Abstract
Increased mortality and overexpression of interleukin-6 (IL-6) during inflammatory stress are well-documented age-associated phenomena; however, the site of IL-6 overexpression is not entirely known. Here, we report that white adipose tissue is a major source of IL-6 in aged animals during lipopolysaccharide (LPS)-induced systemic inflammation. Among the various tissues examined, white adipose tissue from the epididymal fat pad (located in the abdominal cavity) expressed the highest level of IL-6 messenger RNA in both young and aged mice with a 5.5-fold higher level in the aged. Immunohistochemistry revealed that, within the adipose tissue, LPS-induced IL-6 expression is localized to both the adipocytes and stromal cells. Compared with age-matched wild-type mice, aged IL-6(–/–) mice exhibited reduced mortality to LPS suggesting a deleterious effect of IL-6 overexpression in the aged. These results demonstrate that increased vulnerability to systemic inflammation with age is due in part, to augmented IL-6 production by the adipose tissue.
Keywords: Aging, IL-6, Adipose tissue, Sepsis, Endotoxemia
AGING is characterized by an altered stress response that underlies a compromised resistance to disease or injury (1,2). Systemic inflammatory response syndrome (SIRS) results from an uncontrolled immune response to trauma or infection where normally protective responses become deleterious leading to complications such as shock and multiple organ dysfunction (3). SIRS is characterized by elevated levels of circulating cytokines, hypothermia or hyperthermia, tachycardia, and tachypnea (4,5). It is a particularly serious problem in the elderly as severity and mortality are significantly increased with age; however, the mechanisms of this age-related susceptibility remain unclear. Systemic inflammation has been demonstrated in several rodent endotoxemia models by injection with bacterial endotoxin lipopolysaccharide (LPS; 6–10). In these studies, aged mice with systemic inflammation exhibited significantly higher mortality rates as compared with younger mice.
In endotoxemia models, injection with LPS activates a cascade of inflammatory responses resulting in the production of a number of inflammatory cytokines including interleukin-6 (IL-6). IL-6, a multifunctional inflammatory cytokine, stimulates an immune response and is secreted from a wide variety of cell types (11). It is strongly induced in multiple tissues during systemic inflammation (12), and this induction is increased and prolonged with age in the lungs, heart, and plasma of mice (6,8,13). Our previous studies demonstrate that the age-associated high mortality to LPS-induced systemic inflammation is closely associated with overexpression of IL-6 and severe hypothermia (6). A strong correlation between mortality and increased IL-6 induction has also been demonstrated in a murine peritonitis model of sepsis (14). Therefore, plasma IL-6 is good marker for measuring the level of inflammation associated with the severity of sepsis and should be considered as a diagnostic tool for therapy.
Once considered an inert location of energy storage, studies over the last decade identify adipose tissue as a dynamic organ implicated in metabolic, inflammatory, and immune responses. Adipose tissue consists of multiple deposits in several anatomical locations and comprises 20% of the body weight of healthy individuals making it one of the largest organs in the body (15). Several studies suggest that adipose tissue is one of the major sources of chronically produced inflammatory cytokines including IL-6 in healthy, normal weight human participants (16) and obese human participants and mice (17,18). More recent studies have shown an upregulation of inflammatory cytokines in the adipose tissue at basal levels by aging (19) and obesity (19). However, the role of adipose tissue in age-related vulnerability to inflammatory stress has not been reported. In the present study, we investigated LPS-induced expression of IL-6 in the adipose tissues of young and aged mice with endotoxemia.
METHODS
Animals
Young (6–7 months old) and aged (22–27 months old) C57BL/6 male mice were obtained from colonies of the National Institute on Aging (Bethesda, MD). Female IL-6 knockout mice with C57BL/6 background were obtained from The Jackson Laboratory (Bar Harbor, ME) at 2 months of age and maintained at our institute until they were used for study at 26 months of age. Mice were housed in a temperature controlled environment at 22°C and maintained in a 12 hour light–dark cycle. All mice were acclimated for at least 14 days with free access to food (LabDiet, Brentwood, MO) and water. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch. Acute systemic inflammation was induced by intraperitoneal injection with bacterial endotoxin LPS derived from Pseudomonas aeruginosa (Sigma Chemical, St Louis, MO). LPS was dissolved in physiologic saline and administered intraperitoneally with a dose of 2.5 mg/kg throughout the study except for a survival experiment in which a dose of 5 mg/kg was injected. These two doses of LPS are nonlethal to young mice but produce approximately 80% and 100% mortality in aged mice within 5 days (M. E. Starr and H. Saito, unpublished observation). Body temperature was monitored with YSI Precision Thermometer 4600 (Dayton, OH). Six hours after LPS injection, mice were anesthetized with 2% isoflurane in air and blood samples (0.5 mL) were drawn from the inferior vena cava. For RNA analysis, the entire vasculature was perfused with physiological saline (0.9% NaCl) through the cardiac ventricles and tissues were harvested and flash frozen in liquid nitrogen. For histological analysis, mice were sacrificed 3 hours after LPS injection and tissues were harvested and fixed in 10% neutral buffered formalin for 24 hours.
Three different types of adipose tissues were used in the present study. The largest and most easily harvested adipose depot in mice is the epididymal depot (white adipose tissue located in the abdominal cavity around the male reproductive parts). Other large depots include perirenal (or retroperitoneal, mixture of white and brown adipose tissue located around the kidneys) and intrascapular (subcutaneous brown adipose tissue located between the shoulder blades).
RNA Isolation and Northern Blot Analysis
Total RNA was isolated from tissues by a method similar to our previously described protocol (12). Briefly, frozen tissues were processed with a homogenizer, and total RNA was isolated with guanidine/phenol solution (TRIzol reagent, Invitrogen, Carlsbad, CA) using the protocol recommended by the manufacturer. Twenty micrograms of RNA from each tissue was electrophoretically fractionated through 1.2% agarose gels containing 3% formaldehyde buffered in 20 mM 3-(N-morpholino)propanesulfonic acid and 1 mM ethylenediaminetetraacetic acid at pH 7.4. The RNA was transferred to Zeta-Probe GT nylon membranes (Bio-Rad Laboratories, Hercules, CA) overnight and fixed by ultraviolet cross-linking. A radiolabeled IL-6 probe was prepared from mouse complementary DNA using DECAprime II Random-Primed DNA Labeling Kit and NucAway Spin Columns (Ambion, Austin, TX). Hybridization and washing were performed at 65°C as described by Church and Gilbert (20). Membranes were exposed to Blue Lite Autorad Film (ISC Bio Express, Kaysville, UT) in the presence of an intensifying screen at –80°C. Kodak 1D Image Analysis Software, version 3.6 (Carestream Health, New Haven, CT) was used to measure intensity of the bands for statistical analysis.
Cytokine Immunoassays
Plasma IL-6 levels were determined as previously described (21) by enzyme-linked immunosorbent assay using antimouse IL-6 monoclonal antibody and biotinylated antimouse IL-6 antibody (R&D Systems, Minneapolis, MN).
Immunohistochemistry
Epididymal adipose tissue, perirenal adipose tissue, and kidney were fixed in 10% neutral buffered formalin for 24 hours at room temperature and embedded in paraffin. Sections (3 μm) were cut, deparaffinized in xylene, and immunohistochemistry was performed using Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA). Sections were stained with 1:500 diluted anti-IL-6 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C overnight. Sections were counterstained with Mayer's hematoxylin (Poly Scientific, Bay Shore, NY).
Statistical Analysis
Data were analyzed by Student's t test using Sigma Stat Statistical Software version 2.0 (Systat Software, Inc., San Jose, CA). Results from the survival experiment were analyzed using a log rank test from StatView software (SAS Institute, Cray, CA). A p value of less than .05 was considered significant.
RESULTS
Adipose Tissue Is a Major Source of IL-6 During Systemic Inflammation
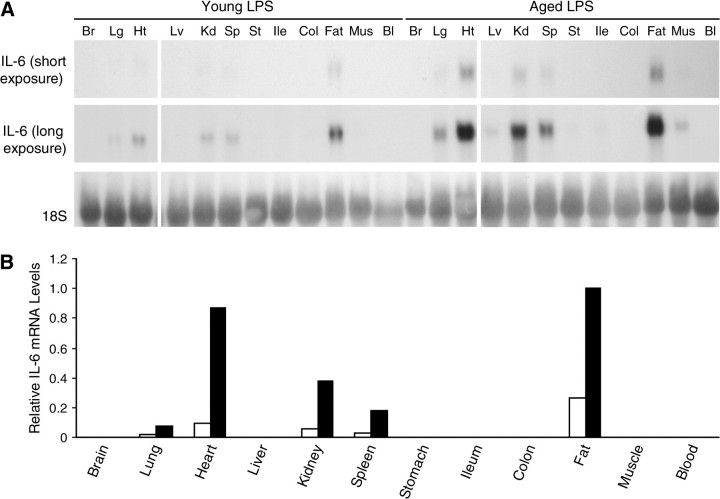
To determine which tissues in the body exhibit an age-associated overexpression of IL-6 during systemic inflammation, messenger RNA (mRNA) levels were examined in the brain, lungs, heart, liver, kidneys, spleen, stomach, ileum, colon, epididymal adipose tissue (white fat), skeletal muscle, and blood samples that were collected from young and aged mice 6 hours after LPS injection. Northern blot analysis showed that IL-6 was strongly expressed in the lungs, heart, kidneys, spleen, and fat of both young and aged mice during endotoxemia and that these five tissues exhibited an age-associated increase in expression of IL-6 (Figure 1A, short exposure). With a longer autoradiograph exposure, IL-6 expression was also detected in the liver, stomach, ileum, and muscle of aged but not young mice (Figure 1A, long exposure). Neither brain nor blood cells showed detectable levels of IL-6 in young or aged mice. Among all the tissues examined, white adipose tissue from the epididymal fat pad expressed the highest level of IL-6 mRNA in both young and aged mice (Figure 1B).
Figure 1.
Tissue distribution of lipopolysaccharide (LPS)-induced interleukin-6 (IL-6) gene expression. Systemic inflammation was induced in young (6–7 mos old) and aged (22–27 mos old) C57BL/6 male mice by LPS injection (2.5 mg/kg, intraperitoneal) and mice were sacrificed 6 h later. All lanes are in a single gel. (A) RNA was isolated from various tissues of young and aged mice and analyzed by Northern blotting. Each lane represents pooled RNA samples from three mice. (B) Densitometric analysis of short exposure from (A). The shorter exposure was used because the bands for heart and fat appear to be saturated in the longer exposed autoradiograph. Young (open bars) and aged (closed bars). Total intensity of each band was normalized to 18S levels. The normalized IL-6 messenger RNA level in aged mouse fat was set at 1.0.
IL-6 Expression Significantly Increases With Age During Systemic Inflammation and Correlates With the Severity of Inflammation
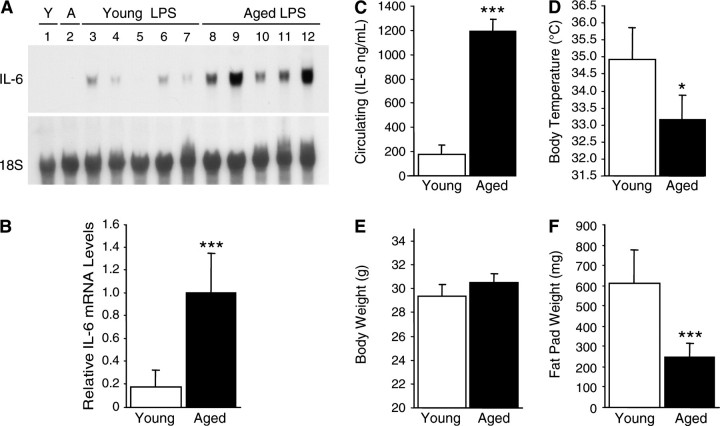
To further clarify the age-associated increase of IL-6 expression in adipose tissue, epididymal adipose tissue was harvested 6 hours after LPS injection from another set of young and aged mice and mRNA analyzed individually (Figure 2A). Whereas the adipose tissues of both young and aged noninjected control mice (Lanes 1 and 2) did not show any IL-6 expression, all adipose tissues of mice injected with LPS showed IL-6 induction (Lanes 3–12). Among these, aged mice showed a 5.5-fold higher level (Figure 2B) of IL-6 mRNA expression in the adipose tissue than young mice (p < .001). These results clearly demonstrate an age-associated increase in IL-6 gene expression in the adipose tissue during LPS-induced systemic inflammation.
Figure 2.
Lipopolysaccharide (LPS)-mediated expression of interleukin-6 (IL-6) in adipose tissue increases with age and correlates with severity of systemic inflammation. Systemic inflammation was induced in young (4–7 mos old) and aged (18–27 mos old) C57BL/6 male mice by LPS injection (2.5 mg/kg, intraperitoneal). (A) RNA was isolated from the epididymal adipose tissue of noninjected controls and mice sacrificed at 6 h after injection and analyzed by Northern blotting. Each lane represents an individual RNA sample from a single mouse. Lane 1: young control mouse without LPS injection; Lane 2: aged control mouse without LPS injection; Lanes 3–7: young mice with LPS injection; and Lanes 8–12: aged mice with LPS injection. (B) Densitometric analysis of (A): young (open bars) and aged (closed bars). Total intensity of each band was normalized to 18S levels. The average normalized IL-6 messenger RNA level in aged mouse fat was set at 1.0. (C) Plasma IL-6 levels 6 h after LPS injection were measured by enzyme-linked immunosorbent assay: young (open bars) and aged (closed bars). (D) Body temperatures 6 h after LPS injection. (E) Body weight at the time of LPS injection (g). (F) Epididymal fat pad weight (mg) at the time of sacrifice. Data are expressed as the mean ± standard deviation, n = 5 each group: *p < .05 and ***p < .001.
Similarly, plasma IL-6 is higher in aged mice when compared with young mice with systemic inflammation (Figure 2C). This corresponds to the increase of IL-6 gene expression in the adipose tissues of aged mice. The body temperatures of all mice were obtained shortly before sacrifice to assess the severity of systemic inflammation (Figure 2D). Compared with the body temperature of healthy mice (approximately 37°C), young mice exhibited a mild hypothermic event where body temperature dropped to approximately 35°C whereas aged mice suffered from a more profound hypothermia with body temperature falling to an average of 33°C (p = .01).
Average body weight of the young and aged mice was not statistically different (29.4 g vs 30.5g, respectively, p = .21; Figure 2E). However, the weight of the epididymal fat pad was significantly smaller in the aged mice as compared with the young mice (p < .001; Figure 2F).
Age-Associated Expression of IL-6 in Various Adipose Tissue Depots
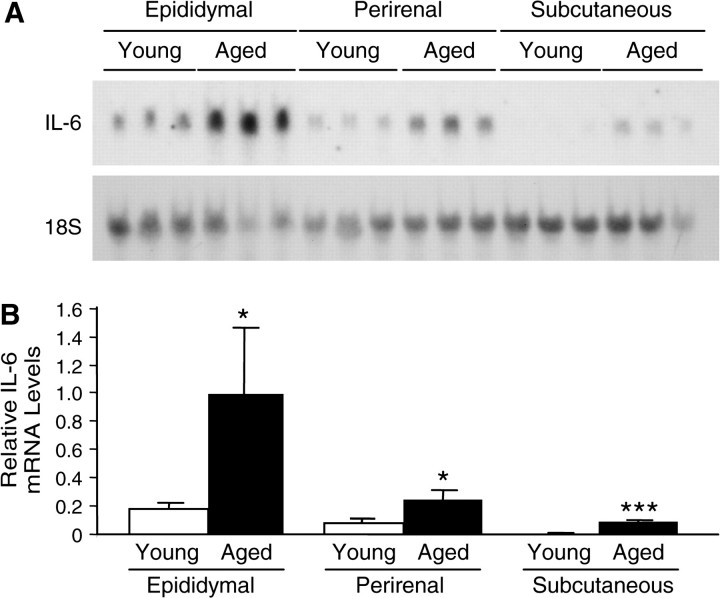
To examine whether the age-associated increase in IL-6 expression occurs in other adipose tissues depots, perirenal and intrascapular fats from young and aged mice were also analyzed. As shown in Figure 3, the age-associated increase in IL-6 mRNA induction occurred in all three types of adipose tissue examined. The magnitude of the age-associated increase was 6-, 3-, and 33-fold in epididymal, perirenal, and intrascapular fat, respectively (p = .04, p = .02, and p < .001). Among these three adipose tissue sites, the epididymal tissue showed significantly higher levels of IL-6 expression in both young and aged mice in comparison with perirenal and intrascapular tissues.
Figure 3.
Age-associated expression of interleukin-6 (IL-6) in various adipose tissue depots. Systemic inflammation was induced in young (4 mos old) and aged (22 mos old) C57BL/6 male mice by lipopolysaccharide injection (2.5 mg/kg, intraperitoneal). (A) RNA was isolated from the epididymal, perirenal, and subcutaneous adipose tissues of both young and aged mice sacrificed 6 h after injection and analyzed by Northern blotting. Each lane represents an individual RNA sample from a single mouse. (B) Densitometric analysis of (A): young (open bars) and aged (closed bars). Total intensity of each band was normalized to 18S levels. The average normalized IL-6 messenger RNA level in aged mouse epididymal fat was set at 1.0. Data are expressed as the mean ± standard deviation, n = 3 each group: *p < .05 and ***p < .001.
IL-6 Is Expressed Within the Adipocytes of the Adipose Tissue
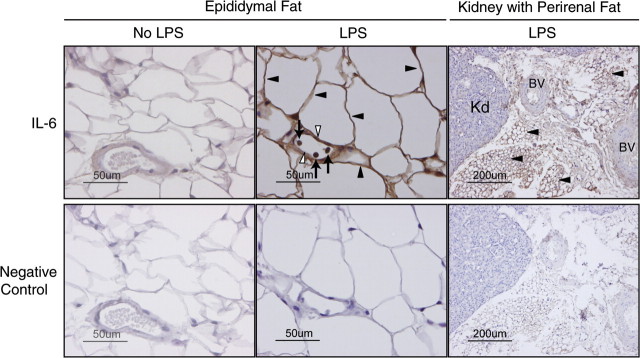
Immunohistochemical analyses of the epididymal fat pad from aged mice were performed to localize IL-6 in the adipose tissue. Within the epididymal adipose tissue, white adipocytes, vascular endothelial cells, and inflammatory cells expressed IL-6 after LPS injection (Figure 4, middle panel) with the majority of the IL-6 being expressed by the adipocytes. Additionally, immunohistochemistry of the kidney with associated perirenal adipose tissue demonstrated that the brown adipose tissue surrounding the kidney also expresses IL-6 (Figure 4, right panel). However, we did not detect IL-6 expression in the vascular endothelial cells of the perirenal adipose tissue.
Figure 4.
Immunohistochemical localization of interleukin-6 (IL-6) in the adipose tissue. Immunohistochemical analysis was performed to localize IL-6 protein in adipose tissue from aged C57BL/6 male mice after lipopolysaccharide (LPS) injection (2.5 mg/kg, intraperitoneal). Strong positive staining with anti-IL-6 antibody is seen in adipocytes (closed arrow heads), vascular endothelial cells (open arrow heads), and inflammatory cells (arrows) of white epididymal adipose tissue from mice injected with LPS (top middle panel). Strong positive staining is seen in brown perirenal adipose tissue (closed arrow heads) associated with the kidney (Kd, top right panel), but not in vascular endothelial cells of the blood vessels (BV) in mice injected with LPS.
Aged IL-6(–/–) Mice Are More Resistant to LPS-Induced Systemic Inflammation
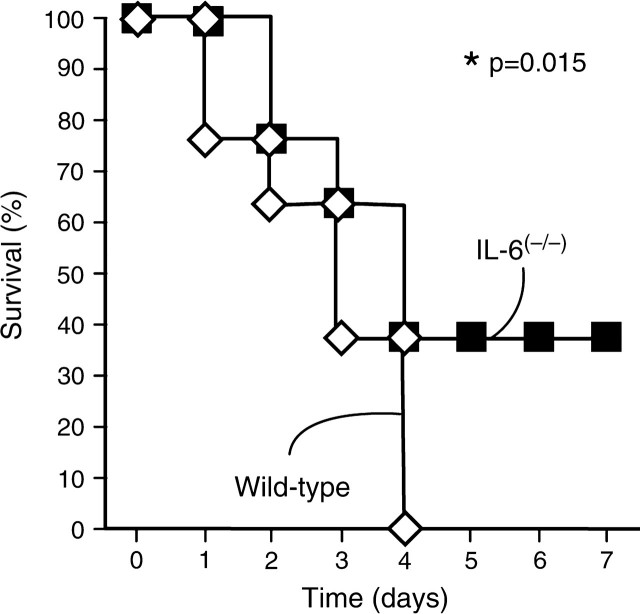
To determine the consequence of the age-associated overexpression of IL-6 during severe systemic inflammation we compared the survival of aged IL-6(–/–) mutant mice with wild-type control mice after injection with LPS (5 mg/kg). Average body weight of these mutant and control mice was 32.9 g and 27.1 g, respectively (p = .01), confirming an earlier report of mature-onset obesity in IL-6(–/–) mice (22). As noted in Figure 5, LPS-injected aged IL-6(–/–) mice showed a 40% increase in survival (p = .015) as compared with age- and sex-matched wild-type mice. All wild-type mice had died by Day 4, whereas 40% of the IL-6(–/–) mice continued to survive beyond Day 7. These findings indicate that induction of IL-6 is deleterious rather than beneficial in aged mice during systemic inflammation.
Figure 5.
Aged interleukin-6 (IL-6) knockout mice are more resistant to lipopolysaccharide (LPS)-induced endotoxemia. Survival of IL-6(–/–) (closed squares) and wild-type control mice (open diamonds) was monitored for 7 days after LPS injection (5 mg/kg, intraperitoneal). All mice were 26-month-old females with C57BL/6 genetic background (n = 8 each group).
DISCUSSION
Previously, we reported that the LPS-induced IL-6 mRNA level in young mice was highest in the heart, followed by kidneys, spleen, and lungs (12); however, adipose tissue was not assessed in this analysis. Here we show that, among 12 tissues examined including the heart, the epididymal adipose tissue of both young and aged mice expresses the highest level of IL-6 mRNA after LPS injection. The heart, kidneys, spleen, and lungs also showed relatively strong IL-6 expression, confirming our previous observation (12). When compared with young mice 6 hours after injection with LPS, aged mice exhibited more than a fivefold increase of IL-6 that correlated with the severity of systemic inflammation. Adipose tissue can be considered the largest organ in the body because it encompasses 20% or more of total body weight, far larger than other IL-6 expressing organs such as spleen, heart, kidney, and lungs that weigh no more than 2% of total body weight. Although we could not calculate the total amount of IL-6 derived from entire fat in the mouse body, adipose tissues appear to produce far more IL-6 than any other organ alone.
A number of cell types reside within the adipose tissue including both white and brown adipocytes, vascular endothelial cells, macrophages, and other immune cells. Our immunohistochemical analysis demonstrated that LPS-induced IL-6 is strongly expressed in white adipocytes although other cell types including inflammatory cells (ie, macrophages) and vascular endothelial cells also express IL-6. This is in contrast with previous studies reporting that, among obese participants, IL-6 expression is increased in the adipose tissue mainly due to secretion by nonadipocyte cells including macrophages (17,23–25). Wu and colleagues (19) showed that the number of macrophages in the adipose tissue is not different between young and aged mice per gram of adipose tissue and that the production of IL-6 by nonadipocyte cells was not significantly different between young and aged mice at basal levels. Our data along with the observations of Wu and colleagues (19) support the suggestion that varied mechanisms exist between obesity- and age-induced adipose tissue inflammation; however, more studies are needed to delineate the differences in aged adipocyte and nonadipocyte cells during LPS-induced systemic inflammation. It is well documented that basal levels of circulatory IL-6 increases with aging (26). Due to a detection limit of Northern blot analysis, we could not detect basal IL-6 mRNA in adipose tissues from mice without LPS stimulation (Figure 2A).
Aging is associated with changes in body composition (27,28), in which muscle mass diminishes and is replaced by fat (27). Body weight often remains stable or increases masking an undesirable redistribution of adipose tissue during this process (27). In our study, the harvested epididymal adipose tissue was smaller in aged mice compared with young mice, whereas total body weights were similar in both age groups. It is intriguing that the epididymal adipose tissue of aged mice, which is significantly smaller in weight than the adipose tissue of young mice, expressed higher levels of IL-6 mRNA (Figure 2). These results suggest that the age-associated overexpression of IL-6 in the adipose tissue is caused by a change in the quality of the adipocytes rather than the size of the tissue.
Adipose tissue is a multidepot organ distributed throughout the body. The most prominent depots in mice are epididymal (white fat pad around reproductive organs), perirenal (both white and brown fat around the kidneys), and intrascapular (subcutaneous brown fat between the shoulder blades). We compared LPS-induced IL-6 expression in these three depots and found that IL-6 induction was higher in aged animals compared with young animals for all three adipose tissue sites. Our results also showed a depot-related difference in the level of IL-6 expression with the epididymal adipose tissue exhibiting the highest level, followed by perirenal and intrascapular adipose tissue depots, respectively. The epididymal fat pad consists of white fat whereas the intrascapular adipose depot consists of brown fat. The perirenal adipose tissues were a mixture of white and brown fat. Thus, our results indicate that inflammation-mediated IL-6 induction occurs more strongly in white fat than brown fat. It is also known that fat redistribution leads to increased ectopic adipose tissue around the heart (pericardial), aorta (periaortal), blood vessels (perivascular), and muscle (29). In aged mice, these adipose tissue depots likely also contribute to increased circulating levels of IL-6 during systemic inflammation. For example, perivascular adipose tissue reportedly secretes a variety of cytokines and chemokines (30) implicating it as an important contributor to adipose tissue–mediated inflammation. Further studies should evaluate perivascular adipose tissue during inflammatory stress.
Our previous study reported a high level of IL-6 induction in the kidney with inflammation (12). As shown in Figure 4, whereas the kidney of aged mice with systemic inflammation expressed a detectable level of IL-6, a far greater level of expression was detected in the surrounding brown adipose tissue. It seems feasible that this small amount of IL-6-expressing adipose tissue may mask the true level of IL-6 mRNA in the kidney. Aged muscle tissue could also pose a problem as a result of adipocyte infiltration. Similarly, any tissue with ectopic adipose tissue deposition in the aged or obese could lead to an overestimation of accurate IL-6 levels. The mechanism for the age-associated change in LPS-induced IL-6 expression in the adipose tissue is unclear at present. LPS signaling is transmitted through Toll-like receptor 4 (TLR4) and activation of the transcription factor NF-ΚB (31,32). TLR4 is expressed by adipocytes in humans (33,34) and mice (35). In vitro experiments have shown that TLR4 levels increase in human adipocytes after treatment with LPS and that this is linked to downstream NF-ΚB activation (33); however, the age-associated difference in adipose TLR4 expression has not yet been reported. IL-6 expression is likely to be induced by early inflammatory cytokines such as TNFα and IL-1β (12).
Previously, Gomez and colleagues (36) showed a reduced mortality in aged IL-6(–/–) mice as compared with wild-type control mice (0% vs 23%) after injection with LPS. In their study, a relatively low dose of LPS (1.5 mg/kg) and an age range of 15–23 months were used (36). Our study used a higher dose of LPS (5 mg/kg) and an older group of mice (26 months old) and demonstrated similar findings as noted by Gomez and colleagues (36), thus providing further evidence that IL-6(–/–) mice are capable of surviving an otherwise lethal dose of LPS. Additionally, whereas Gomez and colleagues used knockout mice of BALB/C background, we used mice of C57BL/6 background showing that the increased survival is not a strain-specific occurrence. Another study reported that IL-6 provides a protective effect in young mice during LPS-induced endotoxemia (37). Though these findings appear to contradict our present study and the study by Gomez and colleagues, these differential results further support our hypothesis that well-regulated modest induction of IL-6 (as noted in young animals) is beneficial for host defense whereas uncontrolled overproduction of IL-6 in aged animals is harmful and contributes to the age-associated increase in mortality (6). It is intriguing that despite their obesity, IL-6(–/–) mice are more resistant to LPS-induced endotoxemia than normal weight wild-type mice, thus supporting our suggestion that the quality of the adipose tissue rather than the size of the depot plays a major role in inflammation. It also indicates that IL-6 is one of the major culprits in age-associated inflammation. IL-6 has an important role in the development of specific immune responses and in the production of a variety of hepatic acute-phase proteins (11). To better understand a role for IL-6 overexpression in age-associated vulnerability to systemic inflammation, further studies including investigation of immune cell responses and hepatic gene expression would be important.
In summary, we have clearly demonstrated that adipose tissue is a major source of IL-6 during LPS-induced systemic inflammation and that the overexpression of IL-6 increases with age. We have further shown that adipocytes in the white adipose tissue largely contribute to IL-6 production in the aged during systemic inflammation. In addition, overexpression of IL-6 in the aged is deleterious and is likely related to increased mortality during systemic inflammation. Taken together, these data support the inflammatory nature of adipose tissue and its involvement in age-associated vulnerability to systemic inflammation.
Acknowledgments
The authors thank Karen Martin for manuscript preparation and Drs Hitoshi Takahashi and Shoji Yamamoto for technical advice. This study was presented, in part, at the 60th Annual Scientific Meeting of the Gerontological Society of America and Marlene E. Starr received the 2007 George Sacher Student Award for best student poster presentation. This work was supported by the National Institutes of Health Grant RO1-AG025908. Additional support came from the UTMB, Claude D. Pepper, Older Americans Independence Center, NIH P30A6024832.
References
- 1.Harman D. Aging: overview. Ann N Y Acad Sci. 2001;928:1–21. doi: 10.1111/j.1749-6632.2001.tb05631.x. [DOI] [PubMed] [Google Scholar]
- 2.Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies MG, Hagen PO. Systemic inflammatory response syndrome. Br J Surg. 1997;84:920–935. doi: 10.1002/bjs.1800840707. [DOI] [PubMed] [Google Scholar]
- 4.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 5.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 6.Saito H, Sherwood ER, Varma TK, Evers BM. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev. 2003;124:1047–1058. doi: 10.1016/j.mad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Chorinchath BB, Kong LY, Mao L, McCallum RE. Age-associated differences in TNF-alpha and nitric oxide production in endotoxic mice. J Immunol. 1996;156:1525–1530. [PubMed] [Google Scholar]
- 8.Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun. 1996;64:769–774. doi: 10.1128/iai.64.3.769-774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horan MA, Brouwer A, Barelds RJ, Wientjens R, Durham SK, Knook DL. Changes in endotoxin sensitivity in ageing. Absorption, elimination and mortality. Mech Ageing Dev. 1991;57:145–162. doi: 10.1016/0047-6374(91)90031-t. [DOI] [PubMed] [Google Scholar]
- 10.Habicht GS. Body temperature in normal and endotoxin-treated mice of different ages. Mech Ageing Dev. 1981;16:97–104. doi: 10.1016/0047-6374(81)90037-3. [DOI] [PubMed] [Google Scholar]
- 11.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 12.Saito H, Patterson C, Hu Z, et al. Expression and self-regulatory function of cardiac interleukin-6 during endotoxemia. Am J Physiol Heart Circ Physiol. 2000;279:H2241–H2248. doi: 10.1152/ajpheart.2000.279.5.H2241. [DOI] [PubMed] [Google Scholar]
- 13.Saito H, Papaconstantinou J. Age-associated differences in cardiovascular inflammatory gene induction during endotoxic stress. J Biol Chem. 2001;276:29307–29312. doi: 10.1074/jbc.M103740200. [DOI] [PubMed] [Google Scholar]
- 14.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Cinti S. The adipose organ. In: Giamila Fantuzzi, Theodore Mazzone., editors. Adipose Tissue and Adipokines in Health and Disease. Totowa, NJ: Humana Press; pp. 3–19. [Google Scholar]
- 16.Flower L, Gray R, Pinkney J, Mohamed-Ali V. Stimulation of interleukin-6 release by interleukin-1beta from isolated human adipocytes. Cytokine. 2003;21:32–37. doi: 10.1016/s1043-4666(02)00495-7. [DOI] [PubMed] [Google Scholar]
- 17.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 18.Harkins JM, Moustaid-Moussa N, Chung YJ, et al. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J Nutr. 2004;134:2673–2677. doi: 10.1093/jn/134.10.2673. [DOI] [PubMed] [Google Scholar]
- 19.Wu D, Ren Z, Pae M, et al. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol. 2007;179:4829–4839. doi: 10.4049/jimmunol.179.7.4829. [DOI] [PubMed] [Google Scholar]
- 20.Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueda J, Starr ME, Takahashi H, et al. Decreased pulmonary extracellular superoxide dismutase during systemic inflammation. Free Radic Biol Med. 2008 doi: 10.1016/j.freeradbiomed.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallenius V, Wallenius K, Ahren B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 23.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 25.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 26.Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher D, Ruts E, Visser M, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279:E366–E375. doi: 10.1152/ajpendo.2000.279.2.E366. [DOI] [PubMed] [Google Scholar]
- 28.Harris TB. Invited commentary: body composition in studies of aging: new opportunities to better understand health risks associated with weight. Am J Epidemiol. 2002;156:122–124. doi: 10.1093/aje/kwf024. discussion 125–126. [DOI] [PubMed] [Google Scholar]
- 29.Thalmann S, Meier CA. Local adipose tissue depots as cardiovascular risk factors. Cardiovasc Res. 2007;75:690–701. doi: 10.1016/j.cardiores.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Henrichot E, Juge-Aubry CE, Pernin A, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 31.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 32.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 33.Vitseva OI, Tanriverdi K, Tchkonia TT, et al. Inducible Toll-like receptor and NF-kappaB regulatory pathway expression in human adipose tissue. Obesity (Silver Spring) 2008;16:932–937. doi: 10.1038/oby.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bes-Houtmann S, Roche R, Hoareau L, et al. Presence of functional TLR2 and TLR4 on human adipocytes. Histochem Cell Biol. 2007;127:131–137. doi: 10.1007/s00418-006-0230-1. [DOI] [PubMed] [Google Scholar]
- 35.Pietsch J, Batra A, Stroh T, et al. Toll-like receptor expression and response to specific stimulation in adipocytes and preadipocytes: on the role of fat in inflammation. Ann N Y Acad Sci. 2006;1072:407–409. doi: 10.1196/annals.1326.021. [DOI] [PubMed] [Google Scholar]
- 36.Gomez CR, Goral J, Ramirez L, Kopf M, Kovacs EJ. Aberrant acute-phase response in aged interleukin-6 knockout mice. Shock. 2006;25:581–585. doi: 10.1097/01.shk.000029553.39081.ec. [DOI] [PubMed] [Google Scholar]
- 37.Xing Z, Gauldie J, Cox G, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]