Abstract
Methylation of the arginine residues of histones by methyltransferases has important consequences for chromatin structure and gene regulation; however, the molecular mechanism(s) of methyltransferase regulation is still unclear, as is the biological significance of methylation at particular arginine residues. Here, we report a novel specific inhibitor of coactivator-associated arginine methyltransferase 1 (CARM1; also known as PRMT4) that selectively inhibits methylation at arginine 17 of histone H3 (H3R17). Remarkably, this plant-derived inhibitor, called TBBD (ellagic acid), binds to the substrate (histone) preferentially at the signature motif, “KAPRK,” where the proline residue (Pro-16) plays a critical role for interaction and subsequent enzyme inhibition. In a promoter-specific context, inhibition of H3R17 methylation represses expression of p21, a p53-responsive gene, thus implicating a possible role for H3 Arg-17 methylation in tumor suppressor function. These data establish TBBD as a novel specific inhibitor of arginine methylation and demonstrate substrate sequence-directed inhibition of enzyme activity by a small molecule and its physiological consequence.
Keywords: Chromatin, Chromatin Histone Modification, Coactivator Transcription, Enzyme Inhibitors, Gene Expression, Arginine Methylation, CARM1, TBBD, Epigenetics, p53
Introduction
Arginine methylation of nonhistone proteins has been known for nearly four decades, regulating various cellular processes such as transcription and RNA processing, and DNA replication and repair (1, 2). However, histone arginine methylation and its role in gene regulation were discovered much more recently (3). Protein arginine methyltransferases (PRMTs)3 are classified into class I and class II enzymes. Class I PRMTs catalyze the formation of asymmetric dimethylarginine (involved in transcriptional activation), whereas class II enzymes are responsible for generating symmetric dimethylarginine (involved in transcriptional repression) (4). The class I enzyme CARM1 (coactivator-associated arginine methyltransferase 1, also known as PRMT4), was first identified as a p160 coactivator-interacting protein in a yeast two-hybrid screen, and later as a histone methyltransferase and transcriptional coactivator (5). All the PRMTs except CARM1 target a glycine arginine recognition motif, whereas CARM1 recognizes XXPRX or XXRPX, where X is any amino acid (6).
CARM1 interacts with GRIP1 (glucocorticoid receptor-interacting protein) and is a secondary coactivator of several nuclear receptors (7–9). CARM1 also interacts with other chromatin-modifying enzymes such as p300/CREB-binding protein and PRMT1 to bring about cooperative transcriptional activation of p53-responsive genes (10). CARM1 and PRMT1 interactions have also been shown to regulate gene expression in different contexts (11, 12). CARM1 is a positive regulator of both cyclin E1 (13) and NF-κB promoter activity (14). CARM1 also participates in various other cellular processes through its ability to methylate nonhistone substrates. Recently, CARM1 has been implicated in muscle (15) and T-cell development (6), stem cell differentiation (16), adipocyte differentiation (17), RNA processing (18), and tumorigenesis (19). Despite such broad functional significance, the exact molecular mechanisms of the enzyme function are not understood, in part due to the unavailability of specific modulators. For example, in the case of lysine methyltransferases, only two specific inhibitors chaetocin (20) and BIX-01294 (21) are known. However, no specific inhibitor for CARM1 with proper characterization is known so far.
There is an intensive ongoing effort to identify specific arginine methylation inhibitors (22–24). Small molecule inhibitors of protein function are powerful tools to probe the physiological roles of enzymes. Furthermore, such modulators are potential lead molecules for therapeutic purposes, as evidenced by the recent clinical trials of histone deacetylase inhibitors. Along with the latter, the recent discovery of specific and nontoxic small molecule modulators of histone acetyltransferases (HATs) and histone methyltransferases (HMTases) may portend a new era of epigenetic-based drugs (25).
We have established a general screening procedure to identify small molecule modulators of chromatin-modifying enzymes present in plant extracts (obtained from bark, stem, root, or fruit). Using this approach we discovered several small molecule modulators of HATs (25). The same extracts from 25 different plant sources were also screened for HMTase modulatory activity, which led to the identification of a molecule (TBBD) having specific activity toward CARM1, as reported here. This small molecule inhibitor, TBBD (ellagic acid) shows substrate sequence dependence for enzyme specific inhibition. Furthermore, the inhibitor is also active physiologically with a significant consequence on p53-dependent gene expression.
MATERIALS AND METHODS
Protein Purifications
The details of the protein purifications are provided in the supplementary data.
Site-directed Mutagenesis
Histone H3 point mutants A25P and P16A were obtained by site-directed mutagenesis. The histone H3 expression clone (Xenopus) was used as the template and mutagenesis was done using a Stratagene site-directed mutagenesis kit according to the manufacturer's instructions. Positive clones were sequenced and transformed into Escherichia coli BL21. Expression and purification of the mutant protein were done as detailed in the supplementary data.
HMTase Assay
HMTase assay has been performed as described elsewhere (26), also see supplementary data.
HAT Assay
HAT assays were performed as described elsewhere (26).
Isothermal Titration Calorimetry (ITC)
ITC experiments were carried out in a VP-ITC system (Microcal LLC, Northampton, MA) at 25 °C. Samples were centrifuged and degassed prior to titration. Titration of TBBD against protein (histone H3/CARM1) was carried out by injecting 0.14 mm TBBD in HMTase assay buffer against 0.007 mm histone H3/CARM1. A 2-min interval was allowed between injections for equilibration, sufficient for the return of the heat signal to baseline. A total of 35 injections were carried out to ensure complete titration. The details of the analysis are provided in the supplementary data.
Kinetic Characterization of TBBD-mediated Inhibition
The HMTase reaction was carried out with CARM1 in the presence of three concentrations of TBBD (10, 20, and 50 μm). The HMTase reaction consisted of two substrates, histone H3 and the tritiated methyl group donor of AdoMet. In the first assay, the concentration of histone H3 was kept constant at 1.467 μm and [3H]AdoMet was varied from 0.39 to 1.98 μm. In the second assay, the concentration of [3H]AdoMet was kept constant at 1.98 μm and histone H3 was varied from 0.026 to 1.467 μm. The incorporation of radioactivity was taken as a measure of the reaction velocity, which was recorded as counts per min (cpm). The obtained values were plotted on a Lineweaver-Burk plot using GraphPad Prism software.
Immunoblotting Analysis
In vitro modified histones were processed for immunoblotting analysis as described below. In vivo modified histones were obtained from TBBD-treated cells. The maximum DMSO concentration used was 0.1%. The quantitated histones were resolved on a 12% SDS-polyacrylamide gel. After electrophoresis, protein on the gel was electrotransferred onto an Immobilon membrane (Millipore Corp., Bedford, MA). The membranes were then blocked in 5% nonfat milk solution in 1× phosphate-buffered saline containing 0.05% Tween 20 and immunoblotted with anti-dimethylated histone H3R17 (Abcam ab8284) and anti-dimethylated histone H3R26 (Upstate 07-215), acetylated H3K14 (Upstate 07-353), and acetylated H3K18 (Upstate 07-354) polyclonal antibodies. Detection was carried out with goat anti-rabbit secondary antibody (Bangalore Genei), and bands were visualized with an ECL detection system (Pierce).
Docking Protocol
Atomic coordinates of histone H3 (chains A and E) from the x-ray structure of the nucleosome core particle complex (Protein Data Bank code 1KX5) were from the PDB. The CARM1 structure used was PDB code 2V74. Polar hydrogens were added, nonpolar hydrogens were merged, and AutoDock 4 atom types and Gasteiger charges were assigned to all atoms of the receptor molecule (i.e. histone H3). Rotatable bonds, AutoDock 4 atom types, and Gasteiger charges were also assigned to the ligand molecule (TBBD) according to AutoDock Tools. To target residues 14 to 18 (KAPRK) of the receptor, “Pro” (proline 16) was assigned as the center of the grid, and an affinity grid box was made with default grid spacing at 0.375 Å using AutoGrid 4. A Lamarckian genetic algorithm was selected to evaluate the ligand binding energies with the receptor using AutoDock 4 with default parameters, except for the number of energy evaluations (ga_num_evals, 25,000,000).
Chromatin Immunoprecipitation Assay (ChIP)
ChIP was carried out for both human embryonic kidney cell line (HEK) 293T and H1299 cell lines as described elsewhere (27). The pulldown was done using antibody against dimethyl histone H3R17 (Abcam ab8284), dimethyl histone H3R26 (Upstate 07-215), and the immunoprecipitated samples were deproteinized and ethanol precipitated to recover the DNA. PCR analysis was performed using primers for the p53-responsive site on the p21 promoter.
Endogenous Gene Expression Assay
HEK 293T was treated with doxorubicin to increase p53 levels and later treated with TBBD for 24 h or left untreated. Following the stipulated treatment time, total RNA was isolated using TRIzol reagent (Invitrogen). cDNA was synthesized with oligo(dT) (28-mer) (Invitrogen) and Moloney murine leukemia virus reverse transcriptase (Sigma), and expression analysis was carried out using iQTM SYBR Green supermix (Bio-Rad) and gene-specific primers of p21 and actin.
Statistical Analysis
All experiments were performed at least as triplicates. The statistical significance of the difference in the means was evaluated by two-tailed paired Student's t test using GraphPad Prism software, version 4.0. Means were considered significant if the p values of the paired t test were less than 0.05.
RESULTS
TBBD Is a Specific Inhibitor of the Arginine Methyltransferase CARM1
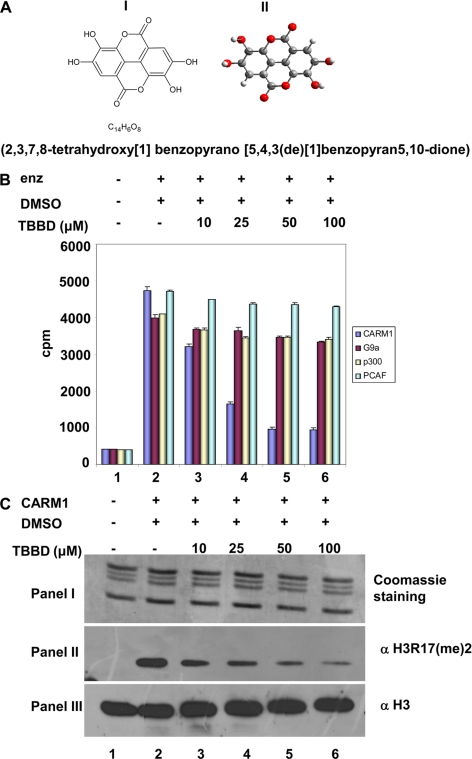
Pomegranate (Punica gratum) extract has been known to possess anticancer activity for several decades, however, the molecular target for these extract components have not yet been identified. The effect of this extract was tested on chromatin modifying enzymes in an in vitro assay. The crude extract of pomegranate fruit skin, processed as described in the supplemental data, was found to inhibit histone arginine methyltransferase activity of CARM1/PRMT4 as well as the acetyltransferase activity of p300/KAT3B (supplemental Fig. S1, lane 2 versus lane 3). Further purification of the active components from the fraction yielded a highly purified compound, TBBD (Fig. 1A), which led to a dose-dependent inhibition of CARM1 methyltransferase activity (Fig. 1B, lanes 3–6), as observed by a filter binding assay. However, when a similar assay was performed with the histone acetyltransferase p300/KAT3B (which was inhibited by the crude extract), a minimal effect was observed even with increasing concentrations of TBBD (Fig. 1B, lanes 3–6). Furthermore, TBBD did not show any significant effect on the HMTase activity of lysine methyltransferase G9a, as well as on the HAT activity of p300/CBP-associated factor (Fig. 1B, lanes 3–6). Although, the activity of all the four enzymes (CARM1, p300, G9a, and p300/CBP-associated factor) were normalized with the histone substrate (Fig. 1B, lane 2), the purified component, TBBD had a significant inhibitory effect on arginine methyltransferase CARM1 alone, indicating its specificity toward CARM1 in an in vitro reaction. This inhibitory effect of TBBD on CARM1 was found to be partial, even at an inhibitor concentration 10-fold above the IC50 of 25 μm (supplemental Fig. S2). TBBD could inhibit CARM1-mediated methylation of recombinant histone H3 (supplemental Fig. S3) as well as histone H3 in the nucleosomal context (data not shown), suggesting that the state (arrangement) of the histone H3 tail is not involved in the mechanism of inhibition by TBBD. This specificity of the inhibitor was further confirmed by subjecting the in vitro reaction to immunoblotting with site-specific histone H3R17 dimethylation antibody. This residue is one of the modification sites of CARM1 on the histone H3 tail. A dose-dependent inhibition of H3R17 methylation was observed with increasing concentrations of TBBD (Fig. 1C, panel II, lanes 3–6). The same reaction was probed with antibody against histone H3, which indicated similar levels of histone H3 (Fig. 1C, panel III, lanes 3–6), despite a drastic decrease in H3 Arg-17 methylation levels. Thus, the small molecule TBBD, purified from pomegranate fruit skin crude extract, is a specific inhibitor of arginine methyltransferase CARM1 in vitro.
FIGURE 1.
TBBD is a specific inhibitor of the arginine methyltransferase CARM1. A, structure of TBBD. Structural formula representation (panel I) and ball and stick model (panel II). B, filter binding assay for inhibition of histone modification. The HMTase assay was performed with CARM1 and G9a, and the HAT assay was performed with p300, p300/CBP-associated factor (PCAF) in the presence or absence of TBBD by using highly purified HeLa core histones and processed for filter binding assay. Lane 1, core histones without enzyme; lane 2, histones with enzyme in the presence of DMSO; lanes 3–6, histones with enzyme in the presence of 10, 25, 50, or 100 μm TBBD. Error bars represent mean ± S.D. of duplicate reactions. C, HMTase assay with CARM1 using core histones as substrate in the presence or absence of TBBD, processed for immunoblotting analysis. Lane 1, core histones without enzyme; lane 2, histones with enzyme in the presence of DMSO; lanes 3–6, histones with enzyme in the presence of 10, 25, 50, or 100 μm TBBD. Panel I represents Coomassie staining; panel II represents immunoblotting with dimethylated H3R17 antibody. Panel III represents the histone loading control using histone H3 antibody.
TBBD Is an Uncompetitive Inhibitor of Arginine Methyltransferase CARM1
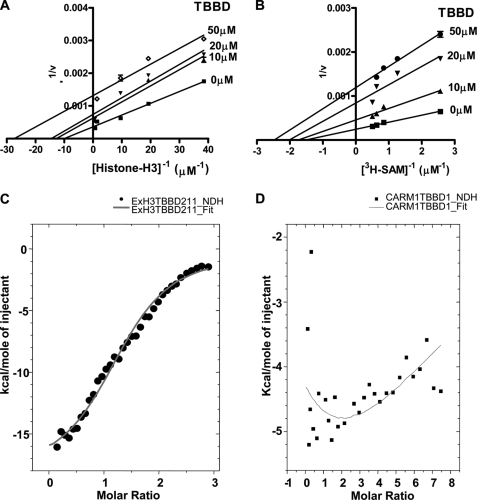
A detailed kinetic characterization of the inhibition was performed using recombinant histone H3 as substrate. The methyltransferase reaction consists of two substrates, histone H3 and the methyl cofactor donor, AdoMet. Hence, the kinetic analysis was done by performing two sets of reactions, the histone H3 concentration was kept constant and the AdoMet concentration varied and vice versa for the second set. It was observed that TBBD-mediated inhibition of CARM1 activity is uncompetitive in nature for both substrates, histone H3 and [3H]AdoMet (Fig. 2, A and B, respectively). These results suggest the involvement of a functional interaction between TBBD and the enzyme-substrate (ES) complex in the process of inhibition. To understand the affinity of the inhibitor toward the two components of the ES complex, we investigated the nature of the interactions of TBBD with CARM1 and histone H3 by employing ITC studies. Titration of the ligand (TBBD) with histone H3 showed a significant heat change, as expected (Fig. 2C). The heat change due to the buffer and buffer-ligand contribution was appropriately subtracted. The resultant heat change could be fitted to a single binding site (n = 1.43 ± 0.0372), which is enthalpy driven ((ΔH = −18.3 kcal/mol) Table 1). Interestingly, the ITC studies showed very minimal interaction between TBBD and the enzyme, CARM1 alone (Fig. 2D). This apparent lack of interaction between the enzyme and inhibitor was verified by surface-enhanced Raman spectroscopy studies (data not shown). Together, the biophysical and kinetic data suggest that partial inhibition of CARM1 by TBBD is mediated through its interaction with the ES complex, and predominantly through the substrate, histone H3 at a single site.
FIGURE 2.
TBBD is an uncompetitive inhibitor of CARM1 with preferential affinity to the substrate histone H3. A, Lineweaver-Burk plot representation of the effect of TBBD on CARM1 activity at a fixed concentration of [3H]AdoMet (0.88 μm) and increasing concentrations of histone H3 in the presence (10, 20, or 50 μm) or absence of TBBD. The results were plotted using GraphPad Prism software. B, Lineweaver-Burk plot representation of the effect of TBBD on CARM1 activity at a fixed concentration of histone H3 (1.467 μm) and increasing concentrations of [3H]AdoMet in the presence (10, 20, or 50 μm) or absence of TBBD. The results were plotted using GraphPad Prism software. C, ITC was carried out by titrating histone H3 (7 μm) against the ligand TBBD (140 μm) at 25 °C. The one-site binding model of the isotherm is shown. D, ITC was carried out by titrating CARM1 (7 μm) against the ligand TBBD (140 μm) at 25 °C.
TABLE 1.
Thermodynamic parameters and stoichiometry of binding for the association of TBBD with histone H3 wild type, and CARM1, histone H3 A25P, and histone H3P16A, in 10 mm Tris-HCl, pH 8.0, at 25 °C
| Kd | No.of binding sites | ΔH | ΔS | |
|---|---|---|---|---|
| μm | kcal/mol | e.u. | ||
| Histone H3 wt | 4 ± 0.736 | 1.43 ± 0.0372 | −18.3 | −35.4 |
| CARM1 | ||||
| Histone H3A25P | 7.9 ± 1.24 | 2.4 ± 0 | −15.8 | −26 |
| Histone H3 P16A | 7.30 ± 1.59 103a |
a Not significant.
TBBD Is a Site-specific Inhibitor of CARM1-mediated Methylation of Histone H3R17
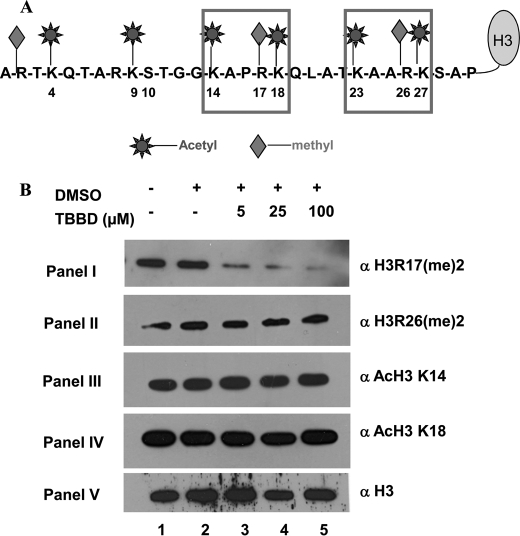
CARM1 can methylate histone H3 at three sites: Arg-2, Arg-17, and Arg-26 (28). Although Arg-2 has so far been reported as an in vitro site of methylation by CARM1, the enzyme has been shown to methylate both Arg-17 and Arg-26 (Fig. 3A) in vivo with several functional consequences. Recent studies have implicated H3R2 as an in vivo site of methylation for PRMT6 (29). Because TBBD-mediated inhibition of H3 methylation by CARM1 was found to be partial even at high concentrations of TBBD, and because the ITC results suggest that one site on histone H3 is favored for TBBD binding, we hypothesized that TBBD could be a site-specific inhibitor of CARM1. To investigate this phenomenon in the physiological condition, HeLa cells were treated with TBBD for 24 h and the acid-extracted histones from the cells were probed with specific antibodies against methylated H3 Arg-17 and H3 Arg-26. Significantly, it was observed that methylation of the H3 Arg-17 residue was inhibited by TBBD in a dose-dependent manner (Fig. 3B, panel I, lanes 3–5). The lower concentration of 5 μm led to more than 50% reduction of H3R17 methylation. As the dose was increased to 25 μm, a drastic reduction of almost 80% was observed. At the higher concentration of 100 μm, there was about 95% reduction in the H3 Arg-17 methylation levels, whereas, H3 Arg-26 methylation was not affected even with 100 μm TBBD (Fig. 3B, panel II, lanes 3–5). Closer examination of the histone H3 tail sequence revealed a characteristic pentapeptide consensus sequence, KAXRK, at the two CARM1 methylation sites, H3 Arg-17 and H3 Arg-26 (Fig. 3A). The pentapeptide motif differs by one amino acid between the two sites. In the case of the residue whose methylation is inhibited (Arg-17), the amino acid (X) preceding Arg-17 is Pro-16. For residue Arg-26 whose methylation is not affected by TBBD, Arg-26 is preceded by Ala-25. Because proline is known to be a conformational disrupter of polypeptide chains, we hypothesized that Pro-16 may be acting as the docking site for TBBD and thus preventing methylation at Arg-17. To confirm whether the inhibition observed is a true enzyme inhibition or an artifact of nonspecific blocking of histone accessibility at a certain region, we examined the acetylation status of histone H3 Lys-14 and Lys-18, which are also adjacent to the H3P16 site. When histones from TBBD-treated HeLa cells were probed with antibodies against acetylated histone H3K14 and Lys-18, interestingly, the acetylation status of both these residues was not affected by TBBD treatment (Fig. 3B, panels III and IV, lanes 4 and 5). The in vivo acetylation status of acetylated H3K14 and acetylated H3K18 were monitored by checking the steady state levels of the total histones. To exclude the possibility of TBBD affecting the total acetylation status by modulating acetyltransferase and deacetylase activities, an in vitro acetylation reaction in the presence of TBBD, using p300/KAT3B, was performed using core histones as substrate. These in vitro modified histones were processed for immunoblotting using the acetylated H3K14 and Lys-18 antibodies. It was observed that histone acetylation was unaffected by TBBD treatment (supplemental Fig. S4, panels III and IV, lanes 2–4) although the H3 Arg-17 methylation was affected at similar concentrations (supplemental Fig. S4, panel I, lanes 2–4). H3 Arg-26 methylation was unaffected (supplemental Fig. S4, panel II, lanes 2–4) as observed earlier. These observations clearly indicate that TBBD is a specific inhibitor of CARM1 even in the in vivo context with preference toward H3 Arg-17 methylation.
FIGURE 3.
TBBD is a site-specific inhibitor of CARM1-mediated methylation of histone H3. A, the possible sites of acetylation and arginine methylation on histone H3 tail sequence are indicated. B, HeLa cells were treated as indicated for 24 h: histones isolated from untreated cells (lane 1); DMSO-treated cells (lane 2); and TBBD-treated cells (lanes 3–5). Histone modifications were probed by Western blotting using the indicated site-specific antibodies.
Docking Studies of TBBD on CARM1-Histone H3 Complex
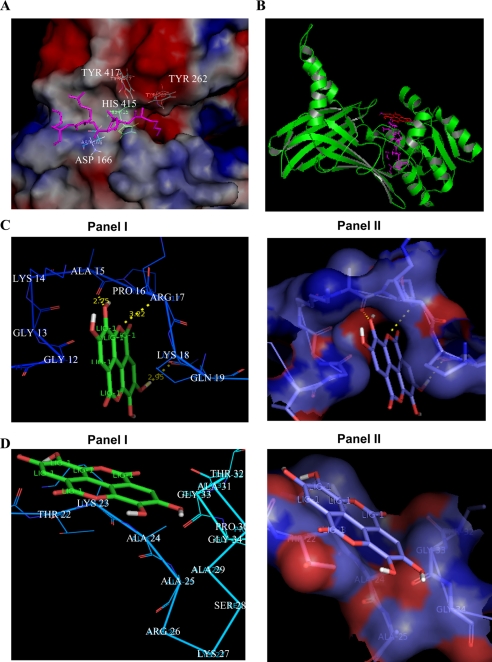
To verify any differential interaction of TBBD with the two motifs, KAPRK and KAARK, we resorted to molecular modeling and docking studies. We docked the pentapeptide sequence from amino acids 14 to 18, KAPRK of histone H3 into the substrate binding site of CARM1 (30, 40) along with TBBD. Because, the reported crystal structure was solved with only the three residues, “PRK,” of the above motif in the substrate binding site, the other two residues, “KA,” were simulated in the substrate binding site of the enzyme (Fig. 4A). It was observed that TBBD docks exactly into the above ES site (Fig. 4B). Interestingly, this arrangement yields a ligand conformation with ΔG = −5.05 kcal/mol, which is the lowest free energy of binding among the analyzed structures. However, to get a better understanding of TBBD interaction with the pentapeptide motif, the subsequent docking analysis was done using the pentapeptide motif and the inhibitor. As per the docking studies, TBBD forms three hydrogen bonds with Ala-15, Arg-17, and Gln-19 of histone H3, with ΔG = −5.36 kcal/mol (Fig. 4C, panels I and II). When the docking experiment was conducted by targeting the grid centered on the motif spanning amino acids 23 to 27, KAARK, of the same receptor molecule (histone H3), a different ligand conformation was obtained with a higher ΔG (Fig. 4D, panels I and II). The only difference between the two motifs is the presence of proline at the third position. This residue, Pro-16, in histone H3 is predicted to form hydrophobic interactions with TBBD, thus giving this conformation the lowest free energy of binding, indicating the possibility of the proline residue playing a critical role in determining the inhibition.
FIGURE 4.
Molecular modeling studies of TBBD with histone H3. A, the H3 (PRL) peptide has already been modeled into CARM1 substrate binding site. The H3 pentapeptide KAPRK has been modeled in the substrate binding site of CARM1. The figure shows the H3 pentapeptide near the active site residues of CARM1 (TYR417, HIS415, ASP166, TYR262). B, TBBD is modeled into this docked complex of CARM1 and H3 pentapeptide. Green, CARM1; magenta, H3 pentapeptide; red, TBBD. C, the docked conformation of TBBD near the KAPRK motif of histone H3. The free energy of binding for this ligand conformation is ΔG = −5.36 kcal/mol. D, the docked conformation of TBBD near the KAARK motif of histone H3. The free energy of binding for this ligand conformation is ΔG = −3.72 kcal/mol, which is higher than the free energy of binding at the KAPRK motif.
The Proline Residue of the Histone H3 Tail Is Essential for TBBD-mediated Inhibition of Arginine Methyltransferase Activity
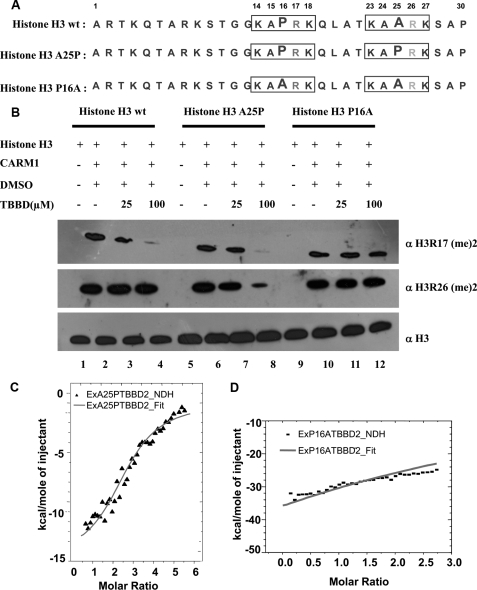
Involvement of the Pro-16 residue of histone H3 in bringing about the inhibition of CARM1 methylation at a single site was further validated by using site-directed mutagenesis. As mentioned above, wild-type histone H3 (Fig. 5A) is methylated by CARM1 at two sites, Arg-17 and Arg-26. As revealed by the docking data, TBBD binds the KAPRK stretch of histone H3, preventing methylation of H3 Arg-17 (Fig. 5B, panel I, lanes 2–4). However, KAARK is not bound by TBBD, thus allowing Arg-26 in this motif to be methylated (Fig. 5B, panel II, lanes 2–4). A mutant histone H3 (Fig. 5A) in which the alanine residue was mutated to proline (A25P) showed inhibition of H3 Arg-17 methylation by TBBD as expected (Fig. 5B, panel I, lanes 6–8). Significantly, we observed that the presence of proline at position 25 (A25P) led to inhibition of Arg-26 methylation by TBBD (Fig. 5B, panel II, lanes 6–8). However, a point mutant, P16A (Fig. 5A), in which proline at residue 16 was mutated to alanine, did not show any inhibition of methylation at both sites (Fig. 5B, panels I and II, lanes 10–12), establishing that it is indeed the proline residue that is responsible for TBBD-mediated inhibition of CARM1 methylation. Thus, in agreement with mechanistic differences in methylation of H3 Arg-17 and Arg-26 as observed by x-ray crystallographic analysis (30), TBBD-mediated inhibition of Arg-17 methylation is also uniquely specific.
FIGURE 5.
The proline residue (Pro-16) of the histone H3 tail is responsible for TBBD-mediated inhibition of arginine methyltransferase activity. A, sequence details of the histone H3 proteins used for the assay. B, in vitro histone methyltransferase assays were performed with CARM1 in the presence or absence of TBBD using histone H3 (lanes 1–4), mutant A25P histone H3 (lanes 5–8), and mutant P16A histone H3 (lanes 9–12) as substrates. Histone H3 in the absence of enzyme (lane 1), presence of enzyme and DMSO (lane 2), or in the presence of 25 μm and 100 μm TBBD (lanes 3 and 4). Histone H3 was probed with antibodies against dimethylated H3R17 (panel I) or dimethylated H3R26 (panel II). As a loading control, histones were probed with antibody against histone H3 (panel III). C, ITC was carried out by titrating histone H3 mutant A25P (7 μm) against the ligand TBBD (140 μm) at 25 °C. The one-site model with two binding sites is shown. D, ITC was carried out by titrating histone H3 mutant P16A (7 μm) against the ligand TBBD (140 μm) at 25 °C. No interaction was observed.
ITC studies using these point mutants confirmed the above observation. Data for the point mutant A25P, which has two proline residues adjacent to the arginine residue, indicates the presence of two binding sites (n = 2.40 ± 0) for TBBD on histone H3 (Fig. 5C). The binding of TBBD to this mutant substrate is also enthalpy driven, ΔH = −15.8 kcal/mol (Table 1). Significantly, the P16A mutant, which lacks the proline residue preceding the arginine, does not show any binding (n = 7.30 ± 1.59E3) with TBBD (Fig. 5D). Taken together, these observations suggest that Pro-16 of histone H3 is the docking site for TBBD, and thus this single amino acid determines the inhibitory effect of TBBD on CARM1 activity.
Histone H3R17 Methylation Regulates p53-responsive Gene Expression
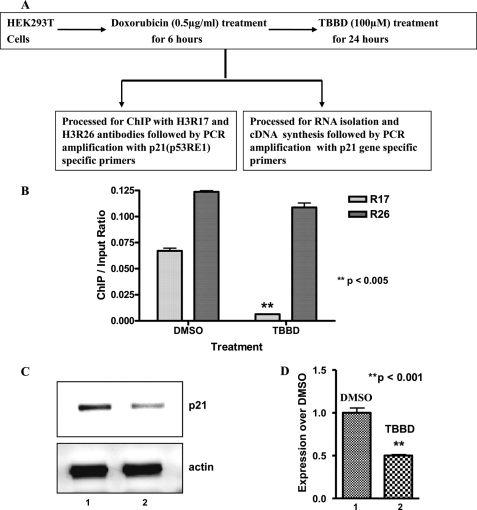
Methylation of Arg-17 and Arg-26 has been linked to transcriptional activation (31). However, the significance of Arg-17 methylation alone has not yet been elucidated. Recent reports have established functional interactions of p300, CARM1, and PRMT1 in p53-responsive gene expression (10). We found that TBBD has a minimal effect on p300 HAT activity and PRMT1 methyltransferase activity on histone H4R3 (data not shown); rather it inhibits CARM1-mediated histone H3 Arg-17 methylation only, as shown above. These unique properties of TBBD provided an opportunity to determine the effect of CARM1 methylation (especially of H3R17) on p53-responsive gene expression. We therefore investigated the effect of TBBD inhibition of H3R17 methylation of histones at the promoter of the p53-responsive gene, p21, in HEK293T cells that have endogenous wild-type p53. HEK293T cells were subjected to genotoxic insult by doxorubicin to induce p53 followed by TBBD treatment for 24 h and processed for ChIP or gene expression analysis (Fig. 6A). H3 Arg-17 methylation at the p21 promoter was verified by ChIP analysis (Fig. 6B, lane 1 versus lane 2). However, H3 Arg-26 methylation at this promoter region was minimally affected by treatment (Fig. 6B, lane 1 versus lane 2). Strikingly, reduced H3 Arg-17 methylation (upon TBBD treatment) at the p21 promoter was significantly correlated with repression of p21 expression (Fig. 6C, lane 1 versus 2). The p21 expression upon TBBD treatment was found to be about 2-fold reduced as compared with the solvent control (Fig. 6D, lane 1 versus 2). Similar results were obtained with H1299 cells transfected with p53 (data not shown). Taken together, these results suggest that H3 Arg-17 methylation by CARM1 is essential for p53-dependent regulation of p21 gene expression.
FIGURE 6.
Histone H3R17 methylation regulates p53-responsive gene expression. A, scheme representing the experimental procedure. HEK293T cells were treated as indicated and processed for either ChIP or gene expression analysis. B, state of histone H3 Arg-17/H3 Arg-26 methylation in the p53-responsive element (p53 RE) of the p21 promoter analyzed by ChIP. ChIP by the input ratio in cells treated with DMSO (lane 1) or TBBD (lane 2); n = 3, p < 0.005. C, repression in p21 expression was observed on TBBD treatment in the HEK293T cell line. Reverse transcriptase-PCR amplification of p21 expression in cells treated with DMSO (lane 1) or TBBD (lane 2). D, the expression of p21 is repressed on TBBD treatment. Real time PCR quantification of fold-change in p21 gene expression in cells treated with DMSO (lane 1) and treated with TBBD (lane 2) (n = 3, p < 0.001).
DISCUSSION
The novel CARM1-specific inhibitor reported here selectively blocks methylation of H3 Arg-17 but not of H3 Arg-26. Remarkably, sequence of the histone H3 tail (specifically the proline residue at position 16) determines the specificity of the inhibition brought about by TBBD. This report also demonstrates a role for H3 Arg-17 methylation in p21 gene expression dependent of p53; thus, TBBD could be used to investigate the roles of methylation in both apoptosis and tumor suppressor pathways. Most importantly, the data establish a new mechanism of specific enzyme inhibition determined by the amino acid sequence of the substrate.
The arginine methyltransferase, CARM1/PRMT4, has been shown to regulate important cellular processes such as pluripotency maintenance (16, 32), differentiation (6, 15, 17), splicing (18), and transcriptional activation (10–14), as well as tumor manifestation and progression (33, 34). However, the methyltransferase activity of this coactivator has been linked with only a few cases of transcriptional activation, muscle and thymocyte differentiation, and tumor progression. In such a scenario, the identification of a specific small molecule modulator targeting the methyltransferase activity would be highly useful in further delineating the essential role of CARM1 methyltransferase in gene expression and other cellular processes.
The small molecule inhibitor, TBBD (ellagic acid), reported here is a major component of pomegranate crude extract, which has been used against various ailments such as parasitic diseases, diarrhea, ulcers, and most importantly as an anticancer agent (35). The antitumor activity has been shown for several cancers especially prostate cancer, breast cancer, and also for colorectal cancer (36, 37). However, the active component from the crude extract and the exact molecular target within the physiological system has not been identified as yet. Possibly, the so far unidentified molecular target for TBBD is CARM1 activity. The crude extract could inhibit both p300 acetyltransferase activity as well as arginine methyltransferase activity of CARM1. This could be because of the various tannins and polyphenolic components of the pomegranate crude extract.
The mechanism of action of TBBD on CARM1 activity is a novel enzyme inhibition effect. Although in classical biochemistry there are examples of the substrate being sequestered by the inhibitor and thereby leading to inhibition (38), TBBD recognizes the substrate sequence and thus brings about inhibition at a single site of modification without affecting the other residues modification. The significance of the ES complex involving the KAPRK motif for Arg-17 methylation inhibition was further confirmed by verifying the modification status of the flanking residues, Lys-14 and Lys-18 acetylation, which were not affected on TBBD treatment. The sequence recognition and subsequent enzyme inhibition seems to require an active site in the context of the residue being recognized. Although characterization of the inhibition reported here, is with respect to histone H3, the protein arginine methyltransferase CARM1 also methylates a few nonhistone proteins including the acetyltransferase CREB-binding protein, HuR, PABP1, and TARPP (4). To have a further understanding of the molecular mechanism of TBBD-mediated inhibition of the arginine methyltransferase activity, these substrates also need to be subjected to similar studies.
CARM1 activity has been shown to be necessary for tumor progression as well as for p53 function (which is a tumor suppressor). The elucidation of the role of CARM1 in maintaining cellular homeostasis is essential. Such studies need to be characterized in light of the various cellular signals. Importantly, TBBD treatment did not affect expression of p21 in HeLa cells, whereas TBBD treatment caused a significant reduction of p21 gene expression in H1299 and HEK293T cells (Fig. 6C). The latter cells were subjected to DNA damage, which leads to activation of p53 downstream targets. Previous work showed that p53-dependent p21 expression is modulated by arginine methylation (39), and because H3 Arg-17 regulates the transcriptional activation of p53-responsive genes, our results also implicate methylation of H3 Arg-17 in p53-dependent gene expression. Fluorescence-activated cell sorter analysis of TBBD-treated HeLa cells (which lack functional p53) showed that there are no cell-cycle defects (supplemental Fig. S5). Possibly, H3 Arg-17 methylation is an important modulator of tumor suppressor function. However, a more intensive analysis of CARM1-mediated H3 Arg-17 methylation in different cell lines under different conditions should be undertaken to elucidate the spatiotemporal importance of H3R17 methylation.
Apart from elucidating the role of H3 Arg-17 methylation in p53 downstream target gene expression and other tumor suppression events, to our knowledge, the present report is the first to establish the molecular target of TBBD as the arginine methyltransferase CARM1, with important physiological consequences. Moreover, the inhibitory action of TBBD seems to be regulated by the substrate sequence itself, thereby highlighting a novel mechanism of enzyme inhibition. Thus, TBBD and its derivatives can be used, not only as probes for elucidating the role of H3 Arg-17 methylation in gene expression but also as therapeutic molecules targeting this modification in pathophysiological conditions.
Supplementary Material
Acknowledgments
We acknowledge Prof. M. R. S. Rao, President, JNCASR, for constant support and encouragement, Prof. Yoichi Shinkai for the G9a baculovirus, and A. Mangaiarkarasi, Molecular Virology Laboratory, JNCASR, for help with fluorescence-activated cell sorter analysis.
This work was supported by the Jawaharlal Nehru Centre for Advanced Scientific Research and the Department of Biotechnology, Government of India.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5 and data.
- PRMT
- protein arginine methyltransferases
- CARM1
- coactivator-associated arginine methyltransferase 1
- TBBD
- 2,3,7,8-tetrahydroxy(1)-benzopyrano(5,4,3(de)(1)-benzopyran-5,10-dione)
- ES complex
- enzyme-substrate complex
- AdoMet
- S-adenosylmethionine
- HEK
- human embryonic kidney cell line
- ITC
- isothermal calorimetry
- CREB
- cAMP-response element-binding protein
- HAT
- histone acetyltransferase
- DMSO
- dimethyl sulfoxide
- ChIP
- chromatin immunoprecipitation
- HMTase
- histone methyltransferase.
REFERENCES
- 1.Liu Q., Dreyfuss G. (1995) Mol. Cell. Biol. 15, 2800–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim S., Park G. H., Paik W. K. (1998) Amino Acids 15, 291–306 [DOI] [PubMed] [Google Scholar]
- 3.Lee D. Y., Teyssier C., Strahl B. D., Stallcup M. R. (2005) Endocr. Rev. 26, 147–170 [DOI] [PubMed] [Google Scholar]
- 4.Bedford M. T., Richard S. (2005) Mol. Cell. 18, 263–272 [DOI] [PubMed] [Google Scholar]
- 5.Schurter B. T., Koh S. S., Chen D., Bunick G. J., Harp J. M., Hanson B. L., Henschen-Edman A., Mackay D. R., Stallcup M. R., Aswad D. W. (2001) Biochemistry 40, 5747–5756 [DOI] [PubMed] [Google Scholar]
- 6.Kim J., Lee J., Yadav N., Wu Q., Carter C., Richard S., Richie E., Bedford M. T. (2004) J. Biol. Chem. 279, 25339–25344 [DOI] [PubMed] [Google Scholar]
- 7.Kang Z., Jänne O. A., Palvimo J. J. (2004) Mol. Endocrinol. 18, 2633–2648 [DOI] [PubMed] [Google Scholar]
- 8.Chen D., Huang S. M., Stallcup M. R. (2000) J. Biol. Chem. 275, 40810–40816 [DOI] [PubMed] [Google Scholar]
- 9.Stallcup M. R., Kim J. H., Teyssier C., Lee Y. H., Ma H., Chen D. (2003) J. Steroid Biochem. Mol. Biol. 85, 139–145 [DOI] [PubMed] [Google Scholar]
- 10.An W., Kim J., Roeder R. G. (2004) Cell 117, 735–748 [DOI] [PubMed] [Google Scholar]
- 11.Hassa P. O., Covic M., Bedford M. T., Hottiger M. O. (2008) J. Mol. Biol. 377, 668–678 [DOI] [PubMed] [Google Scholar]
- 12.Kleinschmidt M. A., Streubel G., Samans B., Krause M., Bauer U. M. (2008) Nucleic Acids Res. 36, 3202–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Messaoudi S., Fabbrizio E., Rodriguez C., Chuchana P., Fauquier L., Cheng D., Theillet C., Vandel L., Bedford M. T., Sardet C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 13351–13356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Covic M., Hassa P. O., Saccani S., Buerki C., Meier N. I., Lombardi C., Imhof R., Bedford M. T., Natoli G., Hottiger M. O. (2005) EMBO J. 24, 85–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S. L., Loffler K. A., Chen D., Stallcup M. R., Muscat G. E. (2002) J. Biol. Chem. 277, 4324–4333 [DOI] [PubMed] [Google Scholar]
- 16.Torres-Padilla M. E., Parfitt D. E., Kouzarides T., Zernicka-Goetz M. (2007) Nature. 445, 214–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav N., Cheng D., Richard S., Morel M., Iyer V. R., Aldaz C. M., Bedford M. T. (2008) EMBO Rep. 9, 193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng D., Côté J., Shaaban S., Bedford M. T. (2007) Mol. Cell. 25, 71–83 [DOI] [PubMed] [Google Scholar]
- 19.Majumder S., Liu Y., Ford O. H., 3rd, Mohler J. L., Whang Y. E. (2006) Prostate 66, 1292–1301 [DOI] [PubMed] [Google Scholar]
- 20.Greiner D., Bonaldi T., Eskeland R., Roemer E., Imhof A. (2005) Nat. Chem. Biol. 1, 143–145 [DOI] [PubMed] [Google Scholar]
- 21.Kubicek S., O'Sullivan R. J., August E. M., Hickey E. R., Zhang Q., Teodoro M. L., Rea S., Mechtler K., Kowalski J. A., Homon C. A., Kelly T. A., Jenuwein T. (2007) Mol. Cell. 25, 473–481 [DOI] [PubMed] [Google Scholar]
- 22.Krause C. D., Yang Z. H., Kim Y. S., Lee J. H., Cook J. R., Pestka S. (2007) Pharmacol. Ther. 113, 50–87 [DOI] [PubMed] [Google Scholar]
- 23.Spannhoff A., Machmur R., Heinke R., Trojer P., Bauer I., Brosch G., Schüle R., Hanefeld W., Sippl W., Jung M. (2007) Bioorg. Med. Chem. Lett. 17, 4150–4153 [DOI] [PubMed] [Google Scholar]
- 24.Osborne T., Roska R. L., Rajski S. R., Thompson P. R. (2008) J. Am. Chem. Soc. 130, 4574–4575 [DOI] [PubMed] [Google Scholar]
- 25.Swaminathan V., Reddy B. A., Selvi B. R., Sukanya. M. S., Kundu T. K. (2007) in Chromatin and Disease (Kundu T. K., Dasgupta D. eds) pp. 397–428, Springer Publications, London, UK [Google Scholar]
- 26.Selvi B. R., Pradhan S. K., Shandilya J., Das C., Sailaja B. S., Shankar G. N., Gadad S. S., Reddy A., Dasgupta D., Kundu T. K. (2009) Chem. Biol. 16, 203–216 [DOI] [PubMed] [Google Scholar]
- 27.Pokholok D. K., Harbison C.T., Levine S., Cole M., Hannett N. M., Lee T. I., Bell G. W., Walker K., Rolfe P. A., Herbolsheimer E., Zeitlinger J., Lewitter F., Gifford D. K., Young R. A. (2005) Cell. 122, 517–527 [DOI] [PubMed] [Google Scholar]
- 28.Teyssier C., Chen D., Stallcup M. R. (2002) J. Biol. Chem. 277, 46066–46072 [DOI] [PubMed] [Google Scholar]
- 29.Guccione E., Bassi C., Casadio F., Martinato F., Cesaroni M., Schuchlautz H., Lüscher B., Amati B. (2007) Nature 449, 933–937 [DOI] [PubMed] [Google Scholar]
- 30.Yue W. W., Hassler M., Roe S. M., Thompson-Vale V., Pearl L. H. (2007) EMBO J. 26, 4402–4412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer U. M., Daujat S., Nielsen S. J., Nightingale K., Kouzarides T. (2002) EMBO Rep. 3, 39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Q., Bruce A. W., Jedrusik A., Ellis P. D., Andrews R. M., Langford C. F., Glover D. M., Zernicka-Goetz M. (2009) Stem Cells 27, 2637–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frietze S., Lupien M., Silver P. A., Brown M. (2008) Cancer Res. 68, 301–306 [DOI] [PubMed] [Google Scholar]
- 34.Hong H., Kao C., Jeng M. H., Eble J. N., Koch M. O., Gardner T. A., Zhang S., Li L., Pan C. X., Hu Z., MacLennan G. T., Cheng L. (2004) Cancer 101, 83–89 [DOI] [PubMed] [Google Scholar]
- 35.Jurenka J. (2008) Altern. Med. Rev. 13, 128–144 [PubMed] [Google Scholar]
- 36.Dorai T., Aggarwal B. B. (2004) Cancer Lett. 215, 129–140 [DOI] [PubMed] [Google Scholar]
- 37.Losso J. N., Bansode R. R., Trappey A., 2nd, Bawadi H. A., Truax R. (2004) J. Nutr. Biochem. 15, 672–678 [DOI] [PubMed] [Google Scholar]
- 38.McGovern S. L., Helfand B. T., Feng B., Shoichet B. K. (2003) J. Med. Chem. 46, 4265–4272 [DOI] [PubMed] [Google Scholar]
- 39.Li P., Yao H., Zhang Z., Li M., Luo Y., Thompson P. R., Gilmour D. S., Wang Y. (2008) Mol. Cell. Biol. 28, 4745–4758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Troffer-Charlier N., Cura V., Hassenboehler P., Moras D., Caverelli J. (2007) EMBO J. 26, 4391–4401 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.