Abstract
Background
The Guided Care Program for Families and Friends (GCPFF) is one component of “Guided Care” (GC), a model of primary care for chronically ill older adults that is facilitated by a registered nurse who has completed a supplemental educational curriculum.
Methods
The GCPFF melds support for family caregivers with the delivery of coordinated and comprehensive chronic care and seeks to improve the health and well-being of both patients and their family caregivers. The GCPFF encompasses (a) an initial meeting between the nurse and the patient's primary caregiver, (b) education and referral to community resources, (c) ongoing “coaching,” (d) a six-session group Caregiver Workshop, and (e) monthly Support Group meetings, all facilitated by the patient's GC nurse.
Results
A cluster-randomized controlled trial of GC is underway in 14 primary care physician teams. Of 904 consented patients, 450 (49.8%) identified a primary caregiver; 308 caregivers met eligibility criteria, consented to participate, and completed a baseline interview. At 6-month follow-up, intervention group caregivers’ mean Center for Epidemiological Studies Depression (CESD) and Caregiver Strain Index (CSI) scores were respectively 0.97 points (p = .14) and 1.14 points (p = .06) lower than control group caregivers’. Among caregivers who provided more than 14 hours of weekly assistance at baseline, intervention group caregivers’ mean CESD and CSI scores were respectively 1.23 points (p = .20) and 1.83 points (p = .04) lower than control group caregivers’.
Conclusions
The GCPFF may benefit family caregivers of chronically ill older adults. Outcomes will continue to be monitored at 18-months follow-up.
Keywords: Caregiving, Chronic disease, Nursing, Primary care
IN light of projected increases in the numbers of older people living with chronic illness and disability, identifying effective mechanisms to improve the quality of chronic care has become an issue of growing importance (1). In parallel, documented burdens imposed by long-term, high-intensity caregiving (2,3) have motivated interventions to better support caregivers (4). Despite growing evidence that involving family caregivers may benefit patients’ quality of health care (5–7) and that improving health care delivery for frail older adults may benefit their caregivers (8–10), intervention research on chronic care and family caregiving has been largely disconnected.
Interventions to support caregivers have typically been offered outside the health care delivery system and have been focused on provision of resources (education, skills, or services) and emotional support to better cope with role-related challenges. We are unaware of interventions that systematically support family caregivers while simultaneously restructuring health care delivery processes to improve the quality of patients’ chronic care, despite the facts that older adults are often accompanied to physician visits (11) and that caregivers frequently assist with medically oriented tasks (12,13).
The Guided Care Program for Families and Friends (GCPFF) was developed to support caregivers of older adults with complex health-related needs, with the joint goals of improving patients’ health and the well-being of their families and friends. The intervention combines “best practice” training and support for caregivers within the context of an innovative model of health care, Guided Care (GC), which facilitates coordinated, comprehensive, evidence-based health care for multimorbid older adults. This article describes the development and structure of the GCPFF as well as preliminary results and lessons learned from an ongoing randomized trial.
MODEL DESIGN
GC was created to improve the quality and outcomes of chronic care for multimorbid adults (14). In GC, a registered nurse, who has completed a supplemental educational curriculum and joined a primary care practice, works closely with several primary care physicians (PCPs) to meet the chronic care needs of 50–60 chronically ill patients who are at high risk for heavy use of health services during the coming year. Using a web-accessible electronic health record (EHR), the Guided Care Nurse (GCN) collaborates with the patient's PCP to facilitate eight clinical processes: (a) assessing the patient at home, (b) creating an evidence-based care plan, (c) promoting patient self-management, (d) proactively monitoring the patient's conditions, (e) coaching the patient to practice healthy behaviors, (f) coordinating patient's transitions between sites and providers of care, (g) facilitating access to community resources, and (h) educating and supporting caregivers (the GCPFF).
The GCPFF: Design
The inclusion of education and support for patients’ families and friends within GC was motivated by compelling results achieved by a spousal dementia caregiving intervention (6,15), although profound differences in intervention scope, target patient population, and discipline of core health professionals inhibited its translation to GC. To develop the GCPFF, we relied on insight from related intervention studies and meta-analyses, consultation with individuals working in the field, focus groups with community- and employer-sponsored caregiver support groups, and an advisory committee of experts. Insights from these activities were incorporated along with the logistical and resource constraints of contemporary primary care practice.
Because limited information was available to guide the development of a program to support family caregivers of high-risk older adults with diverse medical needs within the context of primary care practice, an initial issue was how to define patients’ caregivers. Reflecting our belief that establishing productive working relationships between the GCN and patient's informal supports ultimately benefits both caregivers and patient care, caregivers were identified broadly as relatives or unpaid friends who assisted patients with daily activities or health-related tasks. Because some caregivers may not identify with or may even be offended by the term “caregiver,” (16) the term “Families and Friends” was used, although caregiver is used in this article for the sake of simplicity.
The GCPFF includes five components:
-
An initial meeting between nurses and patients’ caregivers: In light of the benefits of tailored assistance (4), individual consultation is a key aspect of the GCPFF. As a part of developing their caseload of patients, each GCN conducts an in-home initial assessment with the patient, followed by, if relevant, a brief (approximately 30-minute) in-person meeting with the primary caregiver. Goals of this meeting are to initiate a working relationship with patient's informal supports, to provide a forum for the caregiver to state his or her own needs and concerns and to identify and facilitate relevant education and community services referral.
Prior to the meeting, the nurse mails the caregiver a personalized letter, along with a “Family and Friends Intake Form” to be completed before the meeting. The intake form requests caregivers’ demographic and health information, a description of assistance they provide, their desire for information and referral, as well as an opportunity to describe caregiving-related challenges, rewards, and strengths. The intake form was developed to maximize the productivity of the meeting by guiding conversation and reducing time spent documenting information. Information from the form and meeting is summarized and entered by the GCN into the patient's EHR.
Education and referral to community resources: Given the demonstrated importance of caregiver education (4,17), each GCN is provided with disease- and task-specific print and electronic health education materials to disseminate as requested or needed. In addition, the GCN works with representatives from their local Area Administration on Aging office to make referrals to community resources at the time of initial assessments and as issues emerge over time.
Ongoing coaching: The GCN is available by phone and e-mail during business hours to address caregivers’ questions and concerns regarding patients’ health needs. In addition, GCNs interact with caregivers during and after the occurrence of an acute health event or hospitalization or at least quarterly to ascertain their well-being and to inquire about their needs for information and referral.
Workshop: To combine the diversity of GC caregivers’ needs with the efficiency of group interactions, a Workshop for Families and Friends was developed, guided by the philosophy and approach of chronic disease self-management (18), existing programs for family caregivers (19,20), and a lay-led self-management program for amputees, Promoting Amputee Life Skills (21). The Workshop emphasizes how to cope with caregiver concerns by building on strengths, reframing challenges, and developing problem-solving skills. Nurses facilitate the Workshop over the course of six weekly 90-minute sessions (Table 1).
Support groups: GCNs facilitate 1-hour monthly Support Group meetings for families and friends of their patients. Objectives are to reinforce skills and techniques discussed in the Workshop, cultivate relationships and communication between the GCN and caregivers, and provide caregivers the opportunity to share experiences, emotional support, and practical strategies for coping with difficult situations. Previous studies support the use of a trained group leader to facilitate discussion and, if needed, to intervene to inhibit excessive negativity (22,23). Each group self-directs the use of its time (eg, whether to invite speakers or engage in unstructured discussion) and when to hold the meetings.
Table 1.
Guided Care Workshop for Families and Friends
| Topic of Session | Objectives |
| 1. Introduction to self-management | • Be oriented to the program |
| • Understand the benefits of self-management | |
| • Set positive goals | |
| • Create an action plan | |
| • Get to know each other | |
| 2. Taking care of you | • Identify stressors in daily life |
| • Learn how to apply a problem-solving approach for coping with stressful events | |
| • Know several strategies for promoting positive moods | |
| 3. Social support and relationships | • Understand connection between social support, mental, and physical health |
| • Assess personal social needs and goals | |
| • Discuss helpful and unhelpful behaviors and communication | |
| • Know strategies to improve communication | |
| • Identify goals to initiate or improve existing relationships | |
| 4. Communication about health care issues | • Discuss application of listening skills and assertive communication with the medical community |
| • Know strategies for communication with someone who has dementia | |
| • Identify strategies for communicating with medical providers | |
| 5. Planning for the future | • Appreciate the benefits of talking about the future |
| • Know and evaluate options for care | |
| • Discuss strategies for communicating with family about the future, including five wishes | |
| • Understand potential legal issues | |
| 6. Staying on track | • Recognize and appreciate personal strengths and skills |
| • Anticipate set backs | |
| • Discuss strategies for getting back on track and coping with stress | |
| • Recognize each others’ successes |
Preparing GCNs
A two-phase structured educational curriculum was developed to equip GCNs for their role in facilitating the GCPFF. Through a combination of readings, recorded lectures, case-based group seminars, and applied role-playing activities, the objective of the first phase of curriculum is to prepare GCNs to develop their caseloads of patients’ families and friends, to make referrals to community resources, and to deliver ongoing coaching to patients’ families regarding patients’ health needs. The second phase of the curriculum focuses on group facilitation skills and preparation to deliver the GCPFF Workshop and Support Groups.
METHODS
Recruitment
A cluster-randomized trial of GC is underway within 14 PCP teams in three Mid-Atlantic health care delivery systems. As detailed elsewhere, seven nurses were recruited, trained, and integrated into PCP teams during the spring and summer of 2006 (24). Established patients of participating physicians were eligible for the study if they were at least 65 years of age and ranked in the upper quartile of risk for using health services heavily during the coming year using hierarchical condition category predictive model scores from health insurance claims’ diagnoses (25). A total of 904 high-risk patients of participating physicians were identified, provided informed consent, and completed a baseline interview with a professional survey research interviewer. Participants with functional disabilities were asked to identify their primary caregiver as the family member or unpaid friend who helped “the most” with daily activities and/or health-related tasks at the time of the baseline interview. Primary caregivers were screened to confirm eligibility, provided informed consent, and completed a baseline in-person interview. Upon completion of baseline interviews, patient–caregiver dyads were cluster randomized by physician team to receive GC or usual care.
Measures
Baseline interviews were conducted in-person; follow-up interviews (approximately 6 months after each patient's start date) were conducted by telephone by rigorously trained, closely supervised professional interviewers who were masked to group assignment, used computer-assisted interviewing technology, and underwent 10% reliability testing. Primary caregivers were asked about sociodemographic characteristics, employment, health, and the nature of assistance provided to patients. Primary outcomes of interest included depression, as measured by the Center for Epidemiological Studies Depression (CESD) (26), and strain, based on the Caregiver Strain Index (CSI) (27,28). A mailed survey was administered to intervention group caregivers who had participated in the GCPFF Workshop (to ascertain perceived utility) and to those who had not participated (to understand reasons for nonparticipation).
Analysis
As discussed elsewhere (24), chained equations (29,30) were used to impute values for patient responses that were missing at baseline; this procedure was replicated for caregiver responses that were missing at baseline. GC patients, stratified by receipt of assistance, and caregivers, stratified by intervention group, were compared using chi-square or t tests of significance, as appropriate. The effect of the intervention on caregiver strain and depression at 6 months was examined using multivariate linear regression models that accounted for study site, intervention group, and baseline CSI and CESD scores, respectively. Effect sizes were computed as the difference between intervention and control group scores at 6 months, divided by the pooled standard deviation. Given the broad definition used to identify caregivers for this study and the correlation between depression, strain, and hours of care (31), stratified analyses of 6-month outcomes were conducted, dichotomized by the median 14 hours of weekly assistance. Analyses were performed in Stata version 9 statistical software (StataCorp, College Station, TX).
Results
Description of Study Sample
Of 904 patients randomized to participate in the GC study, 449 (49.7%) identified a caregiver. Participants who identified a caregiver were significantly older, poorer, in worse health, and less educated than their counterparts but were comparable in gender and marital status (Table 2). Approximately 16% of patients receiving help also reported providing assistance to another person.
Table 2.
Guided Care Patient Characteristics by Presence of a Caregiver
| No Caregiver (N = 455), M (SD)/Percentage | Caregiver (N = 449), M (SD)/Percentage | |
| Age** | 76.7 (6.0) | 78.6 (7.1) |
| HCC score** | 1.8 (0.8) | 2.3 (1.2) |
| No. of chronic conditions** | 4.0 (1.6) | 4.5 (1.9) |
| SF-36 physical component** | 42.1 (9.8) | 34.7 (10.3) |
| SF-36 mental component** | 52.2 (10.5) | 46.8 (12.9) |
| PACIC score (1 low to 5 high) | 2.6 (0.8) | 2.6 (0.7) |
| Female | 51.7% | 57.9% |
| Married | 45.4% | 48.8% |
| Caucasian* | 53.0% | 47.0% |
| Excellent, very good, or good self-rated health** | 67.9% | 45.9% |
| At least one ADL limitation** | 16.0% | 47.0% |
| Patient has dementia** | 0.9% | 8.3% |
| Could use more support with regular activities** | 19.2% | 33.4% |
| Has someone for emotional support** | 72.5% | 83.7% |
| Could use more emotional support | 24.0% | 27.8% |
| Patient helps care for someone else | 19.4% | 15.5% |
| Has at least a high school education** | 80.0% | 65.9% |
| Not enough money to make ends meet* | 9.0% | 13.8% |
| Enrolled in Medicaid* | 15.6% | 20.9% |
Notes: HCC = hierarchical condition category (1 = average risk of heavy future use of health services); SF-36 = Short-Form 36 (range: 0 [poor function] to 100 [excellent function]); PACIC = Patient Assessment of Chronic Illness Care; and ADL = activity of daily living.
*p < .05; **p < .001.
In total, 308 caregivers were eligible and consented to participate in the study. Primary caregivers were on average 62 years of age and were women (71.4%), married (68.5%), and predominantly spouses/partners (46.1%) or adult children (44.5%) of patients. Nearly two thirds reported helping patients at least daily (61.4%); they provided an average of 25 hours of weekly assistance. There were no statistically significant differences in caregiver characteristics between intervention and control groups at baseline (Table 3).
Table 3.
Caregiver Characteristics by Treatment Group
| Control (N = 152), Percentage/M (SD) | Intervention (N = 156), Percentage/M (SD) | Total (N = 308), Percentage/M (SD) | |
| Age | 63.2 (15.1) | 60.6 (15.1) | 61.8 (15.1) |
| Female gender | 67.8% | 75.0% | 71.4% |
| Married | 69.7% | 67.3% | 68.5% |
| Adult child caregiver | 45.4% | 43.6% | 44.5% |
| Spousal caregiver | 45.4% | 46.8% | 46.1% |
| Employed for pay | 42.8% | 39.1% | 40.9% |
| Helped patient daily | 62.5% | 60.3% | 61.4% |
| Average hours of assistance per week | 23.3 (25.8) | 26.0 (27.7) | 24.7 (26.8) |
| Depression (CESD)† | |||
| Baseline | 7.1 (7.7) | 6.8 (6.1) | 7.0 (7.0) |
| 6-Mo | 6.7 (6.7) | 5.6 (5.5) | 6.1 (6.2) |
| Change in CESD | –0.4 | –1.2 | –0.9 |
| Strain (CSI)‡ | |||
| Baseline | 7.0 (5.8) | 6.6 (5.4) | 6.8 (5.6) |
| 6-Mo | 7.9 (6.1) | 6.5 (5.3) | 7.2 (5.7) |
| Change in CSI | 0.9 | –0.1 | 0.4 |
Notes: CESD = Center for Epidemiological Studies Depression (range: 0–60); CSI = Modified Caregiver Strain Index (range: 0–26).
*p < .05; **p < .001.
CESD scores are presented for the subset of 122 control group and 113 intervention group caregivers with complete responses at both baseline and 6 mos.
CSI scores are presented for the subset of 120 control group and 113 intervention group caregivers with complete responses at both baseline and 6 mos.
Initial Implementation Experiences
After establishing their caseloads of patients (range: 50–60) and primary caregivers (range: 13–26), GCNs invited caregivers to attend the Workshop and monthly Support Groups. Among consented caregivers, 19% expressed an interest and 11% attended at least one of the six workshops. Due to lower than anticipated participation, some nurses jointly led Workshops and cofacilitated Support Groups. In a mailed survey, caregivers who participated in the Workshop (58% response) indicated unanimous endorsement that it was both worth the effort and merited recommendation to others. Among nonparticipating caregivers (40% response), competing demands (42%), inconvenient location (27%) or time (27%), and lack of interest (30%) were the most commonly stated reasons for nonattendance.
Early Effects: Caregiver Outcomes
Outcomes were examined for intervention (n = 115; 89.9% response rate) and control (n = 122; 92.4% response rate) caregivers who completed 6-month surveys and whose care recipients remained alive and enrolled in the study. As shown in Table 3, mean CESD scores declined from 6.8 to 5.6 among intervention caregivers and 7.1 to 6.7 among control caregivers. Mean CSI scores declined from 6.6 to 6.5 among intervention group caregivers but increased from 7.0 to 7.9 among control caregivers. Results from multivariate linear regression models indicated that mean CESD and CSI scores were respectively an average of 0.97 points (p = .14) and 1.14 points lower (p = .06) at 6 months among the intervention group relative to the usual care group.
The 6-month outcomes were then examined by intensity of care provided (Figure 1). Among high-intensity caregivers, mean CESD scores declined from 7.6 to 6.4 in the intervention group and from 7.2 to 7.0 in the control group. CSI scores declined from 7.7 to 7.2 in the intervention group but increased from 8.5 to 9.9 in the control group. Multivariate regression models indicated that mean CESD and CSI scores were on average 1.23 points (p = .20) and 1.83 points (p = .04) lower at 6 months in the intervention group relative to the control group. At 6 months, the intervention yielded aggregate unadjusted effect sizes of –0.16 in depression and –0.25 in strain; effect sizes were respectively –0.09 and –0.47 for high-intensity caregivers.
Figure 1.
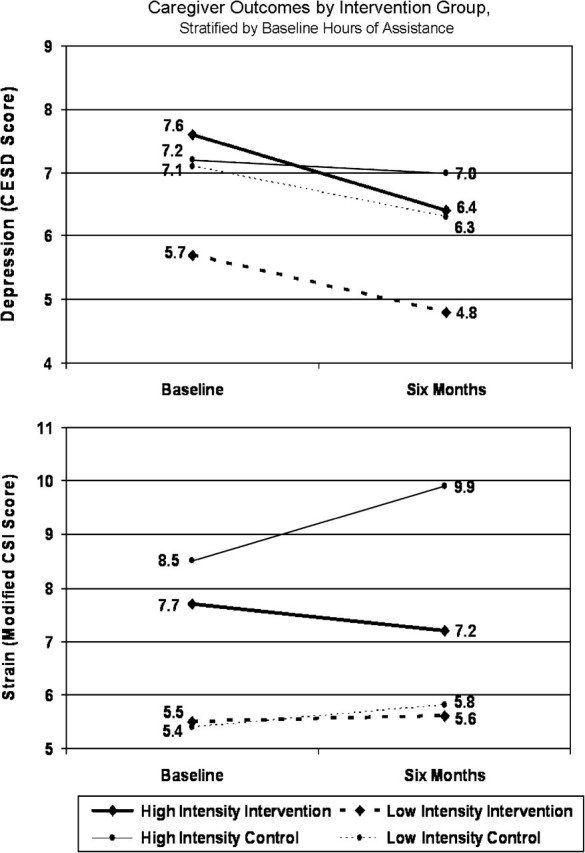
Presents only a portion of the full range of the Center for Epidemiological Studies Depression (CESD, range: 0–60) and Modified Caregiver Strain Index (CSI, range: 0–26) scores. Intensity of care is dichotomized by 14 h of weekly assistance at baseline.
DISCUSSION
The GCPFF was designed to benefit caregivers of high-risk older adults within the context of a new model of comprehensive primary care that combines several of the previous two decades’ most successful chronic disease innovations. Our developmental work and initial implementation experiences substantiate the feasibility of a nurse, based in primary practice, working simultaneously with patients and their caregivers. Moreover, early data from this cluster-randomized controlled trial indicate that some benefit may have been experienced by caregivers who remained enrolled in GC for 6 months.
The GCPFF appears to have provided modest benefit to primary caregivers in terms of reducing depression, and, more notably, strain, at 6 months. At 6 months, intervention caregivers’ mean depression scores trended downward more than controls; mean strain scores remained stable in the intervention group but trended toward an increase in the control group. These findings were amplified among caregivers who were providing more than 14 hours of weekly assistance at baseline, for whom strain at 6 months was significantly lower in the intervention group. That the observed effects of the intervention were both stronger among higher intensity caregivers and consistent across two distinct outcomes suggests that observed effects were due to the intervention.
Relative to other caregiver interventions, this study is unconventional in its approach to identifying caregivers, its primary care orientation, and its explicit recognition of both caregivers and receivers. However, there are important consistencies between this study and other studies of caregiver interventions in terms of the age, gender, identity of caregivers, and intensity of care provided (4). Observed effect sizes during the first 6 months of this study were small but comparable to those achieved in other randomized studies of caregiver interventions in regard to depression (–0.16 in this study vs –0.14 for others) and strain (–0.25 in this study vs –0.07 for others). Consistent with other multicomponent caregiver interventions, the GCPFF had a larger impact on strain than depression. The magnitude of effect on strain that was achieved in this study among caregivers who were providing more hours of care at baseline diverges from other intervention studies, where strain has been less amenable to improvement among high-intensity caregivers. Outcomes of the GCPFF will continue to be monitored for consistency and strength after 18-months of follow-up.
The development and early implementation experiences of the GCPFF have yielded several insights regarding how to structure the support of family caregivers within the context of the health care delivery system.
Ambiguity in Defining Family Caregivers
Our strategy of recruiting and working with caregivers within primary care practice resulted in a surprising challenge regarding how to identify family caregivers and differentiate their needs from those of the patients to whom they provide assistance. We found that some individuals actively provide care but do not identify themselves as “caregivers.” Some caregivers are challenged by health issues that rival or even surpass those of patients, whereas some patients also fulfill the role of caregiver. To address this issue, GCNs are empowered to work with any family member or friend whom they determine to be engaged in helping their patients. In fact, some GC patients with substantial caregiving responsibilities were invited and actively participated in the GCPFF Workshop. It is our impression that the inclusive definition used to identify caregivers and the discretion afforded to GCNs has engendered more family-focused care and greater flexibility in meeting patients’ and families’ needs.
Logistical Challenges of Group Activities
Participation in the GCPFF Workshop and Support Groups was lower than anticipated and may have been impeded by the diversity of caregivers enrolled in this study. Workshop and Support Groups were conducted during workday hours to accommodate GCN schedules but likely impeded participation among working caregivers. Some caregivers may have been unable to participate due to substantial caregiving responsibilities; others may have elected to forego participation even without logistical obstacles.
Integration of Caregivers Within Health Care Delivery Processes
The GCPFF represents only one component of the GC model, which was designed for mainstream health care delivery systems. The separation of family caregivers’ experiences and needs from the broader health care system is a point that has been under-recognized to date. Our experience has been that complexity in how patients and families accommodate to chronic disease and disability defies simplistic notions regarding “patient” and “caregiver” roles and challenges the boundaries of traditional patient care delivery. Although much remains to be learned, GC and GCPFF represent a first step in developing comprehensive models of chronic care delivery to promote partnerships among family caregivers and health professionals.
Acknowledgments
This study was supported by the Jacob and Valeria Langeloth Foundation, The John A. Hartford Foundation, the Agency for Healthcare Research and Quality, the National Institute on Aging, Kaiser Permanente Mid-Atlantic States, Johns Hopkins HealthCare, and the Roger C. Lipitz Center for Integrated Health Care. The authors acknowledge the invaluable contributions to this study made by Johns Hopkins Community Physicians, MedStar, Battelle Centers for Public Health Research, the Centers for Medicare and Medicaid Services, Accumen, ResDAC, the University of Minnesota Survey Research Center, study consultants (Jean Giddens, RN, PhD; Kate Lorig, RN, DrPH), the nurse managers (Lora Rosenthal, RN, and Carol Groves, RN, MPA), and all of the participating patients, caregivers, physicians, and GC nurses. Aspects of this article were presented at the 2007 annual meeting of the Gerontological Society of America and the 2007 Joint Conference of the American Society on Aging and the National Council on Aging, Family Caregiving: State of the Art, Future Trends.
References
- 1.Anderson G, Knickman J. Changing the chronic care system to meet people's needs. Health Aff. 2001;20(6):146–160. doi: 10.1377/hlthaff.20.6.146. [DOI] [PubMed] [Google Scholar]
- 2.Burton L, Newsom J, Schulz R, Hirsch H, German P. Preventive health behaviors among spousal caregivers. Prev Med. 1997;26(2):162–169. doi: 10.1006/pmed.1996.0129. [DOI] [PubMed] [Google Scholar]
- 3.Schulz R, Beach S. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study [see comments] JAMA. 1999;282(23):2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist. 2002;42(3):356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Callahan C, Boustani M, Unverzagt F, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148–2157. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- 6.Mittelman M, Ferris S, Shulman E, Steinberg G, Levin B. A family itervention to delay nursing home placement of patients with Alzheimer disease. A randomized controlled trial. JAMA. 1996;276(21):1725–1731. [PubMed] [Google Scholar]
- 7.Vickrey B, Mittman B, Connor K, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Intern Med. 2006;145(10):713–726. doi: 10.7326/0003-4819-145-10-200611210-00004. [DOI] [PubMed] [Google Scholar]
- 8.Weuve J, Boult C, Morishita L. The effects of outpatient geriatric evaluation and management on caregiver burden. Gerontologist. 2000;40(4):429–436. doi: 10.1093/geront/40.4.429. [DOI] [PubMed] [Google Scholar]
- 9.Silverman M, Musa D, Martin D, Lave J, Adams J, Ricci E. Evaluation of outpatient geriatric assessment: a randomized multi-site trial. J Am Geriatr Soc. 1995;43(7):733–740. doi: 10.1111/j.1532-5415.1995.tb07041.x. [DOI] [PubMed] [Google Scholar]
- 10.Hughes S, Weaver F, Giobbie-Hurder A, et al. Effectiveness of team-managed home-based primary care: a randomized multicenter trial. JAMA. 2000;284(22):2877–2885. doi: 10.1001/jama.284.22.2877. [DOI] [PubMed] [Google Scholar]
- 11.Wolff J, Roter D. Hidden in pain sight: medical visit companions as a quality of care resource for vulnerable older adults. Arch Intern Med. 2008;168(13):1409–1405. doi: 10.1001/archinte.168.13.1409. [DOI] [PubMed] [Google Scholar]
- 12.Wolff J, Kasper J. Caregivers of frail elders: updating a national profile. Gerontologist. 2006;46(3):344–356. doi: 10.1093/geront/46.3.344. [DOI] [PubMed] [Google Scholar]
- 13.Donelan K, Hill C, Hoffman C, et al. Challenged to care: informal caregivers in a changing health system. Health Aff (Millwood) 2002;21(4):222–231. doi: 10.1377/hlthaff.21.4.222. [DOI] [PubMed] [Google Scholar]
- 14.Boyd C, Boult C, Shadmi E, et al. Guided care for multi-morbid older adults. Gerontologist. 2007;47(5):697–704. doi: 10.1093/geront/47.5.697. [DOI] [PubMed] [Google Scholar]
- 15.Mittelman M, Haley W, Clay O, Roth D. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592–1599. doi: 10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- 16.Burridge L, Winch S, Clavarino A. Reluctance to care: a systematic review and development of a conceptual framework. Cancer Nurs. 2007;30(2):E9–E19. doi: 10.1097/01.NCC.0000265298.17394.e0. [DOI] [PubMed] [Google Scholar]
- 17.Acton G, Kang J. Interventions to reduce the burden of caregiving for an adult with dementia: a meta-analysis. Res Nurs Health. 2001;24(5):349–360. doi: 10.1002/nur.1036. [DOI] [PubMed] [Google Scholar]
- 18.Lorig K, Ritter P, Stewart A, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39(11):1217–1223. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Toseland R, McCallion P, Smith T, Huck S, Bourgeois P, Garstka T. Health education groups for caregivers in an HMO. J Clin Psychol. 2001;57(4):551–570. doi: 10.1002/jclp.1028. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher-Thompson D, DeVries H. “Coping with frustration” classes: development and preliminary outcomes with women who care for relatives with dementia. Gerontologist. 1994;34(4):548–552. doi: 10.1093/geront/34.4.548. [DOI] [PubMed] [Google Scholar]
- 21.Wegener S, MacKenzie E, Ephraim P, Ehde D, Williams R. Self-management improves outcomes in persons with limb loss. Arch Phy Med Rehabil. 2009;90(3):373–380. doi: 10.1016/j.apmr.2008.08.222. [DOI] [PubMed] [Google Scholar]
- 22.Helgeson V, Gottlieb B. Support groups. In: Cohen S, Underwood L, Gottlieb B, editors. Social Support Measurement and Intervention. New York: Oxford University Press; 2000. pp. 195–220. [Google Scholar]
- 23.Toseland R, Rossiter C, Labrecque M. The effectiveness of peer-led and professionally led groups to support family caregivers. Gerontologist. 1989;29(4):465–471. doi: 10.1093/geront/29.4.465. [DOI] [PubMed] [Google Scholar]
- 24.Boult C, Reider L, Frey K, et al. Multidimensional geriatric assessment: back to the future early effects of “guided care” on the quality of health care for multimorbid older persons: a cluster-randomized controlled trial. J Gerontol Med Sci. 2008;63A(3):321–327. doi: 10.1093/gerona/63.3.321. [DOI] [PubMed] [Google Scholar]
- 25.Pope G, Kautter J, Ellis R, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25(4):119–141. [PMC free article] [PubMed] [Google Scholar]
- 26.Weissman M, Sholomskas D, Pottenger M, Prusoff B, Locke B. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 27.Robinson B. Validation of a caregiver strain index. J Gerontol. 1983;38(3):344–348. doi: 10.1093/geronj/38.3.344. [DOI] [PubMed] [Google Scholar]
- 28.Thornton M, Travis S. Analysis of the reliability of the modified caregiver strain index. J Gerontol B Psychol Sci Soc Sci. 2003;58(2):S127–S132. doi: 10.1093/geronb/58.2.s127. [DOI] [PubMed] [Google Scholar]
- 29.Royston P. Multiple imputation of missing values: update of ICE. Stata J. 2005;5:527–536. [Google Scholar]
- 30.Rubin D. Multiple Imputation for Nonresponse in Surveys. 1st ed. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 31.Pinquart M, Sorensen S. Associations of stressors and uplifts of caregiving with caregiver burden and depressive mood: a meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2003;58(2):P112–P128. doi: 10.1093/geronb/58.2.p112. [DOI] [PubMed] [Google Scholar]


