Abstract
The recent insight that brown adipocytes and muscle cells share a common origin and in this respect are distinct from white adipocytes has spurred questions concerning the origin and molecular characteristics of the UCP1-expressing cells observed in classic white adipose tissue depots under certain physiological or pharmacological conditions. Examining precursors from the purest white adipose tissue depot (epididymal), we report here that chronic treatment with the peroxisome proliferator-activated receptor γ agonist rosiglitazone promotes not only the expression of PGC-1α and mitochondriogenesis in these cells but also a norepinephrine-augmentable UCP1 gene expression in a significant subset of the cells, providing these cells with a genuine thermogenic capacity. However, although functional thermogenic genes are expressed, the cells are devoid of transcripts for the novel transcription factors now associated with classic brown adipocytes (Zic1, Lhx8, Meox2, and characteristically PRDM16) or for myocyte-associated genes (myogenin and myomirs (muscle-specific microRNAs)) and retain white fat characteristics such as Hoxc9 expression. Co-culture experiments verify that the UCP1-expressing cells are not proliferating classic brown adipocytes (adipomyocytes), and these cells therefore constitute a subset of adipocytes (“brite” adipocytes) with a developmental origin and molecular characteristics distinguishing them as a separate class of cells.
Keywords: Development Differentiation/Adipocyte, Receptors/Nuclear, Subcellular Organelles/Mitochondria, Microscopic Imaging, Mouse, PPARγ, UCP1, Brown Adipocytes, Rosiglitazone, White Adipocytes
Introduction
Primarily because of their shared ability to accumulate lipids, brown and white adipocytes have classically been considered to be closely related cell types, implicating a close common progenitor. However, studies in recent years have completely altered this concept. In 2006, Atit and colleagues (1) observed that cells deriving from the central dermomyotome, molecularly defined as cells that at some time in their development had expressed the homeobox transcription factor Engrailed 1, developed into three types of tissue: dermis, muscle, and brown adipose tissue (BAT),2 thus implying a close developmental relationship between brown adipocytes and myocytes. Because the mice had not developed white adipose tissue (WAT) at the time of investigation, the relationship between the origin of WAT versus BAT could not be elucidated. However, simultaneously, we found that cell cultures of precursors from BAT, but not cultures of WAT precursors, initially demonstrated a remarkable expression of genes that had always been considered to be muscle-specific; thus these brown adipocytes expressed a myogenic signature that was not shared by the white adipocytes, clearly indicating different origins of the two cell types (2). Also, muscle-specific microRNAs (“myomirs”) were expressed and maintained in brown adipocytes but not in white adipocytes (3). Further, it was demonstrated by Seale and coworkers in 2008 in intact mice that cells that had expressed the “myogenic” transcription factor myf5 during development could develop into cells constituting muscle or BAT, but never into the cells found in WAT (4).
Thus, based on this, it would seem that a clear distinction between the white adipocyte and the brown adipocyte (that perhaps should rather be considered as an “adipomyocyte” (5)) could be made; the adipomyocyte should particularly be distinguished from the myocyte by expression of the PRDM16 gene (4, 6, 7).
However, a complicating issue is that a not insignificant expression of the “brown fat-specific” uncoupling protein-1 (UCP1) can be encountered in vivo in adipose tissues that are normally considered WAT depots, either in response to chronic β-adrenergic stimulation (4, 8–11) or in response to chronic PPARγ-agonist stimulation (12–17). This is particularly evident in the inguinal depot, whereas the epididymal depot shows this to the least degree. The basis for this “ectopic” expression of UCP1 is unclear and challenging. Questions that may be formulated include whether this UCP1 is expressed in a few “adipomyocytes” that are resident in the WAT depots, but not normally sufficiently conspicuous to be detected, but that may proliferate and differentiate given the correct stimulus, or whether the UCP1 is found in certain “non-adipomyocyte” cells that thus, despite a different origin from “true” brown adipocytes (the adipomyocytes), can be forced to initiate UCP1 gene expression. Additionally it may be asked whether this expression of UCP1 is an isolated phenomenon, related only to the expression of this particular gene, or do these non-adipomyocytes develop the complete expression profile of a true adipomyocyte? In addition, do all white adipocytes possess this ability or is it only a subset that can respond?
When examined at either the morphological or the molecular level, the brown and the white adipose tissues appear, as indicated above, markedly different, particularly with respect to the expression of UCP1, the ‘brown fat-specific‘ uncoupling protein (e.g. supplemental Fig. 1). However, in situ, the adipocytes will be exposed to distinctive but different external agents (neuronal transmitter substances, hormones, cytokines, etc.), and thus the differences may reflect these external forces rather than be (fully) inherent as a difference between the brown and the white adipocytes. Therefore, primary cultures of white and brown fat precursor cells represent invaluable tools to characterize cell autonomous differentiation of brown and white adipocytes. We have previously shown (2, 18) (supplemental Fig. 2) that white and brown fat precursor cells in culture proliferate and develop into adipocytes, which have distinct, inherent characteristics, resembling (at the molecular level) white and brown adipocytes differentiated in vivo (supplemental Fig. 1). To examine the nature of the UCP1-expressing cells that may be observed in white adipose tissue depots in vivo, we have here treated white adipocyte cultures to enable occurrence of UCP1-expressing cells. Assuming that the most ‘pure‘ white adipocyte cultures would give the most distinct results, we utilized primary cultures of the most pure white fat depot, those obtained from epididymal WAT (9, 19). The most efficient means of inducing ‘browning‘ in white fat depots (15) and even in human white adipocyte cultures (20) is the use of PPARγ activators. Thus, we treated primary cultures of epididymally derived white adipocytes with the potent PPARγ-ligand rosiglitazone and observed that rosiglitazone, besides promoting adipose differentiation, also led to marked UCP1 gene expression even in these pure white adipocyte cultures. We demonstrate here that PPARγ-agonist treatment causes a subset of ‘white‘ adipocytes to express a broad but nonetheless incomplete array of classic brown adipocyte genes, clearly distinguishing these cells molecularly and developmentally from classic brown adipocytes. Despite this, the cells ultimately demonstrate the hallmark of functional brown adipocytes: the ability to perform norepinephrine-induced thermogenesis.
EXPERIMENTAL PROCEDURES
Animals, Cell Isolation, and Cell Culture
Male outbred NMRI mice, purchased from a local supplier (B&K, Stockholm, Sweden), or, where indicated, male UCP1-KO mice (on C57Bl/6 background) and UCP1-wild-type mice (C57Bl/6), bred at the Stockholm University animal facility, were used for the preparation of primary cultures of brown and white adipocytes. BAT was isolated from the interscapular, cervical, and axillary depots, WAT was isolated from epididymal depots, and all isolated depots were processed as described in a previous study (21). The pellet was then suspended in culture medium (0.5 ml/animal for brown preadipocytes, 0.4 ml/animal for white preadipocytes). The cells were cultured in 6-well plates (10 cm2/well, Corning); 2 ml of culture medium was added to each well before 0.2 ml of cell suspension was added. The culture medium was Dulbecco's modified Eagle's medium with 10% (v/v) newborn calf serum (Invitrogen), 2.4 nm insulin, 25 μg/ml sodium ascorbate, 10 mm HEPES, 4 mm glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin, supplemented or not (as indicated) with 1 μm rosiglitazone maleate (Alexis Biochemicals). The cells were grown at 37 °C in an atmosphere of 8% CO2 in air with 80% humidity. The cells were washed in Dulbecco's modified Eagle's medium, and medium was changed on day 1 and then every second day. The medium was not changed on the day the cells were harvested. The experiments were performed on different days of culture, as indicated in each individual experiment. To enable a fair comparison with brown adipocytes, only control white adipocyte cultures exhibiting ∼50% or more differentiated cells were analyzed.
Analysis of mRNA Levels
Northern Blot
After the experiments, the medium was discarded and the cells were harvested from each well with 1 ml of Ultraspec (Biotecx Laboratories, Houston, TX) as described in the manufacturer's protocol. The RNA obtained was examined by Northern blotting as described previously (21).
Quantitative Real-time PCR
For determination of mRNA levels, 1 μg of RNA was reverse-transcribed with a High Capacity cDNA kit (Applied Biosystems, Foster City, CA) in a total volume of 20 μl. Primers (exon-spanning, supplemental Table S1) were pre-mixed with SYBR® Green JumpStartTM Taq ReadyMixTM (Sigma-Aldrich), and aliquots of 11 μl were applied to 96-well MicroAmp Optical plates (Applied Biosystems). cDNA was diluted 1:10, and aliquots of 2 μl were added in triplicates. Thermal cycling conditions were: 2 min at 50 °C, 10 min at 95 °C, and 40 cycles of 15 s at 95 °C and 1 min at 65 °C on an ABI Prism® 7000 Sequence Detection Real-Time PCR System (Applied Biosystems). The ΔCt method was used to calculate relative changes in mRNA abundance. The threshold cycle (Ct) for TATA-binding protein (TBP) was subtracted from the Ct for the target gene to adjust for variations in the cDNA synthesis.
miR-206 expression was determined as in a previous study (3). TBP mRNA was used as endogenous control, as above.
Analysis of UCP1 and VDAC Protein Levels
Immunocytochemistry
White adipocytes were cultured as described above except that 20 × 20-mm coverslips were placed in the wells. Cells were cultured for 7 days in the absence or presence of 1 μm rosiglitazone. The cells were then washed twice with PBS and fixed with 3% paraformaldehyde in PBS for 20 min at room temperature. Cells were washed three times with PBS and then exposed to 5% glycine in PBS to quench unspecific fluorescence. Cells were then washed three times with PBS and permeabilized with 5% acetic acid in ethanol for 10 min at −20 °C. Then the cells were washed three times with PBS and blocked with 8% bovine serum albumin in PBS for 1 h at room temperature. Cells were washed three times with PBS and incubated with 1:3000 diluted anti-UCP1 antibody and 1:3000 diluted anti-VDAC antibody in 8% bovine serum albumin in PBS overnight at 4 °C. Cells were washed three times with PBS and incubated with anti-rabbit-Alexa Fluor 488-labeled and anti-mouse-Texas Red-labeled secondary antibodies (Molecular Probes), diluted 1:1000 in 8% bovine serum albumin in PBS for 1 h at room temperature. Cells were then washed three times with PBS. Nuclei were stained with 1 μg/ml Hoechst 33258 for 15 min. Finally, coverslips were mounted on microscope slides with ProLong Gold Antifade reagent (Molecular Probes). The cells were examined with a Zeiss fluorescence microscope equipped with (Axio Cam HRc) charge-coupled device camera. Wide-field images were acquired with Openlab version 3.1.4 (Improvision). Images were adjusted for brightness/contrast and merged in Photoshop (Adobe Systems Inc.). Images in each figure were processed equally.
Western Blot
Western blotting was performed as described before (21). Antibodies used were UCP1 antibody (rabbit polyclonal, raised against C-terminal decapeptide), diluted 1:3000, and VDAC monoclonal antibody (Calbiochem, 529536), diluted 1:2000.
Oxygen Consumption
White adipocyte cultures were cultured in 6-well plates for 6 days. The cells in 9 wells were then simultaneously trypsinized for 3–5 min, pooled, and centrifuged for 2 min at 700 × g. Oxygen consumption rates of cells were monitored with a Clark-type oxygen electrode (Yellow Springs Instrument) as described before (21).
RESULTS
PPARγ Activation Enables White (Pre)adipocyte Cultures to Acquire Brown Adipocyte-like Characteristics
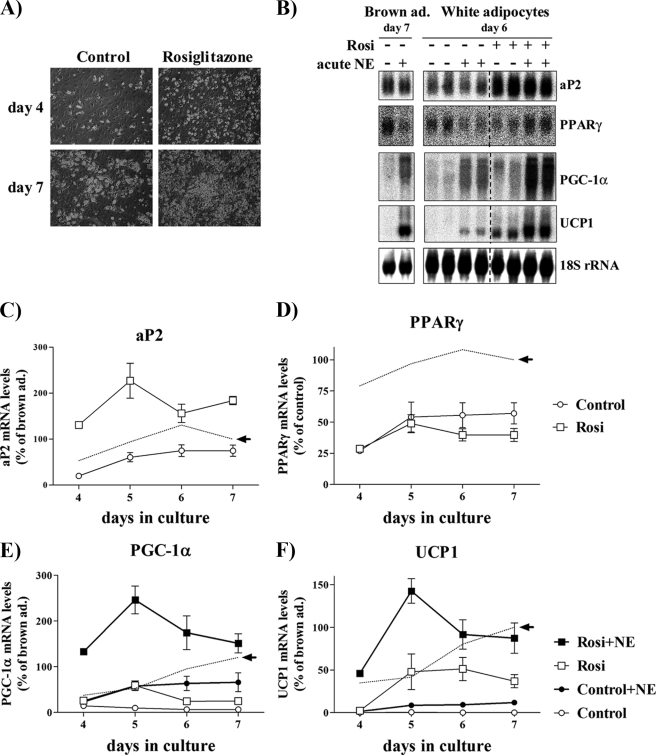
To examine whether the effects of in vivo PPARγ stimulation on the occurrence of UCP1-expressing adipocytes in WAT can be mimicked by similar treatment in vitro, we continuously treated primary cultures of white adipocytes with the potent PPARγ agonist rosiglitazone, starting immediately after plating. We characterized these cultures during differentiation both at the morphological (Fig. 1A) and at the molecular level, examining the expression of general adipogenic genes (aP2 and PPARγ), as well as the brown (as opposed to white) adipocyte-related genes PGC-1α and UCP1 (Fig. 1, B–F).
FIGURE 1.
Expression levels of marker genes during rosiglitazone-stimulated differentiation of white adipocytes. Primary cultures of white adipocytes were grown for the indicated number of days in the absence (control) or presence of 1 μm rosiglitazone. Where indicated, 1 μm norepinephrine (NE) had been added 2 h before harvest. A, white (pre)adipocyte cultures were examined with phase-contrast microscopy after 4 or 7 days in culture. B, representative Northern blot. Total RNA (10 μg) was used per lane, and the blot was hybridized with the aP2, PPARγ, PGC-1α, UCP1, and 18 S rRNA probes. aP2 (C), PPARγ (D), PGC-1α (E), and UCP1 (F) mRNA levels are shown during spontaneous and rosiglitazone-stimulated differentiation of white (pre)adipocyte cultures. The aP2, PPARγ, PGC-1α, and UCP1 mRNA levels were normalized to the 18 S rRNA levels in each sample. The points represent means ± S.E. of four (days 5, 6, and 7) or one (day 4) independent experiments, each performed in duplicate. Stippled lines represent corresponding expression levels in parallel cultures of non-rosiglitazone-treated brown adipocytes (as published previously (21)). The brown adipocyte control levels of aP2 and PPARγ and NE-induced levels of PGC-1α and UCP1 at day 7 were set in each experiment to 100% (indicated by arrow), and the white adipocyte aP2, PPARγ, PGC-1α, and UCP1 mRNA levels on different days were expressed relative to this value in each individual experiment. Rosiglitazone treatment significantly increased the aP2 (p < 0.001), the PGC-1α (p < 0.01), and the UCP1 (p < 0.01) mRNA levels (two-way analysis of variance with replicates; rosiglitazone treatment versus time in culture).
Based on morphology and lipid accumulation (Fig. 1A), the differentiation of the white adipocytes in the chronic presence of rosiglitazone was, as expected, markedly improved, resulting in a significantly increased expression of the late adipogenic marker aP2 (Fig. 1, B and C) and triacylglycerol mass (158 ± 19 pmol/μg of protein in control cultures versus 253 ± 27 in rosiglitazone-treated cultures, n = 3, p < 0.01). PPARγ mRNA levels were unchanged (Fig. 1, B and D), but because activators of PPARγ down-regulate PPARγ gene expression (21), PPARγ is not a suitable adipocyte differentiation marker in rosiglitazone-treated cultures. Importantly, PPARγ mRNA was expressed not only in mature white adipocytes but already in white preadipocytes (Fig. 1, B and D) (22). This indicates that the fibroblast-like undifferentiated precursor cells are already determined for their adipocyte destiny.
In untreated white adipocyte cultures, the brown versus white fat-specific genes PGC-1α and UCP1 were barely or not expressed (Fig. 1, B, E, and F). Unexpectedly, acute NE stimulation of the white adipocyte cultures induced expression of PGC-1α to nearly 50% of the NE-induced levels in brown adipocytes (Fig. 1E, filled circles). Importantly, however, the NE-induced levels of UCP1 in the white adipocyte cultures remained very low (Fig. 1F, filled circles).
In the presence of rosiglitazone, the PGC-1α mRNA levels of the white adipocyte cultures were augmented (Fig. 1E, empty squares). Acute NE stimulation of these rosiglitazone-treated cultures caused a significant further increment of PGC-1α expression (Fig. 1E, filled squares), which, unexpectedly, was even higher than in NE-treated brown adipocytes and reached its maximal level on day 5.
Even more notably, treatment with rosiglitazone resulted in the white adipocytes acquiring the basic property of brown adipocytes: the ability to express UCP1 (Fig. 1, B and F). White adipocyte cultures continuously treated with rosiglitazone expressed UCP1 even in the absence of NE stimulation (Fig. 1F, empty squares). Acute NE treatment further increased UCP1 expression (Fig. 1F, filled squares), to levels that were now even higher than those in NE-treated brown adipocytes. Thus, rosiglitazone-treated white adipocyte cultures became not only fully differentiated but also exhibited brown adipocyte-like characteristics: they expressed PGC-1α and UCP1 at levels that were indistinguishable from those in NE-treated brown adipocyte cultures.
Rosiglitazone Does Not Favor Growth of Brown over White Adipocytes
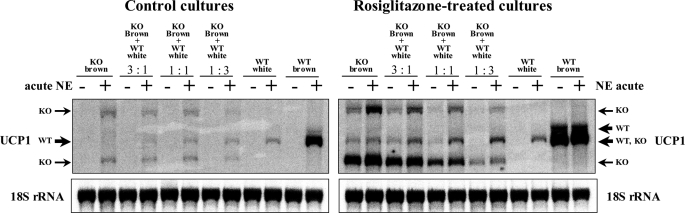
One explanation for the ability of rosiglitazone to turn white adipocyte cultures into cultures resembling brown adipocytes could be that a small number of true brown adipocyte precursors could exist in the precursor preparations (8, 9). As rosiglitazone promotes differentiation of brown adipocytes (21), what is seen during rosiglitazone treatment of cell cultures (or in animals during thiazolidinedione treatment) could therefore be promotion of the proliferation of these brown precursor cells. This would mean that no cell in itself would change its inherent characteristics, but the rosiglitazone treatment would favor the growth of the brown over the white. This hypothesis could be tested by utilization of co-cultures of white and brown adipocytes and by following their destiny after rosiglitazone-induced differentiation. However, this presupposes that the original provenance of the cells from BAT versus WAT is distinguishable, even after treatment. Serendipitously, the availability of UCP1-knock-out (UCP1-KO) mice enables such ‘labeling‘ of the anatomic origin of brown and white adipocytes in mixed cultures. Even in the UCP1-KO mice, the UCP1 promoter is fully functional. After norepinephrine and/or rosiglitazone stimulation, three different transcripts result, which can be identified in Northern blots: two transcripts of lengths different from the length of the UCP1-wild-type transcript (one shorter and one longer) (23) and a third one, very weakly expressed, present only in rosiglitazone-treated cells, of similar length to that of the UCP1-wild-type transcript (Fig. 2 and supplemental Fig. 3). Thus, these different UCP1 transcripts can be employed as markers of adipocyte origin. Consequently, white and brown adipocyte co-cultures can be utilized to investigate whether treatment promotes proliferation of a particular cell subtype and whether brown and white adipocytes influence each other during differentiation.
FIGURE 2.
Rosiglitazone does not favor growth of brown over white adipocytes. Primary cultures of white adipocytes originating from UCP1-wild-type mice (C57Bl/6) and brown adipocytes originating from UCP1-KO mice (on C57Bl/6 background) were grown for 7 days separately or in co-culture in the indicated ratios, in the absence (left panel) or presence of 1 μm rosiglitazone (right panel). Where indicated, 1 μm norepinephrine (NE) had been added 2 h before harvest. Note that, in these rosiglitazone-treated white adipocytes cultures (originating from C57Bl/6 mice), the UCP1 transcripts were detected only after NE stimulation. Representative Northern blots are shown (three additional experiments gave principally the same results). Total RNA (10 μg) was used per lane, and the blot was hybridized with the UCP1 and 18 S rRNA probes. Arrows indicate wild-type (WT) and knockout (KO) UCP1 transcripts. Since rosiglitazone in UCP1-KO cells induces a transcript that co-migrates with the predominant UCP1-WT transcript, the genetic identity of the cells was verified by reverse transcription-PCR (supplemental Fig. 4). An example of a corresponding experiment where the origins of the cells (from wild-type or UCP1-KO mice) were reversed can be seen in supplemental Fig. 3.
We grew primary cultures of white adipocytes originating from UCP1-wild-type mice and brown adipocytes originating from UCP1-KO mice for 7 days separately and in co-culture (Fig. 2) (and vice versa, supplemental Fig. 3), in different ratios as indicated, in the absence or presence of chronic rosiglitazone and/or NE 2 h before harvest. As seen, in control co-cultures, UCP1 transcripts were visible only after NE stimulation. In the presence of NE, both the UCP1-KO transcripts, originating from the brown precursors, and the UCP1-WT transcript, originating from the white precursors, were identified at levels proportional to the number of plated brown and white adipocytes, respectively (Fig. 2, left panel). Thus, the precursor cells from BAT and WAT in co-culture demonstrate similar growth rates.
If rosiglitazone in white adipocyte cultures promoted growth of true, sporadic brown adipocytes, the number of brown adipocytes in co-culture should be significantly higher than that expected based on the initial proportions of mixed brown and white preadipocytes; consequently, the UCP1 transcript tagged from the brown fat cells would be expressed at levels that were also significantly higher than the levels expected based on the initial proportions of mixed brown and white preadipocytes. However, the results obtained in the rosiglitazone-treated cultures were not in agreement with this suggestion. Similarly to the control cultures, both UCP1-KO and UCP1-WT transcripts were identified in the rosiglitazone-treated cultures at levels proportional to the number of plated brown and white adipocytes (Fig. 2, right panel); in co-cultures in which one-quarter, one-half, or three-quarters of the starting cells were of BAT origin, the levels of UCP1-KO transcripts originating from brown adipocytes were not higher than one-quarter, one-half, or three-quarters of the UCP1-KO transcript levels found in the brown adipocyte-cultures grown separately, and vice versa. Thus, each cell type remained faithful to its original destiny, despite the presence of the other adipocyte type, and rosiglitazone did not differentially affect the growth of the white or brown adipocytes. Consequently, the browning effect of rosiglitazone on the white adipocyte cultures cannot be explained by the overgrowth of a few pre-existing brown adipocytes in these cultures. It should be noted that co-cultures in which the white adipocytes came from UCP1-KO animals yielded corresponding results (supplemental Fig. 3).
No Release of a Browning Agent
As seen above, precursor cells isolated from BAT depots spontaneously differentiate in culture into cells that morphologically and biochemically had a high degree of similarity to brown adipocytes matured in vivo, but precursor cells originating from the epididymal WAT depot attained brown adipocyte characteristics only after rosiglitazone treatment. It could therefore be suggested that brown adipocyte cultures inherently possess and/or produce a unique PPARγ ligand, necessary and sufficient to predetermine precursor cells originating from BAT to a brown adipocyte destiny, whereas white adipocyte cultures lack this. Particularly, NE stimulation may induce the brown adipocytes to produce and release such a substance (e.g. a natural PPARγ agonist with enhanced activating properties, i.e. similar to the artificial agonist rosiglitazone used here). The results in Fig. 2 and supplemental Fig. 3 can also be analyzed to elucidate this possibility. As seen, the white adipocytes grown in co-culture with brown adipocytes under control conditions (in the absence of rosiglitazone) (Fig. 2 and supplemental Fig. 3, left panels) did not attain brown adipocyte-like characteristics. Thus, brown adipocytes, by their mere presence, could not alone accomplish an effect similar to the browning effect of rosiglitazone, and, even after the addition of NE, no such effect could be detected. Thus, the existence of an autocrine/paracrine brown adipocyte-specific PPARγ ligand (or similar) could not be the explanation for commitment of precursor cells originating from either WAT or genuine BAT to a brown adipocyte lineage. Thus, brown (pre)adipocytes do not self-promote a brown adipocyte lineage by production/secretion of an agent such as a specific PPARγ ligand.
Rosiglitazone-treated White Adipocyte Cultures Exhibit Distinct Brown Adipocyte-specific Markers
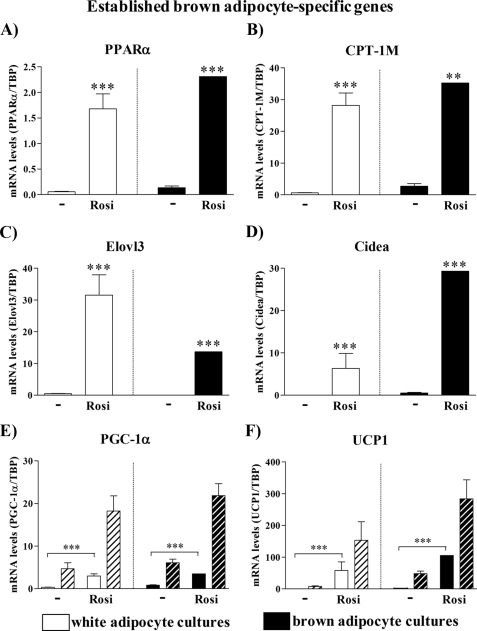
UCP1 is the only protein expressed exclusively in brown adipocytes (24). However, besides UCP1, brown adipocytes are defined at the molecular level by expression of a set of genes that are barely or not expressed in white adipocytes. An important question is whether rosiglitazone in white adipocyte cultures induces manifestation of the entire brown fat-gene expression program or specifically induces only expression of the UCP1 gene (and some thermogenesis-related genes, such as PGC-1α (Fig. 1E)), whereas other brown fat-specific genes remain unaffected. To explore this question, we measured the expression of brown (as opposed to white) adipocyte-specific genes in primary cultures of white and brown adipocytes continuously treated or not with rosiglitazone. We analyzed cells on day 5, when the NE-induced mRNA levels of UCP1 and PGC-1α in rosiglitazone-treated white adipocyte cultures were highest (Fig. 1, E and F).
Established Brown Adipocyte-specific Genes
Gene expression analysis showed that white adipocyte cultures continuously stimulated with rosiglitazone expressed numerous established brown fat-specific genes (Fig. 3). PPARα, associated with the induction of lipid-catabolizing systems (25) and mitochondriogenesis (26), was lower in untreated white adipocytes than in untreated brown adipocytes. After rosiglitazone treatment, the expression levels were increased in both types of cultures, the white adipocyte level now being practically equal to the brown adipocyte level (Fig. 3A). Concerning the mitochondrial carnitine palmitoyl transferases, the liver isoform is normally expressed in WAT and the muscle isoform (CPT-1M) in BAT (27), as confirmed here in the untreated cells. However, after rosiglitazone treatment, a high induction of CPT-1M was observed in both types of adipocytes (Fig. 3B). Elovl3 (Cig30) is expressed in intact animals only in BAT and only after cold exposure (28), but it is remarkably induced even in the white adipocyte cultures by rosiglitazone (Fig. 3C). The exception to this pattern was the expression of Cidea. Cidea (in mice) is highly expressed in BAT but not in WAT (29, 30). Although Cidea gene expression is induced >100 times in white adipocyte cultures continuously treated with rosiglitazone, it was still significantly lower than in corresponding brown adipocyte cultures (Fig. 3D). PGC-1α and UCP1 behaved as in Fig. 1 (E and F).
FIGURE 3.
Expression levels of established brown adipocyte marker genes in control and rosiglitazone-stimulated cultures of white and brown adipocytes. Primary cultures of white and brown adipocytes were grown for 5 days in the absence (control) or presence of 1 μm rosiglitazone. Gene expression was analyzed by quantitative reverse transcription-PCR. PPARα (A), CPT-1M (B), Elovl3 (C), Cidea (D), PGC-1α (E), and UCP1 (F) levels in control and rosiglitazone-stimulated white and brown adipocytes on day 5 of culture. Hatched bars (PGC-1α and UCP1) represent values in the cells treated with 1 μm NE for 2 h. The expression levels of the genes studied were normalized to the TBP levels in each sample. The values represent means ± S.E. of five independent experiments, each performed in duplicate. Expression levels in the rosiglitazone-treated brown adipocyte cultures were set in each experiment to the mean of its normalized values, and the levels in other brown and white adipocytes were expressed relative to this value in each individual experiment. The significance of rosiglitazone treatment was determined with a paired t test performed on the logarithmized expression levels of control and rosiglitazone-stimulated white and brown adipocyte cultures. ***, p < 0.001.
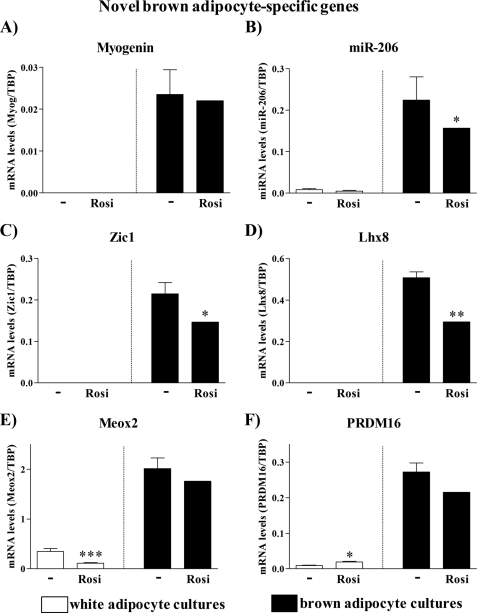
Novel Brown Adipocyte-specific Genes
In our detailed transcriptome analysis of primary brown versus white preadipocyte maturation, we found that brown preadipocytes demonstrate an early transient myogenic transcriptional signature (2) and also maintain expression of classic muscle-specific microRNAs (3). To examine whether the rosiglitazone-treated white adipocytes had also attained these ‘novel‘ but highly origin-specific markers, we measured expression of myogenin, a transcription factor crucial for myogenesis (31), as well as expression of the classic muscle-specific microRNA miR-206 (32). Consistent with previous studies, both myogenin mRNA and miR-206 were expressed in brown (pre)adipocytes and were essentially undetectable in the white adipocytes. However, neither myogenin nor miR-206 were induced by rosiglitazone in white adipocyte cultures (Fig. 4, A and B). Thus, the attainment of brown adipocyte characteristics was not associated with a reprogamming of the adipocytes to fully convert into true brown adipocytes.
FIGURE 4.
Expression levels of novel brown adipocyte marker genes in control and rosiglitazone-stimulated cultures of white and brown adipocytes. Primary cultures of white and brown adipocytes were grown as above (Fig. 3). Gene expression was analyzed by quantitative reverse transcription-PCR. Myogenin (A), miR-206 (B), Zic1 (C), Lhx8 (D), Meox2 (E), and PRDM16 (F) levels in control and rosiglitazone-stimulated white and brown adipocytes on day 5 of culture are shown. The expression levels of the genes studied were normalized to the TBP levels in each sample. The values represent means ± S.E. of four independent experiments, each performed in duplicate. Expression levels in the rosiglitazone-treated brown adipocyte cultures were set in each experiment to the mean of its normalized values, and the levels in other brown and white adipocytes were expressed relative to this value in each individual experiment. Statistical analysis is as in Fig. 3. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
The transcriptome analysis (2) and a global transcription factor screen (33) have identified other novel differences in the transcription factor profile between brown and white adipocytes. Therefore, we examined the expression of the brown (versus white) adipocyte-specific transcription factors Zic1, Lhx8, Meox2, and PRDM16 (Fig. 4, C–F), which were identified in those studies. These transcription factors were not at all or only barely expressed in untreated white adipocyte cultures. Importantly, none of these brown adipocyte-specific genes was induced in white adipocyte cultures upon rosiglitazone treatment (but were somewhat down-regulated in brown adipocytes, in accordance with these factors being implicated in early differentiation). There is particular interest in PRDM16, the expression of which has not earlier been compared in primary cultures of brown versus white adipocytes. PRDM16 has been identified as the key molecular switch determining the development of brown adipocytes from a progenitor that expresses myoblast markers (4). We found that PRDM16 was present in very modest amounts in white adipocytes, and a very modest induction resulted from rosiglitazone treatment. Thus, whereas PRDM16 seems to be the factor that channels classic brown adipocytes into the brown adipocyte versus the myocyte pathway and is thus essential for the cells to acquire the ability to express UCP1, it is apparently not PRDM16 gene expression that confers white adipocytes with the ability to express UCP1. Thus, investigation of the transcription factor profile clearly indicates that the rosiglitazone-induced adipocytes with brown adipocyte characteristics are developmentally qualitatively distinct from classic brown adipocytes and thus should not be referred to as classic brown adipocytes.
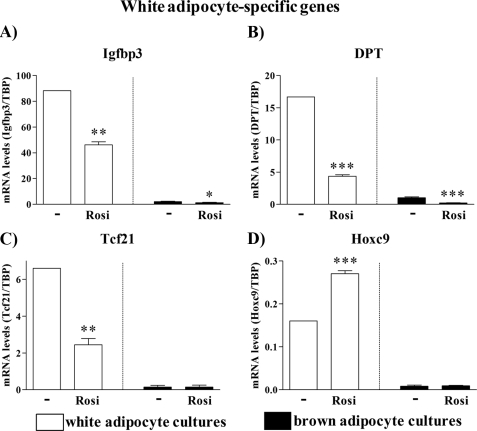
White Adipocyte-specific Genes
In our transcriptome analysis of brown versus white adipocyte gene expression (2), we also identified a series of genes that were practically white adipocyte-specific. In the rosiglitazone-treated cells, the mRNA levels of several of these genes were significantly reduced: the structural proteins Igfbp3 (insulin-like growth factor-binding protein 3) and DPT (dermatopontin) and the transcription factor Tcf21 (Fig. 5, A–C). Thus, in the presence of rosiglitazone, white adipocyte cultures apparently became not only more ‘brown‘ but also less ‘white.‘ Further, we examined the expression of the developmental gene Hoxc9 (Fig. 5D), earlier reported to be expressed in subcutaneous and epididymal white adipocytes but not in brown adipocytes (2, 34). Expression of Hoxc9 was indeed white (versus brown) adipocyte-specific and, importantly, was not reduced by rosiglitazone treatment. Given that Hoxc9 expression was persistent during differentiation of white adipocyte cultures, Hoxc9 fulfills the criteria for being a white adipocyte cell autonomous trait. Therefore, although phenotypically different, rosiglitazone-treated white adipocyte cultures still demonstrate their white fat origin.
FIGURE 5.
Expression levels of white-adipocyte marker genes in control and rosiglitazone-stimulated cultures of white and brown adipocytes. Primary cultures of white and brown adipocytes were grown as above (Figs. 3 and 4). Gene expression was analyzed by quantitative reverse transcription-PCR. Igfbp3 (A), DPT (B), Tcf21 (C), and Hoxc9 (D) levels are shown in control and rosiglitazone-stimulated white and brown adipocytes on day 5 of culture. The expression levels of the genes studied were normalized to the TBP levels in each sample. The values represent means ± S.E. of four independent experiments, each performed in duplicate. Expression levels in the untreated white adipocyte cultures were set in each experiment to the mean of its normalized values, and the levels in other white and brown adipocytes were expressed relative to this value in each individual experiment. Statistical analysis is as in Fig. 3. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Thus, rosiglitazone indeed induced UCP1 and other established brown fat-specific genes but was not capable of driving the full brown adipocyte-differentiation program in white adipocyte cultures: rosiglitazone-treated white adipocyte cultures did not demonstrate either a myogenic or novel brown fat specific transcription factor signature. Importantly, white adipocyte cultures that attained the ability to express UCP1 also persisted in expressing the white adipocyte-marker Hoxc9. Thus, at the molecular level, these cells were qualitatively markedly different from genuine brown adipocytes.
A Subset of Cells Acquires a Brown Fat-specific Phenotype in Rosiglitazone-treated White Adipocyte Cultures
Rosiglitazone-treated white adipocyte cultures acquired many brown fat-specific characteristics that cannot be explained by rosiglitazone-promoted overgrowth of a few classic brown fat precursor cells (Fig. 2 and supplemental Fig. 3). This implies either that another population of cells, endowed with established brown fat-specific characteristics (cf. Fig. 3), is present in white adipocyte cultures or that, alternatively, rosiglitazone transforms all cells in the white culture into a qualitatively different cell type that expresses more brown fat-specific and less white fat-specific genes. To determine whether rosiglitazone reveals a novel subpopulation of brown adipocyte-like cells within the white adipocyte cultures or generally ‘transdifferentiates‘ the white adipocyte cultures, we examined characteristics required for brown adipocyte-specific function at the cellular level in white adipocyte cultures grown in the presence of rosiglitazone or under control conditions.
Mitochondrial biogenesis is an essential part of BAT recruitment (35). Importantly, newly synthesized UCP1 protein is rapidly degraded unless it can be incorporated into mitochondria (36). Rosiglitazone induces mitochondrial biogenesis and mitochondrial remodeling not only in brown adipocytes (21) but also in vivo in white fat of ob/ob mice (17) and in subcutaneous fat of humans (37) and also in fully differentiated 3T3-L1 adipocytes (38).
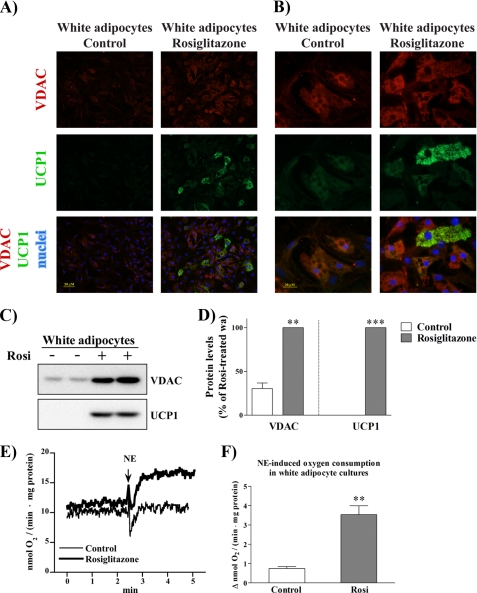
To quantify the mitochondrial content of the cells, we visualized the general mitochondrial marker, the voltage-dependent anion carrier (VDAC) (also known as porin). We compared mitochondrial staining in untreated cultures and in cultures continuously treated with rosiglitazone (Fig. 6, A and B, red color). In untreated white adipocyte cultures, there was only a very faint staining with anti-VDAC antibody (a version of Fig. 6 (A and B) with an altered background for better contrast is found in supplemental Fig. 5). As expected, cultures treated with rosiglitazone demonstrated cells with intense staining, indicating a dramatic enhancement of mitochondrial biogenesis in these cells (Fig. 6, A and B (red color) and C and D). Enhanced mitochondriogenesis in rosiglitazone-treated white adipocyte cultures was already implied above by the markedly increased mRNA levels of both the mitochondrial enzyme CPT-1 M (Fig. 3B) and the mitochondriogenesis-promoting gene PGC-1α (Figs. 1E and 3E), but the present micrographs demonstrate that the transcripts also result in functional structures. Importantly, strong mitochondrial staining was observed in a majority of cells in the rosiglitazone-treated cultures.
FIGURE 6.
Only a subset of cells in rosiglitazone-treated white adipocyte cultures is UCP1-positive and demonstrates NE-induced UCP1-dependent thermogenesis. Primary cultures of white and brown adipocytes were grown for 7 (A–D) or 6 (E and F) days in the absence or presence of 1 μm rosiglitazone. A and B, cells were analyzed by immunofluorescence cell staining using antibodies against VDAC (red) and UCP1 (green). Nuclei were counterstained with Hoechst 33258 (blue). Cells were examined with a fluorescence microscope, and representative images are presented (lower (A) and higher (B) magnification). (A version of A and B, with an altered background, is found in supplemental Fig. 5.) C and D, protein levels of VDAC and UCP1 in control and rosiglitazone-treated white adipocyte cultures. C, representative Western blots. Total cell lysates (30 μg of protein/lane) were examined using antibodies against VDAC and UCP1. D, VDAC and UCP1 protein levels in control and rosiglitazone-treated white adipocyte cultures. The values represent means ± S.E. of four independent experiments, each performed in duplicate. The rosiglitazone-induced level was in each experiment set to 100%, and the levels in untreated cells were expressed relative to this value in each individual experiment. The significance of rosiglitazone treatment was determined with paired t test. **, p < 0.01; ***, p < 0.001. E, representative traces showing the effect of NE on oxygen consumption of control (thin line) and rosiglitazone-treated (thick line) white adipocyte cultures. At the arrow, 1 μm NE was added. F, NE-induced component of respiration (calculated as the difference between the rates of oxygen consumption before and after addition of NE) in control and rosiglitazone-treated white adipocyte cultures. The polarographic output was time-differentiated, sampled, and recalculated per milligram of total cellular protein. The values are means ± S.E. of four independent experiments. The significance of rosiglitazone treatment was determined with paired t test. **, p < 0.01.
We further examined the cellular distribution of UCP1 in control and rosiglitazone-treated cultures. In untreated cultures, UCP1-immunoreactive cells were not detected (Fig. 6, A and B), which is fully in agreement with the absence of UCP1 mRNA (Figs. 1F and 3F) and UCP1 protein (Fig. 6, C and D) in these cultures. However, also in agreement with mRNA and protein data (Figs. 1F, 3F, 6C, and 6D), UCP1-positive cells were induced in the rosiglitazone-treated cultures (Fig. 6, A and B, green color). Importantly, only a subset of cells (∼10%) in these cultures were UCP1-positive. It was noteworthy that mitochondrial amount was not a limiting factor for cells to express UCP1, i.e. numerous mitochondria-rich cells were negative for UCP1 (Fig. 6, A and B, overlay). Thus, rosiglitazone did not transform all cells within white adipocyte cultures into more brown and less white cells but, rather, enabled some precursor cells, which had the potential to develop brown fat-like characteristics, to establish a brown adipocyte-like phenotype.
Rosiglitazone-treated White Adipocyte Cultures Are Competent in Demonstrating Norepinephrine-induced Thermogenesis
Thermogenesis (heat production) takes place in the mitochondria. Thermogenic responses in brown fat mitochondria (39) and brown fat cells (40, 41) are fully UCP1-dependent. Physiologically, UCP1 is the only protein capable of mediating adaptive nonshivering thermogenesis in the cold (42), and it is also the basis for any adaptive adrenergic nonshivering thermogenesis, irrespectively of whether it is cold acclimation-recruited (43) or represents adaptation to certain diets (‘diet-induced thermogenesis‘) (44).
The brown adipocyte-like cells emerging in the rosiglitazone-treated white adipocyte cultures appeared well endowed with the cellular effectors of thermogenesis: numerous mitochondria and the presence of UCP1 protein (Fig. 6, A–D). However, the most crucial test of whether these cells represent functional brown adipocytes must be their ability to display NE-induced thermogenesis. The thermogenic capacity of BAT is reflected in the ability of isolated brown adipocytes to respond to an addition of NE with a large increase in oxygen consumption (thermogenesis) (24). Similarly, upon stimulation with NE, brown fat cells differentiated in culture are also capable of demonstrating UCP1-dependent thermogenesis (21). To examine the thermogenic capacity of brown adipocyte-like cells emerging in white adipocyte cultures, we measured oxygen consumption of control and rosiglitazone-treated cells under basal conditions (before NE addition) and after stimulation with NE, as exemplified in Fig. 6E. There was a qualitative difference between the control cells and the rosiglitazone-treated cells. As expected, control cells (that do not contain UCP1 protein (Fig. 6, C and D)) did not increase oxygen consumption upon stimulation with NE (Fig. 6E, thin line). In contrast, NE addition led to a rapid and marked increase in oxygen consumption in rosiglitazone-treated cells (Fig. 6E, thick line) (compiled in Fig. 6F).
To summarize, upon continuous rosiglitazone treatment, brown adipocyte-like cells differentiating within white adipocyte cultures are capable of demonstrating NE-induced UCP1-dependent thermogenesis despite major differences at the molecular level.
DISCUSSION
In the present investigation, we have shown that although precursors from BAT and WAT are predetermined to develop into distinct cell types, it is possible to convert a subset of precursors found within WAT into cells that display the full functional characteristics of classic brown adipocytes. However, importantly, these cells are molecularly and developmentally distinct from the classic brown adipocytes (the adipomyocytes). These observations thus allow us to identify three types of adipocytes and give new insight into the issue of transdifferentiation. The results may also be relevant to human obesity, as human cells may respond similarly (20).
Effect of PPARγ Agonists
PPARγ agonist treatment of intact animals increases UCP1 expression in different WAT depots, particularly in the inguinal depot (12–17). However, in such in vivo experiments, it is not possible to distinguish between direct and indirect effects of the PPARγ agonist, particularly as, e.g. β3-adrenoreceptor agonist treatment also promotes the occurrence of these ectopic brown adipocytes (4, 8–11), and the effects could therefore be indirect. In contrast, the results presented here are from direct treatment of precursor cells isolated from a white adipose tissue depot considered to be the most pure white depot: the epididymal depot. Thus, the PPARγ agonist effect is directly on the cells, and, notably, the PPARγ agonist promoted precursors in these white cultures to display not only UCP1 expression (as principally shown in (20) for human white adipocytes) but also fundamental features of brown adipocyte functionality, qualitatively practically identical to those of brown adipocytes treated similarly. Thus, whereas the occurrence of brown adipocyte-like cells in WAT depots has earlier been considered to be a rare event, we demonstrate here that a substantial number of the precursors (∼10%) even of this most white depot have this ability, enabling a molecular characterization of the induced cells.
Sympathetically induced emergence of brown adipocytes within WAT depots is under genetic control (9, 45). Notably, the high levels of UCP1 mRNA observed in rosiglitazone-treated white adipocyte cultures of NMRI mice could not be attained in similar cultures of C57Bl6 mice (compare Figs. 1 (B and F) and 3F with Fig. 2 and supplemental Fig. 3). Accordingly, <1% of cells within rosiglitazone-treated white adipocyte cultures originating from C57Bl6 mice were UCP1-positive (not shown). Thus, it seems that the number of brown adipocyte precursors in WAT depots that can respond to PPARγ activation is also genetically determined.
Nature of the Induced Brown Adipocyte-like Cells
From the present studies, it is clear that the induced cells are not brown adipocytes of the classic type: their expression pattern of various genes (the absence of myogenic factors and of specific transcription factors) clearly distinguishes them from the classic brown adipocytes, a conclusion already implied from genetic studies by Kozak and colleagues in 1998 (9) but demonstrated here for the first time. This also means that the brown adipocyte-like cells do not emanate from a few precursors of classic brown adipocytes resident in the precursor population. Indeed, our co-culture experiments very clearly demonstrate that the PPARγ agonist does not promote growth of such putative classic brown adipocyte precursors over that of genuine white adipocytes. These co-cultures also demonstrate that brown adipocytes do not secrete a browning factor to neighboring cells, not even when adrenergically stimulated. It would be natural to suggest (7) that the browning effect would be mediated via an increase in PRDM16, but as PRDM16 is seen today rather as the agent responsible for inhibiting the muscle pathway in the muscle/brown adipocyte common precursors, it is consistent that PRDM16 does not seem to mediate the PPARγ agonist effect on the precursors from the WAT.
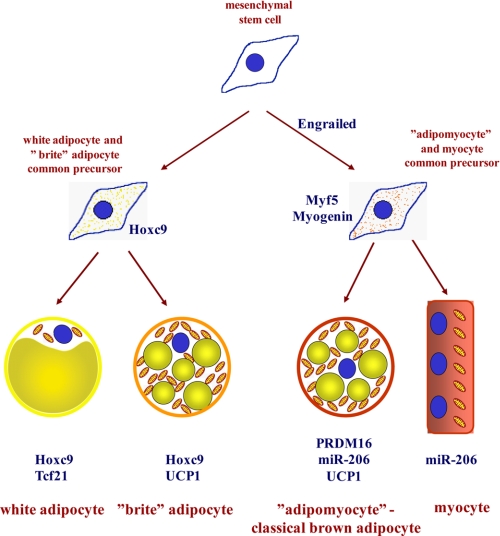
Thus, the results presented here, together with earlier studies, imply that (at least) three types of adipocytes should be distinguished (Fig. 7) as follows. 1) The classic brown adipocytes (the adipomyocytes) are characterized by the transient myogenic and persistent myomir expression signatures. These cells are exclusively found in the classic BAT depots, and they all originate from the dermomyotome. They express Zic1, Lhx8, Meox2, and PRDM16, and the ability to display brown adipocyte characteristics is dependent upon expression of PRDM16. 2) The “brite” (brown-in-white) adipocytes are the adipocytes (precursors) that, given appropriate external stimulus, such as a PPARγ agonist as used here, will differentiate into cells that possess the functional machinery for thermogenesis but never express the myogenic markers or the novel brown fat-specific markers (e.g. Zic1, Lhx8, Meox2, and PRDM16). The origin of these cells is unknown; it is clearly not the dermomyotome. The brite adipocytes could also be of the same lineage as those observed in intermyofibrillar fat depots (46, 47). 3) The true white adipocytes, emerging in vivo independently from the brown adipocytes (48) and molecularly identifiable through expression of Hoxc9 and Tcf21 are adipocytes that, given an external stimulus, e.g. in the form of a PPARγ agonist, display enhanced mitochondriogenesis but do not seem ever to be able to express UCP1 (unless genetically manipulated, e.g. by introduction of PGC-1α (49), PRDM16 (33), or Foxc2 (50) or by functional inactivation of pRb (51) or deletion of the corepressor RIP140 (52)). It would thus seem that a new nomenclature (‘the brite adipocytes‘) is necessary to facilitate the discussion concerning these morphologically similar but developmentally different cell types.
FIGURE 7.
Subtypes of adipocytes and their origins. This study, together with earlier studies, particularly Refs 1, 2, 4, implies that (at least) three types of adipocytes should be distinguished: the classic brown adipocytes (the adipomyocytes), the brite adipocytes (i.e. the brown adipocyte-like adipocytes induced in white adipocyte cultures), and the genuine white adipocytes. The adipomyocytes share their origin with myocytes, whereas brite and white adipocytes have a different origin.
Transdifferentiation or Not?
The observations reported here are related to the issue of possible “transdifferentiation” of white into brown adipocytes (53). However, it is clear that in our studies only a subset of cells within WAT can attain brown characteristics. This is principally in agreement with what has been reported concerning WAT depots where the brown adipocytes (brite adipocytes) occur in islets surrounded by white adipocytes (8–10). It should therefore be pointed out that neither in vivo nor in culture do we seem to observe true transdifferentiation of genuine white adipocytes. Rather, it would seem that the precursors in the tissue represent a mixture of cells, the true nature of each of which is only revealed when induced by an external agent. Secondly, it is also clear that the present experiments do not demonstrate transdifferentiation in the strict sense: that a cell that has first demonstrated white adipocyte characteristics is converted to show brown characteristics. The cells progress directly from the undifferentiated state into the brown adipocyte-like appearance without having been through a phase of being true white adipocytes.
Brite Adipocytes in Humans
An important reason for the interest in the presence of brown adipocyte-like cells in WAT has been based on intentions to treat human obesity by transformation of white adipocytes into brown adipocytes. These ideas were, however, formulated at a time when it was believed that adult humans did not possess active BAT. The realizations, elucidated from positron emission tomography (PET) scans of cancer patients, that adult humans possess active BAT (54), particularly in the subclavicular and cervical adipose tissues, and that even these depots contain UCP1 (55–59), have made it more natural to promote growth of these tissues, and thus of classic brown adipocytes, to combat obesity, especially because these depots also contain precursor cells, even in adults (59). These depots most likely consist of classic brown adipocytes, because they have high levels of PRDM16 (58). There are no indications from published PET scan studies that human classic WAT ever becomes metabolically significantly active.
The rationale for the evolutionary development of BAT is adaptive thermogenesis. However, the rationale behind the development of a second type of UCP1-expressing cells is still not physiologically evident. The brite adipocytes are indeed capable of demonstrating NE-mediated thermogenesis in vitro, but it is not evident whether they participate in adaptive thermogenesis in vivo. It is noteworthy that a high capacity to induce brite adipocytes in areas of traditional white fat in some mice strains does not impact on their ability to gain weight in response to a high fat diet but does correlate with their loss of weight in response to treatment with a β3-adrenergic agonist (9). Thus, a possible involvement of brite adipocytes in regulation of body weight may be implied. Recent data (60) also indicate that brite adipocytes may be involved in the regulation of insulin sensitivity in humans.
Supplementary Material
This work was supported by grants from the Swedish Research Council, the European Union Collaborative Project ADAPT (contract 201100), the Jeansson Foundation, and the COST Action Mitofood.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5 and Table S1.
- BAT
- brown adipose tissue
- WAT
- white adipose tissue
- UCP1
- uncoupling protein-1
- PPARγ
- peroxisome proliferator-activated receptor γ
- TBP
- TATA-binding protein
- VDAC
- voltage-dependent anion carrier
- PBS
- phosphate-buffered saline
- NE
- norepinephrine
- PET
- positron emission tomography.
REFERENCES
- 1.Atit R., Sgaier S. K., Mohamed O. A., Taketo M. M., Dufort D., Joyner A. L., Niswander L., Conlon R. A. (2006) Dev. Biol. 296, 164–176 [DOI] [PubMed] [Google Scholar]
- 2.Timmons J. A., Wennmalm K., Larsson O., Walden T. B., Lassmann T., Petrovic N., Hamilton D. L., Gimeno R. E., Wahlestedt C., Baar K., Nedergaard J., Cannon B. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 4401–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walden T. B., Timmons J. A., Keller P., Nedergaard J., Cannon B. (2009) J. Cell Physiol. 218, 444–449 [DOI] [PubMed] [Google Scholar]
- 4.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H. M., Erdjument-Bromage H., Tempst P., Rudnicki M. A., Beier D. R., Spiegelman B. M. (2008) Nature 454, 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon B., Nedergaard J. (2008) Nature 454, 947–948 [DOI] [PubMed] [Google Scholar]
- 6.Seale P., Kajimura S., Spiegelman B. M. (2009) Genes Dev. 23, 788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farmer S. R. (2008) Genes Dev. 22, 1269–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cousin B., Cinti S., Morroni M., Raimbault S., Ricquier D., Pénicaud L., Casteilla L. (1992) J. Cell Sci. 103, 931–942 [DOI] [PubMed] [Google Scholar]
- 9.Guerra C., Koza R. A., Yamashita H., Walsh K., Kozak L. P. (1998) J. Clin. Invest. 102, 412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Himms-Hagen J., Melnyk A., Zingaretti M. C., Ceresi E., Barbatelli G., Cinti S. (2000) Am. J. Physiol. Cell Physiol. 279, C670–C681 [DOI] [PubMed] [Google Scholar]
- 11.Xue Y., Petrovic N., Cao R., Larsson O., Lim S., Chen S., Feldmann H. M., Liang Z., Zhu Z., Nedergaard J., Cannon B., Cao Y. (2009) Cell Metab. 9, 99–109 [DOI] [PubMed] [Google Scholar]
- 12.Laplante M., Sell H., MacNaul K. L., Richard D., Berger J. P., Deshaies Y. (2003) Diabetes 52, 291–299 [DOI] [PubMed] [Google Scholar]
- 13.Fukui Y., Masui S., Osada S., Umesono K., Motojima K. (2000) Diabetes 49, 759–767 [DOI] [PubMed] [Google Scholar]
- 14.Carmona M. C., Louche K., Lefebvre B., Pilon A., Hennuyer N., Audinot-Bouchez V., Fievet C., Torpier G., Formstecher P., Renard P., Lefebvre P., Dacquet C., Staels B., Casteilla L., Pénicaud L. (2007) Diabetes 56, 2797–2808 [DOI] [PubMed] [Google Scholar]
- 15.Sell H., Berger J. P., Samson P., Castriota G., Lalonde J., Deshaies Y., Richard D. (2004) Endocrinology 145, 3925–3934 [DOI] [PubMed] [Google Scholar]
- 16.Rong J. X., Qiu Y., Hansen M. K., Zhu L., Zhang V., Xie M., Okamoto Y., Mattie M. D., Higashiyama H., Asano S., Strum J. C., Ryan T. E. (2007) Diabetes 56, 1751–1760 [DOI] [PubMed] [Google Scholar]
- 17.Wilson-Fritch L., Nicoloro S., Chouinard M., Lazar M. A., Chui P. C., Leszyk J., Straubhaar J., Czech M. P., Corvera S. (2004) J. Clin. Invest. 114, 1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Néchad M., Kuusela P., Carneheim C., Björntorp P., Nedergaard J., Cannon B. (1983) Exp. Cell Res. 149, 105–118 [DOI] [PubMed] [Google Scholar]
- 19.Loncar D., Afzelius B. A., Cannon B. (1988) J. Ultrastruct. Mol. Struct. Res. 101, 109–122 [DOI] [PubMed] [Google Scholar]
- 20.Digby J. E., Montague C. T., Sewter C. P., Sanders L., Wilkison W. O., O'Rahilly S., Prins J. B. (1998) Diabetes 47, 138–141 [DOI] [PubMed] [Google Scholar]
- 21.Petrovic N., Shabalina I. G., Timmons J. A., Cannon B., Nedergaard J. (2008) Am. J. Physiol. Endocrinol. Metab. 295, E287–E296 [DOI] [PubMed] [Google Scholar]
- 22.Tang W., Zeve D., Suh J. M., Bosnakovski D., Kyba M., Hammer R. E., Tallquist M. D., Graff J. M. (2008) Science 322, 583–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enerbäck S., Jacobsson A., Simpson E. M., Guerra C., Yamashita H., Harper M. E., Kozak L. P. (1997) Nature 387, 90–94 [DOI] [PubMed] [Google Scholar]
- 24.Cannon B., Nedergaard J. (2004) Physiol. Rev. 84, 277–359 [DOI] [PubMed] [Google Scholar]
- 25.Desvergne B., Wahli W. (1999) Endocr. Rev. 20, 649–688 [DOI] [PubMed] [Google Scholar]
- 26.Li P., Zhu Z., Lu Y., Granneman J. G. (2005) Am. J. Physiol. Endocrinol. Metab. 289, E617–E626 [DOI] [PubMed] [Google Scholar]
- 27.Esser V., Brown N. F., Cowan A. T., Foster D. W., McGarry J. D. (1996) J. Biol. Chem. 271, 6972–6977 [DOI] [PubMed] [Google Scholar]
- 28.Tvrdik P., Asadi A., Kozak L. P., Nedergaard J., Cannon B., Jacobsson A. (1997) J. Biol. Chem. 272, 31738–31746 [DOI] [PubMed] [Google Scholar]
- 29.Zhou Z., Yon Toh S., Chen Z., Guo K., Ng C. P., Ponniah S., Lin S. C., Hong W., Li P. (2003) Nat. Genet. 35, 49–56 [DOI] [PubMed] [Google Scholar]
- 30.Nordström E. A., Rydén M., Backlund E. C., Dahlman I., Kaaman M., Blomqvist L., Cannon B., Nedergaard J., Arner P. (2005) Diabetes 54, 1726–1734 [DOI] [PubMed] [Google Scholar]
- 31.Wright W. E., Sassoon D. A., Lin V. K. (1989) Cell 56, 607–617 [DOI] [PubMed] [Google Scholar]
- 32.McCarthy J. J. (2008) Biochim. Biophys. Acta 1779, 682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seale P., Kajimura S., Yang W., Chin S., Rohas L. M., Uldry M., Tavernier G., Langin D., Spiegelman B. M. (2007) Cell Metab. 6, 38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gesta S., Blüher M., Yamamoto Y., Norris A. W., Berndt J., Kralisch S., Boucher J., Lewis C., Kahn C. R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 6676–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skála J., Barnard T., Lindberg O. (1970) Comp. Biochem. Physiol. 33, 509–528 [DOI] [PubMed] [Google Scholar]
- 36.Puigserver P., Herron D., Gianotti M., Palou A., Cannon B., Nedergaard J. (1992) Biochem. J. 284, 393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogacka I., Xie H., Bray G. A., Smith S. R. (2005) Diabetes 54, 1392–1399 [DOI] [PubMed] [Google Scholar]
- 38.Wilson-Fritch L., Burkart A., Bell G., Mendelson K., Leszyk J., Nicoloro S., Czech M., Corvera S. (2003) Mol. Cell Biol. 23, 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shabalina I. G., Jacobsson A., Cannon B., Nedergaard J. (2004) J. Biol. Chem. 279, 38236–38248 [DOI] [PubMed] [Google Scholar]
- 40.Matthias A., Ohlson K. E., Fredriksson J. M., Jacobsson A., Nedergaard J., Cannon B. (2000) J. Biol. Chem. 275, 25073–25081 [DOI] [PubMed] [Google Scholar]
- 41.Shabalina I. G., Backlund E. C., Bar-Tana J., Cannon B., Nedergaard J. (2008) Biochim. Biophys. Acta 1777, 642–650 [DOI] [PubMed] [Google Scholar]
- 42.Golozoubova V., Hohtola E., Matthias A., Jacobsson A., Cannon B., Nedergaard J. (2001) FASEB J. 15, 2048–2050 [DOI] [PubMed] [Google Scholar]
- 43.Golozoubova V., Cannon B., Nedergaard J. (2006) Am. J. Physiol. Endocrinol. Metab. 291, E350–E357 [DOI] [PubMed] [Google Scholar]
- 44.Feldmann H. M., Golozoubova V., Cannon B., Nedergaard J. (2009) Cell Metab. 9, 203–209 [DOI] [PubMed] [Google Scholar]
- 45.Xue B., Rim J. S., Hogan J. C., Coulter A. A., Koza R. A., Kozak L. P. (2007) J. Lipid Res. 48, 41–51 [DOI] [PubMed] [Google Scholar]
- 46.Crisan M., Casteilla L., Lehr L., Carmona M., Paoloni-Giacobino A., Yap S., Sun B., Leger B., Logar A., Penicaud L., Schrauwen P., Cameron-Smith D., Russell A. P., Péault B., Giacobino J. P. (2008) Stem Cells 26, 2425–2433 [DOI] [PubMed] [Google Scholar]
- 47.Almind K., Manieri M., Sivitz W. I., Cinti S., Kahn C. R. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 2366–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moulin K., Truel N., André M., Arnauld E., Nibbelink M., Cousin B., Dani C., Pénicaud L., Casteilla L. (2001) Biochem. J. 356, 659–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiraby C., Tavernier G., Lefort C., Larrouy D., Bouillaud F., Ricquier D., Langin D. (2003) J. Biol. Chem. 278, 33370–33376 [DOI] [PubMed] [Google Scholar]
- 50.Cederberg A., Grønning L. M., Ahrén B., Taskén K., Carlsson P., Enerbäck S. (2001) Cell 106, 563–573 [DOI] [PubMed] [Google Scholar]
- 51.Hansen J. B., Jørgensen C., Petersen R. K., Hallenborg P., De Matteis R., Bøye H. A., Petrovic N., Enerbäck S., Nedergaard J., Cinti S., te Riele H., Kristiansen K. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 4112–4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leonardsson G., Steel J. H., Christian M., Pocock V., Milligan S., Bell J., So P. W., Medina-Gomez G., Vidal-Puig A., White R., Parker M. G. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 8437–8442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cinti S. (2009) Am. J. Physiol. Endocrinol. Metab. 297, E977–E986 [DOI] [PubMed] [Google Scholar]
- 54.Nedergaard J., Bengtsson T., Cannon B. (2007) Am. J. Physiol. Endocrinol. Metab. 293, E444–E452 [DOI] [PubMed] [Google Scholar]
- 55.Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., Kolodny G. M., Kahn C. R. (2009) N. Engl. J. Med. 360, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M., Kemerink G. J., Bouvy N. D., Schrauwen P., Teule G. J. (2009) N. Engl. J. Med. 360, 1500–1508 [DOI] [PubMed] [Google Scholar]
- 57.Saito M., Okamatsu-Ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-Kobayashi J., Iwanaga T., Miyagawa M., Kameya T., Nakada K., Kawai Y., Tsujisaki M. (2009) Diabetes 58, 1526–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N. J., Enerbäck S., Nuutila P. (2009) N. Engl. J. Med. 360, 1518–1525 [DOI] [PubMed] [Google Scholar]
- 59.Zingaretti M. C., Crosta F., Vitali A., Guerrieri M., Frontini A., Cannon B., Nedergaard J., Cinti S. (2009) FASEB J. 23, 3113–3120 [DOI] [PubMed] [Google Scholar]
- 60.Timmons J. A., Pedersen B. K., Stefan N., Pfannenberg C., Häring H. U., Villarroya F., Domingo P., Giralt M., Jacene H. A., Wahl W. L., Lee P., Ho K. K., Fulham M. J., Sacks H. S., van Marken Lichtenbelt W. D., Schrauwen P., Teule G. J., Cypess A. M., Kahn R. C., Enerbäck S., Oksi J., Nuutila P. (2009) N. Engl. J. Med. 361, 415–42119625723 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.