Abstract
Calreticulin (CRT), a chaperone and Ca2+ regulator, enhances wound healing, and its expression correlates with fibrosis in animal models, suggesting that CRT regulates production of the extracellular matrix. However, direct regulation of collagen matrix by CRT has not been previously demonstrated. We investigated the role of CRT in the regulation of fibrillar collagen expression, secretion, processing, and deposition in the extracellular matrix by fibroblasts. Mouse embryonic fibroblasts deficient in CRT (CRT−/− MEFs) have reduced transcript levels of fibrillar collagen I and III and less soluble collagen as compared with wild type MEFs. Correspondingly, fibroblasts engineered to overexpress CRT have increased collagen type I transcript and protein. Collagen expression appears to be regulated by endoplasmic reticulum (ER) calcium levels and intracellular CRT, because thapsigargin treatment reduced collagen expression, whereas addition of exogenous recombinant CRT had no effect. CRT−/− MEFs exhibited increased ER retention of collagen, and collagen and CRT were co-immunoprecipitated from isolated cell lysates, suggesting that CRT is important for trafficking of collagen through the ER. CRT−/− MEFs also have reduced type I procollagen processing and deposition into the extracellular matrix. The reduced collagen matrix deposition is partly a consequence of reduced fibronectin matrix formation in the CRT-deficient cells. Together, these data show that CRT complexes with collagen in cells and that CRT plays critical roles at multiple stages of collagen expression and processing. These data identify CRT as an important regulator of collagen and suggest that intracellular CRT signaling plays an important role in tissue remodeling and fibrosis.
Keywords: Calcium, Cell/Trafficking, Chaperones, Extracellular Matrix/Collagen, Extracellular Matrix/Fibronectin, Protein/Processing, Transcription, Calreticulin
Introduction
Calreticulin (CRT),3 also known as the C1q receptor, is an endoplasmic reticulum (ER) chaperone, and a modulator of intracellular calcium signaling. CRT also is a multifunctional protein that is present in numerous cellular compartments, including the ER, cytoplasm, nucleus, at the cell surface, and as a released protein (1–5). There is evidence to suggest the involvement of CRT in tissue remodeling and wound healing. In a porcine dermal wound healing model, topical application of purified CRT increases the rate of wound healing and wound tensile strength (6). Proteomic data show up-regulation of CRT expression with fibrosis in a rat model of unilateral ureteric obstruction kidney fibrosis and in a mouse model of bleomycin-induced lung fibrosis (7). The mechanisms by which CRT regulates tissue remodeling are not well understood, although fibronectin matrix deposition and modulation of cell adhesion, motility, proliferation, and matrix metalloproteinase expression have been implicated (6, 8–13).
CRT has effects on the extracellular matrix (ECM) and cellular responses to the ECM. Fibroblasts overexpressing CRT have increased fibronectin mRNA, protein, and matrix deposition, and cells lacking CRT express less fibronectin than wild type cells (9, 14). CRT in the ER is thought to regulate the fibronectin matrix through regulation of intracellular calcium signaling (9, 14). In addition, CRT knock-out MEFs have differences in matrix metalloproteinase expression that are regulated through the ERK and phosphoinositide 3-kinase pathways (10). Cytoplasmic CRT stabilizes integrin-mediated adhesion to collagen through binding the cytoplasmic tail of the integrin α subunit and through calcium signaling (15–18). Finally, cell surface or extracellular CRT can potentially modulate ECM remodeling through direct binding to ECM molecules and to cell adhesion receptors, including collagen types I, III, and V, thrombospondin, α2β1 integrin, and glycoprotein VI (18–20).
CRT has multiple cellular functions. CRT is an ER chaperone for N-linked glycoproteins in the CRT/calnexin cycle (21). It also acts as a classical chaperone for nonglycosylated proteins by preventing protein aggregation in an in vitro model (22). CRT is an important regulator of Ca2+ homeostasis within the ER (23, 24). Total CRT expression is up-regulated by many forms of cellular stress, including amino acid deprivation, depletion of Ca2+ stores, oxidative stress, and hypoxia (25–29). Despite its lack of a transmembrane domain, CRT is on the surface of many cell types, including fibroblasts, endothelial cells, and apoptotic cells. From the cell surface, CRT signals multiple cellular processes, including apoptotic clearance of cells, focal adhesion turnover, proliferation, migration, and anoikis resistance (4, 13, 20, 30–33). Many of these responses to cell surface CRT are mediated by CRT binding to LRP1 (low density lipoprotein receptor-related protein 1) (13, 33–35). Recent studies from our laboratory show that engagement of the CRT-LRP1 complex on the cell surface by thrombospondin-1 stimulates fibrillar collagen expression in vitro and in vivo.4 In vitro, exogenous CRT treatment causes proliferation of fibroblasts, endothelial cells, and keratinocytes and increases keratinocyte and fibroblast migration (6).
Given evidence of CRT function in wound healing and its expression in fibrotic tissues, we addressed the role of CRT in regulating fibrillar collagen expression by fibroblasts. Collagen is a major component of the ECM, important for both the provisional matrix during wound healing and in pathologic tissue remodeling in fibrosis (36). Collagen synthesis and deposition are regulated at multiple levels, including transcription, secretion, processing, incorporation into fibrils, and degradation. In this study, we used mouse embryonic fibroblasts deficient in CRT and mouse fibroblasts engineered to overexpress CRT to examine the role of CRT in regulation of fibrillar collagen expression, trafficking, and incorporation into the ECM. These studies show that type I collagen transcription, protein expression, transit through the ER, and incorporation of collagen into the ECM are regulated by CRT expression. Furthermore, we provide evidence for CRT affecting collagen through its role in regulation of ER Ca2+ levels and by mediating protein transit through the ER. In addition, CRT has indirect effects on collagen matrix deposition as a consequence of its role in regulation of fibronectin expression. These data are the first to demonstrate the importance of CRT on multiple processes critical for collagen matrix production.
EXPERIMENTAL PROCEDURES
Materials
Dulbecco's modified Eagle's medium (DMEM) with 4.5 g/liter glucose was purchased from Invitrogen. Ascorbic acid and thapsigargin were purchased from Sigma. Vectashield was purchased from Vector Laboratories (Burlingame, CA), and goat serum was purchased from MP Biomedicals (Solon, OH). Recombinant rabbit calreticulin was a gift from Dr. Leslie Gold (New York University) (6). Calreticulin was stored in 10 mm Tris, 3 mm CaCl2, pH 7.0. Human fibronectin and mouse anti-GM130 antibody were purchased from BD Biosciences. Rabbit anti-CRT (SPA-600), mouse anti-CRT (SPA-601), and rabbit anti-calnexin (SPA-860) were purchased from StressGen (Ann Arbor, MI). Rabbit anti-mouse type I collagen was purchased from MD Biosciences (MD20151, Zurich, Switzerland). Rabbit anti-ERK1/2 was purchased from Cell Signaling (p44/42 MAPK, catalog no. 9102, Beverly, MA). Mouse monoclonal antibody to HSP47 (M16.10A1) was purchased from Abcam (Cambridge, MA). Goat anti-prolyl 4-hydroxylase α-1 antibody was purchased from Novus Biologicals (NB100-57852, Littleton, CO). Rabbit anti-β-tubulin, mouse anti-HA (sc-7392), and goat anti-collagen type I (C-18) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). AlexaFluor 488 goat anti-rabbit IgG, AlexaFluor 488 donkey anti-goat IgG, Texas Red donkey anti-rabbit, AlexaFluor 543 goat anti-mouse IgG, and Hoechst 33342 were purchased from Invitrogen. Rabbit TrueBlotTM HRP anti-rabbit and mouse TrueBlotTM HRP anti-mouse secondary antibodies were purchased from eBioscience (San Diego). Coomassie Plus protein assay reagents were purchased from Pierce. Western Lightning Chemiluminescence Reagent Plus was purchased from PerkinElmer Life Sciences, and ReBlot strong stripping solution was purchased from Chemicon (Billerica, MA). Peptides uses are as follows: CRT-binding TSP1 peptide, hep I (ELTGAARKGSGRRLVKGPD), and control peptide, modified hep I, (ELTGAARAGSGRRLVAGPD) were purchased from AnaSpec, Inc. (San Jose, CA).
Cells
Wild type mouse embryonic fibroblasts (MEFs), calreticulin-null (CRT−/− MEFs), and calreticulin-null MEFs stably transfected with the pcDNA3 expression vector to express rabbit HA-tagged CRT (37) were gifts from Dr. Marek Michalak (University of Alberta, Edmonton, Alberta, Canada). The HA-CRT-reconstituted CRT−/− cells were shown to express HA-tagged CRT and to respond to the CRT-binding peptide of TSP1 in focal adhesion disassembly assays (data not shown). Mouse L fibroblasts (CRT underexpressors, parental cells, and CRT overexpressors) were provided by Dr. Michal Opas (University of Toronto) and Dr. Marek Michalak. These lines were engineered to express either twice the parental levels of CRT (CRT overexpressors) or half of the parental level of CRT expression (CRT underexpressors), as well as the parental L fibroblasts (parental cells) as described previously (38). Cells were maintained in high glucose DMEM with 10% FBS and 2 mm l-glutamine. Mouse L fibroblasts also were cultured in the presence of 100 μg/ml G418 sulfate (Cellgro). Cells routinely were assayed for mycoplasma using the MycoAlert® mycoplasma detection kit (Lonza).
Quantitative Real Time PCR
MEFs were grown in DMEM with 10% FBS and 2 mm l-glutamine for 48 h and then in DMEM with 0.5% FBS and 2 mm l-glutamine for 12 h with or without treatment. RNA was harvested after 12 h in 0.5% FBS using TRIzol® reagent. Mouse L fibroblasts were grown to confluence in 10% FBS before RNA was harvested. RNA was isolated according to the manufacturer's instructions. Quantitative real time PCR was performed using standard protocols with an ABI7500 (Applied Biosystems). The α2(I) chain of collagen I (Col1A2), α1(III) chain of collagen III (Col3A1), thrombospondin-1 (Thbs1), and mitochondrial ribosomal protein S9 (housekeeping gene) were assayed using TaqMan gene expression assay primers designed and optimized by Applied Biosystems. Col1A2 primer ID is Mm01165187_m1; Col3A1 primer ID is Mm00802331_m1; Thbs1 primer ID is Mm01335418_m1; and S9 primer ID is Mm00469845_m1. Col1A2, Col3A1, and Thbs1 levels were normalized to S9 levels. Results are expressed as the mean ± S.D. of 3–8 samples (indicated in figure legend) assayed in triplicate.
Soluble Collagen Assays
Wild type and CRT−/− MEFs were cultured for 48 h in DMEM supplemented with 10% FBS and 2 mm l-glutamine (Glutamax, Invitrogen). Cells were then switched to DMEM with 0.5% FBS, and cells were cultured for another 72 h. Cells were dosed daily with treatments in DMEM with 0.5% FBS. Mouse L fibroblasts were cultured under the same conditions, except that these cells were plated on wells coated with fibronectin and cultured in the presence of 100 μg/ml G418. Conditioned medium was collected in the presence of protease inhibitor mixture (Sigma) and centrifuged at 15,000 × g for 5 min to remove cell debris. For measurement of soluble collagens, the SircolTM assay was used (Biocolor, Ireland). Two hundred microliters of medium were added to 1 ml of SircolTM reagent containing Sirius Red in picric acid. Conditioned medium and SircolTM reagent were rotated for 30 min at room temperature and then pelleted at 12,000 × g for 15 min at room temperature. Excess dye was removed from the tube, and the pellet was reconstituted in the provided alkali reagent. Absorbance was read at 540 nm using a plate reader. A standard curve was made using rat tail collagen (provided by manufacturer). The concentration of soluble collagen in the conditioned media was determined from the standard curve. Results were normalized to cell number determined by counting of trypsinized cells using a hemocytometer.
Protein Concentration Assay
Cells were cultured for 24 h in DMEM with 10% FBS and 2 mm l-glutamine. Cells were then switched to serum and phenol-red free DMEM with 2 mm l-glutamine for 24 h. Conditioned medium was then collected with protease inhibitors, centrifuged to pellet cells, and measured for protein concentration. Protein concentration was measured using the Coomassie Plus reagent (Pierce) according to the manufacturer's instructions and normalized to cell number as determined using a hemocytometer.
Deoxycholate Separation of Cell and Extracellular Matrix Fractions
Cells were cultured for 48 h in DMEM with 10% FBS. Cells were then treated daily over a 72-h period with treatments in DMEM with 0.5% FBS. The protocol for extraction of the deoxycholate (DOC)-insoluble ECM was modified from Midwood et al. (39). Conditioned media were removed, and the cells and ECM were washed with PBS. Cells and ECM were harvested by scraping with 300 μl of 4% DOC (4% DOC in 20 mm Tris-HCl, pH 8.8, with 200 microunits/ml DNase and protease inhibitors), and the extract was homogenized with a 27½-gauge needle. The extract was pelleted at 17,000 × g for 30 min at 4 °C. The supernatant was kept as the DOC-soluble fraction. The pellet was washed with 4% DOC and pelleted again to generate the DOC-insoluble fraction. The DOC-soluble fraction contains cellular material and components not incorporated into the ECM, whereas the DOC-insoluble pellet contains ECM (39). The DOC-insoluble fraction was resolubilized in 1% SDS, 25 mm Tris-HCl, pH 8.0, plus protease inhibitors for analysis by SDS-PAGE and immunoblotting.
SDS-PAGE and Immunoblotting for Collagen in Cell and ECM Fractions
Immunoblotting was performed as described previously (20, 40, 41). Briefly, the total DOC-insoluble fraction and 1/10th of the DOC-soluble fraction were separated by SDS-PAGE (4–15% gradient gel) under reducing conditions. Proteins were transferred to nitrocellulose and blocked with 1% casein, and membranes were probed with rabbit anti-mouse collagen I (MD Biosciences). The anti-collagen I antibody recognizes the procollagen α1(I) chain with the N- and C-propeptides, the pC-propeptide chain (pC-α1(I)), and the α1(I) chain with both the N- and C-propeptides cleaved (42, 43). Membranes were washed with TBS-T (10 mm Tris-HCl, pH 7.6, 100 mm NaCl, and 0.1% Tween 20), probed with appropriate secondary antibody, and developed using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences). Membranes were stripped with ReBlot strong (Chemicon) and reprobed rabbit anti-total ERK (Cell Signaling) to show separation and equal cellular material.
Immunofluorescence
Coverslips were washed by tumbling with 10% SDS with subsequent submersion in 0.5% Triton X-100, deionized water, and stored in 70% ethanol. Cells were plated on coverslips in a 24-well plate at 10,000 cells/well. Cells were cultured in DMEM with 10% FBS and 2 mm l-glutamine for 36 h and then switched to DMEM with 0.5% FBS with or without daily a treatment of 20 μm ascorbic acid. After 48 h, cells were fixed in acetone at −20 °C for 1 min. Cells were blocked in 2.5% goat serum + 4% bovine serum albumin for 10 min at room temperature. Primary antibody was rabbit polyclonal anti-mouse type I collagen used at 1:200 for 2 h at room temperature. For co-localization studies, cells were fixed as described above and blocked with 0.1% fish skin gelatin + 4% bovine serum albumin for 20 min at room temperature. Rabbit anti-type I collagen (1:200) was incubated simultaneously with mouse anti-GM130 IgG (1:200) for Golgi co-localization. Goat anti-type I collagen (1:200) was incubated simultaneously with rabbit anti-calnexin (1:200) as an ER marker for 2 h at room temperature. Secondary antibodies were AlexaFluor 488 goat anti-rabbit IgG and AlexaFluor 543 goat anti-mouse IgG (for Golgi), AlexaFluor 488 donkey anti-goat IgG, and Texas Red donkey anti-rabbit IgG (for ER), all used at 1:300 for 1 h at room temperature. Nuclei were stained using Hoechst 33342. Coverslips were mounted with Vectashield. For analysis of cell collagen and collagen fibrils, cells were imaged using a Nikon Eclipse TE2000-U inverted microscope. Exposure time was below the background threshold of a nonimmune IgG control. Exposure time and intensity range were the same for each image. Scale bar equals 50 μm. Contrast-adjusted post-imaging was equal for all images. For co-localization studies, cells were imaged using a Leitz Orthoplan upright digital microscope. Intensity ranges were the same for each image. Original magnification was ×63. Contrast-adjusted post-imaging was equal for all images.
Focal Adhesion Disassembly Assay
Focal adhesions were measured as described previously (44). For treatments, cells were serum-starved for 30 min, incubated with recombinant CRT (1 μm) for 30 min, and then incubated with the hep I peptide or the modified hep I control peptide (10 μm) for 30 min.
Thapsigargin Treatment
Cells were cultured for 48 h in DMEM supplemented with 10% FBS and 2 mm l-glutamine. Cells were rinsed with PBS and treated for 2 h with 100 nm thapsigargin or vehicle (DMSO) in serum-free medium. Cells were rinsed with PBS and then incubated in DMEM with 10% FBS and 2 mm l-glutamine for 8 h. Cells were washed with PBS, and total cell lysates were obtained as described below.
Total Protein Isolation for Immunoblotting
Total cell lysates were obtained by scraping cells and ECM into Laemmli sample buffer (Bio-Rad) with protease inhibitor (Sigma) and 5% β-mercaptoethanol, sonicated for 5 s, and boiled at 100 °C for 7 min. Equal volumes of sample were loaded onto a 4–15% gradient gel (for type I collagen) or 10% SDS-polyacrylamide gel for CRT, HSP47, and prolyl 4-hydroxylase. Immunoblotting was performed as described previously (20) and above. Rabbit anti-β-tubulin (Santa Cruz Biotechnology) was used for normalization.
Immunoprecipitation
Cells were grown for 2–3 days in DMEM with 10% FBS and 2 mm l-glutamine until confluency. Cells were trypsinized, pelleted, and washed three times with Dulbecco's phosphate-buffered saline with 2 mm l-glutamine. Cells were lysed with 1% Nonidet P-40, 150 mm NaCl, 50 mm Tris, pH 8, with protease inhibitor, and lysates were passed seven times through a 27½-gauge needle. Protein concentration was determined as described above. GammaBindTM G-SepharoseTM beads (GE Healthcare) were blocked overnight at 4 °C in 0.1% ovalbumin in DMEM and then incubated with 10 μg of mouse anti-CRT (StressGen, SPA-601), 10 μg of mouse anti-HA (Santa Cruz Biotechnology), 6 μg of rabbit anti-mouse type I collagen (MD Biosciences), 10 μg of mouse IgG, or 6 μg of rabbit IgG for 1 h at 4 °C in DMEM with 0.1% ovalbumin and 0.1% Triton X-100 (DTO). Beads were washed three to four times with DTO. Cell lysates were precleared with GammaBindTM G-SepharoseTM beads and then incubated with equal amounts of protein from cell lysates for 1 h at 4 °C. Beads were washed four times with DTO, and bound proteins were eluted with reducing Laemmli sample buffer and boiled. Samples were run on an 8% SDS-PAGE and immunoblotted as described, except that the secondary antibodies used for immunoblotting were rabbit TrueBlotTM HRP anti-rabbit IgG and mouse TrueBlotTM HRP anti-mouse IgG.
Fibronectin Substrate Preparation
When indicated, mouse L fibroblasts and MEFs were plated on wells coated with human fibronectin. Wells of a 6-well plate were coated with 1 ml of 10 μg/ml fibronectin in PBS with Mg2+ and Ca2+ for at least 1 h. Excess liquid was removed before cells were plated.
Statistics
The replicates for each SircolTM graph are stated in the figure legends, and results are expressed as the means ± S.D. Quantitative real time PCR graphs represent the means ± S.D. of 3–5 or 8 experiments each performed in triplicate. Data were analyzed for statistical significance using one-way analysis of variance. p < 0.05 was considered significant.
RESULTS
Calreticulin Expression Correlates with Collagen Transcript and Protein Levels
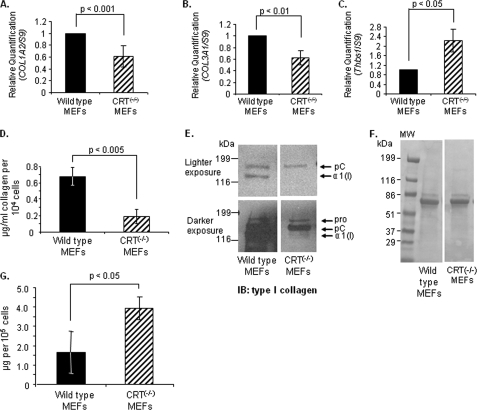
Wild type and CRT knock-out MEFs were compared for relative transcript levels of types I and III collagen using quantitative real time PCR. Transcript levels of both type I and III collagen in CRT−/− MEFs were about 60% of levels measured in wild type MEFs (Fig. 1, A and B). To determine whether all ECM proteins are similarly regulated by CRT expression, transcript levels of TSP1, another ECM protein involved in tissue remodeling, were measured (45). In contrast to collagen, TSP1 was increased in the CRT−/− MEFs (Fig. 1C). To further investigate the role of CRT in regulating collagen expression, we compared soluble collagen protein in the wild type MEFs and CRT−/− MEFs. Fibroblasts were cultured, and conditioned media were collected over the last 72 h for measurement of soluble collagens using the SircolTM assay. CRT−/− MEFs had significantly reduced levels of soluble collagen protein in the conditioned medium as compared with wild type MEFs (Fig. 1D). Because the SircolTM assay measures all soluble fibrillar collagens, conditioned media from wild type and CRT−/− MEFs were analyzed specifically for type I collagen by immunoblotting (Fig. 1E). Immunoblot analysis showed increased type I collagen in the conditioned medium of wild type MEFs as compared with knock-out cells, despite loading of equivalent amounts of protein as determined by Ponceau S staining (Fig. 1F).
FIGURE 1.
Cells deficient in CRT expression have reduced collagen transcript and soluble collagen. A–C, wild type and CRT−/− MEFs were cultured in 10% FBS for 48 h and then 12 h in 0.5% FBS. RNA was harvested, and transcript levels of type I collagen, Col1A2 (A), type III collagen, Col3A1 (B), and Thbs1 (C) were measured by quantitative real time PCR. Values represent the mean expression levels normalized to S9 expression ± S.D. (for Col1A2, n = 8; for Col3A1, n = 5; for Thbs1, n = 4; each “n” was performed in triplicate). Values for wild type cells were set to 1. D–F, wild type and CRT−/− MEFs were cultured in 10% FBS for 48 h and then 72 h in 0.5% FBS. Conditioned media were collected after the 72 h. D, conditioned media were assayed for levels of soluble collagen using the SircolTM assay. Soluble collagen levels were normalized to cell number. Results are expressed as the mean ± S.D. (n = 3 separate experiments performed in triplicate). E, equal volumes of conditioned media were immunoblotted (IB) for type I collagen. A representative blot is shown with a lighter exposure to visualize collagen in the wild type MEFs and a darker exposure to visualize collagen in the CRT−/− MEFs. All four panels are from the same membrane. A representative blot is shown (n = 3 separate experiments). The three forms of the α(I) chain of collagen I recognized by the antibody are indicated as follows: Pro is the unprocessed form with the N- and C-propeptides; pC is collagen with the N-propeptide cleaved; and α1(I) is the fully processed α(I) band. F, membrane from E was stained with Ponceau S to demonstrate protein loading. G, cells were grown for 24 h in 10% FBS and then for 24 h in serum-free and phenol-free DMEM. Conditioned media were collected in the presence of protease inhibitors and centrifuged, and the supernatant was measured for total protein by the Bradford assay. Total protein was normalized to cell number.
To determine whether this decrease in soluble collagen protein expression in CRT−/− MEFS reflects a general defect in protein synthesis or secretion due to CRT deficiency, conditioned media from wild type and CRT−/− cells were assayed for levels of total soluble protein relative to cell number. Interestingly, there is more protein in the conditioned medium of CRT−/− MEFs than in the wild type MEFs (Fig. 1G). This finding was confirmed by analysis of secreted proteins evaluated on silver-stained polyacrylamide gels (data not shown). Together, these results show that type I collagen transcript and protein are reduced in the absence of CRT, despite normal to elevated levels of overall protein expression and secretion.
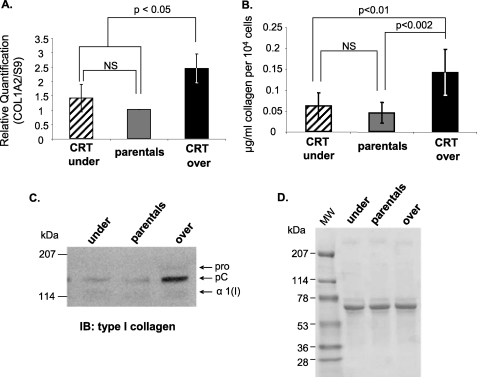
Because increased CRT expression has been reported in fibrotic diseases characterized by excessive collagen production (7), we asked whether overexpression of CRT increases collagen transcript and protein expression. We used mouse L fibroblasts engineered to express either half (CRT underexpressors) or twice parental fibroblast levels of CRT (CRT overexpressors) (38). Transcript levels of type I collagen were evaluated in these variable CRT-expressing fibroblasts using quantitative real time PCR. CRT overexpressors have 2.4-fold higher levels of Col1A2 transcript as compared with parental cells (Fig. 2A). There were no significant differences in transcript levels between the CRT underexpressors and the parental cells. These results demonstrate that elevated CRT expression correlates with increased transcription of type I collagen.
FIGURE 2.
Increased calreticulin expression correlates with increased collagen transcription and expression. A, RNA was harvested from confluent CRT underexpressors, parental cells, and CRT overexpressors. Transcript levels of type I collagen, Col1A2, were analyzed by quantitative real time PCR. Values represent the mean expression levels normalized to S9 expression ± S.D., and values for wild type cells were set to 1 (n = 4, each performed in triplicate). NS, not significant. B–D, cells were cultured on 10 μg/ml fibronectin substrata in 10% FBS for 48 h and then in 0.5% FBS for 72 h. Conditioned media were collected after the 72 h. B, conditioned media from CRT underexpressors, parentals, and CRT overexpressors were assayed for levels of soluble collagen using the SircolTM assay. Soluble collagen levels were normalized to cell number. Results are expressed as the means ± S.D. (underexpressors, n = 6 obtained from three separate experiments; parentals, n = 7 obtained from three separate experiments; overexpressors, n = 4 obtained from two separate experiments). C, equal volumes of conditioned media were immunoblotted (IB) for type I collagen. A representative blot is shown (n = 3 separate experiments). D, membrane from C was stained with Ponceau S to demonstrate equal loading.
Soluble collagen in the conditioned media was evaluated using the SircolTM assay and by immunoblot analysis. CRT overexpressors have increased levels of soluble fibrillar collagens and of type I collagen protein as compared with the parental cells (Fig. 2, B and C). This increase in collagen is observed despite loading of equivalent protein as measured by Ponceau S (Fig. 2D). Consistent with transcript levels, there were no significant differences in soluble collagen levels between CRT underexpressors and parental cells. The combined results obtained from studies with CRT overexpressing and CRT−/− cells suggest that the level of CRT expression by cells is a determining factor in regulating type I collagen transcript and protein in the conditioned media.
Calreticulin Deficiency Is Associated with Increased Intracellular Retention of Type I Collagen, Altered Processing, and Reduced Deposition in the ECM
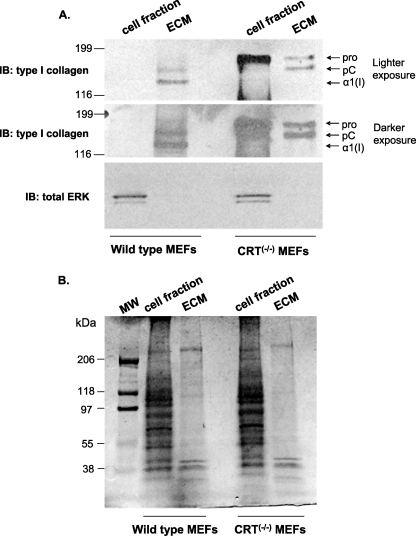
Secretion of collagen and incorporation into the ECM are critical for matrix formation and remodeling. To further investigate the role of CRT in collagen secretion and deposition, we examined the effect of CRT expression on incorporation of type I collagen into the deoxycholate detergent-insoluble ECM. Deoxycholate ECM extraction is a well established method of separating cellular and ECM fractions (39). Analyses of the detergent-insoluble ECM of wild type and CRT−/− MEFs showed that CRT−/− MEFs had decreased type I collagen deposition in the deoxycholate-insoluble ECM as compared with the wild type MEFs (Fig. 3A). Interestingly, despite reduced levels of collagen transcript and soluble protein, CRT−/− MEFs show increased levels of intracellular type I collagen in the detergent-soluble fraction as compared with wild type MEFs (Fig. 3A), suggesting that there is an additional defect in the CRT−/− cells that alters collagen trafficking and secretion. Total protein detected by Coomassie Blue-stained SDS-polyacrylamide gels of the detergent-soluble and -insoluble fractions of wild type and CRT−/− MEFs does not show apparent differences between the two cell types (Fig. 3B), indicating that collagen is specifically regulated by the absence of CRT. Furthermore, CRT−/− MEFs have increased staining for intracellular type I collagen with no apparent collagen matrix fibrils (Fig. 4B), although there is comparatively little intracellular type I collagen staining and more staining for collagen in short extracellular fibrils in the wild type MEFs (Fig. 4A).
FIGURE 3.
Absence of CRT expression reduces type I collagen secretion and ECM incorporation. Wild type and CRT−/− MEFs were cultured for 48 h in 10% FBS and then 72 h in 0.5% FBS. Deoxycholate was used to separate the cell fraction (DOC-soluble) from the ECM fraction (DOC-insoluble). The entire ECM fraction and 1/10th of the cell fraction were separated by SDS-PAGE. A, after transfer, membrane was immunoblotted (IB) for type I collagen. Blots were stripped and reprobed with an antibody to total ERK. A representative blot is shown (n = 3 separate experiments). B, gel was stained with Coomassie Blue to detect total protein in each sample.
FIGURE 4.
CRT−/− MEFs have reduced collagen fibril formation and increased intracellular retention of type I collagen. Wild type and CRT−/− MEFs were cultured on treated coverslips for 36 h in 10% FBS and then for 48 h in 0.5% FBS with or without daily treatments of 20 μm ascorbic acid. Type I collagen was detected with rabbit anti-type I collagen antibody. Exposure time and intensity range were the same for each image. Contrast adjusted post-imaging was equal for all images. Scale bar, 50 μm. Nuclei were detected by Hoechst staining and photographed, and the number of nuclei in each image was counted to evaluate relative cell number. A, wild type MEFs with no treatment, 75 nuclei. B, CRT−/− MEFs with no treatment, 87 nuclei. C, wild type MEFs with ascorbic acid, 69 nuclei. D, CRT−/− MEFs with ascorbic acid, 93 nuclei.
Following secretion of procollagen, the molecule is processed by cleavage of the N- and C-terminal propeptides, which is required for proper fibril assembly (46). The type I collagen in the detergent-insoluble ECM of CRT−/− MEFs shows an absence of the fully processed form of type I collagen, although both uncleaved and N-terminal cleaved forms are present (Fig. 3A). Soluble type I collagen in the conditioned medium of CRT−/− MEFs also is not fully processed (Fig. 1E). This suggests that a CRT deficiency leads to altered collagen processing, possibly involving defects in C-terminal propeptide cleavage.
Exogenous CRT Treatment Does Not Increase Collagen Expression
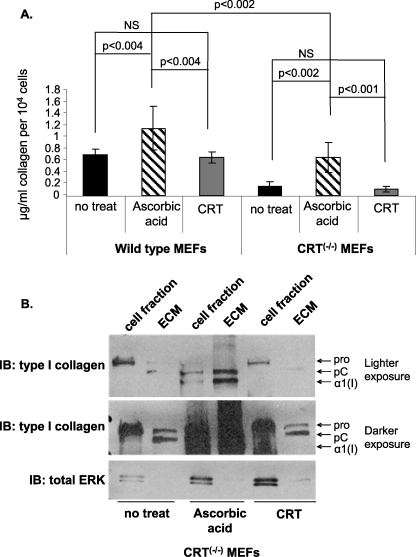
Exogenous CRT can bind to the cell surface and mediate TSP1-stimulated focal adhesion disassembly and increase type I collagen production4 (12). In addition, exogenous CRT treatment enhances wound healing and increases proliferation and migration of fibroblasts (6). Therefore, we asked whether the differences in collagen between the wild type and CRT−/− MEFs were due to intracellular CRT or extracellular/cell surface CRT. Neither wild type nor CRT−/− MEFs treated with recombinant CRT showed increased soluble collagen production in the conditioned media as compared with untreated cells (Fig. 5A). Ascorbic acid treatment increases collagen production and was used as a positive control (36, 47–49). Furthermore, there was no increase in type I collagen in the detergent-insoluble ECM when CRT−/− MEFs were treated with exogenous CRT (Fig. 5B). To verify that the recombinant CRT was functional and can bind to the surface of CRT−/− MEFs, we investigated its ability to mediate TSP1-stimulated focal adhesion disassembly (12). CRT−/− MEFs were incubated with recombinant CRT and then stimulated with the CRT-binding TSP1 peptide, followed by assessment of focal adhesion disassembly. Consistent with previous studies (12), this preparation of recombinant CRT also mediates focal adhesion disassembly (data not shown). Therefore, these data suggest that intracellular CRT, rather than extracellular or cell surface-bound CRT, is primarily involved in regulating collagen expression by these fibroblasts.
FIGURE 5.
Exogenous CRT does not increase soluble collagen or type I collagen deposition into the ECM. A and B, wild type and CRT−/− MEFs were cultured for 48 h in 10% FBS and then treated daily with vehicle control (no treat), 1 μm recombinant CRT, or 20 μm ascorbic acid in DMEM with 0.5% FBS for 72 h. A, conditioned media were assayed for levels of soluble collagen using the SircolTM assay and normalized to cell number. Results are expressed as the mean value ± S.D. (Wild type no treat, wild type + ascorbic acid, CRT−/− MEFs + CRT, CRT−/− MEFs + ascorbic acid: n = 4 from three different experiments; wild type + CRT: n = 3 from two experiments; CRT−/− MEFs no treat, n = 5 from four experiments.) NS, not significant. B, type I collagen deposition was measured by immunoblot (IB) of the DOC-soluble and -insoluble fractions. The entire ECM fraction and 1/10th of the cell fraction were separated by 4–15% SDS-PAGE and immunoblotted for type I collagen. Membranes were stripped and reprobed with an antibody to total ERK. A representative blot for collagen is shown (n = 4 separate experiments).
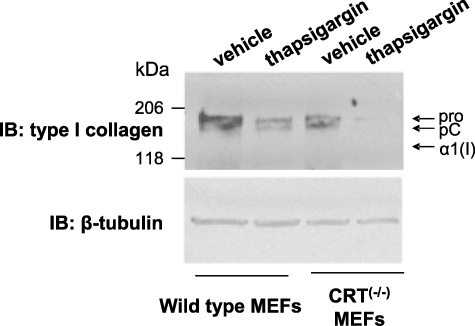
Intracellular Ca2+ Regulates Type I Collagen Expression
Intracellular CRT is a well known regulator of Ca2+ levels within the ER. Increased CRT expression correlates with increased Ca2+ stores in the ER and increased Ca2+ release after stimulation (50). In contrast, CRT−/− MEFs have reduced ER Ca2+ and a lower capacity to store Ca2+ in the ER (37). A high Ca2+ concentration in the ER has been suggested to be necessary for the proper chaperoning of collagen (51). To determine whether CRT regulation of ER Ca2+ release is involved in regulation of collagen expression in these cells, wild type and CRT−/− MEFs were treated for 2 h with 100 nm thapsigargin, an inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, which decreases ER Ca2+ stores available for release. Cells were then returned to growth medium for 8 h (9). Thapsigargin treatment (100 nm) decreased levels of type I collagen in cell lysates in both cell lines (Fig. 6), although the CRT−/− MEFs were more sensitive to changes in calcium, having a proportionally greater decrease in collagen production after treatment. Because of the reduced total Ca2+ levels and the reduced ability to buffer ER Ca2+, the CRT−/− MEFs appear to be more sensitive to Ca2+-dependent regulation of collagen. These data indicate that regulation of collagen expression by CRT potentially involves CRT regulation of ER Ca2+ stores.
FIGURE 6.
Type I collagen expression is dependent on ER Ca2+. Wild type and CRT−/− MEFs were cultured for 48 h in 10% FBS and then treated for 2 h with 100 nm thapsigargin or vehicle control (DMSO) in serum-free medium. Then cells were rinsed and cultured for 8 h in DMEM with 10% FBS. Cells and ECM (total cell lysate) were harvested in reducing Laemmli sample buffer. Equal volumes of cell lysates were separated by 4–15% SDS-PAGE, transferred to nitrocellulose, and immunoblotted (IB) for type I collagen. Blots were stripped and reprobed for β-tubulin. A representative blot is shown (n = 5 in two separate experiments).
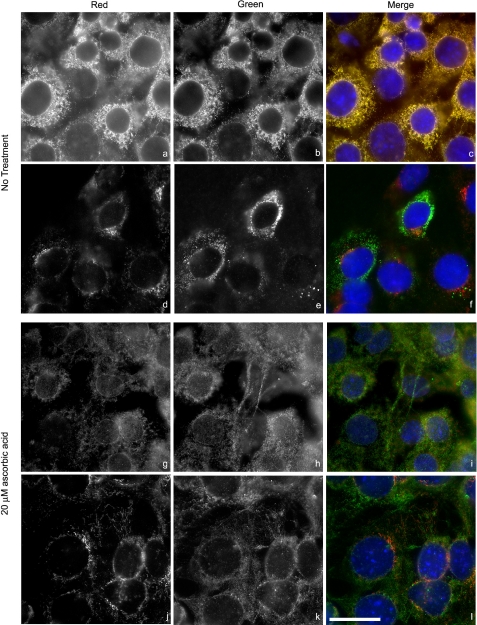
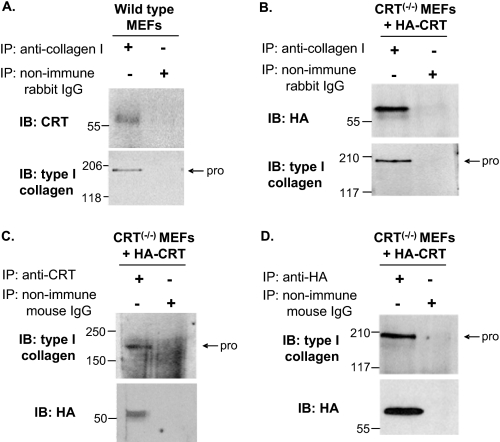
CRT Plays a Role in Type I Collagen Retention in the ER and Associates with Procollagen within the Cell
Intracellular CRT is a key factor involved in mediating protein folding in the ER, an important step for protein trafficking from the ER (50, 52, 53). Based on our observations that CRT−/− cells have increased intracellular pools of collagen, we asked whether the increase in intracellular collagen in the CRT−/− MEFs might reflect an inability to transport collagen out of the ER. It is documented that incorrectly folded proteins are retained in the ER in calreticulin-deficient cells (52). Indeed, collagen co-localized with calnexin, a marker of the ER and a CRT partner in the calnexin-CRT cycle of protein folding (53), in the CRT−/− MEFs but had minimal localization in the Golgi as detected by staining for the Golgi marker GM130 (Fig. 7, a–f). The increased retention of type I collagen in the ER in the absence of CRT suggests that CRT is important for the proper folding of collagen and trafficking of collagen from the ER to the Golgi. If this is the case, then CRT and type I procollagen should be complexed in the ER. Although purified CRT has been shown to bind to purified fibrillar collagens in an enzyme-linked immunosorbent assay (19), binding between CRT and collagen within cells has not been demonstrated. Therefore, wild type MEFs were trypsinized to isolate them from extracellular collagen, lysed, and then immunoprecipitated with antibody to type I collagen. CRT co-immunoprecipitated with type I procollagen in these cell lysates (Fig. 8A). Furthermore, we were able to detect CRT-type I collagen complex formation in CRT−/− MEFs stably transfected with HA-tagged CRT (CRT−/− MEFs + HA-CRT) (37). Production of HA-tagged CRT and type I collagen was confirmed in these cells (data not shown). HA-CRT and type I procollagen co-immunoprecipitated when precipitated by either anti-type I collagen antibody, anti-CRT antibody, or anti-HA antibody (Fig. 8, B–D). These studies show that CRT and type I collagen exist in the cell in a complex that can be immunoprecipitated from isolated cells and that CRT is necessary for trafficking of collagen from the ER, presumably due to its ability to mediate procollagen folding.
FIGURE 7.
Type I collagen is retained in the ER in CRT−/− MEFs. CRT−/− MEFs were cultured on treated coverslips for 36 h in 10% FBS and then for 48 h in 0.5% FBS with (g–l) or without (a–f) daily treatments of 20 μm ascorbic acid. a–c and g–i, ER was detected with rabbit anti-calnexin antibody (a and g, red channel), and type I collagen was detected with goat anti-type I collagen antibody (b and h, green channel). d–f and j–l, Golgi was detected by a mouse anti-GM130 antibody (d and j, red channel), and type I collagen was detected with rabbit anti-type I collagen antibody (e and k, green channel). Merged images showing co-localization are c, f, i, and l. Intensity ranges were the same for each image. Original magnification was ×63. Scale bar, 25 μm.
FIGURE 8.
Type I collagen and CRT are complexed within the cell. A, wild type and B–D, CRT−/− MEFs + HA-CRT MEFS were grown to confluence. Cells were lysed in 1% Nonidet P-40, 150 mm NaCl, 50 mm Tris, pH 8, with protease inhibitor, passed through a syringe, and precleared with GammaBindTM G-SepharoseTM beads. Rabbit anti-mouse type I collagen antibody (A and B), mouse anti-CRT antibody (C), mouse anti-HA antibody (D), or controls rabbit nonimmune IgG or mouse nonimmune IgG (A–D) were preincubated with GammaBindTM G-SepharoseTM beads and then incubated with equal amounts of precleared lysates. Beads were eluted with Laemmli buffer with 5% β-mercaptoethanol and proteins separated by SDS-PAGE (8% gels) and transferred to nitrocellulose membranes. Membranes were immunoblotted (IB) with mouse anti-CRT (A), mouse anti-HA (B), or rabbit anti-type I collagen (C and D) antibodies. Blots were stripped and reprobed with anti-type I collagen (A and B) or anti-HA (C and D) to detect protein input. pro is type I procollagen; IP, immunoprecipitation.
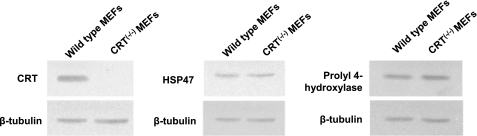
To investigate whether the increased ER retention of collagen in CRT-deficient cells might be the result of indirect effects secondary to deficiencies in known collagen chaperones, we examined levels of prolyl 4-hydroxylase and HSP47 in CRT−/− and wild type MEFs. Prolyl 4-hydroxylase is an ER lumen enzyme that hydroxylates proline on the procollagen peptide and serves as a chaperone for procollagen (54). HSP47 is an ER lumen collagen chaperone that binds the folded triple helical form of procollagen (55). Others have shown that HSP47 expression parallels collagen synthesis, and it has been speculated to be involved in the development of fibrosis (54, 56, 57). Analysis of HSP47 and prolyl 4-hydroxylase protein levels in cell lysates of wild type and CRT−/− MEFs showed no differences in expression (Fig. 9), suggesting that the retention of collagen within the ER in the CRT-deficient cells is not due to a general reduction in collagen chaperone levels, but it appears to be an effect of CRT deficiency.
FIGURE 9.
Absence of CRT does not alter collagen chaperone HSP47 or prolyl 4-hydroxylase expression. Wild type and CRT−/− MEFs were grown to confluence. Cells were harvested in reducing Laemmli sample buffer. Equal volumes of lysates were separated by 10% SDS-PAGE, transferred to nitrocellulose, and immunoblotted with rabbit anti-CRT polyclonal antibody, mouse anti-HSP47 monoclonal antibody, and goat anti-prolyl 4-hydroxylase antibody. Membranes were stripped and reprobed with rabbit anti-β-tubulin.
Role of Fibronectin in CRT-dependent Regulation of Collagen ECM Deposition
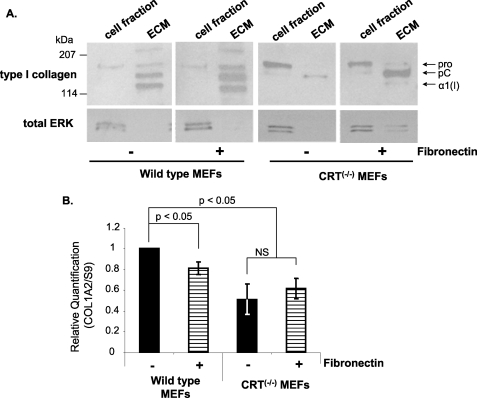
It is known that a fibronectin matrix is necessary for collagen incorporation into the ECM and that fibronectin expression and matrix incorporation are deficient in CRT −/− MEFs (9, 14, 58, 59). To determine whether the reduced collagen ECM deposition in CRT-deficient cells is due to the lack of a fibronectin matrix, we provided fibronectin to both wild type and CRT−/− MEFs cells. Provision of a fibronectin matrix compensated for the lack of CRT and stimulated collagen incorporation into the detergent-insoluble ECM (Fig. 10A). Wild type cells also had increased collagen incorporation into the ECM in the presence of fibronectin (Fig. 10A). The ability of fibronectin to increase collagen ECM deposition by CRT−/− MEFs is not due to enhanced transcription of type I collagen, because quantitative real time PCR analysis showed no increase in Col1A2 transcript after 12 h of fibronectin treatment (Fig. 10B). As would be expected, the increased intracellular retention of collagen in CRT−/− MEFs was not rescued by providing a fibronectin matrix. These data show that the ability of CRT to regulate fibronectin matrix deposition has secondary effects on collagen matrix deposition.
FIGURE 10.
Reduced type I collagen ECM deposition in the CRT−/− MEFs is rescued by fibronectin. A and B, wild type and CRT−/− MEFs were cultured for 48 h in DMEM with 10% FBS and then for 72 h in DMEM with 0.5% FBS. + fibronectin treatments were cultured on a 10 μg/ml fibronectin substrate and treated daily in the last 72 h with 10 μg/ml soluble fibronectin. A, cells were extracted by deoxycholate to separate the cell fraction (DOC-soluble) from the ECM fraction (DOC-insoluble). The entire ECM fraction and 1/10th of the cell fraction were separated by SDS-PAGE and immunoblotted for type I collagen. Blots were stripped and reprobed with an antibody to total ERK. A representative blot for collagen is shown (n = 3 separate experiments). B, RNA was harvested after 12 h of treatment. Transcript levels of Col1A2 in wild type and CRT−/− MEFs were examined by quantitative real time PCR. Values represent the average expression levels normalized to S9 expression ± S.D., and values for wild type cells were set to 1. NS, not significant (n = 3, each performed in triplicate).
Ascorbic Acid Stimulates Collagen Trafficking and Deposition in CRT-deficient Cells
Ascorbic acid increases collagen transcript stabilization and is a cofactor for proline hydroxylation of procollagen, which enhances translation efficiency and secretion (36, 47–49). Because CRT−/− MEFs have apparent defects in post-translational processing of type I collagen, we investigated whether ascorbic acid can rescue these defects in the CRT−/− MEFs. Treatment with ascorbic acid increased soluble fibrillar collagens in the conditioned media of both wild type and CRT-deficient cells, although the difference in total soluble collagen between the wild type and knock-out cells was still significant (Fig. 5A). Ascorbic acid also increased type I collagen fibril formation (Fig. 4, C and D). Importantly, ascorbic acid treatment reduced intracellular retention of type I collagen in CRT−/− MEFs (Fig. 4D) with reduced collagen localization to the ER (Fig. 7, g–i), suggesting that ascorbic acid can compensate for secretion/trafficking defects due to the absence of CRT. In contrast, ascorbic acid did not rescue the reduced COL1A2 transcription in CRT-deficient cells (Fig. 11).
FIGURE 11.
Ascorbic acid has no effect on type I collagen gene transcription in wild type or CRT−/− MEFs. Cells were cultured for 48 h in 10% FBS and then treated with or without 20 μm ascorbic acid in 0.5% FBS. RNA was harvested after 12 h. Transcript levels of Col1A2 in wild type and CRT−/− MEFs were examined by quantitative real time PCR. Values represent the average expression levels normalized to S9 expression ± S.D., and values for wild type cells were set to 1. NS, not significant (n = 4, each performed in triplicate).
DISCUSSION
Previous studies showing CRT stimulation of wound healing and its expression in fibrotic tissues suggested that CRT might be involved in the regulation of collagen (6, 7). However, direct evidence for collagen regulation by CRT and elucidation of potential mechanisms by which CRT might regulate collagens have not been previously defined. This study demonstrates that intracellular CRT has a role in regulating formation of the fibrillar collagen matrix through stimulation of transcription, trafficking, and procollagen processing. Levels of type I collagen transcript and protein varied depending on CRT expression by fibroblasts, indicating regulation at the level of transcription. Collagen expression is regulated by intracellular CRT, rather than by cell surface binding of CRT. CRT regulates collagen in part through regulation of intracellular calcium levels, because thapsigargin treatment decreases type I collagen protein. Importantly, these studies have also identified a role for intracellular CRT in regulation of collagen trafficking through the ER, as CRT−/− MEFs have increased type I collagen retention in the ER and decreased transit to the Golgi. Furthermore, the detection of collagen-CRT complexes within the cell supports the idea that CRT is important for chaperoning collagen through the secretory pathway. We have also identified a previously unappreciated consequence of the deficit in fibronectin matrix production by CRT-deficient cells, the inability to organize a collagen matrix. Together, these data provide a mechanistic basis for the role of CRT in wound healing and fibrogenesis, processes that involve collagen matrix production. This work is the first to show that intracellular CRT regulates multiple steps in type I collagen processing, including expression, trafficking, and deposition into the ECM.
CRT previously has been shown to regulate gene expression. CRT binds to the DNA-binding domains of the glucocorticoid, androgen, and retinoic acid receptors (38, 60). CRT binding to these receptors inhibits them from interacting with their DNA-response elements, thereby inhibiting gene transcription. CRT also affects gene transcription through Ca2+ regulation. CRT has a large storage capacity for Ca2+ within the ER and that storage is diminished in CRT−/− MEFs (37, 50). Papp et al. (14) showed that wild type MEFs have increased fibronectin mRNA levels and protein expression as compared with CRT−/− MEFs. When ER Ca2+ levels were decreased, total fibronectin expression was decreased. Conversely, fibronectin expression was increased when there was an increase in Ca2+ levels (14). Consistent with these observations, our studies suggest that CRT is regulating collagen transcription through regulation of ER Ca2+ stores. Our data show that depleting ER Ca2+ stores with thapsigargin decreased total collagen protein expression. This observation is consistent with other evidence for Ca2+-dependent regulation of type I collagen. Studies show that depletion of ER Ca2+ stores can decrease type I collagen synthesis (51). Sugiura et al. (61) showed that treatment of mesangial cells with Ca2+ channel blockers decreases collagen production and transcript levels of type I collagen and fibronectin. Treatment with Ca2+ channel blockers also decreases the ability of the transcription factor, AP1, to bind to DNA (61). There is an AP1-binding site in the Col1A2 promoter (62), suggesting a possible mechanism for Ca2+ regulation of collagen.
We considered the possibility that increased intracellular retention of collagen could negatively feedback on transcript levels in CRT-deficient cells. It has been shown that the rate-limiting step of ascorbic acid action is secretion of collagen and not its effect on transcript levels (48). In our studies, ascorbic acid did not affect type I collagen gene transcription in the CRT−/− MEFs, although it did increase protein secretion. Therefore, it is unlikely that the reduced transcript levels in CRT-deficient cells are due to negative feedback by increased intracellular collagen levels.
Interestingly, ascorbic acid increased procollagen processing in both the DOC-soluble and -insoluble fractions of CRT−/− cells. This was unexpected, as procollagen processing typically is thought to occur extracellularly. Although the mechanism of the effects of ascorbic acid on procollagen processing in the CRT−/− cells is not clear, one can speculate that ascorbic acid might indirectly affect factors involved in procollagen folding. Alternatively, ascorbic acid might regulate other procollagen processing factors, such as procollagen C-proteinase enhancer, that could potentially be altered in CRT−/− cells (63).
The observation that CRT-deficient cells have increased intracellular collagen retained in the ER, despite reduced transcription, suggests that CRT is important for ER quality control and intracellular trafficking of type I collagen. In contrast, Papp et al. (14) did not see an increase in cellular fibronectin in CRT-deficient cells as compared with the wild type MEFs, suggesting that fibronectin and collagen trafficking are differentially regulated by CRT. In other cases, CRT expression is actually associated with decreased expression of certain proteins such as the cystic fibrosis transmembrane conductance regulator (64). Based on our observations that CRT and procollagen can be co-immunoprecipitated from cell lysates, we suggest that CRT might act to facilitate procollagen transport from the ER to the Golgi by mediating correct folding of the protein. It should be noted that the ER retention of collagen observed in CRT−/− cells occurs despite the presence of normal levels of collagen chaperones HSP47 and prolyl 4-hydroxylase. Our findings are consistent with those of Knee et al. (52) who reported that proteins have increased retention times in the ER-enriched microsomal fractions in the absence of CRT, despite normal or elevated levels of other chaperones such as calnexin.
CRT might also be facilitating collagen trafficking through its effects on Ca2+ within the ER (37, 50). Studies suggest that high ER Ca2+ levels are important for the proper folding of collagen (51). Furthermore, the interaction between CRT and collagen might be Ca2+-dependent; the conformation of CRT is Ca2+-sensitive, and this has been shown to affect CRT binding to other chaperones and proteins in the ER (65, 66).
Interestingly, HSP47 expression has been shown to correlate with fibrosis and is used as a surrogate marker for collagen expression (56, 57). In our studies, HSP47 levels did not differ between cell types, although there were differences in collagen protein expression, suggesting that HSP47 might not be a reliable indicator of relative collagen expression.
Nanney et al. (6) showed that exogenous treatment of CRT increased collagen fibers throughout the wound in a porcine wound healing model. They attributed this change to extracellular CRT stimulation of fibroblast and keratinocyte chemotaxis and proliferation and possibly due to increased transforming growth factor-β3 production. In contrast, we observed no stimulation of collagen by either wild type or CRT−/− MEFs when treated with purified CRT protein. This could reflect the fact that our in vitro data were normalized for cell number and do not reflect increases in collagen due to an increased influx of cells as in the wounds. Furthermore, there could be factors in the wound environment that promote CRT binding to cells, internalization, and/or signaling, which might not be present in our in vitro systems.
CRT expression is elevated in various animal models of fibrosis (7), consistent with our findings that CRT regulates fibrillar collagen. CRT levels are increased during cellular stresses, including oxidative stress and hypoxia (25–28). Oxidative stress is known to be involved in fibrogenesis in a number of disease processes, including liver, lung, and kidney fibrosis (67–71). Hypoxia also increases collagen production and deposition in some systems (72). These findings suggest that CRT might be an important mediator of ECM production and remodeling due to cellular stress, thereby contributing to fibrogenesis. Furthermore, preliminary studies show that the CRT−/− MEFs do not increase soluble type I collagen expression or type I collagen matrix deposition in response to the major pro-fibrogenic growth factor, transforming growth factor-β, despite phosphorylation of Smad3 (data not shown), suggesting that CRT might be an important, heretofore unidentified, downstream mediator of the fibrogenic effects of transforming growth factor-β (73, 74). Further investigation of the possible role of CRT in regulating cellular responses to transforming growth factor-β is warranted.
In summary, these data provide novel insights into how intracellular CRT is potentially involved in extracellular matrix remodeling and fibrogenic processes through its regulation of multiple stages of fibrillar collagen expression, trafficking, and processing into the extracellular matrix. In addition, we provide the first evidence that intracellular CRT complexes with type I procollagen, and our data suggest that these interactions are important for collagen trafficking from the ER. Together, these studies highlight the importance of CRT as a regulatory factor in extracellular matrix production and assembly in the fibrogenic process.
Acknowledgments
We gratefully acknowledge Dr. Marek Michalak (University of Alberta, Edmonton, Alberta, Canada) for providing us with the wild type, CRT−/−, and CRT−/− + HA-CRT MEFs. We thank Dr. Marek Michalak and Dr. Michal Opas (University of Toronto, Canada) for the mouse L fibroblasts. We thank Dr. Amy Bradshaw (Medical University of South Carolina) for helpful advice and characterization of the collagen antibody. We thank Dr. Leslie Gold (New York University) for the recombinant rabbit calreticulin and helpful discussions and Dr. Kedar Vaidya (University of Alabama at Birmingham) for help with quantitative real time PCR and figure presentation. Microscopic images were obtained at the University of Alabama at Birmingham BERM Center FRET Microscopy Core Facility. Facilities were supported by National Institutes of Health Research Facilities Improvement Program Grant C06RR15490 from the National Center for Research Resources.
This work was supported, in whole or in part, by National Institutes of Health Grants HL079644 (to J. E. M.-U.), T32 GM008361 (to L. V. G.), and T32 HL007918 (to M. T. S.) (Medical Scientist Training Program Grants, University of Alabama at Birmingham).
M. T. Sweetwyne, L. Van Duyn Graham, M. A. Pallero, A. Lu, and J. E. Murphy-Ullrich, submitted for publication.
- CRT
- calreticulin
- DOC
- deoxycholate
- DMEM
- Dulbecco's modified Eagle's medium
- ECM
- extracellular matrix
- ER
- endoplasmic reticulum
- ERK
- extracellular signal-regulated kinase
- FBS
- fetal bovine serum
- HSP47
- heat shock protein 47
- MEFs
- mouse embryonic fibroblasts
- HA
- hemagglutinin
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Opas M., Dziak E., Fliegel L., Michalak M. (1991) J. Cell Physiol. 149, 160–171 [DOI] [PubMed] [Google Scholar]
- 2.Holaska J. M., Black B. E., Love D. C., Hanover J. A., Leszyk J., Paschal B. M. (2001) J. Cell Biol. 152, 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eggleton P., Lieu T. S., Zappi E. G., Sastry K., Coburn J., Zaner K. S., Sontheimer R. D., Capra J. D., Ghebrehiwet B., Tauber A. I. (1994) Clin. Immunol. Immunopathol. 72, 405–409 [DOI] [PubMed] [Google Scholar]
- 4.Gray A. J., Park P. W., Broekelmann T. J., Laurent G. J., Reeves J. T., Stenmark K. R., Mecham R. P. (1995) J. Biol. Chem. 270, 26602–26606 [DOI] [PubMed] [Google Scholar]
- 5.Gold L., Eggleton P., Sweetwyne M., Duyn L. V., Greives M., Naylor S., Michalak M., Murphy-Ullrich J. (2010) FASEB J., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nanney L. B., Woodrell C. D., Greives M. R., Cardwell N. L., Pollins A. C., Bancroft T. A., Chesser A., Michalak M., Rahman M., Siebert J. W., Gold L. I. (2008) Am. J. Pathol. 173, 610–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kypreou K. P., Kavvadas P., Karamessinis P., Peroulis M., Alberti A., Sideras P., Psarras S., Capetanaki Y., Politis P. K., Charonis A. S. (2008) Proteomics 8, 2407–2419 [DOI] [PubMed] [Google Scholar]
- 8.Opas M., Szewczenko-Pawlikowski M., Jass G. K., Mesaeli N., Michalak M. (1996) J. Cell Biol. 135, 1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papp S., Fadel M. P., Kim H., McCulloch C. A., Opas M. (2007) J. Biol. Chem. 282, 16585–16598 [DOI] [PubMed] [Google Scholar]
- 10.Wu M., Massaeli H., Durston M., Mesaeli N. (2007) Matrix Biol. 26, 463–472 [DOI] [PubMed] [Google Scholar]
- 11.Fadel M. P., Dziak E., Lo C. M., Ferrier J., Mesaeli N., Michalak M., Opas M. (1999) J. Biol. Chem. 274, 15085–15094 [DOI] [PubMed] [Google Scholar]
- 12.Goicoechea S., Pallero M. A., Eggleton P., Michalak M., Murphy-Ullrich J. E. (2002) J. Biol. Chem. 277, 37219–37228 [DOI] [PubMed] [Google Scholar]
- 13.Orr A. W., Elzie C. A., Kucik D. F., Murphy-Ullrich J. E. (2003) J. Cell Sci. 116, 2917–2927 [DOI] [PubMed] [Google Scholar]
- 14.Papp S., Szabo E., Kim H., McCulloch C. A., Opas M. (2008) Exp. Cell Res. 314, 1313–1326 [DOI] [PubMed] [Google Scholar]
- 15.Coppolino M. G., Woodside M. J., Demaurex N., Grinstein S., St-Arnaud R., Dedhar S. (1997) Nature 386, 843–847 [DOI] [PubMed] [Google Scholar]
- 16.Kwon M. S., Park C. S., Choi K., Ahnn J., Kim J. I., Eom S. H., Kaufman S. J., Song W. K. (2000) Mol. Biol. Cell 11, 1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppolino M., Leung-Hagesteijn C., Dedhar S., Wilkins J. (1995) J. Biol. Chem. 270, 23132–23138 [DOI] [PubMed] [Google Scholar]
- 18.Elton C. M., Smethurst P. A., Eggleton P., Farndale R. W. (2002) Thromb. Haemost. 88, 648–654 [PubMed] [Google Scholar]
- 19.Peerschke E. I., Ghebrehiwet B. (1990) J. Immunol. 145, 2984–2988 [PubMed] [Google Scholar]
- 20.Goicoechea S., Orr A. W., Pallero M. A., Eggleton P., Murphy-Ullrich J. E. (2000) J. Biol. Chem. 275, 36358–36368 [DOI] [PubMed] [Google Scholar]
- 21.Trombetta E. S., Parodi A. J. (2003) Annu. Rev. Cell Dev. Biol. 19, 649–676 [DOI] [PubMed] [Google Scholar]
- 22.Saito Y., Ihara Y., Leach M. R., Cohen-Doyle M. F., Williams D. B. (1999) EMBO J. 18, 6718–6729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Treves S., De Mattei M., Landfredi M., Villa A., Green N. M., MacLennan D. H., Meldolesi J., Pozzan T. (1990) Biochem. J. 271, 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastianutto C., Clementi E., Codazzi F., Podini P., De Giorgi F., Rizzuto R., Meldolesi J., Pozzan T. (1995) J. Cell Biol. 130, 847–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X., Liu X., Zhu X., Tang C. (2007) Shock 27, 572–577 [DOI] [PubMed] [Google Scholar]
- 26.Plakidou-Dymock S., McGivan J. D. (1994) Cell Calcium 16, 1–8 [DOI] [PubMed] [Google Scholar]
- 27.Heal R., McGivan J. (1998) Biochem. J. 329, 389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Núñez M. T., Osorio A., Tapia V., Vergara A., Mura C. V. (2001) J. Cell. Biochem. 82, 660–665 [DOI] [PubMed] [Google Scholar]
- 29.Waser M., Mesaeli N., Spencer C., Michalak M. (1997) J. Cell Biol. 138, 547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogden C. A., deCathelineau A., Hoffmann P. R., Bratton D., Ghebrehiwet B., Fadok V. A., Henson P. M. (2001) J. Exp. Med. 194, 781–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao G., Chung T. F., Fine R. E., Johnson R. J. (1999) J. Neurosci. Res. 58, 652–662 [DOI] [PubMed] [Google Scholar]
- 32.Tufi R., Panaretakis T., Bianchi K., Criollo A., Fazi B., Di Sano F., Tesniere A., Kepp O., Paterlini-Brechot P., Zitvogel L., Piacentini M., Szabadkai G., Kroemer G. (2008) Cell Death Differ. 15, 274–282 [DOI] [PubMed] [Google Scholar]
- 33.Pallero M. A., Elzie C. A., Chen J., Mosher D. F., Murphy-Ullrich J. E. (2008) FASEB J. 22, 3968–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardai S. J., McPhillips K. A., Frasch S. C., Janssen W. J., Starefeldt A., Murphy-Ullrich J. E., Bratton D. L., Oldenborg P. A., Michalak M., Henson P. M. (2005) Cell 123, 321–334 [DOI] [PubMed] [Google Scholar]
- 35.Orr A. W., Pedraza C. E., Pallero M. A., Elzie C. A., Goicoechea S., Strickland D. K., Murphy-Ullrich J. E. (2003) J. Cell Biol. 161, 1179–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prockop D. J., Kivirikko K. I. (1995) Annu. Rev. Biochem. 64, 403–434 [DOI] [PubMed] [Google Scholar]
- 37.Nakamura K., Zuppini A., Arnaudeau S., Lynch J., Ahsan I., Krause R., Papp S., De Smedt H., Parys J. B., Muller-Esterl W., Lew D. P., Krause K. H., Demaurex N., Opas M., Michalak M. (2001) J. Cell Biol. 154, 961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burns K., Duggan B., Atkinson E. A., Famulski K. S., Nemer M., Bleackley R. C., Michalak M. (1994) Nature 367, 476–480 [DOI] [PubMed] [Google Scholar]
- 39.Midwood K., Wierzbicka-Patynowski I., Schwarzbauer J. (2002) in Methods in Cell-Matrix Adhesion (Adams J. ed) pp. 145–161, Academic Press, San Diego: [DOI] [PubMed] [Google Scholar]
- 40.Orr A. W., Pallero M. A., Murphy-Ullrich J. E. (2002) J. Biol. Chem. 277, 20453–20460 [DOI] [PubMed] [Google Scholar]
- 41.Murphy-Ullrich J. E., Gurusiddappa S., Frazier W. A., Höök M. (1993) J. Biol. Chem. 268, 26784–26789 [PubMed] [Google Scholar]
- 42.Rentz T. J., Poobalarahi F., Bornstein P., Sage E. H., Bradshaw A. D. (2007) J. Biol. Chem. 282, 22062–22071 [DOI] [PubMed] [Google Scholar]
- 43.Poobalarahi F., Baicu C. F., Bradshaw A. D. (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H2924–H2932 [DOI] [PubMed] [Google Scholar]
- 44.Murphy-Ullrich J. E., Höök M. (1989) J. Cell Biol. 109, 1309–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kyriakides T. R., Bornstein P. (2003) Thromb. Haemost. 90, 986–992 [DOI] [PubMed] [Google Scholar]
- 46.Canty E. G., Kadler K. E. (2005) J. Cell Sci. 118, 1341–1353 [DOI] [PubMed] [Google Scholar]
- 47.Blanck T. J., Peterkofsky B. (1975) Arch. Biochem. Biophys. 171, 259–267 [DOI] [PubMed] [Google Scholar]
- 48.Chan D., Lamande S. R., Cole W. G., Bateman J. F. (1990) Biochem. J. 269, 175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davidson J. M., LuValle P. A., Zoia O., Quaglino D., Jr., Giro M. (1997) J. Biol. Chem. 272, 345–352 [DOI] [PubMed] [Google Scholar]
- 50.Michalak M., Groenendyk J., Szabo E., Gold L. I., Opas M. (2009) Biochem. J. 417, 651–666 [DOI] [PubMed] [Google Scholar]
- 51.Stefanovic B., Stefanovic L., Schnabl B., Bataller R., Brenner D. A. (2004) Mol. Cell. Biol. 24, 1758–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knee R., Ahsan I., Mesaeli N., Kaufman R. J., Michalak M. (2003) Biochem. Biophys. Res. Commun. 304, 661–666 [DOI] [PubMed] [Google Scholar]
- 53.Molinari M., Eriksson K. K., Calanca V., Galli C., Cresswell P., Michalak M., Helenius A. (2004) Mol. Cell 13, 125–135 [DOI] [PubMed] [Google Scholar]
- 54.Nagata K. (1996) Trends Biochem. Sci. 21, 22–26 [DOI] [PubMed] [Google Scholar]
- 55.Koide T., Nagata K. (2005) Top. Curr. Chem. 247, 85–114 [Google Scholar]
- 56.Taguchi T., Razzaque M. S. (2007) Trends Mol. Med. 13, 45–53 [DOI] [PubMed] [Google Scholar]
- 57.Razzaque M. S., Nazneen A., Taguchi T. (1998) Mod. Pathol. 11, 1183–1188 [PubMed] [Google Scholar]
- 58.Sottile J., Hocking D. C. (2002) Mol. Biol. Cell 13, 3546–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Velling T., Risteli J., Wennerberg K., Mosher D. F., Johansson S. (2002) J. Biol. Chem. 277, 37377–37381 [DOI] [PubMed] [Google Scholar]
- 60.Dedhar S., Rennie P. S., Shago M., Hagesteijn C. Y., Yang H., Filmus J., Hawley R. G., Bruchovsky N., Cheng H., Matusik R. J., Giguere V. (1994) Nature 367, 480–483 [DOI] [PubMed] [Google Scholar]
- 61.Sugiura T., Imai E., Moriyama T., Horio M., Hori M. (2000) Nephron 85, 71–80 [DOI] [PubMed] [Google Scholar]
- 62.Chung K. Y., Agarwal A., Uitto J., Mauviel A. (1996) J. Biol. Chem. 271, 3272–3278 [DOI] [PubMed] [Google Scholar]
- 63.Shalitin N., Schlesinger H., Levy M. J., Kessler E., Kessler-Icekson G. (2003) J. Cell. Biochem. 90, 397–407 [DOI] [PubMed] [Google Scholar]
- 64.Harada K., Okiyoneda T., Hashimoto Y., Ueno K., Nakamura K., Yamahira K., Sugahara T., Shuto T., Wada I., Suico M. A., Kai H. (2006) J. Biol. Chem. 281, 12841–12848 [DOI] [PubMed] [Google Scholar]
- 65.Corbett E. F., Michalak K. M., Oikawa K., Johnson S., Campbell I. D., Eggleton P., Kay C., Michalak M. (2000) J. Biol. Chem. 275, 27177–27185 [DOI] [PubMed] [Google Scholar]
- 66.Corbett E. F., Oikawa K., Francois P., Tessier D. C., Kay C., Bergeron J. J., Thomas D. Y., Krause K. H., Michalak M. (1999) J. Biol. Chem. 274, 6203–6211 [DOI] [PubMed] [Google Scholar]
- 67.Kisseleva T., Brenner D. A. (2007) J. Gastroenterol. Hepatol. 22, S73–S78 [DOI] [PubMed] [Google Scholar]
- 68.Mehta K., Van Thiel D. H., Shah N., Mobarhan S. (2002) Nutr. Rev. 60, 289–293 [DOI] [PubMed] [Google Scholar]
- 69.Kinnula V. L., Fattman C. L., Tan R. J., Oury T. D. (2005) Am. J. Respir. Crit. Care Med. 172, 417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah S. V., Baliga R., Rajapurkar M., Fonseca V. A. (2007) J. Am. Soc. Nephrol. 18, 16–28 [DOI] [PubMed] [Google Scholar]
- 71.Higgins D. F., Biju M. P., Akai Y., Wutz A., Johnson R. S., Haase V. H. (2004) Am. J. Physiol. Renal Physiol. 287, F1223–F1232 [DOI] [PubMed] [Google Scholar]
- 72.Horino Y., Takahashi S., Miura T., Takahashi Y. (2002) Life Sci. 71, 3031–3045 [DOI] [PubMed] [Google Scholar]
- 73.Ignotz R. A., Massagué J. (1986) J. Biol. Chem. 261, 4337–4345 [PubMed] [Google Scholar]
- 74.Raghow R., Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. (1987) J. Clin. Invest. 79, 1285–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]