Abstract
Host cells respond to viral infections by synthesizing and producing antiviral molecules such as type I interferons (IFN). The Kaposi sarcoma-associated herpesvirus (KSHV) encodes multiple proteins expressed during the lytic replication cycle that alter the antiviral response of the host. Considering that in Kaposi sarcoma lesions and primary effusion lymphoma cells KSHV is latent in the vast majority of cells, we were interested in determining whether latently expressed viral proteins have the ability to modulate IFN synthesis. The latency-associated nuclear antigen (LANA-1) is a large nuclear protein that plays a role in the establishment and maintenance of latent KSHV episome in the nucleus of infected cells. LANA-1 is also described to modulate the cellular transcription. Here, we report that LANA-1 inhibits IFN-β transcription and synthesis by competing with the binding of interferon regulatory factor-3 (IRF3) to the IFNB promoter. Using mutants of LANA-1, we have identified the central acidic repeated region as the domain essential for interfering with the binding of IRF3 to the positive regulatory domains I–III of the IFNB promoter. In addition, the nuclear localization of LANA-1 proved essential for IFN-β inhibition. Thus, LANA-1 interferes with the formation of IFN-β enhanceosome by competing with the fixation of IRF3 and by inhibiting the expression of the CREB-binding protein. The ability of LANA-1 to inhibit IFNB gene expression highlights a new role for this protein in cellular gene modulation and immune evasion strategies.
Keywords: Cancer, Immunology, Immunology/Innate Immunity, Protein/DNA Interactions, Viruses/Herpes, Viruses/Tumor
Introduction
Kaposi sarcoma-associated herpesvirus (KSHV),3 also called human herpesvirus 8, is an oncogenic virus associated with the development of Kaposi sarcoma and two lymphoproliferative diseases, primary effusion lymphoma and multicentric Castleman disease (1). Most (>90%) cells recovered from these tumors are latently infected with KSHV and express only a small subset of viral genes (2). One such gene product, encoded by ORF73, is the latency-associated nuclear antigen (LANA-1). LANA-1 is a large (1162 amino acids) multifunctional and constitutively expressed protein that plays critical roles during the KSHV life cycle. LANA-1 is required for viral episome maintenance in proliferating cells (3). LANA-1 modulates the cellular transcription program by altering the functions of various transcription factors, like mSin3A (4), CREB-binding protein (CBP) (5), RING3 (6), GSK-3b (7), and ATF4/CREB2 (8). LANA-1 also functions as a potent transcriptional repressor and can suppress activation of heterologous promoters, suggesting that LANA-1 can act on chromatin structure and/or general transcriptional machinery (9). LANA-1 inhibits p53-mediated apoptosis (10) and stabilizes c-Myc, which is frequently deregulated in cancer (11). Within the KSHV genome, LANA-1 up-regulates its own promoter (12) and represses the transcription of ORF50, a transcriptional activator regulating the switch from latency to lytic replication cycle (13).
The early innate host response to viral infections is characterized by production of interferon (IFN) as well as pro-inflammatory chemokines and cytokines that exert direct antiviral activity and regulate cellular processes important for antiviral host defense (14, 15). The antiviral response is initiated by cellular sensors that recognize viral molecules (RNA or DNA) and activate an intracellular signal transduction cascade leading to activation of genes having antiviral functions (16). Such cellular sensors include the cytoplasmic RNA helicases (retinoic acid-inducible gene I and melanoma differentiation antigen 5), the cytoplasmic dsDNA-dependent activator of IFN regulatory factors, and the Toll-like receptors. Although several pathways are activated following recognition of viral nucleic acids, three principal cellular pathways are known to coordinate the activity of type I IFNs as follows: the nuclear factor (NF)-κB, the mitogen-activated protein kinase, and the IFN regulatory factor (IRF) pathways (16).
Members of Herpesviridae virus are sensitive to the antiviral effects of IFN-α/β. To minimize the antiviral responses triggered following viral entry, these viruses have evolved various mechanisms to counteract the cellular reaction. Microarray analysis performed during early KSHV infection revealed that several IFN-responsive genes were rapidly up-regulated (17). Interestingly, innate immune response gene activation is induced following contact between recombinant KSHV gpK8.1 and cellular receptors, but such an antiviral response was not observed when cells are challenged with infectious KSHV virions (18). This suggests that KSHV possesses mechanisms to differently modulate the expression of IFN. Because KSHV LANA-1 is constantly expressed in infected cells and known to modulate the cellular transcription program, we have examined the effects of LANA-1 expression on the synthesis of type I IFN. We report that by itself LANA-1 has no effect on IFNB gene activation. However, we observed that LANA-1 could efficiently block IFNB gene induction when known inducers of IFN synthesis were used. LANA-1 does not affect the phosphorylation status of IRF3 but prevents the binding of this transcription factor to the IFNB promoter. The central acidic region of LANA-1 is required for the inhibition of IFNB synthesis.
EXPERIMENTAL PROCEDURES
Cells and Virus
HEK-293T cells (ATCC, Manassas, VA) and HEC-IB cells (ATCC), which lack a functional α/β interferon receptor (19), were cultured in Dulbecco's modified Eagle's medium (Sigma) containing 10% fetal bovine serum. HEK-Blue IFN-α/β cells (InvivoGen, San Diego) were cultured in Dulbecco's modified Eagle's medium supplemented with 30 μg/ml blasticidin and 100 μg/ml Zeocin. HEK-293T-E1 were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum supplemented with 150 μg/ml hygromycin (20). A549 cells (ATCC) were cultured in F-12K/Ham's medium (Hyclone, Quebec, Canada) containing 10% fetal bovine serum. Sendai virus (SeV) (Cantell strain) was obtained from Charles River Laboratories (St-Constant, Quebec, Canada).
Plasmids and Constructs
The primers used to generate LANA-1 WT and mutant vectors are listed in supplemental Table 1. Full-length LANA-1 sequence corresponding to nucleotides 123793 to 127300 (GenBankTM U75698) was amplified from genomic DNA of BC3 cells by PCR and digested by EcoRI and XbaI. This PCR product was cloned in-frame with three N-terminal hemagglutinin (HA) tags into the pCMV3T vector digested by EcoRI and XbaI. The pCMV3T vector represents a modified pCMV2N3T (a kind gift from Didier Trouche, University Paul Sabatier, Toulouse, France) in which the two nuclear localization signals (NLS) were removed. Three C-terminal deletion mutants were generated by introducing a stop codon by site-directed mutagenesis according to the manufacturer's instructions (QuikChange site-directed mutagenesis kit, Stratagene, La Jolla, CA) as follows: G996X (LANA-1 1–996), E875X (LANA-1 1–875), and C300X (LANA-1 1–300). Three others mutants were made by PCR amplification of specific LANA-1 domains as follows: LANA-1 319–1162, LANA-1 854–1162, and LANA-1 888–1162. In brief, 50 nm of each primer, 20 μm dNTPs, 1× Expand buffer, and 1 unit of Expand DNA polymerase (Roche Applied Science) were mixed with 50 ng of pCMV3T-LANA-1, followed by PCR amplification. These PCR products were digested by EcoRI and XbaI and cloned into the same restriction enzyme sites in-frame with the three HA tags into pCMV2N3T vector containing two NLS signals. Another mutant was generated by site-directed mutagenesis from LANA-1 319–1162 to create LANA-1 319–892 and was cloned in-frame with the three HA tags into pCMV2N3T vector. pIFN-β-Luc and positive regulatory domain (PRD)-I–III-Luc were obtained from Tom Maniatis (Harvard University) (21). Expression vectors for TBK1 (TANK-binding kinase-1), Myc-IRF3, FLAG-IRF3, and IRF3–5D were obtained from J. Hiscott and Rongtuan Lin (Lady Davis Institute, Canada) (22, 23). Expression vector for p50 was obtained through the National Institutes of Health, AIDS Research and Reference Reagent Program, Division of AIDS, NIAID; pRSV-NF-κB1 (p50) was from Dr. Gary Nabel and Dr. Neil Perkins (24). CBP expression vector was obtained from Didier Trouche (25). The sequence targeting LANA-1 (small interfering RNA-LANA-1) was described by Godfrey et al. (26). One hundred pmol of each LANA-1-specific oligonucleotide (5′-GTC CCA CAG TGT TCA CAT CCG GGC-3′) was phosphorylated using T4 polynucleotide kinase in a total volume of 50 μl for 30 min. To anneal the oligonucleotides, the mixture was incubated at 95 °C for 5 min and allowed to cool to room temperature. One μl of this mixture was ligated into pTER vector (27) that had been digested with BglII and HindIII and treated with shrimp alkaline phosphatase. In the same way, an irrelevant shRNA control (shRNA-NS) was designed from the HHV-6 IE1 mRNA (28). Two others vectors encoding shRNA against LANA-1 (pSUPER-shRNA LANA-1-N and pSUPER-shRNA LANA-1-C) were obtained from Dr. Diane Hayward (29). An unrelated control specific to Renilla luciferase, pSUPER-NS, was obtained from Dr. Shoji Yamaoka (30).
Transfections and Infections
A549 and HEC-1B cells were plated 1 day prior to transfection at 150,000 cells/well of a 6-well plate. Cells were transfected using the TransiT-LT reagent (Mirus Corp.) according to the manufacturer's instructions. 24 h post-transfection, cells were infected with 20 HAU/ml SeV for 8 h. HEK-293T cells were seeded at 75,000 cells/well of a 24-well plate 1 day prior to transfection using the calcium phosphate precipitation procedure. For some experiments, HEK-293T cells were co-transfected with IFNB gene activators, such as TBK1 or IRF3–5D plasmids, together with increasing quantities of LANA-1 expression vector. The level of DNA was kept constant at 1.25 μg by the addition of the empty pCMV3T vector. In some experiments, cells were infected with 10 HAU/ml of SeV or transfected 10 μg of polydeoxyadenosine-deoxythymidine (poly(dA-dT)) DNA (Amersham Biosciences) by using Lipofectamine (Invitrogen) 24 h after transfection with pCMV3T-LANA-1. HEK-293T-E1 were seeded and transfected as HEK-293T cells.
RT-QPCR
Total RNA was extracted from HEK-293T, HEK-293T-E1, or A549 cells using TRIzol reagent (Invitrogen). All RNA samples were treated with DNase I to eliminate residual genomic DNA prior to amplification. cDNA was synthesized, and real time quantitative PCR analysis was performed using a Rotorgene apparatus (Montreal Biotech Inc., Canada) and QuantiTect Multiplex PCR kit (Qiagen, Ontario, Canada) for GAPDH and IFNB gene expressions. The following primer pairs have been used: GAPDH forward (5′-CGA GAT CCC TCC AAA ATC AA-3′), GAPDH reverse (5′-TTC ACA CCC ATG ACG AAC AT-3′), and GAPDH gene probe (5′-hexachloro-6-carboxyfluorescein-TGG AGA AGG CTG GGG CTC AT-black hole quencher 1–3′); IFNB forward (5′-AAA CTC ATG AGC AGT CTG CA-3′), IFNB reverse (5′-AGG AGA TCT TCA GTT TCG GAG G-3′), IFNB gene probe (5′-hexachloro-6-carboxyfluorescein-ATG GTC CAG GCA CAG TGA CTG TCC TC-black hole quencher 1–3′). Levels of ISG15 mRNA were determined by using SYBR Green dye and the following primer pair: ISG15 forward (5′-CAT GGG CTG GGA CCT GAC G-3′) and ISG15 reverse (5′-CGC CAA TCT TCT GGG TGA TCT G-3′).
Interferon β Detection
HEK-293T cells were co-transfected with HA-LANA-1 and TBK1 expression vectors with total DNA levels kept constant at 1.25 μg per well by the addition of pCMV3T control plasmid. Forty eight hours after transfection, supernatant was collected and used for IFN-β detection. According to the manufacturer's instructions, 20 μl of each sample was added to a flat bottom 96-well plate containing 50,000 HEK-Blue IFN-α/β cells per well and incubated at 37 °C for 24 h. Forty μl of induced HEK-Blue IFN-α/β supernatant were then collected and incubated with 160 μl of QUANTI-Blue (InvivoGen) per well for 1 h at 37 °C. The activation of the IFN pathway is made through the inclusion of a reporter gene expressing a secreted form of embryonic alkaline phosphatase under the control of the IFN-responsive ISG54 promoter. Secreted embryonic alkaline phosphatase levels were determined using a spectrophotometer at 650 nm and compared with a standard curve made by serial dilutions of the IFN-β protein.
Nuclear Extracts
HEK-293T were transfected with Myc-IRF3, p50, CBP, and HA-LANA-1 expression vectors in 10-cm plates. One day after transfection, cells were infected with 10 HAU of SeV. After 18 h, cells were collected, and the pellets were resuspended in 400 μl of buffer A (10 mm HEPES, pH 7.9, 10 mm KCl, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm DTT, and 0.5 mm phenylmethylsulfonyl fluoride) and incubated for 10 min on ice. Twenty five μl of 10% Nonidet P-40 were added, and nuclei were sedimented by centrifuging the samples at 12,000 × g for 30 s. The nuclear pellets were resuspended in 50 μl of buffer B (20 mm HEPES, pH 7.9, 0.4 m NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, and 1 mm phenylmethylsulfonyl fluoride) and incubated on a rocker for 15 min at 4 °C. The suspensions were clarified by centrifugation at 12,000 × g for 5 min. The supernatants containing nuclear extracts were collected, and the protein content was determined using the Pierce BCA protein assay kit.
Cell Extracts
A549 cells were transfected with the expression vector of LANA-1 for 36 h, infected with 20 HAU of SeV for 8 h, lysed in 150 μl of lysis buffer (50 mm HEPES, pH 7.4, 150 mm NaCl, 5 mm EDTA, 10% glycerol, 1% Nonidet P-40, Complete-Lysis tablets (Roche Applied Science), 0.1 μm phenylmethylsulfonyl fluoride, and 1 mm DTT) and 50 μl of 3× Laemmli buffer, and resolved by SDS-PAGE followed by immunoblotting. HEC-1B cells were transfected with LANA-1 and IRF3 expression vectors for 36 h and infected with 200 HAU/ml SeV for 6 h. Cells were collected and frozen at −80 °C. Frozen cell pellets were resuspended in 4 volumes of lysis buffer (20 mm HEPES, pH 7.9, 0.2 mm EDTA, 0.2 mm EGTA, 10% glycerol, 10 mm sodium molybdate, 2 mm sodium pyrophosphate, 2 mm sodium orthovanadate, 0.5 mm spermidine, 0.15 mm spermine, 50 μm l-1-tosylamido-2-phenylethyl chloromethyl ketone, 25 μm 1-chloro-3-tosylamido-7-amino-2-heptanone, 1 μg/ml each of aprotinin, pepstatin A, and leupeptin, 0.5 mm benzamidine, 1 mm DTT, and 0.5 mm phenylmethylsulfonyl fluoride). KCl was added to 400 mm final, and the extracts were rotated at 4 °C for 30 min and centrifuged at 10,000 × g for 5 min. The protein content was determined using the Pierce BCA protein assay kit.
Western Blot Analysis
Samples were lysed in Laemmli buffer, boiled, and electrophoresed on SDS-polyacrylamide gel, and separated proteins were transferred onto polyvinylidene difluoride membranes. Membranes were incubated in 5% dry milk in TBS-T saline (0.25 m Tris-HCl, pH 7.6, 0.19 m NaCl, 0.1% Tween 20) for 1 h to block nonspecific sites. Blots were then incubated in 0.2% milk/TBST solution containing either mouse anti-HA (12CA5), mouse anti-Myc (9E10), mouse anti-p50, rabbit anti-ATF2, rabbit anti-c-Jun/AP1, rabbit anti-CBP, mouse anti-actin antibodies (all from Santa Cruz Biotechnology), rat anti-LANA-1 (Advanced Biotechnology), and mouse anti-PARP-1 (31). Some blots were incubated in 5% bovine serum albumin/TBST solution containing anti-rabbit IRF3 serine 396 (a kind gift of Nathalie Grandvaux, University of Montreal, Montreal, Canada). Membranes were washed twice with TBS-T and treated with either a horseradish peroxidase-linked goat anti-mouse or anti-rabbit or anti-rat antibody. Reactive proteins were visualized by enhanced chemiluminescence (PerkinElmer Life Sciences).
DNA Affinity Binding Assay
The DNA affinity binding assay method was described previously by Severa et al. (32) and adapted by Lefort et al. (33). Briefly, biotinylated oligonucleotides containing the IFNB promoter sequence (5′-Bio-CCC CCC AAA TGA CAT AGG AAA ACT GAA AGG GAG AAG TGA AAG TGG GAA ATT CC-3′) or the PRD of the IFNB promoter (PRD-I–III, 5′-Bio-CCC CCC GAA AAC TGA AAG GGA GAA GTG AAA GTG-3′; PRD-II, 5′-Bio-CCC CCC AGT GGG AAA TTC CTC TGA-3′; and PRD-IV, 5′-Bio-CCC CCC AAA TGT AAA TGA CAT AGG-3′) were annealed with the corresponding antisense oligonucleotides in 1× SSC buffer (0.15 m NaCl and 15 mm sodium citrate). Biotinylated DNA oligonucleotides were mixed with 150 μg of nuclear extracts in 500 μl of binding buffer (20 mm Tris-HCl, pH 7.5, 75 mm KCl, 1 mm DTT, 5 mg/ml bovine serum albumin, and 13% glycerol). Twenty μg of poly(dI-dC) were added, and samples were incubated for 25 min at room temperature. Streptavidin magnetic beads (Roche Applied Science), washed three times with binding buffer, were added to the reaction mixtures and incubated for 30 min at 4 °C and for 10 min at room temperature with mixing by rotation. The beads were collected using a magnet and washed three times. The bound proteins were eluted from the beads by boiling in Laemmli sample buffer and were resolved by SDS-PAGE followed by immunoblotting.
Electromobility Shift Assays (EMSA)
The methodology used was as described by Wathelet et al. (34). EMSA were performed using 10 μg of cell extracts from HEC-1B cells transfected with pCMV3T-LANA-1 and FLAG-IRF3 vectors and infected or not with SeV. Extracts were incubated with 40 fmol of 32P-labeled double-stranded oligonucleotides corresponding to the ISG15 promoter (5′-CCT AGG GCC TCG GGA AAG GGA AAC CGA AAC TGA A-3′). For the competition experiments, excess unlabeled ISG15 promoter double-stranded oligonucleotides were used. For supershift experiments, 2 μg of anti-FLAG (IRF3) were added to the binding reactions. Protein-DNA complexes were resolved on 5% polyacrylamide gel (acrylamide/bisacrylamide, 75:1) at 4 °C in 1% glycerol, 0.5× TBE.
Detection of Active IRF3
The amount of IRF3 capable of binding to the consensus IRF3-binding site in the absence or presence of LANA-1 was measured by TransAMTM IRF3 transcription factor ELISA (Active Motif). The amount of LANA-1 capable of binding to consensus IRF3-binding site was measured by substituting the anti-IRF3 primary antibody for a rat anti-LANA-1 (Advanced Biotechnology) diluted 1:1000. Extracts from LANA-1-transfected and/or SeV-infected 293T cells were prepared using the protocol provided (Active Motif).
Reporter Assay
HEK-293T cells were co-transfected with 50 ng of reporter plasmids and expression vectors with total DNA levels kept constant at 1.25 μg per well by the addition of pCMV3T control plasmid. Cells were lysed 48 h after transfection, and the luciferase activity was measured using an MLX Microtiter plate luminometer (Dynex Technologies, Chantilly, VA). Values were normalized using Pierce BCA protein assay (Thermo Fisher Scientific Inc., Rockford, IL).
Immunofluorescence Analysis
HEK-293T cells were cultured on coverslips in 6-well plates for 24 h (600,000 cells/well) and transfected using TransiT-LT reagent (Mirus Corp.) according to the manufacturer's instructions. Twenty four hours post-transfection, coverslips were washed twice in phosphate-buffered saline and fixed with cold acetone for 5 min. The slides were then incubated with mouse anti-HA (12CA5) for 1 h at room temperature, washed twice in phosphate-buffered saline, and incubated with Alexa 488-labeled goat anti-mouse antibody (Invitrogen) in phosphate-buffered saline for 1 h at room temperature. Slides were washed three times in phosphate-buffered saline and mounted with SlowFade antifade with 4′,6-diamidino-2-phenylindole (Invitrogen) before examination. Images were captured by a CoolSNAP HQ camera mounted on an Olympus BX-51 upright microscope (Olympus America, Melville, NY) using a ×60 objective and processed with ImagePro 4.5.1 software (Media Cybernetics, Silver Spring, MD).
Statistical Analysis
Statistical analysis was performed with the aid of GraphPad InStat software using unpaired Student's t test. All experiments were done in triplicate and repeated at least three times.
RESULTS
KSHV LANA-1 Inhibits IFNB Gene Induction
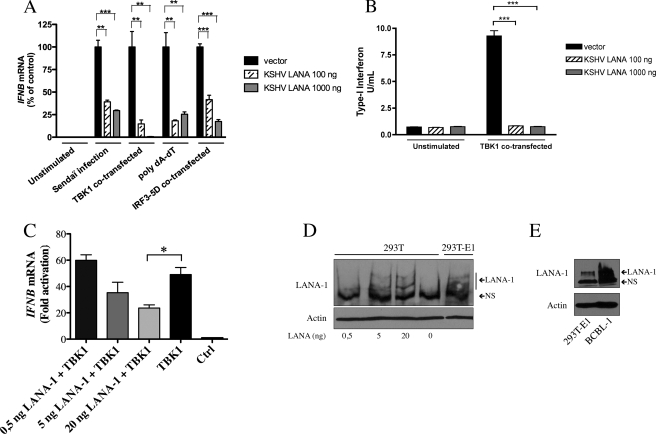
KSHV LANA-1 is described as a nuclear protein able to modulate the transcriptional activity of the human immunodeficiency virus-long terminal repeat promoter and to inhibit KSHV viral lytic replication by inhibiting the expression and the functions of Rta (13). Considering that LANA-1 is constitutively expressed in infected cells during latent as well as lytic infection (36), and the fact that neither IFN-β expression nor a functional antiviral response is observed in cells infected with KSHV virions (18), we hypothesized that LANA-1 protein may interfere with IFNB gene induction. To determine whether LANA-1 influences IFNB gene expression, we transfected a LANA-1 expression vector into HEK-293T cells and studied IFNB gene expression in response to a variety of stimuli, including expression of TBK1 or IRF3–5D, transfection of poly(dA-dT), or infection with Sendai virus. Total RNA was extracted, and endogenous IFNB gene expression was monitored by RT-QPCR. As shown in Fig. 1A, in the absence of LANA-1, IFNB gene induction was efficiently triggered by all of the inducers tested. By itself, LANA-1 did not modulate the IFNB synthesis. In contrast, in LANA-1-expressing cells, IFNB gene activation in response to all IFN-β inducers tested was strongly reduced. These results indicated that LANA-1 could efficiently block IFNB gene induction when the IFN pathway is triggered through different mechanisms. We next confirmed that the reduced IFNB mRNA levels observed correlated with a reduction in biologically active IFN-β protein being secreted. HEK-293T cells were co-transfected with LANA-1, and TBK1 expression vectors and the supernatant were collected and assayed for IFN secretion using HEK-Blue IFN-α/β cells (Fig. 1B). These cells were engineered to monitor the activation of the JAK-STAT pathway upon type I IFN stimulation. In the absence of LANA-1, the JAK-STAT pathway was strongly activated by TBK1 indicating that biologically active IFN was present in the supernatant. In contrast, in LANA-1-expressing cells, the signaling pathway was reduced to basal levels indicating that only a small amount of IFN was present (Fig. 1B). To confirm that physiological levels of LANA-1 were capable of reducing IFNB mRNA expression, we transfected varying quantities of pCMV3T-LANA-1 in HEK-293T cells (0.5, 5, and 20 ng) and studied IFNB gene expression in response to TBK1 (Fig. 1C). With as little as 5 ng of LANA-1 vector, we were able to reduce IFNB gene expression, and statistically significant inhibition was observed following transfection of 20 ng of LANA-1 vector. Levels of LANA-1 expression following transfection with 5 or 20 ng of LANA-1 vector were comparable with LANA-1 expression levels in HEK-293T-E1 cells carrying a recombinant KSHV (Fig. 1D) (20). Expression of LANA-1 in HEK-293T-E1 cells is less abundant than in BCBL-1 cells (Fig. 1E), suggesting that the LANA-1 doses used are physiological and suggest that at physiological levels LANA-1 is capable of inhibiting the expression of IFNB mRNA. Overall, these results indicate that LANA-1 inhibits the expression of IFNB mRNA resulting in reduced synthesis and release of biologically active IFN-β protein.
FIGURE 1.
LANA-1 represses IFNB gene activation triggered by SeV, TBK1, poly(dA-dT), and IRF3–5D. A, HEK-293T cells were transfected with increasing amounts of pCMV3T-LANA-1 (100–1000 ng) and infected by SeV or co-transfected with expression vectors coding for TBK1 and IRF3–5D or transfected 24 h later with poly(dA-dT), as described under “Experimental Procedures.” Forty eight hours post-transfection, total RNA was isolated and processed for IFNB mRNA by RT-QPCR. Results are expressed as mean (triplicate) induction ± S.D. relative to pCMV3T-transfected cells after normalization of samples with GAPDH mRNA expression. Results are representative of three independent experiments. **, 0.001 < p < 0.01, ***, p < 0.0001 as determined using two-tailed t test. B, HEK-293T cells were co-transfected with HA-LANA-1 and TBK1 expression vectors. Forty eight hours after transfection, supernatants were collected, and 20 μl of each sample was used for IFN-β detection using HEK-Blue IFN-α/β cells. Secreted embryonic alkaline phosphatase levels were determined using a spectrophotometer at 650 nm and compared with a standard curve composed of a logarithmic scale between 0.0025 and 250 units/well of IFN-β protein. Results are representative of three experiments performed in triplicate. ***, p < 0.0001 as determined using two-tailed t test. C, HEK-293T were transfected with increasing amounts of pCMV3T-LANA-1 (0.5–5-20 ng) and co-transfected with 25 ng of TBK1 expression vector. Forty eight hours post-transfection, total RNA was isolated and processed for IFNB mRNA by RT-QPCR. Results are expressed as mean (triplicate) induction ± S.D. relative to pCMV3T-transfected cells after normalization of samples with GAPDH mRNA expression. Results are representative of three independent experiments. *, 0.01< p < 0.05 as determined using two-tailed t test. Ctrl, control. D, HEK-293T were transfected with increasing amounts of pCMV3T-LANA-1 (0.5–5-20 ng). Forty eight hours later, cells were lysed and boiled in Laemmli sample buffer and then resolved by SDS-PAGE. Western blots were carried out for the detection of LANA-1 and actin using specific antibodies. LANA-1 expression in transfected HEK-293T cells was compared with that of HEK-293T-E1 cells. E, HEK-293T-E1 and BCBL-1 cells were lysed and boiled in Laemmli sample buffer and then resolved by SDS-PAGE. Western blots were carried out for the detection of LANA-1 and actin using specific antibodies.
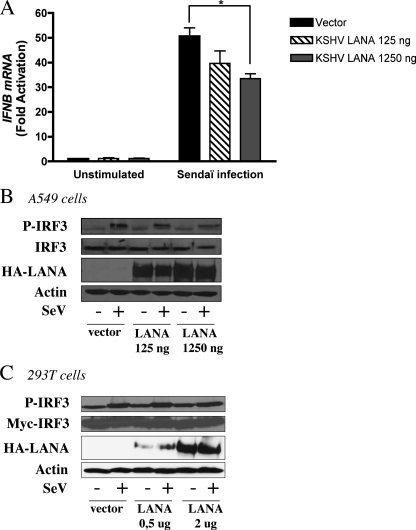
Under resting conditions, IRF3 is located in the cell cytoplasm. Once the cell detects an invading pathogen, IRF3 gets phosphorylated by the IκK-like kinases TBK1 and IKKϵ (21) on serine and threonine residues located within the C terminus (37, 38), and it homodimerizes and enters the nucleus to activate the IFNB gene in cooperation with NF-κB. To determine whether LANA-1 inhibits IFNB gene expression by interfering with the phosphorylation of IRF3, we transfected A549 cells with the LANA-1 expression vector and infected them with Sendai virus. We chose to use A549 cells for two reasons. First, to demonstrate reproducibility in another cell type, and second because the detection of phospho-IRF3 is facilitated in this cell line. A549 cells were transfected with LANA-1 expression vector followed by SeV infection. Total RNA was isolated, and endogenous IFNB gene expression was monitored by RT-QPCR. As shown in Fig. 2A, LANA-1 did not modulate IFNB gene expression by itself, but it effectively inhibited the activation of this gene in response to SeV infection. Lower transfection efficiencies of A549 cells compared with HEK-293T cells explain the slightly diminished potential of LANA-1 at inhibiting IFNB gene expression in response to SeV infection. The phosphorylation status of IRF3 was monitored by Western blot analysis. As shown in Fig. 2B, SeV infection led to the phosphorylation of IRF3, and the presence of LANA-1 did not modify this cellular state. This confirms the results obtained using the phosphomimetic and constitutively active IRF3 (IRF3–5D), which does not rely on cellular kinases for its activation (Fig. 1A). To confirm that the absence of effect of LANA-1 on the phosphorylation of IRF3 was not caused by the lower transfection efficiency of A549 cells, HEK-293T cells were co-transfected with LANA-1 and Myc-IRF3 expression vectors, and phospho-IRF3 levels upon SeV infection were determined. As shown in Fig. 2C, LANA-1 did not interfere with the phosphorylation of IRF3 induced by SeV infection. Using HEK-293T-E1 cells that are infected with a recombinant KSHV, similar levels of phospho-IRF3 were also detected following SeV infection (data not shown). These results suggest that LANA-1 inhibits the synthesis of IFNB, and this effect is not mediated through impaired phosphorylation of IRF3.
FIGURE 2.
LANA-1 does not interfere with the phosphorylation of IRF3. A and B, A549 cells were transfected with increasing amounts of pCMV3T-LANA-1 vector (125–1250 ng). Thirty six hours later, cells were infected or not with SeV (20 HAU) for 8 h. A, total RNA was isolated and processed for IFNB mRNA by RT-QPCR. Results are expressed as mean (triplicate) induction ± S.D. relative to pCMV3T transfected cells after normalization of samples with GAPDH mRNA expression. *, 0.01 < p < 0.05 as determined using two-tailed t test. B, cell lysates were analyzed by Western blotting for P-IRF3, IRF3, LANA-1 (HA), and actin expression. C, HEK-293T were transfected with increasing amounts of pCMV3T-LANA-1 (0.5–2.0 μg) and Myc-IRF3. Thirty six hours later, cells were infected or not by SeV for 8 h. Cell lysates were analyzed by Western blotting for P-IRF3, IRF3 (Myc), LANA-1 (HA), and actin expression. Results are representative of three independent experiments.
LANA-1 Interferes with the Fixation of IRF3 on the IFNB Promoter
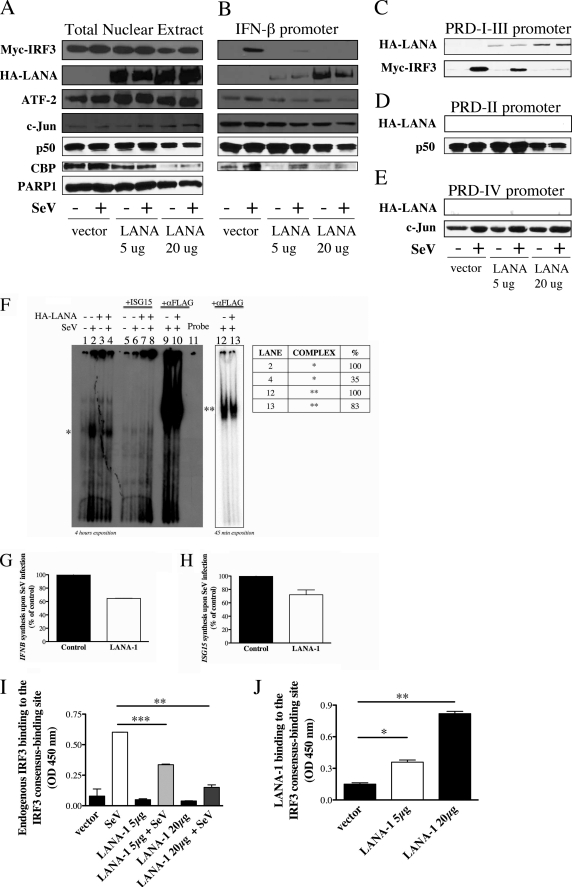
The activation of the IFNB promoter requires the formation of an enhanceosome, a multicomponent complex of transcription factors and enhancers that operate as a functional unit. This promoter is activated by the fixation of p50/p65 on the NF-κB-binding site (PRD-II), the fixation of IRF3 on the PRD-I/III, and the fixation of ATF-2/c-Jun on the PRD-IV domains (39). The local protein-protein contacts suggest that cooperative occupancy of the enhanceosome comes from binding-induced changes in DNA conformation and interactions with additional co-activators such as CBP/p300 (40) and enables the RNA polymerase II machinery access to the promoter. To determine how LANA-1 inhibits IFNB gene activation, we tested whether LANA-1 could affect the binding of these transcription factors to the IFNB promoter. Nuclear extracts of resting and SeV-infected cells co-transfected with expression vectors for IRF3, LANA-1, p50, and CBP were analyzed by Western blot for IRF3 (Myc), LANA-1 (HA), ATF-2, c-Jun, p50, CBP, and PARP-1 to compare the input of the different nuclear extracts (Fig. 3A). The nuclear input is similar between conditions except for HA-LANA-1 and CBP. The expression of LANA-1 was restricted to the conditions where the protein is expressed upon pCMV3T-LANA-1 transfection. The expression of CBP in nuclear extracts is reduced in the presence of LANA-1 confirming the results of Lim et al. (5) describing transcriptional inhibition of CBP by LANA-1. Double-stranded oligonucleotides containing the entire IFNB promoter were incubated with the nuclear extracts, and the proteins bound to the promoter sequence were analyzed by Western blot (Fig. 3B). LANA-1 was able to bind to the promoter in a dose-dependent manner, and SeV infection did not affect LANA-1 attachment. The binding of ATF-2 and c-Jun was neither affected by the presence of LANA-1 nor by SeV infection. The fixation of IRF3 to the IFNB promoter was induced following SeV infection. IRF3 efficiently bound the IFNB promoter in the absence of LANA-1 but much less so when LANA-1 was present. In fact, transfection of cells with 5 μg of LANA-1 expression vector strongly reduced the fixation of IRF3, whereas transfection with 20 μg of LANA-1 abolished the binding of IRF3 to the IFNB promoter. LANA-1 did not affect the fixation of p50 (Fig. 3B) or the binding of p65 (data not shown) to the IFNB promoter. The apparent reduction in CBP binding to the IFNB promoter is a consequence of the reduced levels of CBP in the nuclear extracts (Fig. 3A). These results indicate that LANA-1 negatively affects the binding of IRF3 to the IFNB promoter. Furthermore, the results indicate that LANA-1 can bind (directly or indirectly) to the IFNB promoter and impair the recruitment of IRF3 following activation by SeV infection. These results could explain, at least in part, the inhibition of IFNB gene expression caused by the presence of LANA-1 in stimulated cells.
FIGURE 3.
LANA-1 binds to the PRD-I–III region and interferes with the fixation of IRF3 to the IFNB promoter. A–E, HEK-293T cells were transfected with pCMV3T-LANA-1 (5–20 μg), Myc-IRF3, p50, and CBP vectors. Twenty four hours later, half of the cells were infected with SeV for 18 h. A, nuclear extracts were made and analyzed by Western blot (5% of input used in the binding reaction). The nuclear extracts were incubated with biotinylated oligonucleotides containing either the IFN-β (B), the PRD-I–III (C), PRD-II (D), or the PRD-IV (E) promoter sequences. Streptavidin beads were added for 40 min, washed three times, boiled in Laemmli sample buffer, and resolved by SDS-PAGE. Western blots were carried out for the detection of IRF3 (Myc), LANA-1 (HA), ATF-2, c-Jun, p50, and CBP using specific antibodies. PARP-1 was used as a control to demonstrate the equal amounts of nuclear proteins between the different samples. F, binding of IRF3 on the ISG15 promoter in cell extracts of HEC-1B cells transfected with 500 ng of FLAG-IRF3 and 2 μg of pCMV3T or pCMV3T-LANA-1 and infected or not by 200 HAU of SeV for 6 h was analyzed by EMSA. Specificity of binding was confirmed by homologous competition using excess (25 times) unlabeled ISG15 oligonucleotides. Supershift experiments were done using 2 μg of anti-FLAG (IRF3) antibody. * and ** denote different protein complexes on the ISG15 promoter. G and H, fraction of the cells used for EMSA (F) were kept to analyze the IFNB (G) and ISG15 (H) mRNA content by RT-QPCR. Results are expressed as mean (triplicate) induction ± S.D. relative to pCMV3T- transfected cells after normalization of samples with GAPDH mRNA expression. I and J, HEK-293T cells were transfected with 2 μg of pCMV3T-LANA-1 in a 10-cm dish. Twenty four hours later, half of the cells were infected with 20 HAU of SeV for 18 h. Nuclear extracts were isolated and measured using BCA assay, and 5 μg were used to determine the binding activity. I, endogenous IRF3 binding activity determined using the TransAMTM IRF3 ELISA (Active Motif). Results are expressed mean ± S.D. of IRF3 binding (OD 450 nm). J, LANA-1 binding on the IRF3 consensus binding site was determined using a modified TransAMTM ELISA by substituting the anti-IRF3 primary antibody for anti-LANA-1 diluted 1:1000. *, 0.01 < p < 0.05; **, 0.001 < p < 0.01; ***, p < 0.0001 as determined using two-tailed t test.
To determine on which part of the IFNB promoter LANA-1 was able to bind, we incubated the same nuclear extracts analyzed in Fig. 3A with three different double-stranded oligonucleotides containing each of the three PRDs of the IFNB promoter (PRD-I–III, PRD-II, and PRD-IV) and identified the bound proteins by Western blot (Fig. 3, C–E). As expected, IRF3 efficiently bound to the PRD-I–III domain, p50 bound to the PRD-II domain, and c-Jun bound to the PRD-IV domain. LANA-1 was found capable of binding to the PRD-I–III domain (Fig. 3C) and did not bind to other IFNB promoter domains. The fixation of LANA-1 to the PRD-I–III affected the binding of IRF3. At the highest dose tested, the co-expression of LANA-1 eliminated IRF3 binding to the PRD-I–III promoter. The fixation of p50 to the PRD-II domain seemed to be slightly affected in the presence of the highest dose of LANA-1 (Fig. 3D). However, this diminution was not significant considering the fact that there was also a diminution of p50 in nuclear extracts in the same samples (Fig. 3A). The fixation of c-Jun on PRD-IV was similar under all conditions tested indicating that LANA-1 did not affect the fixation c-Jun to the IFNB promoter (Fig. 3E). These results confirm the results obtained in Fig. 3B. They suggest that LANA-1 binds to the PRD-I–III domain of IFNB promoter and impairs the recruitment of IRF3 when activated by SeV infection.
Next, we confirmed that LANA-1 reduced IRF3 fixation to the promoter by performing EMSA. It is a known fact that the affinity of IRF3 for the IFNB promoter is somewhat low and ∼200-fold lower than that of the ISG15 promoter (34). Our failed attempts to obtain reliable gel shifts using the IFNB promoter sequences support this observation. To alleviate this problem, we used the ISG15 promoter sequence as a dsDNA probe for our EMSA experiments. ISG15 is the first reported target of IRF3 (41). However, knowing that the ISG15 gene can also respond to secreted IFNs, EMSAs were performed using nuclear extracts from HEC-1B, which do not respond to IFN stimulation (42). Upon infection of these cells, phosphorylated IRF3 translocates into the nucleus and activates the expression of IFN-stimulated genes by binding to the ISRE/IRFE sequence (43). HEC-1B cells were transfected with FLAG-IRF3, pCMV3T, or pCMV3T-LANA-1 vectors that were infected or not with SeV for 8 h. As shown in Fig. 3F, a binding complex on ISG15 promoter (*) was observed in SeV-infected pCMV3T cell extracts (lane 2). In the presence of LANA-1, the complex in SeV-infected cells (Fig. 3F, lane 4) was strongly reduced (35% of lane 2). The specificity of the bound complex was ascertained by homologous competition using excess unlabeled (25×) wild type ISG15 (Fig. 3F, lanes 5–8). Then we tested whether this complex (*) could be supershifted by the addition of anti-FLAG (IRF3) antibodies (Fig. 3F, lanes 9 and 10 and lanes 12 and 13 reduced exposition). A new complex (**) with reduced mobility was detected in pCMV3T-SeV-infected cells (Fig. 3F, lanes 9 and 12), and the presence of LANA-1 (lanes 10 and 13) reduced the formation of this complex (100 to 83%). Using the same extracts, we next tested the capacity of LANA-1 to inhibit IFNB and ISG15 expression in HEC-1B cells (Fig. 3, G and H). The inhibitions of IFNB and ISG15 expression in these cells were weak compared with HEK-293T, a likely consequence of lower transfection efficiencies of HEC-1B cells. Overall, these results are in agreement with those obtained using the DNA-affinity beads assay (Fig. 3B) and suggest that LANA-1 interferes with the fixation of IRF3 to consensus DNA sequences.
Binding of LANA-1 to the IFNB promoter and inhibition of IRF3 binding to the IFNB promoter was further confirmed using a commercially available modified ELISA kit allowing the detection and binding of activated IRF3 to consensus binding sites (Fig. 3I). The primary antibody used to detect interferon regulatory factor recognizes an epitope on IRF3 protein upon DNA binding. Incubations of nuclear extracts from resting and SeV-infected HEK-293T cells transfected with HA-LANA-1 expression vector were carried out, and the results obtained indicate that in the presence of LANA-1 endogenous IRF3 binding is severely diminished (Fig. 3I). As control, transfection of an expression vector (20 μg) encoding for an irrelevant HA-tagged protein had no effect on IRF3 binding (data not shown). Furthermore, by substituting the anti-IRF3 primary antibody for anti-LANA-1, we could demonstrate binding of LANA-1 to the IRF3 consensus binding site (Fig. 3J). LANA-1 binding was not modulated by SeV infection (data not shown). Binding of nuclear extracts from control transfected cells yielded background levels of reactivity (data not shown). Taken together, these results are in agreement with our DNA beads binding assay and indicate that LANA-1 binds and affects the binding of IRF3 to consensus sites and subsequent activation of IRF3-responsive genes, such as IFNB.
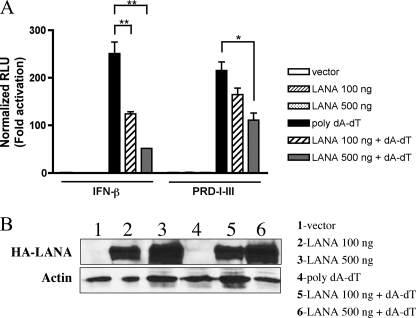
The specificity of LANA-1 for the PRD-I–III element was further confirmed by transfecting HEK-293T cells with expression vector for LANA-1 together with luciferase reporters whose expressions are driven by the IFNB promoter (IFN-β-Luc) or PRD-I–III domain (PRD-I–III-Luc). The IFN-β induction pathway was triggered by transfecting dsDNA (poly(dA-dT)), an IFN inducer relevant to dsDNA viruses such as herpesviruses. Indeed, recent reports indicate that cells possess a DNA-dependent activator of IFN regulatory factors capable of activating innate immune system genes (44, 45). DNA-dependent activator of IFN-regulatory factors recruits TBK1 and IRF3 transcription factor, both of which play critical roles in the induction of type I IFN genes (44). As presented in Fig. 4A, poly(dA-dT) activated both IFNB and PRD-I–III promoters efficiently, whereas LANA-1, by itself, demonstrated no effect on these promoters. A dose-dependent inhibition of both promoters was observed in the presence of increasing amounts of LANA-1. Compared with the PRD-I–III reporter, the IFNB promoter was more severely affected by the presence of LANA-1. Similar results were obtained using SeV infection instead of poly(dA-dT) stimulation (data not shown). PRD-II and PRD-IV promoters were not affected by the presence of LANA-1 (data not shown). Expression of LANA-1 and actin in cell lysates is shown in Fig. 4B confirming that similar quantities of extracts were analyzed.
FIGURE 4.
KSHV LANA-1 inhibits the activation of a reporter gene driven by the IFNB promoter or the PRD-I–III domain. A, HEK-293T cells were transfected with 50 ng of reporter-luciferase (IFN-β-luciferase and PRD-I–III-luciferase) and increasing amounts of pCMV3T-LANA-1 vector (100–500 ng). Twenty four hours later, cells were transfected again or not with poly(dA-dT) as IFN inducer for 24 h. The cells were then lysed in cell lysis buffer and luciferase activity was measured. These values were normalized with the protein concentration determined using BCA protein assay reagent. Results were expressed in fold activation of normalized luciferase units. *, 0.01< p < 0.05; **, 0.001 < p < 0.01 as determined using two-tailed t test. B, Western blot experiments on cell extracts were performed to show expression of LANA-1 (HA) and actin. RLU, relative luciferase units.
Repeated Region of LANA-1 Interferes with the Binding of IRF3 on the IFNB Promoter
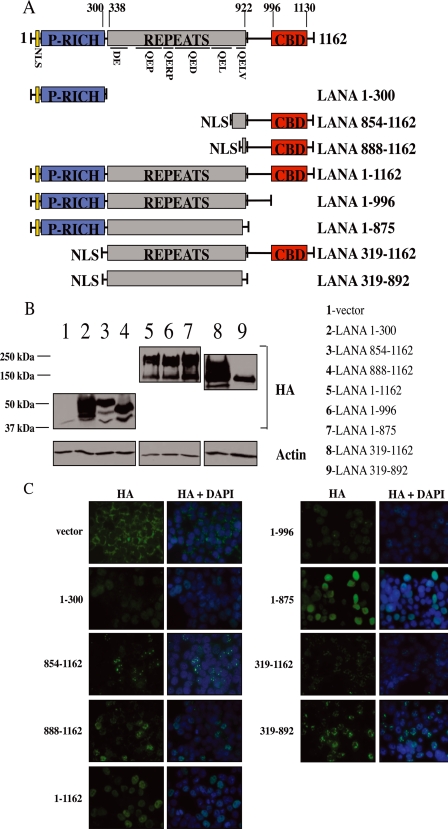
The LANA-1 protein contains three main regions based on amino acid composition as follows: a central region composed of a variable number of highly acidic amino acid repeats, a C-terminal more basic region involved in DNA binding and oligomerization, and an N-terminal region implicated in chromatin attachment and the recruitment of co-repressors (Fig. 5A) (9, 46, 47). To determine which region(s) of LANA-1 is(are) responsible for inhibiting the binding of IRF3 on the IFNB promoter, we generated various deletion mutants of LANA-1 (Fig. 5A). All of these mutants were cloned in-frame with three HA tags at the N terminus. Both the N- and C-terminal regions of the protein contain nuclear localization sequences, but, in accordance with the study of Schwam et al. (9), only mutants containing the N-terminal regions localized in the nucleus suggesting that the C-terminal NLS is nonfunctional in our hands. Consequently, we decided to add NLS at the N-terminal region of these deletion mutants: LANA-1 854–1162, LANA-1 888–1162, LANA-1 319–1162, and LANA-1 319–892. All mutants were sequenced and analyzed for expression by Western blot using anti-HA antibodies (Fig. 5B). We next verified the cellular localization of these mutants, and all were expressed in the nucleus (Fig. 5C). All mutants were expressed uniformly in the nucleus except LANA-1 854–1162, LANA-1 888–1162, and LANA-1 319–1162 that were expressed as nuclear speckles. Schwam et al. (9) described that the deletion of residues 1020–1089 prevents formation of nuclear speckles. Our immunofluorescence results confirm this fact, with the exception that LANA-1 1–1162 (WT) was expressed uniformly in the nucleus in HEK-293T cells. Cell transfected with the pCMV3T (HA) control vector displayed cytoplasmic staining.
FIGURE 5.
A, stick diagram of LANA-1 and the various deletion mutants generated. LANA-1 can be divided into three general regions. The N-terminal portion (aa 1–300) includes an NLS and a Pro-rich domain; the central repeated region (aa 338–922) and the C-terminal region (aa 996–1139) include an NLS and a chromosome-binding domain (aa 996–1130). The repeated region includes a DE repeat (aa 338–421), a QEP repeat (aa 441–551), a QERP repeat (aa 552–594), a QED (aa 595–751), a putative leucine zipper (aa 767–841), and a QELV repeat (aa 861–922). Seven different mutants (1–300, 854–1162, 888–1162, 1–996, 1–875, 319–1162, and 319–892) were generated and compared with LANA-1 WT (1–1162). P-RICH, proline-rich domain; CBD, C-terminal binding domain. B, cellular extracts were made in Laemmli sample buffer from HEK-293T cells transfected with vector, LANA-1 WT, or the seven LANA-1 mutant expression vectors. These cell extracts were analyzed by Western blot using anti-HA (LANA-1) and anti-actin antibodies. C, HEK-293T cells were transfected with the pCMV3T vector, LANA-1 WT or the seven LANA-1 mutant expression vectors and analyzed by immunofluorescence using anti-HA antibodies. Acetone-fixed cells were incubated with anti-HA (LANA-1 WT and mutants) followed by incubation with Alexa 488-conjugated secondary antibody (green). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). The localization of the proteins is presented in the merge pictures (magnification ×60).
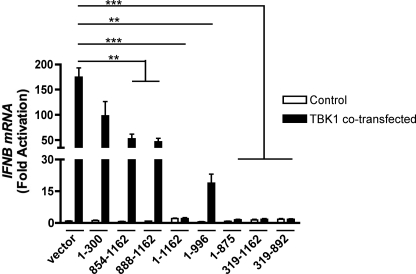
To determine whether these LANA-1 mutants influenced IFNB gene expression, we transfected them into HEK-293T cells alone or in combination with TBK1. Total RNA was extracted, and endogenous IFNB gene expression was monitored by RT-QPCR. As shown in Fig. 6, TBK1 expression induced IFNB gene expression efficiently in the absence of LANA-1. In contrast, in cells expressing LANA-1 1–1162, the activation of the IFNB gene upon TBK1 co-transfection was strongly reduced, confirming the results shown in Fig. 1A. LANA-1 mutants 1–996, 1–875, 319–1162, and 319–892 were equally effective at suppressing the activation of IFNB induced by TBK1. As a control, we performed an experiment using LANA-1 mutant 319–892 cloned into pCMV3T vector. This mutant of LANA-1, which lacks an NLS and does not enter the nucleus, could no longer inhibit IFNB gene induction upon TBK1 expression suggesting an essential role for nuclear localization of LANA-1 (data not shown). LANA-1 mutants 1–300, 854–1162, and 888–1162 displayed weak inhibitory activities toward TBK1-activated IFNB gene expression. These three mutants lack the highly repetitive central domain of LANA-1, suggesting a role for this domain in the inhibition of IFN-β synthesis.
FIGURE 6.
Mapping of LANA-1 domains responsible for IFNB inhibition. HEK-293T cells were co-transfected with pCMV3T vector or expression vectors encoding LANA-1 WT or mutants and TBK1. Forty eight hours post-transfection, total RNA was isolated and processed for IFNB mRNA by RT-QPCR. Results are expressed as mean (triplicate) induction ± S.D. relative to pCMV3T-transfected cells after normalization of samples with GAPDH mRNA expression. Results are representative of three independent experiments. Statistical analysis was done between TBK1-transfected cells and co-transfected with LANA-1 mutants and TBK1. **, 0.001< p < 0.01; ***, p < 0.0001 as determined using two-tailed t test.
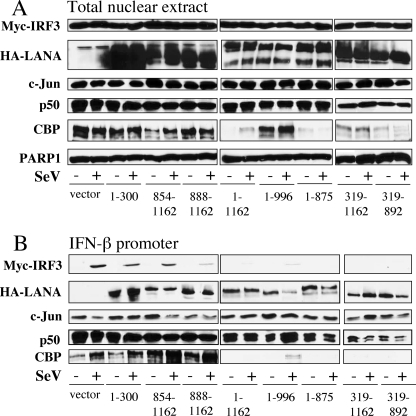
We next tested whether inhibition of IFNB gene expression by the LANA-1 mutants could be correlated with their ability to bind to the PRD-I–III domain. As described above (Fig. 3), nuclear extracts from resting and SeV-infected cells co-transfected with expression vectors of Myc-IRF3, HA-LANA-1 mutants, p50, and CBP were analyzed by Western blot using anti-Myc, anti-HA, anti-c-Jun, anti-p50, anti-CBP, and anti-PARP-1 antibodies to compare nuclear inputs (Fig. 7A). In general, with the exception of LANA-1 expression and CBP, the nuclear inputs were similar between all conditions. CBP was strongly reduced in nuclear extracts in the presence of LANA-1 1–1162 and LANA-1 mutants 1–875, 319–1162, and 319–892. Interestingly, LANA-1 mutant 1–996 did not reduce the expression of CBP. Double-stranded oligonucleotides containing the entire IFNB promoter were incubated with the nuclear extracts, and the proteins bound to this promoter were analyzed by Western blot (Fig. 7B). As shown, all LANA-1 mutants were capable of binding the promoter to some degree, suggesting that multiple domains of LANA-1 can bind the IFNB promoter, and SeV infection did not affect this binding. In response to SeV infection, IRF3 could efficiently attach to the IFNB promoter in pCMV3T nuclear extracts, but this binding varied in the presence of various LANA-1 mutants. LANA-1 1–1162 abolished the fixation of IRF3 to this promoter. LANA-1 mutants 1–996, 1–875, 319–1162, and 319–892 acted like LANA-1 1–1162 and strongly reduced the binding of IRF3 to the promoter. All of these mutants possess the LANA-1 central repeated domain (Fig. 5A) and strongly reduced the IFNB gene induction caused by TBK1 (Fig. 6). The LANA-1 mutants 1–300, 854–1162, and 888–1162 reduced slightly the fixation of IRF3 to the IFNB promoter, but these mutants but were not as effective as LANA-1 1–1162. The binding of c-Jun was not significantly affected by the presence of LANA-1 mutants. The fixation of p50 to the IFNB promoter was minimally affected by the presence of LANA-1 1–1162 and mutants. The binding of CBP to IFNB promoter was affected with LANA-1 1–1162 and LANA-1 mutants 1–996, 1–875, 319–1162, and 319–892. However, considering the varying levels of nuclear CBP, a firm conclusion is difficult to make. These results suggest that the central repeated domain of LANA-1 is responsible for inhibiting the binding of IRF3 to the IFNB promoter. Compared with IRF3, the binding of p50 was only minimally affected. By occupying the IRF3-binding site, LANA-1 prevents the proper assembly of the enhanceosome causing inefficient IFNB gene activation.
FIGURE 7.
Central repeated domain of LANA-1 prevents IRF3 binding to the IFNB promoter. HEK-293T cells were transfected with 20 μg of LANA-1 WT or LANA-1 mutant vectors, Myc-IRF3, p50, and CBP vectors. Twenty four hours later, half of the cells were infected with SeV for 18 h. A, nuclear extracts were made and analyzed by Western blot (5% of input used in the binding reaction). B, nuclear extracts were incubated with biotinylated oligonucleotide containing the entire IFNB promoter sequence, and DNA-bound proteins were isolated using streptavidin beads and resuspended in Laemmli buffer. These samples were resolved by SDS-PAGE and analyzed by Western blot. Protein analyses were carried out for the detection of IRF3 (Myc), LANA-1 WT, or mutants (HA), c-Jun, p50, CBP, and PARP-1 using specific antibodies.
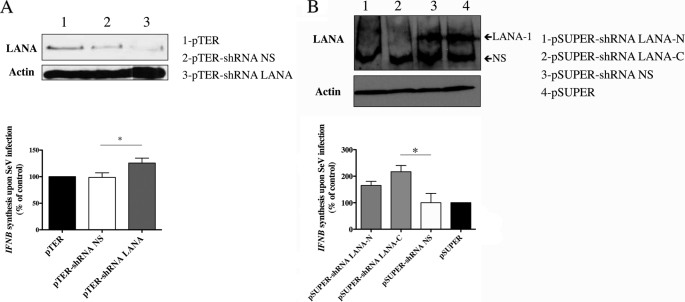
We next attempted to demonstrate the IFN inhibitory role of endogenous LANA-1 in KSHV-infected cells. To do so, we knocked down LANA-1 expression in HEK-293T-E1 cells carrying a recombinant KSHV (20). HEK-293T-E1 cells were transfected with a pTER vector encoding an shRNA against LANA-1 (pTER-shRNA LANA-1) or a nonspecific shRNA (pTER-shRNA NS) (Fig. 8A, top). To confirm these results, two additional LANA-1 shRNAs (pSUPER-shRNA LANA-1-C and -N) and nonspecific shRNA (pSUPER-shRNA NS) were also used (Fig. 8B, top). All these shRNAs, were able to reduce the expression of LANA-1. The control HEK-293T-E1 cells and those with reduced LANA-1 expression were then compared for IFNB gene expression following SeV infection. As shown in Fig. 8A (bottom), cells expressing shRNA LANA-1 (pTER-shRNA LANA-1) had significantly higher IFNB mRNA levels upon SeV infection than vector (pTER) or shRNA NS (pTER-shRNA NS), suggesting that when LANA-1 levels are reduced, IFNB gene expression in response to SeV occurs more efficiently. Similar results were obtained using the second shRNAs LANA-1 (Fig. 8B, bottom). It is noteworthy that HEK-293T-E1 cells transfected by pSUPER-shRNA LANA-1-C expressed 2.63 times (263%) more IFNB mRNA compared with cells expressing the pSUPER control vector (100%). This shRNA is very efficient at reducing expression of LANA-1 as observed in Fig. 8B (top). HEK-293T-E1 cells transfected by pSUPER-shRNA LANA-1-N expressed also increased levels of the IFNB gene, but the results obtained did not reach statistical significance. This could be explained, at least in part, by the weaker reduction in LANA-1 expression observed in Fig. 8B (top). These results support an IFN-β inhibitory role for LANA-1 in the context of KSHV-infected cells.
FIGURE 8.
Knockdown of LANA-1 enhances IFNB gene activation in KSHV-infected cells. A, HEK-293T-E1 were transfected with 500 ng of shRNA against LANA-1 (pTER-shRNA LANA-1) or nonspecific shRNA (pTER-shRNA NS). B, HEK-293T-E1 were transfected with 500 ng of two other shRNAs against LANA-1 (pSUPER-shRNA LANA-1-N and pSUPER-shRNA LANA-1-C) or nonspecific shRNA (pSUPER-shRNA NS). A and B (top), 48 h later, cells were lysed and boiled in Laemmli sample buffer and then resolved by SDS-PAGE. Western blots were carried out for the detection of LANA-1 and actin using specific antibodies. A and B (bottom), 48 h post-transfection, cells were infected with SeV (10 HAU) for 18 h, and total RNA was isolated and processed for IFNB mRNA by RT-QPCR. Results are expressed as mean (triplicate) induction ± S.D. relative to pTER or pSUPER-transfected cells after normalization of samples with GAPDH mRNA expression and are representative of at least three independent experiments. *, p < 0.05 as determined using two-tailed t test between cells expressing shRNA LANA-1 and cells expressing nonspecific shRNA.
DISCUSSION
KSHV deploys several mechanisms to evade the effects of interferon, including four vIRFs sharing homologies with cellular IRFs (46, 48), expression of ORF45 (49), RTA (49), K-bZIP (33), v-FLIP (50), and RIF (encoded by orf10) (51) (for reviews see Refs. 52, 53). The large number of strategies used by KSHV to inhibit interferon synthesis suggests that this antiviral pathway is detrimental to KSHV persistence within the infected host. Lytic genes encode most proteins reported to play a role in the modulation of this pathway, although vIRF1 and vIRF3 have also been detected during latency (54–56). We report here that the major latency-associated protein of KSHV, LANA-1, effectively shunts the interferon pathway. We first demonstrated that LANA-1 is able to repress IFNB gene expression triggered by single strand RNA (SeV), dsDNA (poly(dA-dT)), overexpression of TBK1, or constitutively active IRF3 (IRF3–5D). We used poly(dA-dT) to mimic the dsDNA KSHV genome during the infection or during the reactivation of the virus. Inhibition of IFNB gene expression was correlated with a reduction in IFN-β protein secretion. The result obtained using IRF3–5D, which does not rely on kinases for its activation, suggested that inhibition of IFNB gene activation by LANA-1 is not a consequence of improper phosphorylation of IRF3. There results were confirmed in A549 cells in which the presence of LANA-1 did not affect the phosphorylation of IRF3 induced by SeV infection. We also identified that binding of LANA-1 to the PRD-I–III region of the IFNB promoter affected the binding of IRF3 to this promoter. As described previously (5), LANA-1 inhibited the transcription of CBP. Consequently, the reduced levels of CBP caused reduced binding of CBP to the enhanceosome complex causing aberrant IFNB promoter activation. The fact that IRF3 and CBP were not able to bind the IFNB promoter would prevent the assembly and formation of the enhanceosome resulting in inhibition of IFNB gene transcription. These results were confirmed using three different methods as follows: the DNA affinity binding assay, EMSA, and a modified ELISA. Results obtained using DNA-affinity beads assay suggest that in the absence of LANA-1 and upon SeV infection, multiple transcription factors such as IRF3, p50, ATF-2, c-Jun, and CBP bind to the IFNB promoter, in agreement with previously published results (39). In contrast, in LANA-1-expressing and SeV-infected cell extracts, IRF3 is not bound to the IFNB promoter. Similarly, EMSA results reveal that SeV infection induced the formation of a slower migrating complex (complex * in Fig. 3F) compared with uninfected cell extracts. These results indicate that a nuclear complex binds to the promoter sequence upon SeV infection. This complex is much reduced in LANA-1-SeV-infected cells. We confirmed that IRF3 was part of the complex using antibodies (supershift complex ** in Fig. 3F). The reduction of the supershifted complex (** in Fig. 3F) is smaller than the one obtained in SeV induced complex (* in Fig. 3F) suggesting that LANA-1 might affect the fixation of additional transcriptional factors on this promoter. Finally, impaired fixation of IRF3 in the presence of LANA-1 was confirmed using a modified ELISA that monitors the binding of active IRF3. Furthermore, this assay allowed us to confirm the binding of LANA-1 to the promoter.
To determine which region(s) of LANA-1 is(are) involved in the inhibition of IFNB gene activation, a series of LANA-1 deletion mutants was tested. We have identified that the central repeated region of LANA-1 is responsible for IFNB gene inhibition by preventing IRF3 from binding to the IFNB promoter. We also observed that the transcription of CBP is diminished in nuclear extracts in LANA-1 1–1162 and mutants 1–875, 319–1162, and 319–892 expressing cells. The C terminus (aa 950–1162) and the repeated regions (aa 340–431) of LANA-1 were described to have an inhibitory effect on the transcription of CBP (5). Lim et al. (5) suggest that LANA-1 inhibits the transcriptional activity and the in vitro histone acetyltransferase activity of CBP, suggesting that LANA-1 modulates the global transcriptional activities of infected cells through its interaction with CBP. In this study, we suggest that the central acidic region of LANA-1 is responsible for the inhibition of IFNB gene expression. This is not the first report to give importance to this region. The central acidic region of LANA-1 also abolishes the antigen presentation (57), and most LANA-1-protein interactions (pRb, ATF/CREB, and CBP) have been mapped to the central region containing the leucine-zipper motif (5, 8, 58). These reports and our studies support a role for the repeated domain of LANA-1 in IFNB synthesis inhibition.
Our results indicate that LANA-1 competes with IRF3 for binding to the IFNB promoter. Other KSHV proteins also target IRF3 to inhibit IFNB synthesis. vIRF1 was described to associate with p300 (CBP) and interfere with p300-IRF3 binding on the promoter (59); vIRF2 was described to inhibit the signaling induced by IRF3 (60) and negatively regulate IRF3 stability (52), and K-bZIP was described to suppress the IRF3-driven IFNB gene transactivation by binding to the IFNB promoter (33). As mentioned, all these proteins are expressed during the lytic replication cycle, except for vIRF1 that has also been detected in latently infected cells. Such proteins likely interfere with IFN induction and contribute to the rather modest, but significant, increase in IFNB gene expression observed following LANA-1 knockdown in HEK-293T-E1 cells using two different shRNAs against LANA-1. As discussed by Coscoy (53), KSHV encodes many gene products that affect IFNB synthesis with the possibility that these proteins act at different stages of the viral cycle with some proteins having a role during the lytic replication cycle, although others act during latency. The fact that latency-associated gene products, which are expressed during both the latent and lytic cycles (35, 61), can also inhibit IFNB gene activation, enables KSHV to be protected at all times against IFN effects. The LANA-1 promoter shows characteristics of both immediate-early and latent genes, arguing in favor of a fundamental role for this latency protein in HHV-8 pathogenesis and tumorigenesis (61). Latency genes capable of inhibiting IFNB synthesis may represent the first line of protection against innate immune reactions allowing for the successful establishment of viral persistence.
In a previous study, we reported that another KSHV latent protein, v-FLIP, acts in synergy with classic IFN activators (MAVS, TBK1, and IRF3) in promoting IFNB gene transcription (50). This effect was strictly dependent on NF-κB and is mediated through the PRD-II of the IFNB promoter. During infection, LANA-1, v-cyclin, and v-FLIP are simultaneously transcribed from the same promoter on polycistronic or bicistronic (v-cyclin and v-FLIP) transcripts (61). Our results suggest that LANA-1 and v-FLIP may have opposing effects in IFNB gene modulation. Preliminary results suggest that when LANA-1 and v-FLIP are co-expressed, the synthesis of biologically active IFN-β protein is reduced suggesting that the LANA-1 effects are dominant. The LANA-1 promoter could also achieve a balancing effect between these two proteins through the regulation of the polycistronic/bicistronic transcripts. A cellular model of latency-infected cells using knock-out virus or small interfering RNA will help us to understand more the role of LANA-1 in the synthesis of IFNB. When available, the use of an animal model will be essential to further our understanding of the interplay between KSHV and the type I IFN signaling pathway.
Supplementary Material
Acknowledgments
We thank Didier Trouche, John Hiscott, Rongtuan Lin, Tom Maniatis, Shou-Jiang Gao, Nathalie Grandvaux, and Diane Hayward for generously providing plasmids, antibodies, or cell line.
This work was supported in part by a grant from the National Cancer Institute of Canada (to L. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1.
- KSHV
- Kaposi sarcoma-associated herpesvirus
- CBP
- CREB-binding protein
- CREB
- cAMP-response element-binding protein
- IFN
- interferon
- LANA-1
- latency-associated nuclear antigen
- NF-κB
- nuclear factor-κB
- NLS
- nuclear localization signal
- PRD
- positive-regulatory domain
- SeV
- Sendai virus
- TBK1
- TANK-binding kinase 1
- HAU
- hemagglutination unit
- shRNA
- short hairpin RNA
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- dsDNA
- double strand DNA
- HA
- hemagglutinin
- aa
- amino acid
- EMSA
- electromobility shift assay
- ELISA
- enzyme-linked immunosorbent assay
- DTT
- dithiothreitol
- QPCR
- quantitative PCR
- RT
- reverse transcription
- WT
- wild type
- PARP
- poly(ADP-ribose) polymerase.
REFERENCES
- 1.Cesarman E., Chang Y., Moore P. S., Said J. W., Knowles D. M. (1995) N. Engl. J. Med. 332, 1186–1191 [DOI] [PubMed] [Google Scholar]
- 2.Zhong W., Wang H., Herndier B., Ganem D. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 6641–6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballestas M. E., Chatis P. A., Kaye K. M. (1999) Science 284, 641–644 [DOI] [PubMed] [Google Scholar]
- 4.Krithivas A., Young D. B., Liao G., Greene D., Hayward S. D. (2000) J. Virol. 74, 9637–9645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim C., Gwack Y., Hwang S., Kim S., Choe J. (2001) J. Biol. Chem. 276, 31016–31022 [DOI] [PubMed] [Google Scholar]
- 6.Platt G. M., Simpson G. R., Mittnacht S., Schulz T. F. (1999) J. Virol. 73, 9789–9795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J., Martin H., Shamay M., Woodard C., Tang Q. Q., Hayward S. D. (2007) J. Virol. 81, 4722–4731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim C., Sohn H., Gwack Y., Choe J. (2000) J. Gen. Virol. 81, 2645–2652 [DOI] [PubMed] [Google Scholar]
- 9.Schwam D. R., Luciano R. L., Mahajan S. S., Wong L., Wilson A. C. (2000) J. Virol. 74, 8532–8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friborg J., Jr., Kong W., Hottiger M. O., Nabel G. J. (1999) Nature 402, 889–894 [DOI] [PubMed] [Google Scholar]
- 11.Bubman D., Guasparri I., Cesarman E. (2007) Oncogene 26, 4979–4986 [DOI] [PubMed] [Google Scholar]
- 12.Jeong J. H., Orvis J., Kim J. W., McMurtrey C. P., Renne R., Dittmer D. P. (2004) J. Biol. Chem. 279, 16822–16831 [DOI] [PubMed] [Google Scholar]
- 13.Lan K., Kuppers D. A., Verma S. C., Robertson E. S. (2004) J. Virol. 78, 6585–6594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malmgaard L. (2004) J. Interferon Cytokine Res. 24, 439–454 [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen S. B., Jensen S. B., Nielsen C., Quartin E., Kato H., Chen Z. J., Silverman R. H., Akira S., Paludan S. R. (2009) J. Gen. Virol. 90, 74–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai T., Akira S. (2006) Nat. Immunol. 7, 131–137 [DOI] [PubMed] [Google Scholar]
- 17.Naranatt P. P., Krishnan H. H., Svojanovsky S. R., Bloomer C., Mathur S., Chandran B. (2004) Cancer Res. 64, 72–84 [DOI] [PubMed] [Google Scholar]
- 18.Perry S. T., Compton T. (2006) J. Virol. 80, 11105–11114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuse A., Ashino-Fuse H., Kuwata T. (1984) Gann 75, 379–384 [PubMed] [Google Scholar]
- 20.Zhou F. C., Zhang Y. J., Deng J. H., Wang X. P., Pan H. Y., Hettler E., Gao S. J. (2002) J. Virol. 76, 6185–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzgerald K. A., McWhirter S. M., Faia K. L., Rowe D. C., Latz E., Golenbock D. T., Coyle A. J., Liao S. M., Maniatis T. (2003) Nat. Immunol. 4, 491–496 [DOI] [PubMed] [Google Scholar]
- 22.Lin R., Yang L., Nakhaei P., Sun Q., Sharif-Askari E., Julkunen I., Hiscott J. (2006) J. Biol. Chem. 281, 2095–2103 [DOI] [PubMed] [Google Scholar]
- 23.Sharma S., tenOever B. R., Grandvaux N., Zhou G. P., Lin R., Hiscott J. (2003) Science 300, 1148–1151 [DOI] [PubMed] [Google Scholar]
- 24.Duckett C. S., Perkins N. D., Kowalik T. F., Schmid R. M., Huang E. S., Baldwin A. S., Jr., Nabel G. J. (1993) Mol. Cell. Biol. 13, 1315–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez S., Ait-Si-Ali S., Robin P., Trouche D., Harel-Bellan A., Ait Si Ali S. (1997) J. Biol. Chem. 272, 31016–31021 [DOI] [PubMed] [Google Scholar]
- 26.Godfrey A., Anderson J., Papanastasiou A., Takeuchi Y., Boshoff C. (2005) Blood 105, 2510–2518 [DOI] [PubMed] [Google Scholar]
- 27.van de Wetering M., Oving I., Muncan V., Pon Fong M. T., Brantjes H., van Leenen D., Holstege F. C., Brummelkamp T. R., Agami R., Clevers H. (2003) EMBO Rep. 4, 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefort S., Flamand L. (2009) J. Virol. 83, 5869–5880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujimuro M., Wu F. Y., ApRhys C., Kajumbula H., Young D. B., Hayward G. S., Hayward S. D. (2003) Nat. Med. 9, 300–306 [DOI] [PubMed] [Google Scholar]
- 30.Saitoh T., Tun-Kyi A., Ryo A., Yamamoto M., Finn G., Fujita T., Akira S., Yamamoto N., Lu K. P., Yamaoka S. (2006) Nat. Immunol. 7, 598–605 [DOI] [PubMed] [Google Scholar]
- 31.Lamarre D., Talbot B., de Murcia G., Laplante C., Leduc Y., Mazen A., Poirier G. G. (1988) Biochim. Biophys. Acta 950, 147–160 [DOI] [PubMed] [Google Scholar]
- 32.Severa M., Coccia E. M., Fitzgerald K. A. (2006) J. Biol. Chem. 281, 26188–26195 [DOI] [PubMed] [Google Scholar]
- 33.Lefort S., Soucy-Faulkner A., Grandvaux N., Flamand L. (2007) J. Virol. 81, 10950–10960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wathelet M. G., Lin C. H., Parekh B. S., Ronco L. V., Howley P. M., Maniatis T. (1998) Mol. Cell 1, 507–518 [DOI] [PubMed] [Google Scholar]
- 35.Cloutier N., Gravel A., Flamand L. (2004) J. Virol. Methods 122, 1–7 [DOI] [PubMed] [Google Scholar]
- 36.Dittmer D., Lagunoff M., Renne R., Staskus K., Haase A., Ganem D. (1998) J. Virol. 72, 8309–8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiscott J., Grandvaux N., Sharma S., Tenoever B. R., Servant M. J., Lin R. (2003) Ann. N.Y. Acad. Sci. 1010, 237–248 [DOI] [PubMed] [Google Scholar]
- 38.Yoneyama M., Suhara W., Fujita T. (2002) J. Interferon Cytokine Res. 22, 73–76 [DOI] [PubMed] [Google Scholar]
- 39.Maniatis T., Falvo J. V., Kim T. H., Kim T. K., Lin C. H., Parekh B. S., Wathelet M. G. (1998) Cold Spring Harbor Symp. Quant. Biol. 63, 609–620 [DOI] [PubMed] [Google Scholar]
- 40.Panne D., Maniatis T., Harrison S. C. (2007) Cell 129, 1111–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Au W. C., Moore P. A., Lowther W., Juang Y. T., Pitha P. M. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 11657–11661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wathelet M. G., Clauss I. M., Content J., Huez G. A. (1988) Eur. J. Biochem. 174, 323–329 [DOI] [PubMed] [Google Scholar]
- 43.Hiscott J., Pitha P., Genin P., Nguyen H., Heylbroeck C., Mamane Y., Algarte M., Lin R. (1999) J. Interferon Cytokine Res. 19, 1–13 [DOI] [PubMed] [Google Scholar]
- 44.Takaoka A., Wang Z., Choi M. K., Yanai H., Negishi H., Ban T., Lu Y., Miyagishi M., Kodama T., Honda K., Ohba Y., Taniguchi T. (2007) Nature 448, 501–505 [DOI] [PubMed] [Google Scholar]
- 45.Stetson D. B., Medzhitov R. (2006) Immunity 24, 93–103 [DOI] [PubMed] [Google Scholar]
- 46.Russo J. J., Bohenzky R. A., Chien M. C., Chen J., Yan M., Maddalena D., Parry J. P., Peruzzi D., Edelman I. S., Chang Y., Moore P. S. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 14862–14867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganem D. (2006) Annu. Rev. Pathol. 1, 273–296 [DOI] [PubMed] [Google Scholar]
- 48.Rezaee S. A., Cunningham C., Davison A. J., Blackbourn D. J. (2006) J. Gen. Virol. 87, 1781–1804 [DOI] [PubMed] [Google Scholar]
- 49.Zhu F. X., King S. M., Smith E. J., Levy D. E., Yuan Y. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 5573–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cloutier N., Grandvaux N., Flamand L. (2007) Eur. J. Immunol. 37, 2772–2778 [DOI] [PubMed] [Google Scholar]
- 51.Bisson S. A., Page A. L., Ganem D. (2009) J. Virol. 83, 5056–5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aresté C., Blackbourn D. J. (2009) Trends Microbiol. 17, 119–129 [DOI] [PubMed] [Google Scholar]
- 53.Coscoy L. (2007) Nat. Rev. Immunol. 7, 391–401 [DOI] [PubMed] [Google Scholar]
- 54.Parravicini C., Chandran B., Corbellino M., Berti E., Paulli M., Moore P. S., Chang Y. (2000) Am. J. Pathol. 156, 743–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fakhari F. D., Dittmer D. P. (2002) J. Virol. 76, 6213–6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rivas C., Thlick A. E., Parravicini C., Moore P. S., Chang Y. (2001) J. Virol. 75, 429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaldumbide A., Ossevoort M., Wiertz E. J., Hoeben R. C. (2007) Mol. Immunol. 44, 1352–1360 [DOI] [PubMed] [Google Scholar]
- 58.Radkov S. A., Kellam P., Boshoff C. (2000) Nat. Med. 6, 1121–1127 [DOI] [PubMed] [Google Scholar]
- 59.Lin R., Genin P., Mamane Y., Sgarbanti M., Battistini A., Harrington W. J., Jr., Barber G. N., Hiscott J. (2001) Oncogene 20, 800–811 [DOI] [PubMed] [Google Scholar]
- 60.Fuld S., Cunningham C., Klucher K., Davison A. J., Blackbourn D. J. (2006) J. Virol. 80, 3092–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Talbot S. J., Weiss R. A., Kellam P., Boshoff C. (1999) Virology 257, 84–94 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.