Abstract
p66shc is increased in response to cell stress, and these increases regulate growth factor actions. These studies were conducted to determine how p66shc alters IGF-I-stimulated Src activation, leading to decreased IGF-I actions. Our results show that p66shc binds to Src through a polyproline sequence motif contained in the CH2 domain, a unique domain in p66shc, and IGF-I stimulates this interaction. Disruption of this interaction using a synthetic peptide containing the p66shc polyproline domain or expression of a p66shc mutant containing substitutions for the proline residues (P47A/P48A/P50A) resulted in enhanced Src kinase activity, p52shc phosphorylation, MAPK activation, and cell proliferation in response to IGF-I. To determine the mechanism of inhibition, the full-length CH2 domain and intact p66shc were tested for their ability to directly inhibit Src kinase activation in vitro. The CH2 domain peptide was clearly inhibitory, but full-length p66shc had a greater effect. Deletion of the C-terminal Src homology 2 domain in p66shc reduced its ability to inhibit Src kinase activation. These findings demonstrate that p66shc utilizes a novel mechanism for modulating Src kinase activation and that this interaction is mediated through both its collagen homologous region 2 and Src homology 2 domains.
Keywords: Diseases/Atherosclerosis, Regulation Allosteric, Signal Transduction, Signal Transduction/Phosphotyrosine/SH2 Domains, Signal Transduction/Phosphotyrosine/SH3 Domains, Signal Transduction/Protein Kinases/MAP
Introduction
Shc (Src homology collagen) is an important adaptor protein that couples tyrosine kinase receptor signaling to Ras/MAPK2 activation. As a result of alternative translation initiation sites and mRNA splicing, a single gene produces three isoforms of Shc, p66/p52/p46shc (1, 2). Three important tyrosines, Tyr239, Tyr240, and Tyr317, in p52shc are phosphorylated following growth factor stimulation. These phosphotyrosines provide binding sites for recruiting Grb2 (growth factor receptor-bound 2), which initiates the sequence of events leading to MAPK activation (3, 4). Therefore, p52shc is believed to play a positive growth-stimulatory role in a variety of cell types (4, 5). More recently, p52shc-mediated signaling has been shown to be essential for tumor progression in mouse models of human breast cancer (6). In smooth muscle cells (SMC), exposed to hyperglycemia, p52shc-mediated signaling has been demonstrated to be important for insulin-like growth factor-I (IGF-I) signaling and biological actions. Following IGF-I receptor stimulation, the integral membrane protein SHPS-1 (Src homology 2 domain-containing protein-tyrosine phosphatase substrate-1) is tyrosine-phosphorylated, and a signaling complex composed of SHP-2 (Src homology 2 domain-containing protein-tyrosine phosphatase-2), c-Src, and p52shc is assembled on SHPS-1. This induction of p52shc phosphorylation and subsequent activation of MAPK enhance the sensitivity of smooth muscle cells to the mitogenic actions of IGF-I (7). This has been demonstrated in cultured cells as well as experimental animals (8). In contrast to p52shc, the p66 isoform of Shc has been shown to inhibit both epidermal growth factor- and IGF-I-stimulated MAPK activation and cell proliferation (2, 9, 10). In addition, p66shc has been shown to regulate the oxidative stress response through its unique CH2 domain (11). p66shc expression is increased during states of oxidative stress (12–14) and hyperglycemia-induced oxidative stress (15, 16). This increase in p66shc is believed to be proapoptotic in some cell types, and genetic ablation of p66shc in mice is associated with reduced generation of ROS in response to hyperglycemia and reduced apoptosis (15, 17, 18).
In SMC, hyperglycemic stress and the increase in reactive oxygen species generation can lead to apoptosis, but if growth factors, including IGF-I, are present, the MAPK and cell proliferation responses are increased (19–22). In this cell type, the phosphorylation of p52shc in response to IGF-I is enhanced by hyperglycemia when compared cells are maintained in 5 mm glucose (23). Importantly, this response of p52shc to IGF-I in response to hyperglycemic stress is modulated by c-Src, which directly phosphorylates p52shc (24). Because both p52 and p66shc isoforms are induced in response to hyperglycemic stress yet they have been reported to mediate different actions, we investigated the role of p66shc in modulating p52shc activation and showed that its overexpression attenuated p52shc phosphorylation and inhibited the mitogenic response to IGF-I (10). Therefore, these studies were undertaken to determine the mechanism by which p66shc modulates IGF-I-stimulated p52shc activation and mitogenesis.
EXPERIMENTAL PROCEDURES
Human IGF-I was a gift from Genentech (South San Francisco, CA). Dulbecco's modified Eagle's medium containing 4500 mg of glucose/liter (DMEM-H), penicillin, streptomycin, and anti-phospho-Y419Src antibody were purchased from Invitrogen. The Grb2 polyclonal antibody and the monoclonal phosphotyrosine antibody (PY99) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Polyvinyl difluoride membrane (Immobilon P), SHPS-1 antibody, and activated Src were purchased from Millipore Corp. (Billerica, MA). The Src antibody was purchased from EMD Chemicals, Inc. (San Diego, CA). The Shc antibody and the Grb2 monoclonal antibody were purchased from BD Biosciences. The total Erk1/2, phospho-Erk1/2, and HA antibodies were obtained from Cell Signaling (Danvers, MA). Autoradiographic film was purchased from Danville Scientific, Inc. (South Plainfield, NJ). All other reagents were purchased from Sigma unless otherwise stated. Four synthetic peptides were prepared that contained the TAT sequence that confers cell permeability followed by either the proline-rich region of the CH2 domain of p66shc sequence, termed p189 (YARAAARQARALGPILPPLPGD; the underline indicates residues 42–52 in p66shc) or a control peptide that contained an equal number of prolines (YARAARQARAGPPATPAQPGLH). An additional peptide contained the sequence homologous to Src that contains Tyr328, termed p136 (YARAAARQARAVQLYAVVSEE; the underline indicates residues 325–334 in Src). A tyrosine-phosphorylated form of p136 peptide, termed p226, was used in in vitro binding and Src kinase assays as well as in living cells. The peptides were synthesized by the Protein Chemistry Core Facility (University of North Carolina, Chapel Hill, NC). These sequences were verified by mass spectrometry.
Cell Culture
Porcine smooth muscle cells (pSMC) were isolated from porcine aortas using a method that had been previously described (25). The cells were maintained in DMEM-H (25 mm glucose) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), streptomycin (100 μg/ml), and penicillin (100 units/ml). The cells that were used in these experiments were used between passages 5 and 15. Porcine aortic endothelial cells were isolated from young pigs. The aorta was rinsed through with 37 °C phosphate-buffered solution three times and incubated with medium (M199, Cellgro) containing collagenase (1 mg/ml) for about 5 min. The medium was collected and centrifuged at 3000 rpm for 5 min. The pellet was suspended with the same medium and plated into a 150-cm2 flask (Corning Glass). After attachment (overnight), the medium was refreshed. The purity of the isolated cells was verified by Von Willebrand factor (Dako) staining and morphological observation. Cells processed in this manner formed a homogenous monolayer. For experiments, the cells were plated in either 10-cm or 6-cm dishes (Corning Glass) and fed with M199 containing 20% fetal bovine serum, 25 mm glucose, streptomycin (100 μg/ml), penicillin (100 unit/ml), and sodium pyruvate (100 nm). The cells that were used in these experiments were used between passages 5 and 10.
Construction of cDNAs and Establishment of p66shc Si, EVC, p66shc WT-HA, p66shc 3F-HA, p66shc P3A-HA, p66shc ΔSH2, Src WT-HA, Src 2F-HA, and LacZ Cells
pSMC expressing small hairpin RNA sequence targeting p66shc (p66shc Si) and corresponding control pSMC expressing empty vector (EVC), pSMC expressing wild type (WT) p66shc (p66shc WT-HA), and corresponding control pSMC expressing LacZ (LacZ) have been described previously (10). In addition, pSMC expressing WT Src (Src WT-HA) and Src Y329F/Y360F (Src 2F-HA) also have been previously described (24). The p66shc WT-HA vector was used as a template to generate the p66shc Y349F/Y350F/Y427F (p66shc 3F) by two rounds of PCR. The first round of PCR generated p66shc Y349F/Y350F using forward and reverse primers: 5′-CCACCTGACCATCAGTTCTTTAATGACTTCCCGGGG-3′ and 5′-CCCCGGGAAGTCATTAAAGAACTGATGGTCAGGTGG-3′. The first round PCR product was used as a template for second round PCR to generate p66shc Y349F/Y350F/Y427F using forward and reverse primers: 5′-TTGATGATCCCTCCTTTGTCAACGTCCAGAACC-3′ and 5′-GGTTCTGGACGTTGACAAAGGAGGGATCATCAA-3′. The underlined bases indicate the substitutions that change tyrosine to phenylalanine. The forward and reverse primers that were used to generate the p66shc P47A/P48A/P50A (P3A) mutant were 5′-CATCCCTGGGGGCCATCCTGGCTCCTCTGGCTGGGGACG-3′ and 5′-CGTCCCCAGCCAGAGGAGCCAGGATGGCCCCCAGGGATG-3′. The underlined bases indicated the substitutions that change proline to alanine. Point mutations were introduced using standard method (QuikChange, Stratagene (La Jolla, CA)). The p66shc WT-HA vector was used as a template to generate the p66shc SH2 domain deletion mutant (deleting amino acids 487–579, p66shc ΔSH2) by PCR. The forward and reverse primers that were used to generate the PCR product were 5′-cac cat gga tct cct gcc ccc caa g-3′ and 5′-tta AGCGTAATCTGGAACATCGTATGGGTA ctc ccc tcg gag ctg ctc agc c-3′. The PCR product contained a Kozak sequence (CACC) at the 5′-end and a HA (capitalized) sequence at the 3′-end. All constructions were verified by DNA sequencing. 293FT cells (Invitrogen) were prepared for generation of virus stocks, and pSMC expressing p66shc 3F-HA and p66shc P3A-HA were established using procedures that were described previously (10).
Immunoprecipitation and Immunoblotting
The cell monolayers were lysed in a modified radioimmunoprecipitation assay buffer (1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mm EGTA, 150 mm NaCl, and 50 mm Tris-HCl, pH 7.5) in the presence of protease inhibitors (10 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mm phenylmethylsulfonyl fluoride, and 1 μg/ml pepstatin) and phosphatase inhibitors (25 mm sodium fluoride and 2 mm sodium orthovanadate). Coimmunoprecipitation was typically performed by incubating 0.5 mg of cell lysate with 1 μg of each of the following antibodies: anti-Shc, Grb2, Src, SHPS-1, or PY99 at 4 °C overnight. The immunoprecipitates were immobilized using protein A or G beads for 2 h at 4 °C and washed three times with the same lysis buffer containing protease and phosphatase inhibitors. The precipitated proteins were eluted in 40 μl of 2× Laemmli sample buffer, boiled for 5 min, and analyzed by SDS-PAGE followed by immunoblotting. Immunoblotting was performed as described previously (10) using a dilution of 1:1000 for anti-Shc, phospho-Erk1/2, Erk1/2, p419Src, and Src and 1:5000 for anti-Grb2. The membranes were striped for repeat immunoblotting only for loading controls. The proteins were visualized using enhanced chemiluminescence (Pierce).
In Vitro Binding Assay
The in vitro [35S]methionine (MP Biomedical, Solon, OH)-labeled intact p66shc and p52shc, p66shc P3A mutant, WT CH2 domain, and P3A-mutated CH2 domain of p66shc were generated by a transcription/translation (TNT) reaction (Promega, Madison, WI). An aliquot of the individual TNT mixture (0.4 μm total protein for each) or different combinations of them, as indicated, were incubated with 200 ng of purified active Src (Millipore Corp.) in binding buffer (HEPES, pH 7.6, 50 mm KCl, 1 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride, 0.2% Triton X-100, and 10% glycerol) in the presence or absence of p189 or p226 (1 μg/ml), using a 100-μl final volume and rotation for 2 h at 4 °C. 0.6 μg of anti-Src antibody and 30 μl (50% slurry) of protein A/G-agarose beads were then added and incubated for another 2 h. After extensive washing with the binding buffer, the precipitated proteins were eluted in 30 μl of 2× Laemmli sample buffer, boiled for 5 min, and separated using 10% SDS-PAGE. The images were developed and analyzed using a Storm860 PhosphorImager (Amersham Biosciences).
In Vitro Src Kinase Assay
Src kinase activity was determined in vitro using a Src kinase assay kit (Millipore Corp.). The kinase assay was performed following the manufacturer's instructions. Briefly, the p66shc (WT or P3A mutant), p52shc, and CH2 domain (WT or P3A mutant) were generated by a TNT reaction (Promega). An aliquot of the TNT mixture of each (final concentration 0.4 μm protein) or combinations of them as indicated were incubated with 2.5 units of purified active Src, 150 μm Src kinase substrate peptide (KVEKIGEGTYGVVYK), and 10 μCi of [γ-32P]ATP in Src reaction buffer (25 mm Tris-HCl, pH 7.2, 25 mm MgCl2, 1.25 mm MnCl2, 0.5 mm EGTA, 60 μm sodium orthovanadate, 0.5 mm dithiothreitol, and 100 μm ATP) in the presence or absence of p189 or p226. The mixtures were incubated for 30 min at room temperature. The amount of 32P that was incorporated into the substrate peptide, which reflects the activity of Src kinase, was quantified by a liquid scintillation counter (Packard, Meriden, CT).
Cell Proliferation Assay
Assessment of pSMC proliferation was performed as described previously (26). Briefly, cells were incubated with or without IGF-I (50 ng/ml) in serum-free DMEM-H containing 0.2% platelet-poor plasma for 48 h, and cell number in each well was counted. For assessment of porcine aortic endothelial cell proliferation, cells were plated at 2 × 104 in each well of a 24-well plate in M199 containing 20% fetal bovine serum and 25 mm glucose. The next day, the medium was replaced with M199 containing 0.2% fetal bovine serum and 25 mm glucose. After 14–16 h, the cells were fed with the same medium in the presence or absence of p189 or a control peptide for 2 h of incubation before being exposed or not to IGF-I (50 ng/ml). After incubation for 48 h, the cell number was determined. Each treatment was analyzed in triplicate, and the results represent mean values of three independent experiments.
Cell Migration Assay
Wounding of control and p66shc WT- and p66shc P3A-overexpressing pSMC was performed as described previously (27). The wounded monolayers were exposed to IGF-I (100 ng/ml) for 48 h at 37 °C. The cells were fixed and stained (Diff Quick, Dade Behring, Inc., Newark, DE), and the number of cells migrating into the wound area was counted. At least eight of the previously selected 1-mm areas at the edge of the wound were counted for each data point.
Statistical Analysis
Chemiluminescent images obtained were scanned using a DuoScan T1200 (AGFA, Brussels, Belgium). The band intensities of the scanned images were within the linear range of detection and were analyzed using NIH Image J, version 1.37V. The results that are shown in all experiments are representative of at least three separate experiments unless otherwise stated and expressed as the mean ± S.E. Student's t test was used to compare differences between control and treatment groups or control cells and cells expressing mutant proteins. p ≤ 0.05 was considered statistically significant.
RESULTS
p66shc Inhibits IGF-I Signaling via Disrupting SHPS-1·p52shc·Grb2 Complex Formation
To examine the possibility that p66shc was competitively inhibiting Grb2 binding to p52shc, we substituted the tyrosines in p66shc that have homology to the p52shc-Grb2 binding sites with phenylalanine and expressed this 3F mutant form of p66shc in pSMC. The expression level was comparable with cultures in which WT p66shc had been overexpressed (Fig. 1A). p66shc 3F did not associate with Grb2 after IGF-I stimulation (Fig. 1B). However, overexpression of p66shc 3F, like p66shc WT, impaired IGF-I-stimulated p52shc-Grb2 recruitment (e.g. a 1.20 ± 0.05-fold increase in p66shc 3F cells versus a 2.23 ± 0.17-fold increase in LacZ cells after 10-min IGF-I stimulation; p < 0.05) (Fig. 1B). Similarly, when formation of the SHPS-1 signaling complex was examined following IGF-I stimulation, overexpression of p66shc 3F inhibited IGF-I-dependent p52shc association with SHPS-1 (e.g. a 1.46 ± 0.05-fold increase in p66shc 3F versus a 2.10 ± 0.04-fold increase in LacZ cells after 10-min IGF-I stimulation; p < 0.01) and Grb2 recruitment to the SHPS-1 complex (e.g. a 1.35 ± 0.07-fold increase in p66shc 3F versus a 2.49 ± 0.11-fold increase in LacZ after 10-min IGF-I stimulation; p < 0.01) although it had no effect on IGF-I-stimulated SHPS-1 phosphorylation (Fig. 1C). In addition, we detected increased p66shc association with SHPS-1 after IGF-I stimulation in both the p66shc 3F- and WT p66shc-overexpressing cells, compared with LacZ cells, demonstrating that the ability of p66shc to bind to the SHPS-1 complex was not impaired by the 3F mutation (Fig. 1C). Because our previous studies have shown that Src kinase phosphorylates p52shc (24) and that p52shc phosphorylation is inhibited constitutively by p66shc overexpression (10), these results suggested that p66shc could be inhibiting Src kinase.
FIGURE 1.
Inhibitory effect of p66shc on IGF-I signaling via disrupting SHPS-1·p52shc·Grb2 complex formation does not require its Grb2 binding ability. All three cell types were serum-starved for 16–18 h before adding IGF-I (100 ng/ml) for the indicated times. A, pSMC were transduced with pLenti-LacZ or pLenti-HA p66shc Y349F/Y350F/Y427F (p66shc 3F) or pLenti-HA p66shc (p66shc WT) vector. Cell lysates were immunoblotted (IB) with an anti-HA antibody. B, P66shc WT-, LacZ-, and p66shc 3F-overexpressing cell lysates were immunoprecipitated (IP) with an anti-Grb2 antibody and immunoblotted with an anti-Shc antibody. To control for loading, the blot was reprobed for an anti-Grb2 antibody. p < 0.05 (*) indicates a significant difference between LacZ cells and p66shc WT or p66shc 3F cells at 10 min after IGF-I stimulation. C, the cell lysates were immunoprecipitated with an anti-SHPS-1 polyclonal antibody and immunoblotted with anti-Tyr(P) (PY99), Shc, or Grb2 antibodies, respectively. To control for loading, the blot was immunoblotted with an anti-SHPS-1 antibody. p < 0.05 (*) and p < 0.01 (**) indicate significant differences at 10 and 20 min after IGF-I stimulation between LacZ cells and p66shc WT or p66shc 3F cells. The figure is representative of three independent experiments.
p66shc Binds Directly to Src through Both Polyproline-SH3 and -SH2 Phosphotyrosine Site Interactions
Overexpression of p66shc impaired p52shc recruitment to Src and enhanced p66shc association with Src after IGF-I stimulation (Fig. 2A). Similarly, the p66shc 3F mutant bound to Src, and it also impaired p52shc-Src association after IGF-I stimulation (Fig. 2A). The amino acid sequences of p66shc and p52shc are identical except for the addition of a unique CH2 domain in the N terminus of p66shc. A PPLP motif is present within the CH2 domain; thus, in addition to the phosphotyrosine-SH2 (Tyr(P)-SH2) domain interaction that occurs between p52shc and Src, p66shc could potentially associate with Src through a PXXP-SH3 domain interaction (Fig. 2B). To determine if this domain interacted directly with Src, an in vitro binding assay was performed using a cell-free expression system. Expression of p66shc and p52shc was detected (Fig. 2C, lanes 1 and 7). Co-incubation of activated Src with p66shc resulted in their direct association (Fig. 2C, lane 2). This binding was disrupted by co-incubating with a synthetic peptide, termed p189, which contains the PPLP motif of p66shc (Fig. 2C, lane 3). As predicted, p52shc bound directly to Src (Fig. 2C, lane 4). When both p66shc and p52shc were incubated with activated Src, Src binding to p52shc was impaired (Fig. 2C, lane 5 versus lane 4). This inhibitory effect of p66shc could be reduced by co-incubating with p189 (Fig. 2C, lane 6 versus lane 5). To further address this question, a p66shc P3A mutant was expressed (Fig. 2D, lane 4). Compared with WT p66shc, the p66shc P3A mutant had impaired its binding to Src (Fig. 2D, compare lane 1 with lane 2). In addition, the binding of WT p66shc to Src could not be disrupted by preincubation with peptide 226, which contains the phosphorylated YXXV (pYXXV) motif of Src, the binding site for p52shc (Fig. 2D, lane 2 versus lane 3). Further supporting data were obtained by expressing the full-length WT CH2 domain (Fig. 2E, lane 1) and P3A mutant CH2 domain (Fig. 2E, lane 2) and testing their ability to bind to Src. The results showed that the WT CH2 domain, but not P3A mutant CH2 domain, could directly bind to Src (Fig. 2E, lane 3 versus lane 4). Co-incubation of the CH2 domain peptide with p52shc and Src resulted in a 69 ± 6% decrease in p52shc-Src association (Fig. 2E, lane 6 versus lane 7), but the P3A mutant CH2 domain had no effect (Fig. 2E, lane 8). These in vitro binding assay results indicate that p66shc can directly bind to Src through a PXXP-SH3 domain interaction, and this impairs p52shc-Src association.
FIGURE 2.
p66shc directly binds to Src through a polyproline-SH3 domain interaction in vitro. A, wild type p66shc (p66shc WT)-, LacZ-, and p66shc 3F-overexpressing cells were serum-starved for 16–18 h prior to the addition of IGF-I (100 ng/ml) for the indicated times. The cell lysates were immunoprecipitated (IP) with an anti-Src antibody and immunoblotted (IB) with an anti-Shc antibody. To control for loading, the blots were stripped and reprobed with an anti-Src antibody. B, analysis of potential interactions between Src and p66shc, including SH3 domain-polyproline interaction (SH3-PP) and the phosphotyrosine and SH2 domain interaction (pY-SH2). C and D, the ability of p66shc, p52shc, or the p66shc P3A mutant to bind to Src and of the synthetic peptide p189 or p226 to disrupt Src interaction with p66shc and p52shc was determined using in vitro binding assays as described under “Experimental Procedures.” E, the interaction of intact CH2 domain or P3A-mutated CH2 domain and/or p52shc with Src was determined as described for C and D. 35S-Labeled p66shc, p52shc, p66shc (P3A), CH2, and CH2 (P3A) were visualized by using a PhosphorImager. The figure is representative of at least two independent experiments.
Because this interaction has the potential to lead to a conformational change in Src that could impair either Src activation or its ability to bind to p52shc, we investigated the effect of the Src-p66shc interaction on these signaling events. In p66shc-overexpressing cells, p66shc (HA) binding to Src occurred after 1 min of IGF-I treatment and did not further increase after 3 min (Fig. 3A, 2.6-fold versus 3.0-fold). In LacZ cells, p66shc binding to Src was increased after 1-min IGF-I treatment and further enhanced after 3 min of IGF-I stimulation (Fig. 3A, 2.6-fold versus 5.6-fold). However, the fold increase in LacZ- and p66shc-overexpressing cells cannot be compared because, in order to clearly show the increase in Src-associated p66shc in LacZ cells, more cell lysate had to be used for co-immunoprecipitation. The Src-p66shc interaction at 1 min could be significantly disrupted by incubating with p189 (4.6-fold versus 2.8-fold), whereas the 3-min interaction could only be partially disrupted (Fig. 3B, 9.1-fold versus 5.2-fold). In contrast, p136, a peptide containing the YXXV motif of Src that binds to the SH2 domain in p52shc, only reduces this interaction after 3-min of IGF-I stimulation (Fig. 3B, 9.1-fold versus 4.8-fold) but not after 1 min of IGF-I stimulation. Incubating with both p189 and p136 had an even greater effect after 3-min IGF-I stimulation (Fig. 3B, 9.1-fold versus 2.2-fold). To confirm that the response to p136 was due to the phosphorylated tyrosine, we examined these interactions using a tyrosine-phosphorylated peptide, p226, and nontransduced pSMC. Similar results were obtained (Fig. 3C). Of note, there was a slight variation in the effect of p136 or p226 on p66shc·Src complex formation at 1 min of IGF-I treatment (Fig. 3, B and C, lane 4 versus lane 2). However, after standardization using total Src protein and pooling the results from three independent experiments, no significant difference was detected between IGF-I alone and IGF-I plus p136 or p226. These data suggest that the binding of p66shc to Src is initially mediated through PXXP-SH3 domain interaction after 1 min of IGF-I stimulation and through both PXXP-SH3 and SH2-Tyr(P) interactions after 3 min of IGF-I stimulation in living cells.
FIGURE 3.
p66shc binds to Src through both polyproline-SH3 and SH2-phosphotyrosine interactions in living cells. A, pSMC were transduced with pLenti-LacZ (LacZ) or pLenti-HA p66shc (p66shc WT) vector. Both cell types were serum-starved for 16–18 h before stimulation with IGF-I (100 ng/ml) for the indicated times. Cell lysates (p66shc WT, 500 μg of protein; LacZ, 750 μg of protein) were immunoprecipitated (IP) with an anti-Src antibody and immunoblotted (IB) with an anti-Shc antibody. To control for loading, the blots were reprobed with anti-Src antibody. The arrow denotes the p66shc band. B and C, quiescent LacZ cells (B) or non-transduced wild type pSMC (C) were preincubated with p189 and/or p136 or p226 (5 μm) as indicated for 2 h and treated with IGF-I (100 ng/ml) for the indicated times. The cell lysates were immunoprecipitated with an anti-Src antibody and immunoblotted with an anti-Shc antibody. To control the loading, the blot was reprobed with an anti-Src antibody. The arrow denotes the p66shc band. The ratio represents the densitometry value of Src-associated p66shc divided by the densitometry value of total Src in each individual lane. D and E, cell lysates from quiescent wild type p66shc (p66shc WT)- and P47A/P48A/P50A mutated p66shc (p66shc P3A)-overexpressing cells were analyzed for HA protein expression by immunoblotting with an anti-HA antibody. Quiescent p66shc P3A-overexpressing cells were incubated with or without p189 or p136 (5 μm) as indicated for 2 h before being exposed to IGF-I (100 ng/ml) for the indicated times. The cell lysates were immunoprecipitated with an anti-Src antibody and immunoblotted with an anti-Shc antibody. The arrows denote the p66shc and p52shc bands. p < 0.05 (*) indicates that p136 preincubation significantly impairs p66shc and p52shc binding to Src after IGF-I stimulation, compared with without a peptide or p189 preincubation in p66shc P3A cells (for D). p < 0.01 (**) and p < 0.001 (***) indicate that 3 min of IGF-I stimulation significantly enhances p66shc and p52shc association with Src in p66shc P3A (for E). F, pSMC were transduced with pLenti-wild type Src (Src WT) or pLenti-Y329F/Y360F Src (Src FF) vectors. Quiescent cells were incubated with or without p189 for 2 h and then stimulated with IGF-I (100 ng/ml) for the indicated times. Cell lysates were immunoprecipitated with an anti-Src antibody, followed by immunoblotting with an anti-Shc antibody. To control for loading, the blot was reprobed with an anti-Src antibody. The arrows denote the p66shc and p52shc bands. The figure is representative of three independent experiments.
To further address this question, we utilized mutagenesis of full-length p66shc to disrupt the PXXP-SH3 domain interaction. Three prolines in the CH2 domain of p66shc were substituted with alanines. Expression of this mutant p66shc (p66shc P3A) was verified by HA expression, and the expression level was comparable with WT p66shc (p66shc WT) (Fig. 3D). Overexpression of p66shc P3A did not impair IGF-I-stimulated p66shc binding to Src, which was clearly increased after 10 min (e.g. 4.03 ± 0.52-fold increase above basal level after 10-min IGF-I stimulation; p < 0.05). In contrast to cells overexpressing WT p66shc, Src-p52shc association was increased to a greater extent (e.g. a 2.82 ± 0.10-fold increase in p66shc P3A cells versus a 1.53 ± 0.09-fold increase in p66shc WT cells after 10-min IGF-I stimulation; p < 0.01) (Fig. 3D versus Fig. 2A). This association was not significantly affected by preincubating with p189, which disrupts the PXXP-SH3 domain interaction. However, preincubating with peptide p136, which blocks the SH2-Tyr(P) interaction, inhibited both p66shc-Src (e.g. a 4.03 ± 0.52-fold increase with no peptide preincubation versus a 1.82 ± 0.08-fold increase with p136 preincubation after 10-min IGF-I stimulation; p < 0.05) and p52shc-Src interactions (e.g. a 2.82 ± 0.10-fold increase with no peptide preincubation versus a 1.72 ± 0.10-fold increase with p136 preincubation after 10-min IGF-I stimulation; p < 0.05) (Fig. 3D). This suggested that p66shc bound to Src via a SH2-Tyr(P) domain interaction in p66shc P3A-overexpressing cells. Because analysis of the time course of binding to each of the two sites indicated that the PXXP/SH3 interaction occurred at 1 min after IGF-I stimulation, a detailed time course analysis of this interaction using cells expressing the mutant was undertaken. The results indicated that a significant increase in IGF-I-dependent p66shc binding to Src did not occur until 3 min of IGF-I treatment in cells expressing p66shc P3A (e.g. a 4.73 ± 0.12-fold increase above basal level after 3-min IGF-I treatment; p < 0.001) (Fig. 3E). Similarly, a major increase in p52shc binding to Src could be detected after 3 min of exposure to IGF-I (e.g. a 3.56 ± 0.16-fold increase above basal level after 3-min IGF-I stimulation; p < 0.01) (Fig. 3E). We conclude that this interaction is mediated via the SH2-Tyr(P) domain interaction, which can be disrupted by p136 incubation (e.g. a 3.56 ± 0.16-fold increase with no peptide preincubation versus a 1.66 ± 0.07-fold increase in p136 preincubation after 3-min IGF-I treatment; p < 0.01) but not p189 (e.g. a 3.56 ± 0.16-fold increase with no peptide preincubation versus a 4.16 ± 0.17-fold increase with p189 preincubation after 3-min IGF-I treatment; p, not significant (NS)) (Fig. 3E). To further determine the importance of the YXX(V/L)-SH2 domain interaction for p66shc binding, the two tyrosines in Src that mediate the p52shc-Src interaction were substituted with phenylalanines (Src FF mutant). Overexpression of the Src FF mutant in pSMC significantly impaired both IGF-I-stimulated p52shc and p66shc recruitment to Src, compared with overexpression of Src WT after 5-min treatment (Fig. 3F). This confirms that, like p52shc, p66shc also binds to Src through SH2-Tyr(P) interaction after IGF-I stimulation. However, upon IGF-I stimulation, an increase of p66shc bound to Src was still detected in Src FF cells at the 1-min time point. That this is mediated via the PXXP-SH3 interaction was confirmed by showing that its binding could be disrupted by p189 preincubation. In contrast, there was no change of p52shc-Src interaction after IGF-I stimulation in Src FF cells at the same time (Fig. 3F).
The Inhibitory Effect of p66shc on IGF-I-stimulated Downstream Signaling and Biological Actions Requires Its Binding to Src via PXXP-SH3 Domain Interaction
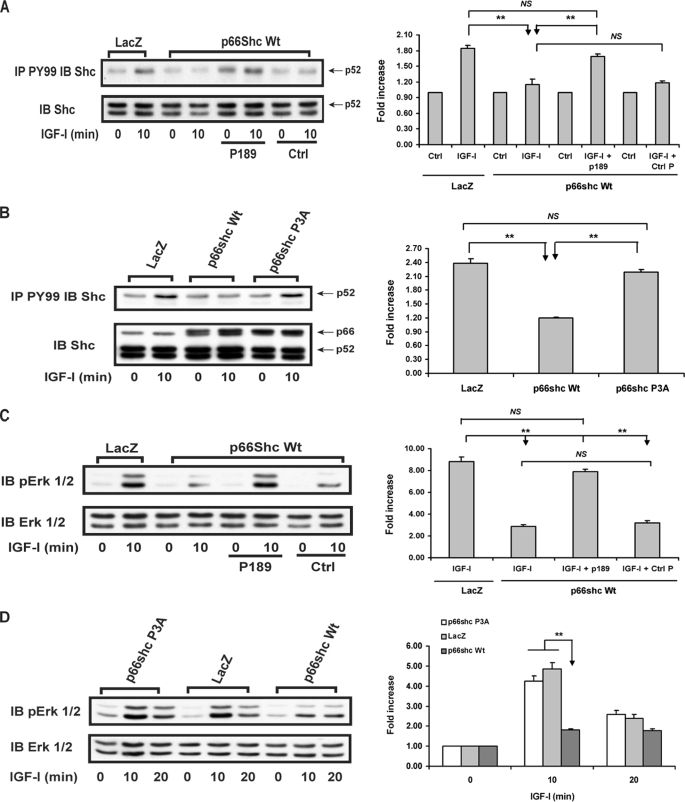
Overexpression of p66shc impaired IGF-I-stimulated p52shc phosphorylation compared with cells expressing LacZ (e.g. a 1.84 ± 0.07-fold increase in LacZ cells versus a 1.15 ± 0.10-fold increase in p66shc WT cells after 10-min IGF-I stimulation; p < 0.01) (Fig. 4A). This inhibition could be reduced by preincubation with p189 (e.g. a 1.69 ± 0.04-fold increase following p189 preincubation versus a 1.15 ± 0.10-fold increase with no peptide preincubation after 10-min IGF-I stimulation; p < 0.01) but not by a control peptide (Fig. 4A). Similarly, overexpression of the p66shc P3A mutant, which was comparable with WT p66shc overexpression, had no inhibitory effect on IGF-I-stimulated p52shc phosphorylation (e.g. a 2.39 ± 0.09-fold increase in LacZ cells versus a 2.19 ± 0.05-fold increase in p66shc P3A cells after 10-min IGF-I stimulation; p, NS) (Fig. 4B). When downstream signaling changes were analyzed using WT p66shc-overexpressing cells, incubation with p189 significantly increased IGF-I-stimulated MAPK activation, compared with no peptide or preincubation with a control peptide (e.g. a 7.88 ± 0.23 fold versus a 2.85 ± 0.20-fold or 3.20 ± 0.21-fold increase after 10-min IGF-I stimulation; p < 0.01) (Fig. 4C). Consistently, overexpression of p66shc P3A, unlike the WT p66shc, had no inhibitory effect on IGF-I-stimulated Erk1/2 phosphorylation (e.g. a 4.25 ± 0.24-fold increase in p66shc P3A cells versus a 4.85 ± 0.32-fold increase in LacZ cells after 10 min of IGF-I stimulation; p < 0.01) (Fig. 4D).
FIGURE 4.
Inhibitory effect of p66shc on IGF-I-stimulated p52shc tyrosine phosphorylation and MAPK activation is dependent on its binding to Src through the polyproline-SH3 domain interaction. Quiescent LacZ-, wild type p66shc (p66shc WT)-, and p66shc P3A mutant-overexpressing cells were incubated with or without the synthetic peptide 189 or control peptide for 2 h before adding IGF-I (100 ng/ml) for the indicated times. A and B, cell lysates were immunoprecipitated (IP) with an anti-Tyr(P) (PY99) antibody and immunoblotted (IB) with an anti-Shc antibody. To control for loading, an equal amount of cell lysate was immunoblotted with an anti-Shc antibody. p < 0.01 (**) indicates a significant difference between two different treatments or the same treatment in two cell types. The arrows denote the p52shc and p66shc bands. C and D, the same cell lysates were used to determine the Erk1/2 phosphorylation by probing with an anti-phospho-Erk1/2 antibody. The blots were reprobed with an anti-Erk1/2 antibody to control for loading differences. p < 0.01 (**) indicates a significant difference between two different treatments or the same treatment in two cell types. The figure is representative of three independent experiments.
Because disruption of p66shc binding to Src through PXXP-SH3 domain interaction significantly attenuated the inhibitory effect of p66shc on IGF-I signal transduction, we determined whether this change could lead to alterations of IGF-I-stimulated biological actions. Overexpression of WT p66shc significantly impaired IGF-I-stimulated cell proliferation, compared with LacZ cells (1.35 ± 0.05-fold versus 2.02 ± 0.08-fold increase (p < 0.01), a 66% reduction compared with the maximal response) (Fig. 5A). However, overexpression of p66shc P3A had no inhibitory effect on the IGF-I-stimulated cell proliferation, compared with LacZ cells (1.90 ± 0.04-fold versus 2.02 ± 0.08-fold increase; p, NS) (Fig. 5A). Of note, for primary pSMC, the maximum response to 10% fetal calf serum is only a 3-fold increase in cell number in 48 h. Similarly, overexpression of p66shc significantly attenuated the ability of IGF-I to stimulate cell migration (1.53 ± 0.09-fold versus 2.07 ± 0.08-fold increase, compared with LacZ cells (p < 0.01), a 50% reduction compared with the maximal response) (Fig. 5B). In contrast, overexpression of p66shc P3A did not inhibit the ability of IGF-I to stimulate cell migration, compared with LacZ cells (1.90 ± 0.03-fold versus 2.07 ± 0.08-fold increase after IGF-I stimulation; p, NS) (Fig. 5B).
FIGURE 5.
Inhibitory effect of p66shc on IGF-I-stimulated proliferation and migration is dependent on its binding to Src through polyproline-SH3 interaction. Cell proliferation and migration were determined as described under “Experimental Procedures.” A, comparison of the cell-proliferative response to IGF-I (50 ng/ml) among wild type p66shc (p66shc WT)-, p66shc P3A-, and LacZ-overexpressing cells, respectively. Each bar indicates the fold increase over basal level and represents the pool of at least three independent experiments. Cells overexpressing wild type p66shc showed a significant impairment of IGF-I-stimulated cell proliferation, compared with control LacZ- and p66shc P3A-overexpressing cells, respectively. B, comparison of cell migration response to IGF-I (100 ng/ml) among p66shc WT-, p66shc P3A-, and LacZ-overexpressing cells, respectively. Each bar indicates the fold increase over basal and represents the pool of at least three independent experiments. Overexpression of wild type p66shc significantly attenuated IGF-I-stimulated cell migration, compared with LacZ- and p66shc P3A-overexpressing cells, respectively.
Determination of the Mechanism by Which p66shc Inhibits Src Activation
We have previously shown that Src activation (phosphorylation of Tyr419, equivalent to Tyr416 in chicken Src), followed by phosphorylation of Tyr329 and Tyr360, is required for Src-p52shc association (24). Because p66shc can bind directly to Src after IGF-I stimulation independently of Tyr329 and Tyr360 phosphorylation and this inhibits p52shc phosphorylation, we determined if the binding of p66shc to Src could alter IGF-I-stimulated Src activation. Cells expressing p66shc short hairpin RNA showed a 87 ± 2% decrease in p66shc (Fig. 6A) compared with empty vector control. IGF-I stimulated Src tyrosine 419 phosphorylation (an index of Src activation) after a 1-min treatment, and this response was enhanced by p66shc knockdown (e.g. a 1.81 ± 0.07-fold increase in EVC versus a 2.53 ± 0.08-fold increase in p66shc Si; p < 0.001) (Fig. 6A). This suggests that constitutive expression of p66shc inhibits IGF-I-dependent Src activation. To determine if p66shc inhibits Src activation directly, we performed an in vitro Src kinase assay. The results showed that p66shc directly inhibited Src activation in a dose-dependent manner (Fig. 6, B and C, lane 2 versus lane 1; p < 0.001). Because we had shown (Fig. 2) that p66shc interacted with Src through its PXXP sequence, we determined if the short peptide containing this sequence p189 could inhibit Src activation. The peptide alone had no effect (Fig. 6C, lane 7 versus lane 1; p, NS). However, p189 completely blocked the inhibitory effect of p66shc on Src activation (Fig. 6C, lane 5 versus lane 1; p, NS). Although p226, the peptide that blocked the SH2-Tyr(P) interaction, was unable to completely abolish the inhibitory effect of WT p66shc on Src activity (Fig. 6C, lane 6 versus lane 1; p < 0.001), it partially rescued Src kinase activity in the presence of WT p66shc (Fig. 6C, lane 6 versus lane 2; p < 0.01). However, it was much less effective than p189 (Fig. 6C, lane 6 versus lane 5; p < 0.001). Interestingly, the CH2 domain alone also impaired Src kinase activity (Fig. 6C, lane 4 versus lane 1; p < 0.01), but its effect was less than that of full-length WT p66shc (Fig. 6C, lane 4 versus lane 2; p < 0.001). Consistent with the in vitro binding assay data, the P3A p66shc mutant and P3A mutant CH2 domain, which do not bind to Src through the PXXP-SH3 interaction, had no effect on Src kinase activity (Fig. 6, lanes 9 and 10 versus lane 1; p, NS). Similarly, p52shc also had no effect on Src kinase activity (Fig. 6C, lane 11 versus lane 1; p, NS). These data confirm that PXXP-SH3 interaction plays a vital role in inhibition of Src activation. However, because full-length p66shc had greater effect than the CH2 domain, this suggests that the SH2-Tyr(P) interaction was also necessary for maximal inhibition.
FIGURE 6.
p66shc impairs Src kinase activation through polyproline-SH3 domain interaction in vitro. A, after IGF-I treatment (100 ng/ml) for the indicated times, the lysates from empty vector (EVC)- and p66shc short hairpin RNA template vector (p66shc Si)-transduced cells were used to determine the activation of Src kinase by immunoblotting (IB) with an anti-phospho-Tyr419 Src antibody. The blot was reprobed with an anti-Src antibody to control for loading differences. p < 0.001 (***) indicates that knockdown of p66shc significantly enhances IGF-I-stimulated Src kinase activation, compared with EVC. B–D, p66shc, p52shc, p66shc (P3A), CH2, and CH2 (P3A) were expressed using a cell-free system and then incubated with activated Src kinase, and the ability of Src to phosphorylate a peptide substrate in vitro was determined as described under “Experimental Procedures.” The effect of p189 or p226 was also determined. p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***) indicate the significant differences between the treatment and control or between two different treatments. The figure is representative of three independent experiments.
To determine the importance of the SH2-Tyr(P) interaction, the SH2 domain of p66shc was deleted, and the mutant form was tested for its ability to inhibit Src kinase activity in vitro. This p66shc mutant was less effective than full-length p66shc (data not shown). However, when the CH2 domain peptide and p52shc, which binds to Src through SH2-Tyr(P) interaction, were added together, there was no additional effect compared with the CH2 domain alone (Fig. 6D, lane 3 versus lane 5; p, NS).
We used cell-permeable peptides to confirm these results using living cells. IGF-I-stimulated Src activation was impaired following overexpression of WT p66shc (e.g. a 2.46 ± 0.15-fold increase in LacZ versus a 1.49 ± 0.10-fold increase in p66shc WT after IGF-I stimulation; p < 0.01), and this could be reduced by preincubation with p189 (e.g. a 1.49 ± 0.10-fold increase in p66shc WT versus a 2.57 ± 0.15-fold increase in p66shc WT with p189 preincubation after IGF-I stimulation; p < 0.01) (Fig. 7A). In addition, we showed that p189 could enhance IGF-I-stimulated Src activation in wild type SMC (data not shown). Although p136 was less effective than p189 in rescuing IGF-I-stimulated Src activation (e.g. a 2.70 ± 0.30-fold increase with p136 preincubation versus a 3.53 ± 0.46-fold increase in p189 preincubation after IGF-I stimulation; p < 0.05, n = 4), it had a significant effect (e.g. a 2.70 ± 0.30-fold increase in p136 preincubation versus a 1.74 ± 0.15-fold increase without preincubation after IGF-I stimulation in p66shc WT cells; p < 0.05, n = 4) (Fig. 7B), suggesting that Tyr(P)-SH2 interaction between Src and p66shc also plays a role in mediating the inhibitory effect of p66shc on Src activation in living cells.
FIGURE 7.
p66shc impairs IGF-I-stimulated Src kinase activation through polyproline-SH3 domain and SH2-phosphotyrosine interactions in living cells. A–C, quiescent LacZ, WT p66shc (p66shc WT)-, and p66shc P3A-overexpressing cells were preincubated with or without p189 or p136 for 2 h before stimulation with IGF-I (100 ng/ml) for the indicated times. The activation of Src kinase was measured by probing with an anti-phospho-Tyr419 Src antibody. The blots were reprobed with an anti-Src antibody to control for loading differences. p < 0.05 (*) and p < 0.01 (**) indicate the significant differences between two different treatments or the same treatment in two cell types. D, pSMC were transduced with pLenti-p66shc WT-HA (p66shc WT) or pLenti-p66shc ΔSH2-HA (p66shc ΔSH2) vector. Both cell types were serum-starved for 16–18 h and analyzed for HA and p66shc protein expression. Cell lysates were immunoblotted with anti-HA and p66shc antibodies. The arrows denote the 66-kDa and SH2-deleted p66shc forms. E, quiescent LacZ, p66shc WT, and p66shc ΔSH2 cells were stimulated with IGF-I (100 ng/ml) for the indicated times. Cell lysates were immunoblotted with an anti-phospho-Tyr419 Src antibody. To control for loading, the blot was reprobed with an anti-Src antibody. p < 0.01 (**) and p < 0.05 (*) indicate the significant differences between two cell types. The figure is representative of at least three independent experiments. IB, immunoblot; Ctrl, control.
To confirm the results obtained using the peptides, the cells expressing the p66shc P3A and p66shc ΔSH2 mutants were utilized. In cells expressing P3A mutant, IGF-I stimulated Src activation to the same extent as cells expressing LacZ (e.g. a 2.00 ± 0.10-fold increase in p66shc P3A cells versus a 2.05 ± 0.10-fold increase in LacZ cells after IGF-I stimulation; p, NS), whereas cells expressing WT p66shc showed the inhibition of Src activation (e.g. a 2.05 ± 0.10-fold increase in LacZ cells versus a 1.23 ± 0.09-fold increase in p66shc WT cells after IGF-I stimulation; p < 0.01) (Fig. 7C). Consistent with peptide data, overexpression of p66shc ΔSH2 (Fig. 7D), which eliminates the Tyr(P)-SH2 interaction between Src and p66shc, still inhibited IGF-I-stimulated Src activation (e.g. a 2.68 ± 0.24-fold increase in LacZ cells versus a 1.86 ± 0.10-fold increase in p66shc ΔSH2; p < 0.01), but it was less effective than full-length p66shc (e.g. a 1.86 ± 0.10-fold increase in p66shc ΔSH2 versus a 1.32 ± 0.13-fold increase in p66shc WT cells after IGF-I stimulation; p < 0.05) (Fig. 7E). Based on these data, we conclude that the full inhibitory effect of p66shc on IGF-I-stimulated Src activation requires both PXXP-SH3 and Tyr(P)-SH2 interactions but that the inhibitory effect is initiated by the PXXP-SH3 interaction.
Examination of the Proposed Mechanism in Porcine Aortic Endothelial Cells
In order to determine whether the proposed mechanism is unique for pSMC or is applicable for all other cell types, we used the porcine aortic endothelial cells to examine several pivotal results. We chose endothelial cells because they express αVβ3, Src, p66shc, and SHPS-1, the major components affecting SHPS-1 complex formation and IGF-I signal transduction, and they have been shown to respond to hyperglycemia by increasing their sensitivity to IGF-I stimulation (28, 29). Similar to pSMC, IGF-I stimulated p66shc association with Src after 1- and 3-min treatment. The interaction at 1 min was disrupted by p189, the polyproline and SH3 domain interaction blocker, but not by tyrosine-phosphorylated p226. Both peptides were able to cause some disruption of the interaction at 3 min after IGF-I stimulation (Fig. 8A), which is consistent with the hypothesis that binding occurs through both sites. In addition, IGF-I stimulated Src activation after a 1-min treatment (2.14 ± 0.08-fold increase above the basal level; p < 0.01), and this stimulation was enhanced by p189 preincubation (additional 1.53 ± 0.12-fold increase compared with IGF-I alone; p < 0.01) but not a control peptide (Fig. 8B). To determine the effect on downstream signaling, we examined the p52shc tyrosine phosphorylation and MAPK activation in response to IGF-I. IGF-I stimulated p52shc tyrosine phosphorylation (2.77 ± 0.22-fold increase above basal; p < 0.01) and MAPK activation (3.18 ± 0.18-fold increase above basal; p < 0.01). Importantly, these responses were enhanced by p189 but not a control peptide (Fig. 8, C and D). To determine the effect of disruption of Src-p66shc, on IGF-I-stimulated biological actions, we examined IGF-I-stimulated cell proliferation in the presence of p189 or a control peptide. IGF-I-stimulated cell proliferation was enhanced by p189 preincubation (1.87 ± 0.09-fold versus 2.38 ± 0.08-fold increase after IGF-I treatment; p < 0.05), but exposure to the control peptide had no effect (1.87 ± 0.09-fold versus 1.76 ± 0.07-fold increase after IGF-I treatment; p, NS) (Fig. 8E).
FIGURE 8.
The proposed mechanism is also utilized in porcine aortic endothelial cells. A, endothelial cells were serum-starved for 16–18 h and preincubated with p189 or p226 (5 μm) for 2 h before adding IGF-I (100 ng/ml) for the indicated times. The cell lysates were immunoprecipitated (IP) with an anti-Src antibody and immunoblotted (IB) with an anti-Shc antibody. To control the loading, the blot was reprobed with an anti-Src antibody. The arrow denotes the p66shc band. B, quiescent endothelial cells were preincubated with p189 or a control peptide (5 μm) for 2 h before adding IGF-I (100 ng/ml) for 1 min. The activation of Src kinase was measured by probing with an anti-phospho-Tyr419 Src antibody. The blots were reprobed with an anti-Src antibody to control for loading differences. C, quiescent endothelial cells were preincubated with p189 or a control peptide (5 μm) for 2 h before adding IGF-I (100 ng/ml) for 5 min. The cell lysates were immunoprecipitated with an anti-Tyr(P) (pY99) antibody and immunoblotted with an anti-Shc antibody. To control the loading, an equal amount of cell lysate was immunoblotted with an anti-Shc antibody. The arrow denotes the p52shc band. D, the same cell lysates were used to determine the Erk1/2 phosphorylation by probing with an anti-phospho-Erk1/2 antibody. The blots were reprobed with an anti-Erk1/2 antibody to control for loading differences. E, cell proliferation was determined as described under “Experimental Procedures”. Each bar indicates the fold increase over basal and represents the pool of at least three independent experiments. p < 0.01 (**) and p < 0.001 (***) indicate the significant differences between two different treatments. The figure is representative of three independent experiments.
DISCUSSION
Our prior studies have shown that in response to hyperglycemia, a signaling complex composed of the scaffolding protein SHPS-1, SHP-2, Src, and p52shc is formed following IGF-I stimulation. This complex is essential for IGF-I-stimulated p52shc and MAPK activation and cell proliferation in vascular cell types (7). p52shc is recruited to this complex by Src, which then phosphorylates three tyrosines (24), leading to Grb2 recruitment and MAPK activation. In contrast to p52shc, the p66shc isoform, which is also increased in response to hyperglycemia, inhibits IGF-I-stimulated SHPS-1 complex formation and p52shc recruitment to this complex, resulting in impaired Grb2 binding and MAPK activation, cell proliferation, and migration in response to IGF-I (10). In the present study, we have determined the mechanism by which p66shc disrupts IGF-I-stimulated p52shc recruitment and activation. The results demonstrate that p66shc sequentially binds to Src via a PXXP-SH3 interaction, followed by a SH2-Tyr(P) interaction, in response to IGF-I, and this leads to impairment of IGF-I-stimulated Src kinase activation, resulting in attenuated IGF-I-stimulated p52shc phosphorylation and impaired MAPK activation, SMC proliferation, and migration.
To understand how p66shc inhibits IGF-I-stimulated p52shc-dependent signaling, several potential mechanisms were analyzed. Previous studies had proposed that EGF-dependent association of Grb2 with p66shc interfered with the ability of Grb2 to recruit SOS (son of sevenless), leading to attenuated activation of MAPK (2), or that overexpression of p66shc competed with p52shc for binding to a limited pool of Grb2, resulting in impaired p52shc association and MAPK activation (9). In contrast, our results showed that p66shc 3F mutant, which does not bind to Grb2, still inhibited the recruitment of Grb2 to p52shc to the same extent as wild type p66shc (Fig. 1C). Therefore, we focused on the role of the unique CH2 domain of p66shc in attenuating p52shc phosphorylation. Because a polyproline sequence was identified in the p66shc CH2 domain and a SH3 domain is contained in Src, we investigated whether this interaction was modulating Src kinase activation. Our in vitro binding assay results showed that p66shc bound directly to the SH3 domain of Src through a PILPPLP sequence contained in the CH2 domain. Further analyses using intact SMC indicated that p66shc bound to Src not only via the PXXP-SH3 interaction but also through a SH2 domain and Tyr(P) interaction in response to IGF-I. Using specific blocking peptides and SMC expressing a variety of mutants, we were able to show that these binding interactions occurred sequentially after IGF-I stimulation, the PXXP-SH3 interaction (detected at the 1 min time point) occurring earlier than the SH2-Tyr(P) interaction (detected at the 3 min time point). Importantly, similar results were obtained in non-p66shc-overexpressing cells (LacZ cells) and non-transduced wild type pSMC (Fig. 3, B and C).
Because extensive studies have shown that Src activation depends on its conformational structure (30, 31), we investigated if p66shc binding to Src would alter IGF-I-stimulated Src kinase activation. The Src kinase domain is maintained in an inactive state by intramolecular interactions (32, 33). Several mechanisms have been proposed for activation of Src kinase, including replacement of either SH2- or SH3-mediated intramolecular interactions by high affinity ligands, such as autophosphorylated platelet-derived growth factor receptor (34), a PXXP motif-containing protein (35); inactivation of Csk (C-terminal Src tyrosine kinase) and Chk (Csk homologous kinase); and dephosphorylation of tyrosine 530 (equivalent to Tyr527 in chick Src) by tyrosine phosphatases (36, 37). Herein, we report a novel mechanism for regulating IGF-I-dependent Src kinase activation. Our findings show that the CH2 domain of p66shc contains a PXXP motif that binds directly to the SH3 domain in Src, and this complex formation initiates a series of events leading to the inhibition of Src activation. To our best knowledge, this study is the first to show that a Src-SH3 domain-PXXP ligand interaction can inhibit Src kinase activation in response to stimuli. In contrast, the PXXP-SH3 interaction has been shown to induce Src family kinase activation (35, 38, 39). Although “turn on by a touch” is a widely accepted mechanism for activation of Src and other members of the Src family kinases, the actual steps leading to kinase activation are more complex, and kinase activation depends on the spatial positions and conformations of the SH3 and SH2 domains, the kinase loop, the activation loop, and the C helix (40). Theoretically, an alteration in any of these steps could prevent the kinase activation. Therefore, it is possible that the SH3 domain binding interaction induces an allosteric change and thereby inhibits Src kinase activation. In support of our observations, prior studies have shown that proteins binding to Src family kinases through non-SH3 domain interactions can inhibit kinase activation. These include the non-catalytic domain of Chk (41), RACK1 (receptor of activated protein C kinase) (42), caveolin (43), and Wiskott-Aldrich syndrome protein (44).
To understand the underlying mechanism of the inhibitory effect of p66shc, several different approaches were employed. In vitro kinase assays showed that full-length WT p66shc directly inhibited Src kinase activity, and this inhibitory effect could be reversed by the PXXP-SH3 blocking peptide. In addition, the CH2 domain of p66shc had an inhibitory effect on Src kinase activity, whereas p66shc P3A mutant and P3A-mutated CH2 domain had no effect on Src activation (Fig. 6). The studies using living cells showed that IGF-I-stimulated Src activation was significantly attenuated by overexpression of WT p66shc, and this effect could be nullified by the addition of a peptide containing the PXXP motif or enhanced by knockdown of p66shc. Additionally, the expression of p66shc P3A mutant had no effect on IGF-I-stimulated Src kinase activation in living cells. These results clearly indicate that PXXP sequences in the CH2 domain of p66shc interact with the SH3 domain in Src, resulting in inhibition of Src kinase activation. Although the PXXP region mediates the inhibition, it is insufficient to inhibit Src kinase activation, which requires the full-length CH2 domain. Of note, full-length p66shc had an even greater effect than the CH2 domain, suggesting that the SH2 domain interaction with Tyr(P)329 or Tyr(P)360 in Src is required for maximal inhibition of Src activation. This prediction is supported by the observation that the Tyr(P)-SH2 blocking peptide partially attenuated the inhibitory effect of p66shc on Src kinase activity in the in vitro kinase assay (Fig. 6C) and that it could partially rescue IGF-I-stimulated Src activation in WT p66shc-overexpressing cells, although it was less effective than the PXXP-SH3 blocking peptide (Fig. 7B). However, once PXXP-SH3 interaction was disrupted, the Tyr(P)-SH2 interaction no longer had an effect on IGF-I-stimulated Src activation. This is consistent with our results showing that expression of the full-length p66shc P3A mutant was unable to inhibit IGF-I-stimulated Src kinase activation. Therefore, we believe that SH3-PXXP interaction serves to initiate the inhibitory effect of p66shc on Src activation in response to IGF-I and that the Tyr(P)-SH2 interaction also contributes to the inhibitory effect. Formation of PXXP-SH3 interaction alone allowed some (but not complete) inhibition by p66shc, even though the SH2-Tyr(P) interaction could not be established (e.g. p66shc SH2 domain deletion) (Fig. 7, D and E). Our data indicate that simply occupying either of the binding sites or both sites did not confer the full inhibitory effect, since neither peptide itself could inhibit Src kinase activity (Fig. 6C), and the addition of the CH2 domain peptide plus p52shc did not have an additional inhibitory effect compared with CH2 peptide alone (Fig. 6D). Based on these results, we postulate that IGF-I treatment triggers the conformation change of inactive (folded) Src and slightly opens its folded structure. This allows the p66shc to bind to it via a PXXP-SH3 interaction. Meanwhile, after IGF-I treatment, Src autophosphorylates Tyr329 and Tyr360, which allows the further binding to p66shc via a SH2-Tyr(P) interaction. When binding is present at both sites, p66shc can significantly inhibit Src activation either by preventing further Src unfolding or by inducing an allosteric change to inactivate kinase activity. Our in vitro Src kinase assay data clearly showed that at least the latter mechanism was present because when p66shc was added into the activated Src, the kinase activity of Src was significantly reduced (Fig. 6C). To support this proposed model, RACK1 has been reported to inhibit Src kinase activation via interaction through multiple binding sites, including SH2 domain of Src, and acts as a molecular chaperone to hold Src in the closed and inactive conformational state (42). Similarly, Hsp90, a chaperone protein, has also been shown to hold v-Src in the inactive state, thereby inhibiting its activation (45). In addition, this model allows us to explain an interesting observation that shows that, in LacZ cells, IGF-I stimulated p66shc·Src complex formation after 1 min of treatment, and the complex formation continued increasing after 3 min of IGF-I treatment, whereas, in p66shc-overexpressing cells, the maximal complex formation was detected after 1 min of IGF-I treatment, and no further increase was detected at the 3 min time point (Fig. 3A). We believe that is because, in p66shc-overexpressing cells, the formation a large amount of the p66shc·Src complex via the PXXP-SH3 interaction at 1 min inhibits Src autophosphorylation. Because Src activation has been shown to be essential for phosphorylation of Src Tyr329 and Tyr360 (24), which provide the binding sites for SH2 domain in p66shc, this inhibition of Src kinase activity limits the further increase of the complex formation via the SH2-Tyr(P) interaction. By contrast, in LacZ cells, the degree of increase in p66shc binding to Src via a PXXP-SH3 interaction at 1 min does not significantly inhibit Src activation in response to IGF-I, and this permits the formation of additional p66shc·Src complex via the SH2-Tyr(P) interaction at the 3 min time point. Because the SH2-Tyr(P) interaction has a higher affinity than PXXP-SH3 domain interaction, it is reasonable to expect the greater p66shc·Src complex formation via the SH2-Tyr(P) interaction at this time point. Consequently, it is not surprising that p136 or p226, both of which inhibit the SH2-Tyr(P) interaction, has a greater inhibitory effect than p189 on the formation of p66shc·Src complex after 3 min of IGF-I treatment (Fig. 3, B and C).
Previous studies have shown that IGF-I activates Src kinase, and this activation is critical for mediating IGF-I downstream signaling, such as MAPK activation (24, 46, 47), Akt activation (48), and IGF-I-stimulated biological actions (24, 49). To understand the mechanism of Src-mediated IGF-I signaling, previous studies focused on the identification of Src kinase substrate, revealing that p52shc was a good candidate. Both in vitro and in vivo data showed that activated Src can phosphorylate p52shc on Tyr239/Tyr240/Tyr317 (50, 51). Our previous study clearly demonstrated that, upon IGF-I stimulation, Src recruited p52shc to the SHPS-1 signaling complex and phosphorylated Tyr239/Tyr240/Tyr317 on p52shc, providing a binding site for Grb2, thus activating downstream signaling in SMC (24). This SHPS-1 complex formation is essential for IGF-I-stimulated MAPK activation and biological actions (7). Disruption of this complex formation by truncation of the SHPS-1 cytoplasmic domain (7) or Src silencing (24) or by overexpression of p66shc (10) leads to impaired IGF-I signaling and biological actions. Our previous and current data have shown that IGF-I-induced Src activation is quite rapid and transient; the highest level is detected at 1 min and then reduced after 3 or 5 min in pSMC (24) (Figs. 6–8). Consistent with our findings, a similar pattern in IGF-I-stimulated Src activation was observed in preadipocytes (46). Consequently, an obvious question is how this rapid and transient activated Src phosphorylates p52shc on the SHPS-1 signaling complex and mediates MAPK activation at the late time points. To address this question, we investigated the kinetic activation of Src in response to IGF-I stimulation, showing that the activation of Src occurred at 1 min but was still greater than the basal activation level at 10 and 30 min after IGF-I stimulation (supplemental Fig. 1A). More importantly, we used co-immunoprecipitation to show that SHPS-1-associated p419Src (which phosphorylates p52shc) increased progressively after 5 and 10 min of IGF-I stimulation (supplemental Fig. 1B). These findings support our previous data indicating that Src is recruited to the SHPS-1 complex and remains active at a time when p52shc is phosphorylated, leading to Erk1/2 activation in response to IGF-I treatment. Because activation of IGF-I signaling has been shown to inhibit hyperglycemia-induced DNA damage (52) and activation of the mitochondrial apoptosis program (53), it is possible that a major increase in p66shc may impair the ability of IGF-I to rescue cells under these conditions. This is consistent with the observation that p66shc can induce apoptosis under conditions of increased oxidative stress or hyperglycemia (54). However, our findings suggest that these responses are dependent upon the level of p66shc and Src activation.
To expand the applicability of our findings, we examined the proposed mechanism in the endothelial cells and confirmed the pivotal molecular events that we observed in pSMC. Endothelial cells express αvβ3, Src, p66shc, and SHPS-1, which are required for studying SHPS-1 signaling complex formation and modulation of IGF-I signal transduction in hyperglycemia. In addition, they have been shown to respond to hyperglycemia by increasing their sensitivity to IGF-I stimulation (28, 29). Therefore, we believe that this mechanism at least is applicable to specific cell types that have the capability to respond to hyperglycemic and/or oxidative stress with an increase in proliferation in response to IGF-I.
In summary, our data demonstrate that p66shc sequentially binds to Src through a PXXP-SH3 and a SH2-Tyr(P) interaction in response to IGF-I. The PXXP-SH3 interaction plays a major role in initiating the inhibition of IGF-I-stimulated Src kinase activation; however, the full inhibitory effect requires the presence of the SH2-Tyr(P) interaction. This inhibition leads to impairment of IGF-I-dependent p52shc tyrosine phosphorylation, MAPK activation, and IGF-I-stimulated cell proliferation and migration. Our findings provide novel information regarding the regulation of Src kinase activation and suggest new possible strategies for inhibiting constitutively activated Src kinase and p52shc signaling.
Supplementary Material
Acknowledgments
We thank Dr. Laura Maile for providing porcine aortic endothelial cells, Dr. Jane Badley-Clarke for help in p66shc 3F construction, and Laura Lindsey for help in preparing the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL56850 and AG02331.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- MAPK
- mitogen-activated protein kinase
- IGF
- insulin-like growth factor
- SMC
- smooth muscle cell(s)
- pSMC
- porcine SMC
- SH2 and -3
- Src homology 2 and 3, respectively
- TNT
- transcription/translation
- P3A
- P47A/P48A/P50A
- 3F
- Y349F/Y350F/Y427F
- WT
- wild type
- HA
- hemagglutinin
- NS
- not significant.
REFERENCES
- 1.Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. (1992) Cell 70, 93–104 [DOI] [PubMed] [Google Scholar]
- 2.Migliaccio E., Mele S., Salcini A. E., Pelicci G., Lai K. M., Superti-Furga G., Pawson T., Di Fiore P. P., Lanfrancone L., Pelicci P. G. (1997) EMBO J. 16, 706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravichandran K. S. (2001) Oncogene 20, 6322–6330 [DOI] [PubMed] [Google Scholar]
- 4.Sasaoka T., Ishiki M., Wada T., Hori H., Hirai H., Haruta T., Ishihara H., Kobayashi M. (2001) Endocrinology 142, 5226–5235 [DOI] [PubMed] [Google Scholar]
- 5.Boney C. M., Gruppuso P. A., Faris R. A., Frackelton A. R., Jr. (2000) Mol. Endocrinol. 14, 805–813 [DOI] [PubMed] [Google Scholar]
- 6.Ursini-Siegel J., Hardy W. R., Zuo D., Lam S. H., Sanguin-Gendreau V., Cardiff R. D., Pawson T., Muller W. J. (2008) EMBO J. 27, 910–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling Y., Maile L. A., Lieskovska J., Badley-Clarke J., Clemmons D. R. (2005) Mol. Biol. Cell 16, 3353–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maile L. A., Capps B. E., Miller E. C., Aday A. W., Clemmons D. R. (2008) Diabetes 57, 2637–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada S., Kao A. W., Ceresa B. P., Blaikie P., Margolis B., Pessin J. E. (1997) J. Biol. Chem. 272, 28042–28049 [DOI] [PubMed] [Google Scholar]
- 10.Xi G., Shen X., Clemmons D. R. (2008) Mol. Endocrinol. 22, 2162–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migliaccio E., Giorgio M., Mele S., Pelicci G., Reboldi P., Pandolfi P. P., Lanfrancone L., Pelicci P. G. (1999) Nature 402, 309–313 [DOI] [PubMed] [Google Scholar]
- 12.Favetta L. A., Robert C., King W. A., Betts D. H. (2004) Exp. Cell Res. 299, 36–48 [DOI] [PubMed] [Google Scholar]
- 13.Favetta L. A., Madan P., Mastromonaco G. F., St John E. J., King W. A., Betts D. H. (2007) BMC Dev. Biol. 7, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orsini F., Migliaccio E., Moroni M., Contursi C., Raker V. A., Piccini D., Martin-Padura I., Pelliccia G., Trinei M., Bono M., Puri C., Tacchetti C., Ferrini M., Mannucci R., Nicoletti I., Lanfrancone L., Giorgio M., Pelicci P. G. (2004) J. Biol. Chem. 279, 25689–25695 [DOI] [PubMed] [Google Scholar]
- 15.Camici G. G., Schiavoni M., Francia P., Bachschmid M., Martin-Padura I., Hersberger M., Tanner F. C., Pelicci P., Volpe M., Anversa P., Lüscher T. F., Cosentino F. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 5217–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chintapalli J., Yang S., Opawumi D., Goyal S. R., Shamsuddin N., Malhotra A., Reiss K., Meggs L. G. (2007) Am. J. Physiol. Renal Physiol. 292, F523–F530 [DOI] [PubMed] [Google Scholar]
- 17.Trinei M., Giorgio M., Cicalese A., Barozzi S., Ventura A., Migliaccio E., Milia E., Padura I. M., Raker V. A., Maccarana M., Petronilli V., Minucci S., Bernardi P., Lanfrancone L., Pelicci P. G. (2002) Oncogene 21, 3872–3878 [DOI] [PubMed] [Google Scholar]
- 18.Menini S., Amadio L., Oddi G., Ricci C., Pesce C., Pugliese F., Giorgio M., Migliaccio E., Pelicci P., Iacobini C., Pugliese G. (2006) Diabetes 55, 1642–1650 [DOI] [PubMed] [Google Scholar]
- 19.Chen Y., Capron L., Magnusson J. O., Wallby L. A., Arnqvist H. J. (1998) Growth Horm. IGF Res. 8, 299–303 [DOI] [PubMed] [Google Scholar]
- 20.Campbell M., Allen W. E., Silversides J. A., Trimble E. R. (2003) Diabetes 52, 519–526 [DOI] [PubMed] [Google Scholar]
- 21.Campbell M., Trimble E. R. (2005) Circ. Res. 96, 197–206 [DOI] [PubMed] [Google Scholar]
- 22.Ruiz E., Gordillo-Moscoso A., Padilla E., Redondo S., Rodriguez E., Reguillo F., Briones A. M., van Breemen C., Okon E., Tejerina T. (2006) Diabetes 55, 1243–1251 [DOI] [PubMed] [Google Scholar]
- 23.Maile L. A., Capps B. E., Ling Y., Xi G., Clemmons D. R. (2007) Endocrinology 148, 2435–2443 [DOI] [PubMed] [Google Scholar]
- 24.Lieskovska J., Ling Y., Badley-Clarke J., Clemmons D. R. (2006) J. Biol. Chem. 281, 25041–25053 [DOI] [PubMed] [Google Scholar]
- 25.Gockerman A., Clemmons D. R. (1995) Circ. Res. 76, 514–521 [DOI] [PubMed] [Google Scholar]
- 26.Nam T. J., Busby W. H., Jr., Rees C., Clemmons D. R. (2000) Endocrinology 141, 1100–1106 [DOI] [PubMed] [Google Scholar]
- 27.Jones J. I., Prevette T., Gockerman A., Clemmons D. R. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 2482–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shigematsu S., Yamauchi K., Nakajima K., Iijima S., Aizawa T., Hashizume K. (1999) Endocr. J. 46, (suppl.) S59–S62 [DOI] [PubMed] [Google Scholar]
- 29.Miller E. C., Capps B. E., Sanghani R. R., Clemmons D. R., Maile L. A. (2007) Invest. Ophthalmol. Vis. Sci. 48, 3878–3887 [DOI] [PubMed] [Google Scholar]
- 30.Yang S., Roux B. (2008) PLoS Comput. Biol. 4, e1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowan-Jacob S. W., Fendrich G., Manley P. W., Jahnke W., Fabbro D., Liebetanz J., Meyer T. (2005) Structure 13, 861–871 [DOI] [PubMed] [Google Scholar]
- 32.Murphy S. M., Bergman M., Morgan D. O. (1993) Mol. Cell Biol. 13, 5290–5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu W., Doshi A., Lei M., Eck M. J., Harrison S. C. (1999) Mol. Cell 3, 629–638 [DOI] [PubMed] [Google Scholar]
- 34.Alonso G., Koegl M., Mazurenko N., Courtneidge S. A. (1995) J. Biol. Chem. 270, 9840–9848 [DOI] [PubMed] [Google Scholar]
- 35.Saksela K., Cheng G., Baltimore D. (1995) EMBO J. 14, 484–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abram C. L., Courtneidge S. A. (2000) Exp. Cell Res. 254, 1–13 [DOI] [PubMed] [Google Scholar]
- 37.Alper O., Bowden E. T. (2005) Curr. Pharm. Des. 11, 1119–1130 [DOI] [PubMed] [Google Scholar]
- 38.Moarefi I., LaFevre-Bernt M., Sicheri F., Huse M., Lee C. H., Kuriyan J., Miller W. T. (1997) Nature 385, 650–653 [DOI] [PubMed] [Google Scholar]
- 39.Chan B., Lanyi A., Song H. K., Griesbach J., Simarro-Grande M., Poy F., Howie D., Sumegi J., Terhorst C., Eck M. J. (2003) Nat. Cell Biol. 5, 155–160 [DOI] [PubMed] [Google Scholar]
- 40.Boggon T. J., Eck M. J. (2004) Oncogene 23, 7918–7927 [DOI] [PubMed] [Google Scholar]
- 41.Chong Y. P., Mulhern T. D., Zhu H. J., Fujita D. J., Bjorge J. D., Tantiongco J. P., Sotirellis N., Lio D. S., Scholz G., Cheng H. C. (2004) J. Biol. Chem. 279, 20752–20766 [DOI] [PubMed] [Google Scholar]
- 42.Chang B. Y., Conroy K. B., Machleder E. M., Cartwright C. A. (1998) Mol. Cell Biol. 18, 3245–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S., Couet J., Lisanti M. P. (1996) J. Biol. Chem. 271, 29182–29190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulte R. J., Sefton B. M. (2003) Biochemistry 42, 9424–9430 [DOI] [PubMed] [Google Scholar]
- 45.Brugge J. S. (1986) Curr. Top. Microbiol. Immunol. 123, 1–22 [DOI] [PubMed] [Google Scholar]
- 46.Boney C. M., Sekimoto H., Gruppuso P. A., Frackelton A. R., Jr. (2001) Cell Growth Differ. 12, 379–386 [PubMed] [Google Scholar]
- 47.Sekimoto H., Eipper-Mains J., Pond-Tor S., Boney C. M. (2005) Mol. Endocrinol. 19, 1859–1867 [DOI] [PubMed] [Google Scholar]
- 48.Cui Q. L., Zheng W. H., Quirion R., Almazan G. (2005) J. Biol. Chem. 280, 8918–8928 [DOI] [PubMed] [Google Scholar]
- 49.Knowlden J. M., Hutcheson I. R., Barrow D., Gee J. M., Nicholson R. I. (2005) Endocrinology 146, 4609–4618 [DOI] [PubMed] [Google Scholar]
- 50.van der Geer P., Wiley S., Gish G. D., Pawson T. (1996) Curr. Biol. 6, 1435–1444 [DOI] [PubMed] [Google Scholar]
- 51.Sato K., Gotoh N., Otsuki T., Kakumoto M., Aoto M., Tokmakov A. A., Shibuya M., Fukami Y. (1997) Biochem. Biophys. Res. Commun. 240, 399–404 [DOI] [PubMed] [Google Scholar]
- 52.Yang S., Chintapalli J., Sodagum L., Baskin S., Malhotra A., Reiss K., Meggs L. G. (2005) Am. J. Physiol. Renal Physiol. 289, F1144–1152 [DOI] [PubMed] [Google Scholar]
- 53.Kang B. P., Frencher S., Reddy V., Kessler A., Malhotra A., Meggs L. G. (2003) Am. J. Physiol. Renal Physiol. 284, F455–F466 [DOI] [PubMed] [Google Scholar]
- 54.Pellegrini M., Pacini S., Baldari C. T. (2005) Apoptosis 10, 13–18 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.