Abstract
It has been postulated that inactivated β1-integrins are involved in the disordered growth of hematopoietic tumor cells. We recently found that TNIIIA2, a peptide derived from tenascin-C, strongly activates β1-integrins through binding with syndecan-4. We show here that Ramos Burkitt's lymphoma cells can survive and grow in suspension but undergo apoptosis when kept adhering to fibronectin by stimulation with TNIIIA2. Other integrin activators, Mg2+ and TS2/16 (an integrin-activating antibody), were also capable of inducing apoptosis. The inactivation of ERK1/2 and Akt and the subsequent activation of Bad were involved in the apoptosis. The results using other hematopoietic tumor cell lines expressing different levels of fibronectin receptors (VLA-4 and VLA-5) showed that potentiated and sustained adhesion to fibronectin via VLA-4 causally induces apoptosis also in various types of hematopoietic tumor cells in addition to Ramos cells. Because TNIIIA2 requires syndecan-4 as a membrane receptor for activation of β1-integrins, it induced apoptosis preferentially in hematopoietic tumor cells, which expressed both VLA-4 and syndecan-4 as membrane receptors mediating the effects of fibronectin and TNIIIA2, respectively. Therefore, normal peripheral blood cells, such as neutrophils, monocytes, and lymphocytes, which poorly expressed syndecan-4, were almost insusceptible to TNIIIA2-induced apoptosis. The TNIIIA2-related matricryptic site of TN-C could contribute, once exposed, to preventing prolonged survival of hematopoietic malignant progenitors through potentiated and sustained activation of VLA-4.
Keywords: Apoptosis, Cell/Adhesion, Cell/Apoptosis, Extracellular Matrix/Fibronectin, Extracellular Matrix/Integrin, Extracellular Matrix/Tenascin, Hematopoietic Tumor Cells
Introduction
Normal hematopoiesis is regulated by the adhesive interactions of hematopoietic stem and progenitor cells with the microenvironment, as well as by hematopoietic growth factors and cytokines (1–3). In addition to stromal cells, extracellular matrix proteins in lymphoid tissues, such as fibronectin (FN),2 collagen, laminin, tenascin (TN), and proteoglycans have been implicated as essential components of the microenvironment that regulates hematopoiesis. Among these extracellular matrix proteins, FN has been proposed to play the most important role in the survival and proliferation of hematopoietic stem and progenitor cells through FN receptors VLA-4 and VLA-5 (4). Like their normal counterparts, transformed hematopoietic progenitor cells remain dependent on signals from the FN for survival and proliferation during their malignant progression (5–7).
Previous studies have shown that adhesion of hematopoietic stem and progenitor cells, including tumor cells, to FN/extracellular matrix inhibits their proliferation but supports their survival by preventing apoptosis (8, 9). Additionally, increasing evidence has demonstrated that adhesion of hematopoietic tumor cells to FN via VLA-4 and VLA-5 confers a multidrug resistance phenotype, commonly referred to as the cell adhesion-mediated drug resistance phenotype (CAM-DR) (10). In contrast, there have been also several reports demonstrating the negative effects of cell adhesion on cell survival. Integrin-mediated adhesive interaction with FN was shown to cause apoptosis in myeloid cell lines (11, 12) and erythroid progenitor cells (13). Although the molecular mechanisms underlying apoptosis were not defined, these studies clearly showed that integrin-mediated adhesion plays a negative role in the survival of hematopoietic progenitor/tumor cells. Thus, it remains controversial as to whether adhesion to FN acts positively or negatively on the survival of hematopoietic progenitor/tumor cells.
TN-C is characterized by its regulated expression and by its cell adhesion modulatory function (14, 15). Constitutive expression of TN-C has been observed in lymphoid tissues, such as adult bone marrow and lymph nodes (16, 17), whereas it is transiently expressed in pathological states, including inflammation and tumorigenesis (18, 19). Therefore, lymphoid tissues of patients with hematopoietic malignancy have highly increased expression of TN-C. TN-C acts as both an adhesive and an antiadhesive substrate, depending on the cellular context (20). We recently found (21) that a 22-mer peptide derived from the TN-C molecule, termed TNIIIA2, has a potent ability to induce conformational change in β1-integrin necessary for its functional activation. The active site of TNIIIA2 appears to be cryptic in the TN-C molecule but is exposed by processing with inflammatory proteinases including matrix metalloproteinase-2. Syndecan-4, a membrane-bound heparan sulfate proteoglycan, serves as a receptor mediating TNIIIA2-induced activation of β1-integrins. Because TNIIIA2 can induce adhesion to FN in hematopoietic tumor cells by activating β1-integrins, this factor is useful for studying the role of adhesion to FN in the survival and growth of hematopoietic progenitor/tumor cells.
Here we show that a variety of hematopoietic tumor cell lines undergo apoptosis when forced to adhere via VLA-4 to FN by stimulation with integrin activators including TNIIIA2. Because TNIIIA2 requires syndecan-4 as a membrane receptor for β1-integrin activation, normal peripheral blood cells such as neutrophils, monocytes, and lymphocytes, which poorly express syndecan-4, are almost insusceptible to TNIIIA2-induced apoptosis. The cryptic functional site TNIIIA2 of the TN-C molecule may play a beneficial role in preventing prolonged survival of hematopoietic tumor cells.
EXPERIMENTAL PROCEDURES
Materials
Peptides derived from human TN-C (TNIIIA2) (RSTDLPGLKAATHYTITIRGVC) and its inactive control peptide, TNIIIA2mutant (TNIIIA2mut) (RSTDLPGLKAATHYTATARGVC) (21), CS-1 (LHPGEILDVPST), and RGD (GRGDSP) peptide were obtained from Operon Biotechnology (Tokyo). The FN fragments derived from the central cell-binding domain (CELL) and the carboxyl-terminal heparin-binding domain II (HepII) of human plasma FN were prepared as described previously (22). The annexin V detection kit, z-VAD-fmk, and z-DEVD-fmk were obtained from Sigma-Aldrich, and the cDNA for constitutively active Akt1 was from Upstate Biotechnology, Inc.
Antibodies
Antibodies (Abs) to phospho-Bad (Ser112) (7E11), phospho-Bad (Ser136), Bad, phospho-ERK1/2 (Tyr202/Tyr204), and phospho-Akt (Ser473) were obtained from Cell Signaling Technology. Abs to Bad (H-18), ERK1/2, and syndecan-4 core protein (5G9) were obtained from Santa Cruz Biotechnology. Function-blocking monoclonal antibodies (mAbs) against integrin subunits α4 (P1H4) and α5 (P1D6) were purchased from Chemicon, and anti-integrin β1 mAb (DE9) was from Upstate Biotechnology, Inc. Anti-Bcl-xL pAb (Upstate Biotechnology, Inc), β1 integrin-activating mAb, TS2/16 (23), mAb recognizing the active conformation of integrin subunit β1, AG89 (MBL) (24), and anti-mouse IgG bridging pAb (Sigma) were obtained as noted. Anti-HLA-ABC (mAb B9.12.1) was purchased from Beckman Coulter.
Cells
Burkitt's lymphoma cell lines Ramos and Raji, erythroleukemia cell line, K562, myeloid leukemia cell lines, U937 and HL-60, T-lymphoma cell line, Jurkat, and human acute monocytic leukemia cell line, THP-1, were grown with RPMI medium containing 10% fetal bovine serum. Monocytic differentiation of THP-1 cells was induced by incubation for 24 h with phorbol 12-myristate 13-acetate (10 ng/ml), as described previously (25). Patient leukemic cells were obtained by iliac bone marrow aspiration and cultured, as described previously (26), and this was accepted by the institutional review boards of Sapporo Medical University and Tokyo University of Science in accordance with the Declaration of Helsinki.
Peripheral blood mononuclear cells and neutrophils were prepared from the blood of healthy volunteers. After Lymphoprep (Axis-Shield Proc AS) density gradient centrifugation, peripheral blood mononuclear cells and neutrophils were collected and subjected to the magnetic cell sorting (Miltenvi Biotec) using anti-human CD14 Ab and anti-human CD3 and CD19 Abs, respectively, according to the manufacturer's instructions. Neutrophil preparation by hypotonic lysis of contaminating erythrocytes was performed as described previously (27).
Cell Adhesion Assay
The cell adhesion assay was performed as described previously (28). Briefly, the cells (3 × 104) suspended with serum-free medium with or without TNIIIA2 were seeded on a 96-well plate coated with FN (10 μg/ml) or its proteolytic fragment (50 μg/ml) derived from the CELL or HepII (22), incubated for 1 h, and then fixed with formalin. Before washing the plate, the total numbers of cells present in four different areas of the well were counted microscopically at ×100 magnification. After washing away the unadhered cells, the numbers of adhered cells in the same areas of the well were also counted. The data represent the percentages of the number of adhered cells relative to the number of cells seeded into the well.
Cell Growth/Survival Assay
Cells (3 × 104) suspended with serum-free medium were seeded on a 96-well plate coated with FN or its fragment (see above) and incubated with or without TNIIIA2 for the indicated period. The assay was also performed on a plate coated with poly(2-hydroxyethyl methacrylate), as described previously (30) with some modifications. The number of living cells was assessed using a cell counting kit (Dojindo, Tokyo, Japan). Absorbance at 450 nm was measured to evaluate the number of viable cells.
Detection of Apoptotic Cells
Apoptotic cells were detected by following two different methods: 1) annexin V detection, in which FITC-labeled annexin V staining of cells was performed using an annexin V detection kit according to the manufacturer's instructions, and 2) DNA degradation, in which the cells (5 × 105) were cultured in the presence of TNIIIA2 for 2 days. The cells collected were stained with propidium iodide using a Cycle Test PLUS DAN reagent kit, and the fluorescence intensity was measured by flow cytometry to quantify the sub-G1 cell fraction. The percentage of cells in sub-G1 was calculated using LYSIS II software (Becton Dickinson).
Western Blotting
The cells were treated under the serum-free conditions used for the cell growth/survival assay. Western blotting was performed as described previously (21).
Protein Knockdown by RNA Interference
siRNAs of Bad (AAGAAGGGACUUCCUCGCCCGtt; GenBankTM accession number NM_004322; Sigma-Aldrich) and the negative control siRNA (Sigma) were obtained as noted. The siRNAs were transfected into cells by using Transmessenger (Qiagen), and the cells were used for experiments 50 h after transfection. RNA interference-mediated knockdown of Bad expression was verified by immunoblot analysis as described previously (21).
Electroporation
Ramos cells (1 × 107) were subjected to electroporation, with 10 μg of empty vector or cDNAs encoding constitutively active Akt1 using Gene Pulser (Bio-Rad) at 300 V and 960 microfarads (6 h). The culture medium was changed to complete medium, and the cells were incubated for an additional 12 h before the cell survival assay.
Immunoprecipitation
Ramos cells (3 × 106) were collected and suspended with lysis buffer (20 mm Tris-HCl, pH 7.6, 2 mm EDTA, 3% Nonidet P-40, 100 mm NaCl, 50 mm NaF, 1 μm phenylmethylsulfonyl fluoride, 1 μm Na2VO4, 5 μg/ml aprotinin). Total cell lysate (1 mg of protein) was incubated with protein A-agarose (Pierce) and anti-Bcl-xL pAb or normal IgG at 4 °C for 6 h. Following centrifugation, the beads were washed with lysis buffer and then subjected to Western blot analysis.
Flow Cytometry
For analysis of integrin subunit expression, cell suspension was incubated with anti-integrin mAb for 30 min and then incubated with each FITC-labeled secondary Ab for 30 min. Alternatively, the activation status of β1-integrins on the cell surface was evaluated using anti-activated β1 integrin mAb, AG89, as described previously (21).
RESULTS
Apoptosis Induction of Ramos Cells through Adhesion to FN
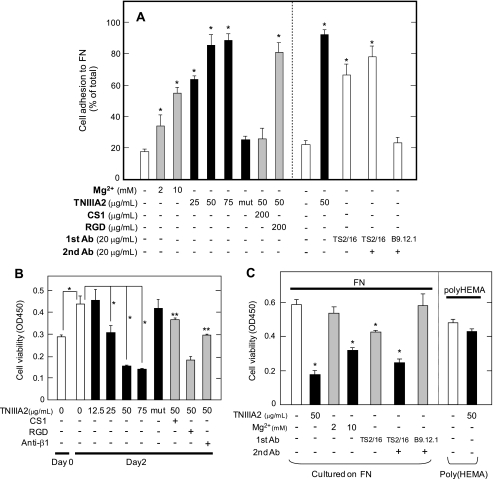
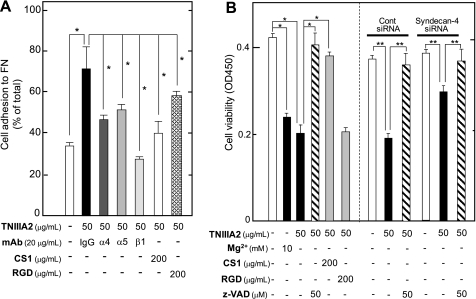
Ramos cells exclusively express VLA-4 as FN receptors but are incapable of adhering to FN because of their inactivated VLA-4 (29). In fact, Ramos cells specifically adhered to the FN only after stimulation with an integrin activator, Mg2+, and β1-integrin-activating mAb TS2/16 (Fig. 1A). Stimulation of Ramos cell adhesion with TS2/16 was slightly enhanced by further addition of a bridging Ab (Fig. 1A). TNIIIA2 also induced adhesion of Ramos cells to FN, in which most Ramos cells (∼90% of total cells) adhered to the FN (Fig. 1A). Ramos cell adhesion to the FN stimulated by TNIIIA2 was blocked by the CS-1 peptide, a VLA-4 antagonist, but not by the RGD peptide, a VLA-5 antagonist (Fig. 1A), indicating that TNIIIA2 induced VLA-4-mediated adhesion of Ramos cells to the FN. TNIIIA2-induced cell adhesion was accompanied by the conformational activation of VLA-4, without changing the expression of VLA-4 (supplemental Fig. S1).
FIGURE 1.
Effect on cell growth of the forced adhesion of Ramos cells to FN. A shows the results of cell adhesion assay. The Ramos cell suspension (3 × 104 cells) with or without Mg2+, TNIIIA2, or the inactive control peptide TNIIIA2mut (represented as mut) was seeded into a 96-well plate coated with FN and then cultured in the absence or presence of the function-blocking anti-integrin β1 subunit mAb or integrin antagonist (CS-1 or GRGDSP (RGD) peptide) at the indicated concentrations (left panel). In the right panel, an adhesion assay was also performed in the presence or absence of TNIIIA2, TS2/16, or TS2/16 in combination with a bridging Ab (anti-mouse IgG rabbit Ab). Instead of TS2/16, anti-HLA class I mAb (B9.12.1) was used as a control. The percentage of adhered cells is shown relative to the total number of cells seeded into the well. Each point represents the mean ± S.E. of triplicate determinations. The data are representative of three individual experiments. *, p < 0.01 compared with control. In B, the effect of induced adhesion to FN on Ramos cell growth was examined. Ramos cell suspension (3 × 104 cells) with the indicated concentrations of TNIIIA2 orTNIIIA2mut (50 μg/ml) was seeded into a 96-well plate coated with FN and then cultured for 2 days in the absence or presence of anti-β1 mAb (20 μg/ml), CS-1 (200 μg/ml), or RGD peptide (200 μg/ml). *, p < 0.01 compared with control. **, p < 0.005 compared with TNIIIA2 (50 μg/ml) without integrin antagonist. C, Ramos cells were cultured as above in the absence or presence of β1-integrin activator, Mg2+ (2 or 10 mm), or TNIIIA2 (50 μg/ml), TS2/16 (20 μg/ml), or TS2/16 in combination with a bridging pAb (20 μg/ml) (left panel). In the right panel, Ramos cell suspension with or without TNIIIA2 was seeded on a plate coated with poly(2-hydroxyethylmethacrylate) (Poly(HEMA)) and cultured for 2 days. The number of viable cells was evaluated using a cell counting kit and represented as the absorbance at 450 nm, as described under “Experimental Procedures.” Each point represents the mean ± S.E. of triplicate determinations. One of four individual experiments is shown. *, p < 0.01 compared with control.
We first examined the effect of induced adhesion of Ramos cells to FN in response to integrin activators on their growth. Ramos cells were cultured with or without TNIIIA2 in plates coated with FN. In the absence of TNIIIA2, Ramos cells continued to survive and grow in suspension even under serum-free conditions. In the presence of TNIIIA2, Ramos cells became adhered and maintained their adhesive state for at least 3 days (data not shown). The growth of Ramos cells was not influenced by the addition of TNIIIA2 at a low concentration (12.5 μg/ml), whereas it was inhibited by TNIIIA2 at higher concentrations (>25 μg/ml) in a dose-dependent manner (Fig. 1B). TNIIIA2-induced inhibition of Ramos cell growth was abrogated by the CS-1 peptide or a β1-integrin-blocking mAb (Fig. 1B). TNIIIA2mut, which showed no proadhesive activity (21) (Fig. 1A), also did not inhibit Ramos cell growth (Fig. 1B). TNIIIA2-induced inhibition of Ramos cell growth was not evident when cells were incubated with TNIIIA2 on a nonadhesive poly(2-hydroxyethylmethacrylate) substrate (30) (Fig. 1C).
The addition of Mg2+ (10 mm as MgCl2) to the culture medium (RPMI 1640), which contains 0.4 mm Mg2+ (as MgSO4), also caused inhibition of Ramos cell growth, whereas the addition of 2 mm Mg2+ did not (Fig. 1C). Similarly, TS2/16 significantly inhibited Ramos cell growth, and this growth inhibition was further enhanced by the addition of a bridging Ab (Fig. 1C).
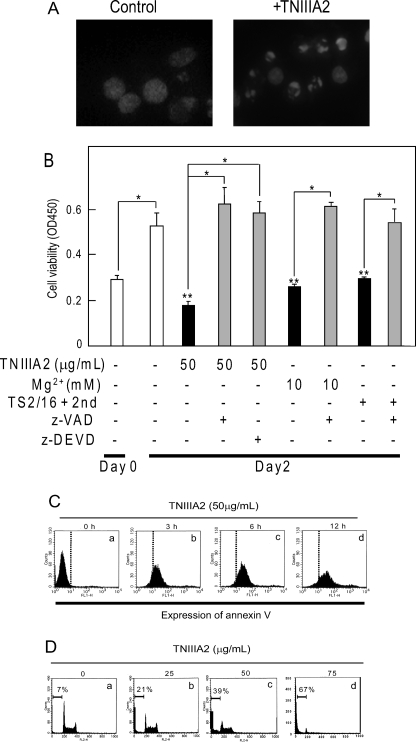
After the sustained adhesion, Ramos cells showed a change in nuclear morphology characteristic of apoptotic cells, as observed with Hoechst staining (Fig. 2A). Supporting this observation, TNIIIA2-induced growth inhibition was reversed almost completely by pretreating cells with z-VAD fmk, a general caspase inhibitor, or z-DEVD fmk, a specific inhibitor for caspase-3 (Fig. 2B). Growth inhibition induced by TS2/16 or Mg2+ was also blocked with z-VAD fmk (Fig. 2B), suggesting that adhesion-dependent growth inhibition may be due to caspase-dependent apoptosis. Apoptosis was further confirmed by the appearance of phosphatidylserine on cell surfaces, detected by binding with annexin V. The cell population that stained with FITC-annexin V antibody became evident as early as 3 h following TNIIIA2-induced adhesion and further increased in a time-dependent manner (Fig. 2C). Additionally, TNIIIA2 treatment resulted in a dose-dependent increase in DNA degradation, and the sub-G1 DNA fraction reached nearly 70% at 75 μg/ml of TNIIIA2 (Fig. 2D). These results suggest that potentiated and sustained adhesion to FN by β1-integrin activators leads Ramos cells to apoptosis.
FIGURE 2.
Apoptosis induction of Ramos cells through β1-integrin activation. A, nuclear morphology of Ramos cells treated with TNIIIA2. Ramos cells were either incubated in suspension (Control) or forced to adhere to the FN by stimulation with TNIIIA2 (50 μg/ml) (+TNIIIA2). After a 40-h incubation, the cells were stained with Hoechst. B, Ramos cell suspension (3 × 104 cells) with or without integrin activator, Mg2+ (10 mm), TS2/16 (25 μg/ml) in combination with a bridging pAb (20 μg/ml), or TNIIIA2 (50 μg/ml) was cultured in the presence or absence of a caspase inhibitor, z-VAD fmk (50 μm) or z-DEVD fmk (50 μm). After 2 days in culture, the viable cells were detected as in Fig. 2. Each point represents the mean ± S.E. of triplicate determinations. One of three individual experiments is shown. * (Day 0), p < 0.005 compared with control. * (Day 2), p < 0.005 compared with untreated. C, Ramos cells (2 × 106) were treated with TNIIIA2 (50 μg/ml) for 0 h (panel a), 3 h (panel b), 6 h (panel c), or 12 h, washed, and then stained with annexin V-FITC antibody, as described under “Experimental Procedures.” D, cells were cultured for 2 days in the absence (panel a) or presence of TNIIIA2 (panel b, 25 μg/ml, panel c, 50 μg/ml; panel d, 75 μg/ml). Sub-G1 events were detected as described under “Experimental Procedures.” The data in C and D are representative of three individual experiments.
Inactivation of the MEK/ERK and Phosphatidylinositol 3-Kinase/Akt Pathways through Adhesion to FN
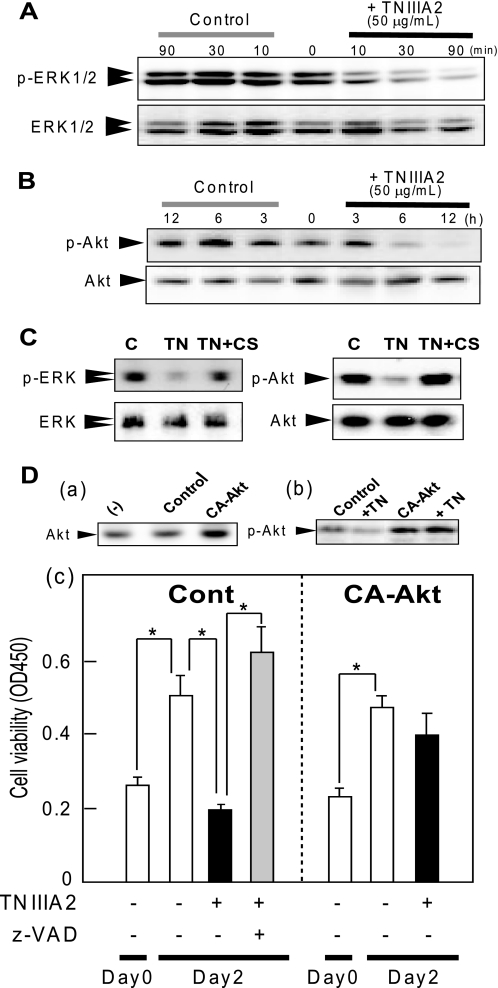
The phosphatidylinositol 3-kinase/Akt and MEK/ERK pathways are involved in cell survival and proliferation. ERK1/2 was detected as the phosphorylated state in Ramos cells in suspension without TNIIIA2 stimulation (Fig. 3A). When Ramos cell adhesion was induced by TNIIIA2 at a lower concentration (12.5 μg/ml), where apoptosis did not occur (Fig. 1B), phosphorylation of ERK1/2 was not changed remarkably (data not shown). When Ramos cells were induced to adhere to FN by stimulating with TNIIIA2 (50 μg/ml), a marked reduction of the phosphorylation of ERK1/2 was observed (Fig. 3A). Phosphorylation of MEK1/2 was also reduced by the TNIIIA2 treatment (supplemental Fig. S2A). Likewise, stimulation with TS2/16 in combination with the bridging pAb exhibited a decrease in the phosphorylation of ERK1/2 (supplemental Fig. S2B). Like ERK1/2, stimulation with TNIIIA2 (50 μg/ml) caused a conspicuous reduction in the phosphorylation of Akt (Fig. 3B). The decrease in the phosphorylation of ERK1/2 and Akt in response to TNIIIA2 stimulation was prevented by treating cells with CS-1 peptide (Fig. 3C). When Ramos cells were transfected with the constitutively active form of Akt cDNA (Fig. 3D, panel a), the decrease in Akt phosphorylation after TNIIIA2 stimulation was inhibited (Fig. 3D, panel b). As a result, apoptosis induced by TNIIIA2 was partially prevented by ectopic expression of active Akt (Fig. 3D, panel c). Thus, adhesion-dependent Ramos cell apoptosis induced by TNIIIA2 occurred in parallel with the reduced phosphorylation of ERK1/2 and Akt.
FIGURE 3.
Inhibition of the MEK/ERK and phosphatidylinositol 3-kinase/Akt pathways by TNIIIA2. Ramos cells (3 × 106 cells) were seeded on a culture plate coated with FN and stimulated with TNIIIA2 (50 μg/ml) for different times at 37 °C. Total cell lysates were prepared and subjected to Western blot analysis using anti-phospho-ERK (A) or anti-phospho-Akt (B), as described under “Experimental Procedures.” In C, the effect of the VLA-4 antagonist, CS-1 peptide, on the phosphorylation of ERK1/2 and Akt was assessed. Cell suspension with (shown as TN) or without (shown as control) TNIIIA2 (50 μg/ml) was cultured in the presence or absence of CS-1 (200 μg/ml) for 2 h. In D, TNIIIA2-induced apoptosis was examined in Ramos cells transiently transfected with constitutively active Akt. Ramos cells were transfected with an empty vector (Cont) or cDNA for constitutively active Akt1 (CA-Akt), as described under “Experimental Procedures.” The cells were stimulated with TNIIIA2 (shown as TN or TNIIIA2) (50 μg/ml) in the presence or absence of z-VAD fmk (50 μm) and then subjected to Western blotting (panels a and b) and the cell proliferation/survival assay (panel c). *, p < 0.005 compared with control. The data are representative of three individual experiments.
Participation of Bad Activation in the Adhesion-dependent Apoptosis
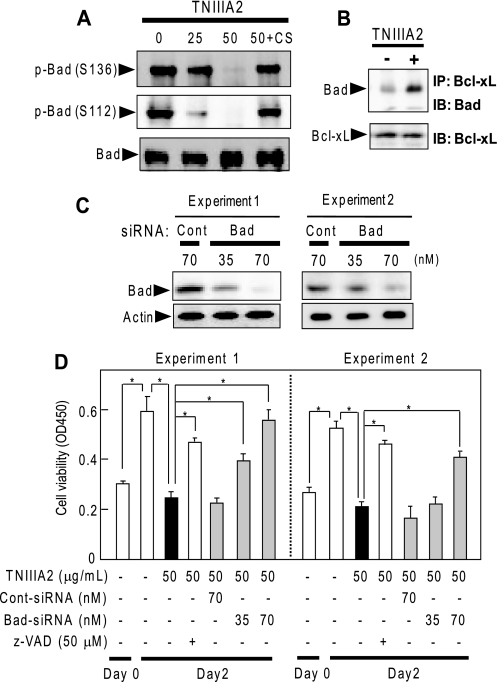
The proapoptotic function of Bad is regulated by its phosphorylation and is a common crucial effector molecule downstream of the phosphatidylinositol 3-kinase/Akt and MAPK/ERK signaling pathways (31, 32). Bad was highly phosphorylated at Ser112 and Ser136 in Ramos cells in suspension without TNIIIA2 stimulation (Fig. 4A). This phosphorylation of Bad was reduced through adhesion of Ramos cells to FN in response to TNIIIA2, whereas pretreatment of cells with CS-1 peptide restored Bad phosphorylation at both sites (Fig. 4A).
FIGURE 4.
Participation of Bad in the induction of adhesion-dependent apoptosis. A, dephosphorylation of Bad through TNIIIA2-induced adhesion of Ramos cells to FN. Ramos cell suspension (3 × 106 cells) with or without TNIIIA2 (25 or 50 μg/ml) was seeded on a plate coated with FN and then incubated in the presence or absence of CS-1 (CS) (200 μg/ml). After incubation for 16 h, cell lysates with equal amounts of protein were subjected to Western blot analysis using anti-phospho-Bad or anti-Bad, as described under “Experimental Procedures.” B, dissociation of Bad from binding with Bcl-xL through TNIIIA2-induced adhesion of Ramos cells to the FN. The cells were cultured on an FN-coated plate in the presence or absence of TNIIIA2 (50 μg/ml) for 16 h. The cell lysates were immunoprecipitated with anti-Bcl-xL Ab, and the resulting precipitate was subjected to Western blot analysis with anti-Bad Ab or anti-Bcl-xL, as described under “Experimental Procedures.” C and D, effect of knockdown of Bad protein expression by siRNA on TNIIIA2-induced apoptosis. Transfection of Bad siRNA (Bad) or control siRNA (Cont) was performed as described under “Experimental Procedures.” Cell proliferation/survival assay was performed as in Fig. 2. Two representative data (Experiments 1 and 2) of four independent experiments are shown. *, p < 0.005 compared with control. IP, immunoprecipitation; IB, immunoblot.
The Akt and ERK can regulate Bcl-xL activity via phosphorylation of Bad, which dissociates from binding with Bcl-xL, resulting in exertion of anti-apoptotic function (31, 32). This was tested by treating Ramos cell lysates with anti-Bcl-xL Ab, and the resulting immunoprecipitates were analyzed. When cells were stimulated with TNIIIA2, Bad protein became detectable in the immunoprecipitate with anti-Bcl-xL (Fig. 4B).
The importance of Bad protein in TNIIIA2-induced apoptosis was examined by transfecting Ramos cells with Bad siRNA. Transfection with Bad siRNA caused a significant decrease in Bad expression in a dose-dependent manner, whereas treatment with control siRNA had no detectable effect (Fig. 4C). Transfection with Bad siRNA, but not with control siRNA, rescued Ramos cells from the TNIIIA2-induced decrease in viable cells (Fig. 4D). Thus, Bad protein played a critical role in TNIIIA2-induced apoptosis in Ramos cells.
Adhesion-dependent Apoptosis in Other Hematopoietic Tumor Cells
We examined whether adhesion-dependent apoptosis is inducible also in other types of hematopoietic tumor cells. The human acute myelogenous leukemia (AML) cell line U937 expressed both of the FN receptors VLA-4 and VLA-5 (supplemental Fig. S3A). Most of the U937 cells became adherent after treatment with either Mg2+ or TNIIIA2 through β1-integrin activation, in which both VLA-4 and VLA-5 were associated with the adhesion (Fig. 5A). Like Ramos cells, U937 cells remained alive and proliferative in suspension under serum-free conditions. U937 cell survival/growth was reduced by forced adhesion to FN and induced by stimulation with either Mg2+ or TNIIIA2 but was restored by pretreating cells with z-VAD fmk (Fig. 5B, left panel), thus suggesting the induction of caspase-dependent apoptosis. This apoptosis was blocked by the addition of CS-1 peptide but not by RGD peptide (Fig. 5B). TS2/16 in combination with the bridging Ab also induced apoptosis in U937 cells (data not shown). Adhesion-dependent apoptosis and its preferential blocking with the CS-1 peptide were similarly observed using another AML cell line, HL-60, and human T-lymphocytic leukemia cell line, Jurkat, which expressed both VLA-4 and VLA-5 (Table 1). These results raised the assumption that adhesion-dependent apoptosis is mediated by VLA-4 but not by VLA-5.
FIGURE 5.
Adhesion-dependent apoptosis induction in U937 cells. A shows results of TNIIIA2-induced adhesion of U937 cells to FN. Adhesion assay under the conditions as indicated was performed as described under “Experimental Procedures.” The data are representative of three individual experiments. Each point represents the mean ± S.E. of triplicate determinations. *, p < 0.01 compared with control. B shows characterization of adhesion-dependent apoptosis of U937 cells. U937 cell suspensions (3 × 104 cells) with or without TNIIIA2 or Mg2+ were seeded on a 96-well plate coated with FN and then cultured for 2 days in the presence or absence of CS-1 or RGD peptide (200 μg/ml) or z-VAD fmk (50 μm). In the right panel, U937 cells were transfected with control siRNA or syndecan-4 siRNA, as described under “Experimental Procedures.” After transfection, the cells were treated with TNIIIA2 as above. The number of viable cells was evaluated as described under “Experimental Procedures.” Each point represents the mean ± S.E. of triplicate determinations. One of three or four individual experiments is shown. *, p < 0.005 compared with control. **, p < 0.01 compared with control.
TABLE 1.
Expression of VLA-4, VLA-5, and syndecan-4 and effects on adhesion-dependent apoptosis of hematopoietic progenitor cell lines, fresh AML cells from patients, and peripheral blood cells from normal adults
The results show that Mg2+ induces apoptosis in cells expressing VLA-4 with moderate levels, whereas TNIIIA2 additionally requires membrane expression of syndecan-4 for the induction of apoptosis. The data show the fraction of total cells that adhered or died. +++, more than ∼70%; ++, ∼50%; +, less than ∼30%; −, almost negative or none. ND, not determined.
| Cells | Expressiona |
Adhesionb |
Apoptosisc |
||||
|---|---|---|---|---|---|---|---|
| VLA-4 | VLA-5 | Syndecan-4 | Induced by Mg2+ | Induced by TNIIIA2 | Induced by Mg2+ | Induced by TNIIIA2 | |
| % | |||||||
| Cell lines | |||||||
| B cells | |||||||
| Ramos | 96.5 | 3.2 | 92.1 | ++ | +++ | ++ | +++ |
| Rajii | 94.3 | 65.3 | 2.3 | ++ | − | + | − |
| T cell (Jurkat) | 92.8 | 96.6 | 42.4 | ++ | +++ | ++ | +++ |
| Erythroid (K562) | 9.3 | 97.2 | 66.7 | ++ | +++ | − | − |
| Myeloid | |||||||
| U937 | 98.1 | 98.2 | 87.5 | ++ | +++ | +++ | ++ |
| HL-60 | 99.8 | 99.7 | 75.3 | ++ | +++ | + | ++ |
| THP-1 | 68.5 | 20.3 | 30.1 | ++ | ++ | + | ++ |
| THP-1 (phorbol 12-myristate 13-acetate) | 10.9 | 18.7 | 99.2 | ++ | ++ | − | − |
| Fresh AML cells | |||||||
| Patient A | 98.2 | 88.8 | 48.8 | ++ | ++ | ++ | + |
| Patient B | 97.5 | 98.5 | 9.5 | + | − | ++ | − |
| Peripheral blood cells | |||||||
| Neutrophil | 6.7 | ND | 3.2 | − | − | − | − |
| Monocyte | 48.6 | ND | 2.0 | ++ | − | ++ | − |
| Lymphocyte | 40.5 | ND | 4.4 | ++ | − | + | − |
a Expression of VLA-4, VLA-5, and syndecan-4 was analyzed by flow cytometry using corresponding Abs.
b The effects of Mg2+ (10 mm) or TNIIIA2 (50 μg/ml) upon cell adhesion to the FN were evaluated by the cell adhesion assay and DNA degradation, respectively, as described under “Experimental Procedures.”
c The effects of Mg2+ (10 mm) or TNIIIA2 (50 μg/ml) upon apoptosis were evaluated by the cell adhesion assay and DNA degradation, respectively, as described under “Experimental Procedures.”
Necessity of VLA-4 and Syndecan-4 for Adhesion-dependent Apoptosis
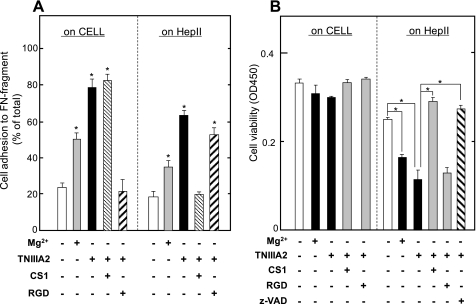
To investigate the role of VLA-4 in adhesion-dependent apoptosis, we examined the cell survival assay using culture plates coated with the CELL and HepII fragments of FN molecule, which include specific binding sites for VLA-5 and VLA-4, respectively (23). Both Mg2+ and TNIIIA2 induced adhesion of U937 cells to either the CELL or HepII fragment (Fig. 6A). An inhibition assay using the RGD and CS-1 peptides showed that U937 cell adhesion to the CELL and HepII fragments was mediated mainly by VLA-5 and VLA-4, respectively (Fig. 6A). Although both Mg2+ and TNIIIA2 were thus capable of inducing U937 cell adhesion to either the CELL or HepII fragment, a decrease in cell viability, which was reversed by z-VAD fmk, was observed only in cells adhering to the HepII fragment (Fig. 6B). This apoptosis induction on the HepII fragment was blocked by the CS-1 peptide (Fig. 6B).
FIGURE 6.
Necessity of VLA-4 for adhesion-dependent apoptosis of U937 cells. In A, U937 cell adhesion to either the CELL or HepII fragment was characterized. U937 cell suspension with or without Mg2+ (10 mm) or TNIIIA2 (50 μg/ml) was seeded in the presence or absence of CS-1 peptide (200 μg/ml) or RGD peptide (200 μg/ml) on a 96-well plate coated with the CELL fragment (left panel) or HepII fragment (right panel). Adhesion assay was performed as described under “Experimental Procedures.” The data are representative of three individual experiments. Each point represents the mean ± S.E. of triplicate determinations. *, p < 0.01 compared with control. B shows results of survival of U937 cells adhered on the CELL and HepII fragment. U937 cell suspension with or without TNIIIA2 (50 mg/ml) or Mg2+ (10 mm) were seeded on a 96-well plate coated with the CELL or HepII fragment and then cultured for 2 days in the presence or absence of CS-1, RGD peptide (200 μg/ml), or z-VAD fmk (50 μm). The number of viable cells was evaluated as described under “Experimental Procedures.” Each point represents the mean ± S.E. of triplicate determinations. One of three or four individual experiments is shown. *, p < 0.01 compared with control.
Next, the human chronic myelogenous leukemia cell line K562, which expresses VLA-5 exclusively as an FN receptor, was examined by the cell survival assay. Either Mg2+ or TNIIIA2 induces K562 cell adhesion to FN by activating VLA-5 (21), whereas K562 cell survival was not influenced by stimulation with either Mg2+ or TNIIIA2 (Table 1). Furthermore, THP-1 cells that express VLA-4 and VLA-5 also underwent apoptosis through adhesion to FN by stimulation with TNIIIA2 (Table 1). However, THP-1 cells became resistant to adhesion-dependent apoptosis after the induction of monocytic differentiation with phorbol 12-myristate 13-acetate, which was accompanied by a marked reduction in VLA-4 expression (Table 1). These results support the assumption that apoptosis may be induced depending on the VLA-4-mediated adhesion of hematopoietic tumor cells to FN.
Next, to verify the necessity of syndecan-4 for TNIIIA2-induced apoptosis, we examined the susceptibility to TNIIIA2-induced apoptosis using Epstein-Barr virus-positive Burkitt's lymphoma cell line, Raji, which reportedly exhibits no endogenous cell surface heparan sulfate proteoglycan (33). In fact, syndecan-4 was hardly detected in Raji cells (Table 1). The Raji cells were almost insusceptible to TNIIIA2 in the induction of not only adhesion to FN but also apoptosis (Table 1). Furthermore, we examined the effect of syndecan-4 knockdown on TNIIIA2-induced apoptosis in U937 cells. Flow cytometric analysis showed that syndecan-4 expression on U937 cells was reduced (∼60% of control) by the introduction of siRNA targeted against syndecan-4 core protein but not by control siRNA (data not shown). Syndecan-4 knockdown partially prevented TNIIIA2-induced apoptosis, whereas control siRNA had no remarkable effect (Fig. 5B, right panel).
On the other hand, fresh leukemic cells from AML patients, which express VLA-4 at high levels but syndecan-4 at different levels, were examined by the cell survival assay. Fresh leukemic cells from two AML patients (patients A and B) underwent apoptosis in response to treatment with Mg2+ (Table 1). TNIIIA2 induced apoptosis in AML cells from patient A, which moderately expressed syndecan-4, but not in AML cells from patient B, which showed only weak expression (Table 1). These results suggest that VLA-4-mediated forced adhesion to FN causes apoptosis in a variety of hematopoietic tumor cell lines and fresh leukemic cells from AML patients, whereas TNIIIA2 additionally requires membrane expression of syndecan-4 for the induction of apoptosis.
Effect of TNIIIA2 on Nontransformed Peripheral Blood Cells
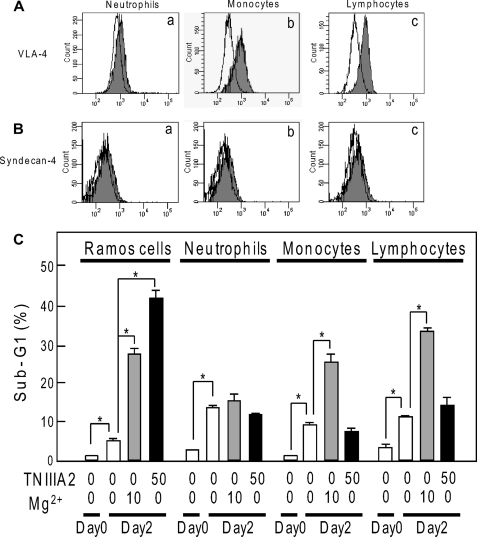
We finally examined the effect of TNIIIA2 on normal peripheral blood cells, such as neutrophils, monocytes, and lymphocytes. By flow cytometric analysis, expression of VLA-4 was clearly detected on monocytes and lymphocytes but was detected only slightly on neutrophils (Fig. 7A and Table 1). On the other hand, these blood cells had very low expression of syndecan-4 (Fig. 7B and Table 1). We then evaluated the susceptibility of these peripheral blood cells to apoptosis (Fig. 7C). Mg2+ treatment increased the sub-G1 DNA fraction in monocytes and lymphocytes. In contrast, TNIIIA2 treatment induced little or no increase in the sub-G1 DNA fraction in either monocytes or lymphocytes. Neutrophils, which had very little expression of VLA-4, did not undergo apoptosis by stimulation with either Mg2+ or TNIIIA2. Thus, TNIIIA2 hardly exhibits proapoptotic effects on normal peripheral blood cells, such as neutrophils, monocytes, and lymphocytes, probably because of their low expression of syndecan-4.
FIGURE 7.
Expression of VLA-4 and syndecan-4 on peripheral blood cells and effects of TNIIIA2 on these cells. A and B, flow cytometric analysis of VLA-4 (A) and syndecan-4 (B) expression on peripheral blood neutrophils (panel a), monocytes (panel b), and lymphocytes (panel c). The data are representative of two individual experiments. C, effects of treatment with TNIIIA2 (50 μg/ml) or Mg2+ (10 mm) on peripheral blood cell survival, as determined by the fraction of sub-G1 DNA. Each point represents the mean value of three experiments. *, p < 0.05 compared with control.
DISCUSSION
In the present study, we showed that potentiated and sustained adhesion to FN induces apoptosis in hematopoietic tumor cells. The following results suggest that adhesion to fibronectin via VLA-4 is responsible for the apoptosis: 1) TNIIIA2, which kept cells adhering to fibronectin by activating β1-integrins, induced apoptosis, whereas its control peptide TNIIIA2mut, which had no proadhesive activity, did not; 2) other integrin activators, Mg2+ and TS2/16, were also capable of inducing apoptosis; 3) apoptosis induced by the integrin activators was specifically abrogated by antagonists for VLA-4 but not for VLA-5; 4) hematopoietic tumor cell lines and fresh leukemic cells, which express VLA-4 with a functional level, underwent apoptosis, whereas those cells poorly expressing VLA-4 did not; and 5) U937 cells, expressing both VLA-4 and VLA-5, underwent apoptosis only when adhered to fibronectin fragments containing VLA-4-binding sites, and this apoptosis was specifically abrogated by the VLA-4 antagonist.
In contrast to Mg2+ and TS2/16, which can activate β1-integrins through their direct binding to integrin, TNIIIA2 requires syndecan-4 as a membrane receptor for activation of β1-integrin (21). In fact, TNIIIA2 also required syndecan-4 expression, in addition to VLA-4, for the induction of apoptosis (summarized in Table 1). Syndecan-4 probably contributes to the sustained activation of VLA-4 through a lateral association with it (21). The results showing that knockdown of membrane syndecan-4 by siRNA caused a reduction of TNIIIA2-induced apoptosis support the necessity of syndecan-4. Interestingly, TNIIIA2 exhibited no remarkable proapoptotic effects on normal peripheral blood cells, such as neutrophils, monocytes, and lymphocytes. The results using hematopoietic tumor cell lines with various expression levels of VLA-4 and syndecan-4 suggest that this may be due to low expression of syndecan-4 on these blood cells. In fact, TNIIIA2 could not so much as induce specific adhesion to the FN in these peripheral blood cells. Syndecans are highly regulated with respect to developmental expression and cell type specificity. It has been reported that very little syndecan-4 is present on polymorphonuclear leukocytes and peripheral blood mononuclear cells (27, 34). The effects of TNIIIA2 on other adherent and nonadherent cell types originating from normal tissues, as well as tumors, should be investigated more precisely.
Increasing evidence has demonstrated that the adhesion of hematopoietic tumor cells to FN via VLA-4 and VLA-5 confers a multidrug resistance phenotype, called CAM-DR (35). The CAM-DR of hematopoietic tumor cells appears to be in conflict with our conclusion in the present study. However, there may be an explanation for this discrepancy. A weak or moderate adhesion to FN may be favorable for continuous survival or for the acquisition of chemoresistance in hematopoietic tumor cells. We previously demonstrated (36) that leukemic cell adhesion to bone marrow FN via VLA-4 generated CAM-DR, which could be a cause of minimal residual disease in bone marrow (37). Additionally, we recently demonstrated using in vitro and in vivo experiments (26) that combination therapy with an anti-cancer drug and a FN peptide, FNIII14, which is capable of inactivating β1-integrins (38), effectively overcomes CAM-DR of AML. In this report, we showed that CAM-DR was induced in U937 and HL-60 cells through forced adhesion of these AML cells to FN by stimulating with 1 mm of Mg2+, which corresponds to mild conditions for β1-integrin activation defined in the present study. In fact, under these conditions U937 and HL-60 cells did adhere to FN but did not undergo apoptosis in either the previous (36) or the present study. Also in a series of previous reports investigating CAM-DR, hematopoietic tumor cells acquired chemoresistance through spontaneous adhesion to FN without the addition of integrin activators (10, 35, 37, 39). It appears likely that CAM-DR may be induced through weak or moderate adhesion to FN.
TN-C, which is expressed constitutively in lymphoid tissues, is also transiently expressed during tumorigenesis. Therefore, expression of TN-C becomes prominently high in hematopoietic malignancies. On the other hand, the extracellular matrix proteins often harbor functional sites within their molecular structure, and these cryptic active sites (matricryptic sites) are disclosed by proteolytic degradation with inflammatory proteinases including matrix metalloproteinases (40, 41). TNIIIA2 as a matricryptic site can be disclosed by at least matrix metalloproteinase-2 (21). It has been known that spontaneous adhesion of hematopoietic tumor cells is induced mainly by β1-integrin activation through the interaction between cytokine and G protein-coupled receptor (42). However, activation of β1-integrin (VLA-4) mediated by G protein-coupled receptor activation is reportedly of lower magnitude compared with the integrin activating mAb TS2/16 (43). Additionally, it has been reported that cytokine-stimulated adhesion via VLA-4 and VLA-5 to FN is rapid (reaching a max within 30 min) but transient (returning to basal levels after several hours) (43). In sharp contrast, TNIIIA2 has the ability to strongly activate β1-integrins and to sustain this activated status, probably because of stabilization of the active β1 conformation through lateral association with syndecan-4 (21). TNIIIA2 activity of the TN-C molecule could contribute, once exposed, to preventing prolonged survival of hematopoietic malignant progenitors. Further study is needed to examine whether the TNIIIA2-related matricryptic site is exposed at its functional level in lymphoid tissues with hematopoietic malignancy.
Supplementary Material
This work was supported in part by the High-Tech Research Center Project for Private Universities with a matching fund subsidy from Ministry for Education, Culture, Sports, Science and Technology of Japan (2004–2008) and by the Vehicle Racing Commemorative Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- FN
- fibronectin
- Ab
- antibody
- mAb
- monoclonal antibody
- pAb
- polyclonal antibody
- ERK
- extracellular signal-regulated kinase
- TN
- tenascin
- CAM-DR
- cell adhesion-mediated drug resistance phenotype
- CELL
- central cell-binding domain
- HepII
- heparin-binding domain II
- z-VAD
- benzyloxycarbonyl-Val-Ala-Asp(OMe)
- fmk
- fluoromethyl ketone
- FITC
- fluorescein isothiocyanate
- siRNA
- small interfering RNA
- MAPK
- mitogen-activated protein kinase
- MEK
- MAPK/ERK kinase
- AML
- acute myelogenous leukemia.
REFERENCES
- 1.Clark B. R., Gallagher J. T., Dexter T. M. (1992) Bailleres Clin. Hematol. 5, 619–652 [DOI] [PubMed] [Google Scholar]
- 2.Coulombel L., Vuillet M. H., Leroy C., Tchernia G. (1988) Blood 71, 329–334 [PubMed] [Google Scholar]
- 3.Murti K. G., Brown P. S., Kumagai M., Campana D. (1996) Exp. Cell Res. 226, 47–58 [DOI] [PubMed] [Google Scholar]
- 4.Williams D. A., Rios M., Stephens C., Patel V. P. (1991) Nature 352, 438–441 [DOI] [PubMed] [Google Scholar]
- 5.Shain K. H., Landowski T. H., Dalton W. S. (2000) Curr. Opin. Oncol. 12, 557–563 [DOI] [PubMed] [Google Scholar]
- 6.Sachs L. (1995) Adv. Cancer Res. 66, 1–40 [PubMed] [Google Scholar]
- 7.Bradstock K. F., Gottlieb D. J. (1995) Leuk. Lymphoma 18, 1–16 [DOI] [PubMed] [Google Scholar]
- 8.Hurley R. W., McCarthy J. B., Verfaillie C. M. (1995) J. Clin. Invest. 96, 511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molla A., Block M. R. (2000) Cell Growth & Differ. 11, 83–90 [PubMed] [Google Scholar]
- 10.Damiano J. S., Hazlehurst L. A., Dalton W. S. (2001) Leukemia 15, 1232–1239 [DOI] [PubMed] [Google Scholar]
- 11.Sugahara H., Kanakura Y., Furitsu T., Ishihara K., Oritani K., Ikeda H., Kitayama H., Ishikawa J., Hashimoto K., Kanayama Y., Matsuzawa Y. (1994) J. Exp. Med. 179, 1757–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terui Y., Furukawa Y., Sakai T., Kikuchi J., Sugahara H., Kanakura Y., Kitagawa S., Miura Y. (1996) J. Immunol. 156, 1981–1988 [PubMed] [Google Scholar]
- 13.Kapur R., Cooper R., Zhang L., Williams D. A. (2001) Blood 97, 1975–1981 [DOI] [PubMed] [Google Scholar]
- 14.Tsunoda T., Inada H., Kalembeyi I., Imanaka-Yoshida K., Sakakibara M., Okada R., Katsuta K., Sakakura T., Majima Y., Yoshida T. (2003) Am. J. Pathol. 162, 1857–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lightner V. A., Slemp C. A., Erickson H. P. (1990) Ann. N. Y. Acad. Sci. 580, 260–275 [DOI] [PubMed] [Google Scholar]
- 16.Chilosi M., Lestani M., Benedetti A., Montagna L., Pedron S., Scarpa A., Menestrina F., Hirohashi S., Pizzolo G., Semenzato G. (1993) Am. J. Pathol. 143, 1348–1355 [PMC free article] [PubMed] [Google Scholar]
- 17.Ocklind G., Talts J., Fässler R., Mattsson A., Ekblom P. (1993) J. Histochem. Cytochem. 41, 1163–1169 [DOI] [PubMed] [Google Scholar]
- 18.Atula T., Hedström J., Finne P., Leivo I., Markkanen-Leppänen M., Haglund C. (2003) Anticancer Res. 23, 3051–3056 [PubMed] [Google Scholar]
- 19.Leins A., Riva P., Lindstedt R., Davidoff M. S., Mehraein P., Weis S. (2003) Cancer 98, 2430–2439 [DOI] [PubMed] [Google Scholar]
- 20.Joshi P., Chung C. Y., Aukhil I., Erickson H. P. (1993) J. Cell Sci. 106, 389–400 [DOI] [PubMed] [Google Scholar]
- 21.Saito Y., Imazeki H., Miura S., Yoshimura T., Okutsu H., Harada Y., Ohwaki T., Nagao O., Kamiya S., Hayashi R., Kodama H., Handa H., Yoshida T., Fukai F. (2007) J. Biol. Chem. 282, 34929–34937 [DOI] [PubMed] [Google Scholar]
- 22.Fukai F., Suzuki H., Suzuki K., Tsugita A., Katayama T. (1991) J. Biol. Chem. 266, 8807–8813 [PubMed] [Google Scholar]
- 23.van de Wiel-van Kemenade E., van Kooyk Y., de Boer A. J., Huijbens R. J., Weder P., van de Kasteele W., Melief C. J., Figdor C. G. (1992) J. Cell Biol. 117, 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takagi J., Isobe T., Takada Y., Saito Y. (1997) J. Biochem. 121, 914–921 [DOI] [PubMed] [Google Scholar]
- 25.Drexler H. G., Otsuka K., Gaedicke G., Minowada J. (1986) Cancer Res. 46, 6078–6082 [PubMed] [Google Scholar]
- 26.Matsunaga T., Fukai F., Miura S., Nakane Y., Owaki T., Kodama H., Tanaka M., Nagaya T., Takimoto R., Takayama T., Niitsu Y. (2008) Leukemia 22, 353–360 [DOI] [PubMed] [Google Scholar]
- 27.Kaneider N. C., Egger P., Dunzendorfer S., Wiedermann C. J. (2001) Biochem. Biophys. Res. Commun. 287, 42–46 [DOI] [PubMed] [Google Scholar]
- 28.Fukai F., Kamiya S., Ohwaki T., Goto S., Akiyama K., Goto T., Katayama T. (2000) Cell. Mol. Biol. 46, 145–152 [PubMed] [Google Scholar]
- 29.Sánchez-Aparicio P., Dominguez-Jiménez C., Garcia-Pardo A. (1994) J. Cell Biol. 126, 271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raz A., Ben-Ze'ev A. (1983) Science 221, 1307–1310 [DOI] [PubMed] [Google Scholar]
- 31.Datta S. R., Dudek H., Tao X., Masters S., Fu H., Gotoh Y., Greenberg M. E. (1997) Cell 91, 231–241 [DOI] [PubMed] [Google Scholar]
- 32.Scheid M. P., Schubert K. M., Duronio V. (1999) J. Biol. Chem. 274, 31108–31113 [DOI] [PubMed] [Google Scholar]
- 33.Lebakken C. S., Rapraeger A. C. (1996) J. Cell Biol. 132, 1209–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamashita Y., Oritani K., Miyoshi E. K., Wall R., Bernfield M., Kincade P. W. (1999) J. Immunol. 162, 5940–5948 [PubMed] [Google Scholar]
- 35.Wang M. W., Consoli U., Lane C. M., Durett A., Lauppe M. J., Champlin R., Andreeff M., Deisseroth A. B. (1998) Cell Growth & Differ. 9, 105–112 [PubMed] [Google Scholar]
- 36.Matsunaga T., Takemoto N., Sato T., Takimoto R., Tanaka I., Fujimi A., Akiyama T., Kuroda H., Kawano Y., Kobune M., Kato J., Hirayama Y., Sakamaki S., Kohda K., Miyake K., Niitsu Y. (2003) Nat. Med. 91, 1158–1165 [DOI] [PubMed] [Google Scholar]
- 37.Hazlehurst L. A., Dalton W. S. (2001) Cancer Metastasis Rev. 20, 43–50 [DOI] [PubMed] [Google Scholar]
- 38.Kamiya S., Kato R., Wakabayashi M., Tohyama T., Enami I., Ueki M., Yajima H., Ishii T., Nakamura H., Katayama T., Takagi J., Fukai F. (2002) Biochemistry 41, 3270–3277 [DOI] [PubMed] [Google Scholar]
- 39.Damiano J. S., Cress A. E., Hazlehurst L. A., Shtil A. A., Dalton W. S. (1999) Blood 93, 1658–1667 [PMC free article] [PubMed] [Google Scholar]
- 40.Sage E. H. (1997) Trends Cell Biol. 7, 182–186 [DOI] [PubMed] [Google Scholar]
- 41.Davis G. E., Bayless K. J., Davis M. J., Meininger G. A. (2000) Am. J. Pathol. 156, 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lévesque J. P., Leavesley D. I., Niutta S., Vadas M., Simmons P. J. (1995) J. Exp. Med. 181, 1805–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chigaev A., Blenc A. M., Braaten J. V., Kumaraswamy N., Kepley C. L., Andrews R. P., Oliver J. M., Edwards B. S., Prossnitz E. R., Larson R. S., Sklar L. A. (2001) J. Biol. Chem. 276, 48670–48678 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.