Abstract
β-Defensins are small antimicrobial polypeptides that are mainly expressed by epithelial cells and play an important role in the antimicrobial innate immune response. In addition to the direct microbicidal effects of these polypeptides, members of the β-defensin super family have the capacity to promote local innate inflammatory and systemic adaptive immune responses, which are in part mediated by the CC-chemokine receptor CCR6. Here we report the expression of recombinant mBD4 and its human orthologue hBD2 fused to the constant domain of human IgG1 to obtain correct folding and to increase stability and solubility using the Drosophila S2 expression system. Purified recombinant mBD4:Ig and hBD2:Ig fusion proteins retained potent antimicrobial activity against Gram-negative and Gram-positive bacteria. Furthermore, these β-defensin fusion proteins showed specific binding to CCR6-expressing cells as revealed by flow cytometry. Interestingly, although hBD2:Ig bound to both human and mouse CCR6-expressing cells, mBD4:Ig did only bind to mCCR6-expressing cells but not to hCCR6-expressing cells. Both β-defensin fusion proteins demonstrated chemotactic activity for cells expressing the mouse CC-chemokine receptor CCR6. The chemokine ligand CCL20 competed with the β-defensin fusion proteins for specific binding to CCR6 as analyzed by fluorescence-activated cell sorter analysis. Both β-defensin fusion proteins demonstrated chemotactic activity for cells expressing the mouse CCR6 receptor, but mBD4:Ig did not induce chemotactic activity of cells expressing human CCR6. This result supports our finding that mBD4 does not interact with human CCR6-expressing cells. Further evidence for specific interaction of the β-defensin fusion proteins with CCR6-expressing cells is demonstrated by the observation that CCL20 and β-defensin fusion proteins desensitize each other in inducing chemotactic activity. In addition both mBD4:Ig and hBD2:Ig demonstrated CCR6-independent chemotaxis of freshly isolated mouse resident peritoneal cells and human peripheral blood mononuclear cells, indicating the interaction with another chemotaxis-inducing receptor. Thus, the β-defensin fusion proteins used in this study retained their biological activity and are a feasible tool to identify and analyze specific β-defensin receptor interactions.
Keywords: Chemokines, Chemotaxis, Defensins, Receptor Desensitization, Receptors
Introduction
β-Defensins are cationic, antimicrobial peptides contributing to host defense against bacterial, fungal, and viral infections (1). Mouse β-defensin 4 (mBD4,2 Defb4) is recognized as an orthologue of human β-defensin 2 (hBD2 or DEFB102). Based on the primary structural analysis, mBD4 demonstrates about 45% homology to its human orthologue on the protein level, containing three conserved cysteine linkages, characteristic for the β-defensin super family. Expression of mBD4-mRNA has been detected in a number of tissues, e.g. trachea, tongue, and epithelial cells lining various organs, and can be induced by Toll-like receptor agonists such as lipopolysaccharide and by proinflammatory stimuli (2). Immunohistochemical staining revealed a strongly induced expression of mBD4 protein in bronchial epithelial cells of the lung during the course of experimental tuberculosis infection (3). A recent report demonstrated an enhanced expression of mBD4 protein in the upper and lower airway mucosa in mice after infection with human influenza A virus (4). These results strongly suggest that mBD4 expression is also inducible in response to microbial organisms and proinflammatory stimuli as described for other members of the mouse β-defensin super family. The expression of its human orthologue hBD2 is induced by various proinflammatory stimuli, e.g. tumor necrosis factor, interleukin-1, and interferon-γ (5), and in response to pathogen-associated molecular patterns (PAMPs) after infection with Gram-positive and Gram-negative bacteria (6, 7). At the transcriptional level, induction of hBD2-mRNA was detected in epithelial cells, peripheral blood, monocytes, and keratinocytes (8–10).
In addition to having potent antimicrobial effects, previous reports indicate that mouse β-defensin 2 (mBD2) activates mouse dendritic cells through interacting with Toll-like receptor 4 (TLR4) and many human and mouse β-defensins, e.g. human β-defensin 2 (hBD2), hBD3, mBD2, mBD3 and mBD29, are chemotactic for dendritic cells and memory T cells via the chemokine receptor CCR6, thus, providing a link between innate and adaptive immune responses (11–14). Although β-defensin usage of CCR6 as a chemotactic receptor is documented in many reports, it has not been shown whether β-defensins can specifically bind to CCR6. Furthermore, a more recent study using chemically synthesized β-defensins concluded that CCR6 was not involved in β-defensin-induced migration of leukocytes (15). Therefore, it remains somewhat controversial whether CCR6 can bind to β-defensins and mediate its chemotactic effects.
To determine whether β-defensins can interact with CCR6, we generated fusion proteins in which hBD2 or its mouse orthologue mBD4 is fused to the Fc portion of human IgG1. Here we report the successful expression and purification of both β-defensin fusion proteins hBD2 and mBD4, which retained their potent antimicrobial activity. Functional testing by fluorescence-activated cell sorter analysis revealed specific binding to the CC-chemokine receptor CCR6, which was paralleled by induction of chemotactic activity for CCR6-expressing cells.
EXPERIMENTAL PROCEDURES
Expression and Purification of the mBD4:Ig, hBD2:Ig, and mCCL20:Ig Fusion Proteins
All fusion proteins were generated by insertion of the mBD4, hBD2, and mCCL20 cDNA encoding for the mature polypeptides into the Signal Ig plus vector (R&D Systems, Wiesbaden, Germany). The cDNAs were subcloned into the pMTBiP/V5-His A expression vector (Invitrogen) after PCR amplification of the mBD4:Ig, hBD2:Ig, and mCCL20:Ig cDNAs using the following primers: 5′-CCC AGA TCT AAT CCA ATA ACA TGC ATG-3′ for mBD4–5′; 5′-CCC AGA TCT GTT ACG TGC CTG AAA AGC GG-3′ for hBD2–5′; 5′-CCC AGA TCT ATG GCC TGC GGT GGC AAG CG-3′ for mCCL20–5′; 5′-CG CGG CCG CCA TCA TTT ACC CGG AGA CAG G-3′ for human IgG1-Fc-3′. Stable expressing S2 cells were selected and maintained in hygromycin (0.3 mg/ml; Invitrogen). The β-defensin fusion proteins were purified from the culture medium using HiTrap Protein G HP columns (GE Healthcare) according to the manufacturer's instructions. Expression and purification of the fusion proteins were confirmed by Western blotting using peroxidase-conjugated donkey anti-human IgG monoclonal antibody (Dianova, Hamburg, Germany). The constant domain of human IgG1 without β-defensin fusion (hIgG1) was expressed and purified as described above and used as a negative control.
Antibacterial Assay
Bacteria (Escherichia coli, ATCC 25922; Bacillus subtilis, ATCC 6633) were grown in LB broth (USB Corp., Staufen, Germany) to 1.0 A600 at 37 °C with vigorous shaking (220 rpm) and diluted with MT-LB buffer (16 mm disodium hydrogen phosphate, 5 mm sodium dihydrogen phosphate, 150 mm sodium chloride, and 1% LB broth) to a final concentration of 1 × 104 colony forming units/ml. Bacteria were incubated on a shaker (220 rpm) for 3 h at 37 °C with 0–5 μg/ml mBD4:Ig, hBD2:Ig, hIgG1 in a volume of 100 μl and plated at an appropriate dilution on LB-agar plates. LB-agar plates were incubated overnight at 37 °C, and colony forming units were determined. Antibacterial assays were performed in triplicate. Results were presented as the mean ± S.D. of triplicate. Three independent experiments were performed, and representative data are shown.
Flow Cytometric Analysis
All staining and washing steps were performed for 20 min on ice in phosphate-buffered saline containing 2% fetal calf serum and 0.05% azide. Mouse and human CCR6 expressing HEK293 (mCCR6/293, hCCR6/293) cells were washed and incubated with 10% rat serum to prevent nonspecific binding. 1 × 106 cells were incubated with 1 μg/ml control Ig (hIgG1), mCCL20:Ig, or β-defensin fusion protein. After washing, the cells were labeled with phycoerythrin-conjugated anti-human IgG-Fc antibody (Dianova), washed, and analyzed by flow cytometry. In competitive inhibition assays 1 μg/ml mCCL20 or hCCL20 (Peprotech, Rocky Hill, NJ) was added 20 min before the addition of mBD4:Ig or hBD2:Ig fusion protein. Three separate experiments were performed, and representative data are shown.
Target Cell Preparation and Chemotaxis Assay
HEK293 cells expressing mCCR6 (mCCR6/293) or hCCR6 (hCCR6/293) were maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum and 800 μg/ml G418 and used in a chemotaxis assay when they reached 60∼70% confluence. Human peripheral blood enriched in mononuclear cells was obtained from healthy donors by leukapheresis (Transfusion Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD, with the approved human subjects' agreements). The blood was centrifuged through Histopaque-1077 (Sigma), and peripheral blood mononuclear cells collected at the interface were washed with phosphate-buffered saline. After centrifugation through an iso-osmotic Percoll (GE Healthcare) gradient, the enriched monocytes (PBMs) were obtained from the top of the gradient. Mouse resident peritoneal cells (RPCs) were obtained by lavage of the peritoneum of 8-week-old C57BL/6 mice with 5 ml of ice-cold phosphate-buffered saline containing heparin (20 units/ml) and EDTA (5 mm). Both HEK293 cells and primary cells were suspended in chemotaxis medium (RPMI 1640 containing 1% bovine serum albumin, 20 mm HEPES, 2 mm l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin) at 1 × 106/ml. The migration of both types of cells in response to chemoattractants (β-defensins or control chemotactic factors) was determined using the 48-well microchemotaxis chamber assay. In brief, chemoattractants diluted in chemotaxis medium at the indicated concentrations were put into the lower wells of a 48-well microchemotaxis chamber (Neuro Probe, Cabin John, MA), and cell suspension was added into the upper wells. The lower and upper compartments were separated by a 5-μm uncoated (for primary cells) or a 10-μm collagen-coated (for HEK293 cells) polycarbonate filter membrane (Osmonics, Livermore, CA). In certain experiments primary cells were preincubated with 100 ng/ml chemoattractant (as indicated) for 30 min at 37 °C in humidified air containing 5% CO2. After incubation at 37 °C for 1.5 h for primary cells and 5 h for HEK293 cells in humidified air with 5% CO2, the filters were removed, scraped, and stained, and the number of cells migrating across the filter was counted under a light microscope. The results (mean ± S.D. of triplicate wells) were presented as the number of cells per high power field. Three independent experiments were performed, and representative data are shown.
Sequence Alignment
The alignment of amino acid sequences was performed using the ClustalW algorithm.
RESULTS
Expression and Antimicrobial Activity of the mBD4:Ig and hBD2:Ig Fusion Proteins
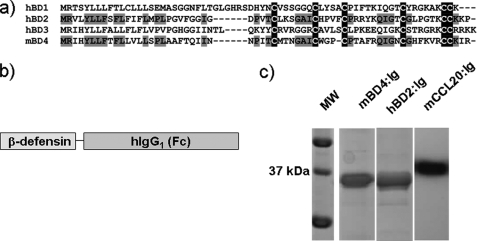
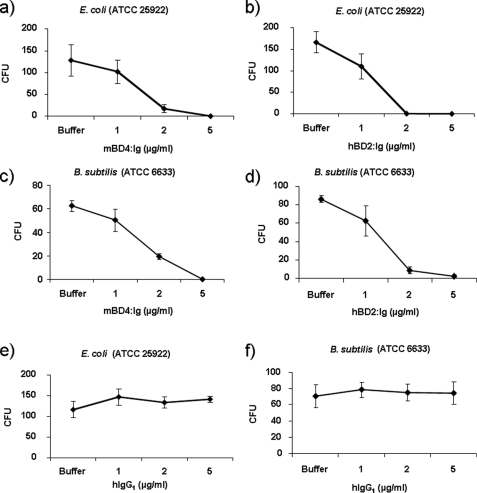
The mBD4 (Defb4) protein demonstrated 45% homology to hBD2 on the protein level, with significantly lower homology to hBD1 (31%), hBD3 (20%), and hBD4 (20%). Furthermore, the characteristic cysteine residues of the β-defensin super family were completely conserved (Fig. 1a). To obtain correct folding and increased stability and solubility of the recombinant β-defensins, we expressed the mature mBD4 and hBD2 polypeptides as fusion proteins fused to the constant domain (Fc) of human IgG1 (mBD4:Ig, hBD2:Ig) in the Drosophila S2 system. As a positive control, we also expressed mouse CCL20 fused to human IgG1 (mCCL20:Ig) (Fig. 1b). Stable expression of the mBD4:Ig, hBD2:Ig, and mCCL20:Ig fusion proteins followed by purification and Western blot analysis using an anti-human IgG antibody resulted in detection of a single protein band with the expected apparent molecular mass of ∼37kDa (Fig. 1c). The recombinant expressed fusion proteins retained antimicrobial activity against Gram-negative and Gram-positive bacteria. The mBD4:Ig and hBD2:Ig killed E. coli and B. subtilis with a concentration as low as 1 μg/ml at physiological salt concentrations (Fig. 2, a–d). The antimicrobial effect of mBD4:Ig and hBD2:Ig is comparable with previously reported recombinant β-defensins (6, 17, 18). In contrast, recombinant human IgG1 without β-defensin fusion (hIgG1) did not exhibit any antimicrobial activity against E. coli or B. subtilis (Fig. 2, e and f), demonstrating that the β-defensin component of the hIgG1 fusion proteins seems to be responsible for the antimicrobial effect.
FIGURE 1.
Expression of mBD4 and its human orthologue hBD2 as IgG1-Fc fusion proteins. a, alignment is shown of the predicted amino acid sequence of mBD4 with other human β-defensins using the ClustalW algorithm. Conserved residues among mBD4 and hBD2 are shown in gray, and conserved cysteine residues are highlighted in black. b, schematic drawing of the β-defensin fusion proteins is shown. c, shown is a Western blot analysis of the recombinant expressed mBD4:Ig, hBD2:Ig, and mCCL20:Ig fusion proteins using a peroxidase-conjugated donkey anti-human IgG monoclonal antibody for detection. MW, molecular mass marker.
FIGURE 2.
Antimicrobial activity of the mBD4:Ig and hBD2:Ig fusion proteins. Antimicrobial assays were performed as described under “Experimental Procedures” using E. coli (ATCC 25922) and B. subtilis (ATCC 6633). To determine the number of colony forming units (CFU), serial dilutions of samples with or without the indicated concentrations of the fusion proteins were plated, and colony counts were performed 24 h later. Data are the means ± S.D. of one representative experiment of three, each done in triplicate.
Binding of mBD4:Ig and hBD2:Ig to CCR6-expressing Cells
β-Defensins have been shown to be chemotactic by interacting with the chemokine receptor CCR6. Thus, CCR6 is able to act as a receptor for β-defensins as well as the chemokine CCL20. Interaction of β-defensins with the CCR6 receptor has so far only been detected indirectly based on the induction of chemotactic activity for CCR6-expressing cells. We, therefore, proposed to show the direct binding of mBD4:Ig and hBD2:Ig to CCR6-expressing HEK293 cells.
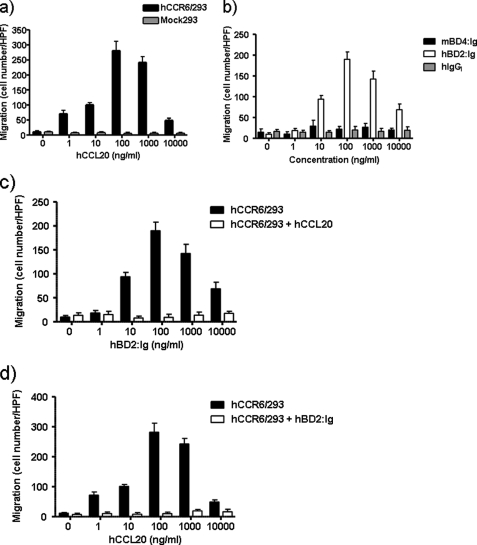
Incubation of mouse CCL20:Ig (mCCL20:Ig) with mouse (mCCR6/293)- or human CCR6- (hCCR6/293)-expressing HEK293 cells, as detected with an anti-human IgG-phycoerythrin conjugate, resulted in consistent binding of mCCL20:Ig to mCCR6- or hCCR6-expressing HEK293 cells (Fig. 3, a and d). No binding was observed when mCCR6- or hCCR6-expressing HEK293 cells were incubated with recombinant human IgG1 without β-defensin fusion (hIgG1). HBD2:Ig fusion protein demonstrated binding to both mCCR6- and hCCR6-expressing cells (Fig. 3, c and f). In contrast, mBD4:Ig fusion protein was species-specific and bound to mCCR6-expressing cells but not to cells expressing hCCR6 (Fig. 3, b and e).
FIGURE 3.
Binding analysis of mBD4:Ig, hBD2:Ig, and mCCL20:Ig to CCR6-expressing cells using flow cytometry. Shown is fluorescence-activated cell sorter analysis as described under “Experimental Procedures” of mCCL20:Ig, mBD4:Ig, and hBD2:Ig (black line) using mouse CCR6 (a–c) and human CCR6 expressing HEK293 cells (d–f), respectively, and hIgG1 as a negative control (filled gray) in each experiment. Data represent one experiment of three independent experiments.
To further verify specific interaction of the β-defensin fusion proteins with their respective CCR6 receptor, we performed desensitization assays by preincubation of mCCR6-expressing cells with mCCL20- or hCCR6-expressing cells with hCCL20 before adding the β-defensin fusion protein (mBD4:Ig and hBD2:Ig) followed by detection with an anti-human IgG-phycoerythrin conjugate. The binding of mBD4:Ig to mCCR6 as well as the binding of hBD2 to hCCR6 was partially inhibited by the prior addition of an equal amount of mCCL20 or hCCL20 (Fig. 4), providing additional evidence to support specific binding of the β-defensin fusion proteins to their respective CCR6 receptor.
FIGURE 4.
Competition assays for binding of mBD4:Ig and hBD2:Ig to mCCR6- and hCCR6-expressing cells. HEK293 cells stably transfected with mCCR6 were incubated with 1 μg/ml mBD4:Ig (black line) or 1 μg/ml hIgG1 (filled gray) (a), and cells transfected with hCCR6 were incubated with 1 μg/ml hBD2:Ig (black line) or 1 μg/ml hIgG1 (filled gray) (b). For competition analysis transfected HEK293 cells were pretreated with mCCL20 (a) or hCCL20 (b) 20 min before the addition of β-defensin fusion proteins (dashed line). Binding was determined by flow cytometry using a phycoerythrin (PE)-conjugated anti-human IgG-Fc antibody. Data show one representative experiment of three independent experiments.
MBD4 and hBD2 Induced Chemotactic Activity of CCR6-expressing Cells
Previous studies have revealed that hBD2 is chemotactic for CD45RO+ memory T cells and for immature, but not mature dendritic cells. The chemotactic activity has been shown to be mediated by the human CC chemokine receptor 6 (CCR6), (11). Furthermore, mBD2 and mBD3 are chemotactic for immature mouse dendritic cells as well as HEK293 cells transfected to express mouse CCR6 (11, 13). Recently, we have demonstrated that the mBD14:Ig fusion protein is chemotactic for CCR6-expressing cells as well as for freshly isolated mouse RPCs (19). In line with these experiments, we tested mBD4:Ig and its human orthologue hBD2:Ig for their chemotactic effect on HEK293 cells expressing mCCR6 or hCCR6. As shown in Fig. 5a, mCCL20 induced dose-dependent chemotactic activity of cells expressing mCCR6. Recombinant mBD4:Ig and hBD2:Ig fusion proteins also induced migration of mCCR6-expressing HEK293 cells in a dose-dependent and typical bimodal manner. Recombinant human IgG1 did not induce any chemotactic activity of HEK293 cells expressing mCCR6 (Fig. 5b). The dose-dependent chemotactic activity of mBD4:Ig and hBD2:Ig toward mCCR6-expressing cells was abrogated after preincubation of the mCCR6-expressing cells with mCCL20, indicating that CCR6-specific desensitization resulted in an unresponsiveness of the cells toward β-defensin fusion proteins (Fig. 5, c and d). Furthermore, desensitization of mCCR6-expressing cells using mBD4:Ig or hBD2:Ig resulted in unresponsiveness of the cells toward mCCL20 (Fig. 5e). These results clearly demonstrate that mBD4 and hBD2 are capable of 1) binding to and 2) inducing chemotactic activity of cells using the mouse CC chemokine receptor 6. Based on our binding results using hCCR6-expressing cells and mBD4:Ig and hBD2:Ig fusion proteins, we tested mBD4:Ig and hBD2:Ig for their chemotactic effect on HEK293 cells expressing hCCR6. Human CCL20 induced dose-dependent chemotactic activity of cells expressing hCCR6 (Fig. 6a). Although recombinant hBD2:Ig fusion protein also induced migration of hCCR6-expressing HEK293 cells in a dose-dependent and typical bimodal manner, mBD4:Ig failed to induce any chemotactic activity of cells expressing hCCR6 (Fig. 6b). This result is in accordance with our observation that mBD4:Ig did not bind to hCCR6-expressing cells as revealed by fluorescence-activated cell sorter analysis. Recombinant human IgG1 did not show any chemotactic activity for HEK293 cells expressing hCCR6 (Fig. 6b). The dose-dependent chemotactic activity of hBD2:Ig toward hCCR6-expressing cells was abrogated after preincubation of the hCCR6-expressing cells with hCCL20 (Fig. 6c), and conversely, the chemotactic activity of hCCL20 was abrogated by preincubating hCCR6-expressing cells with hBD2:Ig (Fig. 6d), indicating that specific desensitization of cells expressing hCCR6 could be achieved by β-defensins.
FIGURE 5.
Chemotactic activity of mBD4:Ig and hBD2:Ig for mouse CCR6-expressing cells. The migration of mock transfected HEK293 cells and HEK293 cells stably expressing mCCR6 in response to various concentrations of mCCL20 (a) and recombinant mBD4:Ig, hBD2:Ig and hIgG (b) was tested using a 48-well chemotaxis chamber assay. The migration of HEK293 cells stably expressing mCCR6 in response to various concentrations of recombinant mBD4:Ig (c) and hBD2:Ig (d) after preincubation with mCCL20 (100 ng/ml) or in response to various concentrations of mCCL20 after preincubation with mBD4:Ig or hBD2:Ig (e) was tested using a 48-well chemotaxis chamber assay as described under “Experimental Procedures.” Data are the means ± S.D. of one representative experiment of three, each done in triplicate. HPF, high power field.
FIGURE 6.
Chemotactic activity of mBD4:Ig and hBD2:Ig for human CCR6-expressing cells. The migration of mock-transfected HEK293 cells and HEK293 cells stably expressing human CCR6 in response to various concentrations of hCCL20 (a) and recombinant mBD4:Ig, hBD2:Ig, and hIgG (b) was tested using a 48-well chemotaxis chamber assay. The migration of HEK293 cells stably expressing human CCR6 in response to various concentrations of recombinant hBD2:Ig after pre-incubation with human CCL20 (100 ng/ml) (c) or in response to various concentrations of human CCL20 after pre-incubation with hBD2:Ig (d) was tested using a 48-well chemotaxis chamber assay as described under “Experimental Procedures.” Data are the means ± S.D. of one representative experiment of three, each done in triplicate. HPF, high power field.
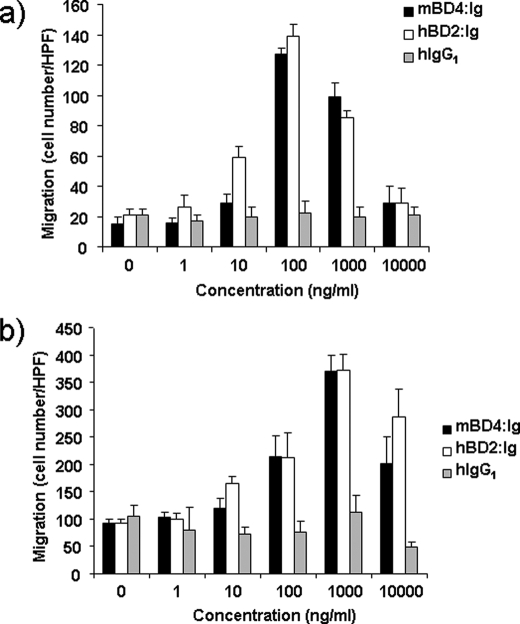
Furthermore, we analyzed the chemotactic activity of recombinant mBD4:Ig and hBD2:Ig for mouse resident peritoneal cells and human peripheral blood mononuclear cells. In line with previous experiments using mBD14:Ig (19), both mBD4:Ig and hBD2:Ig fusion proteins were chemotactic for freshly isolated mouse RPCs and for human PBMs (Fig. 7, a and b). Because these cells do not express CCR6, mBD4 and hBD2 presumably use at least one additional as yet undescribed chemotactic receptor besides CCR6.
FIGURE 7.
Chemotactic activity of mBD4:Ig and hBD2:Ig for freshly isolated mouse RPCs and human PBMs. The migration of freshly isolated mouse RPCs (a) and freshly isolated human PBMs in response to various concentrations of recombinant mBD4:Ig, hBD2:Ig and hIgG1 (b) was tested using a 48-well chemotaxis chamber assay as described under “Experimental Procedures.” Data are the means ± S.D. of one representative experiment of three, each done in triplicate. HPF, high power field.
DISCUSSION
We expressed mBD4 and hBD2 fused to the constant region of human IgG1 in Drosophila S2 cells. The secretion of these fusion proteins by S2 cells circumvents the problem of recovering a bactericidal polypeptide from bacterial host cells in sufficient biologically active amounts. In addition, correct folding is often a problem in polypeptides with a higher number of cysteine residues (20) and is achieved more readily using insect than bacterial cells. Although the antimicrobial activity of hBD2 either synthesized or produced in E. coli has been extensively tested using various Gram-positive and Gram-negative bacterial strains, fungi, and viruses (21–23), little is known about the antimicrobial activity of its mouse orthologue mBD4. The recombinant mBD4:Ig and hBD2:Ig fusion proteins both retained potent bactericidal activity against both Gram-positive and Gram-negative bacteria at physiological salt concentrations.
Taking advantage of the human IgG1-Fc tail fused to the β-defensin moiety, we tested the binding ability of these β-defensin fusion proteins to mCCR6- and hCCR6-positive cells by using an anti-human Fc specific antibody for detection. Mouse CCL20:Ig as well as mBD4:Ig and hBD2:Ig demonstrated specific binding to mCCR6-expressing cells. No binding was observed using the unfused hIgG1 protein or HEK293 cells that do not express mCCR6 or hCCR6. Binding specificity of the β-defensin fusion proteins was verified by the addition of equal amounts of human or mouse CCL20, resulting in competitive inhibition of binding to human or mouse CCR6, respectively. Although both human and mouse β-defensins bound mCCR6-expressing HEK293 cells, interestingly, mBD4:Ig failed to bind to cells expressing the human CCR6 receptor. The molecular basis of this species-specific binding to CCR6 is yet unclear and needs to be addressed in the near future. This to our knowledge is the first report demonstrating direct species-specific binding of mBD4:Ig and cross-species binding of hBD2:Ig to the chemokine receptor CCR6 as revealed by fluorescence-activated cell sorter analysis.
Several studies demonstrated that β-defensins can induce chemotactic activity by interacting with CCR6 and that the chemotactic activity can be inhibited by pertussis toxin, suggesting that this is mediated by Gα1 protein-coupled receptors (24). Additionally, it has been demonstrated that intramolecular disulfide bonding is important for the chemotactic activity of β-defensins and that alterations of the cysteine residues affects the chemotactic activity of the polypeptide (25). Recently, we were able to demonstrate that mBD14, the functional orthologue of human β-defensin 3 (hBD3), is chemotactic for cells expressing mCCR6 (19). These results were independently verified by Taylor et al. (16), demonstrating that single cysteine residues are needed for the chemotactic function of hBD3 and mBD14 but also that additional residues within the correct folded β-defensins are important for their chemotactic activity. Incorrect folding probably accounts for the recent report suggesting that β-defensins do not act through CCR6 (15). Our results clearly demonstrate that the specific binding of β-defensins to CCR6, as revealed by flow cytometry analysis, correlates with the induction of chemotactic activity in cells expressing CCR6. Furthermore, preincubation with the functional chemokine ligand for CCR6 specifically induced homologous desensitization and inhibited subsequent chemotactic responses to the tested β-defensins. However, the chemotactic activity of mBD4:Ig and hBD2:Ig toward freshly isolated primary mouse RPCs and human PBMs, as reported for other β-defensins, is independent of CCR6 as these cells do not express CCR6.
Consequently, both of these β-defensins use at least one additional chemotactic receptor besides CCR6. We have recently identified CCR2 as responsible for the chemotactic effect on these defensins on monocytes.3 The chemotactic activities of β-defensins predict that they contribute to the recruitment of immune cells to the sites of infection, thereby supporting innate and adaptive immune responses.
This work was authored, in whole or in part, by National Institutes of Health staff. This work was supported by Bayerische Forschungsstifung Grant PDOK-62-08 (to T. H.).
J. Röhrl, D. Yang, J. J. Oppenheim, and T. Hehlgans, unpublished observations.
- BD
- β-defensin
- PBM
- peripheral blood monocyte
- RPC
- resident peritoneal cells
- CCR6
- CC chemokine receptor 6.
REFERENCES
- 1.Lehrer R. I., Ganz T. (2002) Curr. Opin. Immunol. 14, 96–102 [DOI] [PubMed] [Google Scholar]
- 2.Jia H. P., Wowk S. A., Schutte B. C., Lee S. K., Vivado A., Tack B. F., Bevins C. L., McCray P. B., Jr. (2000) J. Biol. Chem. 275, 33314–33320 [DOI] [PubMed] [Google Scholar]
- 3.Rivas-Santiago B., Sada E., Tsutsumi V., Aguilar-Leon D., Contreras J. L., Hernandez-Pando R. (2006) J. Infect. Dis. 194, 697–701 [DOI] [PubMed] [Google Scholar]
- 4.Chong K. T., Thangavel R. R., Tang X. (2008) Virology 380, 136–143 [DOI] [PubMed] [Google Scholar]
- 5.Joly S., Organ C. C., Johnson G. K., McCray P. B., Jr., Guthmiller J. M. (2005) Mol. Immunol. 42, 1073–1084 [DOI] [PubMed] [Google Scholar]
- 6.Harder J., Bartels J., Christophers E., Schroder J. M. (2001) J. Biol. Chem. 276, 5707–5713 [DOI] [PubMed] [Google Scholar]
- 7.Harder J., Meyer-Hoffert U., Wehkamp K., Schwichtenberg L., Schröder J. M. (2004) J. Invest Dermatol. 123, 522–529 [DOI] [PubMed] [Google Scholar]
- 8.Harder J., Schröder J. M. (2005) Chem. Immunol. Allergy 86, 22–41 [DOI] [PubMed] [Google Scholar]
- 9.Fang X. M., Shu Q., Chen Q. X., Book M., Sahl H. G., Hoeft A., Stuber F. (2003) Eur. J. Clin. Invest. 33, 82–87 [DOI] [PubMed] [Google Scholar]
- 10.Tsutsumi-Ishii Y., Nagaoka I. (2003) J. Immunol. 170, 4226–4236 [DOI] [PubMed] [Google Scholar]
- 11.Yang D., Chertov O., Bykovskaia S. N., Chen Q., Buffo M. J., Shogan J., Anderson M., Schröder J. M., Wang J. M., Howard O. M., Oppenheim J. J. (1999) Science 286, 525–528 [DOI] [PubMed] [Google Scholar]
- 12.Yang D., Biragyn A., Kwak L. W., Oppenheim J. J. (2002) Trends Immunol. 23, 291–296 [DOI] [PubMed] [Google Scholar]
- 13.Biragyn A., Surenhu M., Yang D., Ruffini P. A., Haines B. A., Klyushnenkova E., Oppenheim J. J., Kwak L. W. (2001) J. Immunol. 167, 6644–6653 [DOI] [PubMed] [Google Scholar]
- 14.Conejo-Garcia J. R., Benencia F., Courreges M. C., Kang E., Mohamed-Hadley A., Buckanovich R. J., Holtz D. O., Jenkins A., Na H., Zhang L., Wagner D. S., Katsaros D., Caroll R., Coukos G. (2004) Nat. Med. 10, 950–958 [DOI] [PubMed] [Google Scholar]
- 15.Soruri A., Grigat J., Forssmann U., Riggert J., Zwirner J. (2007) Eur. J. Immunol. 37, 2474–2486 [DOI] [PubMed] [Google Scholar]
- 16.Taylor K., Clarke D. J., McCullough B., Chin W., Seo E., Yang D., Oppenheim J., Uhrin D., Govan J. R., Campopiano D. J., MacMillan D., Barran P., Dorin J. R. (2008) J. Biol. Chem. 283, 6631–6639 [DOI] [PubMed] [Google Scholar]
- 17.Hinrichsen K., Podschun R., Schubert S., Schröder J. M., Harder J., Proksch E. (2008) Antimicrob. Agents Chemother. 52, 1876–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishimoto H., Mukae H., Date Y., Shimbara T., Mondal M. S., Ashitani J., Hiratsuka T., Kubo S., Kohno S., Nakazato M. (2006) Eur. Respir. J. 27, 253–260 [DOI] [PubMed] [Google Scholar]
- 19.Röhrl J., Yang D., Oppenheim J. J., Hehlgans T. (2008) J. Biol. Chem. 283, 5414–5419 [DOI] [PubMed] [Google Scholar]
- 20.Piers K. L., Brown M. H., Hancock R. E. (1993) Gene 134, 7–13 [DOI] [PubMed] [Google Scholar]
- 21.Pazgier M., Hoover D. M., Yang D., Lu W., Lubkowski J. (2006) Cell. Mol. Life Sci. 63, 1294–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin C., Dang H. N., Gazor F., Huang G. T. (2006) Int. J. Antimicrob. Agents 28, 352–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vargues T., Morrison G. J., Seo E. S., Clarke D. J., Fielder H. L., Bennani J., Pathania U., Kilanowski F., Dorin J. R., Govan J. R., Mackay C. L., Uhrín D., Campopiano D. J. (2009) Protein Pept. Lett. 16, 668–676 [DOI] [PubMed] [Google Scholar]
- 24.Yang D., Chen Q., Chertov O., Oppenheim J. J. (2000) J. Leukoc. Biol. 68, 9–14 [PubMed] [Google Scholar]
- 25.Wu Z., Hoover D. M., Yang D., Boulègue C., Santamaria F., Oppenheim J. J., Lubkowski J., Lu W. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 8880–8885 [DOI] [PMC free article] [PubMed] [Google Scholar]