMPK stimulation. This finding highlights the relevance of exercise to ameliorate molecular defects in type 2 diabetes.
Acknowledgments
This study was supported by grants from the American Diabetes Association (N.M., E.C., and R.A.D.), the UTHSCSA Executive Research Committee (N.M.), the South Texas Health Research Center (N.M.), the U.S Department of Veterans Affairs (R.A.D.), the Siriraj Hospital Mahidol University of Thailand and the Endocrine Fellow Foundation (A.S.), and the National Institutes of Health (DK24092 to R.A.D. and DK067690 to C.P.J). K.S. received research support from Diabetes U.K., the British Medical Research Council, Astra-Zeneca, Boehringer-Ingelheim, GlaxoSmith-Kline, Merck and Company, Merck Germany, and Pfizer. We thank Laurie Goodyear for providing AMPKα2, and γ3 antibodies, and all the volunteers who participated in the study.
- ACC
acetyl CoA carboxylase
- AICAR
5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside
- AMPK
AMP-activated protein kinase
- FFA
free fatty acid
- IL
interleukin
- NRF
nuclear respiratory factor
- OGTT
oral glucose tolerance test
- PAS
phospho-Akt substrate
- PGC
peroxisome proliferator–activated receptor coactivator
References
- 1.Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- 2.Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, Witters LA, Ruderman NB. Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J Biol Chem. 1997;272:13255–13261. doi: 10.1074/jbc.272.20.13255. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 4.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 5.Musi N, Hayashi T, Fujii N, Hirshman MF, Witters LA, Goodyear LJ. AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:E677–E684. doi: 10.1152/ajpendo.2001.280.5.E677. [DOI] [PubMed] [Google Scholar]
- 6.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates: Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277:22115–22118. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- 7.Zeigerer A, McBrayer MK, McGraw TE. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol Biol Cell. 2004;15:4406–4415. doi: 10.1091/mbc.E04-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thong FS, Dugani CB, Klip A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology (Bethesda) 2005;20:271–284. doi: 10.1152/physiol.00017.2005. [DOI] [PubMed] [Google Scholar]
- 9.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067–2076. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- 10.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes. 2006;55:2051–2058. doi: 10.2337/db06-0175. [DOI] [PubMed] [Google Scholar]
- 11.Deshmukh A, Coffey VG, Zhong Z, Chibalin AV, Hawley JA, Zierath JR. Exercise-induced phosphorylation of the novel Akt substrates AS160 and filamin A in human skeletal muscle. Diabetes. 2006;55:1776–1782. doi: 10.2337/db05-1419. [DOI] [PubMed] [Google Scholar]
- 12.Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005;54:41–50. doi: 10.2337/diabetes.54.1.41. [DOI] [PubMed] [Google Scholar]
- 13.Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–E1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- 14.Sriwijitkamol A, Ivy JL, Christ-Roberts C, DeFronzo RA, Mandarino LJ, Musi N. LKB1-AMPK signaling in muscle from obese insulin-resistant Zucker rats and effects of training. Am J Physiol Endocrinol Metab. 2006;290:E925–E932. doi: 10.1152/ajpendo.00429.2005. [DOI] [PubMed] [Google Scholar]
- 15.Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun. 2002;296:350–354. doi: 10.1016/s0006-291x(02)00881-1. [DOI] [PubMed] [Google Scholar]
- 16.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 17.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 18.Musi N, Fujii N, Hirshman MF, Ekberg I, Froberg S, Ljungqvist O, Thorell A, Goodyear LJ. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes. 2001;50:921–927. doi: 10.2337/diabetes.50.5.921. [DOI] [PubMed] [Google Scholar]
- 19.Koistinen HA, Galuska D, Chibalin AV, Yang J, Zierath JR, Holman GD, Wallberg-Henriksson H. 5-amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes. 2003;52:1066–1072. doi: 10.2337/diabetes.52.5.1066. [DOI] [PubMed] [Google Scholar]
- 20.Barnes BR, Ryder JW, Steiler TL, Fryer LG, Carling D, Zierath JR. Isoform-specific regulation of 5′ AMP-activated protein kinase in skeletal muscle from obese Zucker (fa/fa) rats in response to contraction. Diabetes. 2002;51:2703–2708. doi: 10.2337/diabetes.51.9.2703. [DOI] [PubMed] [Google Scholar]
- 21.Bergeron R, Previs SF, Cline GW, Perret P, Russell RR, 3rd, Young LH, Shulman GI. Effect of 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 2001;50:1076–1082. doi: 10.2337/diabetes.50.5.1076. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- 25.Stephens TJ, Chen ZP, Canny BJ, Michell BJ, Kemp BE, McConell GK. Progressive increase in human skeletal muscle AMPKalpha2 activity and ACC phosphorylation during exercise. Am J Physiol Endocrinol Metab. 2002;282:E688–E694. doi: 10.1152/ajpendo.00101.2001. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 27.Sellevold OF, Jynge P, Aarstad K. High performance liquid chromatography: a rapid isocratic method for determination of creatine compounds and adenine nucleotides in myocardial tissue. J Mol Cell Cardiol. 1986;18:517–527. doi: 10.1016/s0022-2828(86)80917-8. [DOI] [PubMed] [Google Scholar]
- 28.Sahlin K, Harris RC, Nylind B, Hultman E. Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch. 1976;367:143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- 29.Frederich M, Balschi JA. The relationship between AMP-activated protein kinase activity and AMP concentration in the isolated perfused rat heart. J Biol Chem. 2002;277:1928–1932. doi: 10.1074/jbc.M107128200. [DOI] [PubMed] [Google Scholar]
- 30.Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose metabolism and energy homeostasis in mouse hearts overex-pressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR, Vanoverschelde JL, Ashworth A, Jovanovic A, Alessi DR, Bertrand L. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1. Am J Physiol Endocrinol Metab. 2006;290:E780–E788. doi: 10.1152/ajpendo.00443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson DK, Kashyap S, Bajaj M, Cusi K, Mandarino SJ, Finlayson J, DeFronzo RA, Jenkinson CP, Mandarino LJ. Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J Biol Chem. 2005;280:10290–10297. doi: 10.1074/jbc.M408985200. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Payen JF, Wuyam B, Reutenauer H, Laurent D, Levy P, Le Bas JF, Benabid AL. Impairment of muscular metabolism in chronic respiratory failure: a human 31P MRS study. NMR Biomed. 1991;4:41–45. doi: 10.1002/nbm.1940040108. [DOI] [PubMed] [Google Scholar]
- 36.Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- 37.Sakamoto K, Hirshman MF, Aschenbach WG, Goodyear LJ. Contraction regulation of Akt in rat skeletal muscle. J Biol Chem. 2002;277:11910–11917. doi: 10.1074/jbc.M112410200. [DOI] [PubMed] [Google Scholar]
- 38.Sakamoto K, Arnolds DE, Ekberg I, Thorell A, Goodyear LJ. Exercise regulates Akt and glycogen synthase kinase-3 activities in human skeletal muscle. Biochem Biophys Res Commun. 2004;319:419–425. doi: 10.1016/j.bbrc.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 39.Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- 40.Hojlund K, Mustard KJ, Staehr P, Hardie DG, Beck-Nielsen H, Richter EA, Wojtaszewski JF. AMPK activity and isoform protein expression are similar in muscle of obese subjects with and without type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;286:E239–E244. doi: 10.1152/ajpendo.00326.2003. [DOI] [PubMed] [Google Scholar]
- 41.Derave W, Ai H, Ihlemann J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. doi: 10.2337/diabetes.49.8.1281. [DOI] [PubMed] [Google Scholar]
- 42.Wojtaszewski JF, Jorgensen SB, Hellsten Y, Hardie DG, Richter EA. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes. 2002;51:284–292. doi: 10.2337/diabetes.51.2.284. [DOI] [PubMed] [Google Scholar]
- 43.Wojtaszewski JF, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, Kiens B, Richter EA. Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E813–E822. doi: 10.1152/ajpendo.00436.2002. [DOI] [PubMed] [Google Scholar]
- 44.Akerstrom TC, Birk JB, Klein DK, Erikstrup C, Plomgaard P, Pedersen BK, Wojtaszewski J. Oral glucose ingestion attenuates exercise-induced activation of 5′-AMP-activated protein kinase in human skeletal muscle. Biochem Biophys Res Commun. 2006;342:949–955. doi: 10.1016/j.bbrc.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 45.Lee-Young R, Palmer M, Linden KC, Leplastrier K, Canny BJ, Hargreaves M, Wadley GD, Kemp BE, McConell GK. Carbohydrate ingestion does not alter skeletal muscle AMPK signaling during exercise in humans. Am J Physiol Endocrinol Metab. 2006;291:E566–E573. doi: 10.1152/ajpendo.00023.2006. [DOI] [PubMed] [Google Scholar]
- 46.Witters LA, Kemp BE. Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J Biol Chem. 1992;267:2864–2867. [PubMed] [Google Scholar]
- 47.Wadley GD, Lee-Young RS, Canny BJ, Wasuntarawat C, Chen ZP, Har-greaves M, Kemp BE, McConell GK. Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am J Physiol Endocrinol Metab. 2006;290:E694–E702. doi: 10.1152/ajpendo.00464.2005. [DOI] [PubMed] [Google Scholar]
- 48.Roepstorff C, Thiele M, Hillig T, Pilegaard H, Richter EA, Wojtaszewski JF, Kiens B. Higher skeletal muscle alpha2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol. 2006;574:125–138. doi: 10.1113/jphysiol.2006.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christopher MJ, Chen ZP, Rantzau C, Kemp BE, Alford FP. Skeletal muscle basal AMP-activated protein kinase activity is chronically elevated in alloxan-diabetic dogs: impact of exercise. J Appl Physiol. 2003;95:1523–1530. doi: 10.1152/japplphysiol.00199.2003. [DOI] [PubMed] [Google Scholar]
- 50.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 52.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 53.MacDonald C, Wojtaszewski JF, Pedersen BK, Kiens B, Richter EA. Interleukin-6 release from human skeletal muscle during exercise: relation to AMPK activity. J Appl Physiol. 2003;95:2273–2277. doi: 10.1152/japplphysiol.00242.2003. [DOI] [PubMed] [Google Scholar]
- 54.Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, Giralt M, Hidalgo J, Saha AK, Pedersen BK, Ruderman NB. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320:449–454. doi: 10.1016/j.bbrc.2004.05.188. [DOI] [PubMed] [Google Scholar]
- 55.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 56.Raney MA, Yee AJ, Todd MK, Turcotte LP. AMPK activation is not critical in the regulation of muscle FA uptake and oxidation during low-intensity muscle contraction. Am J Physiol Endocrinol Metab. 2005;288:E592–E598. doi: 10.1152/ajpendo.00301.2004. [DOI] [PubMed] [Google Scholar]
- 57.Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem. 2005;280:39033–39041. doi: 10.1074/jbc.M504208200. [DOI] [PubMed] [Google Scholar]
- 58.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimida-zole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 59.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 60.Kennedy JW, Hirshman MF, Gervino EV, Ocel JV, Forse RA, Hoenig SJ, Aronson D, Goodyear LJ, Horton ES. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes. 1999;48:1192–1197. doi: 10.2337/diabetes.48.5.1192. [DOI] [PubMed] [Google Scholar]
- 61.Chen ZP, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes. 2003;52:2205–2212. doi: 10.2337/diabetes.52.9.2205. [DOI] [PubMed] [Google Scholar]
- 62.Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]