Abstract
The L-shaped anterior zone of the lateral hypothalamic area’s subfornical region (LHAsfa) is delineated by a pontine nucleus incertus input. Function evidence suggests the subfornical region and nucleus incertus modulate foraging and defensive behaviors, although subfornical region connections are poorly understood. A high resolution Phaseolus vulgaris-leucoagglutinin (PHAL) structural analysis is presented here of the LHAsfa neuron population’s overall axonal projection pattern. The strongest LHAsfa targets are in the interbrain and cerebral hemisphere. The former include inputs to anterior hypothalamic nucleus, dorsomedial part of the ventromedial nucleus, and ventral region of the dorsal premammillary nucleus (defensive behavior control system components), and to lateral habenula and dorsal region of the dorsal premammillary nucleus (foraging behavior control system components). The latter include massive inputs to lateral and medial septal nuclei (septo-hippocampal system components), and inputs to bed nuclei of the stria terminalis posterior division related to the defensive behavior system, intercalated amygdalar nucleus (projecting to central amygdalar nucleus), and posterior part of the basomedial amygdalar nucleus. LHAsfa vertical and horizontal limb basic projection patterns are similar, although each preferentially innervates certain terminal fields. Lateral hypothalamic area regions immediately medial, lateral, and caudal to the LHAsfa each generate quite distinct projection patterns. Combined with previous evidence that major sources LHAsfa neural inputs include the parabrachial nucleus (nociceptive information), defensive and foraging behavior system components, and the septo-hippocampal system, the present results suggest that the LHAsfa helps match adaptive behavioral responses (either defensive or foraging) to current internal motivational status and external environmental conditions.
Indexing terms: amygdala, behavioral activation, defensive behavior, hypothalamus, lateral habenula, motivation, nucleus incertus
INTRODUCTION
The lateral hypothalamic area’s anterior zone of the subfornical region (LHAsfa) lies ventral to the fornix at a transverse level that includes the caudal end of the anterior hypothalamic nucleus and the rostral end of the ventromedial hypothalamic nucleus. The LHAsfa was recognized and delineated in rats by a dense, restricted input from the nucleus incertus (NI) of the pons (Goto et al., 2001; Olucha-Bordonau et al., 2003). In those studies it was also shown that the NI forms a highly interconnected neural network with two other midline cell populations of the brainstem—the interpeduncular nucleus (IPN) and superior central nucleus (CS, also known as the median raphé nucleus)—that are thought to play a role in modulating behavioral activity levels. Functional evidence suggests that the IPN and CS are involved in controlling behavioral inhibition, whereas several analyses indicate that Fos expression in the NI is upregulated in certain challenging situations (see Goto et al., 2001).
Two experiments from our own group also suggest that Fos expression in the NI reflects at least in part the general level of an animal’s exploratory or foraging behavior, although the relationship is not necessarily simple (Goto, 1998). First, naive rats placed in a water tank for 10 minutes initially display vigorous swimming, followed by a characteristic immobile posture, and they show much less Fos-immunoreactivity in the NI as compared to animals that simply swim for 10 minutes searching for a platform they have learned to expect. And second, rats trained on a restricted food access paradigm display impressive Fos increases in the NI immediately before food delivery, coinciding with a period of hyperactivity and increased aggressiveness.
One way the midline brainstem NI-CS-IPN network could influence goal-oriented or motivated responses during behavioral activation is through the LHAsfa. In addition to receiving direct axonal inputs from all three brainstem network components—NI, CS, and IPN (Groenewegen et al., 1986; Vertes et al., 1999; Goto et al., 2001)—the LHAsfa is also innervated by the parabrachial nucleus (Bester et al., 1997); the ventral region of the dorsal premammillary nucleus (M. Goto and N.S. Canteras, personal observations) and dorsal division of the periaqueductal gray (S.R. Mota-Ortiz and N.S. Canteras, personal observations), both components of the defensive behavior system mediating innate fear responses (Canteras, 2002); and the septohippocampal system via an input from the dorsolateral zone of the rostral part of the lateral septal nucleus (Risold and Swanson, 1977).
Here we analyze systematically with the anterograde tracer, PHAL, the overall pattern of LHAsfa axonal projections, which are essentially unknown, although a broader lateral hypothalamic region including the LHAsfa was examined previously (Roeling et al., 1994). The results demonstrate that the LHAsfa projection pattern to interbrain (diencephalon) and cerebral hemisphere is quite distinct from those generated by adjacent LHA regions, thus providing additional evidence that the LHAsfa is an anatomically distinct LHA cell group. Of particular interest, the results indicate that the LHAsfa neuron population generates topographically ordered inputs to the septal and amygdalar regions of the cerebral hemisphere, and to the hypothalamus and thalamus. The LHAsfa’s ventrolateral or horizontal limb preferentially innervates the posterior basomedial and intercalated amygdalar nuclei, and the medial hypothalamic defensive behavior control network (anterior hypothalamic nucleus, dorsomedial part of the ventromedial nucleus, and ventral region of the dorsal premammillary nucleus), whereas the dorsomedial or vertical limb innervates preferentially the lateral habenula and dorsal region of the dorsal premammillary nucleus. Overall, the results suggest that the LHAsfa is positioned to influence neural activity in the septo-hippocampo-amygdalar system, and to modulate a variety of defensive (fight or flight) and exploratory or foraging behavioral responses.
MATERIALS AND METHODS
Adult male Wistar rats (n=25) weighing about 280–300g were used in this study. The animals were kept under controlled temperature (23°C) and illumination (12-hour cycle) conditions in the animal quarters and had free access to water and standard laboratory diet (Purina). Conditions of animal housing and all experimental procedures were conducted under institutional guidelines, and in compliance with NIH Guidelines for the Care and Use of Laboratory Animals.
Each animal received a single injection of PHAL (Vector) into the LHAsfa or adjacent LHA regions. They were anesthetized with a mixture of ketamine and xylazine (v/v; 1 ml/kg body weight), and the iontophoretic injection of a 2.5% solution of PHAL in 0.1 M sodium phosphate-buffered saline, pH 7.4 (Gerfen and Sawchenko, 1984), was made over a 10 min period through a stereotaxically positioned glass micropipette (tip diameter 10 μm) by applying a +5 μA current, pulsed at 7 second intervals, with a constant-current source (Midgard Electronics, model CS3). After a survival time of 14 to 16 days, the animals were perfused and the brains were processed according to procedures described in detail elsewhere (Canteras et al., 1992). Briefly, each animal was deeply anesthetized with sodium pentobarbital (40 mg/kg, intraperitoneal) and perfused transcardially with a solution of 4.0% paraformaldehyde in 0.1 M sodium borate buffer at pH 9.5%; the brains were removed immediately and postfixed for 3 hours in the same fixative containing 20% sucrose. The brains were then frozen, and 6 series of 25 μm-thick sections were cut on a sliding microtome in the transverse (frontal) plane and collected from the caudal medulla through the rostral tip of the prefrontal cortex. One complete series was processed for immunohistochemistry with an antiserum directed against PHAL (Dako; Carpintera, CA) at a dilution of 1:5,000. The antigen-antibody complex was localized with a variation of the avidin-biotin complex system (ABC; Hsu and Raine, 1981), with a commercially available kit (ABC Elite kit, Vector). The sections were mounted on gelatin-coated slides and then treated with osmium tetroxide to enhance visibility of the reaction product. Slides were then dehydrated and coverslipped with DPX. An adjacent series was always stained with thionin to serve as a reference for cytoarchitecture.
Sections were examined under a microscope with bright- and darkfield illumination. PHAL-labeled cells in the injection sites, and the distribution of anterogradely labeled fibers, were plotted with the aid of a camera lucida onto maps prepared from adjacent thionin-stained sections. For illustration purposes the distribution of anterogradely labeled fibers was transferred onto a reference atlas of the rat brain (Swanson, 2004). The figures were prepared for publication using Adobe Photoshop (version 5.5; Adobe Systems, San Jose, CA) for photomicrographs, and Adobe Illustrator (version 10, Adobe Systems) for line drawings. Unless otherwise indicated, parcellation of the rat brain follows Swanson (2004). Terminology for describing morphological features of PHAL-labeled axons, and mapping strategies and procedures, are detailed by Swanson (2004).
RESULTS
Nomenclature
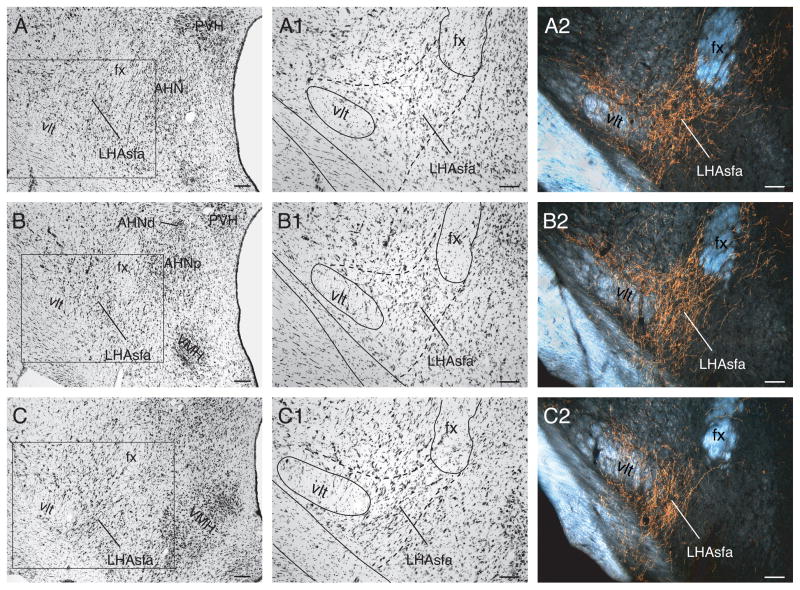
In transverse sections, the LHAsfa is an L-shaped differentiation of the LHA that descends just below the fornix and then extends laterally, just dorsal to the supraoptic commissure (Fig. 1). It is delimited precisely by a heavy input from the NI (Goto et al., 2001) (Fig. 1A2–C2), and it extends rostrocaudally for about 400–600 μm, from the rostral pole of the ventromedial nucleus (Fig. 1A) to the anterior part of the dorsomedial hypothalamic nucleus (Fig. 1C). The LHAsfa reaches its maximal extent at the level of the lateral parvicellular part of the paraventricular nucleus and the tiny dorsal part of the anterior hypothalamic nucleus (Fig. 1B; Atlas Level 27 in Swanson, 2004). The LHAsfa is not homogeneous when examined in Nissl-stained preparations. Instead, there are clear dorsomedial (vertical) and ventrolateral (horizontal) limbs. The former is characterized by small to medium-sized fusiform neurons with a vertical orientation that are somewhat more darkly stained than those in surrounding areas (Fig. 1A1–C1). The latter is characterized by larger multipolar neurons with a somewhat greater packing density than in its surroundings, although it is difficult to distinguish in Nissl preparations (Fig. 1A1–C1), darkfield illumination being more helpful than brightfield. The LHA’s subfornical region also has separate posterior (LHAsfp) and then premammillary (LHAsfpm) zones (see Swanson, 2004).
Fig. 1.
Low (A–C) and high (A1–C1) power brightfield photomicrographs of transverse Nissl-stained sections showing cytoarchitectonic features of the left LHAsfa and surrounding parts if the hypothalamus. The boxed areas in A, B, and C are shown at higher magnification in A1, B1, and C1, respectively. (A2–C2) Darkfield photomicrographs of adjacent sections to show the LHAsfa terminal field after a PHAL injection in the NI. Scale bars = 100 μm.
Injection sites
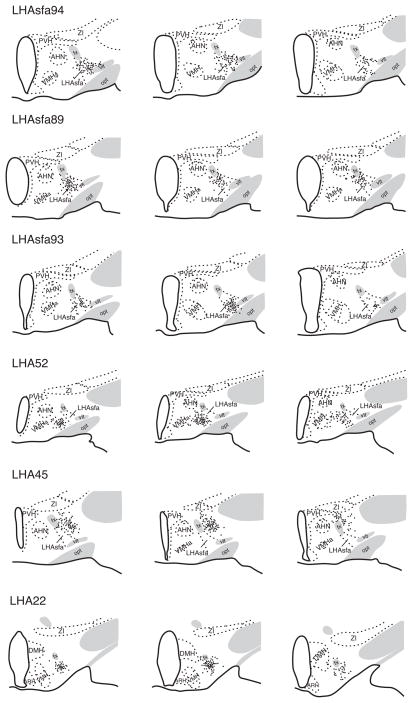
In 15 experiments the PHAL deposit is confined almost entirely to the LHAsfa. In three experiments (LHA57, 88, and 94) the PHAL injection is relatively large and labels neurons throughout much of the LHAsfa. In nine other experiments the injection site is smaller and most PHAL-labeled neurons are centered either dorsomedially (LHA43, 89, and 95) or ventrolaterally (LHA53, 54, 56, 63, 65, and 93). Figures 2 and 3 illustrate a representative example of each group.
Fig. 2.
Camera lucida plots of PHAL-labeled neurons following injections centered in the LHAsfa (Experiments LHAsfa94, LHAsfa89, LHAsfa93), LHA region medial to the LHAsfa (the LHAjv; Experiment LHA52), LHA region lateral to the LHAsfa (LHAa/d; Experiment LHA45), and LHA region caudal to the LHAsfa (the LHAsfp; Experiment LHA22). In Experiments LHAsfa89 and LHAsfa93 the PHAL injection is centered in the vertical (dorsomedial) and horizontal (ventrolateral) limbs of the LHAsfa, respectively. Transverse sections are arranged from rostral (left) to caudal (right).
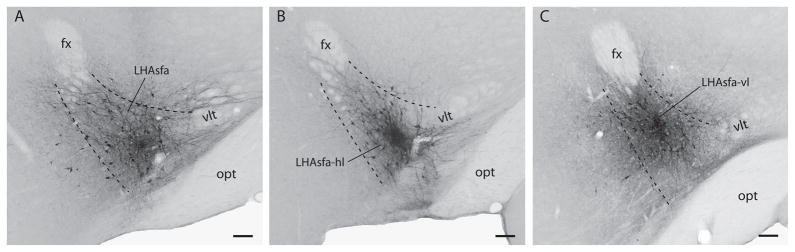
Fig. 3.
Brightfield photomicrographs showing PHAL deposits in the LHAsfa. (A) A large PHAL injection in the LHAsfa, Experiment LHA94; (B) a PHAL injection centered in the horizontal (ventrolateral) limb of the LHAsfa, Experiment LHA93; (C) a PHAL injection centered in the vertical (dorsomedial) limb of the LHAsfa, Experiment LHA 89. Scale bars = 100 μm.
The projections labeled in Experiment LHA94 are described in detail because it labels neurons throughout most of the LHAsfa, and the projection pattern is similar to that labeled by other injections centered in the LHAsfa. The projection pattern seen in Experiment LHA94 is summarized in Table 1. Because a few labeled neurons spread outside the borders of the LHAsfa as defined here, major projection differences seen with injections centered medial (LHA52), lateral (LHA45) or caudal (LHA22) to the LHAsfa (Fig. 2) are also considered.
TABLE 1.
Semiquantitative analysis of PHAL labeling in the cerebrum and cerebrospinal trunk after a PHAL injection in the LHAsfa and adjacent LHA. The cell group hierarchy is from Swanson (2004).
| Cell group | LHAsfa (LHA94) | LHAjv LHA52) | LHAa/d (LHA45) | LHAsfp (LHA22) |
|---|---|---|---|---|
| 1 CEREBRUM | ||||
| 1.1 CEREBRAL CORTEX | ||||
| 1.1.1 Cortical Plate | ||||
| Visceral sensory-motor areas | ||||
| infralimbic área | + | −/+ | −/+ | −/+ |
| agranular insular | − | + | − | − |
| Olfactory áreas | ||||
| cortical amygdalar area | + | −/+ | − | − |
| Postpiriform amygdalar area | − | + | − | − |
| Polymodal association cortex | ||||
| prelimbic | −/+ | −/+ | −/+ | |
| anterior Cingulate | − | − | − | − |
| Hippocampal formation | ||||
| entorhinal area (medial) | + | ++ | − | − |
| subiculum (ventral part) | − | +/++ | − | − |
| field CA1 | + | ++ | − | − |
| 1.1.2 Cortical subplate | ||||
| Lateral amygdalar nucleus | + | + | − | − |
| Basolateral amygdalar nucleus | − | − | − | − |
| Basomedial amygdalar nucleus | ||||
| anterior part | − | − | − | − |
| posterior part | ++/+++ | ++/+++ | − | − |
| Posterior amygdalar nucleus | −/+ | +++ | − | ++/+++ |
| 1.2 CEREBRAL NUCLEI | ||||
| 1.2.1 Striatum | ||||
| Nucleus accumbens | − | + | + | ++ |
| Lateral septal complex | ||||
| Lateral septal nucleus | ||||
| caudal part | ||||
| dorsal zone (LSc.d) | −/+ | − | +++ | − |
| ventral zone (LSc.v) | ++/+++ | ++ | ++/+++ | ++/+++ |
| rostral part | ||||
| medial zone (LSr.m) | − | ++ | −/+ | ++ |
| ventrolateral zone (LSr.vl) | − | ++ | ++ | + |
| dorsolateral zone | ||||
| medial region (LSr.dl.m) | ++ | +++ | + | −/+ |
| lateral region (LSr.dl.l) | +++ | + | −/+ | −/+ |
| ventral part (LSv) | − | − | − | + |
| Septofimbrial nucleus | − | + | + | − |
| Anterior amygdaloid area | − | −/+ | + | − |
| Central amygdalar nucleus | ||||
| medial part | − | − | − | +++ |
| lateral part | − | − | − | − |
| capsular part | −/+ | + | ++ | − |
| Medial amygdalar nucleus | − | + | − | − |
| Intercalated amygdalar nuclei | +++ | − | + | + |
| 1.2.2 Pallidum | ||||
| Substantia innominata | +/++ | + | ++ | ++ |
| Medial septal complex | ||||
| Medial septal nucleus | +++ | ++ | ++/+++ | − |
| Diagonal band nucleus | ++ | − | −/+ | − |
| Bed nuclei of the stria terminalis | ||||
| Anterior division | ||||
| anterolateral area (BSTal) | − | −/+ | − | ++/+++ |
| anteromedial area (BSTam) | − | ++/+++ | + | +++ |
| oval nucleus (BSTov) | − | − | − | − |
| juxta-capsular nucleus (BSTju) | − | − | − | − |
| rhomboid nucleus (BSTrh) | − | + | − | +++ |
| dorsomedial nucleus (BSTdm) | − | + | − | +++ |
| fusiform nucleus (BSTfu) | − | − | − | |
| ventral nucleus (BSTv) | − | + | − | ++/+++ |
| magnocellular nucleus (BSTmg) | − | + | − | ++/+++ |
| Posterior division | ||||
| principal nucleus (BSTpr) | − | − | − | −/+ |
| interfascicular nucleus (BSTif) | ++ | ++/+++ | ++ | ++ |
| transverse nucleus (BSTtr) | ++ | ++ | + | ++ |
| 2. CEREBROSPINAL TRUNK | ||||
| 2.1. SENSORY SYSTEM | ||||
| 2.1.1 Thalamus | ||||
| Sensory-motor cortex related | ||||
| Polymodal association cortex related | ||||
| Medial group of the thalamus | ||||
| Mediodorsal nucleus of the thalamus | ||||
| medial part | ++ | ++/+++ | + | + |
| central part | − | − | − | − |
| lateral part | − | − | ++ | − |
| Midline group of the dorsal thalamus | ||||
| paraventricular thalamic nucleus | + | ++ | ++ | ++ |
| parataenial nucleus | ++ | ++ | − | − |
| nucleus reuniens | ++ | +−/++ | ++ | − |
| Intermediodorsal nucleus (?) | −/+ | − | − | + |
| Intralaminar group of the dorsal thalamus | ||||
| rhomboid nucleus | − | − | −/+ | − |
| central medial nucleus of the thalamus | + | + | ++ | − |
| paracentral nucleus of the thalamus | − | − | − | − |
| central lateral nucleus of the thalamus | − | − | − | − |
| parafascicular nucleus | − | − | − | − |
| 2.2 BEHAVIORAL STATE | ||||
| Subparaventricular zone | − | +−/++ | − | − |
| Hypothalamic lateral zone | ||||
| lateral hypothalamic area, dorsal region | ++ | −/+ | ++ | ++ |
| Supramammillary nucleus | − | + | ++ | + |
| Raphé nuclei | ||||
| interfascicular nucleus raphé | − | − | − | + |
| interpeduncular nucleus | −/+ | − | − | − |
| dorsal nucleus raphé | ++ | −/+ | ++ | ++ |
| superior central nucleus raphé | ++ | ++ | ++ | + |
| nucleus incertus | + | − | −/+ | − |
| 2.3 MOTOR SYSTEM | ||||
| 2.3.1 Behavioral Control Column | ||||
| Medial preoptic nucleus | ||||
| lateral part | − | + | − | + |
| medial part | − | − | − | − |
| central part | − | − | − | − |
| Anterior hypothalamic nucleus | ||||
| anterior part | ++ | +/++ | + | ++ |
| Central | ++ | ++ | + | − |
| posterior part | ++ | + | − | − |
| Paraventricular hypothalamic nucleus, descending division | ||||
| medial parvicellular part | − | − | − | +++ |
| dorsal parvicellular part | − | − | − | + |
| lateral parvicellular part | − | − | − | +++ |
| forniceal part | − | − | − | ++/+++ |
| Ventromedial nucleus | ||||
| anterior | + | +++ | − | − |
| dorsomedial part | ++ | +++ | − | − |
| central part | − | ++/+++ | − | − |
| ventrolateral part | − | +/+++ | − | − |
| Ventral premammillary nucleus | − | +/++ | − | − |
| Dorsal premammillary nucleus | ||||
| dorsal part | +++ | ++ | +++ | − |
| ventral part | +++ | ++/+++ | ++ | − |
| Ventral tegmental area | − | − | ++ | ++ |
| Midbrain reticular nucleus, retrorubral area | − | ++ | ++ | ++ |
| Midbrain reticular nucleus | − | ++ | −/+ | − |
| 2.3.2 Central Gray | ||||
| Epithalamus | ||||
| Medial habenula | − | − | + | − |
| Lateral habenula | ||||
| medial part | ++ | ++ | +++ | ++ |
| lateral part | + | + | ++++ | − |
| Posterior hypothalamic nucleus | ++ | ++ | ++/+++ | ++/+++ |
| Periaqueductal gray | ||||
| precommissural nucleus | + | + | ++/+++ | − |
| rostromedial division | − | + | ++/+++ | −/+ |
| medial division | − | + | ++/+++ | −/+ |
| dorsal division | −/+ | ++ | +/++ | −/+ |
| dorsolateral division | + | −/+ | − | −/+ |
| lateral division | −/+ | ++/+++ | ++ | + |
| ventrolateral division | ++ | ++/+++ | ++ | ++/+++ |
| pontine central gray | −/+ | + | + | ++/+++ |
| Barrington’s nucleus | − | −/+ | − | ++/+++ |
| 2.3.3 Hypothalamic Periventricular Region | ||||
| Median preoptic nucleus | − | − | − | + |
| Anteroventral periventricular nucleus | − | − | − | −/+ |
| Preoptic periventricular | − | − | − | + |
| Anterodorsal preoptic nucleus | − | − | − | +++ |
| Anteroventral preoptic nucleus | − | ++ | + | +++ |
| Parastrial nucleus | − | − | − | +++ |
| Medial preoptic area | −/+ | ++/+++ | −/+ | +++ |
| Anterior hypothalamic area | + | ++ | −/+ | ++ |
| Dorsomedial hypothalamic nucleus | ||||
| anterior part | ++ | ++ | + | ++/+++ |
| posterior part | − | −/+ | − | − |
| ventral part | − | −/+ | − | ++/+++ |
| Periventricular hypothalamic nucleus, posterior part | − | −/+ | − | ++ |
| 2.3.4 Reticular Formation | ||||
| Hypothalamic lateral zone, motor related | ||||
| Lateral preoptic area | ++ | −/+ | +++ | +++ |
| Lateral hypothalamic area, motor related | ||||
| juxtaparaventricular region | ++/+++ | +++ | ++ | +/++ |
| juxtadorsomedial region | + | +/++ | − | + |
| juxtaventromedial | + | − | ++/+++ | |
| anterior region | ||||
| dorsal zone | ++ | ++/+++ | ++ | ++/+++ |
| intermediate zone | ++/+++ | ++ | ||
| ventral zone | ++ | + | + | ++/+++ |
| retrochiasmatic area | −/+ | ++ | −/+ | +/++ |
| tuberal nucleus | − | −/+ | − | ++ |
| suprafornical region | − | − | ++/+++ | ++/+++ |
| subfornical | ||||
| anterior zone | − | − | − | |
| posterior zone | + | + | + | |
| premammillary zone | ++ | ++ | −/+ | ++ |
| magnocellular nucleus | − | − | − | − |
| parvicellular region | − | − | + | + |
| ventral region | ++ | − | ++ | +/++ |
| posterior region | −/+ | − | ++ | ++/+++ |
| Preparasubthalamic nucleus | − | − | − | − |
| Parasubthalamic nucleus | − | − | − | + |
| Zona incerta | −/+ | + | −/+ | −/+ |
| Cuneiform nucleus | ++ | ++ | + | − |
| 2.3.5 Motoneuron Groups | ||||
| Neuroendocrine motor zone | ||||
| Parvicellular | ||||
| paraventricular hypothalamic nucleus | ||||
| anterior parvicellular part | −/+ | ++ | + | +++ |
| medial parvicellular part, dorsal zone | − | + | − | +++ |
| periventricular part | − | + | − | ++ |
| periventricular hypothalamic nucleus, anterior part | − | + | − | + |
| periventricular hypothalamic nucleus, posterior part | − | −/+ | − | + |
| arcuate nucleus | − | − | − | − |
(−) absence of labeling; (−/+) very light labeling; (+) light labeling; (++) moderate labeling; (++/+++) moderately dense labeling; (+++) dense labeling.
Projections from the LHAsfa
Axons labeled from PHAL injections centered in the LHAsfa (exemplified by experiment LHA94, Figs. 2 and 3) follow six main pathways to innervate cell groups in the cerebral hemispheres and brainstem (Fig. 4). Individual pathways are distinguished by the spatial distribution of labeled axons.
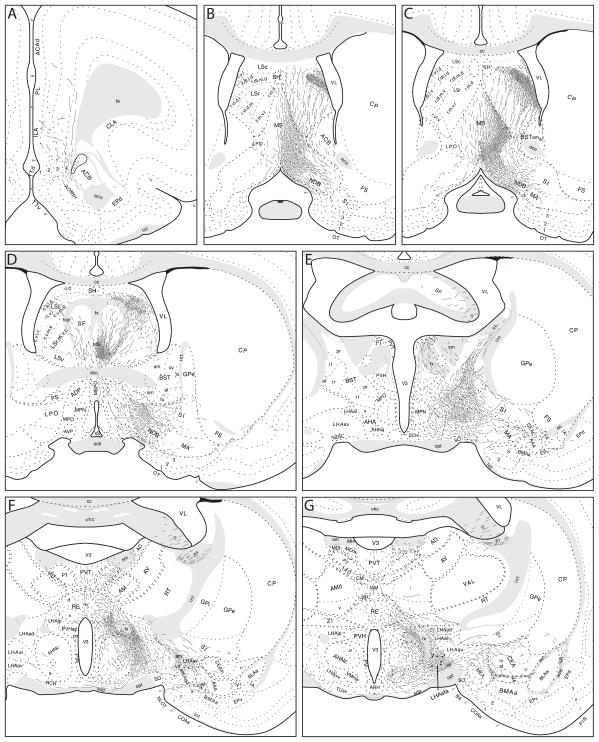
Fig. 4.
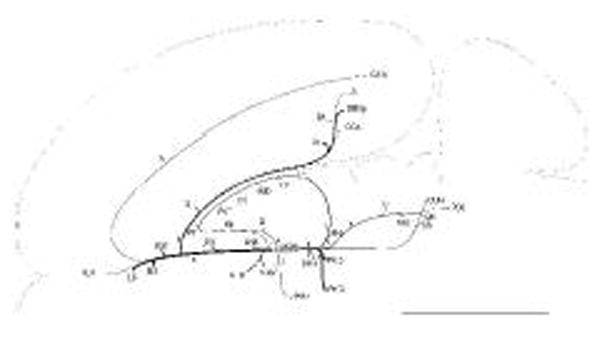
General organization of projections from the LHAsfa. The magnitude of each pathway is roughly proportional to the thickness of the line representing it. The numbers 1–6 refer to main axonal pathways described in the text. The flatmap is adapted from Swanson (2004).
Ascending projections
Rostrally directed axons leaving the LHAsfa may be divided conveniently into dorsomedial, dorsal, ventromedial, and ventrolateral pathways, as well as those coursing through the medial forebrain bundle.
Dorsomedial ascending pathway
Many PHAL-labeled axons extend dorsomedially from the injection site (Figs. 3-1 and 5G–H). Most of them end within the hypothalamus, although some continue into the thalamus, along with axons in the dorsal and ventromedial pathways (see below). The former generate a moderate terminal field in the anterior hypothalamic nucleus (Fig. 5F–H) and dorsomedial part of the ventromedial nucleus (Fig. 5H). Axons that extend slightly rostrally in the dorsomedial pathway richly innervate the LHA juxtaparaventricular region along with axons in the dorsal pathway (Fig. 5F,G). Only a very light input to the paraventricular nucleus itself is observed.
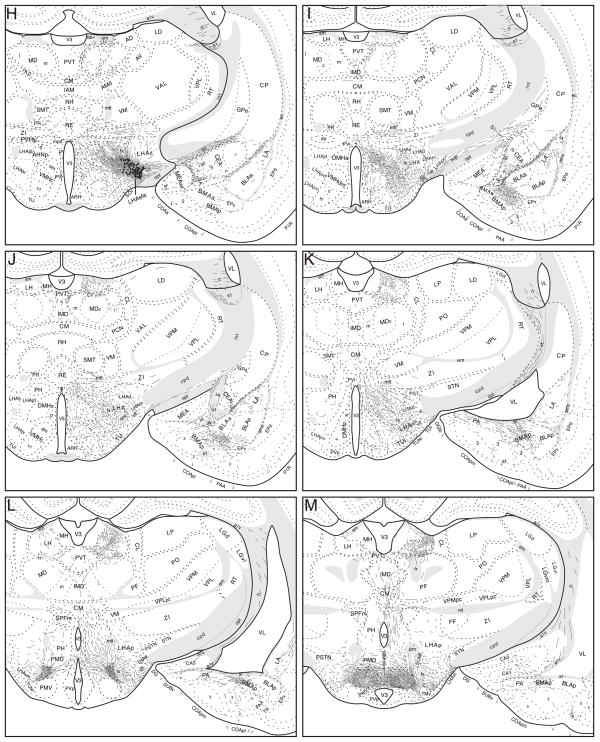
Fig. 5.
Projections from the LHAsfa. The distribution of PHAL-labeled axons (thin black lines) in experiment LHAsfa94 was plotted onto a series of rat brain reference templates derived from Swanson (2004), arranged from rostral to caudal (A-S). The black dots in G and H indicate the injection site.
Dorsal ascending pathway
Moderate numbers of PHAL-labeled axons course dorsally from the LHAsfa through and to the zona incerta (Figs. 4-2, 5G–I). Next, some of the axons continue dorsally into the dorsal thalamus, whereas others veer medially into the dorsal thalamic midline nuclei, along with axons in the dorsomedial and ventromedial pathways (Fig. 5F–I). A larger number of dorsal pathway axons enter rostral levels of the dorsal thalamus, along with axons in the dorsomedial and ventromedial ascending pathways. Together, they innervate moderately the paratenial and mediodorsal nuclei and ventral part of the nucleus reuniens (Fig. 5F–J), and lightly the paraventricular (Fig. 5E–M), central medial (Fig. 5G–M), and intermediodorsal (Fig. 5I–M) nuclei.
Other dorsal pathway axons enter the stria medullaris to innervate the lateral habenula (especially caudally), along with axons entering the thalamus caudally from the posterior hypothalamic nucleus. According to the parceling of Andres and colleagues (1999) moderate terminal fields are established in the caudal magnocellular, parvicellular, and marginal subnuclei of the lateral habenula lateral division, and in the parvicellular and central subnuclei of the medial division (Figs. 5H–M and 6).
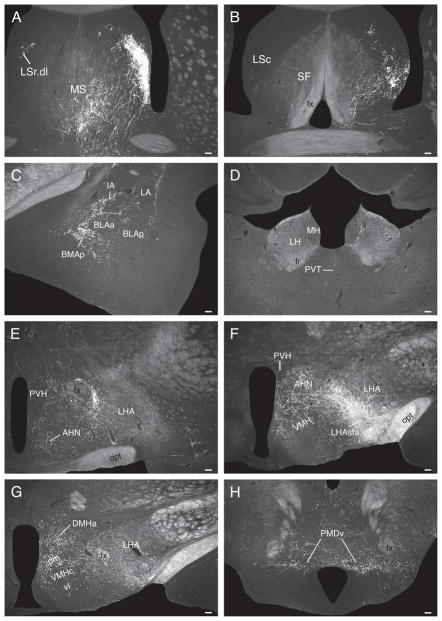
Fig. 6.
Darkfield photomicrographs showing the distribution of PHAL-labeled axons in experiment LHAsfa94, with a large injection centered in the LHAsfa. (A) Dorsolateral zone of the lateral septal nucleus’s rostral part, with an abundance of pericellular baskets; (B) intercalated and posterior basomedial nuclei of the temporal lobe’s amygdalar region; (C) lateral habenula; and (D) dorsal premammillary nucleus. Scale bars = 100 μm.
Ventromedial ascending pathway
A small group of LHAsfa axons courses ventrally and medially to innervate the ventromedial hypothalamic nucleus (Figs. 4-3 and 5G,H). Some of them continue dorsally to enter the paraventricular hypothalamic nucleus, where they generate a few branches with terminal boutons, and then enter the thalamus, where they merge with the dorsomedial and dorsal ascending pathways. Another component of the ventromedial ascending pathway courses through the tuberal nucleus and retrochiasmatic area, where it crosses the midline to reach the contralateral LHAsfa (Figs. 4-3 and 5F,G).
Ventrolateral ascending pathway
These axons run through the caudal ansa peduncularis to the amygdalar region (Fig. 5G,H), where they are joined by axons coursing through more rostral levels of the ansa peduncularis and the stria terminalis (Fig. 4--4). These axons generate a moderately dense input to the posterior part of the basomedial nucleus (Figs. 5I–N and 6B). Many labeled axons with terminal boutons accumulate near the dorsal border of the main intercalated nucleus and the central nucleus (Fig. 5G,H), and in the paracapsular part of the intercalated nucleus (Millhouse, 1986), between the central nucleus and the basolateral and lateral nuclei (Figs. 5H,J and 6B). The lateral nucleus itself receives a light input (Figs. 5G–L and 6B), along with the posterior amygdalar nucleus (Fig. 5K–N). A few axons and terminal boutons are observed in caudoposterolateral (Fig. 5K–M) and posteromedial (Fig. 5L–N) regions of the anterior cortical nucleus and in rostral regions of the medial nucleus (Fig. 5F–H) of the amygdalar region.
Ascending medial forebrain bundle
A clear majority of LHAsfa ascending axons course through the medial forebrain bundle (Fig. 4–5). They heavily innervate more anterior regions of the LHA (Fig. 5E–F) and moderately innervate the lateral preoptic area (Fig. 5D).
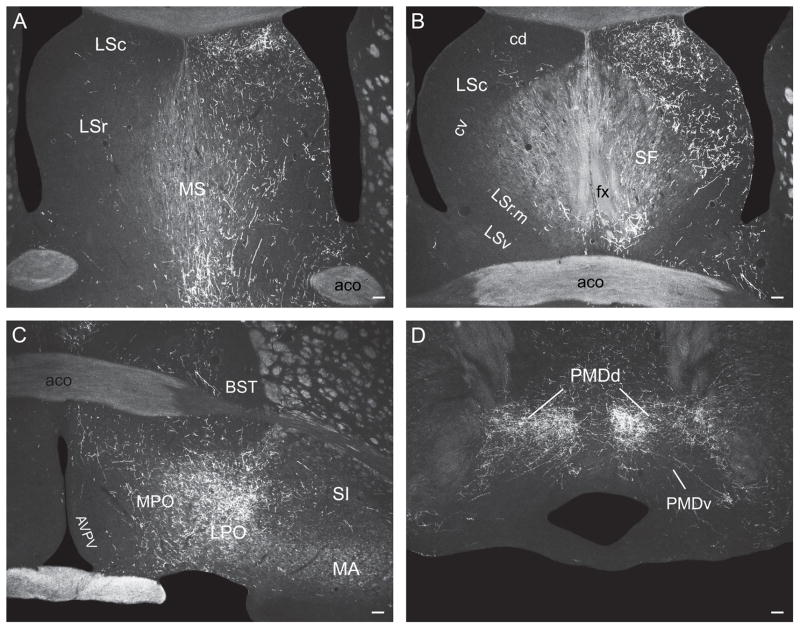
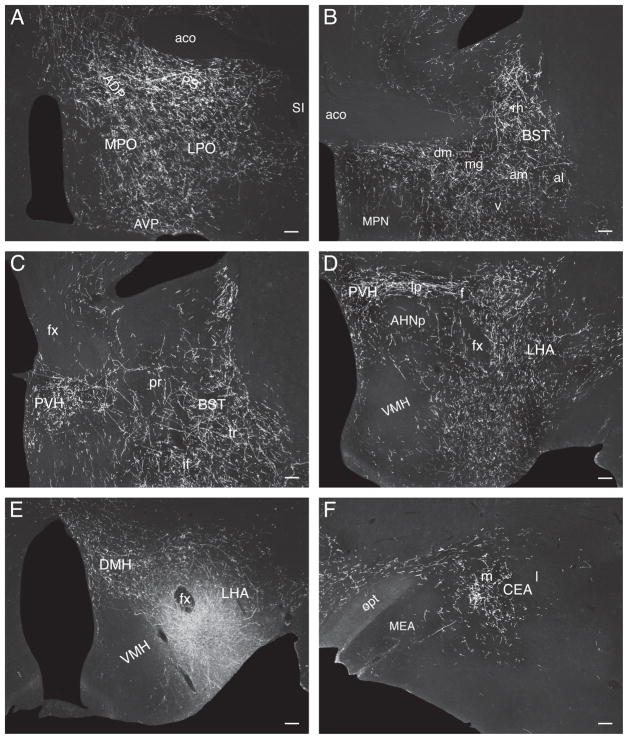
At the anterior hypothalamic nucleus level, ascending LHAsfa axons in the medial forebrain bundle diverge to follow different routes. One fiber group enters the bed nuclei of the stria terminalis (Fig. 5E), where they split into three smaller bundles: a) moderately innervating the interfascicular and medial transverse nuclei of the bed (Fig. 5E), b) entering the stria terminalis (Fig. 5E), and c) entering the fornix (Fig. 5D,E) to innervate caudal regions of the lateral septal nucleus (Fig. 5D) along with axons coursing through rostral levels of the medial forebrain bundle. A second fiber group courses through and to the substantia innominata, in the ansa peduncularis, to reach the amygdalar region (Fig. 5E–I), along with axons in the ventrolateral ascending pathway that reach the amygdalar region through the caudal ansa peduncularis and stria terminalis (see above). A third ascending medial forebrain bundle group of axons enters the lateral preoptic area, where it generates a moderately dense terminal field (Fig. 5D). This bundle then splits, with one component coursing dorsomedially to enter and innervate densely caudal regions of the medial septal nucleus and the ventral zone of the caudal part of the lateral septal nucleus (Fig. 5D). From there a few axons course through the septofimbrial nucleus and enter the fimbria to generate light inputs to ventral regions of hippocampal field CA1 (Fig. 5M,N). The other component enters the substantia innominata and diagonal band nucleus before extending rostrally (Fig. 5D) and richly innervating the medial septal nucleus (Figs. 5B,C and 6C). A group of axons in the third ascending component of the medial forebrain bundle also extends dorsally to generate very dense branching and terminal boutons in the dorsolateral zone of the rostral part of the lateral septal nucleus (LSr.dl), where many pericellular baskets of terminal boutons are observed (Fig. 5B,C).
A few labeled axons in the medial forebrain bundle proceed more rostrally, passing through the anterior olfactory nucleus and ventral regions of the nucleus accumbens to enter the tenia tecta and eventually reach the infralimbic area of medial prefrontal cortex, where some branching and terminal boutons are observed (Figs. 5A and 6A).
Descending projections
Most descending PHAL-labeled axons from the LHAsfa course initially through the medial forebrain bundle (Fig. 4–6), before diverging to specific terminal fields or to branches of the bundle. One group of axons extends dorsomedially and many of them enter the dorsomedial hypothalamic nucleus (Fig. 4–6a), where they generate a moderate terminal field in the rostral tip of its anterior part (Fig. 5I). Another group of medial forebrain bundle fibers enters the posterior hypothalamic nucleus (Fig. 4–6b), where there is also a relatively moderate terminal field (Fig. 5J–M) and some labeled axons cross the midline to enter the contralateral LHA. Other labeled fibers in the posterior hypothalamic nucleus extend dorsally into the thalamus (Fig. 4–6c). They branch abundantly and generate many boutons in the lateral habenula, especially caudally, along with axons from the dorsomedial ascending pathway described above (Figs. 5K,L and 6C).
Many LHAsfa axons extend farther caudally. At mammillary levels they generate a dense terminal field in the dorsal premammillary nucleus (Figs. 4–6d, 5L,M, and 6D). Some axons then extend laterally from the dorsal premammillary nucleus region to innervate moderately the LHAsfpm, and even farther laterally the LHA lateral zone’s ventral region (LHAvl; Fig. 5M). Slightly more caudally, axons in the medial forebrain bundle enter the supramammillary nucleus, where some cross the midline to enter the contralateral medial forebrain bundle (Fig. 5N). Some axons in the supramammillary nucleus turn dorsally and join axons in the posterior hypothalamic nucleus to enter the periaqueductal gray (Figs. 4–6e and 5N). This dorsal branch of the medial forebrain bundle lightly innervates the precommissural nucleus (Fig. 5N)—and the medial, dorsolateral, and lateral periaqueductal gray divisions—and relatively moderately innervates the ventrolateral division (Fig. 5O–R), along with the dorsal raphé nucleus (Fig. 5Q,R). Terminal fields in the latter two regions seem to be reinforced by axons ascending from the ventral continuation of the medial forebrain bundle (see below), and together these axons generate a moderate input to the cuneiform nucleus (Fig. 5Q,R).
Caudal to the supramammillary nucleus, LHAsfa axons in the medial forebrain bundle proper enter the ventral tegmental area (Figs. 4–6f and 5O,P). Some of them course laterally through and near the compact substantia nigra to then arch dorsomedially through the midbrain reticular nucleus and its retrorubral area differentiation to enter the periaqueductal gray, as mentioned above (Fig. 5O,P). Other axons in the ventral tegmental area extend dorsally through the rostral raphé region to enter the periaqueductal gray (Fig. 5O,P). However, most labeled axons in the ventral tegmental area continue caudally through the raphé region, where they provide moderately dense inputs to the superior central nucleus (Fig. 5Q,R), and then the dorsal raphé nucleus and adjacent periaqueductal gray ventrolateral region, together with axons described above that course through the dorsal branch of the medial forebrain bundle (Fig. 5Q,R). A few labeled axons in the midbrain ventral extension of the medial forebrain bundle take a dorsolateral course to enter the retrorubral area and cuneiform nucleus (Fig. 5Q,R), and it should also be mentioned that a few labeled axons cross the midline to innervate the contralateral periaqueductal gray (Fig. 5Q,R).
Finally, some LHAsfa axons reach the caudal brainstem central gray where they generate relatively light inputs to the region of pontine central gray bordering the dorsal tegmental nucleus and the nucleus incertus (Fig. 5S).
Projections from LHAsfa vertical and horizontal limbs
Analysis of experiments with PHAL injections centered in either the dorsomedial or ventrolateral limbs of the LHAsfa indicates that whereas the overall projection pattern from each is similar, relative terminal field strengths reveal a certain topographic organization to projections innervating septal, amygdalar, hypothalamic, and thalamic cell groups.
The LHAsfa horizontal limb
As mentioned above, six PHAL injections are centered in ventrolateral regions of the LHAsfa, and Experiment 93 (Figs. 2, 3, and 7F) was chosen to illustrate projection patterns typical of all six.
Fig. 7.
Darkfield photomicrographs showing the distribution of PHAL-labeled axons in experiment LHAsfa93, with a PHAL injection centered in the LHAsfa’s horizontal limb. (A) Dorsolateral zone of the lateral septal nucleus’s rostral part, with abundant pericellular baskets, and medial septal nucleus; (B) caudal part of the lateral septal nucleus and septofimbrial nucleus; (C) intercalated and posterior basomedial nuclei of the amygdalar region; (D) lateral habenula; (E) LHA’s juxtaparaventricular region and anterior hypothalamic nucleus; (F) anterior and ventromedial hypothalamic nuclei and the PHAL deposit centered in the LHAsfa’s ventrolateral or horizontal limb; (G) dorsomedial and ventromedial hypothalamic nuclei; (H) ventral region of the dorsal premammillary nucleus. Scale bars = 100 μm.
The lateral septal nucleus receives a massive input to the ventrolateral two-thirds of the LSr.dl (Fig. 7A) and an only slightly less dense input to ventral regions of the LSc.v (Fig. 7B). Ventral regions of the septofimbrial nucleus also receive a moderately dense terminal field, and as in experiment LHA94 there is a dense input to the medial septal nucleus (Fig. 7A).
In the amygdalar region, the paracapsular part of the intercalated nucleus, and the posterior basomedial nucleus, are densely innervated (Fig. 7C), whereas the lateral nucleus has a sparser terminal field (Fig. 7C). The hypothalamus displays a rich terminal field in the LHA’s juxtaparaventricular region (Fig. 7E), and substantial terminal fields are also observed in the anterior hypothalamic nucleus (Fig. 7E,F), dorsomedial part of the ventromedial nucleus (Fig. 7F,G), and dorsomedial nucleus (Fig. 7G). These amygdalar and hypothalamic neuron populations are only lightly labeled by PHAL injections centered in the LHAsfa dorsal limb.
More caudally, abundant PHAL-labeled branches and boutons are observed in and around the ventral region of the dorsal premammillary nucleus, whereas the dorsal region is more lightly innervated (Fig. 7H).
In the thalamus, a sparse terminal field is observed in lateral regions of the medial habenula, and in the parvicellular and superior parts of the lateral habenula’s medial division (Fig. 7D).
The LHAsfa vertical limb
Experiment LHA89 (Figs. 2, 3, and 8F) was chosen to illustrate the three PHAL injections mentioned above that are centered in dorsomedial regions of the LHAsfa.
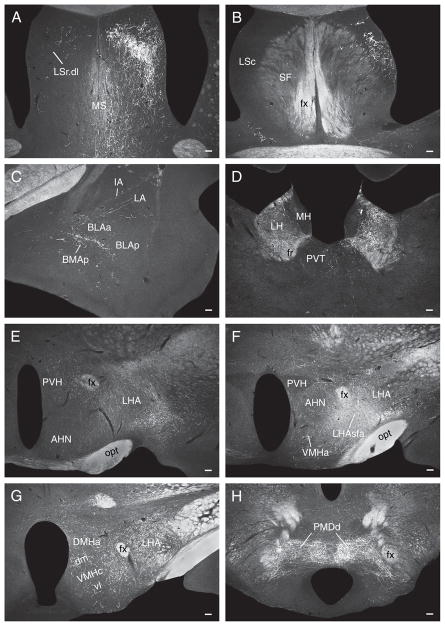
Fig. 8.
Darkfield photomicrographs showing the distribution of PHAL-labeled axons in experiment LHAsfa89, with a PHAL injection centered in the LHAsfa’s vertical limb. (A) Dorsolateral zone of the lateral septal nucleus’s rostral part, with abundant pericellular baskets, and medial septal nucleus; (B) caudal part of the lateral septal nucleus; (C) intercalated and posterior basomedial amygdalar nuclei; (D) lateral habenula; (E) juxtaparaventricular region of the LHA and anterior hypothalamic nucleus; (F) anterior and ventromedial hypothalamic nuclei, and the PHAL deposit in the dorsomedial or vertical limb of the LHAsfa; (G) dorsomedial and ventromedial hypothalamic nuclei; (H) dorsal region of the dorsal premammillary nucleus. Note dense terminal field in the lateral habenula and in dorsal regions of the dorsal premammillary nucleus, in contrast to very little labeling in the intercalated and posterior basomedial nuclei, LHA’s juxtaparaventricular region, and anterior, ventromedial, and dorsomedial hypothalamic nuclei. Ventral regions of the dorsal premammillary nucleus are almost free of labeling as well. Scale bars = 100 μm.
In the lateral septal nucleus a dense input is observed in the dorsal third of the LSr.dl (Fig. 8A). More ventrally, anterograde labeling intensity decreases and is concentrated in more medial regions of the LSr.dl—avoiding more lateral regions that are massively labeled in experiments with injections centered in the LHAsfa horizontal limb (Fig. 8A). In the LSc, anterograde labeling is concentrated in the intermediate third of the ventral zone (Fig. 8B), like that described above for experiment LHA94—and just dorsal to the region labeled in experiments with injections centered in the LHAsfa horizontal limb. PHAL labeling in the medial and septofimbrial nuclei is lighter than that observed in horizontal limb injections (Fig. 8A,B).
In the amygdalar region, anterograde labeling in the posterior basomedial nucleus and paracapsular part of the intercalated nucleus is much lighter than that observed in experiments with injections centered in the horizontal limb (Fig. 8C).
In the hypothalamus only very light inputs are seen in the anterior, ventromedial, and dorsomedial nuclei, and LHA juxtaparaventricular region, all of which are densely labeled by horizontal limb injections (Fig. 8E–G). In the dorsal premammillary nucleus, the dorsal region and areas surrounding the ventral region are richly innervated—very few boutons are observed within the latter (Fig. 8H). In contrast, the ventral region of the dorsal premammillary nucleus is densely innervated by the LHAsfa horizontal limb.
In the thalamus, the marginal and parvicellular parts of the lateral habenular medial division are densely innervated, along with the magnocellular and parvicellular parts of the lateral division (Fig. 8D).
Control injections
PHAL injections centered in LHA regions outside the border of the LHAsfa were obtained and the main differences with LHAsfa experiments are described briefly because they control for scattered labeled neurons in some experiments with injections centered in the LHAsfa, and because they help clarify the organization of projections from LHA regions surrounding the LHAsfa. In these experiments, very distinct projection pattern are observed to the septal region, amygdalar region, hypothalamus, thalamus, and lower brainstem, as described below and summarized in Table 1.
The LHA juxtaventromedial region (LHAjv)
In Experiment LHA52 the PHAL injection is centered in the rostral end of the LHAjv (just medial to the LHAsfa), although some PHAL-labeled neurons also lie within the LHAsfa (Fig. 2).
At intermediate levels of the lateral septal nucleus a dense terminal field is labeled in the ventral third of the medial region of the LSr.dl (Fig. 9A), with lighter labeling in lateral regions of the LSr.dl that are massively labeled by PHAL injections centered in the LHAsfa horizontal limb. Labeled axons with boutons are also observed in the LSr.m (Fig. 9A), which is only lightly labeled in experiments with injections centered in the LHAsfa. A substantial terminal field is also observed in the interfascicular nucleus of the bed nuclei of the stria terminalis’s posterior division, as well as in the anteromedial area of the anterior division (Fig. 9B). The latter is not labeled in experiments with injections centered in the LHAsfa.
Fig. 9.
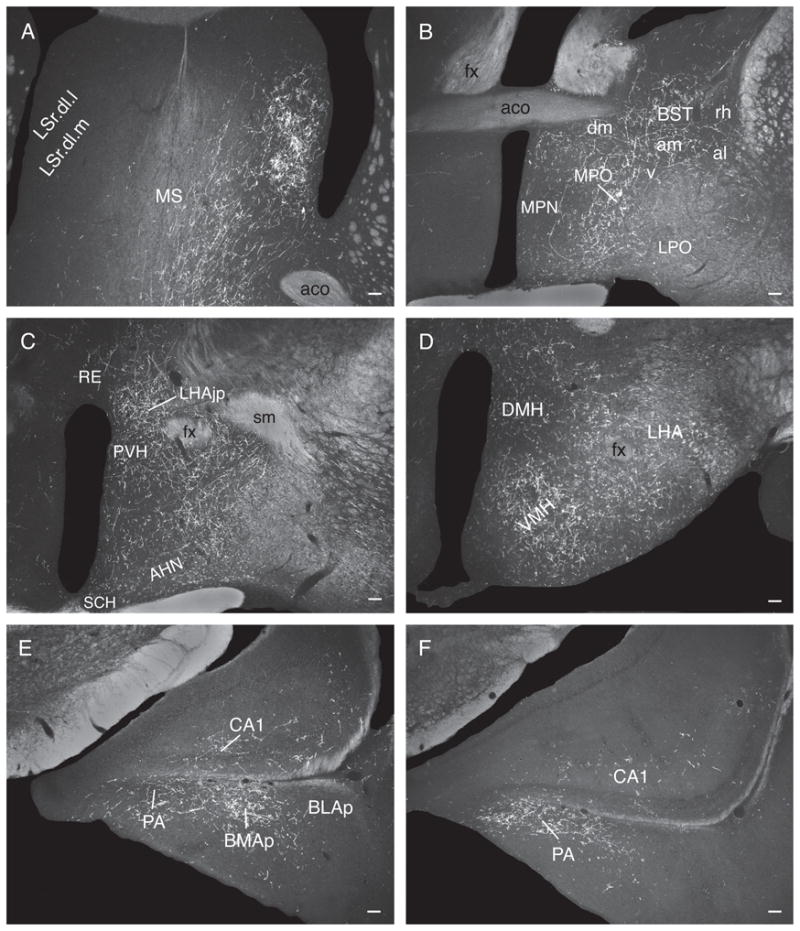
Darkfield photomicrographs showing the distribution of PHAL-labeled axons in experiment LHA52, with a PHAL injection centered just medial to the LHAsfa, rostrolaterally in the LHA’s juxtaventromedial region. (A) LSr.dl.l and LSr.dl.m, and the medial septal nucleus; (B) bed nuclei of the stria terminalis’s anterior division and the medial preoptic area; (C) LHA juxtaparaventricular region; (D) ventromedial hypothalamic nucleus; (E) caudal region of the posterior basomedial nucleus, posterior amygdalar nucleus, and field CA1 of Ammon’s horn; (F) caudal regions of the posterior amygdalar nucleus and field CA1. Note labeling in the bed nuclei’s anterior division, and in the ventromedial nucleus’s central and ventrolateral parts, which are not labeled in LHAsfa experiments—and a dense terminal field in the posterior amygdalar nucleus, which is only lightly labeled in LHAsfa experiments. Scale bars = 100 μm.
In the amygdalar region, a dense terminal field is labeled in the posterior nucleus (Fig. 9F), which is only lightly labeled in LHAsfa experiments, and in caudal regions of the posterior basomedial nucleus (Fig. 9F). The intercalated nucleus is free of labeling, starkly contrasting with the dense terminal fields labeled after LHAsfa injections. Furthermore, a moderate input is observed in temporal regions of hippocampal field CA1 (Fig. 9E,F) and the medial entorhinal area.
In the hypothalamic preoptic region, moderately dense anterograde labeling is seen in the medial preoptic area (Fig. 9B), near the border with the lateral preoptic area (which only contains what appear to be fibers of passage). In LHAsfa experiments only a few axons and boutons are observed in the medial preoptic area, and a moderate to dense terminal field is found in the lateral preoptic area. More caudally, the LHA juxtaparaventricular region is heavily labeled (Fig. 9C), and substantial labeling is also observed in the dorsal zone of the anterior region of the LHA. Moderate labeling is seen in the anterior parvicellular part of the paraventricular nucleus. In the ventromedial nucleus, the rostral pole and the dorsomedial, central, and ventrolateral parts are all heavily labeled (Fig. 9D), whereas in LHAsfa experiments the anterograde labeling is restricted to the rostral pole and dorsomedial part. A moderately dense terminal field occupies the ventral region of the dorsal premammillary nucleus.
In the thalamus, conspicuous anterograde labeling is observed in far medial regions of the mediodorsal nucleus’s medial part (bordering the paraventricular nucleus), and moderate labeling is observed in the paraventricular nucleus and rostral regions of the paratenial nucleus.
Finally, inputs to the periaqueductal gray are denser than after LHAsfa injections. Moderately dense terminal fields occupy the lateral and ventrolateral divisions and moderately dense labeling is also found in the midbrain reticular, cuneiform, and superior central raphé nuclei.
The LHA anterior and dorsal regions (LHAa/d)
In experiment LHA45 the PHAL deposit is centered just lateral to the LHAsfa, in a region that appears to include the caudomedial tip of the LHA anterior region and rostromedial tip of the LHA dorsal region (Fig. 2; Atlas Levels 26 and 27 of Swanson, 2004).
In the lateral septal nucleus, anterograde labeling is densest in the caudal part (Fig. 10A,B), and moderate labeling is seen in the ventrolateral zone of the rostral part. The LSr.dl, which is heavily labeled in LHAsfa experiments, is only lightly innervated. A moderately dense terminal field is also observed in the medial septal nucleus (Fig. 10A).
Fig. 10.
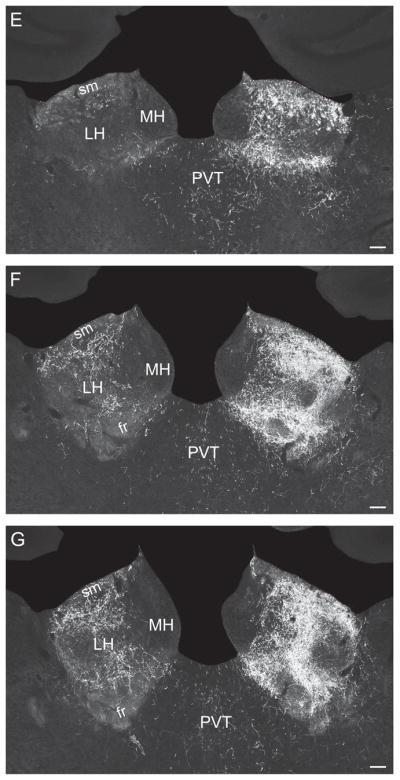
Darkfield photomicrographs showing the distribution of PHAL-labeled axons in experiment LHA45, with a PHAL injection centered just lateral to the LHAsfa, in the LHA. (A) Caudal part of the lateral septal nucleus and medial septal nucleus, (B) caudal part of the lateral septal nucleus and septofimbrial nucleus, (C) lateral preoptic area, (D) dorsal region of the dorsal premammillary nucleus, and (E–G) lateral habenula, arranged from rostral to caudal. Note dense labeling in dorsal regions of the dorsal premammillary nucleus, contrasting with very sparse labeling in ventral regions of the nucleus, and a massive projection to the lateral habenula. Scale bars = 100 μm.
In the amygdalar region, a moderately dense input is observed in the central nucleus’s capsular part, whereas no labeling is seen in the posterior part of the basomedial nucleus, and only light labeling is found in the intercalated nucleus (both heavily labeled by LHAsfa injections).
Moving to the hypothalamus, there is a moderately dense terminal field in the lateral preoptic area, which is most obvious rostrally (Fig. 10A). A moderately dense input occupies the suprafornical region of the LHA, and moderate labeling is seen in the dorsal part of the anterior region of the LHA. Caudally, there is a massive input to the dorsal region of the dorsal premammillary nucleus (Fig. 10D). The posterior hypothalamic nucleus is moderately densely innervated, whereas the ventral and posterior regions of the LHA, and the supramammillary nucleus, receive moderate inputs.
In the thalamus, a massive input is observed in the lateral habenula’s medial and lateral divisions. In the former, the marginal and anterior parts are especially involved, whereas labeling is much lighter in the parvicellular and central parts (Fig. 10E–G). In the lateral division massive terminal fields are labeled in the magnocellular, parvicellular, marginal, and basal parts, with the oval part being avoided (Fig. 10F,G). Moderate inputs are seen in the nucleus reuniens, lateral part of the mediodorsal nucleus, central medial and paraventricular nuclei, and caudal regions of the intermediodorsal nucleus.
Finally, the precommissural nucleus, and the rostromedial, medial, lateral, and ventrolateral divisions, of the periaqueductal gray receive moderately dense inputs.
The LHAsfp
In Experiment LHA22 the PHAL injection is centered just caudal to the LHAsfa, in the LHAsfp, although some labeled neurons extend beyond the limits of the LHAsfp.
The dorsolateral zone of the LSr (heavily labeled in the LHAsfa experiments) contains very few terminals in this experiment, whereas moderate labeling is observed in the medial zone, which is not labeled in LHAsfa experiments. In addition, a moderate terminal field is labeled in caudomedial regions of the nucleus accumbens, commencing a short distance but clear distance below the dorsomedial pole of the nucleus at this level; it is not labeled in LHAsfa experiments. In the bed nuclei of the stria terminalis, the posterior division’s interfascicular and transverse nuclei are clearly labeled (Fig. 11C), and the anterior division’s anteromedial and anterolateral areas, along with the rhomboid, dorsomedial, magnocellular, and ventral nuclei, are densely innervated (Fig. 11B). The anterior division is not labeled in our LHAsfa experiments. In the amygdala, the medial part of the central nucleus is densely innervated (Fig. 11F), and a substantial terminal field is observed in the posterior nucleus. Neither amygdalar terminal field is labeled in the LHAsfa experiments. The intercalated nucleus is only lightly labeled, also in contrast to the LHAsfa experiments.
Fig. 11.
Darkfield photomicrographs showing the distribution of PHAL-labeled axons in experiment LHA22 with a PHAL injection centered caudal to the LHAsfa, in the LHAsfp. (A) Medial preoptic area, and the parastrial, anterodorsal preoptic, and anteroventral preoptic nuclei; (B) anteromedial and anterolateral areas and the rhomboid, dorsomedial, and ventral nuclei of bed nucleus of the stria terminalis’s anterior division; (C) interfascicular and transverse nuclei of bed nuclei’s the posterior division, and the paraventricular hypothalamic nucleus’s anterior parvicellular part; (D) paraventricular hypothalamic nucleus and lateral hypothalamic area; (E) dorsomedial hypothalamic nucleus and PHAL deposit centered in, but not restricted to, the LHAsfp; (F) medial part of the central amygdalar nucleus. Scale bars = 100 μm.
In the hypothalamic preoptic region, heavy terminal fields are labeled in the medial preoptic area and in medial and rostral regions of the lateral preoptic area (Fig. 11A), and substantial inputs are also seen in the anteroventral preoptic, anterodorsal preoptic, and parastrial nuclei (Fig. 11A). Only the lateral preoptic area appears significantly innervated by the LHAsfa. Farther caudally, the paraventricular hypothalamic nucleus’s anterior, medial, and lateral parvicellular parts, and the forniceal part, are densely labeled (Fig. 11C,D), in clear distinction to injections centered in the LHAsfa. The dorsomedial nucleus’s anterior (Fig. 11E) and ventral parts contain rich terminal plexuses. A moderate terminal field is seen in the juxtaventromedial, anterior, suprafornical, and ventral regions of the LHA, as well as in the posterior hypothalamic nucleus. Little or no anterograde labeling is observed in the anterior (Fig. 11D), ventromedial (Fig. 11D), and dorsal premammillary nuclei.
Moderately dense terminal fields are observed in the paraventricular thalamic nucleus and in the lateral habenula medial division’s parvicellular and central parts. More caudally, the periaqueductal gray’s medial and ventrolateral divisions are innervated considerably more substantially than after LHAsfa PHAL injections, and moderately dense inputs are seen in the pontine central gray and in Barrington’s nucleus. In addition, moderate anterograde labeling is observed in the retrorubral area and dorsal raphé nucleus.
Experiments involving the dorsal premammillary nucleus
The results described above indicate that the LHAsfa horizontal and vertical limbs project differentially to the dorsal premammillary nucleus, with the former innervating preferentially the ventral region of the nucleus, and, in contrast, the latter preferentially generating terminals in the dorsal region and around the ventral region. Because we previously analyzed projections from the dorsal premammillary nucleus as a whole (Canteras and Swanson, 1992b), and demonstrated an important role for it in the expression of defensive behavior (Canteras et al., 1997), we next made tiny PHAL injections centered in either dorsal or ventral regions of the nucleus to determine whether they generate distinguishable projection patterns that are subsets of the overall projection pattern.
Figure 12 illustrates two PHAL injection sites, one centered in the dorsal region (Fig. 12A), and the other in the ventral region (Fig. 12B), of the dorsal premammillary nucleus.
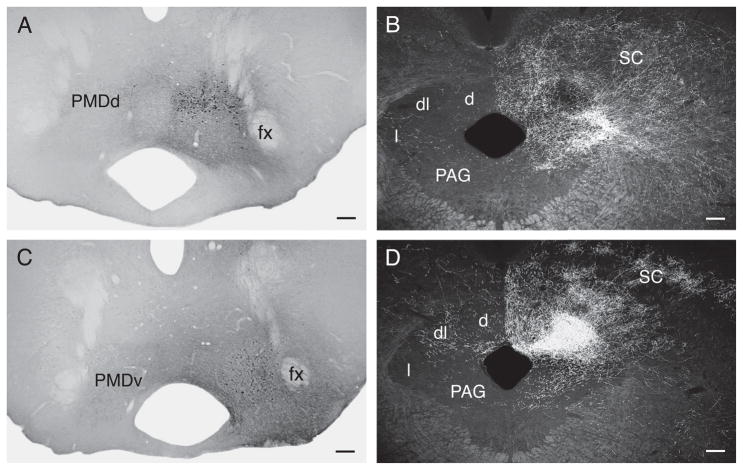
Fig. 12.
(A,C) Brightfield photomicrographs showing the PHAL deposit centered in dorsal (A) and ventral (B) regions of the dorsal premammillary nucleus. (B,D) Darkfield photomicrographs showing the periaqueductal gray terminal fields labeled from the dorsally and ventrally centered injections, respectively. Note heavy labeling in the lateral division, and light labeling in the dorsolateral division, in the experiment with an injection centered dorsally in the nucleus (B), as compared to massive terminal labeling in the dorsolateral division, and lighter labeling in the lateral division, in the experiment with a PHAL deposit centered in ventral regions of the nucleus (D). Scale bars = 100 μm.
The clearest difference between these two experiments is in the periaqueductal gray. Injections centered dorsally label a very dense terminal field in the lateral division, especially in far lateral regions, and in the dorsal division (Fig. 12B). Inputs to the dorsolateral division are lighter and restricted to medial regions (Fig. 12B). In contrast, injections centered ventrally in the dorsal premammillary nucleus label a massive terminal field in the periaqueductal gray’s dorsolateral division, a dense terminal field in the dorsal division, and a lighter terminal field in the lateral division (Fig. 12D).
Finally, ventral regions of the dorsal premammillary project very heavily back to the LHAsfa.
DISCUSSION
Overall, the structural results presented here indicate that the LHAsfa, which was initially distinguished by a circumscribed input from the pontine NI, generates an axonal projection pattern that is distinct from LHA regions immediately lateral, medial, and caudal to it—and that the LHAsfa vertical and horizontal limbs generate topographically organized projections to the septal and amygdalar regions of the cerebral hemisphere on one hand, and to the hypothalamus and thalamus on the other.
An earlier PHAL study (Roeling et al., 1994) analyzed the projections of a related LHA region, Nieuwenhuys and colleagues’s (1982) intermediate hypothalamic area, or hypothalamic “aggression area,” as defined by electrical stimulation. However, the latter is more extensive and only its rostrodorsal region overlaps with the LHAsfa as defined here. Two experiments (207 and 334) mentioned by Roeling et al. (1994) may have been centered in the LHAsfa’s vertical limb, but comparisons with our data are difficult because their results were not illustrated. Nevertheless, comparisons with their results will be made where feasible.
Projections from LHAsfa and adjacent regions
As summarized in Figure 4, a majority of LHAsfa axons innervate forebrain structures, whereas a minority innervate cell groups in the midbrain and pons.
Projections to cerebral hemisphere
Our results indicate that by a wide margin the septal region is the most heavily innervated target of the LHAsfa. Furthermore, the LHAsfa, along with LHA regions immediately medial and lateral to it (the LHAjv and LHAa/d), generate a topographically organized projection to the lateral septal nucleus. The LHAsfa itself massively innervates the LSr.dl, and less densely the LSc—with the horizontal and vertical limbs targeting ventral and dorsal regions of the LSr.dl, respectively. The LHAa/d regions (in our experiments including the caudomedial anterior region and the adjacent rostromedial dorsal region) innervate densely the LSc and less densely the LSr. The LHAjv (in our experiment the rostrolateral LHAjv) innervates more medial regions of the LSr.dl and the LSr.m. The latter is also innervated by the LHAsfp (just caudal to the LHAsfa), which also innervates the ventral zone of the LSc. Roeling and colleagues (1994) described a dense projection from their intermediate LHA to dorsolateral regions of the intermediate part of the lateral septal nucleus. Our results extend their observation by showing that the projections from the LHA to the lateral septal nucleus are topographically organized.
It is clear that the region of the LHAsfa and the LSr.dl share strong bidirectional connections (Luiten et al., 1982; Veening et al., 1987; Staiger and Nürnberger, 1989; Risold et al., 1994; Roeling et al., 1994; Risold and Swanson, 1997b; present results), and it is interesting to note that the LHAsfa projection forms pericellular baskets around LSr.dl neurons (Fig. 5A), which are presumably GABAergic medium spiny stellate neurons (Risold and Swanson, 1997a). A projection from the region of the LHAsfa to the LSc was demonstrated with retrograde labeling (Risold and Swanson, 1997b).
The LHAsfa also projects densely to the medial septal nucleus, which may also project “back” to the LHAsfa (Swanson and Cowan, 1979). A moderately dense projection to the nucleus was observed in PHAL experiments with injections centered in the LHAa/d and LHAjv. The LHAsfp does not seem to innervate the medial septal nucleus. Roeling and colleagues (1994) also reported a dense projection to the medial septal nucleus after injections centered in dorsal regions of their intermediate hypothalamic area.
In the bed nuclei of the terminalis’s posterior division, the LHAsfa innervates moderately the interfascicular and transverse nuclei. The latter are heavily innervated by the LHAjv, which also innervates the bed nuclei anterior division’s anteromedial area. The LHAsfp also heavily innervates the bed nuclei’s anterior division, which is not innervated by the LHAsfa. It should also be mentioned that the LHAsfa generates a moderately dense terminal field in the subjacent substantia innominata, like the LHAa/d and LHAsfp. Roeling and colleagues (1994) reported projections to the BST from their intermediate hypothalamic area that were denser after PHAL injections centered medially.
In the amygdalar region, the LHAsfa clearly innervates the posterior basomedial nucleus and paracapsular region of the intercalated nucleus, with less dense terminal fields in the lateral nucleus, and a few terminal boutons in the medial and central nuclei. The LHAsfa horizontal limb innervates the posterior basomedial and paracapsular intercalated nuclei more heavily than the vertical limb. Ottersen (1980) demonstrated retrograde labeling in the vicinity of the LHAsfa after retrograde tracer injections in an area that included ventral regions of the central and intercalated nuclei. In contrast, the rostrolateral LHAjv does not innervate the paracapsular intercalated nucleus. Instead, dense anterograde labeling is observed in the posterior amygdalar nucleus and caudal regions of the posterior basomedial nucleus. In striking contrast, PHAL injections in the LHAsfp richly innervate the medial part of the central amygdalar nucleus. Similarly, Roeling and colleagues (1994) described projections from medial regions of their intermediate hypothalamic area to the posterior amygdalar nucleus. However, they did not report projections from dorsal regions to the intercalated and basomedial nuclei.
Projections to hypothalamus
In the preoptic region, the LHAsfa innervates moderately the lateral preoptic area. PHAL injections in the LHAa/d label a dense input to the lateral preoptic area, although, in contrast, it is most dense rostrally. Importantly from a topographic point of view, the LHAjv innervates the medial rather than lateral preoptic area—as does the LHAsfp, which also innervates the anteroventral preoptic, anterodorsal preoptic, and parastrial nuclei. In agreement, Roeling and colleagues (1994) described a projection to the medial preoptic area from medial regions of their intermediate LHA.
The major intrahypothalamic target of the LHAsfa is the dorsal premammillary nucleus, bilaterally. Moderately dense terminal fields are also established in the LHA juxtaparaventricular region, anterior hypothalamic nucleus, dorsomedial part of the ventromedial nucleus, and anterior part of the dorsomedial nucleus—and they arise preferentially in the LHAsfa horizontal limb. The LHAsfa-dorsal premammillary nucleus projection was described with anterograde and retrograde methods (Roeling et al., 1994; Comoli et al., 2000). The observations are extended here by showing a topographic organization in the projection. The LHAsfa vertical limb innervates preferentially the dorsal region, and an area surrounding the ventral region, of the dorsal premammillary nucleus, whereas the LHAsfa horizontal wing innervates preferentially the ventral region of the dorsal premammillary nucleus. This is interesting because, as shown here with PHAL, the dorsal and ventral regions of the dorsal premammillary nucleus display a complementary projection pattern to the periaqueductal gray, with the former preferentially innervating the lateral division and the latter preferentially innervating the dorsolateral division (see below). Curiously, the LHAa/d projects densely to the dorsal region of the dorsal premammillary nucleus, whereas the LHAjv projects densely to areas surrounding the ventral region of the dorsal premammillary nucleus.
The LHAsfa projects selectively to the dorsomedial part of the ventromedial nucleus, whereas the LHAjv innervates the entire nucleus. In contrast, the LHAsfp avoids the ventromedial and anterior hypothalamic nuclei and instead richly innervates the paraventricular nucleus, including its anterior, medial, and lateral parvicellular parts—which are not labeled by LHAsfa injections. The LHAa/d only very sparsely innervates the ventromedial and anterior hypothalamic nuclei, instead projecting substantially to the LHA suprafornical region. The latter also receives substantial inputs from the LHAsfp, which also innervates the juxtaventromedial, and the dorsal and ventral zones of the anterior, regions of the LHA. The dorsal zone of the LHA’s anterior region is also an important target of the LHAjv. Roeling and colleagues (1994) reported only sparse to moderate inputs to the LHA from their intermediate hypothalamic area.
A projection from LHAsfa to dorsomedial hypothalamic nucleus was observed with retrograde labeling (Thompson and Swanson, 1998). The LHAsfp projects substantially to the anterior and ventral parts of the dorsomedial hypothalamic nucleus, whereas the LHAjv sends moderate inputs to the anterior part, both of which are only lightly labeled by the LHAa/d. Essentially the same findings were reported by Roeling and colleagues (1994).
Finally, the LHAsfa and LHAjv provide moderate terminal fields to the posterior hypothalamic nucleus, as do the LHAa/d and LHAsfp.
Projections to thalamus
The LHAsfa sends moderately dense inputs to caudal levels of the lateral habenula, somewhat lighter inputs to the paratenial and mediodorsal nuclei, and ventral regions of the nucleus reuniens; and even lighter inputs to the paraventricular, intermediodorsal, and central medial nuclei. The heaviest projection to the lateral habenula arises in the LHAsfa vertical limb. Projections from LHAsfa to the paratenial, paraventricular, mediodorsal, and reuniens nuclei were identified with retrograde tracer methods (Groenewegen, 1988; Chen and Su, 1990; McKenna and Vertes, 2004). Roeling and colleagues (1994) reported dense projections from dorsal regions of their intermediate hypothalamic area to the mediodorsal and paratenial nuclei.
Heavier, topographically organized projections to the lateral habenula’s lateral division arise from the LHAa/d, where they innervate massively more lateral regions of the magnocellular and marginal parts.
Curiously, the region of the LHAjv injected here innervates a conspicuous region lying between the paratenial and mediodorsal nuclei.
Projections to midbrain and pons
The LHAsfa generates light projections to the precommissural nucleus, and to the dorsal, medial, dorsolateral, and lateral divisions of the periaqueductal gray; and moderate projections to caudal regions of the periaqueductal gray’s ventrolateral division, the dorsal and superior central nuclei of the raphé, and the cuneiform nucleus. The periaqueductal gray is innervated more densely by LHA regions immediately surrounding the LHAsfa. Thus, the LHAjv generates moderately dense terminal fields in the lateral and ventrolateral periaqueductal gray divisions; the LHAa/d innervates substantially the precommissural nucleus, and the PAG medial and rostromedial divisions; and the LHAsfp projects heavily to the PAG ventrolateral division and pontine central gray, as well as to Barrington’s nucleus. Moderate projections to the retrorubral area arise in the LHAjv, LHAa/d, and LHAsfp. The latter two also provide moderate innervation to the dorsal raphé nucleus.
Evidence for some of the projections reported here has already been adduced. Following noxious visceral stimulation and retrograde tracer injections in the periaqueductal gray’s ventrolateral division, labeled neurons also exhibiting Fos immunoreactivity were identified in the LHAsfa region (Snowball et al., 2000). Projections from the LHAsfa to the dorsal and superior central nuclei of the raphé were reported with retrograde tracer methods (Behzadi et al., 1990; Peyron et al., 1998), and electrical stimulation of the cuneiform nucleus increases c-fos mRNA levels in the region of the LHAsfa (Lam et al., 1997).
Roeling and colleagues (1994) observed dense projections to the precommissural nucleus and other regions of the periaqueductal gray after PHAL injections in medial regions of their intermediate hypothalamic area. Our experiments confirm that these midbrain cell groups are substantially labeled after PHAL injections centered in LHA regions immediately medial to the LHAsfa.
Functional implications of LHAsfa circuitry
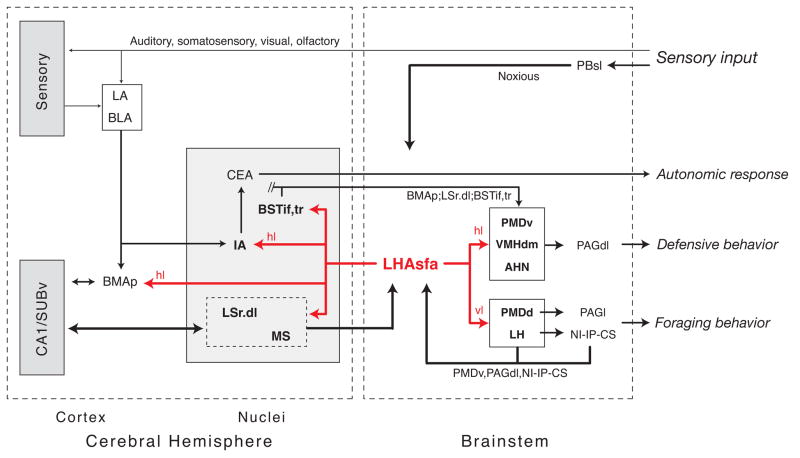
It is instructive to start a brief consideration of the most obvious possible LHAsfa functional significance with an overview of what is known about sources of axonal inputs, which fall into three broad categories (see Fig. 13). First, the LHAsfa was defined originally by a dense, circumscribed input from the NI (Goto et al., 2001; Swanson, 2004), and in this context it is significant that two other brainstem midline nuclei that share bidirectional connections with the NI, and with each other—the superior central (median raphé) and interpeduncular nuclei—also provide clear inputs to the LHAsfa (Groenewegen et al., 1986; Vertes et al., 1999). These three midline nuclei form a highly interconnected network (they are all bidirectionally connected) involved in modulating levels of behavioral activity, arousal, or foraging behavior (for references see Goto et al, 2001).
Fig. 13.
Summary diagram illustrating the major axonal inputs and outputs of the LHAsfa, arranged to emphasize their possible functional significance, as discussed in the text. The overall projection pattern arising from the LHAsfa’s horizontal limb (hl) and vertical limb (vl) is similar, although one or the other obviously projects more densely to certain terminal fields in a topographically ordered way, as indicated. For clarity, all of the connections of cell groups receiving inputs from, or projecting to, the LHAsfa are not shown.
The midline network integrates neural inputs from prefrontal cortex (directly) and hippocampal formation (via a septo-habenular pathway), and is strategically placed to control behavior-gating mechanisms through its axonal projections to mediodorsal thalamic nucleus and prefrontal cortex, hippocampal formation, and nucleus accumbens. In addition, midline network activity may be influenced by corticotropin-releasing hormone (CRH) in the cerebrospinal fluid, through abundant type 1 CRH receptors expressed on NI neurons. It is therefore likely that under certain challenging conditions, the LHAsfa receives information from a midline brainstem neural network involved in modulating behavioral arousal. The LHAsfa may also receive important affective information via direct axonal projections from the ventral region of the dorsal premammillary nucleus (here) and the periaqueductal gray’s dorsolateral division (S.R. Mota-Ortiz and N.S. Canteras, personal observations), which are integral parts of the neural network that mediates innate fear responses (see Canteras, 2002).
To summarize, the strongest “descending” projections of the LHAsfa are to neural networks that control the expression of defensive (also see below) and foraging behaviors, and key parts of this circuitry (the nucleus incertus, dorsolateral periaqueductal gray, and dorsal premammillary nucleus) provide major “feedback” inputs to the LHAsfa.
The only evidence to date for a relatively direct source of sensory information to the LHAsfa involves a direct projection from the parabrachial nucleus’s superior lateral part. It probably relays noxious information, at least in part (Bester et al., 1997).
A third—and strongest—source of input to the LHAsfa is the septal region (Fig. 13). The LSr.dl is the single greatest source of this projection to the LHAsfa (Risold and Swanson, 1997b), and it is important to note that the LSr.dl in turn is the single densest target of LHAsfa projections (Swanson and Cowan, 1979; Roeling et al., 1994; Risold and Swanson, 1997b; present results). The LSr.dl “relays” neural activity from specific regions of hippocampal field CA1, and to a somewhat lesser extent field CA3, that may be related to environmental, spatial, and navigational cues (see Risold and Swanson, 1997b). Thus, specific regions of Ammon’s horn project to the LSr.dl, which then establishes massive bidirectional connections with the LHAsfa. The LHAsfa in turn is positioned to influence septo-hippocampal system activity (see Swanson et al., 1987) by way of a dense projection to the medial septal nucleus, and a less dense projection to the diagonal band nucleus.
LHAsfa interactions with the septo-hippocampal system are complemented by LHAsfa projections to the amygdalar region, in particular the posterior basomedial and intercalated nuclei (Fig. 13). On one hand, the posterior basomedial nucleus shares bidirectional connections with hippocampal field CA1 and the subiculum (Canteras and Swanson, 1992a; Petrovich et al., 1996, 2001; Cenquizca, 2004), and on the other it shares bidirectional connections with the lateral amygdalar nucleus (Krettek and Price, 1978; Pitkanen et al., 1995; Petrovich et al., 1996), thus integrating auditory, visual, and polymodal sensory information from various sources (Canteras and Swanson, 1992a; Romanski and LeDoux, 1993; McDonald and Jackson, 1987; McDonald, 1998). Finally, the posterior basomedial nucleus projects massively to virtually the entire ventromedial hypothalamic nucleus and the medial and capsular parts of the central amygdalar nucleus (Petrovich et al., 1996), positioning it to influence the expression of various reproductive and defensive behaviors (see Swanson, 2000) and autonomic responses (see Loewy, 1991), respectively.
The intercalated nucleus (Millhouse, 1986), another major target of LHAsfa projections, is formed by tiny GABAergic neurons (Nitecka and Ben-Ari, 1987; McDonald and Augustine, 1993; Pare and Smith, 1993; Royer et al., 1999) that receive presumed sensory-related inputs from the basolateral amygdalar complex (Royer et al., 1999), and orbitofrontal, visual, and auditory regions of cerebral cortex (Ghashghaei and Barbas, 2002)—and project to the central amygdalar nucleus (Royer et al., 1999).
The final set of connections with possible functional relevance, based on data currently available, involves intrahypothalamic LHAsfa projections, mentioned above in relation to defensive and foraging behaviors. Interestingly, the experimental PHAL analysis suggests that the LHAsfa horizontal (ventrolateral) and vertical (dorsomedial) limbs preferentially innervate functionally different neural networks.
The LHAsfa horizontal limb preferentially innervates the anterior hypothalamic nucleus, dorsomedial ventromedial nucleus, and ventral dorsal premammillary nucleus—integral components of the medial hypothalamic circuit controlling defensive (fight or flight) behavior (Canteras, 2002), which in turn is part of the rostral behavior control column (Swanson, 2000). As reviewed by Canteras (2002), the LHAsfa is a primary intrahypothalamic source of neural inputs to the behavior control column defensive behavior component, which also receives major inputs from the bed nuclei of the stria terminalis posterior division’s interfascicular and transverse nuclei (Dong and Swanson, 2004) and posterior basomedial amygdalar nucleus (Petrovich et al., 1996)—both of which receive inputs from the LHAsfa. Thus, the LHAsfa is positioned to influence the medial hypothalamic defensive behavior circuit, which appears to mediate behavioral responses to innate threats like exposure to natural predators (Canteras, 2002). Correspondingly, selective lesions including the LSr.dl (which shares strong bidirectional connections with the LHAsfa) are followed by hyperdefensiveness characterized by agonistic responses to the experimenter and hyperreactivity to a variety of sensory stimuli (Albert and Walsh, 1984).
In contrast, the LHAsfa vertical limb projects preferentially to medial regions of the lateral habenula and the dorsal part of the dorsal premammillary nucleus. Lateral habenular lesions disrupt integration of contextual cues and selection of adaptive behavioral responses under stress (Thornton and Evans, 1982, 1984; Thorton et al., 1990), perhaps through projections to the ventral tegmental area, the NI-CS-IPN midline network (see Goto et al, 2001), and other areas. As shown here, dorsal regions of the dorsal premammillary nucleus project strongly to the periaqueductal gray’s lateral division, which has been implicated in the modulation of foraging or exploratory responses (Comoli et al., 2005). In line with this possibility, NMDA lesions of the periaqueductal gray’s lateral division appear to disrupt foraging activity during predatory hunting (S.R. Mota-Ortiz and N.S. Canteras, personal observations).
Overview
Clearly, the LHAsfa is embedded within exceptionally complex circuitry, both structurally and functionally, and only highlights of the apparently relevant literature available today can be reviewed here. As summarized in Figure 13, the LHAsfa is positioned to integrate a complex set of input information from the nociception system, brainstem networks involved in coordinating behavioral responses (particularly foraging and defensive behaviors), and the septo-hippocampal system. On the output side, LHAsfa projections to cerebral hemisphere target especially the hippocampal formation and amygdalar region, and its projections to interbrain target neural networks controlling a variety of behavioral responses related most directly to defense (fight or flight) and foraging (exploration).
Supporting this view, a number of studies implicate LHA regions ventral to the fornix, including the LHAsfa, in controlling defensive behavior because either attack or escape responses can be evoked there by electrical stimulation (Roberts, 1969; Kruk et al., 1983; Lammers et al., 1988a; Kruk, 1991; Roberts and Nagel, 1996). Furthermore, the same general LHA region also has been associated with nondefensive behaviors like upward flight and exploratory behavior that includes sniffing, nosing, looking around, and locomotion (Lammers et al., 1988b; Roberts and Nagel, 1996).
Overall, the present analysis supports the hypothesis that the LHAsfa helps match adaptive autonomic and behavioral responses (either defensive or foraging) to current internal motivational status and external environmental conditions.
Acknowledgments
Grant sponsors: National Institutes of Health (2R01NS16686), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP # 2001/14039-9).
ABBREVIATIONS
- AAA
anterior amygdalar area
- ab
angular bundle
- ACAd
anterior cingulate area, dorsal part
- ACB
nucleus accumbens
- aco
anterior commissure, olfactory limb
- AD
anterodorsal nucleus
- ADP
anterodorsal preoptic nucleus
- AHA
anterior hypothalamic area
- AHN
anterior hypothalamic nucleus
- AHNa
anterior hypothalamic nucleus, anterior part
- AHNc
anterior hypothalamic nucleus, central part
- AHNd
anterior hypothalamic nucleus, dorsal part
- AHNp
anterior hypothalamic nucleus, posterior part
- alv
alveus
- AM
anteromedial nucleus thalamus
- amc
amygdalar capsule
- AMd
anteromedial nucleus thalamus, dorsal part
- AMv
anteromedial nucleus thalamus, ventral part
- AONpv
anterior olfactory nucleus, posteroventral part
- APN
anterior pretectal nucleus
- AQ
cerebral aqueduct
- ARH
arcuate nucleus hypothalamus
- AV
anteroventral nucleus thalamus
- AVP
anteroventral preoptic nucleus
- AVPV
anteroventral periventricular nucleus
- BA
bed nucleus accessory olfactory tract
- BLA
basolateral nucleus amygdala
- BLAa
basolateral nucleus amygdala, anterior part
- BLAp
basolateral nucleus amygdala, posterior part
- BMAa
basomedial nucleus amygdala, anterior part
- BMAp
basomedial nucleus amygdala, posterior part
- bsc
brachium of the superior colliculus
- BST
bed nuclei of the stria terminalis
- BSTal
bed nuclei of the stria terminalis, anterior division, anterolateral area
- BSTam
bed nuclei stria terminalis, anterior division, anteromedial area
- BSTdm
bed nuclei of the stria terminalis, anterior division, dorsomedial nucleus
- BSTfu
bed nuclei of the stria terminalis, anterior division, fusiform nucleus
- BSTif
bed nuclei of the stria terminalis, posterior division, interfascicular nucleus
- BSTju
bed nuclei of the stria terminalis, anterior division, juxtacapsular nucleus
- BSTmg
bed nuclei of the stria terminalis, anterior division, magnocellular nucleus
- BSTov
bed nuclei of the stria terminalis, anterior division, oval nucleus
- BSTpr
bed nuclei of the stria terminalis, posterior division, principal nucleus
- BSTrh
bed nuclei of the stria terminalis, anterior division, rhomboid nucleus
- BSTtr
bed nuclei of the stria terminalis, posterior division, transverse nucleus
- BSTv
bed nuclei of the stria terminalis, anterior division, ventral nucleus
- CA1
field CA1, Ammon’s horn
- CA1slm
field CA1, stratum lacunosum-moleculare
- CA1so
field CA1, stratum oriens [Sala]
- CA1sp
field CA1, pyramidal layer
- CA1spd
field CA1, pyramidal layer, deep
- CA1sps
field CA1, pyramidal layer, superficial
- CA1sr
field CA1, stratum radiatum
- CA2
field CA2, Ammon’s horn
- CA3
field CA3, Ammon’s horn
- CA3slu
field CA3, stratum lucidum
- CA3so
field CA3, stratum oriens
- CA3sp
field CA3, pyramidal layer
- CA3sr
field CA3, stratum radiatum
- cc
corpus callosum, genu
- CEA
central nucleus amygdala
- CEAc
central nucleus amygdala, capsular part
- CEAl
central nucleus amygdala, lateral part
- CEAm
central nucleus amygdala, medial part
- cic
inferior colliculus commissure
- CL
central lateral nucleus thalamus
- CLA
claustrum
- CLI
central linear nucleus raphé
- CM
central medial nucleus thalamus
- COA
cortical amygdalar nucleus
- COAa
cortical amygdalar nucleus, anterior part
- COApl
cortical nucleus amygdala, posterior part, lateral zone
- COApm
cortical nucleus amygdala, posterior part, medial zone
- COM
commissural nucleus, periaqueductal gray
- CP
caudoputamen
- cpd
cerebral peduncle
- csc
commissure superior colliculus
- CS
superior central nucleus raphé
- CSl
superior central nucleus raphé, lateral part
- CSm
superior central nucleus raphé, medial part
- CUN
cuneiform nucleus
- DG
dentate gyrus
- DGlb
dentate gyrus, lateral blade
- DGlb-mo
dentate gyrus, lateral blade-molecular layer
- DGlb-po
dentate gyrus, lateral blade-polymorph layer
- DGlb-sg
dentate gyrus, lateral blade-granule cell layer
- DGmb
dentate gyrus, medial blade
- DMH
dorsomedial hypothalamic nucleus
- DMHa
dorsomedial hypothalamic nucleus, anterior part
- DMHp
dorsomedial nucleus hypothalamus, posterior part
- DMHv
dorsomedial hypothalamic nucleus, ventral part
- DR
dorsal nucleus raphé
- dtd
dorsal tegmental decussation
- DTN
dorsal tegmental nucleus
- ec
external capsule
- em
external medullary lamina thalamus
- ENTm1-6
entorhinal area, medial part, layers 1–6
- ENTmv
entorhinal area, medial part, ventral zone
- EPd
endopiriform nucleus, dorsal part
- EPv
endopiriform nucleus, ventral part
- EW
Edinger-Westphal nucleus
- fa
corpus callosum, anterior forceps
- FF
fields of Forel
- fi
fimbria
- fr
fasciculus retroflexus
- FS
fundus of the striatum
- fx
columns of the fornix
- fxpr
precommissural fornix
- GPe
globus pallidus, external segment
- GPi
globus pallidus, internal segment
- hf
hippocampal fissure
- IA
intercalated nuclei amygdala
- IAD
interanterodorsal nucleus thalamus
- IAM
interanteromedial nucleus thalamus
- IC
inferior colliculus
- IF
interfascicular nucleus raphé
- IGL
lateral geniculate complex, intergeniculate leaflet
- III
oculomotor nucleus
- ILA
infralimbic cortical area
- IMD
intermediodorsal nucleus thalamus
- INC
interstitial nucleus of Cajal
- int
internal capsule
- IPN
interpeduncular nucleus
- IPNc
interpeduncular nucleus, central subnucleus
- IPNlr
interpeduncular nucleus, lateral subnucleus, rostral part
- IPNr
interpeduncular nucleus, rostral subnucleus
- IV
trochlear nucleus
- IVn
trochlear nerve
- LA
lateral amygdalar nucleus
- LC
locus ceruleus
- LD
lateral dorsal nucleus thalamus
- LDT
laterodorsal tegmental nucleus
- LGd
lateral geniculate complex, dorsal part
- LGvl
lateral geniculate complex, ventral part, lateral zone
- LGvm
lateral geniculate complex, ventral part, medial zone
- LH
lateral habenula
- LHA
lateral hypothalamic area
- LHAad
lateral hypothalamic area, anterior region, dorsal zone
- LHAai
lateral hypothalamic area, anterior region, intermediate zone
- LHAav
lateral hypothalamic area, anterior region, ventral zone
- LHAd
lateral hypothalamic area, dorsal region
- LHAjd
lateral hypothalamic area, juxtadorsomedial region
- LHAjp
lateral hypothalamic area, juxtaparaventricular region
- LHAjv
lateral hypothalamic area, juxtaventromedial region
- LHAjvd
lateral hypothalamic area, juxtaventromedial region, dorsal zone
- LHAjvv
lateral hypothalamic area, juxtaventromedial region, ventral zone
- LHAm
lateral hypothalamic area, magnocellular nucleus
- LHAp
lateral hypothalamic area, posterior region
- LHApc
lateral hypothalamic area, parvicellular region
- LHAs
lateral hypothalamic area, suprafornical region
- LHAsf
lateral hypothalamic area, subfornical region
- LHAvl
lateral hypothalamic area, ventral region, lateral zone
- LHAvm
lateral hypothalamic area, ventral region, medial zone
- LM
lateral mammillary nucleus
- lot
lateral olfactory tract
- LP
lateral posterior nucleus thalamus
- LPO
lateral preoptic area
- LS
lateral septal nucleus
- LSc
lateral septal nucleus, caudal part
- LSc.d
lateral septal nucleus, caudal part, dorsal zone
- LSc.v
lateral septal nucleus, caudal part, ventral zone
- LSc.v.l
lateral septal nucleus, caudal part, ventral zone, lateral region
- LSc.v.l.d
lateral septal nucleus, caudal part, ventral zone, lateral region, dorsal domain
- LSc.v.l.v
lateral septal nucleus, caudal part, ventral zone, lateral region, ventral domain
- LSc.v.m.d
lateral septal nucleus, caudal part, ventral zone, medial region, dorsal domain
- LSc.v.m.v
lateral septal nucleus, caudal part, ventral zone, medial region, ventral domain
- LSr
lateral septal nucleus, rostral part
- LSr.dl
lateral septal nucleus, rostral part, dorsolateral zone
- LSr.dl.l
lateral septal nucleus, rostral part, dorsolateral zone, lateral region
- LSr.dl.l.d
lateral septal nucleus, rostral part, dorsolateral zone, lateral region, dorsal domain
- LSr.dl.l.v
lateral septal nucleus, rostral part, dorsolateral zone, lateral region, ventral domain
- LSr.dl.m
lateral septal nucleus, rostral part, dorsolateral zone, medial region
- LSr.dl.m.d
lateral septal nucleus, rostral part, dorsolateral zone, medial region, dorsal domain
- LSr.dl.m.v
lateral septal nucleus, rostral part, dorsolateral zone, medial region, ventral domain
- LSr.m
lateral septal nucleus, rostral part, medial zone
- LSr.m.d
lateral septal nucleus, rostral part, medial zone, dorsal region
- LSr.m.v.c
lateral septal nucleus, rostral part, medial zone, ventral region, caudal domain
- LSr.m.v.r
lateral septal nucleus, rostral part, medial zone, ventral region, rostral domain
- LSr.vl.d.l
lateral septal nucleus, rostral part, ventrolateral zone, dorsal region, lateral domain
- LSr.vl.d.m
lateral septal nucleus, rostral part, ventrolateral zone, dorsal region, medial domain
- LSr.vl.v
lateral septal nucleus, rostral part, ventrolateral zone, ventral region
- LSv
lateral septal nucleus, ventral part
- MA
magnocellular preoptic nucleus
- MD
mediodorsal nucleus thalamus
- MDc
mediodorsal nucleus thalamus, central part
- MDl
mediodorsal nucleus thalamus, lateral part
- MDm
mediodorsal nucleus thalamus, medial part
- MEA
medial nucleus amygdala
- MEAad
medial amygdalar nucleus, anterodorsal part
- MEAav
medial amygdalar nucleus, anteroventral part
- MEPO
median preoptic nucleus
- MEV
midbrain nucleus of the trigeminal
- MGm
medial geniculate complex, medial part
- MGv
medial geniculate complex, ventral part
- MH
medial habenula
- ml
medial lemniscus
- MM
medial mammillary nucleus, body
- MMme
medial mammillary nucleus, median part
- MOp
primary motor area
- moV
motor root of the trigeminal nerve
- mp
mammillary peduncle
- MPN
medial preoptic nucleus
- MPO
medial preoptic area
- MPT
medial pretectal area
- MRN
midbrain reticular nucleus
- MRNp
midbrain reticular nucleus, parvicellular part
- MS
medial septal nucleus
- mtt
mammillothalamic tract
- NB
nucleus brachium inferior colliculus
- ND
nucleus of Darkschewitsch
- NDB
diagonal band nucleus
- NI
nucleus incertus
- NIc
nucleus incertus, compact part
- NId
nucleus incertus, diffuse part
- NLL
nucleus of the lateral lemniscus
- NPC
nucleus of the posterior commissure
- och
optic chiasm
- OP
olivary pretectal nucleus
- opt
optic tract
- OT
olfactory tubercle
- PA
posterior nucleus amygdala
- PAA
piriform-amygdalar area
- PAG
periaqueductal gray
- PAGd
periaqueductal gray, dorsal division
- PAGdl
periaqueductal gray, dorsolateral division
- PAGl
periaqueductal gray, lateral division
- PAGm
periaqueductal gray, medial division
- PAGrl
periaqueductal gray, rostrolateral division
- PAGrm
periaqueductal gray, rostromedial division
- PAGvl
periaqueductal gray, ventrolateral division
- PAR1-6
parasubiculum, layers 1–6
- PARN
parvicellular reticular nucleus
- PBl
parabrachial nucleus, lateral part
- PBlc
parabrachial nucleus, central lateral part
- PBlv
parabrachial nucleus, ventral lateral part
- PBmm
parabrachial nucleus, medial medial part
- pc
posterior commissure
- PCG
pontine central gray
- PCN
paracentral nucleus thalamus
- PF
parafascicular nucleus
- PH
posterior hypothalamic nucleus
- PIR
piriform area
- PL
prelimbic cortical area
- pm
principal mammillary tract
- PMD
dorsal premammillary nucleus
- PMDd
dorsal premammillary nucleus, dorsal region
- PMDv
dorsal premammillary nucleus, ventral region
- PMv
ventral premammillary nucleus
- PO
posterior complex thalamus
- PP
peripeduncular nucleus
- PPN
pedunculopontine nucleus
- PR
perireuniens nucleus
- PRC
precommissural nucleus, periaqueductal gray
- PRE1-6
presubiculum, layers 1–6
- PRNc
pontine reticular nucleus, caudal part
- PRNr
pontine reticular nucleus, rostral part
- PS
parastrial nucleus
- PST
preparasubthalamic nucleus
- PSTN
parasubthalamic nucleus of the lateral hypothalamic area
- PSV
principal sensory nucleus of the trigeminal
- PT
paratenial nucleus
- PVa
periventricular hypothalamic nucleus, anterior part
- PVH
paraventricular hypothalamic nucleus
- PVHap
paraventricular hypothalamic nucleus, anterior parvicellular part
- PVHf
paraventricular nucleus hypothalamus, forniceal part
- PVHlp
paraventricular hypothalamic nucleus, lateral parvicellular part
- PVHmpd
paraventricular hypothalamic nucleus, medial parvicellular part, dorsal zone
- PVHpml
paraventricular hypothalamic nucleus, posterior magnocellular part, lateral zone
- PVHpv
paraventricular hypothalamic nucleus, periventricular part
- PVi
periventricular hypothalamic nucleus, intermediate part
- PVp
periventricular hypothalamic nucleus, posterior part
- PVT
paraventricular thalamic nucleus
- py
pyramid
- RCH
retrochiasmatic area, lateral hypothalamic area
- RE
nucleus reuniens
- REd
nucleus reuniens, rostral division, dorsal part
- REm
nucleus reuniens, rostral division, median part
- REv
nucleus reuniens, rostral division, ventral part
- RH
rhomboid nucleus
- RL
rostral linear nucleus raphé
- RM
nucleus raphé magnus
- RN
red nucleus
- RPO
nucleus raphé pontis
- RR
midbrain reticular nucleus, retrorubral area
- RT
reticular nucleus thalamus
- rust
rubrospinal tract
- SC
superior colliculus
- SCdg
superior colliculus, deep gray layer
- SCdw
superior colliculus, deep white layer
- SCH
suprachiasmatic nucleus
- SCig
superior colliculus, intermediate gray layer
- SCiw
superior colliculus, intermediate white layer
- SCop
superior colliculus, optic layer
- scp
superior cerebellar peduncle
- SCsg
superior colliculus, superficial gray layer
- sctv
ventral spinocerebellar tract
- SCzo
superior colliculus, zonal layer
- SF
septofimbrial nucleus
- SGN
suprageniculate nucleus
- SH
septohippocampal nucleus
- SI
substantia innominata
- SLC
subceruleus nucleus
- sm
stria medullaris
- smd
supramammillary decussation
- SMT
submedial nucleus thalamus
- SNc
substantia nigra, compact part
- SNr
substantia nigra, reticular part
- SO
supraoptic nucleus
- SPFm
subparafascicular nucleus thalamus, magnocellular part
- SPFpl
subparafascicular nucleus thalamus, parvicellular part, lateral division
- SPFpm
subparafascicular nucleus thalamus, parvicellular part, medial division
- st
stria terminalis
- STN
subthalamic nucleus
- SUBv
subiculum, ventral part
- SUBv-sp
subiculum, ventral part, pyramidal layer
- SUMl
supramammillary nucleus, lateral part
- SUMm
supramammillary nucleus, medial part
- sup
supraoptic commissures
- SUT
supratrigeminal nucleus
- SUV
superior vestibular nucleus
- TMv
tuberomammillary nucleus, ventral part
- TR
postpiriform transition area
- tsp
tectospinal pathway
- TTd
tenia tecta, dorsal part
- TTv
tenia tecta, ventral part
- TU
tuberal nucleus
- TUi
tuberal nucleus, intermediate part
- TUl
tuberal nucleus, lateral part
- TUsv
tuberal nucleus, subventricular part
- TUte
tuberal nucleus, terete subnucleus
- V3
third ventricle
- V4
fourth ventricle
- VAL
ventral anterior-lateral complex thalamus
- vhc
ventral hippocampal commissure
- VL
lateral ventricle
- vlt
ventrolateral hypothalamic tract
- VM
ventral medial nucleus thalamus
- Vma
motor nucleus of the trigeminal, magnocellular part
- VMH
ventromedial nucleus hypothalamus
- VMHa
ventromedial nucleus hypothalamus, anterior part
- VMHc
ventromedial nucleus hypothalamus, central part
- VMHdm
ventromedial nucleus hypothalamus, dorsomedial part
- VMHvl
ventromedial nucleus hypothalamus, ventrolateral part
- VPL
ventral posterolateral nucleus thalamus
- VPLpc
ventral posterolateral nucleus thalamus, parvicellular part
- VPM
ventral posteromedial nucleus thalamus
- VPMpc
ventral posteromedial nucleus thalamus, parvicellular part
- VTA
ventral tegmental area
- vtd
ventral tegmental decussation
- VTN
ventral tegmental nucleus
- ZI
zona incerta
- ZIda
zona incerta, dopaminergic group
LITERATURE CITED
- Albert DJ, Walsh ML. Neural systems and the inhibitory modulation of agonistic behavior: a comparison of mammalian species. Neurosci Biobehav Rev. 1984;8:5–24. doi: 10.1016/0149-7634(84)90017-4. [DOI] [PubMed] [Google Scholar]
- Andres KH, Andres KH, von During M, Veh RW. Subnuclear organization of the rat habenular complexes. J Comp Neurol. 1999;407:130–150. doi: 10.1002/(sici)1096-9861(19990428)407:1<130::aid-cne10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Behzadi G, Kalén P, Behzadi G, Kalen P, Parvopassu F, Wiklund L. Afferents to the median raphe nucleus of the rat: retrograde cholera toxin and wheat germ conjugated horseradish peroxidase tracing, and selective D-[3H]aspartate labelling of possible excitatory amino acid inputs. Neuroscience. 1990;37:77–100. doi: 10.1016/0306-4522(90)90194-9. [DOI] [PubMed] [Google Scholar]
- Bester H, Besson JM, Bernard JF. Organization of efferent projections from the parabrachial area to the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1997;383:245–281. doi: 10.1002/(sici)1096-9861(19970707)383:3<245::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Chiavegatto S, Ribeiro do Valle LE, Swanson LW. Severe reduction of defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res Bull. 1997;44:297–305. doi: 10.1016/s0361-9230(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Connections of the posterior nucleus of the amygdala. J Comp Neurol. 1992;302:143–179. doi: 10.1002/cne.903240203. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992a;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. The dorsal premammillary nucleus: an unusual component of the mammillary body. Proc Natl Acad Sci USA. 1992b;89:10089–10093. doi: 10.1073/pnas.89.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenquizca LA. PhD thesis. Los Angeles, CA: Neuroscience Program, University of Southern California; 2004. A PHAL analysis of the efferent connections of the hippocampal field CA1. [Google Scholar]
- Chen S, Su HS. Afferent connections of the thalamic paraventricular and parataenial nuclei in the rat—a retrograde tracing study with iontophoretic application of Fluoro-Gold. Brain Res. 1990;522:1–6. doi: 10.1016/0006-8993(90)91570-7. [DOI] [PubMed] [Google Scholar]
- Comoli E, Ribeiro-Barbosa ER, Canteras NS. Afferent connections of the dorsal premammillary nucleus. J Comp Neurol. 2000;423:83–98. doi: 10.1002/1096-9861(20000717)423:1<83::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Comoli E, Ribeiro-Barbosa ER, Negrao N, Goto M, Canteras NS. Functional mapping of the prosencephalic systems involved in organizing predatory behavior in rats. Neuroscience. 2005;130:1055–1067. doi: 10.1016/j.neuroscience.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004;471:396–433. doi: 10.1002/cne.20002. Erratum in: J Comp Neurol 474:603–604. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1984;290:219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Goto M, Swanson LW, Canteras NS. Connections of the nucleus incertus. J Comp Neurol. 2001;438:86–122. doi: 10.1002/cne.1303. [DOI] [PubMed] [Google Scholar]
- Goto M. PhD thesis. São Paulo, Brazil: Institute of Psychology, University of São Paulo; 1998. Hodological and functional study of the nucleus incertus and pars alpha of the pontine periventricular gray. [Google Scholar]
- Groenewegen HJ, Ahlenius S, Haber SN, Kowall NW, Nauta WJH. Cytoarchitecture, fiber connections, and some histochemical aspects of the interpeduncular nucleus in the rat. J Comp Neurol. 1986;249:65–102. doi: 10.1002/cne.902490107. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L. Protein A, avidin and biotin in immunohistochemistry. J Histochem Cytochem. 1981;29:1349–1353. doi: 10.1177/29.11.6172466. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observation on intra-amygdaloid axonal connections. J Comp Neurol. 1978;178:255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- Kruk MR. Ethology and pharmacology of hypothalamic aggresssion in the rat. Neurosci Biobehav Rev. 1991;15:527–538. doi: 10.1016/s0149-7634(05)80144-7. [DOI] [PubMed] [Google Scholar]
- Kruk MR, van der Poel AM, Meelis W, Hermans J, Mostert PG, Mos J, Lohman AHM. Discriminant analysis of the localization of aggression-inducing electrode placements in the hypothalamus of the male rats. Brain Res. 1983;260:61–79. doi: 10.1016/0006-8993(83)90764-3. [DOI] [PubMed] [Google Scholar]
- Lam W, Gundlach AL, Verberne AJM. Neuronal activation in the forebrain following electrical stimulation of the cuneiform nucleus in the rat: hypothalamic expression of c-fos and ngfi-a messenger RNA. Neuroscience. 1997;78:1069–1085. doi: 10.1016/s0306-4522(96)00527-1. [DOI] [PubMed] [Google Scholar]
- Lammers JHCM, Kruk MR, Meelis W, van der Poel AM. Hypothalamic substrates for brain stimulation-induced attack, teeth-chattering and social grooming in the rat. Brain Res. 1988a;449:311–327. doi: 10.1016/0006-8993(88)91046-3. [DOI] [PubMed] [Google Scholar]
- Lammers JHCM, Kruk MR, Meelis W, van der Poel AM. Hypothalamic substrates for brain stimulation-induced patterns of locomotion and escape jumps in the rat. Brain Res. 1988b;449:294–310. doi: 10.1016/0006-8993(88)91045-1. [DOI] [PubMed] [Google Scholar]
- Loewy AD. Forebrain nuclei involved in autonomic control. Prog Brain Res. 1991;87:253–268. doi: 10.1016/s0079-6123(08)63055-1. [DOI] [PubMed] [Google Scholar]
- Luiten PG, Kuipers F, Schuitmaker H. Organization of diencephalic and brainstem afferent projections to the lateral septum in the rat. Neurosci Lett. 1982;30:211–216. doi: 10.1016/0304-3940(82)90401-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience. 1993;52:281–294. doi: 10.1016/0306-4522(93)90156-a. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Jackson TR. Amygdaloid connections with posterior insular and temporal cortical areas in the rat. J Comp Neurol. 1987;262:59–77. doi: 10.1002/cne.902620106. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol. 2004;480:115–142. doi: 10.1002/cne.20342. [DOI] [PubMed] [Google Scholar]
- Millhouse OE. The intercalated cells of the amygdala. J Comp Neurol. 1986;247:246–271. doi: 10.1002/cne.902470209. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Geeraedts LM, Veening JG. The medial forebrain bundle of the rat. I. General introduction. J Comp Neurol. 1982;206:49–81. doi: 10.1002/cne.902060106. [DOI] [PubMed] [Google Scholar]
- Nitecka L, Ben-Ari Y. Distribution of GABA-like immunoreactivity in the rat amygdaloid complex. J Comp Neurol. 1987;266:45–55. doi: 10.1002/cne.902660105. [DOI] [PubMed] [Google Scholar]
- Olucha-Bordonau FE, Teruel V, Barcia-Gonzalez J, Ruiz-Torner A, Valverde-Navarro AA, Martinez-Soriano F. Cytoarchitecture and efferent projections of the nucleus incertus of the rat. J Comp Neurol. 2003;464:62–97. doi: 10.1002/cne.10774. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Afferent connections to the amygdaloid complex of the rat and cat: II. Afferents from the hypothalamus and the basal telencephalon. J Comp Neurol. 1980;194:267–289. doi: 10.1002/cne.901940113. [DOI] [PubMed] [Google Scholar]
- Pare D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57:1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Risold PY, Swanson LW. Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1996;374:387–420. doi: 10.1002/(SICI)1096-9861(19961021)374:3<387::AID-CNE6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Forebrain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Stefanacci L, Farb CR, Go GG, LeDoux JE, Amaral DG. Intrinsic connections of the rat amygdaloid complex: projections originating in the lateral nucleus. J Comp Neurol. 1995;356:288–310. doi: 10.1002/cne.903560211. [DOI] [PubMed] [Google Scholar]
- Risold PY, Canteras NS, Swanson LW. Organization of projections from the anterior hypothalamic nucleus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:1–40. doi: 10.1002/cne.903480102. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Chemoarchitecture of the rat lateral septal nucleus. Brain Res Rev. 1997;24:91–113. doi: 10.1016/s0165-0173(97)00008-8. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Rev. 1997;24:115–195. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Roberts WW. Are hypothalamic motivational mechanisms functionally and anatomically specific? Brain Behav Evol. 1969;2:317–342. [Google Scholar]
- Roberts WW, Nagel J. First order projections activated by stimulation of hypothalamic sites eliciting attack and flight in rats. Behav Neuroscience. 1996;110:509–527. doi: 10.1037//0735-7044.110.3.509. [DOI] [PubMed] [Google Scholar]
- Roeling TA, Veening JG, Kruk MR, Peters JP, Vermelis ME, Nieuwenhuys R. Efferent connections of the hypothalamic “aggression area” in the rat. Neuroscience. 1994;59:1001–1024. doi: 10.1016/0306-4522(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticortical and corticoamygdaloid projections of temporal cortex in the rat. Cerebral Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowball RK, Semenenko FM, Lumb BM. Visceral inputs to neurons in the anterior hypothalamus including those that project to the periaqueductal gray: a functional anatomical and electrophysiological study. Neuroscience. 2000;99:351–361. doi: 10.1016/s0306-4522(00)00203-7. [DOI] [PubMed] [Google Scholar]
- Staiger JF, Nurnberger F. Pattern of afferents to the lateral septum in the guinea pig. Cell Tissue Res. 1989;257:471–490. doi: 10.1007/BF00221457. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. 3. Amsterdam: Elsevier; 2004. [Google Scholar]
- Swanson LW, Cowan WM. The connections of the septal region in the rat. J Comp Neurol. 1979;186:621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Köhler C, Björklund A. The limbic region. I. The septo-hippocampal system. In: Hökfelt T, Björklund A, Swanson LW, editors. Handbook of Chemical Neuroanatomy, vol. 5, Integrated systems of the CNS, part 1. Amsterdam: Elsevier; 1987. pp. 125–277. [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Thornton EW, Evans JA. The role of the habenular nuclei in the selection of behavioral strategies. Physiol Psychol. 1982;10:361–367. [Google Scholar]
- Thornton EW, Evans JA. The effects of lesions of the habenula nucleus on lever press behaviour during a tandem operant schedule with contrasting response requirements. Behav Brain Res. 1984;12:327–334. doi: 10.1016/0166-4328(84)90158-x. [DOI] [PubMed] [Google Scholar]
- Thornton EW, Bradbury GE, Davies C. Increased immobility in an automated forced swimming test following lesion of the habenula in rats: absence of evidence for a contribution from motor impairment. Behav Neurosci. 1990;104:37–43. doi: 10.1037//0735-7044.104.1.37. [DOI] [PubMed] [Google Scholar]
- Veening JG, Te Lie S, Posthuma P, Geeraedts LM, Nieuwenhuys R. A topographical analysis of the origin of some efferent projections from the lateral hypothalamic area in the rat. Neuroscience. 1987;22:537–551. doi: 10.1016/0306-4522(87)90351-4. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]