Abstract
The role of iron in the pathogenesis of Parkinson’s disease (PD) has been implicated strongly due to generation of oxidative stress leading to dopamine cell death. In our overall goal to develop bifunctional/multifunctional drugs, we designed dopamine D2/D3 agonist molecules with a capacity to bind to iron. Binding assays were carried out with HEK-293 cells expressing either D2 or D3 receptors with tritiated spiperone to evaluate inhibition constants (Ki). Functional activity of selected compounds was carried out with GTPγS binding assay. SAR results identified compounds (+)-19a and (−)-19b as two potent agonists for both D2 and D3 receptors (EC50 (GTPγS); D2 = 4.51 and 1.69 nM and D3 = 1.58 and 0.74 nM for (−)-19b and (+)-19a, respectively). In vitro complexation studies with 19b demonstrated efficient chelation with iron. Furthermore, the deoxyribose assay with 19b demonstrated potent antioxidant activity. In PD animal model study, (−)-19b exhibited potent in vivo activity in reversing locomotor activity in reserpinized rats and also in producing potent rotational activity in 6-OHDA lesioned rats. This reports initial development of unique lead molecules which might find potential use in symptomatic and neuroprotective treatment of PD.
Introduction
Parkinson’s disease (PD) is a progressive disorder of the central nervous system that mainly affects motor and other functions1. The cardinal clinical features of Parkinson’s disease (PD) include resting tremor, rigidity, difficulty in initiating movement and postural instability.1, 2 These symptoms develop as a result of slow degeneration of dopamine neuron in the substantia niagra resulting in production of less and less dopamine. The loss of dopaminergic neurons in the pars compacta region of the substantia nigra and the inhibition of nigrostriatal dopaminergic pathway results in development of dysfunction of movements. Even though the pathogenesis of PD is poorly understood, studies of the genetic mutations, neuropathology and other factors of PD have provided much insight into the pathophysiology of PD and the progression of this disease.3, 4 Both oxidative stress and mitochondrial dysfunction have been strongly implicated in cell death5–7. The presence of Lewy bodies (LBs) in the surviving neurons of the substantia nigra is the neuropathological hallmark of PD.8, 9 The physical characteristics of LBs are round, eosinophilic, intracytoplasmic proteinaceous inclusions and they are found to contain principally polymeric α-synuclein proteins.9
Evidence from various studies have consistently implicated iron in the pathophysiology of PD. Iron being the most abundant metal in human body is particularly found in higher level in the brain and lever. The role of iron in the pathogenesis of PD has been strengthened by several observations 10–12. Higher levels of iron is generally found in the brains of PD patients compared to normal brain13. Additionally, iron accumulation observed is higher in the substantia nigra region of people afflicted with PD.12, 14 It is well known that free iron plays a role in generating of oxidative stress leading to dopamine cell death. The generation of hydroxyl radical from free iron occurs by the Fenton reaction (Equation 1). It has also been shown that the presence of iron can initiate aggregation of alpha-synuclein in LBs, implicated in dopamine cell death. The aggregation of alpha-synuclein possibly takes place via conversion of this molecule into β-pleated sheet. Recent studies have shown that overexpression of α-synuclein can form toxic aggregates in the presence of iron.15 This is believed to contribute to the formation of LBs via production of oxidative stress.16 Iron released from neuromelanin has also been reported to cause mitochondrial dysfunction and to reduce proteasomal function.17 All these evidences have further been corroborated by the fact that iron chelators are neuroprotective18. Thus, a crucial role of iron in PD pathogenesis has been emphasized because of its capacity to enhance the production of oxygen radicals and accelerate neuronal degeneration.19 Oxidative stress can facilitate mutant protein aggregation, mimicking proteasomal malfunction.20 Thus, iron chelators can possibly sequester free iron and thereby prevent its ability to induce oxidative stress as a consequence of reactive hydroxyl radical generation21.
| (Equation 1) |
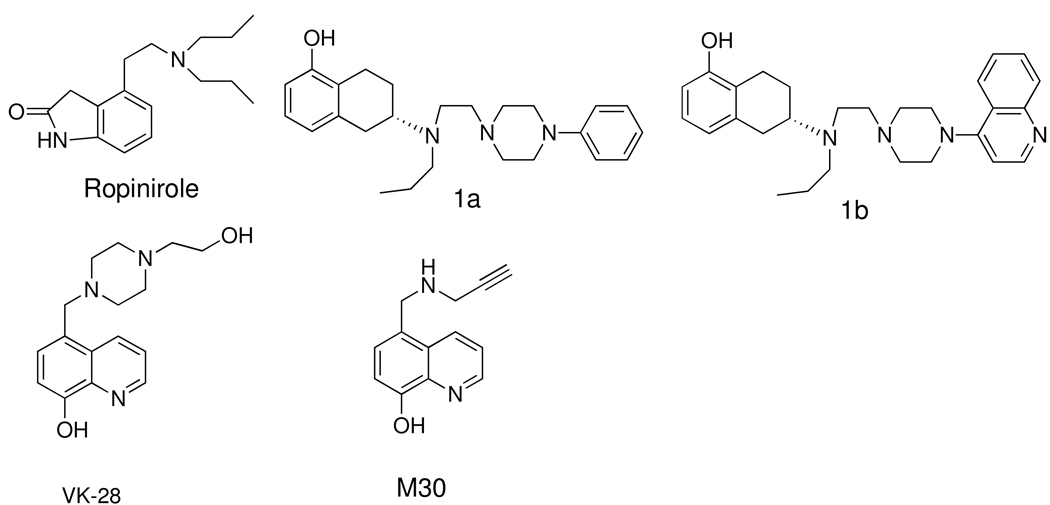
Recently, bifunctional iron chelators were developed where an iron binding 8-hydroxy quinoline moiety was attached to N-propargyl amine and to the piperazine moiety to provide neuprotective property via reduction of oxidative stress. Two such compounds, 5-((4-(2-hydroxyethyl)piperazin-1-yl)methyl)quinolin-8-ol (VK 28) and 5-((prop-2-ynylamino)methyl)quinolin-8-ol (M30), were shown to be antioxidant and neuroprotective in animal experiments.22, 23
Given the important role of iron in PD, our overall goal is to develop bifunctional/multifunctional dopamine D2/D3 agonist molecules with a capacity to bind to iron. This paper reports on introducing a metal binding 8-hydroxy quinoline moiety into the piperazine ring of the hybrid template which we developed previously.24–26 Compounds e.g. 1a (D-237) and 1b (D-354), derived from this hybrid template displayed agonist interaction with dopamine D2/D3 receptors. Our hypothesis behind designing these bivalent molecules originates from the idea that by maintaining the hybrid template we should be able to retain affinity for dopamine receptors while the hydroxy quinoline moiety, being located at a distal position with respect to the agonist binding moiety, will participate in binding to iron in the brain without having an impact on agonist activity. In this regard, extensive data is available on metal binding capacity of 8-hydroxy quinoline and its derivatives.27, 28 For proof of concept, following their initial design, these molecules were prepared as described in synthesis Scheme 1– Scheme 4. We hypothesized that compound with dopamine D2/D3 agonist activity along with a capacity to chelate with iron will not only alleviate motor dysfunction in PD but will also reduce oxidative stress leading to greater survival of dopamine neurons. Therefore, this approach might provide more desirable therapeutic agents which may slow or even halt the progression of dopamine cell death in PD along with restoration of motor dysfunction.
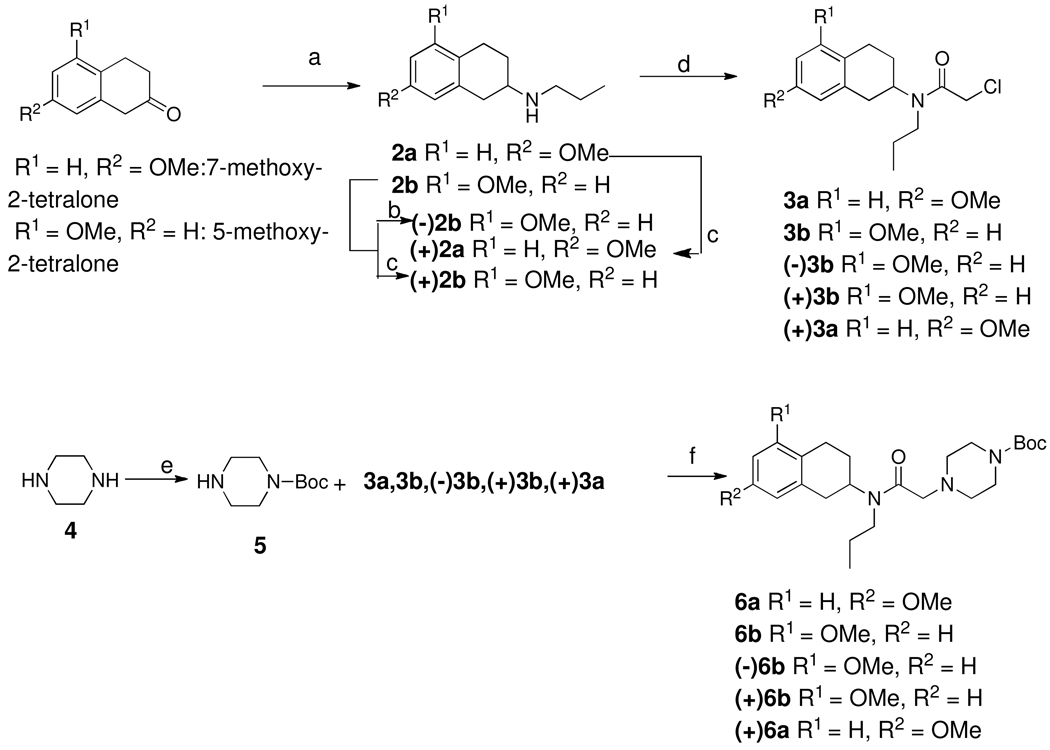
Scheme 1.
a. n-Propylamine, NaCNBH3, CH3COOH, dichloroethane, RT, overnight; b. (+)-chlocyphos, EtOH; c. (−)-chlocyphos, EtOH; d. chloroacetyl chloride, TEA, dichloromethane, 0 °C, 30 min; e. (Boc)2O, CH2Cl2, 0 °C, 2 h; f. K2CO3, CH3CN, 80 °C, 2 h; g. TFA/DCM (1/1), RT, overnight; h. LiAlH4, THF, reflux, 2 h; i. BBr3, −40 °C, CH2Cl2, overnight
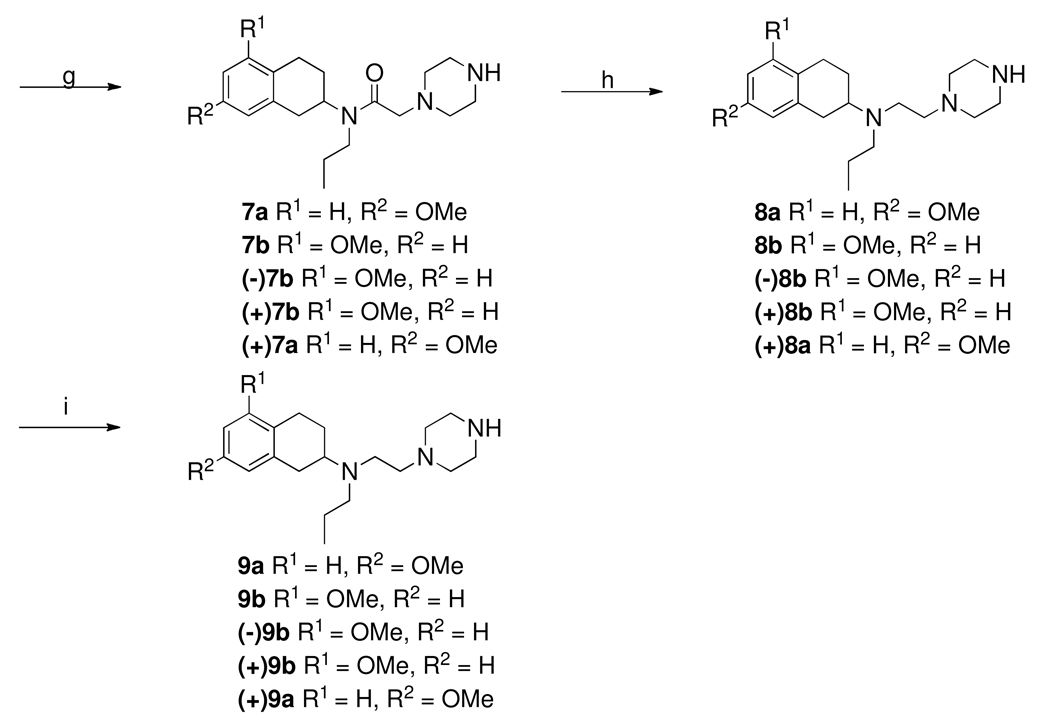
Scheme-4.
a. DIPEA, 2-propanol, relux, overnight; b. 48% aqueous HBr, reflux, overnight
Chemistry
Scheme 1 outlines the syntheses of 9a, 9b and their enantiomers. The starting materials for these compounds were appropriately substituted 7- and 5-methoxy-2-tetralones. These were condensed with propyl amine under standard reductive amination condition to give secondary amines 2a–b. Enantiomerically pure amines were made by using a synthetic chiral resolving agent as described previously by us25, 29. N-alkylation of amines using chloroacetyl chloride in presence of triethyl amine produced intermediate α-chloro amides 3a, 3b and their enantiomers. N-acylation with mono Boc-protected piperazine gave amides,30 which were then reduced by lithium aluminum hydride followed by deprotection with trifluoroacetic acid yielded 8. Demethylation in the presence of boron tribromide afforded phenols 9a, 9b and their enantiomers.
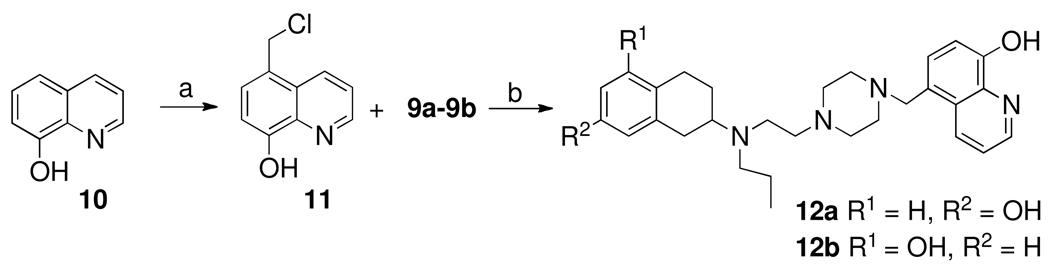
Scheme 2 depicts the synthesis of two final compounds 12a and 12b. Here the starting material is 8-hydroxyquinoline which was converted into 5-methylenechloride derivative 11 by treating it with formaldehyde and hydrogen chloride gas. N-alkylation of 11 with two different piperazine fragments 9a and 9b provided two final compounds 12a and 12b which were then purified by recrystallization of their hydrochloride salts from ethanol.23
Scheme-2.
a. HCl (32% water), HCHO (37% in water), 0 °C, RT, 8 h; b. N-substitued piperazine (9a or 9b), (Me2CH)2-NEt, CHCl3, RT, 1 day.
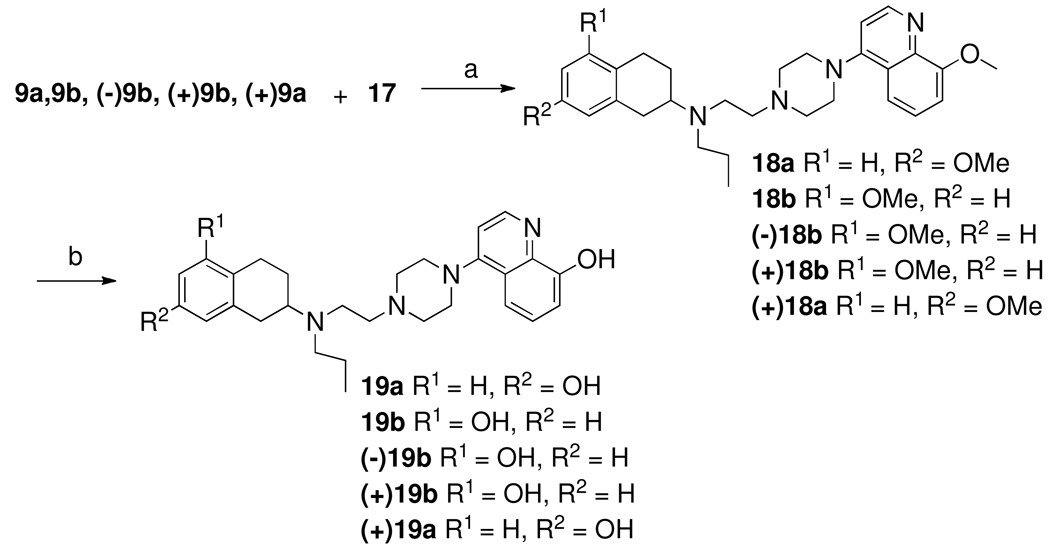
Scheme 3 describes the syntheses of chloride derivative of quinoline component 17. Most of the current methods for synthesis of quinoline rings are variations of the Skraup method in which an aniline derivative is heated with glycerol and an acid catalyst to form a stable intermediate that undergoes cyclization after a high-temperature Friedel–Crafts acylation. We used a modified method that uses methoxymethylene Meldrum’s acid31. This has the advantage of giving the same products in two steps rather than four steps. The first step in this sequence is the condensation of O-anisidine with Meldrum’s acid and trimethyl-orthoformate. The Meldrum’s acid was refluxed in trimethyl-ortho-formate to form methoxymethylene Meldrum’s acid 14 in situ. Addition of anisidine into the reaction mixture initiated addition–elimination reaction with the methoxy-methylene moiety to afford an ene–amine intermediate 15 for cyclization. Addition of an equal volume of DMF to the reaction with increase of reaction temperature overcame sluggishness of the reaction to facilitate the formation of 15. The ene–amine intermediate was then refluxed at 300 °C in phenyl ether as solvent for 15 min. The cyclized product was isolated by cooling and subsequent precipitation by mixing with hexane followed by filtration, washing with additional hexane, and drying. This compound (16) was then purified by column chromatography. The 4-hydroxy-8-methoxy quinoline (16) was dissolved in phosphorus oxychloride and heated to reflux for 2 h to give the desired 4-chloroquinoline derivative32.
Scheme-3.
a. Trimethylorthoformate, reflux, 1 h; b. o-anisidine, DMF (cat. amt), relux, 2 h; c. Diphenyl ether, 300 °C, 15 min; d. POCl3, reflux, 2h.
Scheme 4 describes the syntheses of final compounds 19a, 19b and their enantiomers. Intermediate 9a, 9b and their enantiomers were condensed with quinoline component (17) under refluxing condition in 2-propanol in presence of diisopropylethylamine as base gave 18 which were then demethylated under refluxing condition with 48% aqueous HBr. The final compounds 19a, 19b and their enantiomers were purified by recrystalization of their HCl salt from ethanol.
Discussion
In our approach to design multifunctional ligands targeting dopamine D2/D3 receptors as agonist and at the same time binding iron to reduce oxidative stress,an hydroxy quinoline moiety was introduced in our hybrid template for D2/D3 receptors. Numerous studies have shown that 8-hydroxy quinoline binds to iron.27 SAR studies on the hybrid template have indicated that bulky aromatic substitutions in the piperazine ring in a distal location from the aminotetralin moiety are well tolerated by D2/D3 receptors. Our recent SAR studies indicated that different quinoline moieties are not only well tolerated but also produced high agonist activity33. Thus, it was reasoned that replacement of the quinoline moiety by 8-hydroxy quinoline should not only retain high affinity for the D2/D3 receptor but also provide potent agonist activity. Preliminary compounds were designed and synthesized to test this rationale.
The two compounds that were synthesized first were 12a and 12b. In these two compounds, a 5-methylquinoline-8-ol moiety was attached to the piperazine ring. Both compounds exhibited low nanomolar potency for D3 receptor (5.57 and 3.71 nM for 12a and 12b, respectively). Compound 12b was twice as potent at D2 receptors compared to 12a (41 vs. 86 nM for 12b and 12a, respectively). These results indicated that the 5-methylquinoline-8-ol moiety was well tolerated by D2/D3 receptors.
In our next step, we introduced an 8-hydroxy quinoline moiety directly attached to the piperazine ring, which resulted in the development of next five compounds. The two racemic compounds, 19a and 19b, designed based on 5-hydroxy and 7-hydroxy templates, exhibited high affinity for both D2 and D3 receptors (Ki; 15.9 and 0.81 nM for 19a and 13.8 and 1.35 nM for 19b, respectively). Both these compounds showed moderate preferential affinity at D3 receptor compared to the D2 receptor (D2/D3; 19.62 and 10.22 for 19a and 19b, respectively). These results indicated that introduction of the 8-hydroxy quinoline moiety retained high affinity activity at both D2 and D3 receptors. In our next effort to prepare enantiomerically pure compounds of racemic 19b, compounds (−)19b and (+)19b were synthesized. As expected from our previous data from 5-hydroxy series hybrid compounds, higher affinity at both D2 and D3 was observed in (−)-19b compared to (+)-19b (Ki; 3.75 and 1.28 nM for (−)19b and 20.7 and 7.73 nM for (+)19b, respectively). Based on our results from activity of enantiomers of 7-hydroxy series hybrid compounds, we selectively synthesized (+)-19a. This compound like its 5-hydroxy counterpart exhibited high affinity for D2 and D3 receptors (Ki; 4.55 and 1.27 nM, respectively).
Following binding analysis, selected compounds (−)19b and (+)19a were subjected to the [35S]GTPγS functional assay for D2 and D3 receptors and compared with the full agonists dopamine and ropinirole. The assays were carried out with the cloned human D2 and D3 receptors expressed in CHO cells25. The results indicate that both compounds are quite active in stimulating both D2 and D3 receptors with similar potency: No appreciable selectivity was displayed by these compounds. Both showed full or near-complete agonism at D2 and D3 when compared against the reference substance dopamine, as did ropinirole. Thus, our binding and functional assay results indicated that introduction of an 8-hydroxy quinoline moiety retained not only high affinity for binding to D2/D3 receptors but also potent agonist activity at both receptors. Compound (−)-19b was selected for animal study as 5-hydroxy aminotetralin derived compound e.g. 1a, was previously shown to exhibit potent in vivo activity with long duration of action.25
pH-Dependent complexation studies with iron (III)
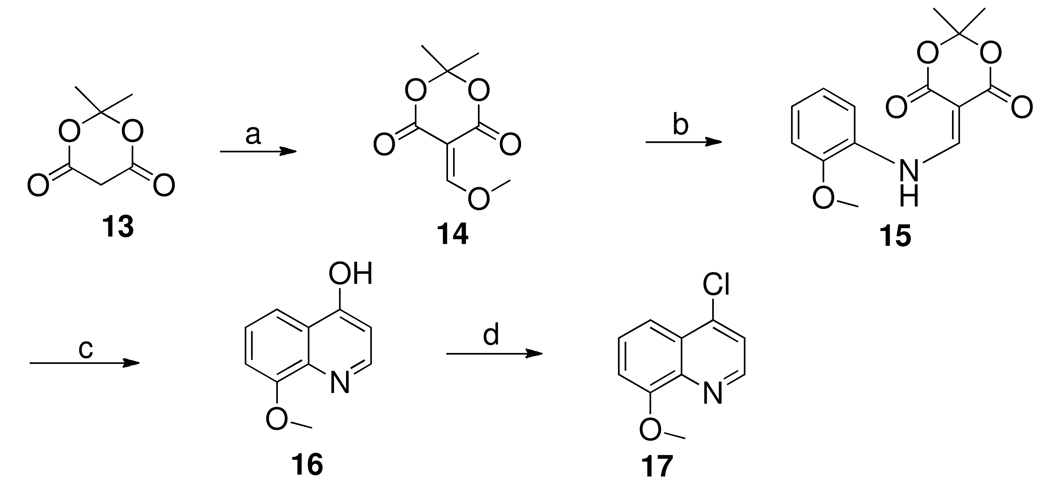
Equimolar amounts (600 µM) of 19b and FeCl3 were mixed together and complex formation was followed by UV absorption scanned from 200–800 nm at different pHs. It is evident from the plot (Figure 2) that 19b in presence of ferric chloride produced distinctly different UV absorption profiles compared to UV absorption of the compound 19b alone in the absence of iron chloride. This is indicative of a complex formation. In general a shift in λmax to the left takes place with increase of pH in the solution. Thus, at pH 3.76 the λmax was 647 nm (ε = 567 M−1 cm−1 ) and at pH 7.4 λmax it was 580 nm (ε =1300 M−1 cm−1). This observation agrees with the reported data on complexation of 8-hydroxy related compound with iron.28 Also the absorption spectra of the solution at pH 7.4 exhibited higher intensity at 580 nm than the spectra at lower pHs. This pH-dependent Fe(III)-19b complexation study indicated the amount of complex formation was favored by neutral pH. Since brain maintains physiologically a neutral pH, compound 19b might be a potential candidate to chelate iron in the PD brain.
Figure 2.
UV-visible absorption spectra of complex formation between racemic 19b (0.6 mM) and FeCl3 in water at various pH.
Evaluation of Fe (II) chelating potency of 19b in Ferrozine assay
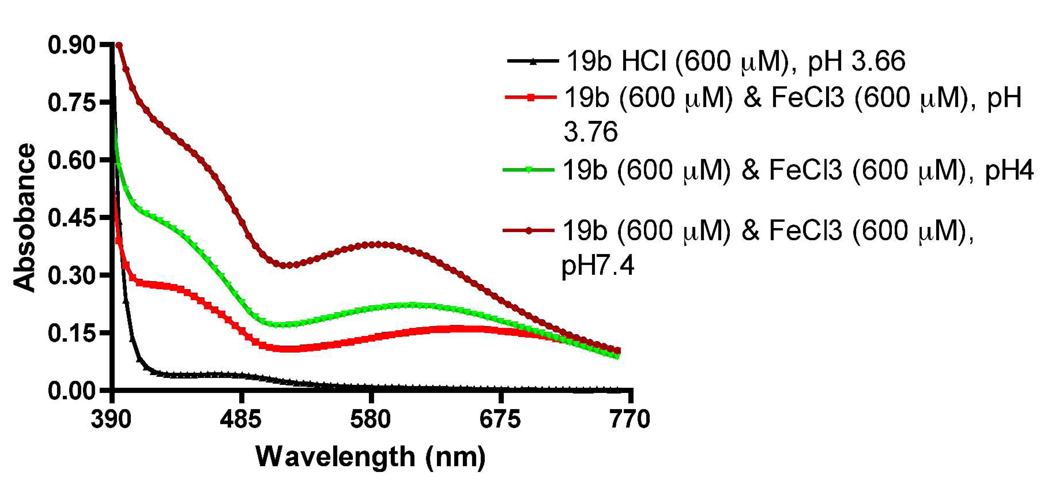
We performed an in vitro biochemical iron binding assay, known as the Ferrozine Assay, to determine the chelation potency of molecule 19b. We envisioned that potent iron binding compound will arrest iron in the PD brain from taking part in the Fenton reaction and thereby reduce the formation of hydroxyl radical and oxidative stress. Chelating potency of 19b was evaluated by Ferrozine assay method which is a colorimetric assay. Ferrozine is known to bind to iron (II) and forms a characteristics color upon complexation with Fe(II) which can be quantitated at 562 nm.34, 35 In this assay displacement of ferrozine from its complexation to Fe (II) by 19b in a concentration dependent manner was measured by absorption at 562 nm. It is evident from Figure 3 that at higher concentration of 19b complexation almost exclusively takes place with Fe (II). It has been shown in the past that ligands such as DFO complex to Fe (II) to undergo aerobic oxidation to Fe (III).36 Thus, this process of chelation potentially measures complexation to both Fe (II) and Fe(III). The chelating effect is expressed as a percent of control [80 µM ferrozine, 20 µM ferrous ammonium sulfate in pH 6.9 ammonium acetate buffer (5%)] by using the known equation (shown in the experimental section). Inhibition constant (IC50) of this compound was calculated to be 155.56 ± 0.73 µM (n=3).
Figure 3.
Chelating potency of 19b via displacement of ferrozine complexed to FeSO4 and is expressed as percent of control. Each point represents value from experiment done in triplicate and is expressed as mean ± SEM.
Mass spectroscopy evidence of complex formation
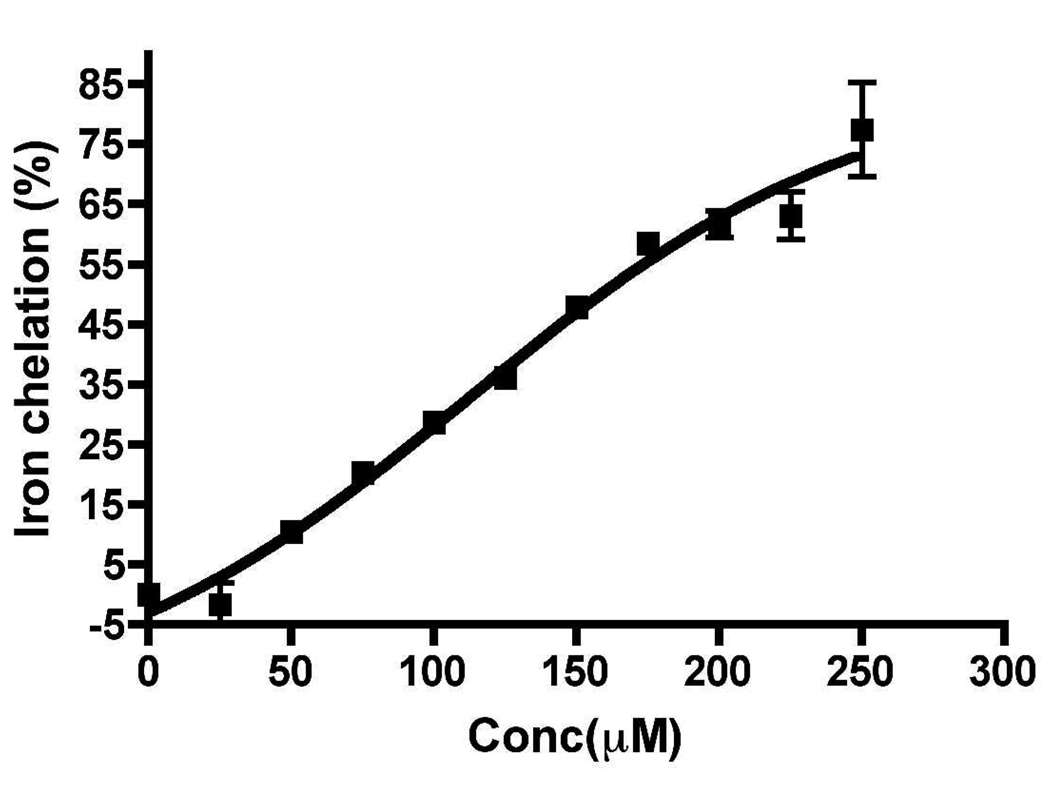
In an effort to demonstrate further formation of iron complexes, we carried out mass spectral analysis of 19b and FeCl3 solutions used in UV analysis above to detect any molecular ion peak corresponding to complex molecular ions. As shown in Figure 4, we indeed observed molecular ion peaks corresponding to L2-Fe3+ (M/z: 975 ) and L3-Fe3+ (M/z: 1434, 1435) complexes formation (L = ligand molecule). Thus, these results give clear evidence of formation of iron complexes with compound 19b.
Figure 4.
Molecular ion peaks of complexes formed from racemic 19b and FeCl3 at pH 7.4. Peaks at m/z 975 and 1434 correspond to 19b-Fe complex stoichiometry 2:1 and 3:1.
Deoxyribose antioxidant assay
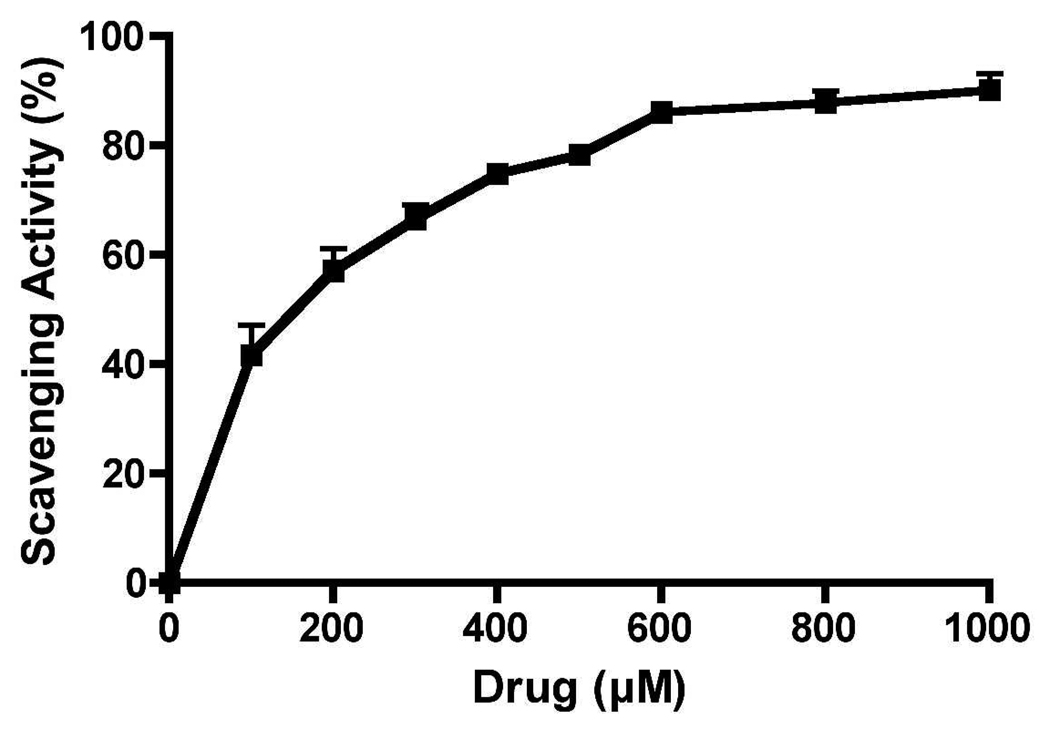
Antioxidant activity of 19b was analyzed by this assay. This is a test tube assay which measures hydroxyl radical scavenging capacity of a test compound by competing with deoxyribose37. In this assay, hydroxyl radical is generated by reaction of iron (Fe3+)-EDTA complex with H2O2 (by Fenton reaction) in presence of ascorbic acid which then react with deoxyribose to form smaller molecular fragment which upon heating with 2-thiobarbituric acid under acid conditions yields a pink color dye. Test compound (s) with potential radical scavenging capacity will compete with deoxyribose for hydroxyl radical generated by Fenton reaction. Thus, formation of fragment from deoxyribose and the pink color formation will be dictated by the capacity of a test compound to quench hydroxyl radical. This is a colorimetric assay and the absorbance is measured at 532 nm. Hydroxyl radical formation by Fenton reaction in the substantia nigral (SN) region of the PD brain might take place as H2O2 is generated in SN by dopamine metabolism and SN area is rich in iron in case of PD. It is apparent from Figure 5 that compound 19b dose dependently inhibited decomposition of deoxyribose by OH˙ with the highest dose exhibiting 80% scavenging activity with respect to control containing deoxyribose alone.
Figure 5.
Hydroxyl radical scavenging capacity of 19b in deoxyribose-containing solution. Values are reported as percentage versus a blank ± SD
Reversal of reserpine-Induced hypolocomotion in rats by (−)19b and ropinirole
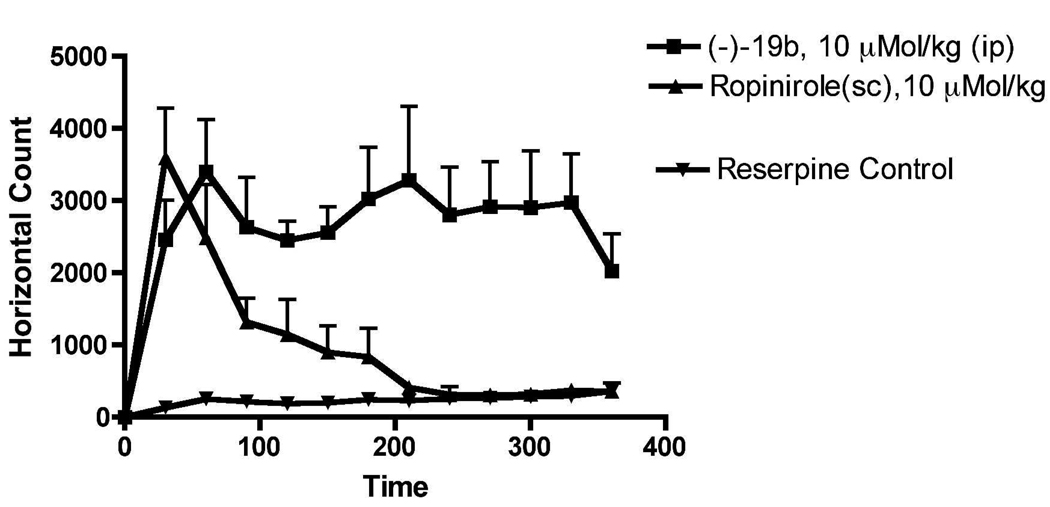
Reserpine induces depletion of catecholamines in nerve terminals resulting in a cataleptic condition in rats, which is a well established animal model for PD 38. Significant reduction of locomotion of the rats was observed 18 h after the administration of reserpine (5 mg/kg, s.c.) which indicated the development of akinesia in rats. Compound (−)19b was highly efficacious in reversing the locomotor activity of reserpinized rats. The locomotor activity of (−)19b at the end of 6 h remained very high. The reference drug ropinirole on the other hand exhibited much shorter duration of action compared to (−)19b. Compound (−)19b at a dose of 10 µmol/kg i.p. not only reversed reserpine induced hypokinesia to the normal level of locomotion found in control animals (vehicle treated reserpinized rats), but also demonstrated significant enhancement of locomotion for the entire duration of study. The mechanism of the locomotor stimulation in the reserpine model is likely to be mediated by postsynaptic D2/D3 receptor activation by (−)19b. Thus, the results suggest that the compound is a potent agonist, which crosses the blood brain barrier effectively and possesses excellent in vivo stability.
In vivo effect of (−)-19b in 6-OHDA lesioned rats
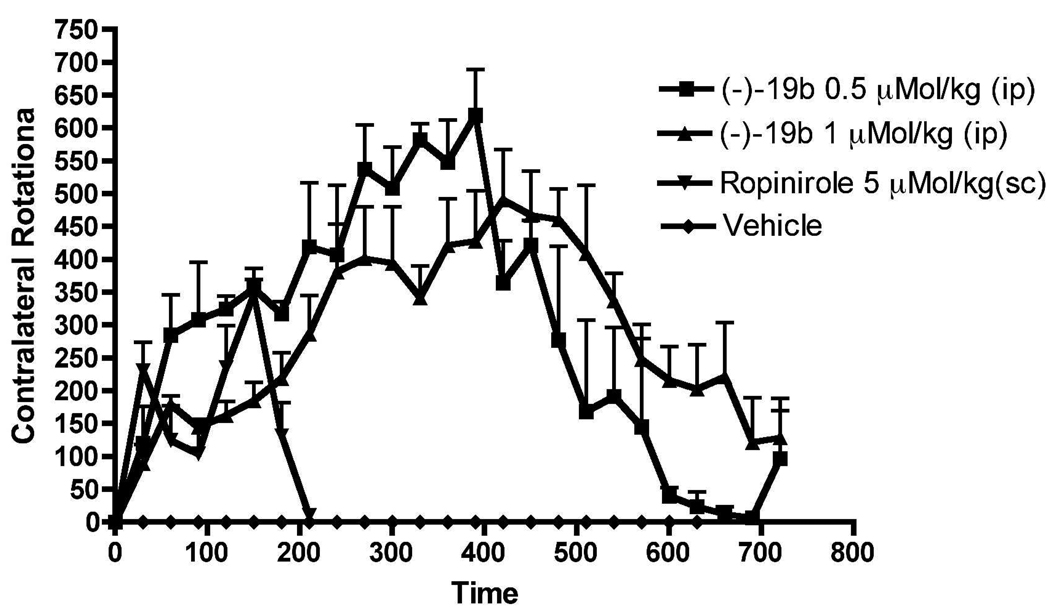
Compound (−)19b was tested in vivo in rats carrying an unilateral lesion in the medial forebrain bundle induced by application of the neurotoxin 6-hydroxydopamine (6-OHDA). Development of supersensitivity of dopamine receptors takes place resulting from destruction of dopamine neurons in these surgically modified rats. When these rats are challenged with direct acting dopamine agonists, they produced contralateral rotations away from the lesioned side. This rat model is considered to be one of the standard models for preclinical screening of drugs for possible antiparkinsonian property.39 Compound (−)19b was highly efficacious in producing large number of rotations at a smaller dose of 0.5 µMol/kg (0.3 mg/kg) and the activity lasted more than 11 h (Number of rotation = 7081). At a higher dose of 1 µMol/kg (0.61 mg/kg), the rotational activity produced by (−)-19b was initially less compared to the lowest dose (0.5 µMol/kg) but the activities increased gradually and remained high at the termination of the experiments after 11 h (Figure 7). On the other hand, the reference ropinirole at 5 µMol/kg produced much less rotations with shorter duration of action. The efficacy of this compound in producing rotations indicated its excellent brain penetration under i.p. administration condition.
Figure 7.
Effect on turning behavior of two different doses of (−)19b (i.p.) and vehicle in lesioned rats studied for maximum 12 h. Each point is the mean ± SEM of 3–4 rats. The drugs were administered i.p. One way ANOVA analysis demonstrates significant effect among treatments: F (4,95) = 21.12 (P< 0.0001). Dunnett’s analysis showed that the effect of (−)19b on rotations at two doses was significantly different compared to vehicle (P< 0.01) and the effect of ropinirole was significant compared to vehicle (p<0.05).
Conclusion
In this initial report, we describe the development of unique multifunctional dopamine D2/D3 agonist compounds with a capacity to chelate with iron (Fe2+/Fe3+). Our design of the preliminary compounds originated from observations collected in our earlier SAR studies, which demonstrated existence of a flexible binding pocket for substitutions on the piperazine ring located in a distal position with respect to the aminotetralin moiety. Thereby, a known iron chelating moiety 8-hydroxy quinoline was introduced in the hybrid structure, which resulted in the development of first-generation multifunctional molecules. Such molecules are not only expected to relieve motor dysfunction in PD but also will have potential to reduce oxidative stress in the PD brain by chelating with iron. Two lead molecules (−)19b and (+)19a identified from the binding study were subjected to the GTPgS functional assay which demonstrated their potent agonist property. Complexation studies with 19b demonstrated chelation with iron efficiently. Furthermore, the deoxyribose assay with 19b demonstrated potent antioxidant activity in these compounds. One of the lead molecules was then tested in PD animal models. Compound (−)19b not only reversed the reserpine-induced hypolocomotion in rats but also maintained a significant level of higher activity throughout the study session. In this regard efficacy of (−)19b at an equivalent dose was far greater than the standard reference ropinirole, which exhibited a shorter duration of action. In rotational experiments with 6-OH-DOPA-lesioned rats, two doses of (−)19b were efficacious in producing extensive rotational activity. Compound (−)-19b will be subjected to neuroprotection study to evaluate its potential in protecting against cell death in near future.
Experimental
Analytical silica gel-coated TLC plates (Silica Gel 60 F254) were purchased from EM Science and were visualized with UV light or by treatment with phosphomolybdic acid (PMA). Flash chromatography was carried out on Baker Silica Gel 40 mM. 1H NMR spectra were routinely obtained on GE-300 MHz and Varian 400 MHz FT NMR. The NMR solvent used was either CDCl3 or CD3OD or DMSO-d6 as indicated. TMS was used as an internal standard. Elemental analyses were performed by Atlantic Microlab, Inc and were within ± 0.4% of the theoretical value.
Procedure A. Preparation of 7-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propylamine (2a)
7-Methoxy-2-tetralone (10 g, 56.75 mmol) and acetic acid (13.5 ml, 226.9 mmol) were dissolved in dichloroethane (150 ml) and cooled to 0° C. n-propyl amine (11.7 ml, 141.87 mmol) was added and the mixture stirred under a N2 atmosphere for 30 min. NaCNBH3 (8.915 g, 141.87 mmol) in anhydrous MeOH (15 ml) was then added to the mixture and allowed to stir overnight at ambient temperature. The volatiles were then evaporated and saturated NaHCO3 solution was added. It was then extracted with dichloromethane, dried over Na2SO4, filtered, and concentrated. The crude residue was then taken up in EtOAc, at which time ethereal HCl was added, and the crude salt was filtered and dried over vacuum oven. The crude salt was then recrystalized in ethanol to yield 9.5 g (65%) white solid and used in the subsequent transformations. 1H NMR (free base) (400 MHz, CDCl3) δ ppm 0.91–0.95 (t, 3H, J = 7.6 Hz), 1.38 (bs, 1H), 1.48–1.60 (m, 3H), 2.04–2.09 (m, 1H), 2.67–2.71 (t, 3H, J = 7.6 Hz), 2.88–2.92 (m, 2H), 2.97–3.04 (m, 1H), 3.81 (s, 3H), 6.60–6.62 (dd, 1H, J1 = 1.6 Hz, J2 = 4.2 Hz), 6.65–6.78 (m, 1H), 6.95–6.98 (d, 1H, J = 8.8 Hz).
(5-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-propyl-amine (2b)
Compound 2b was prepared following procedure A using 5-methoxy 2-tetralone and purified by recrystalization of its hydrochloride salt from ethanol to get white salt of 2b (yield is 64%). 1H NMR (free base) (400 MHz, CDCl3) δ ppm 0.92–0.964 (t, 3H, J = 7.6 Hz), 1.39 (bs, 1H), 1.49–1.61 (m, 3H), 2.05–2.10 (m, 1H), 2.66–2.70 (t, 3H, J = 7.6 Hz), 2.87–2.94 (m, 2H), 2.98–3.03 (m, 1H), 3.81 (s, 3H), 6.65–6.67 (d, 1H, J = 8 Hz), 6.96−6.71 (d, 1H, J = 8 Hz), 7.07–7.11 (t, 1H, J = 8 Hz).
Procedure B. Resolution of 5-Methoxy-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine
Racemic (±)-2b was resolved into its (+) and (−) isomers by using the both (−) and the (+) isomers of the synthetic resolving agent 4-(2-chlorophenyl)-5,5-dimethyl-2-hydroxy-1,3,2-dioxaphosphorinane 2-oxide. This optically active resolving agents were prepared according to the published procedure40,. 2b (free base 14.77 g, 67.36 mmol) and (+)-4-(2-chlorophenyl)-5,5-dimethyl-2-hydroxy-1,3,2-dioxaphosphorinane 2-oxide (20.5 g, 74.1 mmol) were dissolved by warming in 100 ml of ethanol. The solution was cooled to room temperature and then at 0°C. The precipitated crystals were filtered off, washed with cold ether to yield 17.4 g of the salt ([α]D= (−)1.2°, c =1 in methanol). Further recrystallization two times from hot ethanol yielded the salt (12.9 g, [α] D= (−) 14.1°, c =1 in methanol). Further crystallization of the salt from hot ethanol did not change the optical rotation to a significant extent. The salt was then neutralized in presence of 20% NaOH solution in water under stirred condition for 2 h at room temperature. The aqueous layer was extracted with dichloromethane (3×100 ml), dried over Na2SO4 and evaporated to dryness to yield thick transparent liquid (−)2b (5.8 g, [α] D of the HCl salt = (−)71.5°, (c =1 in methanol) Yield. 78.5 %.
(±)-2b (18.5 g, 84.35 mmol) was similarly treated using (−)-4-(2-chlorophenyl)-5,5-dimethyl-2-hydroxy-1,3,2-dioxaphosphorinane 2-oxide (24.5 g, 88.57 mmol). Recrystallization from hot ethanol yielded the salt (16.2 g, [α] D = (+)-13.0, c = 1 in methanol). Yield is 78%. Further crystallization of the salt from hot ethanol did not change the optical rotation to a significant extent. Hydrolysis of the chlocyphos salt following above mentioned procedure yielded thick transparent liquid (+)2b. ([α]D of the HCl salt is (+)-69.8°, c =1 in methanol).
Resolution of (R)-7-methoxy-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine
This resolution was done according to procedure B, in which (±)-2a (5.99 g, 27.31 mmol) was similarly treated using (−)-4-(2-chlorophenyl)-5,5-dimethyl-2-hydroxy-1,3,2-dioxaphosphorinane 2-oxide (7.93 g, 28.68 mmol). Recrystallization from hot ethanol yielded the salt (5.4 g, [α] D = (+)-12.9°, c = 1 in methanol). Yield is 80 %. Further crystallization of the salt from hot ethanol did not change the optical rotation to a significant extent. Hydrolysis of the chlocyphos salt following above mentioned procedure yielded R(+)2a, [α]D of the HCl salt is (+)-68.6° (c =1 in methanol).
Procedure C. Preparation of 2-Chloro-N-(7-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-N-propyl-acetamide (3a)
Compound 2a (HCl salt, 3.117 g, 12.18 mmol) and Et3N (8.4 ml, 60.9 mmol) was stirred at 0° C in CH2Cl2 (75 ml) for 15 min. Chloroacetylchloride (1.94 ml, 24.37 mmol) was added dropwise and the resulting solution was stirred at room temperature for 30 min, at which time the reaction mixture was poured into a 1M solution of NaOH (50 ml) and the product was extracted with dichloromethane, dried (Na2SO4), filtered, and concentrated. The crude material was purified by column chromatography (Hex:EtOAc, 3:1) to give 3.42 g (95%) of 3a as transparent liquid. 1H NMR (400 MHz, CDCl3) δ ppm 0.90–0.98 (m, 3H), 1.64–1.72 (m, 2H), 1.83–2.12 (m, 2H), 2.58–2.70 (m, 1H), 2.84–2.89 (dd, 1H, J1 = 4.8 Hz, J2 = 16 Hz), 3.00–3.10 (m, 2H), 3.15– 3.26 (m, 2H), 3.82 (s, 3H), 3.95–4.03 (m, 1H), 4.08–4.12 (m, 2H), 6.61–6.62 (dd, 1H, J1 = 1.6 Hz, J2 = 4.8 Hz), 6.64–6.77 (m, 1H), 6.96–6.99 (d, 1H, J = 12 Hz).
2-Chloro-N-(5-methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-N-propyl-acetamide (3b)
This compound was prepared from 2b following the procedure C (yield is 93 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.92–0.96 (t, 3H, J = 8 Hz), 1.64–1.72 (m, 2H), 1.83–2.12 (m, 2H), 2.58–2.70 (m, 1H), 2.84–2.89 (m 1H), 3.00–3.10 (m, 2H), 3.19–3.27 (m, 2H), 3.86 (s, 3H), 3.95–4.03 (m, 1H), 4.08–4.12 (m, 2H), 6.61–6.68 (m, 2H), 7.07–7.11 (t, 3H, J = 8 Hz).
(−)-2-chloro-N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propylacetamide, (−)3b
Compound (−)2b (HCl salt, 6.0 g, 23.46 mmol) was reacted under similar conditions as reported in procedure C to afford the optically pure (−)3b as transparent liquid (6.52 g, 94%): 1H NMR (400 MHz, CDCl3) δ ppm 0.92–0.96 (t, 3H, J = 8 Hz), 1.64–1.72 (m, 2H), 1.83–2.12 (m, 2H), 2.58–2.70 (m, 1H), 2.84–2.89 (m, 1H), 3.00–3.10 (m, 2H), 3.19–3.27 (m, 2H), 3.86 (s, 3H), 3.95–4.03 (m, 1H), 4.08–4.12 (m, 2H), 6.61–6.68 (m, 2H), 7.07–7.11 (t, 1H, J = 8 Hz).
(+)-2-chloro-N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propylacetamide, (+)-3b
Compound (+)2b (HCl salt, 3.5 g, 13.68 mmol) was reacted under similar conditions as in procedure C to afford the optically pure (+)3b as transparent liquid (3.9 g, 90.7%): 1H NMR (400 MHz, CDCl3) δ ppm 0.92–0.96 (t, 3H, J = 8 Hz), 1.64–1.72 (m, 2H), 1.83–2.12 (m, 2H), 2.58–2.70 (m, 1H), 2.84–2.89 (m, 1H), 3.00–3.10 (m, 2H), 3.19–3.27 (m, 2H), 3.86 (s, 3H), 3.95–4.03 (m, 1H), 4.08–4.12 (m, 2H), 6.61–6.68 (m, 2H), 7.07–7.11 (t, 1H, J = 8 Hz).
(+)-2-Chloro-N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-N-propylacetamide, (+)-3a
Compound (+)2a (1.12 g, 5.1 mmol) was prepared following procedure C to afford the optically pure (+)3a as transparent liquid (1.44 g, 95%): 1H NMR (400 MHz, CDCl3) δ ppm 0.90–0.98 (m, 3H), 1.64–1.72 (m, 2H), 1.83–2.12 (m, 2H), 2.58–2.70 (m, 1H), 2.84–2.89 (m, 1H), 3.00–3.10 (m, 2H), 3.15– 3.26 (m, 2H), 3.82 (s, 3H), 3.95–4.03 (m, 1H), 4.08–4.12 (m, 2H), 6.59–6.61 (dd, 1H, J1 = 1.6 Hz, J2 = 4.8 Hz), 6.64–6.77 (m, 1H), 6.96–6.99 (d, 1H, J = 8.8 Hz).
Procedure D. Preparation of t-butyl 4-(2-((7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)-2-oxoethyl)piperazine-1-carboxylate (6a)
Compound 3a (3.17 g, 10.72 mmol) and compound 5 (1.397 g, 7.5 mmol), anhydrous K2CO3 powder (7.4 g, 53.58 mmol) were refluxed in acetonitrile (100 ml) for 2 h. The solution was cooled, filtered, and concentrated. The crude material was then partitioned between EtOAc and H2O, and the organic layer was separated, dried (Na2SO4), and concentrated. The crude mixture was purified by column chromatography (EtOAc:Hex;1:1) to give 3.87 g (81 %) of 6a as thick yellow liquid. 1H NMR (400 MHz, CDCl3) δ ppm 0.86–0.90 (t, 3H, J = 8 Hz), 1.45 (s, 9H), 1.59–1.82 (m, 2H), 1.87–1.94 (m, 1H), 1.95–1.97 (m, 3H), 2.420 (bs, 2H), 2.50–2.63 (m, 2H), 2.76–2.82 (m, 4H), 2.96–3.20 (m, 3H), 3.88−3.47 (m, 4H), 3.81 (s, 3H), 6.60–6.61 m, 1H), 6.65–6.78 (m, 1H), 6.95–6.98 (d, 1H, J = 8.8 Hz).
t-butyl-4-(2-((5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)-2-oxoethyl)piperazine-1-carboxylate (6b)
This compound was prepared from 3b according to the procedure D which yielded 6b as thick yellow liquid (77 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.87–0.91 (t, 3H, J = 8 Hz), 1.44 (s, 9H), 1.58–1.81 (m, 2H), 1.88–1.95 (m, 1H), 1.96–1.98 (m, 3H), 2.40 (bs, 2H), 2.51–2.63 (m, 2H), 2.78–2.83 (m, 4H), 2.95–3.29 (m, 3H), 3.38–3.47 (m, 4H), 3.81 (s, 3H), 6.63–6.70 (m, 2H), 7.10–7.14 (t, 1H, J = 8 Hz).
(−)-t-butyl-4-(2-((5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)-2-oxoethyl)piperazine-1-carboxylate, (−)-6b
This compound was prepared from (−)3b (3.5 g, 11.83 mmol) following procedure D to get (−)6b as thick yellow liquid (4.6 g, 87.3 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.88–0.92 (t, 3H, J = 8 Hz), 1.45 (s, 9H), 1.59–1.82 (m, 2H), 1.88–1.95 (m, 1H), 1.96–1.98 (m, 3H), 2.40 (bs, 2H), 2.52–2.64 (m, 2H), 2.81–2.84 (m, 4H), 2.95–3.29 (m, 3H), 3.39–3.48 (m, 4H), 3.82 (s, 3H), 6.64–6.71 (m, 2H), 7.11–7.14 (t, 1H, J = 6 Hz).
(+)-t-butyl-4-(2-((5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)-2-oxoethyl)piperazine-1-carboxylate, (+)-6b
This compound was prepared from (+)3b (4.0 g, 13.52 mmol) according to the procedure D to afford (+)6b as thick yellow liquid (4.44 g, 73.69 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.86–0.90 (t, 3H, J = 8 Hz), 1.42 (s, 9H), 1.58–1.81 (m, 2H), 1.88–1.95 (m, 1H), 1.95–1.97 (m, 3H), 2.39 (bs, 2H), 2.51–2.63 (m, 2H), 2.78–2.83 (m, 4H), 2.94–3.28 (m, 3H), 3.88−3.47 (m, 4H), 3.80 (s, 3H), 6.63–6.70 (m, 2H), 7.09–7.13 (t, 1H, J = 8.0 Hz).
(+)-tert-butyl-4-(2-((7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)-2-oxoethyl)piperazine-1-carboxylate, (+)-6a
This compound was prepared from (+)3a (2.32 g, 7.8mmol) according to the procedure D to afford (+)6a as thick yellow liquid (2.76 g, 79 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.86–0.90 (t, 3H, J = 8 Hz), 1.45 (s, 9H), 1.59–1.82 (m, 2H), 1.87–1.94 (m, 1H), 1.95–1.97 (m, 3H), 2.420 (bs, 2H), 2.50–2.63 (m, 2H), 2.76–2.82 (m, 4H), 2.96–3.20 (m, 3H), 3.88−3.47 (m, 4H), 3.81 (s, 3H), 6.60–6.62 (dd, 1H, J1 = 2.2 Hz, J2 = 5.2 Hz), 6.65–6.78 (m, 1H), 6.95–6.97 (d, 1H, J = 8 Hz).
Procedure E. Preparation of N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-2-(piperazin-1-yl)-N-propylacetamide (7a)
Compound 6a (3.87 g, 8.68 mmol) was dissolved in 40 ml of dichloromethane and 40 ml of trifluoroacetic acid was added. The mixture was stirred for overnight, at which time the solution was concentrated to dryness, dissolved in saturated NaHCO3 solution and extracted with EtOAc. The organic layer was dried over Na2SO4, filtered, and concentrated to yield 2.91 g (97 %) of 7a as yellow wax, which was used in the next reaction. 1H NMR (400 MHz, CDCl3) 0.93–0.96 (t, 3H, J = 12 Hz), 1.62 (m, 3H), 1.85–1.97 (m, 3H), 2.56 (m, 1H), 2.81–2.88 (m, 5H), 2.98–3.34 (m, 9H), 3.81 (s, 3H), 6.60 (s, 1H), 6.65–6.68 (d, 1H, J = 12 Hz), 6.96–6.98 (d, 1H, J = 8 Hz).
N-(5-Methoxy-1,2,3,4-tetrahydro-naphthalen-2-yl)-2-piperazin-1-yl-N-propylacetamide (7b)
This compound was prepared from 6b as yellow wax (3.87g, 8.6 mmol) following the procedure E and yield of this reaction is 2.76 g (92 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.92–0.964 (t, 3H, J = 7.6 Hz), 1.63 (m, 3H), 1.84–1.97 (m, 3H), 2.55 (m, 1H), 2.80–2.87 (m, 5H), 2.97–3.34 (m, 9H), 3.82 (s, 3H), 6.64–6.71 (m, 2H), 7.06–7.15 (m, 1H).
(−)-N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-2-(piperazine-1-yl)-N-propylacetamide, (−)7b
This compound was prepared from (−)6b (4.6 g, 10.32 mmol) following the procedure E to get (−)7b as yellow wax (3.2 g, 89.6 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.90–0.93 (t, 3H, J = 6.0 Hz), 1.61 (m, 3H), 1.84–1.97 (m, 3H), 2.54 (m, 1H), 2.79–2.86 (m, 5H), 2.97–3.34 (m, 9H), 3.81 (s, 3H), 6.65–6.72 (m, 2H), 7.09–7.16 (m, 1H).
(+)-N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-2-(piperazin-1-yl)-N-propylacetamide, (+)7b
Following the procedure E this compound was prepared from (+)6b (4.4 g, 9.87 mmol) to afford (+)7b as yellow wax (3.0 g, 88.23 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.90–0.94 (t, 3H, J = 8.0 Hz), 1.66 (m, 3H), 1.84–1.97 (m, 3H), 2.52 (m, 1H), 2.79–2.87 (m, 5H), 2.97–3.34 (m, 9H), 3.84 (s, 3H), 6.65–6.72 (m, 2H), 7.10–7.16 (m, 1H).
(+)-N-(7-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)-2-(piperazin-1-yl)-N-propylacetamide, (+)7a
This compound was prepared from R(+)6a (3.17 g, 7.11 mmol) following the procedure E that gave quantitative yield R(+)7a as yellow wax (2.46 g). 1H NMR (400 MHz, CDCl3) δ ppm 0.93–0.96 (t, 3H, J = 6 Hz), 1.62 (m, 3H), 1.85–1.97 (m, 3H), 2.56 (m, 1H), 2.81–2.88 (m, 5H), 2.98–3.34 (m, 9H), 3.81 (s, 3H), 6.60 (s, 1H), 6.65–6.68 (d, 1H, J = 12 Hz), 6.95–6.98 (d, 1H, J = 12.0 Hz).
Procedure F. Preparation of 7-methoxy-N-(2-(piperazin-1-yl)ethyl)-N-propyl-1,2,3,4-tetrahydronaphthalen -2-amine (8a)
To a suspension of LiAlH4 (1.735 g, 45.73 mmol) in THF (100 ml) at 0 °C was added 7a (3.16 g, 9.15 mmol) in a solution of THF (25 ml). After addition, the mixture was refluxed for 2 h and cooled to 0 °C. 15% NaOH was added dropwise to quench the reaction and deactivate the LiAlH4, and the mixture stirred for 20 min, and filtered, washed with ethyl acetate. The solution was dried over Na2SO4, filtered, and concentrated. This crude product was then purified through column chromatography using 20% MeOH in ethyl acetate to get 8a as transparent thick liquid (2.23 g, 74%). 1H NMR (400 MHz, CDCl3) δ ppm 0.91–0.96 (t, 3H, J = 10 Hz), 1.40–1.6 (m, 3H), 1.97–2.01 (m, 1H), 2.139 (bs, 1H) 2.41–3.11 (m, 18H), 3.82 (s, 3H), 6.61 (s, 1H), 6.66–6.79 (d, 1H, J = 6 Hz), 6.95–6.98 (d, 1H, J = 8.8 Hz).°
5-Methoxy-N-(2-(piperazin-1-yl)ethyl)-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine (8b)
This compound was prepared from 7b following the procedure F to get 8b as transparent thick liquid (yield is 72.5 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.92–0.96 (t, 3H, J = 7.6 Hz), 1.41–1.59 (m, 3H), 1.98–2.22 (m, 1H), 2.41–3.1 (m, 19H), 3.81 (s, 3H), 5.2 (bs, 1H), 6.62–6.64 (d, 1H, J = 8 Hz), 6.68–6.69 (d, 1H, J = 4 Hz), 7.05–7.09 (t, 1H, J = 8 Hz).
(−)-5-methoxy-N-(2-(piperazin-1-yl)ethyl)-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine, (−)8b
This compound was prepared from (−)7b (3.8 g, 11 mmol) following the procedure F to get (−)8b as transparent thick liquid (yield is 3.26 g, 89 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.87–0.91 (t, 3H, J = 8.0 Hz), 1.16–1.19 (t, 3H, J = 6 Hz), 1.98–2.1 (m, 1H), 2.46–3.01 (m, 19H), 3.81 (s, 3H), 5.93 (bs, 1H), 6.64–6.66 (d, 1H, J = 8.0 Hz), 6.70–6.71 (d, 1H, J = 4.0 Hz), 7.07–7.11 (t, 1H, J = 8.0 Hz).
(+)-5-methoxy-N-(2-(piperazin-1-yl)ethyl)-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine, (+)8b
This compound was prepared from (+)7b (3.5 g, 10.13 mmol) following the above procedure F that yielded 2.48 g (74 %) of (+)8b as transparent thick liquid. 1H NMR (400 MHz, CDCl3) δ ppm 0.88–0.91 (t, 3H, J = 6.0 Hz), 1.17–1.20 (t, 3H, J = 6 Hz), 1.97–2.00 (m, 1H), 2.46–3.01 (m, 19H), 3.81 (s, 3H), 5.71 (bs, 1H), 6.65–6.67 (d, 1H, J = 8.0 Hz), 6.71–6.72 (d, 1H, J = 4.0 Hz), 7.06–7.10 (t, 1H, J = 8.0 Hz).
(+)-7-methoxy-N-(2-(piperazin-1-yl)ethyl)-N-propyl-1,2,3,4-tetrahydronaphthalen-2-amine, (+)8a
This compound was prepared from (+)7a (2.46 g, 7.1 mmol) following procedure F for to make (+)8a as transparent thick liquid (yield is 2.15 g, 91 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.91–0.96 (t, 3H, J = 10 Hz), 1.40–1.6 (m, 3H), 1.97–2.01 (m, 1H), 2.139 (bs, 1H) 2.41–3.11 (m, 18H), 3.82 (s, 3H), 6.61 (s, 1H), 6.66–6.79 (d, 1H, J = 12 Hz), 6.96–6.98 (d, 1H, J = 8 Hz).
Procedure G. Preparation of 7-[(2-Piperazin-1-yl-ethyl)-propyl-amino]-5,6,7,8-tetrahydro-naphthalen-2-ol (9a)
Compound 8a (2.23 g, 6.73 mmol) was dissolved in 80 ml of CH2Cl2 and cooled to −78 °C. 1M boron tribromide solution in dichloromethane (20 ml) was added dropwise and the mixture was allowed to warm to ambient temperature and was stirred overnight. Sat. NaHCO3 was added and the product extracted with dichloromethane, dried over Na2SO4, filtered, and concentrated to yield the crude product. Column chromatography (7:2:1;CH2Cl2:MeOH:Et3N) afforded 1.62 g (76 %) of 9a as brown wax. 1H NMR (400 MHz, CDCl3) δ ppm 0.84–0.87 (t, 3H, J = 6 Hz), 1.27–1.31 (m, 2H), 1.70–1.82 (m, 3H), 2.25–2.28 (m, 1H), 2.58−2.06 (m, 1H), 2.73–2.78 (m, 4H), 2.97–3.09 (m, 5H), 3.16–3.20 (m, 7H), 6.45 (s, 1H), 6.53–6.55 (d, 1H, J = 9.2 Hz), 6.84–6.86 (d, 1H, J = 8 Hz).
6-[(2-Piperazin-1-yl-ethyl)-propyl-amino]-5,6,7,8-tetrahydro-naphthalen-1-ol (9b)
This compound was prepared from 8b following the procedure G (yield is 78 %) as brown wax. 1H NMR (400 MHz, CDCl3) δ ppm 0.99–1.02 (t, 3H, 7.6 Hz), 1.28–1.30 (m, 2H), 1.71–1.83 (m, 3H), 2.26–2.29 (m, 1H), 2.57–2.60 (m, 1H), 2.72–2.77 (m, 4H), 2.97–3.07 (m, 5H), 3.16–3.21 (m, 7H), 6.5–6.52 (d, 1H, J = 8 Hz), 6.55–6.57 (d, 1H, J = 8 Hz), 6.9–6.95 (t, 1H, J = 10 Hz).
(−)-6-((2-(piperazin-1-yl)ethyl)(propyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol, (−)9b
This compound was prepared from (−)8b (3.26 g, 9.8 mmol) following the procedure G to make (−)9b as brown wax (yield is 2.23, 71.5 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.86–0.89 (t, 3H, 6.0 Hz), 1.08–1.11 (t, 2H, J = 6 Hz), 1.40–1.51 (m, 3H), 1.98–2.01 (m, 1H), 2.46–2.54 (m, 9H), 2.62–2.67 (m, 4H), 2.87–2.96 (m, 4H), 6.47–6.49 (d, 1H, J = 8 Hz), 6.54–6.56 (d, 1H, J = 8 Hz), 6.91–6.95 (t, 1H, J = 8 Hz).
(+)-6-((2-(piperazin-1-yl)ethyl)(propyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol, (+)9b
This compound was prepared from (+)8b (2.48 g, 7.48 mmol) following the procedure G which afforded (+)9b as brown wax (yield 1.74 g, 73.2 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.88–0.91 (t, 3H, 6.0 Hz), 1.10–1.13 (t, 2H, J = 6 Hz), 1.40–1.51 (m, 3H), 1.98–2.01 (m, 1H), 2.48–2.56 (m, 9H), 2.64–2.69 (m, 4H), 2.87–2.96 (m, 4H), 6.48–6.50 (d, 1H, J = 8 Hz), 6.55–6.56 (d, 1H, J = 4 Hz), 6.92–6.95 (t, 1H, J = 6 Hz).
(+)-7-((2-(piperazin-1-yl)ethyl)(propyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol, (+)9a
This compound was prepared from (+)8a (2.15 g, 6.5 mmol) following the procedure G to yield (+)9a as brown wax (0.39 g, 20 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.84–0.87 (t, 3H, J = 6 Hz), 1.70–1.82 (m, 3H), 2.25–2.28 (m, 1H), 2.58−2.06 (m, 1H), 2.73–2.78 (m, 4H), 2.97–3.09 (m, 5H), 3.16–3.20 (m, 9H), 6.45 (s, 1H), 6.53–6.55 (d, 1H, J = 8 Hz), 6.84–6.86 (d, 1H, J = 8 Hz).
5-(chloromethyl)quinolin-8-ol (11)
A mixture of commercially available compound 10, 8-quinolinol (7.3 g, 50.29 mmol), 8 mL of 32% HCl in water, and 8 mL of 37% formaldehyde in water at 0 °C was treated with hydrogen chloride gas for 6h. The solution was allowed to stand at room temperature for 2 h without stirring. The yellow solid obtained was collected on a filter, washed with 90% alcohol, and dried under vacuum to give 5- chloromethyl-8-quinolinol hydrochloride 11 as yellow solid (9.0 g, 77.78 %). 1H NMR of HCl salt (400 MHz, DMSO) δ ppm 5.30 (s, 2H), 7.49–7.51 (d, 1H, J = 8 Hz), 7.83–7.85 (d, 1H, J = 8 Hz), 8.08–8.12 (dd, 1H, J1 = 5.6 Hz, J2 = 8.8), 9.098–9.110 (d, 1H, J = 4.8 Hz), 9.193– 9.213 (d, 1H, J = 8 Hz)
Procedure H. Preparation of 5-((4-(2-((7-hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)ethyl)piperazin-1-yl)methyl) quinolin-8-ol (12a)
To a mixture of 5-chloromethyl-8-quinolinol hydrochloride, 11 (0.20 g, 0.86 mmol) and diisopropylethylamine (0.3 mL, 1.73 mmol, 2.2 equiv) in 25 mL CHCl3 at 0 °C was added 9a (0.25 g, 0.78 mmol). The mixture was stirred for 24 h at room temperature. CHCl3 (100mL) was added and the solution obtained was washed with 10% NaHCO3, brine, and then dried over Na2SO4. The solution was filtered and evaporated to dryness. The residue was made hydrochloride salt and crystallized from ethanol to yield 12a as yellow solid (0.28 g, 57.3 %). 1H NMR of HCl salt (400 MHz, DMSO-d6) δ ppm 0.88–0.99 (m, 3H), 1.79–1.81 (bs, 4H), 2.298 (bs, 1H), 2.476–2.48 (m, 2H), 2.74 (s, 2H), 2.96–3.02 (t, 1H, J = 12 Hz), 3.122 (bs, 3H), 3.34–3.65 (m, 12H), 6.51–6.56 (m, 2H), 6.86–6.88 (d, 1H, J = 8 Hz), 7.50–7.52 (d, 1H, J = 8 Hz), 7.997–8.008 (m, 2H); 9.06–9.07 (d, 1H, J = 4 Hz); 9.49–9.51 (d, 1H, J = 8 Hz). The hydrochloride salt mp 242-242 °C. Analysis calculated for (C29H40.6N4O3.3, 4HCl) C, H, N
5-((4-(2-((5-hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl) amino)ethyl)piperazin-1-yl)methyl)quinolin-8-ol (12b)
This compound was prepared following the procedure H in which compound 11 (0.18 g, 0.8 mmol) and 9b (0.23 g, 0.72 mmol) were used to afford 12b as yellow solid (0.265 g, 59 %).1H NMR of salt (400 MHz, DMSO-d6) δ ppm 0.84– 0.87 (t, 3H, J = 6 Hz), 1.79–1.81 (bs, 4H), 2.298 (bs, 1H), 2.476–2.48 (m, 2H), 2.74 (s, 2H), 2.96–3.02 (t, 1H, J = 12 Hz), 3.122 (bs, 3H), 3.34–3.65 (m, 12H), 6.50–6.52 (d, 1H, J = 8 Hz), 6.55–6.57 (d, 1H, J = 8 Hz), 6.93–6.96 (t, 1H, J = 6 Hz), 7.06–7.08 (d, 1H, J = 8 Hz), 7.32–7.34 (d, 1H, J = 8 Hz), 7.44–7.46 (m, 1H), 8.66–8.68 (d, 1H, J = 8 Hz), 8.77–8.78 (d, 1H, J = 4 Hz). The hydrochloride salt mp 252–255 °C. Analysis calculated for (C29H43N4O4.5, 4HCl) C, H, N
8-methoxyquinolin-4-ol (16)
Trimethyl orthoformate (190mL, 1.7 mol) and isopropylidine malonate (Meldrum’s acid, 5 gm, 34.7 mmol) were refluxed for 1 h, and then cooled slightly. O-anisidine (3.9 ml, 34.7 mmol) was added to the mixture along with 8 mL of dimethylformamide (DMF) and the resulting mixture was reheated to reflux for 2 h. The mixture was cooled to room temperature, poured into cold water (150 mL), upon which a crystalline solid formed and was filtered and allowed to dry in open air. It was then recrystalized from methanol to get pure white solid 15. The solid material was then poured into warm diphenyl ether (50 ml) and heated to 300 °C for 15 minutes under reflux condensor, then cooled down to room temperature. The cyclized product was isolated by cooling and subsequent precipitation by mixing with hexane followed by filtration, washing with additional hexane, and drying. It was the purified by column chromatograpy (EtOAc:MeOH;95:5) to give 2.65 g (overall 44 %) of 16 as off white solid. 1H NMR (400 MHz, CDCl3) δ ppm 3.99 (s, 3H), 6.31–6.33(d, 1H, J = 8 Hz), 7.03–7.05 (d, 1H, J = 8 Hz), 7.23–7.27 (t, 1H, J = 8 Hz), 7.64–7.67 (t, 1H, J = 6 Hz), 7.92–7.94 (d, 1H, 8 Hz).
4-Chloro-8-methoxyquinoline (17)
Compound 16 (2.65 g, 15.1 mmol) was dissolved in POCl3 (4.2 ml, 45.3 mmol) under nitrogen atmosphere and refluxed for 2 h. It was then cooled and concentrated under vacuum. The solid concentrate was taken in a beaker with 200 ml water neutralized with NaHCO3 powder and extracted with EtOAc. The solution was dried over Na2SO4, filtered, and concentrated. This crude product was then purified through column chromatography using ethyl acetate to get 17 as brown solid (2.113 g, 72%). 1H NMR (400 MHz, CDCl3) δ ppm 4.079 (s, 3H), 7.08–7.1 (d, 1H, J = 8 Hz), 7.49–7.56 (m, 2H), 7.76–7.78 (d, 1H, J = 8 Hz), 8.76–8.77 (d, 1H, J =4 Hz).
Procedure I. Preparation of 7-((2-(4-(8-methoxyquinolin-4-yl)piperazin-1-yl)ethyl) (propyl) amino)-5,6,7,8-tetrahydronaphthalen-2-ol (18a)
To a mixture of 9a (0.6 g, 1.89 mmol) and diisopropylethylamine (0.4 mL, 2.08 mmol, 1.1 equiv) in 15 mL isopropanol was added 4-chloro-8-methoxyquinoline (17) (0.366 g, 1.89 mmol, 1 equiv). The mixture was refluxed with stirring for overnight. It was then evaporated and concentrate was dissolved in CH2Cl2 (50 mL) and the solution obtained was washed with 5% NaHCO3 (3 × 50 mL), brine (2 × 50 mL), and then dried over Na2SO4. The solution was filtered and evaporated to dryness. The residue was purified through column chromatography using 20% methanol in ethyl acetate to give 18a as white wax (0.66 g, 73% yield). 1H NMR (400 MHz, CDCl3) δ ppm 0.93–0.95 (t, 3H, 4 Hz), 1.75 (m, 3H), 2.11–2.21 (m, 2H), 2.69–3.07 (m, 13H), 3.23 (s, 4H), 3.47 (s, 1H), 4.02 (s, 3H), 6.60 (s, 1H), 6.66–6.68 (d, 1H, J = 8 Hz), 6.82–6.84 (t, 2H, J = 4 Hz), 6.98–7.00 (d, 1H, J = 8 Hz), 7.36–7.42 (t, 1H, J = 12 Hz), 7.50–7.52 (d, 1H, J = 8 Hz), 8.67–8.68 (d, 1H, J = 4 Hz).
Preparation of 6-((2-(4-(8-methoxyquinolin-4-yl)piperazin-1-yl)ethyl)(propyl) amino)-5,6,7,8-tetrahydronaphthalen-1-ol (18b)
This compound was prepared from 9b (0.668 g, 2.1 mmol) and 17 (0.4 g, 2.1 mmol) following the procedure I to get 18b as white wax (yield 0.76 g, 75 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.92–0.95 (t, 3H, J = 6 Hz), 1.58–1.65 (m, 3H), 2.04–2.14 (m, 2H), 2.73–2.96 (m, 13H), 3.25 (bs, 4H), 3.48 (s, 1H), 4.04 (s, 3H), 6.57–6.59 (d, 1H, J = 8 Hz ), 6.66–6.68 (d, 1H, J = 8 Hz), 6.86–6.87(d, 1H, J = 4 Hz), 6.92–6.96 (t, 1H, J = 8 Hz), 6.99–7.01 (d, 1H, J = 8 Hz), 7.37–7.41 (t, 1H, J = 8 Hz), 7.54–7.56 (d, 1H, J = 8 Hz), 8.72–8.73 (d, 1H, J = 4 Hz) .
Preparation of (−)-6-((2-(4-(8-methoxyquinolin-4-yl)piperazin-1-yl)ethyl) (propyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol, (−)18b
This compound was prepared from (−)9b (0.50 g, 1.6 mmol) and 17 (0.305 g, 1.6 mmol) following the procedure I to afford (−)18b as white wax (0.419 g, 56 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.98–1.00 (t, 3H, J = 4 Hz), 1.58–1.65 (m, 3H), 2.04–2.14 (m, 2H), 2.85–3.12 (m, 13H), 3.25 (bs, 4H), 3.48 (s, 1H), 4.06 (s, 3H), 6.57–6.59 (d, 1H, J = 8 Hz ), 6.87–6.88 (d, 1H, J = 4 Hz), 6.92–6.97(m, 2H), 7.01–7.03 (d, 1H, J = 8 Hz), 7.39–7.43 (t, 1H, J = 8 Hz), 7.54–7.57 (t, 1H, J = 6 Hz), 8.73–8.74 (d, 1H, J = 4 Hz).
(+)-6-((2-(4-(8-methoxyquinolin-4-yl)piperazin-1-yl)ethyl) (propyl)amino)-5,6,7,8-tetrahydronaphthalen-1-ol (+)18b
This compound was prepared from (+)9b (0.62 g, 1.95 mmol) and 17 (0.378 g, 1.95 mmol) following the procedure I to make (+)18b as white wax (0.565 g, 61 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.97–0.99 (t, 3H, J = 4 Hz), 1.57–1.64 (m, 3H), 2.04–2.14 (m, 2H), 2.85–3.12 (m, 13H), 3.25 (bs, 4H), 3.48 (s, 1H), 4.06 (s, 3H), 6.57–6.59 (d, 1H, J = 8 Hz ), 6.87–6.88 (d, 1H, J = 4 Hz), 6.93–6.97(m, 2H), 7.01–7.03 (d, 1H, J = 8 Hz), 7.38–7.42 (t, 1H, J = 8 Hz), 7.53–7.56 (t, 1H, J = 6 Hz), 8.72–8.73 (d, 1H, 4 Hz)
(+)-7-((2-(4-(8-methoxyquinolin-4-yl)piperazin-1-yl)ethyl) (propyl)amino)-5,6,7,8-tetrahydronaphthalen-2-ol, (+)18a
This compound was prepared from (+)9a (0.39 g, 1.22 mmol) and 17 (0.214 g, 1.11 mmol) following the procedure I to get (+)18a as white wax (0.110 g, 19 %). 1H NMR (400 MHz, CDCl3) δ ppm 0.93–0.95 (t, 3H, 4 Hz), 1.75 (m, 3H), 2.11–2.21 (m, 2H), 2.69–3.07 (m, 13H), 3.23 (s, 4H), 3.47 (s, 1H), 4.02 (s, 3H), 6.60 (s, 1H), 6.66–6.68 (d, 1H, J = 8 Hz), 6.82–6.84 (t, 2H, J = 4 Hz), 6.98–7.00 (d, 1H, J = 8 Hz), 7.36–7.42 (t, 1H, J = 12 Hz), 7.50–7.52 (d, 1H, J = 8 Hz), 8.67–8.68 (d, 1H, J = 4 Hz).
Procedure J. Preparation of 4-(4-(2-((7-hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl) amino)ethyl) piperazine-1-yl)quinolin-8-ol (19a, hydrochloride salt)
Compound 18a (0.5 g, 1.05 mmol) and 48% aqueous HBr (10 ml) was refluxed for overnight. It was then cooled and concentrated under vacuum and 50 ml water was added to the crude residue and made freebase with NaHCO3 powder which was then extracted with CH2Cl2, dried over Na2SO4, filtered and evaporated. The greenish solid crude product was dissolved in minimum amount of ethanol at which time ethereal HCl was added, and the crude salt was filtered and dried over vacuum oven. The crude salt was then purified by recrystallization in ethanol. The HCl salt was filtered and dried to yield 0.329 g (26%) of the final compound as off white solid. 1H NMR of HCl salt (400 MHz, DMSO-d6) δ ppm 0.93–0.95 (t, 3H, J = 4 Hz), 1.75 (m, 3H), 2.11–2.21 (m, 2H), 2.69–3.07 (m, 13H), 3.23 (s, 4H), 3.47 (s, 1H), 6.58 (s, 1H), 6.60–6.61 (d, 1H, J = 4 Hz), 6.91–6.93 (d, 1H, J = 8 Hz), 7.35–7.40 (m, 2H), 7.60–7.68 (m, 2H), 8.60–8.62 (d, 1H, J = 8 Hz). The hydrochloride salt, mp 244–246 °C. Analysis calculated for (C28H38.8N4O3.4, 4HCl) C, H, N.
4-(4-(2-((5-hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl) amino)ethyl) piperazin-1-yl)quinolin-8-ol (19b, hydrochloride salt)
This compound was prepared from 18b following the procedure J that gave 19b off white solid (31 %). 1H NMR of HCl salt (400 MHz, DMSO-d6) δ ppm 0.92–0.95 (t, 3H, J = 6 Hz), 1.58–1.65 (m, 3H), 2.04–2.14 (m, 2H), 2.73–2.96 (m, 13H), 3.25 (bs, 4H), 3.48 (s, 1H), 6.55–6.57 (d, 1H, J = 8 Hz ), 6.62–6.64 (d, 1H, J = 8 Hz), 6.92–6.96 (t, 1H, J = 8 Hz), 7.31–7.33 (d, 1H, J = 8 Hz), 7.39–7.41 (d, 1H, J = 8 Hz), 7.55–7.57 (m, 2H), 8.57–8.59 (d, 1H, J = 8 Hz). The hydrochloride salt, mp 249–251 °C. Analysis calculated for (C28H38.4N4O3.2, 4HCl) C, H, N.
(−)-4-(4-(2-((5-hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl) (propyl)amino)ethyl)piperazin-1-yl)quinolin-8-ol ((−)19b, hydrochloride salt)
This compound was prepared from (−)18b (0.480 g, 1.01 mmol) following the procedure J to afford (−)19b off white solid (0.214.65 g, 35 %). 1H NMR (free base, 400 MHz, CDCl3) δ ppm 0.95–0.99 (t, 3H, J = 8 Hz), 1.46–1.53 (m, 3H), 2.24–2.50 (m, 2H), 2.84–3.06 (m, 13H), 3.25 (bs, 4H), 3.49–3.67 (m, 1H), 6.52–6.54 (d, 1H, J = 8 Hz ), 6.60–6.62 (d, 1H, J = 8 Hz), 6.85–6.86 (d, 1H, J = 4 Hz), 6.95–6.99 (t, 1H, J = 8 Hz), 7.10–7.12 (d, 1H, J = 8 Hz), 7.35–7.39 (t, 1H, J = 8 Hz), 7.44–7.46 (d, 1H, 8 Hz), 8.57–8.58 (d, 1H, J = 4 Hz). [α]25D= (−)36° (c=0.5, CH3OH). The hydrochloride salt, mp 237–240 °C. Analysis calculated for (C28H37N4O2.5, 4HCl) C, H, N.
(+)-4-(4-(2-((5-hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl) (propyl)amino)ethyl)piperazin-1-yl)quinolin-8-ol ((+)19b, hydrochloride salt)
This compound was prepared from (+)18b (0.520 g, 1.10 mmol) following the procedure J to make (+)19b off white solid (0.259 g, 39 %). 1H NMR (free base, 400 MHz, CDCl3) 1H NMR (400 MHz, CDCl3) δ ppm 0.96–0.99 (t, 3H, J = 6 Hz), 1.47–1.52 (m, 3H), 2.24–2.50 (m, 2H), 2.85–3.05 (m, 13H), 3.25 (bs, 4H), 3.49–3.67 (m, 1H), 6.53–6.55 (d, 1H, J = 8 Hz ), 6.61–6.63 (d, 1H, J = 8 Hz), 6.86–6.88 (d, 1H, J = 8 Hz), 6.95–6.99 (t, 1H, J = 8 Hz), 7.10–7.12 (d, 1H, J = 8 Hz), 7.34–7.38 (t, 1H, J = 8 Hz), 7.46–7.48 (d, 1H, 8 Hz), 8.56–8.57 (d, 1H, J = 4 Hz). [α]25D= (+)33.6° (c=0.5, CH3OH). The hydrochloride salt, mp 230–232 °C. Analysis calculated for (C28H41N4O4.5, 4HCl) C, H, N.
(+)-4-(4-(2-((7-hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl) (propyl)amino)ethyl)piperazin-1-yl)quinolin-8-ol ((+)19a, hydrochloride salt)
This compound was prepared from (+)18a (0.110 g, 0.232 mmol) following the procedure J that yielded (+)19a off white solid (0.055 g, 43 %).1H NMR of HCl salt (400 MHz, DMSO-d6) δ ppm δ ppm 0.89–0.91 (t, 3H, J = 4 Hz), 1.71 (m, 3H), 2.09–2.19 (m, 2H), 2.67–3.06 (m, 13H), 3.20 (s, 4H), 3.47 (s, 1H), 6.56 (s, 1H), 6.59–6.60 (d, 1H, J = 4 Hz), 6.91–6.92 (d, 1H, J = 4 Hz), 7.32–7.36 (m, 2H), 7.64–7.68 (m, 2H), 8.59–8.61 (d, 1H, J = 8 Hz). [α]25D= (+)32.4° (c=0.5, CH3OH). The hydrochloride salt, mp 239–241 °C. Analysis calculated for (C28H37.4N4O2.7, 4HCl) C, H, N.
Complexation of 19b with iron (III) chloride
Compound 19b (4HCl salt) was dissolved in water to make 600 µM solution and the pH of the solution was found to be 3.66. The UV scanned spectra of the solution was taken from 200 nm to 760nm. FeCl3, 6H2O was next dissolved in water to make 600 µM colorless solution. The two solutions were mixed together in equal volume which gave green color solution at pH 3.76. The solution was subjected to UV scan as before. Then pH of the solution was next increased to 4.0 by adding base diisopropylethylamine (DIPEA) (diluted with H2O) base which produced deep green color which was followed by UV spectra scan. The pH of the solution was next increased to 7.4 by adding additional amount of DIPEA which produced light brown color solution followed by scanning in UV spectra from 390 nm to 770 nm.
Iron-chelating capacity
The iron-binding capacity of the test compound 19b (iron chelating compounds) was determined by assessing their ability to compete with ferrozine (3-[2-pyridyl]-5,6-diphenyl-1,2,4-triazine-4,4'-disulfonic acid) for ferrous ions which resultis in reduced absorbance of the ferrozine-Fe(II) complex at 562 nm. Complexation reactions were carried out in 5% ammonium acetate buffer (pH 6.9). Various conc. of the test compound 19b (25–250 µM) were incubated with {[NH4]2[Fe][SO4]2·6H2O} (20 µM) for 30 min, followed by the addition of 80 µM ferrozine. After incubation at ambient temperature for 1 h, the UV absorbance of the resulting solutions at 562 nm was read. The limitation of this assay is that, chelating compound forms complex with iron at higher drug concentration which also gives absorbance reading at 562 nm. We subtracted the absorbance by a blank solution containing only drug and iron without ferrozine (corrected absorbance). The Fe (II)-chelating effect was calculated as follows: Chelating effect (%) = [1 − (Abs562nm of sample)/ (Abs562nm of control)] × 100 The chelating effect is expressed as percent of control [80 uM ferrozine, 20 uM Ferrous ammonum sulfate in pH 6.9 ammonium acetate buffer (5%)].
Deoxyribose antioxidant assay procedure
The assay was carried out by following the published procedure37. Briefly, the reaction mixture contained in a final volume of 1.0 ml, which contained the following reagents: deoxyribose (2.8 mM), potassium phosphate buffer, pH 7.4 (100 mM), increasing concentration of test drug (0–1000 µM), ferric chloride (100 µM), ascorbic acid (100 µM), EDTA (100 µM) and H2O2 (1 mM). Fresh solution of deoxyribose, ferric chloride and H2O2 were made prior to use. All solutions were made in degassed water. H2O2 solution was added at the end to initiate the reaction to form hydroxyl radical. The reaction was continued for 30 minutes at 37°C. The extent of deoxyribose degradation was monitored by formation of malondialdehyde ( a pink color chromogen) determined by the addition of 1 ml of 1% (w/v) 2-Thiobarbituric acid in 50 mM NaOH (TBA) and 1 ml of 2.8 % (w/v) trichloroacetic acid (TCA). After addition of TBA and TCA, the solutions were heated at 85 °C for 20 minutes. Pink chromogen was formed. The solutions were cooled and the resultant absorbance was read at 532 nM against appropriate blanks. The intensity of pink color decreases in presence of increased concentration of hydroxyl radical scavenger.
Dopamine receptor affinity and agonism
Binding potency was monitored by inhibition of [3H]spiperone (15.0 Ci/mmole, Perkin-Elmer) binding to dopamine rD2 and rD3 receptors expressed in HEK-293 cells, in a buffer containing 0.9% NaCl as described by us previously.26 Functional activity of test compounds in activating dopamine hD2 and hD3 receptors expressed in CHO cells was measured by stimulation of binding of [35S]GTPγS (1250 Ci/mmole, Perkin-Elmer) in comparison to stimulation by the full agonist dopamine as described by us previously.26
Animal Experiment
Drugs and chemicals
The following commercially available drugs were used in the experiment: reserpine hydrochloride (Alfa Aesar), Ropinirole (Sigma), The hydrochloride salts of compounds (−)-19b and ropinirole were dissolved in water for both locomotor and 6-OH-DA rotational experiments. Reserpine was dissolved in 10–25 µL of glacial acetic acid and further diluted with 5.5% glucose solution. All compounds for this study were administered in a volume of 0.1 to 0.2 mL for subcutaneous administration and 0.7 to 0.9 mL for intraperitoneal administration into each rat.
Animals
In rodent studies, animals were male Sprague-Dawley rats from Harlan (Indianapolis, IN) weighing 220–225 g unless otherwise specified. The lesioned rats (290–320 g) were purchased from Taconic Biotechnology (Rensselaer, NY) and their unilateral lesion was checked twice by apomorphine challenge following the surgery. Animals were maintained in sawdust-lined cages in a temperature and humidity controlled environment at 22 ± 1°C and 60 ± 5 % respectively, with a 12-h light/dark cycle, with lights on from 6:00 AM to 6:00 PM. They were group housed with unrestricted access to food and water. All experiment was performed during the light component. All animal use procedures were in compliance with the Wayne State University Animal Investigation Committee consistent with AALAC guidelines.
Reversal of Reserpine-Induced Hypolocomotion in Rats
Administration of reserpine induces catalepsy in rodents primarily by blocking the vesicular monoamine trasporter (VMAT) which helps in the internalization of monoamines into vesicles, resulting in metabolism of unprotected monoamines in the cytosol that ultimately causes depletion of monoamines in the synapse of the peripheral sympathetic nerve terminals 38, 41. The ability of the compound (−)-19b to reverse the reserpine induced hypolocomotion was investigated 42. Ropinirole was used as standard reference compound in this study. Reserpine (5.0 mg/kg, s.c.) or saline (s.c.) were administered 18h before the injection of drug or vehicle (i.p.). The rats were placed individually in chambers for 1 h for acclimatization purpose before the administration of the test drug, standard drug or vehicle. Immediately after administration of drug or vehicle, animals were individually placed in versamax animal activity monitor chamber (45×30×20 cm) (AccuScan Instruments, Inc. Columbus, OH) to start measuring locomotor activity.. Locomotion was monitored for 6 h. Consecutive interruption of two infrared beams situated 24 cm apart and 4 cm above the cage floor in the monitor chamber recorded movement. The data were presented as horizontal counts (HACTV). The effect of the individual doses of drugs on locomotor activity was compared with respect to saline treated controls (mean ± S.E.M.). The data were analyzed by one way analysis of variance (ANOVA) followed by Dunnett’s post-hoc test. The effect was considered significant if the difference from control group was observed at p<0.05.
In vivo Rotational experiment with 6-OH-DA lesioned rats
The first 14 days post-lesion challenge with apomorphine was done with lesioned animals to observe a complete rotation session post administration. In the second challenge with apomorphine (0.05 mg/kg) 21 days post lesion, contralateral rotations were recorded for 30 min; apomorphine produced rotations in all four rats (average rotation > 250) indicating successful unilateral lesion. In these rats, lesion was performed on the left side of the medial forebrain bundle in the brain and the coordinates used from Bregma are: AP-4.3, ML +1.2, DV −8.3. The rotations produced upon agonist challenge occurring clockwise. In this study, apomorphine was also used as a reference compound. The test drugs including ropinirole were dissolved in saline and were administered SC. The rotations were measured over 7–10 hours. For control, vehicle was administered alone. Rotations were measured in the Rotomax Rotometry System (AccuScan Instruments, Inc. Columbus, Ohio) equipped with Rotomax Analyser, high resolution sensor and animal chambers with harnesses. Data were analyzed with Rotomax Window software program. Test drug (−)-19b (0.5 and 1 µMol/kg) and ropinirole (5 µMol/kg) dissolved in saline were administered sc. The rotations were measured in a rotational chamber immediately after administration of drugs. The data were collected at every 30 min. Data were analyzed by Graph Pad (Version 4, San Diego) program. All drugs produced contralateral rotations in all lesioned rats which lasted over 3–10 hours.
Supplementary Material
Figure 1.
Molecular structures of D3 preferring compounds and iron chelators.
Figure 6.
Effect of different drugs upon reserpine (5.0 mg/Kg, s.c.)-induced hypolocomotion in rats. Data are means ± S.E.M, n = 4 per value. Horizontal activity was measured as described under materials and methods. Panel A is the representation of horizontal locomotor activity at discrete 30-min intervals after the administration of (−)19b (i.p.) and ropinirole (s.c.) at the dose of 10 µMol/kg compared to control rats in 18 h post reserpine treatment. One way ANOVA analysis demonstrates significant effect among treatments F (3,95) = 31.36 (P< 0.0001). Dunnett’s analysis following ANOVA showed that the effects of (−)19b (P< 0.01) and ropinirole (P< 0.05) were significantly different compared to reserpine control.
Table 1.
Inhibition constants for competing for [3H]spiperone binding to cloned D2L and D3 receptors expressed in HEK cells. Results are means ± SEM for 3–7 experiments each performed in triplicate.
| Compound | Ki, (nM), D2L [3H]Spiperone |
Ki, (nM), D3 [3H]Spiperone |
D2L/D3 |
|---|---|---|---|
| 7-OH-DPAT | 202 ± 34 | 2.35 ± 0.29 | 86.0 |
| (−)-5-OH-DPAT | 58.8 ± 11.0 | 1.36 ± 0.28 | 43.2 |
| 1aa | 26.0 ± 7.5 | 0.825 ± 0.136 | 31.5 |
| 1b | 3.74 ± 0.70 | 0.186 ± 0.030 | 19.7 |
| 12a | 86.0 ± 4.1 | 5.57 ± 1.15 | 15.4 |
| (+)19b | 20.7 ± 1.5 | 7.73 ± 0.64 | 2.67 |
| (−)19b | 3.75 ± 0.63 | 1.28 ± 0.08 | 2.92 |
| 19a | 15.9 ± 2.1 | 0.818 ± 0.165 | 19.6 |
| 19b | 13.8 ± 0.6 | 1.35 ± 0.22 | 10.2 |
| (+)19a | 4.55 ± 0.59 | 1.27 ± 0.15 | 3.58 |
| 12b | 41.4 ± 7.1 | 3.71 ± 0.48 | 11.2 |
Table 2.
Stimulation of [35S]GTPγS binding to hD2 and hD3 receptors expressed in CHO cells. EC50 values (nM) are means ± SEM for 3–6 experiments each performed in triplicate.
| CHO-D2 | CHO-D3 | ||||
|---|---|---|---|---|---|
| Compound | EC50 (nM)a [35S]GTPγS |
%Emax | EC50 (nM)a [35S]GTPγS |
%Emax | D2/D3 |
| Dopamine | 209 ± 29 | 100 | 4.76 ± 0.87 | 100 | 43.9 |
| Ropinirole | 304 ± 11 | 73.9 ± 0.9 | 10.3 ± 1.5 | 66.6 ± 8.1 | 29.5 |
| (−)19b | 4.51 ± 0.93 | 106 ± 4 | 1.58 ± 0.31 | 92.6 ± 3.6 | 2.85 |
| (+)19a | 1.69 ± 0.16 | 55.3 ± 4.8 | 0.74 ± 0.15 | 99.8 ± 1.4 | 2.26 |
EC50 is the concentration producing half-maximal stimulation; for each compound, maximal stimulation (Emax) is expressed as percent of the Emax observed with 1 mM (D2) or 100 uM (D3) of the full agonist DA (%Emax). Results are the means +/− SEM for 3–6 experiments each performed in triplicate.
Acknowledgements
This work is supported by National Institute of Neurological Disorders and Stroke/ National Institute of Health (NS047198, AKD). We are grateful to Dr. K. Neve, Oregon Health and Science University, Portland, USA, for D2L and D3 expressing HEK cells. We are also grateful to Dr. J. Shine, Garvan Institute for Medical Research, Sydney, Australia, for D2L expressing CHO cells.
Abbreviations
- GTPγS
Guanosine 5’-[g-thio]triphosphate
- 5-OH-DPAT
5-Hydroxy-2-(dipropylamino)tetralin
- 6-OHDA
6-Hydroxy dopamine
- CHO
Chinese Hamster Ovary
- HEK
Human embryonic kidney
- L-DOPA
(S)-(3,4 -Dihydroxyphenyl) Alanine
- PD
Parkinson’s Disease
Footnotes
Supporting Information Available: Elemental analysis data for all final targets is available. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Wooten GF. Movement Disorders. Neurologic Principles and Practice. New York, NY, USA: McGraw-Hill; 1997. pp. 153–160. [Google Scholar]
- 2.Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson's disease. J Neuropathol Exp Neurol. 1991;50:743–755. doi: 10.1097/00005072-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Mouradian MM. Recent advances in the genetics and pathogenesis of Parkinson disease. Neurology. 2002;58:179–185. doi: 10.1212/wnl.58.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 5.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53 Suppl 3:S26–S36. doi: 10.1002/ana.10483. discussion S36–S38. [DOI] [PubMed] [Google Scholar]
- 6.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 7.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 8.Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 10.Berg D, Gerlach M, Youdim MB, Double KL, Zecca L, Riederer P, Becker G. Brain iron pathways and their relevance to Parkinson's disease. J Neurochem. 2001;79:225–236. doi: 10.1046/j.1471-4159.2001.00608.x. [DOI] [PubMed] [Google Scholar]
- 11.Jellinger KA. The role of iron in neurodegeneration: prospects for pharmacotherapy of Parkinson's disease. Drugs Aging. 1999;14:115–140. doi: 10.2165/00002512-199914020-00004. [DOI] [PubMed] [Google Scholar]
- 12.Wolozin B, Golts N. Iron and Parkinson's disease. Neuroscientist. 2002;8:22–32. doi: 10.1177/107385840200800107. [DOI] [PubMed] [Google Scholar]
- 13.Faucheux BA, Martin ME, Beaumont C, Hunot S, Hauw JJ, Agid Y, Hirsch EC. Lack of up-regulation of ferritin is associated with sustained iron regulatory protein-1 binding activity in the substantia nigra of patients with Parkinson's disease. J Neurochem. 2002;83:320–330. doi: 10.1046/j.1471-4159.2002.01118.x. [DOI] [PubMed] [Google Scholar]
- 14.Zecca L, Gallorini M, Schunemann V, Trautwein AX, Gerlach M, Riederer P, Vezzoni P, Tampellini D. Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: consequences for iron storage and neurodegenerative processes. J Neurochem. 2001;76:1766–1773. doi: 10.1046/j.1471-4159.2001.00186.x. [DOI] [PubMed] [Google Scholar]
- 15.Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B. The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci. 2000;20:6048–6054. doi: 10.1523/JNEUROSCI.20-16-06048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbull S, Tabner BJ, El-Agnaf OM, Moore S, Davies Y, Allsop D. alpha-Synuclein implicated in Parkinson's disease catalyses the formation of hydrogen peroxide in vitro. Free Radic Biol Med. 2001;30:1163–1170. doi: 10.1016/s0891-5849(01)00513-5. [DOI] [PubMed] [Google Scholar]
- 17.Shamoto-Nagai M, Maruyama W, Yi H, Akao Y, Tribl F, Gerlach M, Osawa T, Riederer P, Naoi M. Neuromelanin induces oxidative stress in mitochondria through release of iron: mechanism behind the inhibition of 26S proteasome. J Neural Transm. 2006;113:633–644. doi: 10.1007/s00702-005-0410-5. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Xie W, Qu S, Pan T, Wang X, Le W. Neuroprotection by iron chelator against proteasome inhibitor-induced nigral degeneration. Biochem Biophys Res Commun. 2005;333:544–549. doi: 10.1016/j.bbrc.2005.05.150. [DOI] [PubMed] [Google Scholar]
- 19.Gaeta A, Hider RC. The crucial role of metal ions in neurodegeneration: the basis for a promising therapeutic strategy. Br J Pharmacol. 2005;146:1041–1059. doi: 10.1038/sj.bjp.0706416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goswami A, Dikshit P, Mishra A, Mulherkar S, Nukina N, Jana NR. Oxidative stress promotes mutant huntingtin aggregation and mutant huntingtin-dependent cell death by mimicking proteasomal malfunction. Biochem Biophys Res Commun. 2006;342:184–190. doi: 10.1016/j.bbrc.2006.01.136. [DOI] [PubMed] [Google Scholar]
- 21.Kaur D, Andersen JK. Ironing out Parkinson's disease: is therapeutic treatment with iron chelators a real possibility? Aging Cell. 2002;1:17–21. doi: 10.1046/j.1474-9728.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 22.Youdim MB, Fridkin M, Zheng H. Novel bifunctional drugs targeting monoamine oxidase inhibition and iron chelation as an approach to neuroprotection in Parkinson's disease and other neurodegenerative diseases. J Neural Transm. 2004;111:1455–1471. doi: 10.1007/s00702-004-0143-x. [DOI] [PubMed] [Google Scholar]
- 23.Zheng H, Weiner LM, Bar-Am O, Epsztejn S, Cabantchik ZI, Warshawsky A, Youdim MB, Fridkin M. Design, synthesis, and evaluation of novel bifunctional iron-chelators as potential agents for neuroprotection in Alzheimer's, Parkinson's, and other neurodegenerative diseases. Bioorg Med Chem. 2005;13:773–783. doi: 10.1016/j.bmc.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 24.Dutta AK, Venkataraman SK, Fei XS, Kolhatkar R, Zhang S, Reith ME. Synthesis and biological characterization of novel hybrid 7-[[2-(4-phenyl-piperazin-1-yl)-ethyl]-propyl-amino]-5,6,7,8-tetrahydro-na phthalen-2-ol and their heterocyclic bioisosteric analogues for dopamine D2 and D3 receptors. Bioorg Med Chem. 2004;12:4361–4373. doi: 10.1016/j.bmc.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Biswas S, Zhang S, Fernandez F, Ghosh B, Zhen J, Kuzhikandathil E, Reith ME, Dutta AK. Further structure-activity relationships study of hybrid 7-{[2-(4-phenylpiperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphtha len-2-ol analogues: identification of a high-affinity D3-preferring agonist with potent in vivo activity with long duration of action. J Med Chem. 2008;51:101–117. doi: 10.1021/jm070860r. [DOI] [PubMed] [Google Scholar]
- 26.Biswas S, Hazeldine S, Ghosh B, Parrington I, Kuzhikandathil E, Reith ME, Dutta AK. Bioisosteric heterocyclic versions of 7-{[2-(4-phenyl-piperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphth alen-2-ol: identification of highly potent and selective agonists for dopamine D3 receptor with potent in vivo activity. J Med Chem. 2008;51:3005–3019. doi: 10.1021/jm701524h. [DOI] [PubMed] [Google Scholar]
- 27.Pierre JL, Baret P, Serratrice G. Hydroxyquinolines as iron chelators. Curr Med Chem. 2003;10:1077–1084. doi: 10.2174/0929867033457584. [DOI] [PubMed] [Google Scholar]
- 28.d'Hardemare Adu M, Alnaga N, Serratrice G, Pierre JL. Oxinobactin, a siderophore analogue to enterobactin involving 8-hydroxyquinoline subunits: synthesis and iron binding ability. Bioorg Med Chem Lett. 2008;18:6476–6478. doi: 10.1016/j.bmcl.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 29.Hoeve WWH. The design of resolving agents. Chiral cyclic phosphoric acids. J. Org. Chem. 1985;50:4508–5414. [Google Scholar]
- 30.Park JH, Choi JK, Lee E, Lee JK, Rhim H, Seo SH, Kim Y, Doddareddy MR, Pae AN, Kang J, Roh EJ. Lead discovery and optimization of T-type calcium channel blockers. Bioorg Med Chem. 2007;15:1409–1149. doi: 10.1016/j.bmc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Briehl H, Lukosch A, Wentrup C. Reactive nitrogenous molecules from Meldrum's acid derivatives, pyrrole-2,3-diones, and isoxazolones. J. Org. Chem. 1984;49:2772–2779. [Google Scholar]
- 32.Madrid PB, Sherrill J, Liou AP, Weisman JL, Derisi JL, Guy RK. Synthesis of ring-substituted 4-aminoquinolines and evaluation of their antimalarial activities. Bioorg Med Chem Lett. 2005;15:1015–1018. doi: 10.1016/j.bmcl.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh B, Antonio T, Zhen J, Kharkar P, Reith ME, Dutta AK. Development of (S)-N6-(2-(4-(Isoquinolin-1-yl)piperazin-1-yl)ethyl)-N6-propyl-4,5,6,7-tetrahydrobenzo[d]-thiazole-2,6-diamine and its analogue as a D3 receptor preferring agonist: Potent in vivo activity in Parkinson’s disease animal models. J. Med. Chem. doi: 10.1021/jm901184n. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stookey L. Ferrozine-A New Spectrophotometric Reagent for Iron. ANALYTICAL CHEMISTRY. 1970;42:779–781. [Google Scholar]
- 35.Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine) Anal Biochem. 1971;40:450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- 36.Farkas E, Enyedy EA, Zekany L, Deak G. Interaction between iron(II) and hydroxamic acids: oxidation of iron(II) to iron(III) by desferrioxamine B under anaerobic conditions. J Inorg Biochem. 2001;83:107–114. doi: 10.1016/s0162-0134(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 37.Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: a simple "test-tube" assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 38.Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180:1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- 39.Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. European journal of pharmacology. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- 40.Ten Hoeve W, Wynberg H. The design of resolving agents. Chiral cyclic phosphoric acids. The Journal of Organic Chemistry. 2002;50:4508–4514. [Google Scholar]
- 41.Millan MJ, Di Cara B, Hill M, Jackson M, Joyce JN, Brotchie J, McGuire S, Crossman A, Smith L, Jenner P, Gobert A, Peglion JL, Brocco M. S32504, a novel naphtoxazine agonist at dopamine D3/D2 receptors: II. Actions in rodent, primate, and cellular models of antiparkinsonian activity in comparison to ropinirole. J Pharmacol Exp Ther. 2004;309:921–935. doi: 10.1124/jpet.103.062414. [DOI] [PubMed] [Google Scholar]
- 42.McCall RB, Lookingland KJ, Bedard PJ, Huff RM. Sumanirole, a highly dopamine D2-selective receptor agonist: in vitro and in vivo pharmacological characterization and efficacy in animal models of Parkinson's disease. J Pharmacol Exp Ther. 2005;314:1248–1256. doi: 10.1124/jpet.105.084202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.